Background: β-Secretase BACE1 is elevated in Alzheimer disease (AD) during pathogenesis through an unknown mechanism.
Results: Aβ42 increases BACE1 in primary neurons via a post-transcriptional mechanism and is synergized by Cdk5 inhibitors.
Conclusion: Aβ42 increases neuronal BACE1 translation through a Cdk5-independent pathway.
Significance: Amyloid may initiate a feed-forward mechanism of BACE1 elevation and Aβ production in AD, and Cdk5 inhibitor drugs may exacerbate this.
Keywords: Alzheimer Disease, Amyloid, Caspase, CDK (Cyclin-dependent kinase), Secretases, beta-secretase, Abeta, BACE1, Caspase 3, Cdk5
Abstract
The β-secretase enzyme BACE1 initiates production of the amyloid-β (Aβ) peptide that comprises plaques in Alzheimer disease (AD) brain. BACE1 levels are increased in AD, potentially accelerating Aβ generation, but the mechanisms of BACE1 elevation are not fully understood. Cdk5/p25 has been implicated in neurodegeneration and BACE1 regulation, suggesting therapeutic Cdk5 inhibition for AD. In addition, caspase 3 has been implicated in BACE1 elevation. Here, we show that the Cdk5 level and p25:p35 ratio were elevated and correlated with BACE1 level in brains of AD patients and 5XFAD transgenic mice. Mouse primary cortical neurons treated with Aβ42 oligomers had increased BACE1 level and p25:p35 ratio. Surprisingly, the Aβ42-induced BACE1 elevation was not blocked by Cdk5 inhibitors CP68130 and roscovitine, and instead the BACE1 level was increased greater than with Aβ42 treatment alone. Moreover, Cdk5 inhibitors alone elevated BACE1 in a time- and dose-dependent manner that coincided with increased caspase 3 cleavage and decreased Cdk5 level. Caspase 3 inhibitor benzyloxycarbonyl-VAD failed to prevent the Aβ42-induced BACE1 increase. Further experiments suggested that the Aβ42-induced BACE1 elevation was the result of a post-transcriptional mechanism. We conclude that Aβ42 may increase the BACE1 level independently of either Cdk5 or caspase 3 and that Cdk5 inhibition for AD may cause BACE1 elevation, a potentially negative therapeutic outcome.
Introduction
The β-secretase, β-site APP2-cleaving enzyme1 (BACE1), is the enzyme that cleaves APP to initiate the production of the β-amyloid (Aβ) peptide involved in Alzheimer disease (AD) (1). Reduction of BACE1 levels by gene targeting (2–4) or RNA interference (5–7) inhibits Aβ generation and amyloid plaque formation. Conversely, modest overexpression of BACE1 in transgenic mice increases Aβ production and amyloid deposition, demonstrating that Aβ production and amyloid pathology can be modulated by BACE1 level (8–11). Familial AD (FAD) cases caused by the APP Swedish mutation (K670N,M671L) (12) that enhances cleavage by BACE1 (13) suggest that increased activity of BACE1 may be sufficient to induce Alzheimer disease in humans. In addition, BACE1 levels are increased ∼1.5–3-fold in the brains of AD patients, suggesting that BACE1 elevation contributes to disease (14–18). These results imply that preventing the BACE1 increase may be beneficial for AD.
Inhibition of BACE1 should effectively lower Aβ levels in AD, but therapeutically useful BACE1 inhibitors have proved challenging to design. Moreover, concerns have been raised over potential mechanism-based side effects of BACE1 inhibition, as suggested by phenotypes observed in BACE1 null mice, including memory and emotional deficits (19–23) and hypomyelination (24, 25). Blocking the BACE1 increase observed in AD could be therapeutically useful in slowing or preventing AD, while at the same time allowing normal levels of BACE1 to perform BACE1 functions and thus minimize side effects. However, the mechanisms that regulate BACE1 levels in the brain are not fully understood.
Using immunostaining on brain sections from AD patients and mouse models of AD, we have demonstrated that BACE1 levels are elevated in neurons surrounding amyloid plaques (18), a result that was recently confirmed by another group with a different anti-BACE1 antibody (26). These results suggest that Aβ42, the main constituent of amyloid plaques, may increase BACE1 levels in neurons near plaques. As such, a feed-forward mechanism may be initiated where BACE1 elevation increases Aβ42 generation, which further raises the BACE1 level.
Recent evidence implicates the calcium/calpain/Cdk5 kinase signaling pathway in AD pathogenesis, suggesting it could be responsible for an Aβ42-induced BACE1 increase. Aβ42 increases intracellular calcium in neurons, which activates calpain to cleave the Cdk5 regulatory subunit, p35 to p25 (27). Overexpression of the p25 subunit causes cytoskeletal disruption, altered phosphorylation patterns, and neurotoxicity (28). Mice with elevated cerebral Cdk5/p25 activity were reported to have increased BACE1 levels and Aβ production (29–31). Based on these data suggesting a connection between Cdk5 dysregulation and AD in humans and mice, Cdk5 inhibitors are being developed as potential AD therapeutics (30).
Caspase 3 activation may also increase BACE1 levels. Levels of activated caspase 3 are elevated in AD brains (32–35). Caspase 3 cleavage of the adaptor protein GGA3, which may traffic BACE1 to the lysosome for degradation, appears to result in BACE1 accumulation in vitro and in vivo (36). It may also play a role in AD, as the GGA3 level appears to be decreased in the brains of Alzheimer patients (36).
Here, we investigated the potential roles of Cdk5 and caspase 3 activation in Aβ42 oligomer-induced BACE1 elevation in mouse primary neuronal cultures. We show that Aβ42 oligomers increase the BACE1 level and p25:p35 ratio in primary neurons. Although p35/25-Cdk5 signaling may be increased in AD patients and in the 5XFAD mouse model of AD, inhibition of Cdk5 activity in primary neurons did not prevent the Aβ42-induced increase in BACE1 level nor did caspase inhibition. Our results have important implications for both the molecular mechanism of the Aβ42-induced BACE1 elevation and for therapeutic approaches involving Cdk5 inhibition for AD.
EXPERIMENTAL PROCEDURES
Human Brain Samples
Post-mortem frontal cortex tissues were obtained from AD (n = 9; 88.3 ± 4.1 years) and noncognitively impaired (n = 13; 88.0 ± 4.8 years) participants in the Rush Hospital Memory and Aging Project (R01AG17917; David A. Bennett) following Rush University IRB approval (supplemental Table 1). Frozen tissues (0.2–0.4 g) were homogenized in 1× PBS with 1% Triton X-100, supplemented with protease inhibitors (Calbiochem) and Halt Phosphatase Inhibitor Mixture (Thermo Scientific). BACE1, Cdk5, and p35/25 levels in frontal cortex samples were measured by immunoblot analysis as described below. To compensate for any difference in transfer between the two blots, 2–4 samples were loaded on both blots and used to normalize the signal. The normalized BACE1 or Cdk5 signal was then normalized to actin to account for any differences in loading. p35/25 signals were also normalized between blots and then the ratio was calculated directly. Linear regressions and comparisons of means using the t test were performed using GraphPad Prism and InStat software, respectively (GraphPad Software, Inc., San Diego).
Mice
5XFAD mice were generated and maintained as described (37). Animals were sacrificed at 2 months of age, and one hemibrain was snap-frozen in liquid nitrogen and then homogenized in 1× PBS with 1% Triton X-100 supplemented with protease inhibitors (Calbiochem) and Halt Phosphatase Inhibitor Mixture (Thermo Scientific). Homogenates were sonicated and protein was quantified using the BCA assay (Pierce). All animal work was done in accordance with Northwestern University IACUC approval.
Immunoblotting
10 μg of brain homogenate or 15 μg of neuronal cell lysate was resolved with 4–12% BisTris NuPAGE mini gels (Invitrogen). Protein was transferred to a 0.45-μm PVDF membrane and probed with anti-BACE1 antibody (3D5 1:1000) (18), anti-Cdk5 (Abcam ab40773, 1:3000), anti-p35/25 (Santa Cruz Biotechnology C-19, 1:3000), anti-β-actin (Sigma clone AC-15, A5441, 1:30,000), anti-caspase 3 (Cell Signaling 9662, 1:1000), anti-APP (Millipore 22C11, 1:5000), anti-APPThr(P)-668 (Cell Signaling 2451, 1:1000), followed by washing and 1 h of incubation with secondary HRP-conjugated anti-mouse or anti-rabbit secondary antibody (Jackson ImmunoResearch, 1:10,000). Blots were visualized using ECL+ chemiluminescent substrate (Amersham Biosciences), and signals were quantified using a Kodak Image Station 4000R phosphorimager. Signals were normalized to actin or tubulin, except the ratio of p25:p35, phospho/total APP, and cleaved/total caspase 3. Triplicate neuronal cultures were averaged, and comparison with control was done using Student's two-tailed t test using InStat software (GraphPad Software, Inc., San Diego).
