Background: The essentiality of an antioxidant enzyme for the development and maturation of photoreceptor cells has remained unclear.
Results: While GPx4-abrogated photoreceptor cells develop and differentiate into rod and cone cells, their outer segments are structurally disorganized and they undergo rapid apoptosis in vivo.
Conclusion: GPx4 is essential for the maturation of photoreceptor cells.
Significance: A novel role of an antioxidant enzyme for photoreceptor cells is disclosed in this study.
Keywords: Antioxidants, Oxidative Stress, Photoreceptors, Retina, Retinal Degeneration
Abstract
Oxidative stress is implicated in the pathologies of photoreceptor cells, and the protective role of antioxidant enzymes for photoreceptor cells have been well understood. However, their essentiality has remained unknown. In this study we generated photoreceptor-specific conditional knock-out (CKO) mice of glutathione peroxidase 4 (GPx4) and showed the critical role of GPx4 for photoreceptor cells. In the wild-type retina the dominant GPx4 expression was in the mitochondria, indicating the mitochondrial variant was the major GPx4 in the retina. In the GPx4-CKO mice, although photoreceptor cells developed and differentiated into rod and cone cells by P12, they rapidly underwent drastic degeneration and completely disappeared by P21. The photoreceptor cell death in the GPx4-CKO mice was associated with the nuclear translocation of apoptosis-inducing factor (AIF) and TUNEL-positive cells. Photoreceptor cells before undergoing apoptosis (P11) exhibited decreased mitochondrial biomass, decreased number of connecting cilia, as well as disorganized structure of outer segments. These findings indicate that GPx4 is a critical antioxidant enzyme for the maturation and survival of photoreceptor cells.
Introduction
Oxidative stress and antioxidant enzymes have been an intensively discussed topic in the pathologies of photoreceptor cells. Especially in inherited retinal degenerations that have been untreatable and a leading cause of blindness worldwide for humans, a number of studies have indicated the involvement of oxidative stress in the progression of retinal degenerations and the potential of antioxidant therapy. First, oxidative stress including photo-oxidative stress (1–3), paraquate, and hyperoxia (4, 5), can induce retinal degeneration and has been used as common methods to induce retinal degeneration in animal models. Second, the accumulation of peroxidized lipids has been observed in the animal models of retinal degenerations (6–8). Third, enhancing antioxidant enzymes, including thioredoxin supplementation (1), forced expressions of glutathione peroxidase 4 (GPx4)2 (5), and catalase and superoxide dismutase 2 (9), and treatment with antioxidant nutrients (10) can ameliorate retinal degenerations in mouse models.
Therefore, the protective role of antioxidant enzymes for photoreceptor cells has been well established. However, their essentiality has remained unclear. Although several studies have reported the knock-out mice of antioxidant enzymes, they have not shown severe photoreceptor cell abnormality or degeneration (11, 12). This is probably because multiple antioxidant enzymes share target substrates; thus compensating for each other.
GPx4 is a ubiquitously expressed selenoprotein that directly reduces peroxidized phospholipids produced in cell membranes. In contrast to other GPx isoforms, GPx4 reduces complex lipid hydroperoxides, even if they are incorporated in biomembranes or lipoproteins (13). GPx4 consists of three splicing variants that have different subcellular localization; cytosolic, mitochondrial, and nucleolar GPx4 (14). A knock-out of GPx4 gene in mice led to embryonic lethality at around P8 (15). A knock-out of mitochondrial GPx4 (16) and spermatocyte-specific depletion of GPx4 (17) showed mitochondrial dysfunction and structural abnormality in spermatozoa, which led to male infertility. Neuron-specific ablation of GPx4 has been reported to show partial degeneration although the phenotype was milder than that seen in mice whose neuronal selenoproteins were totally abrogated (18). The aim of the present study was to elucidate the essentiality of GPx4 for photoreceptor cells.
EXPERIMENTAL PROCEDURES
Animal Care
All procedures were in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Research Committee of the University of Tokyo. Mice were housed in a temperature-controlled room with fresh water and fed a rodent-specific diet. Mice were kept in a usual 12 h light/dark cycle at 22 °C until P7. From P7 to P21, mice were kept in the dark with feeding mothers to rule out the effect of photo-oxidative stress on the retina.
Generation of Mice with Photoreceptor-specific Conditional Knock-out (CKO) of GPx4
To examine whether GPx4 is essential for photoreceptor cells, mice with photoreceptor-specific CKO of GPx4 were generated by crossing Crx-Cre transgenic mice (19) and loxP/GPx4 mice (17, 20). Because the Crx-Cre transgenic mice exhibit Cre-transgene expression beginning in the outer neuroblastic layer of the embryo where the precursors of photoreceptor cells are located (19), the resultant observations of the CKO mouse retina in this study were due to GPx4 deficit from an early developmental stage. As for the floxed mice, we used the previously established loxP/GPx4 mice which were loxP-GPx4 transgene-rescued GPx4-knock-out mice (17, 20). Therefore, in this study, GPx4−/−: Tg(loxP-GPx4): Crx-Cre+ mice served as the CKO mice, and their littermates, i.e. GPx4−/−: Tg(loxP-GPx4): Crx-Cre− mice, served as the control mice.
Immunohistochemistry and Terminal Deoxynucleotidyl Transferase dUTP Nick-end Labeling (TUNEL) Assay
Enucleated eyeballs were fixed in 4% paraformaldehyde in phosphate-buffered saline. The samples were paraffin-embedded and 5-μm thick sections were cut. Slides were first incubated with blocking solution (2% normal goat serum) overnight, and then with primary antibodies at room temperature for 2 h and with secondary antibodies for 1 h. The sections were then coverslipped with mounting medium.
