Background: Increased amyloid precursor protein (APP) contributes to Alzheimer disease (AD), but how its protein stability is regulated is unclear.
Results: Pin1 inhibits glycogen synthase kinase-3β (GSK3β) and thereby reduces APP phosphorylation and promotes its protein turnover.
Conclusion: Pin1 promotes APP protein turnover by inhibiting GSK3β activity.
Significance: This study provided a novel mechanism for Pin1 to protect against AD.
Keywords: Alzheimer Disease, Glycogen Synthase Kinase-3, Neurodegeneration, Prolyl Isomerase, Protein Stability
Abstract
Alzheimer disease (AD) is characterized by the presence of senile plaques of amyloid-β (Aβ) peptides derived from amyloid precursor protein (APP) and neurofibrillary tangles made of hyperphosphorylated Tau. Increasing APP gene dosage or expression has been shown to cause familial early-onset AD. However, whether and how protein stability of APP is regulated is unclear. The prolyl isomerase Pin1 and glycogen synthase kinase-3β (GSK3β) have been shown to have the opposite effects on APP processing and Tau hyperphosphorylation, relevant to the pathogenesis of AD. However, nothing is known about their relationship. In this study, we found that Pin1 binds to the pT330-P motif in GSK3β to inhibit its kinase activity. Furthermore, Pin1 promotes protein turnover of APP by inhibiting GSK3β activity. A point mutation either at Thr-330, the Pin1-binding site in GSK3β, or at Thr-668, the GSK3β phosphorylation site in APP, abolished the regulation of GSK3β activity, Thr-668 phosphorylation, and APP stability by Pin1, resulting in reduced non-amyloidogenic APP processing and increased APP levels. These results uncover a novel role of Pin1 in inhibiting GSK3β kinase activity to reduce APP protein levels, providing a previously unrecognized mechanism by which Pin1 protects against Alzheimer disease.
Introduction
Alzheimer disease (AD)4 is characterized by the accumulation of senile plaques made of amyloid-β peptides (Aβ) derived from amyloid precursor protein (APP) and neurofibrillary tangles composed of hyperphosphorylated Tau. It has been shown that duplication of human APP gene causes familial early-onset AD (1), and familial early-onset AD-linked genetic mutations in APP gene promoter cause 2-fold increase in APP transcriptional activity resembling the effect of APP triplication in Down syndrome (2). Almost all Down syndrome patients over the age of 40 develop AD neuropathology (3), but importantly, Down syndrome patients with partial trisomy 21 excluding the APP gene region showed no AD pathology (4). In addition, overexpression of APP results in amyloid deposition, memory deficit, and Aβ elevation in mice (5). These results suggest that APP gene dosage is the key detrimental factor in the pathogenesis of AD. Although APP processing has been well studied (6, 7), little is known about the regulation of APP protein stability.
Glycogen synthase kinase-3 (GSK3) is involved in a variety of cellular processes. GSK3β, also known as Tau kinase I, is widely expressed in all tissues, with high expression in brain, and is important for normal neuronal growth (9). Aberrant regulation of GSK3β has been linked to diseases such as AD (10), cancer, and diabetes (8). Hyperactivity of GSK3β increases Aβ production and toxicity and Tau phosphorylation (11). Mice with conditional overexpression of GSK3β in forebrain neurons have a reversible condition that resembles AD neuropathology (12). Thus, tight GSK3β regulation is important in AD pathology.
The primary mechanism for the regulation of GSK3β is through inhibition (8), well recognized by Ser-9 phosphorylation that inhibits kinase activity. An additional inhibitory phosphorylation site is regulated by p38 mitogen-activated protein kinase (MAPK) (13). However, so far, how GSK3β activity is regulated in AD is unclear.
Pin1 is a unique prolyl isomerase that binds to and isomerizes certain phosphorylated Ser/Thr-Pro (pS/T-P) motifs (14). Pin1 aberrations contribute to a growing number of diseases, notably AD, cancer, and aging (15). Pin1 acts on the pT231-P in Tau to restore its microtubule function and to promote its dephosphorylation and degradation (14, 16, 17). Pin1 also acts on the T668-P motif in APP to promote non-amyloidogenic APP processing (7). Importantly, Pin1 is inhibited in human AD neurons via multiple mechanisms. In contrast, the Pin1 SNP that prevents its down-regulation in the brain is associated with delayed onset of AD (19). These and other results suggest a neuroprotective role for Pin1 in AD.
Previous studies have shown that APP and Tau can be phosphorylated by GSK3β (11, 20). Notably, Pin1 and GSK3β have the opposite effects on APP processing and Tau hyperphosphorylation in AD. However, nothing is known about their relationship. In this study, we reveal for the first time that Pin1 promotes APP protein turnover by binding and inhibiting GSK3β kinase activity, providing a novel mechanism for Pin1 to protect against AD.
EXPERIMENTAL PROCEDURES
Cell Lines
Human H4 cells were treated for 72 h with an oligonucleotide for silencing Pin1 (siRNA-Qiagen) with HiPerFect (Qiagen). GSK3β stable knockdown H4 cells were generated using human GSK3β shRNA (RNAi Consortium), as described (19).