Neuronal Culture
Cortical neurons were isolated from day 15.5 mouse embryos via dissociation at 37 °C in 0.25% trypsin. Brains were plated at the density of about 0.05 brains per well in 12-well plates previously coated with 1 mg/ml poly-l-lysine in borate buffer. Neurons were maintained in neurobasal media supplemented with 2% B-27 supplement and 500 μm glutamine. Plating media also contained 10% horse serum and 2.5 μm glutamate. All cell culture reagents were from Invitrogen. After 7 or 14 days in vitro, neurons were exposed to 10 μm human Aβ42 for 24–96 h. Neurons were pretreated 1–3 h with either DMSO vehicle (control cultures) CP681301 (generous gift of Pfizer, Inc.), ZVAD (Sigma), roscovitine (Sigma), or 10 μg/ml cycloheximide (Sigma) before the addition of Aβ42. All treatment conditions were done in triplicate. Neurons were lysed on ice in RIPA buffer (150 mm NaCl, 1% IGEPAL CA-630, 0.5% sodium deoxycholate, 0.1% SDS, 50 mm Tris, pH 8, 1 mm PMSF) with protease inhibitors (Calbiochem) and Halt Phosphatase Inhibitor Mixture (Thermo Scientific), and spun down for 10 min at 10,000 rpm at 4 °C, and protein in the supernatant was quantified by BCA assay (Pierce).
Aβ42 Preparation
Aβ42 oligomers and fibrils were prepared as described previously (38). Briefly, lyophilized recombinant Aβ42 peptide was hexafluoroisopropanol-treated, dried down, then resuspended to 5 mm in dry DMSO, and brought to 100 μm in cold phenol-free F-12 media for oligomers or 10 mm HCl for fibrils. Oligomers were incubated on ice at 4 °C for 24 h, and fibrils were incubated at 37 °C for 24 h before adding to neuron cultures. For control cultures, DMSO alone was added to F-12 or HCl and incubated as described.
mRNA Analysis
mRNA was isolated from cell lysates using RNeasy mini kit (Qiagen). 1 μg of RNA was used for first strand cDNA synthesis using SuperScript III (Invitrogen). The resulting cDNA was subjected to real time PCR analysis on an ABI 7900HT machine (Applied Biosystems) using TaqMan murine BACE1 primer Mm00478664_m1 and 18 S rRNA primer 433760F (Applied Biosystems). The BACE1 signal was normalized, and the 18 S rRNA signal was quantified using the relative quantification method. Aβ42-treated neurons were expressed as percent of control.
Statistical Analysis
Instat and GraphPad Prism were used for statistical analysis. Comparisons between control and experimental conditions were done using a nonpaired two-tailed t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001. All error bars represent means ± S.E.
RESULTS
Levels of Cdk5, p25, and p35 Are Dysregulated in AD and the 5XFAD Mouse Model of AD
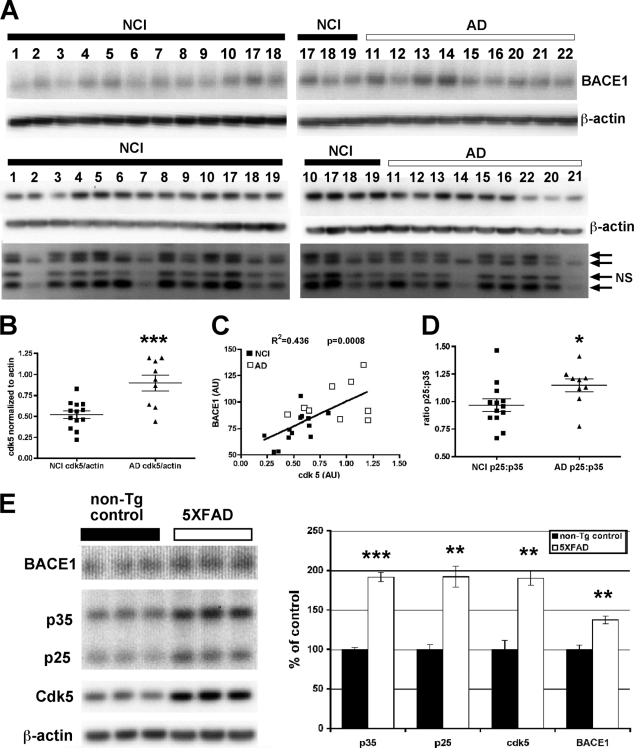
There are conflicting data on whether levels of the p25 cleavage fragment, and of Cdk5 protein and activity, are elevated in the brains of AD patients. Two papers from Tsai and co-workers (28, 39) indicate that levels of the p25 fragment are increased, although others report that it is either unchanged or decreased (40–45). Because of this controversy, we investigated Cdk5 and p25/p35 levels in a set of AD and control brains from the Rush University Memory and Aging Project (46). We previously reported that BACE1 level was elevated in this set of brains (17). Here, we found that Cdk5 protein levels were significantly elevated in AD compared with noncognitively impaired controls and that Cdk5 levels were significantly correlated with BACE1 levels (Fig. 1, A–C). These results suggested that elevated Cdk5 could lead to increases in BACE1 level. In addition, the ratio of p25:p35 was significantly increased in the brains of AD patients (Fig. 1D), but this ratio did not show a statistically significant correlation with BACE1 (data not shown). A doublet for the p35 band was observed, which was previously reported in human brain by Tandon et al. (43), and was attributed to differential phosphorylation of p35. Both bands were included in this quantification. A band between p35 and p25 was also observed, but it has not been reported previously; it did not appear to correlate with either p35 or p25 and therefore is likely to be a nonspecific band (indicated by arrow and NS in Fig. 1A).
FIGURE 1.
BACE1 and Cdk5/p35/p25 pathway components are increased in the brains of AD patients and the 5XFAD mouse model of AD. A, brain samples from 13 noncognitively impaired controls (NCI) and nine Alzheimer patients (AD) were homogenized in PBS, 1% Triton X-100 and 15 μg of protein per lane were analyzed by immunoblot. Signals were normalized across blots using duplicate samples run on both gels (samples 17 and 18 for BACE1 and samples 10, 17, 18, and 19 for Cdk5 and p25/p35). BACE1 and Cdk5 signals were first normalized across blots and then were finally normalized using actin signals. The p25 and p35 signals were normalized across blots and then the p25:p35 ratio was calculated directly. The doublet at 35 kDa in A may be due to differential phosphorylation of p35 and has been reported in human brain previously (43). We also observed a nonspecific band (labeled NS in A) near 25 kDa. B and C, Cdk5 levels were elevated in AD patients (B) and correlated significantly with BACE1 levels (C), which were also significantly elevated in the AD patients (17). AU, arbitrary units. D, there was a small but significant increase in the ratio of p25/p35, which could also contribute to elevated Cdk5 activity. E, hemibrains from 2-month-old 5XFAD mice and nontransgenic littermate controls were homogenized in PBS, 1% Triton X-100, and 15 μg of protein were analyzed by immunoblot. BACE1 and Cdk5 signals were normalized using actin signals. Levels of BACE1 and Cdk5 were elevated in 5XFAD brains, as were those of both p25 and p35. Error bars = mean ± S.E.; *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001.
Because Aβ42 is the principal constituent of amyloid plaques in AD, it appeared plausible that Aβ42 might induce the BACE1 elevation in AD brain. Moreover, if increased Cdk5 activity played a role in the Aβ42-induced BACE1 increase, we hypothesized that Cdk5 activity may be elevated early in the pathogenesis of a mouse model of AD. The 5XFAD transgenic mouse model carries five mutations associated with familial Alzheimer disease and begins generating high levels of Aβ42 at 6 weeks of age, soon after the onset of transgene expression (37). By immunoblot, we observed that BACE1, Cdk5, p25, and p35 were all elevated at 2 months of age, only 2 weeks after transgenic Aβ42 was first detected (Fig. 1E). These results in 5XFAD mice were similar to those in human AD, except that an increased p25:p35 ratio was not observed. Instead, p25 and p35 were both increased 2-fold, resulting in no change in ratio (Fig. 1E). Because altered levels of Cdk5 pathway components were observed soon after the onset of Aβ42 generation in 5XFAD mice, and were coincident with BACE1 elevation, it appeared plausible that Cdk5 could have been involved in an Aβ42-associated BACE1 increase in these mice.