For immunostaining, the primary antibodies used were mouse monoclonal antibodies specific to rhodopsin (Santa Cruz Biotechnology, Santa Cruz, CA), acrolein (NOF Corporation, Tokyo, Japan), superoxide dismutase 1 (SOD1) (Assay Designs, Ann Arbor, MI), SOD2 (Assay Designs, Ann Arbor, MI), prohibitin (Santa Cruz Biotechnology) and GPx4 (21). Alexa Fluor 488 and 594 (1:400)-conjugated secondary antibodies (Sigma-Aldrich) were used. 4,6-Diamidino-2-phenylindole (DAPI; Sigma-Aldrich) was used for staining nuclei. Fluorescent conjugates of peanut agglutinin lectin (PNA) (Molecular Probes, Invitrogen, Carlsbad, CA, 25 μg/ml) were used to label cone cells.
DNA strand breaks were labeled with digoxigenin-deoxyuridine triphosphate using terminal deoxynucleotidyl transferase (0.18 units/μl) according to the manufacturer's protocol (DeadEnd Fluorometric TUNEL System, Promega). Immunohistochemical slides were observed under a fluorescent microscope (BZ-9000, Keyence, Osaka, Japan) or a confocal microscope (LSM510, Carl Zeiss, Oberkochen, Germany).
Transmission Electron Microscopy (TEM)
For TEM, mouse eyes were processed for histology, and transverse sections, 70 nm in thickness, were cut through the central retina and mounted onto grids. The sections were stained with lead citrate and uranyl acetate. The images were obtained on a transmission electron microscope (80 kV, model JEM-1200EX; JEOL Ltd., Tokyo, Japan) via a CCD digital camera (model VELETA; JEOL Ltd., Tokyo, Japan).
Quantification of mRNA and Mitochondrial DNA by Real-time PCR
RNA from homogenized samples of the retina was extracted using TRIzol reagent (Invitrogen). RNA was reverse-transcribed (RT) using Superscript III for RT-PCR (Invitrogen) to make cDNA. PCR was carried out by LightCycler 480 Sybr Green (Roche). Values for each gene were normalized to expression levels of GAPDH. The sequences of the primers used in the real-time RT-PCR for cDNA samples were as follows: mouse GAPDH (Fwd: 5′-CACATTGGGGGTAGGAACAC-3′ and Rev: 5′-AACTTTGGCATTGTGGAAGG-3′), mitochondrial transcription factor A (Tfam) (Fwd: CCAAAAAGACCTCGTTCAGC 5′-CTTCAGCCATCTGCTCTTCC-3′ and Rev: 5′-AACTTTGGCATTGTGGAAGG-3′).
Relative amounts of nuclear and mitochondrial DNA were determined by GAPDH and cytochrome b, respectively, and the ratio of mitochondrial DNA to nuclear DNA reflects the tissue concentration of mitochondrial biomass. Samples used in this experiment were the whole retina, not only photoreceptor cells. DNA of the retina from the CKO and control mice were isolated using Wizard® SV Genomic DNA Purification System (Promega). The primer sequences used in the real-time PCR for DNA samples were as follows: cytochrome b (Fwd: 5′-TATTCCTTCATGTCGGACGA-3′ and Rev: 5′-AAATGCTGTGGCTATGACTG-3′), and GAPDH (Fwd: 5′- CAAGGTCATCCATGACAACTTTG-3′ and Rev: 5′- ACCACAGTCCATGCCATCACTGCCA-3′).
Western Blotting
After making eye-cups, the retina and RPE/choroid were dissected. The cerebral hemispheres were collected as brain samples. Samples were homogenized in mild RIPA buffer containing a protease inhibitor mixture (Roche). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electroblotted to nitrocellulose membranes using a semi-dry transfer cell (Bio-Rad). Membranes were blocked with 5% milk in Tris-buffered saline/Tween 20 buffer. The aforementioned anti-GPx4 antibody and anti- β-actin mouse monoclonal antibody (Sigma-Aldrich) were used as primary antibodies. Blots were next incubated with horseradish peroxidase-conjugated secondary antibody (GE Healthcare) for 1 h. Bound antibodies were detected by ECL plus Western blotting Detection System (GE Healthcare) and visualized by a luminescent image analyzer (Fujifilm Corporation, Tokyo, Japan).
Statistics
All statistical analyses were carried out by the JMP 9 software (SAS Institute Inc.). For comparison between unpaired 2 groups, two-tailed t test was used. For comparison among 3 or more groups, one-way analysis of variance (ANOVA) was conducted, followed by Tukey's post hoc test. p < 0.05 meant statistical significance.
RESULTS
GPx4 Protein Expression in Wild-type (WT) Mouse Retina
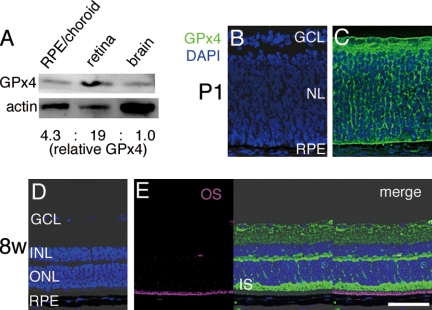
First, we examined the expression of GPx4 protein in the WT mouse retina by Western blotting. GPx4 was expressed in the retina at relatively high concentration in comparison with the brain, the other central nerves organ, and retinal pigment epithelium, the adjacent tissue of the retina (Fig. 1A) in adult mice. The physiological distribution of GPx4 protein expression in the WT retina was evaluated by immunuhistochemistry (Fig. 1, B–E). GPx4 protein was expressed abundantly in the retina both at developing stage (P1) and mature stage (8-week-old), especially in the inner segments (IS) of photoreceptor cells in adult retina. In contrast, in the outer segments (OS) of photoreceptor cells or in the nucleus, there was not detectable GPx4 expression. GPx4 has three distinct splicing variants; mitochondrial, cytosolic, and nucleolar. Because IS of photoreceptor cells is the area where mitochondria localizes while there are no mitochondria in OS, we speculated that the majority of GPx4 in the retina might be the mitochondrial variant of GPx4. First we confirmed that SOD1, a major antioxidant enzyme in cytosol, was expressed more abundantly in OS than in IS (Fig. 2A) and that SOD2, a major antioxidant enzyme in mitochondria, was expressed abundantly in IS, but not in OS (Fig. 2B). Furthermore, we confirmed co-localization of GPx4 and prohibitin, a mitochondrial marker, in the WT retina on confocal immunohistochemistry (Fig. 2, C–E). In contrast, in the retinal pigment epithelium majority of GPx4 and prohibitin did not co-localize (Fig. 2F).
