Background: The mechanism of PLB inhibition of SERCA2a activity remains unresolved.
Results: Cross-linking Lys27 of PLB to SERCA2a in human SR vesicles is conformationally dependent and correlates closely with enzyme inhibition.
Conclusion: PLB stabilizes the E2·ATP state, thereby blocking formation of E1.
Significance: Mutually exclusive binding of PLB and Ca2+ regulates SERCA2a activity in normal and failed human myocardium.
Keywords: Calcium, Calcium ATPase, Calcium Transport, Heart Failure, Sarcoplasmic Reticulum (SR), Human Heart, SERCA, Cross-linking, Phospholamban
Abstract
Chemical cross-linking was used to study protein binding interactions between native phospholamban (PLB) and SERCA2a in sarcoplasmic reticulum (SR) vesicles prepared from normal and failed human hearts. Lys27 of PLB was cross-linked to the Ca2+ pump at the cytoplasmic extension of M4 (at or near Lys328) with the homobifunctional cross-linker, disuccinimidyl glutarate (7.7 Å). Cross-linking was augmented by ATP but abolished by Ca2+ or thapsigargin, confirming in native SR vesicles that PLB binds preferentially to E2 (low Ca2+ affinity conformation of the Ca2+-ATPase) stabilized by ATP. To assess the functional effects of PLB binding on SERCA2a activity, the anti-PLB antibody, 2D12, was used to disrupt the physical interactions between PLB and SERCA2a in SR vesicles. We observed a tight correlation between 2D12-induced inhibition of PLB cross-linking to SERCA2a and 2D12 stimulation of Ca2+-ATPase activity and Ca2+ transport. The results suggest that the inhibitory effect of PLB on Ca2+-ATPase activity in SR vesicles results from mutually exclusive binding of PLB and Ca2+ to the Ca2+ pump, requiring PLB dissociation for catalytic activation. Importantly, the same result was obtained with SR vesicles prepared from normal and failed human hearts; therefore, we conclude that PLB binding interactions with the Ca2+ pump are largely unchanged in failing myocardium.
Introduction
Phospholamban (PLB)2 is a 52-amino acid, single-span membrane protein that regulates the activity of SERCA2a, the Ca2+-ATPase in cardiac SR (1). SERCA2a is a major Ca2+-handling protein in the heart that actively transports cytosolic Ca2+ into the lumen of the SR, inducing myofilament relaxation (2). The dephosphorylated PLB monomer binds to and inhibits Ca2+-ATPase activity by decreasing the apparent Ca2+ affinity of the enzyme (3, 4), having little or no effect on the Vmax of the enzyme measured at saturating Ca2+ concentration (1, 5). PLB monomers also oligomerize into noninhibitory homopentamers in the SR membrane (6) (stabilized by transmembrane Leu/Ile zipper interactions) (7), but the functional significance of the pentamer remains unclear. The inhibitory effect of PLB on SERCA2a activity is reversed when PLB is phosphorylated at Ser16 (by protein kinase A) or Thr17 (by calmodulin-dependent protein kinase) during β-adrenergic receptor stimulation of the heart, increasing the size of the contractile-dependent SR Ca2+-store and the rate of cardiac relaxation (1, 2). Similarly, anti-PLB monoclonal antibodies binding near the phosphorylation site also reverse Ca2+ pump inhibition by PLB (5, 8), with functional effects indistinguishable from those of β-adrenergic stimulation (9). As a key regulator of myocardial contractile kinetics, the physical basis of PLB inhibition of SERCA2a activity has long been an active area of investigation, both as it relates to normal cardiac function and to pathophysiological states such as in human heart failure (10).
One obstacle to solving the molecular mechanism of PLB action has been the inability monitor protein binding interactions between PLB and SERCA2a in SR membranes. Numerous biochemical and biophysical techniques have been employed to investigate physical interactions between PLB and SERCA, but nearly all have required cellular expression systems with mutated proteins (11–13) or fusion proteins (14, 15), and/or protein solubilization and reconstitution (16–19) for implementation. Techniques assessing protein-protein interactions have included co-immunoprecipitation (11), chemical cross-linking (12, 13, 17), FRET (14, 16, 18), and two-dimensional co-crystallization (2, 19), which have frequently given conflicting results. For example, some studies (including our own) suggest that the Ca2+ pump with bound PLB is catalytically inactive, requiring PLB dissociation prior to enzyme activation (11, 12, 13, 15, 17), whereas others maintain that PLB is a subunit of the Ca2+ pump that remains attached throughout the catalytic cycle (14, 16, 18). Due to the wide array of techniques utilized, each with its inherent limitations, it has been difficult to reconcile some of these contradictory results. Therefore, a method to study physical interactions between the naturally occurring proteins in their native environment in the SR membrane seems highly desirable.
Chemical cross-linking has been used by our group to study protein binding interactions between PLB and SERCA2a co-expressed in insect cell membranes (13). Using the heterobifunctional (sulfhydryl to amine) cross-linker, EMCS, for example, we showed that the N27C mutant of canine PLB cross-links exclusively to Lys328 of the 110-kDa Ca2+ pump at the cytoplasmic extension of M4 (20). Notably, only the Ca2+-free, E2 conformation of SERCA2a cross-linked to PLB, and the extent of cross-linking was increased significantly by ATP (13, 20). Other cross-linking interactions at different sites between SERCA2a and PLB were subsequently identified, all of which were similarly conformationally dependent (21, 22). Based upon these results, it was proposed that mutually exclusive binding of PLB and Ca2+ to the Ca2+ pump was the underlying mechanism by which PLB decreases the Ca2+ affinity of the enzyme (23, 24). In a series of studies by our group (13, 20–22) and MacLennan and co-workers (12, 25), it was further demonstrated that PLB cross-linking to E2 occurred in the groove formed between transmembrane helices M2, M4, and M9 of the Ca2+-ATPase. Residues extending from Gln23 (25) to Leu52 (21) of PLB were cross-linked to SERCA2a within this groove, providing structural insight into how PLB binding to E2 might interfere with the conformational transition to E1 required for formation of the high affinity Ca2+ binding sites (5). Although these cross-linking studies have provided valuable information with regard to the factors regulating PLB association with the Ca2+ pump and effects on enzyme activity, it has yet to be determined whether the naturally occurring proteins in native SR vesicles interact identically to their recombinant counterparts.
Importantly for the work here, human PLB is unique among mammals in that it contains a lysine residue at position 27 instead of asparagine (26). Based upon the studies above with recombinant canine PLB and SERCA2a, we reasoned that it should be possible to cross-link the naturally occurring Lys27 of human PLB to SERCA2a directly in native human SR vesicles. Indeed, using human cardiac SR vesicles, we demonstrate highly specific cross-linking of Lys27 of PLB to SERCA2a at the cytoplasmic extension of M4. Cross-linking was achieved with the 7.7-Å long, homobifunctional cross-linking reagent, DSG. We went on to investigate the conformational specificity of the PLB to SERCA2a cross-linking interaction and whether normal protein-protein interactions between PLB and SERCA2a are maintained in SR membranes from failing myocardium.
EXPERIMENTAL PROCEDURES
Materials
Cross-linkers were obtained from Pierce. TG was purchased from Sigma. Endo Asp-N sequencing grade was obtained from Roche Applied Science. Protein iodination was performed with Pierce Iodination Tubes. 125I− and 45Ca2+ were purchased from New England Nuclear. Nitrocellulose membranes and ImmobilonPSQ sheets were obtained from Bio-Rad and Millipore, respectively.