Aβ42 Elevates BACE1 Level in Cultured Primary Neurons
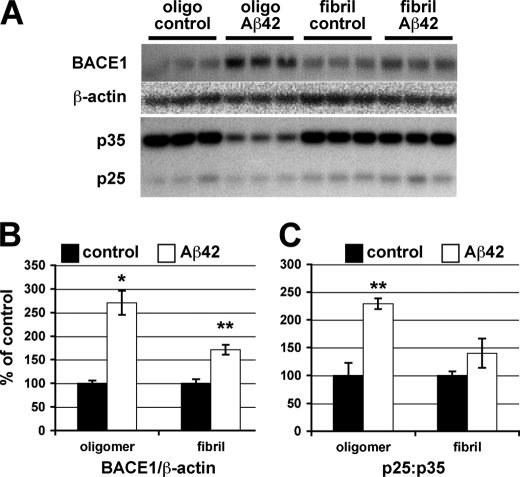
To gain insight into the potential role of Cdk5 in Aβ42-induced BACE1 elevation at a molecular and cellular level, we determined whether we could elicit a BACE1 increase in response to Aβ42 in a primary neuron culture system amenable to pharmacological and genetic manipulation. Primary cortical neurons cultured from day 15.5 embryonic mice were treated with either oligomeric or fibrillar forms of Aβ42 (10 μm), and protein levels in neuron lysates were analyzed by immunoblot (Fig. 2A). After 24 h of treatment, both oligomeric and fibrillar Aβ42 caused BACE1 levels to increase in neurons, with oligomeric Aβ42 showing a greater BACE1 elevation (Fig. 2B). Treatment with 5 μm Aβ42 produced a smaller BACE1 increase, suggesting a dose response (data not shown). We also examined these cultures for changes in the ratio of p25:p35 because of cleavage of p35. In the presence of oligomeric Aβ42, p35 was reduced, resulting in a higher p25:p35 ratio that may increase Cdk5 activity (Fig. 2C). This is congruent with data presented by Lee et al. (27) showing that 20 μm Aβ42 led to p35 cleavage by calpain. Because oligomeric Aβ42 had a greater effect on BACE1 level and on p35 cleavage, and because of the growing consensus that oligomeric forms of Aβ are a major cause of toxicity in AD (47–54), we focused on the effects of oligomeric Aβ42 in subsequent experiments.
FIGURE 2.
Oligomeric Aβ42 causes BACE1 elevation and increases p25:p35 ratio in primary neurons. Murine primary embryonic neurons were cultured for 7 days and then treated in triplicate with 10 μm oligomeric (Oligo) or fibrillar Aβ42 or appropriate vehicle controls for 24 h. A, neurons were lysed in RIPA buffer, and 15 μg of protein per lane were analyzed by immunoblot. BACE1 signals were normalized using actin signals (B), whereas the p25:p35 ratio was calculated directly from raw signals (C). The triplicates for each condition were averaged, and S.E. was calculated. Fibrillar Aβ42 had a similar but smaller effect on the BACE1 elevation as oligomeric Aβ42. Note that oligomeric Aβ42 treatment significantly decreased p35 level resulting in an increased p25:p35 ratio. Error bars, mean ± S.E.; *, p ≤ 0.05; **, p ≤ 0.01.
Cdk5 Inhibitors Do Not Prevent Aβ42-induced BACE1 Elevation in Primary Neurons
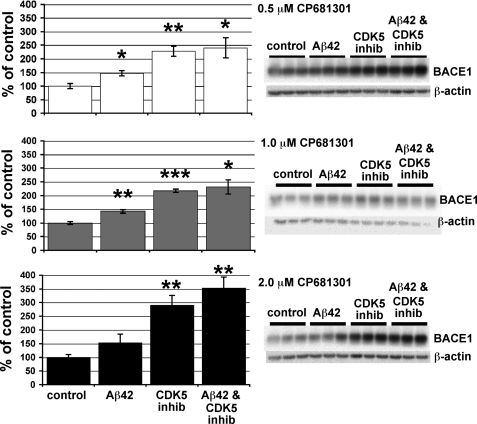
To determine whether Cdk5 activity is a mediator of Aβ42-induced BACE1 elevation, we pretreated primary neuron cultures with three different concentrations (0.5, 1.0, and 2.0 μm) of the selective Cdk5 inhibitor CP681301 (31) and then exposed cultures to 10 μm oligomeric Aβ42. Although Aβ42 elevated BACE1 levels to ∼150% of control, Aβ42 in conjunction with CP681301 increased BACE1 even more to ∼250–350% of control (Fig. 3). Rather than inhibiting the BACE1 elevation, CP681301 appeared to potentiate it. When CP681301 was used at 1.0 and 2.0 μm, BACE1 level was significantly higher in Aβ42- plus CP681301-treated cultures than in cultures treated with Aβ42 alone. This is probably due to the fact that, unexpectedly, the Cdk5 inhibitor alone elevated BACE1 protein to ∼200–300% of control (Fig. 3). These data suggested that Cdk5 did not mediate the Aβ42-induced BACE1 increase, as Cdk5 inhibition failed to attenuate the BACE1 elevation, at least in primary neuron cultures.
FIGURE 3.
Selective Cdk5 inhibitor CP681301 does not block the Aβ42-induced BACE1 elevation and increases BACE1 levels when used alone on primary neurons. After 7 days in culture, primary murine embryonic neurons were pretreated with CP681301 at 0.5, 1.0, 2.0 μm or DMSO (control) for 3 h and then were exposed to 10 μm oligomeric Aβ42 or vehicle for 24 h. Neurons were lysed in RIPA buffer, and 15 μg of protein per lane were analyzed by immunoblot (right panels). Similar to our previous results (Fig. 2), BACE1 levels were increased to ∼150% of control by Aβ42 treatment (left panels). Surprisingly, CP681301 not only failed to prevent the Aβ42-induced BACE1 elevation at every concentration, but instead appeared to cause an even greater BACE1 increase when in combination with Aβ42 (∼250–350% of control). In addition, CP681301 treatment alone elevated BACE1 levels to ∼200–300% of control at all concentrations. These results suggest that Cdk5 activation is not responsible for the Aβ42-induced BACE1 elevation in primary neuron cultures. Error bars, mean ± S.E.; *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001.
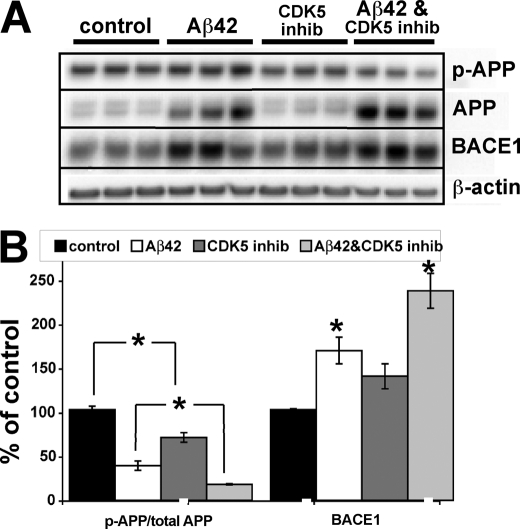
To determine whether CP681301 treatment had effectively blocked Cdk5 activity in our neuronal cultures, we verified that CP681301 could inhibit phosphorylation at threonine 668 of APP, a known Cdk5 target (Fig. 4) (55). Treatment with oligomeric Aβ42 greatly elevated total APP levels in neurons, possibly by increasing generation or decreasing degradation of APP, although the marked increase in the lower immaturely glycosylated APP immunoblot band suggested greater APP synthesis (Fig. 4A). As a result of increased APP levels, the ratio of phosphorylated to total APP was markedly reduced in Aβ42-treated cultures, complicating comparison of phosphorylation between the different culture conditions. To assess whether CP681301 had inhibited Cdk5 kinase activity, we compared the ratios of phosphorylated to total APP only between cultures with equivalent amounts of total APP. When examined in this manner, CP681301 significantly reduced the ratio of phosphorylated to total APP in CP681301-only treated compared with control neurons, and in Aβ42 + CP681301-treated compared with Aβ42-only treated cultures (Fig. 4B). The concentration used in this experiment, 0.5 μm, was the IC50 of CP681301 in culture.3 In Aβ42-treated neuron cultures, CP681301 reduced APP phosphorylation by about one-half, from 38% of control to 17% of control (Fig. 4B). Importantly, BACE1 levels were still elevated in Aβ42 + CP681301-treated neurons, despite significant Cdk5 inhibition, demonstrating that blocking Cdk5 activity did not prevent the Aβ42-induced BACE1 elevation. As we observed before (Fig. 3), the cultures treated with both Aβ42 and the Cdk5 inhibitor exhibited greater BACE1 levels than those treated with Aβ42 alone, but in this experiment the difference was not statistically significant (p = 0.052).
FIGURE 4.
CP681301 inhibits Cdk5 phosphorylation of APP but fails to attenuate Aβ42-induced BACE1 elevation in primary neurons. A, murine primary embryonic neurons were cultured for 7 days, pretreated with CP681301 at 0.5 μm or DMSO vehicle for 3 h, and then exposed to 10 μm oligomeric Aβ42 for 48 h. Neurons were lysed in RIPA buffer, and 15 μg of protein per lane were analyzed by immunoblot. Note that both BACE1 and total APP levels were markedly elevated by Aβ42 treatment. B, immunoblot signals in A were quantified on a phosphorimager, normalized using actin signals, and graphed as the percent of vehicle-treated control cultures. Aβ42 treatment increased BACE1 levels to ∼175% of control, whereas Aβ42 +CP681301 raised BACE1 levels to ∼250% of control. CP681301-alone treated cultures showed a nonsignificant trend toward BACE1 elevation. Because total APP levels were markedly increased by Aβ42 treatment, the ratio of phosphorylated to total APP (p-APP/total APP) was significantly reduced in Aβ42-treated neurons even though absolute levels of phosphorylated APP were slightly higher. As expected, Aβ42 + CP681301 treatment decreased the p-APP/total APP ratio compared with Aβ42-alone treatment. CP681301 alone also lowered the p-APP/total APP ratio compared with vehicle control. These results demonstrate that BACE1 levels were dramatically elevated in the presence of Aβ42 plus CP681301 despite the fact that Cdk5 activity was significantly inhibited by CP681301, indicating a mechanism other than Cdk5 was involved in the Aβ42-induced BACE1 elevation in primary neuron cultures. Error bars, mean ± S.E.; *, p ≤ 0.05.