FIGURE 1.
GPx4 expression in the wild-type mouse retina. A, lysates of the retina, retinal pigment epithelium (RPE)/choroid, and brain were immunoblotted for GPx4 and β-actin. GPx4 protein level in the retina was higher than that in RPE/choroid and brain. B–E, immunohistochemistry of the retina for GPx4 (green) at P1 and 8-week-old mice. GPx4 was abundantly expressed in the retina, especially in the inner segments (IS) of photoreceptor cells while there was no detectable expression of GPx4 protein in the outer segments (OS) depicted by autofluorescence. B and D, negative controls. GCL (ganglion cell layer), INL (inner nuclear layer), ONL (outer nuclear layer), NL (neuroblastic layer). Scale bar, 50 μm.
FIGURE 2.
Mitochondrial GPx4 is the major variant of GPx4 in the retina. A, SOD1, a cytosolic antioxidant enzymes was expressed more dominantly in OS than in IS. B, SOD2, a mitochondrial antioxidant enzyme was expressed in IS, not in OS. C–E, GPx4 and prohibitin, a mitochondrial marker, co-localized in the retina. F, in contrast, in RPE GPx4 and prohibitin did not co-localize. Scale bar, 50 μm.
Lipid Peroxidation in the Retina of Photoreceptor-specific CKO Mice of GPx4
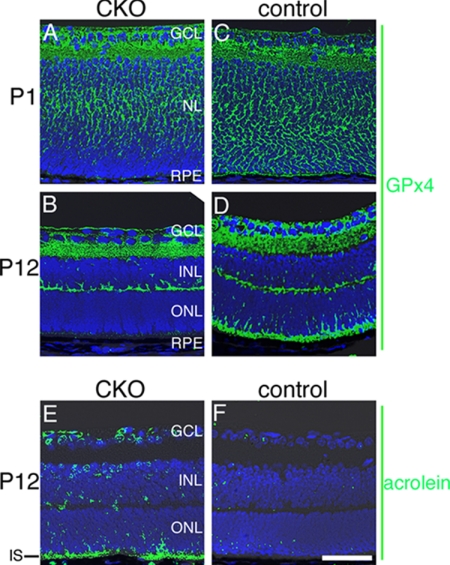
The adequacy of the generated CKO and control littermates was evaluated by immunohistochemistry. At P1 when photoreceptor cells were still at a precursor stage in the neuroblastic layer, GPx4 expression was already diminished in the outer area of the neuroblastic layer (Fig. 3, A versus C). This is consistent with the previous report of early Cre expression in the Crx-Cre system (19). At P12 when photoreceptor cells have developed and formed the outer nuclear layer (ONL), GPx4 was completely abrogated in photoreceptor cells (Fig. 3, B versus D). In other layers of the retina GPx4 expression was similar between CKO and control mice. These results confirmed the adequacy of the CKO and control mice in this study.
FIGURE 3.
Generation of the photoreceptor-specific CKO mice of GPx4. A–D, immunohistochemistry of the retina for GPx4. A, at P1 the CKO mouse did not express GPx4 in the outer area of NL. B, at P12 photoreceptor cells of the CKO mice comprised ONL, but GPx4 was not expressed. C and D, the control mice expressed GPx4 in photoreceptor cells both at P1 and P12. E and F, consistent with the deletion of GPx4 in photoreceptor cells, significant amount of acrolein, a marker of lipid peroxidation, was found in IS of photoreceptor cells in the CKO mice at P12 compared with the control. Scale bar, 50 μm.
In the CKO mouse retina, increased lipid peroxidation in photoreceptor cells was expected as an early effect of abrogation of GPx4 that normally reduces peroxidized lipid. Lipid peroxidation is also known as the initial step of cell death induced by GPx4 silence (22) and is also observed in the process of retinal degenerations (7, 8). In this study lipid peroxidation was evaluated using an antibody against acrolein, an aldehyde marker of lipid peroxidation (23). At P12 a marked increase of lipid peroxidation was observed in IS of photoreceptor cells in the CKO mouse retina compared with IS of the control mice retina (Fig. 3, E and F). There was also a modest increase in the immunoreactivity against acrolein in the other layers of retina including inner nuclear layer (INL) and ganglion cell layer (GCL) in CKO mouse retinas.
GPx4 Abrogation in Photoreceptor Cells Led to Rapid and Massive Retinal Degeneration after P12
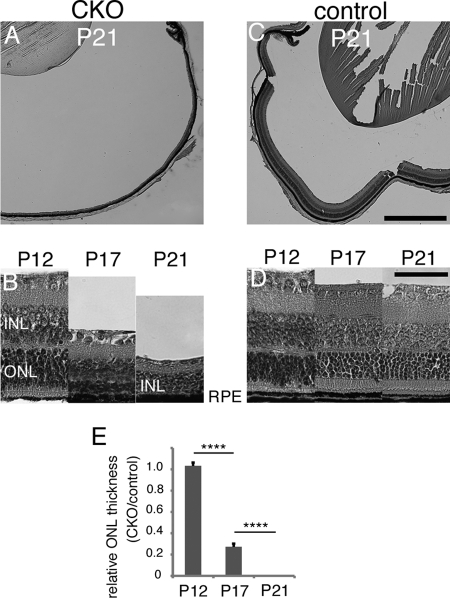
Histological analysis by hematoxylin-eosin staining was performed. Up to P12 (Fig. 4, B and D) there was not a significant difference in ONL thickness between the CKO and control mouse retina. This indicates photoreceptor cells can develop without GPx4. However, after P12, when photoreceptor cells are supposed to elongate their OS and become mature, the retina of the CKO mice underwent drastic retinal degeneration (Fig. 4, B, D, E). By P21 none of the photoreceptor cells retained while the retinal phenotype of the control mice was normal with mature OS formation (Fig. 4, A and C).