Preparation of Human SR Vesicles
Cardiac microsomes enriched in SR (45,000 × g pellets) were prepared from the left ventricles of normal and failed human hearts according to our standard method for dog hearts (27). Preparations from individual human hearts were made over a period of 2 years from 1993 to 1994 and stored frozen in small aliquots at −40 °C in 0.25 m sucrose, 30 mm histidine (pH 7.2). Protein concentrations were determined by the Lowry method. Since the date of preparation, enzyme activities have remained stable in the freshly thawed aliquots assayed. Nonfailing left ventricular myocardium was obtained from four unmatched organ donors. Failed left ventricular myocardium was obtained from three transplant recipients with idiopathic dilated cardiomyopathy, who exhibited ejection fractions of 9% or less. Ca2+-ATPase activities, PLB regulatory effects on Ca2+ pump activity, and cross-linking results were similar in SR membranes from the different preparations, and results from one representative normal preparation and one representative failed preparation are presented here unless otherwise stated.
PLB and SERCA2a Protein Expression in Sf21 Cells
Sf21 insect cells were co-infected with baculoviruses encoding canine PLB and SERCA2a as described previously (4). Mutagenesis of canine wild-type PLB to N27K-PLB was conducted as described previously (20). Cells were harvested 60 h after co-infection with baculoviruses and homogenized, and membranes were stored frozen in small aliquots at −40 °C at a protein concentration of 6–10 mg/ml in 0.25 m sucrose, 10 mm MOPS (pH 7.0).
PLB Cross-linking to SERCA2a
Cross-linking reactions were conducted with 11 μg of human SR vesicles or insect cell membranes in 12 μl of buffer. Cross-linking buffer contained 50 mm MOPS (pH 7.0), 3.0 mm MgCl2, 100 mm KCl, 3 mm ATP, and 1 mm EGTA. In some experiments the ATP concentration was varied or the Ca2+ pump inhibitor, TG, was added. Ionized Ca2+ concentrations were set by addition of CaCl2 to a final concentration of 0.1–1.0 mm. Cross-linking reactions were started by adding cross-linking reagents from concentrated stock solutions in dimethyl sulfoxide. The final cross-linker concentrations were 0.1 mm for EMCS and 5 mm for DSG, except where indicated in the figure legends. Cross-linking was conducted at room temperature for 15 min with EMCS and for 5 min at room temperature with DSG, which gave the maximal cross-linking of PLB to SERCA2a in both cases. Reactions were stopped with 7.5 μl of gel loading buffer containing 15% SDS and 100 mm DTT. Samples were heated to 50 °C for 10 min unless otherwise stated and then subjected to SDS-PAGE and immunoblotting for detection of PLB cross-linked to SERCA2a. In the experiment of Fig. 9, cross-linking was carried out simultaneously with the Ca2+ uptake assay and measurement of 125I-2D12 binding to PLB in SR vesicles, as described in more detail below.
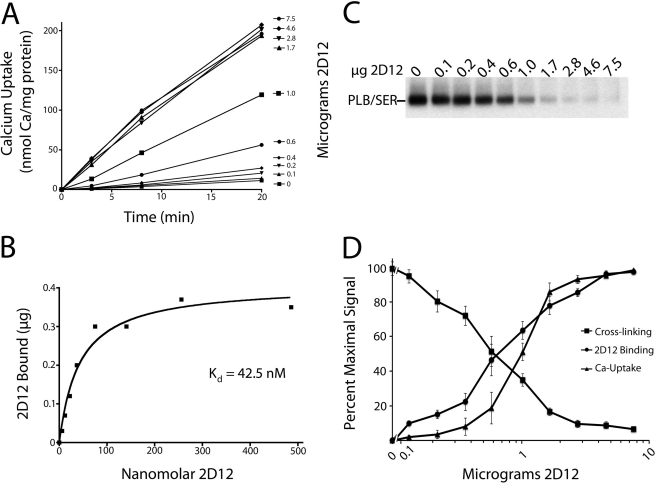
FIGURE 9.
2D12 Binding to PLB in human SR vesicles measured simultaneously with 2D12 effects on PLB cross-linking and Ca2+ uptake. A, 45Ca2+ uptake into human cardiac SR vesicles (14.5 μg) was measured at 37 °C in the presence of 1 mm EGTA and 0.1 mm CaCl2, and 0–7.5 μg of 2D12 antibody (indicated in right margin). B, 125I-2D12 binding to PLB was measured in assay tubes prepared and handled identically to those described for Ca2+ uptake, only trace 45Ca2+ was omitted. On the abscissa, the free 2D12 (non–protein-bound) concentration is plotted. C, 2D12 effect on PLB cross-linking to SERCA2a measured in assay tubes prepared and handled identically to those described for Ca2+ uptake (A) and 2D12 binding (B), only omitting radionuclides. D, composite plot showing averaged results conducted as in A–C (means ± S.E. (error bars) from five separate experiments conducted with normal and failed membranes).
Purification of Human SERCA2a Cross-linked to PLB
SERCA2a cross-linked to PLB was purified to homogeneity from human SR vesicles via sequential monoclonal antibody affinity chromatographies, as described previously (13). The total population of SERCA2a molecules was purified first using the anti-SERCA2a antibody column (2A7-A1), then only those SERCA2a molecules cross-linked to PLB were isolated using the anti-PLB (2D12) antibody column. 50 mg of human SR membranes were first cross-linked in bulk with DSG as described above. Cross-linking was terminated by addition of 200 mm ethanolamine, membranes were spun down, and the pellet was resuspended by addition of 1% SDS followed by 3.5% Triton X-100. Solubilized membrane proteins were then applied to the anti-SERCA2a column. The purified Ca2+ pumps that eluted at acid pH were then applied to the anti-PLB column for purification of Ca2+ pumps exclusively cross-linked to PLB, exactly as described in Ref. 13.
SDS-PAGE, Immunoblotting, and Antibodies
SDS-PAGE (using 8% polyacrylamide) and immunoblotting were performed as described previously (13). Membrane proteins were transferred to nitrocellulose sheets or ImmobilonPSQ with similar results. For detection of PLB, blots were probed with anti-PLB monoclonal antibody, 2D12, which recognizes residues 7–13 of PLB. For detection of SERCA2a, blots were probed with anti-SERCA2a monoclonal antibody, 2A7-A1, which recognizes residues 386–396 of SERCA2a (13). Antibody-bound protein bands were visualized with 125I-protein A followed by autoradiography and quantified using a Bio-Rad Personal Fx PhosphorImager. In the experiments of Figs. 8 and 9, antibody-bound bands were visualized directly with 125I-2D12.
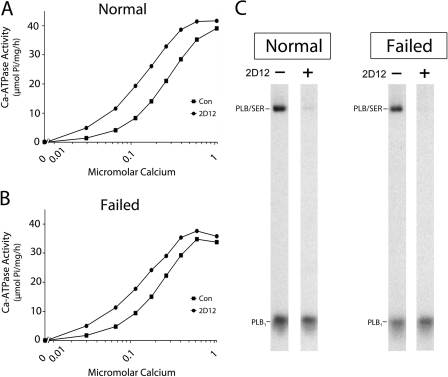
FIGURE 8.
2D12 effects on Ca2+-ATPase activity and PLB cross-linking. A and B, Ca2+-ATPase activity was measured in SR vesicles from normal (A) and failed (B) hearts in the presence and absence of the 2D12 antibody. C, effect of 2D12 on PLB cross-linking to SERCA2a at 0.03 μm Ca2+ concentration. Cross-linking samples contained 11 μg of SR vesicles in the presence or absence of 5 μg of 2D12.
For quantification of PLB and SERCA2a in human SR vesicles, pure SERCA2a and PLB protein standards were electrophoresed and processed in parallel with SR membrane samples. Purified, recombinant canine PLB was isolated from insect cell membranes as described previously (28), and the purified SERCA2a standard was isolated from canine cardiac SR vesicles by 2A7-A1 affinity chromatography (13). The protein concentrations of the purified protein standards were determined by absorbance at 280 nm with knowledge of the molar extinction coefficients calculated from the amino acid sequences (29). The results were in good agreement with those determined by the Lowry protein assay.