CP681301 Alone Elevates BACE1, Decreases Cdk5 Protein, and Causes Caspase 3 Cleavage
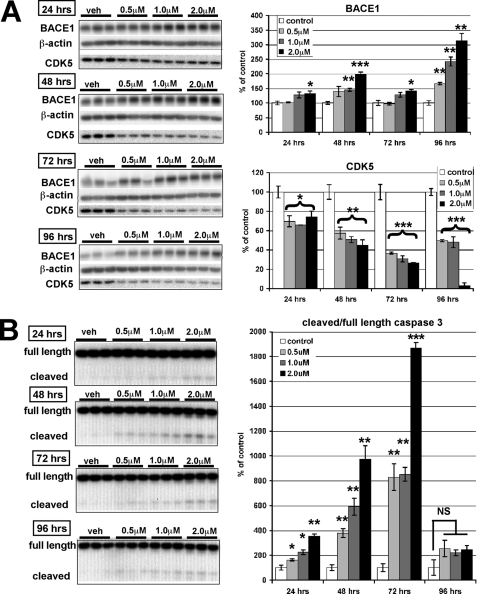
As noted above, we typically observed a significant increase in BACE1 level in neurons exposed to CP681301 alone. This effect could be problematic with regard to Cdk5 inhibition as an AD therapeutic approach, as Cdk5 inhibitors may need to be taken long term and perhaps started before symptoms appear. Using cultured primary neurons, we tested whether longer exposures to Cdk5 inhibition had an effect on BACE1 levels. Indeed, we found a clear time- and dose-dependent increase in BACE1 levels in response to Cdk5 inhibition (Fig. 5A). Surprisingly, we also observed a decrease in Cdk5 levels with exposure to CP681301. For some doses and incubation times, the BACE1 increase caused by CP681301-alone treatment was not as marked as in our previous experiment (Fig. 4), perhaps because these neurons had been cultured for 14 days rather than 7 days. Even at the lowest concentration (0.5 μm), long term exposure (96 h) to CP681301 resulted in significant BACE1 elevation (Fig. 5A).
FIGURE 5.
CP681301 has dose- and time-dependent effects on BACE1, Cdk5, and caspase 3 cleavage in primary neurons. A, after 14 days in culture, murine primary embryonic neurons were treated with CP681301 at 0.5, 1.0, and 2.0 μm or DMSO vehicle (veh) for 24, 48, 72, or 96 h and then lysed in RIPA buffer. Immunoblot analysis of treated neuron lysates showed that BACE1 levels increased in a time- and dose-dependent fashion, whereas Cdk5 levels were significantly reduced. B, cleaved 17-kDa caspase 3 fragment in lysates of treated primary neuron cultures in A was analyzed by immunoblot. The ratio of cleaved to full-length caspase 3 was maximal at 72 h with all concentrations of CP681301, slightly anticipating the maximum BACE1 increase observed at 96 h (A). Error bars, mean ± S.E.; *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001; NS, not significant.
We next investigated whether CP681301 treatment could have increased the BACE1 level by activating caspase 3. In primary neuron cultures exposed to CP681301, levels of the cleaved 17-kDa caspase 3 fragment (indicative of caspase 3 activation) increased starting at 24 h of 0.5 μm treatment (Fig. 5B), whereas the BACE1 elevation did not become significant until 96 h in this experiment (Fig. 5A). Clearly, a large lag existed between caspase 3 cleavage and the increase in BACE1 level in response to CP681301 treatment.
To determine whether the effects of CP681301 were specific to this compound or in fact related to Cdk5 inhibition in general, we tested the effects of the widely used Cdk5 inhibitor roscovitine on the Aβ42-induced BACE1 increase (supplemental Fig. 1). Neurons were treated for 48 h with 10 μm roscovitine (56), with similar outcome to CP681301 treatment. Like CP681301, roscovitine in combination with Aβ42 increased BACE1 levels above that of Aβ42 alone, and roscovitine-only treatment also elevated BACE1 levels (supplemental Fig. 1). In addition, roscovitine plus Aβ42 and roscovitine alone caused increased caspase 3 cleavage. Finally, roscovitine reduced both APP phosphorylation and Cdk5 protein levels, similar to CP681301. These results suggested that the effects of the Cdk5 inhibitors on BACE1 and caspase 3 cleavage were likely consequences of Cdk5 inhibition and not merely off-target effects of CP681301. The decrease in Cdk5 protein was unexpected. It has been shown that various apoptotic agents decrease Cdk5 and p35 levels (57), and we observed caspase 3 cleavage, suggesting that Cdk5 inhibition reduces Cdk5 levels through a caspase 3/apoptosis-dependent mechanism.
Inhibition of Caspase 3 Does Not Block the Aβ42-induced BACE1 Increase
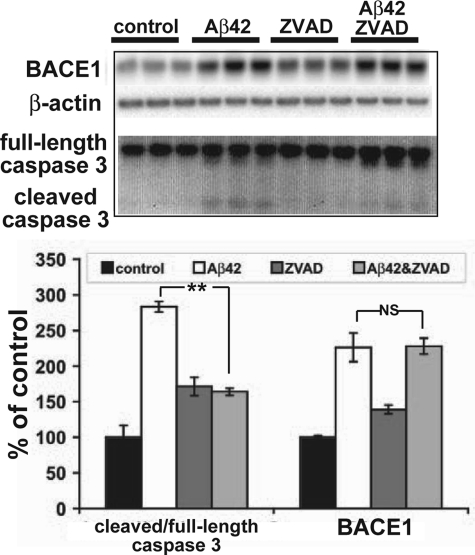
Because our results with CP681301 and roscovitine treatment allowed us to exclude our first hypothesis that inhibition of Cdk5 activity would attenuate the Aβ42-induced BACE1 increase, we tested the possibility that inhibition of caspase 3 could block the BACE1 elevation. Primary neuronal cultures were pretreated with 100 μm ZVAD, a general caspase inhibitor, or vehicle for 1 h and then were exposed to 10 μm oligomeric Aβ42. After 24 or 48 h, caspase 3 cleavage was significantly reduced in the Aβ42- plus ZVAD-treated cultures compared with the cultures treated with Aβ42 alone, but BACE1 levels still remained elevated (Fig. 6). ZVAD alone caused a small increase in BACE1 levels. These results suggested that caspase 3 activation was not responsible for increased BACE1 levels in response to Aβ42, at least in primary neuron cultures.
FIGURE 6.
Inhibition of caspase 3 cleavage does not block Aβ42-induced BACE1 elevation in primary neurons. Primary neuron cultures were pretreated with 100 μm ZVAD for 24 h and then exposed to 10 μm oligomeric Aβ42 or vehicle for 48 h. Neurons were lysed in RIPA buffer and analyzed for BACE1, caspase 3, and β-actin by immunoblot (15 μg/lane; upper panel). BACE1 ECL signals were quantified by phosphorimager and normalized to actin signals, whereas the ratio of cleaved 17-kDa fragment to full-length caspase 3 was calculated directly from ECL signals. Data were displayed as percentage of appropriate vehicle controls (lower panel). Pretreatment with ZVAD significantly reduced the ratio of cleaved to full-length caspase 3 cleavage caused by Aβ42, but it had no effect on the Aβ42-induced BACE1 elevation in primary neurons. Error bars, mean ± S.E.; **, p ≤ 0.01; NS, not significant.
Lower Concentrations of Aβ42 Increase BACE1 Levels without Inducing Caspase 3 Cleavage in Primary Neurons
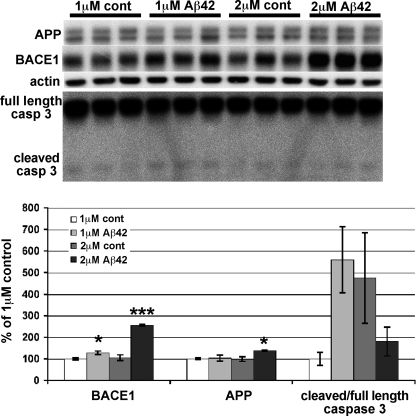
To investigate whether concentrations of Aβ42 lower than 10 μm could also increase BACE1 levels in primary neurons, we treated cultures with 1 or 2 μm oligomeric Aβ42 for 5 days and analyzed lysates for BACE1, APP, and caspase 3 by immunoblot (Fig. 7). Neurons treated with lower concentrations of Aβ42 also had elevated BACE1 levels. Although the BACE1 increase for 1 μm Aβ42 was modest (∼130% of control), the BACE1 elevation for the 2 μm Aβ42 treatment reached levels similar to those for 1–2 days of 10 μm Aβ42 treatment (∼250% of control). Under these conditions, the APP level increased slightly (2 μm Aβ42) or not at all (1 μm Aβ42). Importantly, although the cleaved/full-length caspase 3 ratio exhibited a high degree of variability in this experiment, it did not show a statistically significant increase as a result of 1 or 2 μm Aβ42 treatment, compared with controls. These results suggested that caspase 3 cleavage was not required for the Aβ42-induced BACE1 increase in primary neurons.