FIGURE 4.
Phenotype comparison between the CKO and control mice by hematoxylin-eosin staining. A and C, massive retinal degeneration was seen in the central and peripheral retina of the CKO mice, but not in the retina of the control mice. B and D, sequential observations revealed that at P12, there was no apparent difference between the retina of the CKO and control mice. However, at P17, ONL of the CKO mice progressively thinned and by P21 completely disappeared. E, there is a clear statistical significance in this process (mean ± S.E.; n = 3; ****, p < 0.0001, by ANOVA, followed by Turkey's post hoc test). Scale bar, 500 μm (C) and 50 μm (D).
Besides the rapid loss of ONL there was also milder thinning or degeneration in INL. Because we observed a mild degeneration in INL in rd1/rd1 mice (supplemental Fig. S1) whose retinal degeneration is known to originate from rod photoreceptor cells, the milder degeneration of INL in the CKO mice was considered a secondary effect following the primary loss of photoreceptor cells as in the case of rd1/rd1 mice.
Cone Photoreceptor Cells Are Lost Earlier than Rod Photoreceptor Cells in the CKO Mice
The subclasses of photoreceptor cells, i.e. rod and cone photoreceptor cells, were examined by immunohistochemistry with an anti-rhodopsin antibody and PNA labeling, respectively (Fig. 5). At P12, both markers for rod and cone photoreceptor cells were detected at similar levels in the retina of the CKO and control mice (Fig. 5, A and D), confirming that a lack of GPx4 did not interfere with the development and differentiation of photoreceptor cells. However, at P15 the PNA labeling was lost and rhodopsin expression was abnormally localized within ONL of the CKO mice (Fig. 5, B versus E). Abnormal localization of rhodopsin is also found during other retinal degenerations (24). Rhodopsin protein was not detected in the retina of the CKO mice by P21 (Fig. 5, C versus F). In contrast, in the control mice, rhodopsin expression and PNA labeling patterns were normal at P15 and P21, indicating a normal development and maturation of cone and rod cells in the retina.
FIGURE 5.
The photoreceptor cell loss in the CKO mice started in cone photoreceptor cells, followed by rod cells. Rod and cone photoreceptor cells were labeled with anti-rhodopsin antibody (red) and PNA (light blue), respectively. A and D, at P12 photoreceptor cells in the retina of the CKO and control mice presented a similar staining pattern for rod and cone cells. B and E, however, PNA-labeled cone cells disappeared by P15 in the CKO mice while rhodopsin localized abnormally in ONL. C and F, cone or rod photoreceptor cells were absent in the CKO mouse retina by P21. Normal maturation and OS formation was observed in the CKO mouse retina. Scale bar, 50 μm.
Apoptosis-inducing factor (AIF)-mediated Apoptosis in the Loss of GPx4-abrogated Photoreceptor Cells
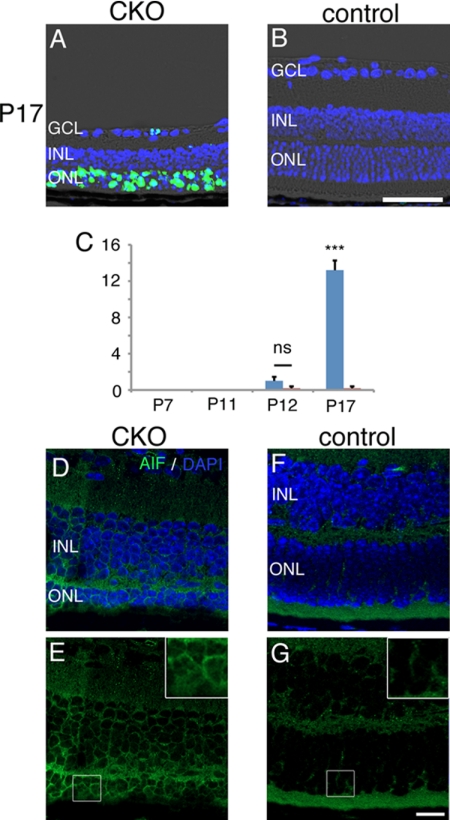
To explore the mechanism of retinal degeneration in the CKO mice, we first evaluated TUNEL in the CKO and control mouse retina (Fig. 6, A–C). Although during P7–11 no TUNEL-positive cell was observed, a progressively increasing number of TUNEL-positive cells were observed in ONL after P12. In literature both caspase-mediated (17) and AIF-mediated (22) mechanism of apoptosis was suggested in the GPx4-null cells or tissues. In photoreceptor cell death of the CKO mice we found a nuclear translocation of AIF on confocal immunohistochemistry (Fig. 6, D–G).
FIGURE 6.
TUNEL-positive cells and nuclear translocation of AIF in photoreceptor cells of the CKO mice. A–C, significant number of TUNEL-positive photoreceptor cells were observed in the CKO mouse retina at P17, but not in the control mouse retina. The TUNEL-positive cells appeared after P12. D–G, nuclear translocation of AIF was observed in photoreceptor cells of the CKO mice at P15, but not in the control mice (mean ± S.E.; n = 4; ns, not significant; ***, p < 0.001 by two-tailed t test for CKO versus control mice). Scale bar, 50 μm (B) and 10 μm (G).