Three new SERCA2a antibodies were used in this study for peptide mapping purposes. Site-specific antibodies were raised in rabbits against synthetic peptides corresponding to residues 106–120 and 522–533 of SERCA2a. Mouse monoclonal antibody, 2D6, was raised to a synthetic peptide corresponding to residues 319–332 of SERCA2a. All of the sequences above are identical in canine and human SERCA2a (4). Peptides were synthesized with an N-terminal cysteine residue for coupling to maleimide-activated BSA or keyhole limpet hemocyanin (Pierce). The epitope of SERCA2a recognized by 2D6 was further localized to residues 319–323 of the Ca2+ pump using PepSpotsTM (Jerini Bio Tools) (13).
Peptide Mapping
Approximately 1 μg of purified SERCA2a cross-linked to PLB was incubated with 0.04 μg (1 μl) or 0.24 μg (6 μl) of endo Asp-N for 30 min at 23 or 37 °C, respectively. Replicate proteolyzed samples were then subjected to SDS-PAGE and immunoblotted with the anti-PLB and the anti-SERCA2a antibodies above.
Ca2+-ATPase Assay
Ca2+-ATPase activities were measured at 37 °C in buffer containing 50 mm MOPS (pH 7.0), 100 mm KCl, 3 mm MgCl2, 3.0 mm ATP, 5 mm NaN3, 3 μg/ml Ca2+ ionophore A23187, and 1 mm EGTA. Ionized Ca2+ concentrations were set by varying the CaCl2 concentration from 0 to 1.0 mm. Assays were conducted in the presence and absence of 15 μg of the anti-PLB monoclonal antibody, 2D12, which reverses PLB inhibition of SERCA2a activity (5). Ca2+-dependent ATPase activities were determined in a reaction volume of 1 ml containing 32 μg of membrane protein during a 60-min incubation. Pi release from ATP was measured colorimetrically (24).
2D12 Binding to PLB in Human SR Vesicles Measured Simultaneously with 2D12 Effects on PLB Cross-linking and Ca2+ Uptake
Ca2+ uptake into human cardiac SR vesicles (86.6 μg) was conducted at 37 °C in 450 μl of buffer containing 50 mm MOPS, 100 mm KCl, 3 mm MgCl2, 10 mm potassium oxalate, 5 mm NaN3, 0–46 μg of 2D12, 1 mm EGTA, and 0.1 mm CaCl2 with a trace amount of 45Ca. After a 10-min preincubation at 37 °C, Ca2+ uptake reactions were started by adding 45 μl of ATP to a final concentration of 3 mm. 100-μl samples were then taken at 3, 8, and 20 min after initiation of Ca2+ uptake and filtered over glass fiber filters to determine the degree of Ca2+ oxalate accumulated (27). Filters were rinsed twice with 5 ml of ice-cold saline prior to scintillation counting.
2D12 binding to PLB in SR vesicles was conducted in assay tubes prepared and handled identically to those described for Ca2+ uptake, only tracer 125I-2D12 (13) was included with the cold 2D12 and 45Ca2+ was omitted. After addition of ATP, incubations were conducted for 10 min at 37 °C. For determination of 125I-2D12 binding, three 100-μl aliquots were taken from each reaction tube and filtered over glass fiber filters preblocked with 1% BSA and rinsed as above. 125I retained by the filters was determined by scintillation counting. Control experiments indicated that presoaking the filters in 1% BSA was essential in reducing nonspecific binding of 125I-2D12 to background levels comparable with blank filters receiving no SR vesicle protein. Rabbit skeletal muscle SR vesicles devoid of PLB also retained insignificant 125I-2D12 in control experiments. Antibody binding data analysis was carried out using GraphPad Prism for Macintosh, Version 4.0c (GraphPad Software).
To measure 2D12 effects on PLB cross-linking in the same assay, a third series of identical reaction tubes was prepared only without 125I-2D12 or 45Ca2+. 10 min after Ca2+ uptake initiation by the addition of ATP, the samples were cross-linked with 5 mm DSG for 5 min at room temperature, and cross-linking was stopped with gel loading buffer (15% SDS and 100 mm DTT). Samples were heated to 50 °C for 10 min prior to SDS-PAGE, and immunoblots were probed directly with 125I-2D12, and protein A was omitted (13).
RESULTS
SR vesicles were prepared from the left ventricles of three failing and five nonfailing human hearts, and the protein content of SERCA2a and PLB in the preparations was measured via quantitative immunoblotting. Results obtained from one representative normal and one failed heart are shown in Fig. 1. Similar levels of SERCA2a (anti-SER) and PLB (anti-PLB) were detected in the two preparations (Fig. 1A), consistent with our earlier reports (33–35). The characteristic heat-induced dissociation of the PLB pentamer into monomers in the presence of SDS is also apparent in the leftmost lanes of the anti-PLB immunoblot in Fig. 1A.
FIGURE 1.
SDS-PAGE and immunoblotting of SR vesicles prepared from normal (N) and failed (F) human hearts. A, 20 μg of membrane protein was electrophoresed per gel lane, and the samples were transferred to nitrocellulose for protein staining or incubation with antibodies. One replicate set was stained with Amido Black (left). A second set was probed with the anti-SERCA2a antibody, 2A7-A1 (middle), and the third set was probed with the anti-PLB antibody, 2D12 (right), followed by 125I-protein A and autoradiography. In the third set, the last two samples were heated to 50 °C for 10 min prior to SDS-PAGE. PLB5 and PLB1 designate the pentameric and monomeric forms of PLB, respectively. B, autoradiograph depicting typical immunoblotting results used for SERCA2a and PLB quantification in human SR vesicles. Micrograms of pure PLB and SERCA2a protein standards run are indicated in the upper margins.
Using purified PLB and SERCA2a protein standards (Fig. 1B), the molar ratio of PLB to SERCA2a was determined to be 1.37 ± 0.3 and 1.18 ± 0.2 mol of PLB/mol of Ca2+ pump in normal and failed membranes, respectively (mean ± S.E. from 10 determinations). For either type of preparation, the ∼110-kDa Ca2+-ATPase accounted for ∼10% of the total SR protein and is therefore clearly visible on the Amido Black-stained nitrocellulose transfer in Fig. 1A (lanes 1 and 2). However, despite being approximately equimolar with the Ca2+ pump, due to its small molecular mass (∼6 kDa), PLB accounted for only ∼0.7% of the total SR protein and is not visible on the protein-stained nitrocellulose. No evidence for a lower mobility SUMOylated form of the Ca2+-ATPase (∼150 kDa) (30) was noted in SR vesicles prepared from any of the human hearts.
Cross-linking PLB to SERCA2a in Human SR Vesicles
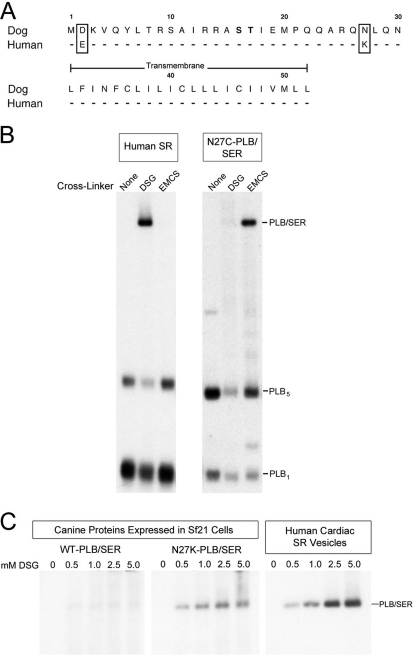
Human PLB differs from canine PLB at only two residues; in human PLB, Asp2 is a glutamic acid, and Asn27 is a lysine (Fig. 2A). Based upon earlier co-expression studies in which canine N27C-PLB cross-linked to Lys328 of SERCA2a with the cysteine to lysine cross-linker EMCS (20), we predicted that the natural Lys27 of human PLB should also be cross-linkable to Lys328 of SERCA2a with a homobifunctional, lysine-specific cross-linking reagent. To test this hypothesis, human cardiac microsomes and insect cell microsomes co-expressing canine N27C-PLB and SERCA2a, were incubated with the homobifunctional, amine-specific, NHS ester, DSG (7.7 Å), or the heterobifunctional (sulfhydryl to amine) cross-linker, EMCS (9.4 Å). Cross-linking was conducted in the absence of Ca2+ and presence of ATP, shown previously to yield optimal chemical coupling between PLB and SERCA2a. Cross-linked PLB/SERCA2a heterodimers (PLB/SER) were detected using the anti-PLB monoclonal antibody, 2D12. Strong cross-linking between PLB and SERCA2a was observed in the human SR vesicles using DSG, whereas no cross-linking occurred with EMCS (Fig. 2B). Alternately, canine N27C-PLB cross-linked to SERCA2a only with EMCS and not with DSG. In both the human and insect cell microsomes, PLB cross-linked exclusively to the Ca2+ pump, indicating that the protein-protein interaction between PLB and SERCA2a detected by cross-linking is highly specific.