Plasmid Constructs and Transfection
Human wild-type (WT) GSK3β was from Addgene, and its mutants were prepared using the QuikChange mutagenesis kit (Stratagene, La Jolla, CA). They were transfected into GSK3β knockdown H4 cells followed by harvesting 24 h later.
Immunoprecipitation and Western Blots
αAPPs and βAPPs were measured at the steady state (7). Briefly, culture media of growing cells were immunoprecipitated using 6E10 antibody (Covance), which specifically identifies αAPPs, followed by Western blot using the 22C11 antibody (Chemicon), or using 22C11 antibody, followed by Western blot using the sAPPβ antibody (Covance). Western blots were performed (16) using APP C-terminal antibody (Sigma), GSK3β (BD Transduction Laboratories), p-GSK-3β (S9) (Cell Signaling, Danvers, MA), β-catenin (6B3, Cell Signaling), or laboratory-generated pT668-APP antibody or using a monoclonal Pin1 antibody (7) followed by semiquantification using ImageJ (National Institutes of Health).
Co-immunoprecipitation (Co-IP) and Glutathione S-Transferase (GST) Pulldown Assay
Co-IP experiments were performed as described (19) with Pin1 monoclonal antibody (Epitomics) on brain lysates from wild-type (Pin1-WT), Pin1 knock-out (Pin1-KO), and Pin1 transgenic (Pin1-Tg) mice followed by Western blot with anti-GSK3β antibody. To further determine the S/T-P motifs of GSK3β critical for the binding of Pin1, GSK3β stable knockdown H4 cells were transfected with GSK3β (WT or mutants) followed by GST pulldown assay and Western blot with anti-GSK3β.
Protein Stability Assay
Cells were transfected with GSK WT or T330A constructs for 24 h followed by protein stability assay, as described (17).
In Vitro GSK Kinase Assay
Cells transfected with HA-tagged GSK WT or T330A constructs were lysed and followed by immunoprecipitation using HA antibody. Alternatively, brain lysate was added to the kinase buffer. The kinase reactions were performed using a GSK peptide substrate, GSM (62.5 μm) (Millipore, Temecula, CA) as described (13). To examine the effect of Pin1 on GSK3β kinase activity, His-tagged Pin1 wild-type or mutant protein was preincubated with Pin1 knock-out mouse brain lysates on ice prior to performing GSK3β kinase activity.
RESULTS AND DISCUSSION
Pin1 Binds to pT330-P Motif in GSK3β
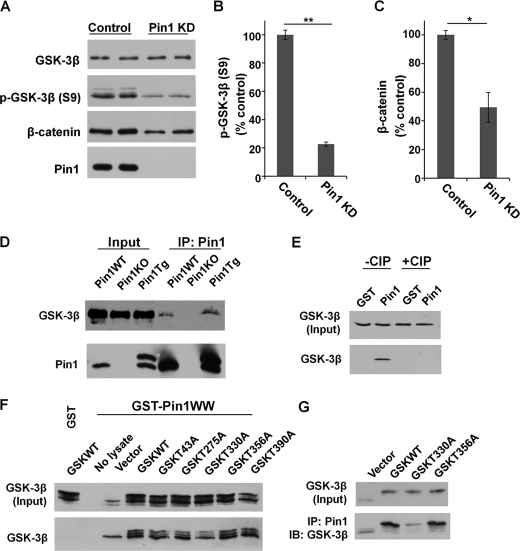
Our results showed that knockdown of Pin1 significantly decreased the level of p-GSK-3β (S9) and β-catenin as a result of reduced inhibition of GSK3β activity (Fig. 1, A–C). Co-IP experiments showed the endogenous interaction between GSK3β and Pin1 in mouse brain lysates (Pin1-WT or Pin1-Tg) (Fig. 1D). The binding between GSK3β and Pin1 was abolished by calf intestinal phosphatase (CIP) treatment (Fig. 1E), indicating a phosphorylation-dependent interaction.
FIGURE 1.
Pin1 binds to phosphorylated Thr-330-P motif in GSK3β. A–C, Pin1 knockdown (Pin1 KD) reduced p-GSK-3β (S9), leading to higher GSK3β activity and a higher rate of β-catenin degradation. Error bars indicate S.D. D, Co-IP confirmed the endogenous interaction of GSK3β and Pin1 in Pin1-WT and Pin1-Tg mouse brains. E, Pin1 binding to GSK3β is dependent on phosphorylation and is abolished after CIP treatment, as assayed by GST-Pin1 pulldown assay. F and G, Pin1 binding to GSK3β point mutants was assayed by GST-Pin1 pulldown (F) and Co-IP (G). *, p < 0.05; **, p < 0.005. IB, immunoblotting.