Decreased Mitochondrial Biomass in GPx4-abrogated Photoreceptor Cells
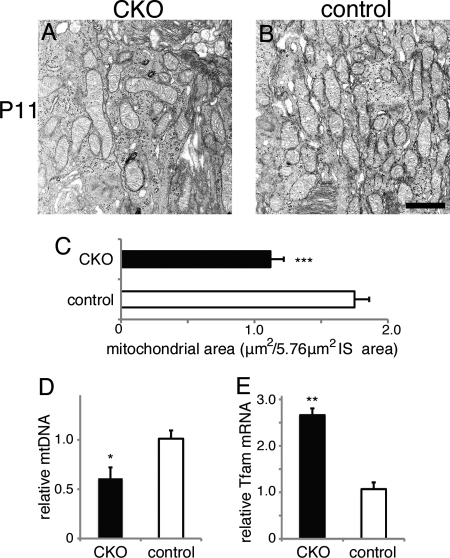
To further pursue the mechanism of the GPx4-abrogated photoreceptor cell death, we examined the retina at P11, at the timing before photoreceptor cell death occurred. Because mitochondrial GPx4 was considered the major variant of GPx4 expressed in the retina and IS was the main location of mitochondria in photoreceptor cells, we evaluated the mitochondrial biomass in IS of photoreceptor cells in the CKO and control mice. On TEM, mitochondrial area in IS of photoreceptor cells was significantly decreased in the CKO mice compared with the control mice (Fig. 7, A–C). Moreover, although GPx4 was abrogated only in photoreceptor cells of the CKO mice, mitochondrial DNA replication in the whole retina was significantly lower in the whole retina of the CKO mice than in that of the control mice (Fig. 7D). The decreased mitochondrial biomass by the loss of GPx4 in the CKO mice was accompanied by an increased expression of Tfam mRNA (Fig. 7E), which might suggest that PGC-1/Tfam pathway was activated in a compensatory manner (25).
FIGURE 7.
Decreased mitochondrial biomass in photoreceptor cells of the CKO mice at P11. A–C, in IS of photoreceptor cells of the CKO mice, mitochondrial area on TEM was significantly smaller than in IS of the control mice (mean ± S.E.; n = 4; ***, p < 0.001 by two-tailed t test). D, in the whole retina, mitochondrial DNA (mtDNA) replication was decreased in the CKO mice in comparison to the control mice (mean ± S.E.; n = 3; *, p < 0.05, by two-tailed t test). E, a paradoxical increase in Tfam mRNA in the whole retina of the CKO mice, suggesting a compensatory aspect of Tfam against the mitochondrial decrease by GPx4 deficit (mean ± S.E.; n = 3; **, p < 0.01 by two-tailed t test). Scale bar, 1 μm.
Decreased Number of Connecting Cilia and OS Malformation in the GPx4-abrogated Photoreceptor Cells
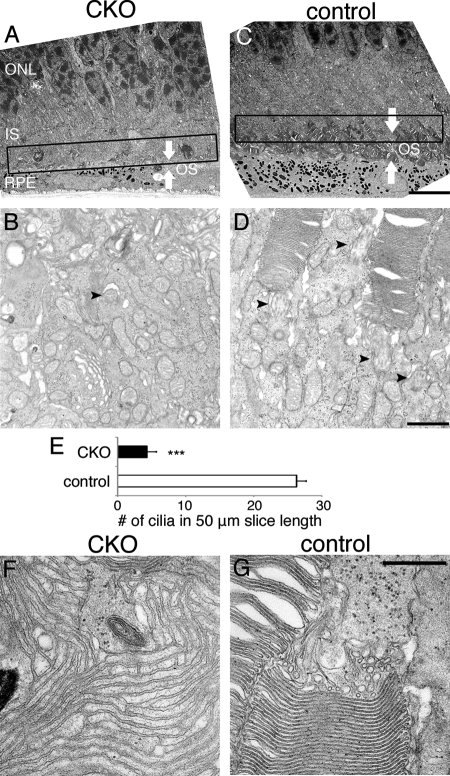
Cilia, including primary cilia, constantly transport molecules along the structure, which needs profound amount of energy supply. Because ciliated cells contain many mitochondria at the base of cilia, importance of mitochondria for cilia is a generally accepted notion. In line with this, disruption of mitochondrial GPx4 resulted in impaired spermatic structure and motility (16). Proper ciliogenesis, followed by OS formation and elongation, as well as their maintenance/renewal are critical for photoreceptor cell survival (26, 27). Therefore we evaluated the structural integrity of cilia in photoreceptor cells of the CKO and control mice at P11, before the photoreceptor cell death occurred in the CKO mice. We found a 80% decrease in the number of connecting cilia in photoreceptor cells of the CKO mice compared with those of the control mice (Fig. 8, B, D, E), indicating that many photoreceptor cells in the CKO mice did not have connecting cilia. In addition, OS layer, formed by transport from IS through the connecting cilium, existed, but it was very thin in the CKO mice compared with its counterpart in the control mice (Fig. 8, A and C). Furthermore, OS produced through the decreased number of connecting cilia of the CKO mice had morphological abnormalities. Compared with OS of the control mice that was composed of a pile of double membrane discs, OS of the CKO mice often branched and formed a large web-like membrane network without apparent disc rim. Intra- and inter- OS membrane space was also abnormally wide (Fig. 8, F and G).
FIGURE 8.