FIGURE 2.
Lys27 of human PLB facilitates cross-linking to SERCA2a in SR vesicles. A, amino acid sequences of human and canine PLB. Residues 1–30, containing the two phosphorylation sites (bold), project into the cytoplasm. B, 11 μg of human SR vesicles (left) or Sf21 membranes co-expressing canine SERCA2a and N27C-PLB (right) were cross-linked with DGS or EMCS. Samples were then subjected to SDS-PAGE and immunoblotting with 2D12. The high molecular mass band (PLB/SER) is SERCA2a cross-linked to the PLB monomer. Samples were not heated to 50 °C prior to SDS-PAGE to allow visualization of the PLB pentamer. C, human SR vesicles (right), or Sf21 membranes co-expressing canine SERCA2a and either wild-type canine PLB (left) or canine PLB with Asn27 mutated to lysine (N27K) (middle) were cross-linked with 0–5 mm DSG, subjected to SDS-PAGE, and probed with 2D12.
Next, to verify that Lys27 of PLB cross-links to SERCA2a with DSG, we tested for cross-linking of canine PLB with Asn27 changed to Lys (N27K-PLB). Fig. 2C shows that under the same cross-linking conditions with a range of cross-linker concentrations (0.5–5 mm DSG), wild-type canine PLB did not cross-link to the Ca2+ pump, whereas canine PLB substituted with Lys27 did. This result confirms that Lys27 allows cross-linking of human PLB to SERCA2a in native SR vesicles.
Cross-linking Quantification and Localization
To quantify the extent of PLB cross-linking to SERCA2a in human SR vesicles and to gain information on the site cross-linked in SERCA2a, we purified the cross-linked PLB/SERCA2a heterodimers. Sequential monoclonal antibody chromatographies were used for the purification (13, 20). 50 mg of human SR vesicles were cross-linked with DSG, solubilized in detergent, and then passed over an anti-SERCA2a (2A7-A1) monoclonal antibody column. This allowed purification of the Ca2+ pump protein to homogeneity while removing the free PLB monomers not cross-linked to the enzyme (Fig. 3). Fig. 3A shows the Coomassie Blue-stained gel of selected fractions from the first purification and demonstrates that the purified Ca2+ pump was eluted from the column at acidic pH (pH 2.4). Immunoblotting the same fractions with the anti-SERCA2a antibody (Fig. 3B, 2A7-A1) demonstrates that all the Ca2+-ATPase was bound to the column prior to acid elution at pH 2.4. The anti-PLB antibody panel (Fig. 3C, 2D12) shows that the uncross-linked PLB monomers (PLB1) passed freely through the column and were recovered in the column flow-through fraction, whereas all of the PLB cross-linked to SERCA2a (PLB/SER) was retained and eluted at pH 2.4.
FIGURE 3.
Purification of SERCA2a from human SR vesicles using anti-SERCA2a monoclonal antibody (2A7-A1) affinity chromatography. PLB and SERCA2a in human SR vesicles were cross-linked with DSG on large scale, solubilized in detergent, and then the Ca2+-ATPase was purified by monoclonal antibody affinity chromatography using 2A7-A1. Par, parent microsomal fraction; Sup, detergent supernatant fraction; FT, column flow-through fraction; pH 2.4, peak acid-elution fraction. A, Coomassie Blue-stained gel showing selected fractions from the purification. B and C, immunoblots of the same fractions probed with the anti-SERCA2a antibody, 2A7-A1, or the anti-PLB antibody, 2D12, respectively.
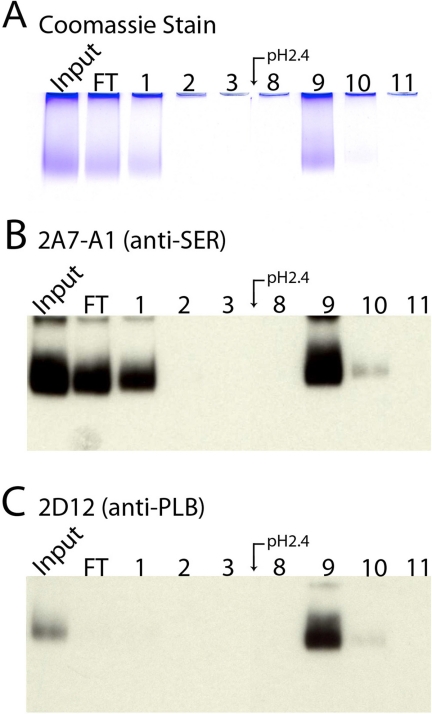
Next, to separate PLB-free Ca2+ pumps from Ca2+ pumps cross-linked to PLB, a second round of purification was done with the anti-PLB (2D12) column. The purified Ca2+-ATPase from the first purification (Input) was loaded onto the anti-PLB column. The PLB-free Ca2+ pumps passed though the column and were recovered in the flow-through fraction and fraction 1, as visible on both the Coomassie Blue-stained gel (Fig. 4A) and the 2A7-A1 immunoblot (Fig. 4B). All of the Ca2+ pump molecules cross-linked to PLB were retained by the 2D12 column and eluted at pH 2.4, visible on the 2D12 immunoblot (Fig. 4C). Quantification of immunoblots from four separate purifications, which included SR vesicles isolated from both normal and failed hearts, indicated that 23.6 ± 5.1% of the Ca2+-ATPase molecules in human SR vesicles were cross-linked to PLB with DSG (mean ± S.E.).
FIGURE 4.
Purification of SERCA2a cross-linked to PLB using anti-PLB monoclonal antibody affinity chromatography. SERCA2a purified as indicated in Fig. 3 (Input) was subjected to anti-PLB monoclonal antibody affinity chromatography. A, Coomassie Blue stained gel of selected fractions. B and C, immunoblots of the same fractions probed with 2A7-A1 and 2D12, respectively.
To gain information on the region of SERCA2a cross-linked to Lys27 of PLB, peptide mapping of the purified PLB/SERCA2a heterodimers was performed. PLB/SERCA2a was digested with endo Asp-N, and the proteolytic fragments were analyzed by immunoblotting using the anti-PLB antibody (2D12) and four different anti-SERCA2a antibodies recognizing different regions of the Ca2+ pump (Fig. 5). Because human PLB contains no aspartic acid residues, any Ca2+ pump fragment generated that is cross-linked to PLB will remain anchored to the intact PLB molecule.
FIGURE 5.