FIGURE 7.
Longer exposure to lower concentrations of Aβ42 elevates BACE1 level but not caspase 3 cleavage in primary neurons. Mouse primary cortical neurons were cultured for 7 days and treated with 1 or 2 μm oligomeric Aβ42 or appropriate vehicle controls for 5 days. Neurons were lysed in RIPA buffer and analyzed for BACE1, APP, caspase 3, and β-actin by immunoblot (5 μg/lane; upper panel). ECL signals were quantified by phosphorimager, and BACE1 and APP signals were normalized to actin signals and displayed as percentage of 1 μm Aβ42 vehicle control culture (lower panel). The ratio of cleaved 17-kDa fragment to full-length caspase 3 was calculated directly from ECL signals. Both 1 and 2 μm Aβ42 treatment significantly elevated BACE1 level in primary neurons compared with controls. A large variation in the ratio of cleaved 17-kDa fragment to full-length caspase 3 was observed, primarily because of low signals for the 17-kDa fragment, but no statistically significant changes were present for any condition. APP level was not altered by 1 μm Aβ42 and was only slightly increased with 2 μm Aβ42 treatment. Error bars, mean ± S.E.; *, p ≤ 0.05; ***, p ≤ 0.001.
Aβ42 Treatment Increases BACE1 via a Post-transcriptional Mechanism in Primary Neurons
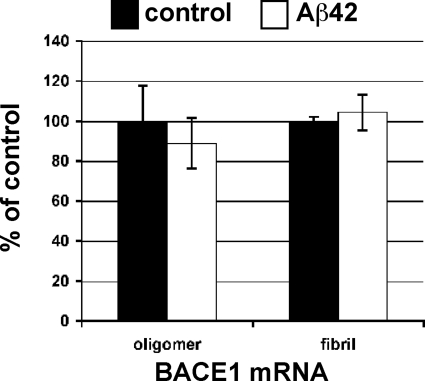
Because the Aβ42-induced BACE1 elevation in primary neurons was not attenuated by either Cdk5 or caspase 3 inhibition, other molecular mechanisms appeared responsible. Aβ is known to exert pleiotropic effects on neurons, such as disrupting calcium signaling (58), altering kinase signaling pathways (59), and affecting NMDA receptor-mediated signaling and synaptic plasticity (53, 60). Although our results appeared to exclude Cdk5, which may activate BACE1 gene expression via Stat3 (31), we explored the possibility that Aβ42 could raise BACE1 levels through increased gene transcription in primary neurons. To do so, we treated mouse primary cortical neuron cultures with 10 μm oligomeric or fibrillar Aβ42 or the appropriate vehicle controls and quantified BACE1 mRNA by TaqMan quantitative real time PCR (Fig. 8). We observed no difference in BACE1 mRNA levels in neurons under treatment with either form of Aβ42, as compared with controls. We performed additional experiments in which we treated primary neuron cultures with 1 or 2 μm oligomeric Aβ42, and again we found no statistically significant difference in BACE1 mRNA levels between Aβ42-treated and control groups (data not shown).
FIGURE 8.
BACE1 mRNA levels are unaffected by Aβ42 treatment in primary neurons. Triplicate wells of mouse primary cortical neurons were cultured for 7 days and treated with 10 μm oligomeric or fibrillar Aβ42 or appropriate vehicle controls for 24 h. Neurons were lysed and mRNA was isolated and analyzed by TaqMan quantitative RT-PCR for levels of BACE1 mRNA, which was normalized to 18 S rRNA and displayed as percentage of vehicle control. No statistically significant difference between BACE1 mRNA levels in Aβ42-treated or control primary neuron cultures was observed.
To determine whether protein synthesis was required for the Aβ42-induced BACE1 elevation, primary neurons were exposed to 10 μm oligomeric Aβ42 with or without 10 μg/ml cycloheximide (61) for 24 h. Neurons were lysed in RIPA and lysates analyzed by immunoblot with anti-BACE1 antibody (supplemental Fig. 2). Compared with vehicle, Aβ42 treatment produced a significant increase in BACE1 levels, as observed previously. As expected, treatment with cycloheximide alone reduced BACE1 levels in primary neurons. Importantly, cycloheximide plus Aβ42 treatment prevented the Aβ42-induced increase in neuronal BACE1 levels. This result indicates that BACE1 mRNA translation is required for Aβ42-induced BACE1 elevation. Taken together, our TaqMan and cycloheximide experiments suggest that Aβ42 increases BACE1 level through a post-transcriptional mechanism in primary neuron cultures. These results also support the conclusion that the Aβ42-induced BACE1 elevation in primary neurons is not the result of Cdk5-mediated BACE1 gene expression.
DISCUSSION
Studies from several groups have determined that BACE1 levels are elevated in AD brain and suggest that the BACE1 increase may play a role in AD pathogenesis. Therefore, understanding the molecular mechanism of the BACE1 elevation may provide new insights of therapeutic value for AD. Cdk5 has been implicated in neurodegeneration and AD, and our results here with human post-mortem and 5XFAD transgenic mouse brain samples (Fig. 1) provide additional data supporting an association of Cdk5 with AD (28, 39) and corroborate previous studies reporting elevated BACE1 levels and/or activity associated with AD (14–18).
Availability of an in vitro model to investigate Aβ42-induced BACE1 elevation in neurons would facilitate elucidation of the molecular mechanisms responsible for the BACE1 increase, which may represent novel therapeutic targets. This is the first study that oligomeric and fibrillar Aβ42 elevate BACE1 in murine primary cortical neurons. Both fibrillar and oligomeric Aβ42 raised BACE1 levels in primary neurons, although oligomeric Aβ42 had a more potent effect (Fig. 2). We used our primary neuron culture model to investigate the potential involvement of Cdk5 and caspase 3 in the Aβ42-induced BACE1 elevation, two molecules that have been previously implicated in regulation of BACE1 level and AD (31, 36).
Although we showed that levels of Cdk5 and its activator proteins p25 and p35 were dysregulated in post-mortem AD brain, 5XFAD transgenic mouse brain, and Aβ42-treated primary neurons, our results suggest that increased Cdk5 levels or activity may not be responsible for the Aβ42-induced BACE1 increase, at least in primary neuron culture. Treatment of primary neurons with the Cdk5 inhibitor CP681301 at its IC50 concentration of 0.5 μm3 decreased APP phosphorylation by ∼50% but did not attenuate the BACE1 increase (Fig. 4B). Unexpectedly, CP681301 appeared to potentiate the Aβ42-induced BACE1 increase in neurons and was able to elevate BACE1 levels when administered alone. CP681301 treatment also increased caspase 3 cleavage (Fig. 5B), suggesting it may induce apoptosis at certain doses. Results similar to those of CP681301 treatment were obtained with a different, well studied Cdk5 inhibitor, roscovitine (supplemental Fig. 1), demonstrating that the effects of CP681301 were likely caused by Cdk5 inhibition and were not the result of compound-specific side effects.
In addition, inhibition of caspase 3 cleavage with ZVAD also failed to block the BACE1 increase in Aβ42-treated primary neurons (Fig. 6). Lower concentrations (1–2 μm) of Aβ42 elevated BACE1 levels in primary neurons without increasing caspase 3 cleavage (Fig. 7), suggesting a caspase 3-independent mechanism for the Aβ42-induced increase.
Finally, although 10 μm oligomeric or fibrillar Aβ42 treatment both significantly increased BACE1 levels, these conditions had no effect on the BACE1 mRNA level in primary neurons (Fig. 8). Similarly, treatment of primary neurons with lower concentrations (1–2 μm) of oligomeric Aβ42 did not increase BACE1 mRNA levels (data not shown). In addition, the BACE1 increase appeared to require protein synthesis (supplemental Fig. 2). Taken together, our results suggest that neither Cdk5 nor caspase 3 is responsible for the Aβ42-induced BACE1 elevation in primary neurons, but they point toward a post-transcriptional mechanism, such as an increase in BACE1 mRNA translation. Recently, we have described a translational control mechanism of BACE1 that involves phosphorylation of the translation initiation factor eIF2α (17). In future work it will be of interest to explore the potential role of eIF2α phosphorylation in the Aβ42-induced BACE1 elevation in our primary neuron model.
We deliberately chose to use micromolar (1–10 μm) concentrations of Aβ42 to treat primary neuron cultures in this study. It has been suggested that such concentrations are much higher than those found in the brain, even in Alzheimer patients. This may be true for the concentration of Aβ42 when averaged over the entire brain. However, we were interested in modeling the effects of high concentrations of Aβ42 in the immediate vicinity of amyloid plaques. Previously, we reported that BACE1 elevation is observed in dystrophic neurites in close proximity to plaques in the brains of AD patients and two independent APP transgenic mouse strains (18). A third APP transgenic strain also exhibited BACE1 elevation around plaques (62). Given that the BACE1 elevation correlates with amyloid plaques in APP transgenic and AD brains, it is likely that the major constituent of plaques, Aβ42, rather than other APP processing products, is responsible for the BACE1 increase. The 1–10 μm Aβ42 concentrations that we used in our experiments here produced increases of BACE1 levels in primary neuron cultures comparable with those observed in APP transgenic and AD brains. Clearly, a very high concentration of fibrillar Aβ42 exists in plaques, and evidence suggests that plaques are surrounded by halos of Aβ42 oligomers associated with decreased synaptic density (63). This synaptic loss may be responsible for memory impairment in AD (48, 52) before frank neuronal loss, so understanding detrimental changes in synapses and neurites exposed to high levels of oligomeric and/or fibrillar Aβ42 near plaques may suggest novel therapeutic approaches to slow memory loss.