The decreased number of connecting cilia and disorganized OS in photoreceptor cells of the CKO mice. A and C, in these overview, OS layer (indicated by white arrows) existed in the CKO mice but profoundly thinner than OS of the control mice. B, D, E, the number of connecting cilia at the junction of IS and OS was significantly smaller in the CKO mice than in the control mice. The number of cilia was counted at the IS/OS junction with 50 μm span, shown as the boxed area in A and C. (mean ± S.E.; n = 3; ns, not significant; ***, p < 0.001 by two-tailed t test). F, OS of the CKO mice was disorganized and consists of cell membranes that branched and formed a large web-like network structure without apparent disc rim. Intra- and inter- OS membrane spaces were abnormally wide. G, OS of the control mice was composed of a pile of double membrane discs. Scale bar, 10 μm (C), 1 μm (D), and 0.5 μm (G).
DISCUSSION
Involvement of oxidative stress and protective role of antioxidant enzymes have been intensively studied in the pathologies of photoreceptor cells to date. Among all, anti-oxidative therapy has been expected to be a useful strategy for retinal degenerations, since it might suppress the progression of retinal degenerations independent of diverse genetic causes (10). In line with this, enhancement of numerous antioxidant enzymes has been reported to modulate oxidative stress and ameliorate retinal degenerations in animal models. In addition, several neurotrophic factors have also been discussed to modulate the oxidative stress and retard the progression of retinal degeneration. These anti-oxidative and neurotrophic factors reported in the past include; thioredoxin (1), GPx4 (5), catalase, and SOD2 (9), pigment epithelium-derived factor (27), glial cell line-derived neurotrophic factor (28, 29), ciliary neurotrophic factor (30), rod-derived cone viability factor (31), and brain-derived neurotrophic factor (4, 32). These previous reports have substantiated the protective role of antioxidant molecules against the pathologies of photoreceptor cells.
However, the essentiality of antioxidant enzymes for the retina has remained unknown. Knock-out mice of antioxidant enzymes created to date include SOD1−/− mice (12) and GPx1−/− mice (11). Both SOD1 and GPx1 localize in the cytosol, not mitochondria. SOD1 knock-out mice presented a mild thinning of the retina at 15 months old, while GPx1 knock-out mice exhibited a rather resistance against oxidative stress. Based on the present results, GPx4 is not only protective for photoreceptor cells (5) along with other antioxidant enzymes, but also is critical for the survival of photoreceptor cells. This is notable because evidence that the lack of a single antioxidant enzyme leading to a drastic abnormality in phenotype has been rare. A unique role of GPx4 in mitochondria compared with other mitochondrial antioxidant enzymes is that it can reduce complex lipid hydroperoxides that are incorporated in biomembranes (13), which might contribute to the drastic abnormality in the CKO mice. Furthermore, this critical role of GPx4 in photoreceptor cells is in contrast to a previous study of the neuron-specific ablation of GPx4 (18), in which neuronal degeneration was limited to the CA3 region of the hippocampus and a reduced number of parvalbumin-positive interneurons in the somatosensory cortex at P13. The authors of the study discussed that in addition to GPx4 other selenoproteins are critical for neuronal development, because the neuronal degeneration was severed in mice whose neuronal selenoproteins were totally abrogated.
Although in the present study GPx4 had already been silenced at P1 in the photoreceptor precursor cells, obvious photoreceptor loss was not observed before P12. By P12 differentiation into rod and cone photoreceptor cells to form ONL was observed in the retina of the CKO mice. These results suggest that GPx4 was not essential for the development and differentiation of photoreceptor cells. This is in contrast to the previous studies that GPx4-null fibroblast cells underwent prompt apoptosis (22) and knock-down of mitochondrial GPx4 resulted in developmental retardation in the brain of the embryo (33). In our previous study, the spermatocyte-specific CKO mice of GPx4 still have spermatozoa although the number and quality were severely impaired (17). Because photoreceptor cells of the CKO mice underwent apoptosis after P12, when normal photoreceptor cells elongate their OS and become mature, GPx4 may be critical for OS formation and maturation of photoreceptor cells.
By observing the ultrastracture of photoreceptor cells at P11, when apoptosis of photoreceptor cells in the CKO mice had not yet occurred, we found decreased mitochondrial biomass that might result in the decreased number of connecting cilia and OS malformation, which might be a reason of the loss of GPx4-abrogated photoreceptor cells. We also elucidated that mitochondrial GPx4 was the dominant variant of GPx4 in the retina. The importance of mitochondria for the cilia has been a widely accepted notion although the evidence is relatively scarce. However, a recent report of impaired structures of spermatozoa in the knock-out mice of mitochondrial GPx4 (16) is considered to indicate the importance of mitochondria for the cilium. In the present study a decrease in mitochondrial biomass by the loss of GPx4 was also indicated by the measurement of mitochondrial DNA replication and mitochondrial area on TEM. We also observed an increase in Tfam mRNA expression, a well-known transcriptional factor promoting mitochondrial biogenesis (25), suggesting a compensatory activation against the mitochondrial loss. Because decreased mitochondria as well as impaired structures and functions of cilia were observed not only in photoreceptor cells but also in the spermatozoa (16, 17), mitochondrial GPx4 seems to be important for the cilium. However, the phenotype of the retina in the specific knock-out mice of mitochondrial GPx4 has remained to be elucidated. Although mitochondrial GPx4 was the dominant GPx4 variant in the retina, a small amount of cytosolic or nucleolar GPx4 might partially contribute to the drastic degeneration of photoreceptor cells in the CKO mice in the present study.
The present results showed that GPx4-abrogated photoreceptor cell death began in the cone cells, not in the rod cells, indicating that cone cells are more susceptible to oxidative stress than rod cells in the absence of GPx4. This is in line with the literature that emphasizes the pathogenic role of oxidative stress, especially in the loss of cone cells in retinal degeneration (5, 7). Furthermore, in contrast to previous animal models of rod-cone dystrophy, in which the loss of rod cells preceded that of cone cells, mice with the photoreceptor-specific CKO of GPx4 provide a unique opportunity to study cone-rod dystrophy, another important clinical manifestation of retinal degeneration.