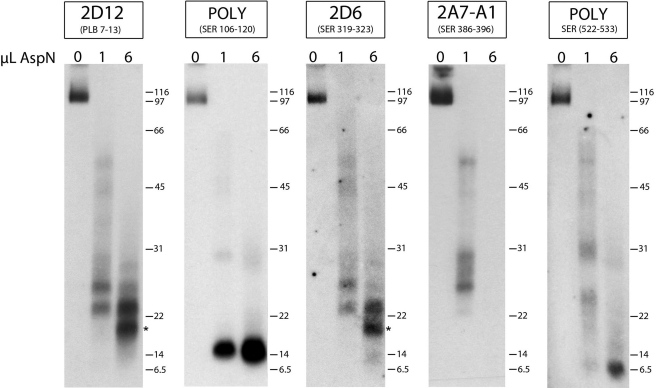
Proteolytic mapping of PLB cross-linked to SERCA2a in human SR vesicles. Purified SERCA2a cross-linked to PLB (1 μg) was proteolyzed with 0.04 μg (1 μl) or 0.24 μg (6 μl) of endo Asp-N. Replicate proteolyzed samples were subjected to SDS-PAGE and immunoblotted with the anti-PLB antibody, 2D12, or one of four different anti-SERCA2a antibodies recognizing different regions of the Ca2+ pump. POLY, polyclonal rabbit antiserum.
Immunoblotting with the 2D12 antibody shows that digestion with the highest endo Asp-N concentration (6 μl) generated two major proteolytic fragments with molecular masses of ∼23 and 19 kDa (Fig. 5, 2D12 panel). The Ca2+ pump fragments detected using the anti-SERCA2a monoclonal antibody, 2A7-A1 (to residues 386–396), as well as the polyclonal antibodies recognizing residues 106–120 or 522–533, were distinctly different in mobility from those detected by 2D12, indicating that PLB was not cross-linked to the Ca2+ pump near these sites. However, the SERCA2a fragments detected by the 2D6 antibody, recognizing residues 319–323 of the Ca2+ pump, were identical in mobility to those detected by 2D12. Therefore, Lys27 of PLB is cross-linked to the same fragments recognized by the 2D6 antibody.
Analysis of the human Ca2+ pump sequence indicates that digestion with endo Asp-N could generate a fragment spanning residues 254–369 (containing the 2D6 epitope), which when combined with the molecular mass of PLB (6 kDa), results in a cross-linked peptide with molecular mass of ∼18.6 kDa. This is consistent with the size of the lower, major cross-linked peptide detected by both the 2D12 and 2D6 antibodies (asterisks). Importantly, in an earlier study we showed that proteolytic digestion of canine SERCA2a cross-linked to N27C-PLB generated the same two major fragments detected in the present study, and protein sequencing confirmed that the smaller fragment (∼18.6 kDa) started at residue Asp254 of the Ca2+ pump (20). Residues 254–369 of human SERCA2a, which includes transmembrane segments M3 and M4, contain four Lys residues at which PLB could potentially cross-link: Lys262, Lys328, Lys329, and Lys352. (Lys297 is also contained within this fragment, but is not is not a likely candidate as it is located on the opposite face of the membrane from Lys27 of PLB.) These peptide mapping results are consistent with our earlier findings with recombinant N27C-PLB, which was shown to cross-link exclusively to Lys328 of the Ca2+ pump (20). Based upon these results we conclude that Lys27 of human PLB cross-links at (or near) Lys328 of the Ca2+ pump located within the cytoplasmic extension of M4.
Effects of Ca2+, TG, and ATP on PLB Cross-linking
To test whether cross-linking interactions between PLB and SERCA2a in SR vesicles are conformationally dependent, we measured the effects of Ca2+, TG, and ATP on protein coupling by DSG. SR vesicles from normal and failed human ventricles were analyzed.
Ca2+ effects on PLB cross-linking to SERCA2a are shown in Fig. 6, A and B. In the absence of Ca2+, comparable cross-linking of PLB to SERCA2a was detected in both normal and failed SR vesicles. Cross-linking was completely inhibited by increasing the Ca2+ concentration over the micromolar range at which SERCA2a binds Ca2+ (Fig. 6B). The mean Ki values for Ca2+ inhibition of cross-linking were 0.11 ± 0.01 and 0.10 ± 0.01 μm Ca2+ for SR membranes from normal and failed hearts, respectively (mean ± S.E. from seven determinations). These results demonstrate that PLB does not cross-link to SERCA2a in the E1 conformation in SR vesicles.
FIGURE 6.
Effect of Ca2+ and TG on PLB cross-linking to SERCA2a in SR membranes from normal and failed human ventricles. A and C, autoradiographs showing concentration dependence of Ca2+ (A) and TG (C) inhibition of PLB cross-linking to SERCA2a in normal and failed human SR vesicles. B and D, results depicted graphically.
TG effects on cross-linking, measured in the absence of Ca2+, are shown in Fig. 6, C and D. Cross-linking was completely inhibited by TG in normal and failed membranes (Fig. 6C). The Ki values for TG inhibition of cross-linking were 0.18 ± 0.04 and 0.16 ± 0.04 μm, respectively (mean ± S.E. from four determinations). It should be noted that the concentration of SERCA2a protein present in the cross-linking assays was 0.6–0.8 μm, within the range of TG concentration required to inhibit cross-linking completely.
Fig. 7 shows the effect of increasing ATP concentration on PLB cross-linking to the Ca2+ pump (measured in the absence of Ca2+). For both normal and failed SR preparations, cross-linking was stimulated 2–3-fold by ATP; half-maximal stimulation of cross-linking occurred at 109.4 ± 17.1 μm ATP for membranes from normal hearts and 99.4 ± 15.4 μm ATP for membranes from the failed hearts (mean ± S.E. from five determinations). Thus, the affinity of SERCA2a for ATP at the modulatory ATP binding site (13, 31) is comparable in membranes from normal and failed hearts.
FIGURE 7.
ATP effect on cross-linking. A, ATP stimulation of PLB cross-linking to SERCA2a in normal and failed human SR vesicles in the absence of Ca2+. B, percentage of ATP stimulation of cross-linking versus ATP concentration plotted graphically.
Effect of 2D12 on Ca2+-ATPase Activity and PLB Cross-linking
The anti-PLB antibody, 2D12, reverses PLB inhibition of SERCA2a activity in SR vesicles (5, 9) and has been shown to disrupt the physical interaction between the two proteins in our recombinant system (23). It was of interest to know whether 2D12 inhibits PLB cross-linking to SERCA2a in SR vesicles.
Fig. 8 shows Ca2+ stimulation of Ca2+-ATPase activity measured in SR vesicles prepared from normal (A) and failed (B) human ventricles, determined in the presence and absence of the 2D12 antibody. In the absence of 2D12, the KCa values for half-maximal enzyme activation were 0.24 and 0.20 μm Ca2+ for normal and failed membranes, respectively. In the presence of 2D12, the KCa values were leftward-shifted about 2-fold, from 0.24 to 0.13 μm Ca2+ for the normal SR vesicles and from 0.20 to 0.12 μm Ca2+ for the failed SR vesicles. No effect of 2D12 on maximal Ca2+-ATPase activity, measured at saturating Ca2+ concentration (∼1 μm), was noted for SR membranes prepared from normal and failed hearts, consistent with previous results by Movsesian et al. (32).
Fig. 8C shows the effect of 2D12 on PLB cross-linking to SERCA2a measured at 0.03 μm Ca2+, the lowest Ca2+ concentration used in the Ca2+-ATPase assay. 2D12 abolished PLB cross-linking to SERCA2a in membranes prepared from both normal and failed hearts. These results demonstrate that 2D12 activation of Ca2+-ATPase activity is associated with PLB dissociation from the Ca2+ pump in SR vesicles.
Effect of 2D12 on Ca2+ Transport and PLB Cross-linking in Human SR Vesicles
Functional effects of 2D12 were analyzed in greater detail using the more sensitive Ca2+ uptake assay and correlated with PLB cross-linking. The effect of increasing 2D12 concentration on Ca2+ uptake into human SR vesicles, measured at 0.03 μm Ca2+, is shown in Fig. 9A. In the absence of 2D12, Ca2+ uptake was low and barely detectable at 30 nm Ca2+ (filled squares). Addition of 2D12 strongly stimulated Ca2+ uptake. For example, 0.6 μg of 2D12 added to 14.5 μg of SR vesicles (filled circles) gave a 5-fold stimulation of Ca2+ uptake, producing 20% of the maximal response. At 1.7–7.5 μg of added 2D12, stimulation of Ca2+ uptake reached saturation, giving a striking 18-fold increase in Ca2+ uptake.