Inhibition of caspase 3 cleavage with ZVAD blocks the BACE1 increase induced by the apoptotic agents staurosporine and etoposide in cultured human H4 neuroglioma cells (36). The inability of ZVAD to inhibit the Aβ42-induced BACE1 elevation in murine primary neurons suggests that Aβ42 may elevate BACE1 through a mechanism independent of caspase 3 cleavage. Because staurosporine and etoposide increased BACE1 level 6–9-fold (36), and Aβ42 elevated BACE1 ∼150–250%, it seems plausible that apoptotic agents and Aβ42 may increase the BACE1 levels by different mechanisms, at least in cell culture.
We report that Cdk5 level is elevated in AD brain, although other publications have reported that Cdk5 levels appear unchanged (28, 40, 43, 44). This may be related to the degree of neuronal loss in the AD brain samples we analyzed, as another group reported that when normalized to synaptophysin levels, which are decreased in AD, Cdk5 level was actually increased (64). In 2-month-old 5XFAD mice, which may represent an early stage of the disease, both p35 and p25 levels were elevated equally and to the same degree as Cdk5, suggesting that the Cdk5-p35/25 system may be up-regulated early in AD pathogenesis. In post-mortem AD brains, which represent a late end-stage of the disease, we observed that although p35 was not elevated, the levels of p25 were slightly raised leading to an increased p25:p35 ratio, suggesting that increased cleavage of p35 may be associated with AD (28).
Whether the p25:p35 ratio is increased in the brains of AD patients remains controversial. Tsai and co-workers (28, 39) reported an elevated p25:p35 ratio in AD, although others observe no change in ratio (40–44) or even a decrease (45). It has been shown in rat brain that p25 cleavage increases with post-mortem interval (44), so it has been asserted that the post-mortem interval should be short to measure an accurate p25:p35 ratio (39, 40). Variability of control cases and sample handling also affect the accuracy of p25:p35 ratio measurements, as post-mortem ischemia and freeze-thaw cycles may lead to cleavage of p35 (39). Our AD and control groups had an average post-mortem interval of 6.5 h, ranging from 3.5 to 14.75 h (see supplemental Table 1). Only the studies by Tseng et al. (39), reporting an increase in p25:p35, and by Taniguchi et al. (44), reporting no change, had shorter average post-mortem intervals than our own. Perhaps the relatively short post-mortem interval of our sample group, along with use of protease inhibitors and careful sample handling, allowed us to detect a small increase in p25:p35 ratio in the AD group, although other studies did not.
We did not expect Cdk5 inhibition to elevate BACE1 in primary neuron cultures, as another group reported that the compound reduced BACE1 levels in the brains of treated mice (31). A single injection of CP681301 reduced Stat3 phosphorylation and BACE1 levels within 4 h in 4–6-day-old wild type and p25 transgenic mice (31). This coincides with a time of high BACE1 expression in the early post-natal mouse brain. BACE1 levels peak between P0 and P8 at 8–10 times the adult level and then decrease during postnatal weeks 2 and 3 (25). It is possible that Cdk5 and Stat3 may regulate BACE1 transcription during this post-natal period of high BACE1 expression, although the BACE1 increase observed in AD, APP transgenic mice, and Aβ42 treated primary neurons may be controlled by another mechanism. CP681301 was able to decrease cerebral BACE1 levels in the brains of wild type adult mice (31); however, the effect of the compound in a mouse model of AD has not yet been reported. In addition, chronic in vivo administration of CP681301 has not been addressed, which would be necessary for its validation as an AD therapeutic. Although we currently do not understand the mechanism of the BACE1 increase following CP681301 and roscovitine treatment of primary neurons, our results suggest that in vivo evaluation of Cdk5 inhibitors should include monitoring for BACE1 elevation and caspase 3 cleavage during chronic administration. Finally, because we have only tested the effects of CP681301 treatment on primary neurons in vitro, it is possible that the mechanisms of Aβ42-induced BACE1 elevation differ between neurons in culture versus neurons in the brain so that Cdk5 may still play a role in the AD-associated BACE1 increase in vivo.
In conclusion, we present data demonstrating that Aβ42 oligomers elevate BACE1 levels in cultured primary neurons through a post-transcriptional mechanism. Although Cdk5 and p25/p35 are dysregulated in AD brains and in the 5XFAD mouse model of AD and have been shown to influence BACE1 levels in other contexts, our data suggest that Cdk5 may not be responsible for Aβ42-induced BACE1 elevation, at least not in primary neurons in vitro. In addition, although caspase 3 cleavage is triggered by oligomeric Aβ42 and has been demonstrated to increase BACE1 in other systems, caspase 3 cleavage does not appear to be required for Aβ42-induced BACE1 elevation in primary neurons. Although Cdk5 inhibitors are under investigation as potential AD therapeutics, our data suggest that Cdk5 inhibition may potentially increase levels of BACE1 in neurons exposed to Aβ42 oligomers. Because Cdk5 inhibitors and Aβ42 synergize to elevate BACE1 levels above each treatment alone, and given the high cerebral amyloid loads of AD patients, the possibility exists that therapeutic inhibition of Cdk5 for AD may exacerbate disease progression rather than reduce it.
Supplementary Material
Acknowledgments
We acknowledge Dr. David A. Bennett and the Rush Memory and Aging Project (National Institutes of Health Grant R01AG17917) for the generous gift of human brain samples, Dr. Lit-Fui Lau (Pfizer, Inc.) for the donation of the selective Cdk5 inhibitor CP681301, and the laboratories of Drs. Linda Van Eldik, D. Martin Watterson, and Mary Jo Ladu for Aβ42 preparation and helpful advice.
This work was supported, in whole or in part, by National Institutes of Health Grants 5T32AG00026 and F32AG033445 (to K. R. S.) and R01AG030142 (to R. V.).

This article contains supplemental Table 1 and Figs. 1 and 2.
Lit-Fui Lau, Pfizer Inc., personal communication.
- APP
- amyloid precursor protein
- AD
- Alzheimer disease
- Aβ
- amyloid-β
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- FAD
- familial AD
- Z
- benzyloxycarbonyl.