In conclusion, the present study clearly demonstrated that GPx4 is critical for photoreceptor cells. To the best of our knowledge, this is the first study to demonstrate that a single anti-oxidative factor is critical for the survival of photoreceptor cells.
Supplementary Material
Acknowledgment
We thank the University of Tokyo Center for NanoBio Integration for technical support in confocal microscopy.
This work was supported by Grants-in-Aid for Scientific Research from Japan Society for the Promotion of Science (21791669) (to Y. Y.), and (20390446 and 23659806) (to Y. T.).

This article contains supplemental Fig. S1.
- GPx4
- glutathione peroxidase 4
- CKO
- conditional knockout
- AIF
- apoptosis-inducing factor
- SOD
- superoxide dismutase
- TUNEL
- terminal deoxynucleotidyl transferase dUTP nick-end labeling
- TEM
- transmission electron microscopy
- Tfam
- mitochondrial transcription factor A
- IS
- internal segment
- OS
- outer segment
- GCL
- ganglion cell layer
- INL
- inner nuclear layer
- ONL
- outer nuclear layer
- PGC-1
- peroxisome proliferator-activated receptor γ coactivator-1.
REFERENCES
- 1. Tanito M., Masutani H., Nakamura H., Ohira A., Yodoi J. (2002) Cytoprotective effect of thioredoxin against retinal photic injury in mice. Invest. Ophthalmol. Vis. Sci. 43, 1162–1167 [PubMed] [Google Scholar]
- 2. Tanito M., Kwon Y. W., Kondo N., Bai J., Masutani H., Nakamura H., Fujii J., Ohira A., Yodoi J. (2005) Cytoprotective effects of geranylgeranylacetone against retinal photooxidative damage. J. Neurosci. 25, 2396–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haruta M., Bush R. A., Kjellstrom S., Vijayasarathy C., Zeng Y., Le Y. Z., Sieving P. A. (2009) Depleting Rac1 in mouse rod photoreceptors protects them from photo-oxidative stress without affecting their structure or function. Proc. Natl. Acad. Sci. U.S.A. 106, 9397–9402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Okoye G., Zimmer J., Sung J., Gehlbach P., Deering T., Nambu H., Hackett S., Melia M., Esumi N., Zack D. J., Campochiaro P. A. (2003) Increased expression of brain-derived neurotrophic factor preserves retinal function and slows cell death from rhodopsin mutation or oxidative damage. J. Neurosci. 23, 4164–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lu L., Oveson B. C., Jo Y. J., Lauer T. W., Usui S., Komeima K., Xie B., Campochiaro P. A. (2009) Increased expression of glutathione peroxidase 4 strongly protects retina from oxidative damage. Antioxid. Redox. Signal. 11, 715–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Osborne N. N., Wood J. P. (2004) Metipranolol blunts nitric oxide-induced lipid peroxidation and death of retinal photoreceptors: a comparison with other anti-glaucoma drugs. Invest. Ophthalmol. Vis. Sci. 45, 3787–3795 [DOI] [PubMed] [Google Scholar]
- 7. Shen J., Yang X., Dong A., Petters R. M., Peng Y. W., Wong F., Campochiaro P. A. (2005) Oxidative damage is a potential cause of cone cell death in retinitis pigmentosa. J. Cell. Physiol. 203, 457–464 [DOI] [PubMed] [Google Scholar]
- 8. Cronin T., Raffelsberger W., Lee-Rivera I., Jaillard C., Niepon M. L., Kinzel B., Clérin E., Petrosian A., Picaud S., Poch O., Sahel J. A., Léveillard T. (2010) The disruption of the rod-derived cone viability gene leads to photoreceptor dysfunction and susceptibility to oxidative stress. Cell Death Differ. 17, 1199–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Usui S., Komeima K., Lee S. Y., Jo Y. J., Ueno S., Rogers B. S., Wu Z., Shen J., Lu L., Oveson B. C., Rabinovitch P. S., Campochiaro P. A. (2009) Increased expression of catalase and superoxide dismutase 2 reduces cone cell death in retinitis pigmentosa. Mol. Ther. 17, 778–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Komeima K., Rogers B. S., Lu L., Campochiaro P. A. (2006) Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc. Natl. Acad. Sci. U S A. 103, 11300–11305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gosbell A. D., Stefanovic N., Scurr L. L., Pete J., Kola I., Favilla I., de Haan J. B. (2006) Retinal light damage: structural and functional effects of the antioxidant glutathione peroxidase-1. Invest. Ophthalmol. Vis. Sci. 47, 2613–2622 [DOI] [PubMed] [Google Scholar]
- 12. Hashizume K., Hirasawa M., Imamura Y., Noda S., Shimizu T., Shinoda K., Kurihara T., Noda K., Ozawa Y., Ishida S., Miyake Y., Shirasawa T., Tsubota K. (2008) Retinal dysfunction and progressive retinal cell death in SOD1-deficient mice. Am. J. Pathol. 172, 1325–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomas J. P., Geiger P. G., Maiorino M., Ursini F., Girotti A. W. (1990) Enzymatic reduction of phospholipid and cholesterol hydroperoxides in artificial bilayers and lipoproteins. Biochim. Biophys. Acta 1045, 252–260 [DOI] [PubMed] [Google Scholar]
- 14. Maiorino M., Scapin M., Ursini F., Biasolo M., Bosello V., Flohé L. (2003) Distinct promoters determine alternative transcription of gpx-4 into phospholipid-hydroperoxide glutathione peroxidase variants. J. Biol. Chem. 278, 34286–34290 [DOI] [PubMed] [Google Scholar]