2D12 binding to PLB in SR vesicles, measured in the same assay, is shown in Fig. 9B. Antibody binding saturated over the same concentration range at which Ca2+ uptake was stimulated. The Kd determined for 2D12 binding to PLB (42.5 nm) corresponds to an added 2D12 concentration of 0.8 μg. Fig. 9C shows the effect of 2D12 on PLB cross-linking in the same assay. 2D12 inhibited PLB cross-linking to SERCA2a over the identical concentration range at which Ca2+ uptake was stimulated and 2D12 binding to PLB occurred. Fig. 9D is a composite plot showing the average results from five separate assays conducted with both nonfailing and failing human cardiac SR preparations. Clearly, there is a strong correlation between 2D12 binding to PLB, stimulation of Ca2+ uptake, and inhibition of cross-linking. These results strongly suggest that dissociation of PLB from the Ca2+ pump is associated with SECA2a activation.
DISCUSSION
In the present study, we demonstrated highly specific, robust cross-linking between PLB and SERCA2a in SR vesicles prepared from normal and failed human hearts. Cross-linking was monitored for the first time between the naturally occurring proteins in the SR membrane, concurrently with PLB effects on Ca2+-ATPase activity and Ca2+ transport. Our results provide important insights into the physical mechanism of Ca2+ pump inhibition by PLB.
Mechanism of PLB Inhibition in SR Vesicles
It is largely accepted that PLB inhibits SERCA2a activity by lowering Ca2+ affinity, having little or no effect on the Vmax of the enzyme measured at saturating Ca2+ concentration (1). This inhibitory effect of PLB is manifested as a rightward shift in the Ca2+ curve of enzyme activation and has been observed in SR vesicles prepared from all mammalian species examined to date, including SR vesicles prepared from canine (5) and human hearts (32). As a mechanism to explain this effect of PLB on Ca2+ affinity, it has long been proposed that PLB competes with Ca2+ for binding to SERCA2a (1). However, due to an inability to easily monitor protein binding interactions between the wild-type proteins, it was, until now, difficult to test this hypothesis directly in SR vesicles. Here, we took advantage of the observation that Lys27 of human PLB is cross-linkable to SERCA2a at the cytoplasmic extension of M4, to test for competition between PLB and Ca2+ for binding to SERCA2a in human SR vesicles. Indeed, PLB cross-linking to SERCA2a was completely inhibited by Ca2+ over the same micromolar concentration range required for formation of E1 and activation of ATP hydrolysis. This result confirms in human SR vesicles that PLB cross-links exclusively to E2. In our present work, Ca2+-sensitive cross-linking of PLB to SERCA2a in SR vesicles was demonstrated at a single point of interaction, but it should be pointed out that identical effects of Ca2+ were demonstrated previously at numerous cross-linking sites between PLB and SERCA2a, located on either side of the lipid bilayer (12, 13, 20–25).
To demonstrate directly in human SR vesicles that SERCA2a with PLB bound is catalytically inactive (12, 13, 24, 25), we used the 2D12 antibody to disrupt the physical interaction between the two proteins at very low Ca2+ concentration. Importantly, we observed a strong correlation between 2D12-induced dissociation of PLB from E2, and 2D12 stimulation of Ca2+-ATPase activity and Ca2+ uptake (Figs. 8 and 9). Collectively, these results strongly support the model of mutually exclusive binding of PLB to E2 and Ca2+ to E1 as the molecular mechanism of PLB inhibition.
To verify further the specificity of the Lys27 cross-link identified in human SR vesicles, we tested the effects of other allosteric modifiers on the cross-linking interaction. As demonstrated previously with the recombinant proteins (13, 20, 24), TG binding to the Ca2+ pump induced a conformational state that was incompatible with PLB binding (E2·TG), whereas ATP binding to the modulatory nucleotide binding site induced a state that favored PLB binding. Therefore, we conclude that PLB competes with Ca2+ for binding to SERCA2a in SR vesicles by stabilizing the enzyme in a unique E2·ATP state.
Molar Content of PLB and SERCA2a in SR Vesicles and Role of Pentamer
In this work, we determined that PLB is present in slight molar excess over SERCA2a in human SR vesicles. The molar ratio of PLB to SERCA2a determined here (about 1.3:1) is considerably lower than the 5:1 ratio (PLB to SERCA2a) previously reported for mammalian SR vesicles (36).
The molar stoichiometry determined here allows us to draw two new conclusions about the protein-protein interactions occurring between PLB and SERCA2a in the SR membrane. First, the affinity of the PLB monomer for E2 is much stronger than previously appreciated (12); and second, it is likely that an important function of the PLB pentamer is to aid in complete reversal of SERCA2a inhibition at high Ca2+ concentration.
These two conclusions are supported by the following reasoning: at low Ca2+ concentration, SERCA2a is almost completely catalytically inactive due to the fact that nearly all of the Ca2+ pumps have PLB bound (as evidenced by the dramatic stimulation of Ca2+ uptake induced by the 2D12 antibody at nanomolar Ca2+ concentration, Fig. 9). Because most of the PLB is bound to SERCA2a at very low Ca2+ concentration, the pool of PLB pentamers in the SR membrane must be nearly depleted. This suggests that the transmembrane contacts stabilizing the PLB-E2 interaction are actually much stronger than the Leu/Ile zipper contacts stabilizing the PLB pentamer (7). However, despite the high affinity of the PLB monomer for E2 (in the range of vanadate binding to E2) (24), PLB inhibition of SERCA2a activity is still completely reversed at saturating Ca2+ concentration (Fig. 8), when Ca2+ binding to E1 induces the complete dissociation of PLB from the Ca2+ pump (Fig. 6). Therefore, self-assembly of PLB monomers into noninhibitory pentamers may help to reverse Ca2+ pump inhibition when Ca2+ is not limiting and pentamer formation is expected to be at its greatest.
Physical Association between PLB and SERCA2a in SR Membranes from Failed and Nonfailed Hearts
In the present study, we found no difference in KCa values for activation of Ca2+-ATPase activity in SR membranes prepared from normal and failed human hearts. Using chemical cross-linking, we went on to demonstrate that physical interactions between PLB and SERCA2a are identical in SR membranes prepared from normal and failed hearts. In both cases, PLB binds exclusively to E2, and dissociation of PLB from E2 is required for SERCA2a activation. Moreover, the molar ratio of PLB to SERCA2a is similar in SR membranes from normal and failed human hearts. These results suggest that PLB is a reasonable protein target for developing drugs to treat heart failure. A drug that acts like 2D12, preventing PLB binding to SERCA2a, might be expected to produce large increases in cardiac contraction and relaxation in failed human myocardium (9).
Although not the focus of this study, in light of our current results, we feel it important to address the very recent study by Kho et al. (30) in which it was suggested that SUMOylation of SERCA2a in human hearts by SUMO1 increases Ca2+ pump stability and Ca2+-ATPase activity by enhancing ATP binding affinity (30). The authors also suggested that the level of SUMOylation of SERCA2a is reduced in failing human hearts and that restoration of SERCA2a SUMOylation may be a viable means of treating heart failure (30). However, in our present study with normal and failed human hearts, no 150-kDa mobility form that might correspond to SUMOylated SERCA2a was detected. Nor was a slower mobility form of SERCA2a detected in numerous previous immunoblotting studies quantifying the Ca2+-ATPase in normal and failed human myocardium (35). Also in contrast to Kho et al. (30), here we observed similar ATP binding affinities for SERCA2a in membranes from normal and failed myocardium (Fig. 7). It should be pointed out that in the study by Kho et al. (30), ATP affinity of SERCA2a was assessed to an enzyme solubilized in Triton X-100 and 10 mm EDTA, a condition known to promote rapid denaturation of the Ca2+ pump. Therefore, we believe the role of SERCA2a SUMOylation in normal and failing human myocardium should be viewed cautiously.