REFERENCES
- 1. Vassar R., Bennett B. D., Babu-Khan S., Kahn S., Mendiaz E. A., Denis P., Teplow D. B., Ross S., Amarante P., Loeloff R., Luo Y., Fisher S., Fuller J., Edenson S., Lile J., Jarosinski M. A., Biere A. L., Curran E., Burgess T., Louis J. C., Collins F., Treanor J., Rogers G., Citron M. (1999) β-Secretase cleavage of Alzheimer amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741 [DOI] [PubMed] [Google Scholar]
- 2. Cai H., Wang Y., McCarthy D., Wen H., Borchelt D. R., Price D. L., Wong P. C. (2001) BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat. Neurosci. 4, 233–234 [DOI] [PubMed] [Google Scholar]
- 3. Luo Y., Bolon B., Kahn S., Bennett B. D., Babu-Khan S., Denis P., Fan W., Kha H., Zhang J., Gong Y., Martin L., Louis J. C., Yan Q., Richards W. G., Citron M., Vassar R. (2001) Mice deficient in BACE1, the Alzheimer β-secretase, have normal phenotype and abolished β-amyloid generation. Nat. Neurosci. 4, 231–232 [DOI] [PubMed] [Google Scholar]
- 4. Roberds S. L., Anderson J., Basi G., Bienkowski M. J., Branstetter D. G., Chen K. S., Freedman S. B., Frigon N. L., Games D., Hu K., Johnson-Wood K., Kappenman K. E., Kawabe T. T., Kola I., Kuehn R., Lee M., Liu W., Motter R., Nichols N. F., Power M., Robertson D. W., Schenk D., Schoor M., Shopp G. M., Shuck M. E., Sinha S., Svensson K. A., Tatsuno G., Tintrup H., Wijsman J., Wright S., McConlogue L. (2001) BACE knockout mice are healthy despite lacking the primary β-secretase activity in brain. Implications for Alzheimer disease therapeutics. Hum. Mol. Genet. 10, 1317–1324 [DOI] [PubMed] [Google Scholar]
- 5. Kao S. C., Krichevsky A. M., Kosik K. S., Tsai L. H. (2004) BACE1 suppression by RNA interference in primary cortical neurons. J. Biol. Chem. 279, 1942–1949 [DOI] [PubMed] [Google Scholar]
- 6. Laird F. M., Cai H., Savonenko A. V., Farah M. H., He K., Melnikova T., Wen H., Chiang H. C., Xu G., Koliatsos V. E., Borchelt D. R., Price D. L., Lee H. K., Wong P. C. (2005) BACE1, a major determinant of selective vulnerability of the brain to amyloid-β amyloidogenesis, is essential for cognitive, emotional, and synaptic functions. J. Neurosci. 25, 11693–11709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Singer O., Marr R. A., Rockenstein E., Crews L., Coufal N. G., Gage F. H., Verma I. M., Masliah E. (2005) Targeting BACE1 with siRNAs ameliorates Alzheimer disease neuropathology in a transgenic model. Nat. Neurosci. 8, 1343–1349 [DOI] [PubMed] [Google Scholar]
- 8. Bodendorf U., Danner S., Fischer F., Stefani M., Sturchler-Pierrat C., Wiederhold K. H., Staufenbiel M., Paganetti P. (2002) Expression of human β-secretase in the mouse brain increases the steady-state level of β-amyloid. J. Neurochem. 80, 799–806 [DOI] [PubMed] [Google Scholar]
- 9. Chiocco M. J., Kulnane L. S., Younkin L., Younkin S., Evin G., Lamb B. T. (2004) Altered amyloid-β metabolism and deposition in genomic-based β-secretase transgenic mice. J. Biol. Chem. 279, 52535–52542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee E. B., Zhang B., Liu K., Greenbaum E. A., Doms R. W., Trojanowski J. Q., Lee V. M. (2005) BACE overexpression alters the subcellular processing of APP and inhibits Aβ deposition in vivo. J. Cell Biol. 168, 291–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mohajeri M. H., Saini K. D., Nitsch R. M. (2004) Transgenic BACE expression in mouse neurons accelerates amyloid plaque pathology. J. Neural Transm. 111, 413–425 [DOI] [PubMed] [Google Scholar]
- 12. Mullan M., Crawford F., Axelman K., Houlden H., Lilius L., Winblad B., Lannfelt L. (1992) A pathogenic mutation for probable Alzheimer disease in the APP gene at the N terminus of β-amyloid. Nat. Genet. 1, 345–347 [DOI] [PubMed] [Google Scholar]
- 13. Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. (1992) Mutation of the β-amyloid precursor protein in familial Alzheimer disease increases β-protein production. Nature 360, 672–674 [DOI] [PubMed] [Google Scholar]
- 14. Fukumoto H., Cheung B. S., Hyman B. T., Irizarry M. C. (2002) β-Secretase protein and activity are increased in the neocortex in Alzheimer disease. Arch. Neurol. 59, 1381–1389 [DOI] [PubMed] [Google Scholar]
- 15. Holsinger R. M., McLean C. A., Beyreuther K., Masters C. L., Evin G. (2002) Increased expression of the amyloid precursor β-secretase in Alzheimer disease. Ann. Neurol. 51, 783–786 [DOI] [PubMed] [Google Scholar]
- 16. Li R., Lindholm K., Yang L. B., Yue X., Citron M., Yan R., Beach T., Sue L., Sabbagh M., Cai H., Wong P., Price D., Shen Y. (2004) Amyloid β peptide load is correlated with increased β-secretase activity in sporadic Alzheimer disease patients. Proc. Natl. Acad. Sci. U.S.A. 101, 3632–3637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Connor T., Sadleir K. R., Maus E., Velliquette R. A., Zhao J., Cole S. L., Eimer W. A., Hitt B., Bembinster L. A., Lammich S., Lichtenthaler S. F., Hébert S. S., De Strooper B., Haass C., Bennett D. A., Vassar R. (2008) Phosphorylation of the translation initiation factor eIF2α increases BACE1 levels and promotes amyloidogenesis. Neuron 60, 988–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao J., Fu Y., Yasvoina M., Shao P., Hitt B., O'Connor T., Logan S., Maus E., Citron M., Berry R., Binder L., Vassar R. (2007) β-Site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques. Implications for Alzheimer disease pathogenesis. J. Neurosci. 27, 3639–3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dominguez D., Tournoy J., Hartmann D., Huth T., Cryns K., Deforce S., Serneels L., Camacho I. E., Marjaux E., Craessaerts K., Roebroek A. J., Schwake M., D'Hooge R., Bach P., Kalinke U., Moechars D., Alzheimer C., Reiss K., Saftig P., De Strooper B. (2005) Phenotypic and biochemical analyses of BACE1- and BACE2-deficient mice. J. Biol. Chem. 280, 30797–30806 [DOI] [PubMed] [Google Scholar]
- 20. Harrison S. M., Harper A. J., Hawkins J., Duddy G., Grau E., Pugh P. L., Winter P. H., Shilliam C. S., Hughes Z. A., Dawson L. A., Gonzalez M. I., Upton N., Pangalos M. N., Dingwall C. (2003) BACE1 (β-secretase) transgenic and knockout mice. Identification of neurochemical deficits and behavioral changes. Mol. Cell. Neurosci. 24, 646–655 [DOI] [PubMed] [Google Scholar]
- 21. Ohno M., Chang L., Tseng W., Oakley H., Citron M., Klein W. L., Vassar R., Disterhoft J. F. (2006) Temporal memory deficits in Alzheimer mouse models. Rescue by genetic deletion of BACE1. Eur. J. Neurosci. 23, 251–260 [DOI] [PubMed] [Google Scholar]
- 22. Ohno M., Sametsky E. A., Younkin L. H., Oakley H., Younkin S. G., Citron M., Vassar R., Disterhoft J. F. (2004) BACE1 deficiency rescues memory deficits and cholinergic dysfunction in a mouse model of Alzheimer disease. Neuron 41, 27–33 [DOI] [PubMed] [Google Scholar]
- 23. Savonenko A. V., Melnikova T., Laird F. M., Stewart K. A., Price D. L., Wong P. C. (2008) Alteration of BACE1-dependent NRG1/ErbB4 signaling and schizophrenia-like phenotypes in BACE1-null mice. Proc. Natl. Acad. Sci. U.S.A. 105, 5585–5590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hu X., Hicks C. W., He W., Wong P., Macklin W. B., Trapp B. D., Yan R. (2006) Bace1 modulates myelination in the central and peripheral nervous system. Nat. Neurosci. 9, 1520–1525 [DOI] [PubMed] [Google Scholar]
- 25. Willem M., Garratt A. N., Novak B., Citron M., Kaufmann S., Rittger A., DeStrooper B., Saftig P., Birchmeier C., Haass C. (2006) Control of peripheral nerve myelination by the β-secretase BACE1. Science 314, 664–666 [DOI] [PubMed] [Google Scholar]
- 26. Zhang X. M., Cai Y., Xiong K., Cai H., Luo X. G., Feng J. C., Clough R. W., Struble R. G., Patrylo P. R., Yan X. X. (2009) β-Secretase-1 elevation in transgenic mouse models of Alzheimer disease is associated with synaptic/axonal pathology and amyloidogenesis. Implications for neuritic plaque development. Eur. J. Neurosci. 30, 2271–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee M. S., Kwon Y. T., Li M., Peng J., Friedlander R. M., Tsai L. H. (2000) Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405, 360–364 [DOI] [PubMed] [Google Scholar]
- 28. Patrick G. N., Zukerberg L., Nikolic M., de la Monte S., Dikkes P., Tsai L. H. (1999) Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402, 615–622 [DOI] [PubMed] [Google Scholar]
- 29. Cruz J. C., Kim D., Moy L. Y., Dobbin M. M., Sun X., Bronson R. T., Tsai L. H. (2006) p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid β in vivo. J. Neurosci. 26, 10536–10541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wen Y., Planel E., Herman M., Figueroa H. Y., Wang L., Liu L., Lau L. F., Yu W. H., Duff K. E. (2008) Interplay between cyclin-dependent kinase 5 and glycogen synthase kinase 3β mediated by neuregulin signaling leads to differential effects on Tau phosphorylation and amyloid precursor protein processing. J. Neurosci. 28, 2624–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wen Y., Yu W. H., Maloney B., Bailey J., Ma J., Marié I., Maurin T., Wang L., Figueroa H., Herman M., Krishnamurthy P., Liu L., Planel E., Lau L. F., Lahiri D. K., Duff K. (2008) Transcriptional regulation of β-secretase by p25/cdk5 leads to enhanced amyloidogenic processing. Neuron 57, 680–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Louneva N., Cohen J. W., Han L. Y., Talbot K., Wilson R. S., Bennett D. A., Trojanowski J. Q., Arnold S. E. (2008) Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer disease. Am. J. Pathol. 173, 1488–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Selznick L. A., Holtzman D. M., Han B. H., Gökden M., Srinivasan A. N., Johnson E. M., Jr., Roth K. A. (1999) In situ immunodetection of neuronal caspase-3 activation in Alzheimer disease. J. Neuropathol. Exp. Neurol. 58, 1020–1026 [DOI] [PubMed] [Google Scholar]