- 15. Imai H., Hirao F., Sakamoto T., Sekine K., Mizukura Y., Saito M., Kitamoto T., Hayasaka M., Hanaoka K., Nakagawa Y. (2003) Early embryonic lethality caused by targeted disruption of the mouse PHGPx gene. Biochem. Biophys. Res. Commun. 305, 278–286 [DOI] [PubMed] [Google Scholar]
- 16. Schneider M., Förster H., Boersma A., Seiler A., Wehnes H., Sinowatz F., Neumüller C., Deutsch M. J., Walch A., Hrabé de Angelis M., Wurst W., Ursini F., Roveri A., Maleszewski M., Maiorino M., Conrad M. (2009) Mitochondrial glutathione peroxidase 4 disruption causes male infertility. FASEB J. 23, 3233–3242 [DOI] [PubMed] [Google Scholar]
- 17. Imai H., Hakkaku N., Iwamoto R., Suzuki J., Suzuki T., Tajima Y., Konishi K., Minami S., Ichinose S., Ishizaka K., Shioda S., Arata S., Nishimura M., Naito S., Nakagawa Y. (2009) Depletion of selenoprotein GPx4 in spermatocytes causes male infertility in mice. J. Biol. Chem. 284, 32522–32532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wirth E. K., Conrad M., Winterer J., Wozny C., Carlson B. A., Roth S., Schmitz D., Bornkamm G. W., Coppola V., Tessarollo L., Schomburg L., Köhrle J., Hatfield D. L., Schweizer U. (2010) Neuronal selenoprotein expression is required for interneuron development and prevents seizures and neurodegeneration. FASEB J. 24, 844–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nishida A., Furukawa A., Koike C., Tano Y., Aizawa S., Matsuo I., Furukawa T. (2003) Otx2 homeobox gene controls retinal photoreceptor cell fate and pineal gland development. Nat. Neurosci. 6, 1255–1263 [DOI] [PubMed] [Google Scholar]
- 20. Imai H. (2010) New strategy of functional analysis of PHGPx knockout mice model using transgenic rescue method and Cre-LoxP system. J. Clin. Biochem. Nutr. 46, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imai H., Suzuki K., Ishizaka K., Ichinose S., Oshima H., Okayasu I., Emoto K., Umeda M., Nakagawa Y. (2001) Failure of the expression of phospholipid hydroperoxide glutathione peroxidase in the spermatozoa of human infertile males. Biol. Reprod. 64, 674–683 [DOI] [PubMed] [Google Scholar]
- 22. Seiler A., Schneider M., Förster H., Roth S., Wirth E. K., Culmsee C., Plesnila N., Kremmer E., Rådmark O., Wurst W., Bornkamm G. W., Schweizer U., Conrad M. (2008) Glutathione peroxidase 4 senses and translates oxidative stress into 12/15-lipoxygenase dependent- and AIF-mediated cell death. Cell. Metabolism 8, 237–248 [DOI] [PubMed] [Google Scholar]
- 23. Uchida K., Kanematsu M., Morimitsu Y., Osawa T., Noguchi N., Niki E. (1998) Acrolein is a product of lipid peroxidation reaction. Formation of free acrolein and its conjugate with lysine residues in oxidized low density lipoproteins. J. Biol. Chem. 273, 16058–16066 [DOI] [PubMed] [Google Scholar]
- 24. Roof D. J., Adamian M., Hayes A. (1994) Rhodopsin accumulation at abnormal sites in retinas of mice with a human P23H rhodopsin transgene. Invest. Ophthalmol. Vis. Sci. 35, 4049–4062 [PubMed] [Google Scholar]
- 25. Ross C. A., Thompson L. M. (2006) Transcription meets metabolism in neurodegeneration. Nat. Med. 12, 1239–1241 [DOI] [PubMed] [Google Scholar]
- 26. Ramamurthy V., Cayouette M. (2009) Development and disease of the photoreceptor cilium. Clin. Genet. 76, 137–145 [DOI] [PubMed] [Google Scholar]
- 27. Cayouette M., Smith S. B., Becerra S. P., Gravel C. (1999) Pigment epithelium-derived factor delays the death of photoreceptors in mouse models of inherited retinal degenerations. Neurobiol. Dis. 6, 523–532 [DOI] [PubMed] [Google Scholar]
- 28. Frasson M., Picaud S., Léveillard T., Simonutti M., Mohand-Said S., Dreyfus H., Hicks D., Sabel J. (1999) Glial cell line-derived neurotrophic factor induces histologic and functional protection of rod photoreceptors in the rd/rd mouse. Invest. Ophthalmol. Vis. Sci. 40, 2724–2734 [PubMed] [Google Scholar]
- 29. Dong A., Shen J., Krause M., Hackett S. F., Campochiaro P. A. (2007) Increased expression of glial cell line-derived neurotrophic factor protects against oxidative damage-induced retinal degeneration. J. Neurochem. 103, 1041–1052 [DOI] [PubMed] [Google Scholar]
- 30. Harada T., Harada C., Kohsaka S., Wada E., Yoshida K., Ohno S., Mamada H., Tanaka K., Parada L. F., Wada K. (2002) Microglia-Müller glia cell interactions control neurotrophic factor production during light-induced retinal degeneration. J. Neurosci. 22, 9228–9236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang Y., Mohand-Said S., Danan A., Simonutti M., Fontaine V., Clerin E., Picaud S., Léveillard T., Sahel J. A. (2009) Functional cone rescue by RdCVF protein in a dominant model of retinitis pigmentosa. Mol. Ther. 17, 787–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang M., Mo X., Fang Y., Guo W., Wu J., Zhang S., Huang Q. (2009) Rescue of photoreceptors by BDNF gene transfer using in vivo electroporation in the RCS rat of retinitis pigmentosa. Curr. Eye. Res. 34, 791–799 [DOI] [PubMed] [Google Scholar]
- 33. Ufer C., Wang C. C., Fähling M., Schiebel H., Thiele B. J., Billett E. E., Kuhn H., Borchert A. (2008) Translational regulation of glutathione peroxidase 4 expression through guanine-rich sequence-binding factor 1 is essential for embryonic brain development. Genes Dev. 22, 1838–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.