Functionality of Lys27
An earlier mutagenesis study demonstrated that lysine substitution at Asn27 of PLB (creating human PLB) modestly augments PLB inhibitory function, increasing the KCa for SERCA2a activation slightly more than PLB with Asn27 (37). Subsequently, in a study in which human PLB was expressed in transgenic mice, Kranias and co-workers concluded that lysine at position 27 makes PLB superinhibitory, inducing heart failure and cardiac remodeling in transgenic mice (38, 39). Because of these effects observed in mice, novel functions for PLB in human myocardium were proposed (38, 39). However, in the present study, we determined that human PLB (with Lys27) inhibits SERCA2a activity to the same extent as canine PLB, which has Asn27. In human SR vesicles, the KCa value for Ca2+-ATPase activation was determined to be 0.22 ± 0.03 μm; in dog SR vesicles, the KCa value for Ca2+-ATPase activation was 0.27 ± 0.01 μm. In the presence of the 2D12 antibody, the KCa values were 0.13 ± 0.01 and 0.12 ± 0.01 μm for human and canine cardiac SR vesicles, respectively (mean ± S.E. from five separate preparations). Clearly, no superinhibitory effect of human PLB was noted. Likewise, using our co-expression system with Sf21 cells, we observed that human PLB (with Lys27) decreased the apparent Ca2+ affinity of SERCA2a to the same extent as canine PLB (with Asn27) (data not shown). Therefore, we conclude that PLB with Asn27 or Lys27 behaves virtually identically with respect to its ability to inhibit SERCA2a activity in SR membranes.
CONCLUSIONS
In summary, we have demonstrated with the simple system of human SR vesicles that PLB cross-links exclusively to the E2 state of SERCA2a. Cross-linking occurs at the inhibitory binding site originally characterized in more complicated recombinant systems (12, 13, 20–25). Importantly, because the naturally occurring Lys27of human PLB (unique to human PLB) is cross-linkable near the natural Lys328 (at the cytoplasmic extension of M4) of SERCA2a, we were able, for the first time, to characterize PLB to SERCA2a binding interactions using native proteins in the SR milieu, without the need for mutant or fusion proteins (11–15), or protein solubilization and reconstitution (16–19). Cross-linking PLB to SERCA2a in human SR vesicles exhibited conformational constraints identical to those observed previously using recombinant proteins, including sensitivity to Ca2+, TG, ATP, and the 2D12 antibody. Most importantly, stimulation of Ca2+-ATPase activity and Ca2+ ion transport correlated closely with inhibition of PLB cross-linking to SERCA2a, which strongly suggests that enzyme activation requires PLB dissociation. It was recently suggested that when the enzyme binds Ca2+, PLB may leave the M2/M4/M9 binding site and move to an alternate binding location in the Ca2+ pump (14, 19), but an accessory binding site has yet to be identified with the cross-linking approach (12, 13, 20–25). Regardless of whether or not PLB binds at a secondary, noninhibitory site, it seems clear that the cross-linking interactions detected here and previously reflect genuine protein-protein interactions that are that closely associated with functionality of the Ca2+ pump.
Acknowledgments
We thank Glen Schmeisser for excellent technical assistance and Zhenhui Chen for helpful comments.
This work was supported, in whole or in part, by National Institutes of Health Grant HL49428.
- PLB
- phospholamban
- DSG
- disuccinimidyl glutarate
- E1
- high Ca2+-affinity conformation of Ca2+-ATPase
- E2
- low Ca2+ affinity conformation of Ca2+-ATPase
- EMCS
- N-(ϵ-maleimidocaproyloxy)sulfosuccinimide ester
- endo Asp-N
- endoproteinase Asp-N
- KCa
- Ca2+ concentration required for half-maximal effect
- Ki
- concentration giving half-maximal inhibition
- M
- transmembrane domain
- SERCA
- sarco(endo)plasmic reticulum Ca2+-ATPase
- SERCA2a
- isoform of Ca2+-ATPase in cardiac SR
- SR
- sarcoplasmic reticulum
- SUMO
- small ubiquitin-like modifier
- TG
- thapsigargin.
REFERENCES
- 1. Simmerman H. K., Jones L. R. (1998) Phospholamban: protein structure, mechanism of action, and role in cardiac function. Physiol. Rev. 78, 921–947 [DOI] [PubMed] [Google Scholar]
- 2. Young H. S., Stokes D. L. (2004) The mechanics of calcium transport. J. Membr. Biol. 198, 55–63 [DOI] [PubMed] [Google Scholar]
- 3. Kimura Y., Kurzydlowski K., Tada M., MacLennan D. H. (1997) Phospholamban inhibitory function is activated by depolymerization. J. Biol. Chem. 272, 15061–16064 [DOI] [PubMed] [Google Scholar]
- 4. Autry J. M., Jones L. R. (1997) Functional co-expression of the canine cardiac Ca2+ pump and phospholamban in Spodoptera frugiperda (Sf21) cells reveals new insights on ATPase regulation. J. Biol. Chem. 272, 15872–15880 [DOI] [PubMed] [Google Scholar]
- 5. Cantilina T., Sagara Y., Inesi G., Jones L. R. (1993) Comparative studies of cardiac and skeletal sarcoplasmic reticulum ATPases: effect of a phospholamban antibody on enzyme activation by Ca2+. J. Biol. Chem. 268, 17018–17025 [PubMed] [Google Scholar]
- 6. Wegener A. D., Jones L. R. (1984) Phosphorylation-induced mobility shift in phospholamban in sodium dodecyl sulfate-polyacrylamide gels: evidence for a protein structure consisting of multiple identical phosphorylatable subunits. J. Biol. Chem. 259, 1834–1841 [PubMed] [Google Scholar]
- 7. Simmerman H. K., Kobayashi Y. M., Autry J. M., Jones L. R. (1996) A leucine zipper stabilizes the pentameric membrane domain of phospholamban and forms a coiled-coil pore structure. J. Biol. Chem. 271, 5941–5946 [DOI] [PubMed] [Google Scholar]
- 8. Morris G. L., Cheng H. C., Colyer J., Wang J. H. (1991) Phospholamban regulation of cardiac sarcoplasmic reticulum (Ca2+-Mg2+)-ATPase: mechanism of regulation and site of monoclonal antibody interaction. J. Biol. Chem. 266, 11270–11275 [PubMed] [Google Scholar]
- 9. Sham J. S., Jones L. R., Morad M. (1991) Phospholamban mediates the β-adrenergic-enhanced Ca2+ uptake in mammalian ventricular myocytes. Am. J. Physiol. 261, H1344–1349 [DOI] [PubMed] [Google Scholar]
- 10. Vinge L. E., Raake P. W., Koch W. J. (2008) Gene therapy in heart failure. Circ. Res. 102, 1458–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Asahi M., McKenna E., Kurzydlowski K., Tada M., MacLennan D. H. (2000) Physical interactions between phospholamban and sarco(endo)plasmic reticulum Ca2+-ATPases are dissociated by elevated Ca2+, but not by phospholamban phosphorylation, vanadate, or thapsigargin, and are enhanced by ATP. J. Biol. Chem. 275, 15034–15038 [DOI] [PubMed] [Google Scholar]
- 12. Toyoshima C., Asahi M., Sugita Y., Khanna R., Tsuda T., MacLennan D. H. (2003) Modeling of the inhibitory interaction of phospholamban with the Ca2+-ATPase. Proc. Natl. Acad. Sci. U.S.A. 100, 467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jones L. R., Cornea R. L., Chen Z. (2002) Close proximity between residue 30 of phospholamban and cysteine 318 of the cardiac Ca2+ pump revealed by intermolecular thiol cross-linking. J. Biol. Chem. 277, 28319–28329 [DOI] [PubMed] [Google Scholar]