- 34. Stadelmann C., Deckwerth T. L., Srinivasan A., Bancher C., Brück W., Jellinger K., Lassmann H. (1999) Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer disease. Evidence for apoptotic cell death. Am. J. Pathol. 155, 1459–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Su J. H., Zhao M., Anderson A. J., Srinivasan A., Cotman C. W. (2001) Activated caspase-3 expression in Alzheimer and aged control brain. Correlation with Alzheimer pathology. Brain Res. 898, 350–357 [DOI] [PubMed] [Google Scholar]
- 36. Tesco G., Koh Y. H., Kang E. L., Cameron A. N., Das S., Sena-Esteves M., Hiltunen M., Yang S. H., Zhong Z., Shen Y., Simpkins J. W., Tanzi R. E. (2007) Depletion of GGA3 stabilizes BACE and enhances β-secretase activity. Neuron 54, 721–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Oakley H., Cole S. L., Logan S., Maus E., Shao P., Craft J., Guillozet-Bongaarts A., Ohno M., Disterhoft J., Van Eldik L., Berry R., Vassar R. (2006) Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer disease mutations. Potential factors in amyloid plaque formation. J. Neurosci. 26, 10129–10140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stine W. B., Jr., Dahlgren K. N., Krafft G. A., LaDu M. J. (2003) In vitro characterization of conditions for amyloid-β peptide oligomerization and fibrillogenesis. J. Biol. Chem. 278, 11612–11622 [DOI] [PubMed] [Google Scholar]
- 39. Tseng H. C., Zhou Y., Shen Y., Tsai L. H. (2002) A survey of Cdk5 activator p35 and p25 levels in Alzheimer disease brains. FEBS Lett. 523, 58–62 [DOI] [PubMed] [Google Scholar]
- 40. Borghi R., Giliberto L., Assini A., Delacourte A., Perry G., Smith M. A., Strocchi P., Zaccheo D., Tabaton M. (2002) Increase of cdk5 is related to neurofibrillary pathology in progressive supranuclear palsy. Neurology 58, 589–592 [DOI] [PubMed] [Google Scholar]
- 41. Nguyen K. C., Rosales J. L., Barboza M., Lee K. Y. (2002) Controversies over p25 in Alzheimer disease. J. Alzheimer's Dis. 4, 123–126 [DOI] [PubMed] [Google Scholar]
- 42. Takashima A., Murayama M., Yasutake K., Takahashi H., Yokoyama M., Ishiguro K. (2001) Involvement of cyclin-dependent kinase5 activator p25 on Tau phosphorylation in mouse brain. Neurosci. Lett. 306, 37–40 [DOI] [PubMed] [Google Scholar]
- 43. Tandon A., Yu H., Wang L., Rogaeva E., Sato C., Chishti M. A., Kawarai T., Hasegawa H., Chen F., Davies P., Fraser P. E., Westaway D., St George-Hyslop P. H. (2003) Brain levels of CDK5 activator p25 are not increased in Alzheimer or other neurodegenerative diseases with neurofibrillary tangles. J. Neurochem. 86, 572–581 [DOI] [PubMed] [Google Scholar]
- 44. Taniguchi S., Fujita Y., Hayashi S., Kakita A., Takahashi H., Murayama S., Saido T. C., Hisanaga S., Iwatsubo T., Hasegawa M. (2001) Calpain-mediated degradation of p35 to p25 in postmortem human and rat brains. FEBS Lett. 489, 46–50 [DOI] [PubMed] [Google Scholar]
- 45. Yoo B. C., Lubec G. (2001) p25 protein in neurodegeneration. Nature 411, 763–764 [DOI] [PubMed] [Google Scholar]
- 46. Bennett D. A., Schneider J. A., Buchman A. S., Mendes de Leon C., Bienias J. L., Wilson R. S. (2005) The Rush Memory and Aging Project. Study design and base-line characteristics of the study cohort. Neuroepidemiology 25, 163–175 [DOI] [PubMed] [Google Scholar]
- 47. Lacor P. N., Buniel M. C., Chang L., Fernandez S. J., Gong Y., Viola K. L., Lambert M. P., Velasco P. T., Bigio E. H., Finch C. E., Krafft G. A., Klein W. L. (2004) Synaptic targeting by Alzheimer-related amyloid β oligomers. J. Neurosci. 24, 10191–10200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lacor P. N., Buniel M. C., Furlow P. W., Clemente A. S., Velasco P. T., Wood M., Viola K. L., Klein W. L. (2007) Aβ oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer disease. J. Neurosci. 27, 796–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lambert M. P., Velasco P. T., Chang L., Viola K. L., Fernandez S., Lacor P. N., Khuon D., Gong Y., Bigio E. H., Shaw P., De Felice F. G., Krafft G. A., Klein W. L. (2007) Monoclonal antibodies that target pathological assemblies of Aβ. J. Neurochem. 100, 23–35 [DOI] [PubMed] [Google Scholar]
- 50. Lesné S., Koh M. T., Kotilinek L., Kayed R., Glabe C. G., Yang A., Gallagher M., Ashe K. H. (2006) A specific amyloid-β protein assembly in the brain impairs memory. Nature 440, 352–357 [DOI] [PubMed] [Google Scholar]
- 51. Lesné S., Kotilinek L., Ashe K. H. (2008) Plaque-bearing mice with reduced levels of oligomeric amyloid-β assemblies have intact memory function. Neuroscience 151, 745–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shankar G. M., Bloodgood B. L., Townsend M., Walsh D. M., Selkoe D. J., Sabatini B. L. (2007) Natural oligomers of the Alzheimer amyloid-β protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J. Neurosci. 27, 2866–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shankar G. M., Li S., Mehta T. H., Garcia-Munoz A., Shepardson N. E., Smith I., Brett F. M., Farrell M. A., Rowan M. J., Lemere C. A., Regan C. M., Walsh D. M., Sabatini B. L., Selkoe D. J. (2008) Amyloid-β protein dimers isolated directly from Alzheimer brains impair synaptic plasticity and memory. Nat. Med. 14, 837–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Snyder E. M., Nong Y., Almeida C. G., Paul S., Moran T., Choi E. Y., Nairn A. C., Salter M. W., Lombroso P. J., Gouras G. K., Greengard P. (2005) Regulation of NMDA receptor trafficking by amyloid-β. Nat. Neurosci. 8, 1051–1058 [DOI] [PubMed] [Google Scholar]
- 55. Lee M. S., Kao S. C., Lemere C. A., Xia W., Tseng H. C., Zhou Y., Neve R., Ahlijanian M. K., Tsai L. H. (2003) APP processing is regulated by cytoplasmic phosphorylation. J. Cell Biol. 163, 83–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zheng Y. L., Li B. S., Amin N. D., Albers W., Pant H. C. (2002) A peptide derived from cyclin-dependent kinase activator (p35) specifically inhibits Cdk5 activity and phosphorylation of Tau protein in transfected cells. Eur. J. Biochem. 269, 4427–4434 [DOI] [PubMed] [Google Scholar]
- 57. Kerokoski P., Suuronen T., Salminen A., Soininen H., Pirttilä T. (2001) The levels of cdk5 and p35 proteins and Tau phosphorylation are reduced during neuronal apoptosis. Biochem. Biophys. Res. Commun. 280, 998–1002 [DOI] [PubMed] [Google Scholar]
- 58. Zempel H., Thies E., Mandelkow E., Mandelkow E. M. (2010) Aβ oligomers cause localized Ca(2+) elevation, missorting of endogenous Tau into dendrites, Tau phosphorylation, and destruction of microtubules and spines. J. Neurosci. 30, 11938–11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ma Q. L., Yang F., Rosario E. R., Ubeda O. J., Beech W., Gant D. J., Chen P. P., Hudspeth B., Chen C., Zhao Y., Vinters H. V., Frautschy S. A., Cole G. M. (2009) β-Amyloid oligomers induce phosphorylation of Tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling. Suppression by omega-3 fatty acids and curcumin. J. Neurosci. 29, 9078–9089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yamin G. (2009) NMDA receptor-dependent signaling pathways that underlie amyloid β-protein disruption of LTP in the hippocampus. J. Neurosci. Res. 87, 1729–1736 [DOI] [PubMed] [Google Scholar]
- 61. Mengesdorf T., Jensen P. H., Mies G., Aufenberg C., Paschen W. (2002) Down-regulation of Parkin protein in transient focal cerebral ischemia. A link between stroke and degenerative disease? Proc. Natl. Acad. Sci. U.S.A. 99, 15042–15047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sheng J. G., Price D. L., Koliatsos V. E. (2003) The β-amyloid-related proteins presenilin 1 and BACE1 are axonally transported to nerve terminals in the brain. Exp. Neurol. 184, 1053–1057 [DOI] [PubMed] [Google Scholar]
- 63. Koffie R. M., Meyer-Luehmann M., Hashimoto T., Adams K. W., Mielke M. L., Garcia-Alloza M., Micheva K. D., Smith S. J., Kim M. L., Lee V. M., Hyman B. T., Spires-Jones T. L. (2009) Oligomeric amyloid β associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc. Natl. Acad. Sci. U.S.A. 106, 4012–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jacobs E. H., Williams R. J., Francis P. T. (2006) Cyclin-dependent kinase 5, Munc18a, and Munc18-interacting protein 1/X11α protein up-regulation in Alzheimer disease. Neuroscience 138, 511–522 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.