- 14. Bidwell P., Blackwell D. J., Hou Z., Zima A. V., Robia S. L. (2011) Phospholamban binds with differential affinity to calcium pump conformers. J. Biol. Chem. 286, 35044–35050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Z. (2011) A phospholamban-tethered cardiac Ca2+ pump reveals stoichiometry and dynamic interactions between the two proteins. Biochem. J. 439, 313–319 [DOI] [PubMed] [Google Scholar]
- 16. Mueller B., Karim C. B., Negrashov I. V., Kutchai H., Thomas D. D. (2004) Direct detection of phospholamban and sarcoplasmic reticulum Ca-ATPase interaction in membranes using fluorescence resonance energy transfer. Biochemistry 43, 8754–8765 [DOI] [PubMed] [Google Scholar]
- 17. James P., Inui M., Tada M., Chiesi M., Carafoli E. (1989) Nature and site of phospholamban regulation of the Ca2+ pump of sarcoplasmic reticulum. Nature 342, 90–92 [DOI] [PubMed] [Google Scholar]
- 18. Li J., Bigelow D. J., Squier T. C. (2004) Conformational changes within the cytosolic portion of phospholamban upon release of Ca-ATPase inhibition. Biochemistry 43, 3870–3879 [DOI] [PubMed] [Google Scholar]
- 19. Glaves J. P., Trieber C. A., Ceholski D. K., Stokes D. L., Young H. S. (2011) Phosphorylation and mutation of phospholamban alter physical interactions with the sarcoplasmic reticulum calcium pump. J. Mol. Biol. 405, 707–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen Z., Stokes D. L., Rice W. J., Jones L. R. (2003) Spatial and dynamic interactions between phospholamban and the canine cardiac Ca2+ pump revealed with use of heterobifunctional cross-linking agents. J. Biol. Chem. 278, 48348–48356 [DOI] [PubMed] [Google Scholar]
- 21. Chen Z., Akin B. L., Stokes D. L., Jones L. R. (2006) Cross-linking of C-terminal residues of phospholamban to the Ca2+ pump of cardiac sarcoplasmic reticulum to probe spatial and functional interactions within the transmembrane domain. J. Biol. Chem. 281, 14163–14172 [DOI] [PubMed] [Google Scholar]
- 22. Chen Z., Stokes D. L., Jones L. R., (2005) Role of leucine 31 of phospholamban in structural and functional interactions with the Ca2+ pump of cardiac sarcoplasmic reticulum. J. Biol. Chem. 280, 10530–10539 [DOI] [PubMed] [Google Scholar]
- 23. Chen Z., Akin B. L., Jones L. R. (2007) Mechanism of reversal of phospholamban inhibition of the cardiac Ca2+-ATPase by protein kinase A and by anti-phospholamban monoclonal antibody 2D12. J. Biol. Chem. 282, 20968–20976 [DOI] [PubMed] [Google Scholar]
- 24. Akin B. L., Chen Z., Jones L. R. (2010) Superinhibitory phospholamban mutants compete with Ca2+ for binding to SERCA2a by stabilizing a unique nucleotide-dependent conformational state. J. Biol. Chem. 285, 28540–28552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morita T., Hussain D., Asahi M., Tsuda T., Kurzydlowski K., Toyoshima C., Maclennan D. H. (2008) Interaction sites among phospholamban, sarcolipin, and the sarco(endo)plasmic reticulum Ca2+-ATPase. Biochem. Biophys. Res. Commun. 369, 188–194 [DOI] [PubMed] [Google Scholar]
- 26. Fujii J., Zarain-Herzberg A., Willard H. F., Tada M., MacLennan D. H. (1991) Structure of the rabbit phospholamban gene, cloning of the human cDNA, and assignment of the gene to human chromosome 6. J. Biol. Chem. 266, 11669–11675 [PubMed] [Google Scholar]
- 27. Jones L. R., Cala S. E. (1981) Biochemical evidence for functional heterogeneity of cardiac sarcoplasmic reticulum vesicles. J. Biol. Chem. 256, 11809–11818 [PubMed] [Google Scholar]
- 28. Reddy L. G., Jones L. R., Cala S. E., O'Brian J. J., Tatulian S. A., Stokes D. L. (1995) Functional reconstitution of recombinant phospholamban with rabbit skeletal Ca2+-ATPase. J. Biol. Chem. 270, 9390–9397 [DOI] [PubMed] [Google Scholar]
- 29. Gill S. C., von Hippel P. H. (1989) Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 182, 319–326 [DOI] [PubMed] [Google Scholar]
- 30. Kho C., Lee A., Jeong D., Oh J. G., Chaanine A. H., Kizana E., Park W. J., Hajjar R. J. (2011) SUMO1-dependent modulation of SERCA2a in heart failure. Nature 477, 601–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Clausen J. D., McIntosh D. B., Woolley D. G., Andersen J. P. (2008) Critical interaction of actuator domain residues arginine 174, isoleucine 188, and lysine 205 with modulatory nucleotide in sarcoplasmic reticulum Ca2+-ATPase. J. Biol. Chem. 283, 35703–35714 [DOI] [PubMed] [Google Scholar]
- 32. Movsesian M. A., Colyer J., Wang J. H., Krall J. (1990) Phospholamban-mediated stimulation of Ca2+ uptake in sarcoplasmic reticulum from normal and failing hearts. J. Clin. Invest. 85, 1698–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Movsesian M. A., Mohsen K., Green K., Jones L. R. (1994) Circulation Ca2+ transporting ATPase, phospholamban, and calsequestrin levels in non-failing and failing human myocardium. J. Clin. Invest. 90, 653–657 [DOI] [PubMed] [Google Scholar]
- 34. Linck B., Bokník P., Eschenhagen T., Müller F. U., Neumann J., Nose M., Jones L. R., Schmitz W., Scholz H. (1996) Messenger RNA expression and immunological quantification of phospholamban and SR-Ca2+-ATPase in failing and nonfailing human hearts. Cardiovasc. Res. 31, 625–632 [PubMed] [Google Scholar]
- 35. Hasenfuss G. (1998) Alterations of calcium-regulatory proteins in heart failure. Cardiovasc. Res. 37, 279–289 [DOI] [PubMed] [Google Scholar]
- 36. Ferrington D. A., Yao Q., Squier T. C., Bigelow D. J. (2002) Comparable levels of Ca-ATPase inhibition by phospholamban in slow-twitch skeletal and cardiac sarcoplasmic reticulum. Biochemistry 41, 13289–13296 [DOI] [PubMed] [Google Scholar]
- 37. Kimura Y., Asahi M., Kurzydlowski K., Tada M., MacLennan D. H. (1998) Phospholamban domain lb mutations influence functional interactions within the Ca2+-ATPase isoform of cardiac sarcoplasmic reticulum. J. Biol. Chem. 273, 14238–14241 [DOI] [PubMed] [Google Scholar]
- 38. Zhao W., Yuan Q., Qian J., Waggoner J. R., Pathak A., Chu G., Mitton B., Sun X., Jin J., Braz J. C., Hahn H. S., Marreez Y., Syed F., Pollesello P., Annila A., Wang H. S., Schultz Jel J., Molkentin J. D., Liggett S. B., Dorn G. W., 2nd, Kranias E. G. (2006) The presence of Lys-27 instead of Asn-27 in human phospholamban promotes sarcoplasmic reticulum Ca2+-ATPase superinhibition and cardiac remodeling. Circulation 113, 995–1004 [DOI] [PubMed] [Google Scholar]
- 39. Wang H. S., Arvanitis D. A., Dong M., Niklewski P. J., Zhao W., Lam C. K., Kranias E. G., Sanoudou D. (2011) SERCA2a superinhibition by human phospholamban triggers electrical and structural remodeling in mouse hearts. Physiol. Genomics 43, 357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]