Pin1 only binds to certain phosphorylated pS/T-P motifs (14), and GSK3β was phosphorylated at the T-P motif, as shown by Western blot using pT-P-specific antibody (supplemental Fig. 1A). The substitution of Ala at GSK3β Thr-330 showed greatly reduced binding to Pin1, whereas other GSK3β mutants showed no significant difference in their ability to bind to Pin1 (Fig. 1, F and G). Thus, the pT330-P motif in GSK3β is a major binding site for Pin1.
Pin1 Inhibits GSK3β Activity in Vivo and in Vitro
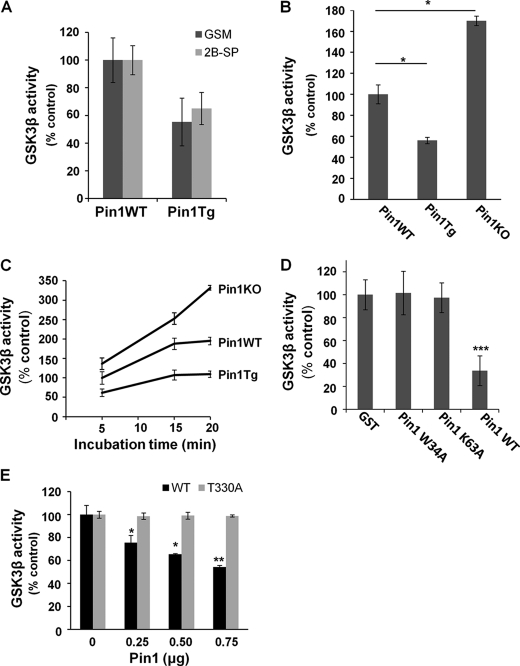
Given the phosphorylation-dependent interaction between Pin1 and GSK3β, a key question is whether Pin1 affects GSK3β kinase activity. To address this question, crude brain lysates from age-matched Pin1-Tg mice and Pin1-KO mice and their respective Pin1-WT littermates were subjected to an in vitro GSK kinase assay using [γ-32P]ATP with two commonly used GSK3β-specific substrates, 2B-SP and GSM, as described (13). GSM was used for subsequent experiments based on its high sensitivity (Fig. 2A). When compared with the age-matched Pin1-WT controls, the GSK3β kinase activity was significantly increased in Pin1-KO mice but dramatically decreased in Pin1-Tg mice (Fig. 2B). Time course experiments revealed that in the absence of Pin1, the rate of GSK3β kinase activity increased significantly when compared with Pin1-WT mice (Fig. 2C). These results suggested that Pin1 might inhibit GSK3β kinase activity. To examine whether this Pin1 inhibitory function requires the ability of Pin1 to bind to and isomerize its substrate, recombinant Pin1 WT, Pin1 W34A that cannot bind to its substrate and Pin1 K63A that cannot isomerize its substrate were incubated in Pin1-KO mouse brain lysates prior to GSK3β kinase assay. Our results showed that GSK3β kinase activity was significantly inhibited only by functional Pin1 (Fig. 2D).
FIGURE 2.
Pin1 inhibits GSK3β activity in vivo and in vitro. A, comparison of GSK kinase activity between Pin1-WT and Pin1-Tg mouse brains using GSM and 2B-SP as substrates for in vitro kinase assay. B, comparison of the GSK3β activity among Pin1-WT, Pin1-Tg and Pin1-KO mice. Pin1-KO and Pin1-Tg mice showed an increase and decrease in GSK3β activity, respectively, when compared with their respective Pin1-WT mice. C, time course analysis of GSK3β activity showed differential GSK3β activity among Pin1-WT, Pin1-Tg, and Pin1-KO mice at 5 min and a higher and faster rate of GSK3β activity in Pin1-KO mice when compared with Pin1-WT controls. D, only Pin1-WT, but not its mutants lacking the substrate binding or substrate isomerization ability, inhibited GSK3β activity. E, GSK3β WT or T330A constructs were transfected in GSK3β knockdown cells and immunoprecipitated by HA antibody followed by preincubation with different amounts of Pin1 and in vitro GSK kinase assay, showing that Pin1 inhibited GSK3β activity of GSK3β WT, but not its T330A mutant, in a dose-dependent manner. *, p < 0.05; **, p < 0.005, ***, p < 0.001. Error bars indicate S.D.
To further confirm that the inhibitory effects of Pin1 on GSK3β kinase activity are due to Pin1 binding to GSK3β on pT330, GSK3β knockdown cells were transfected with HA-GSK3β WT or its T330A constructs followed by the GSK3β kinase activity after immunoprecipitation with anti-HA antibodies. A dose-dependent inhibitory effect of Pin1 on GSK3β kinase activity was observed in GSK3β WT, but not T330A mutant-transfected cells (Fig. 2E). In addition, no difference in basal GSK3β kinase activity was observed between GSK3β WT or T330A transfected cells, suggesting that T330A mutation has no major effects on basal kinase activity. These results indicate that the binding of Pin1 and GSK3β at Thr-330 is critical for the regulation of GSK3β by Pin1.
GSK3β Thr-330 Is Critical for Pin1 to Promote Non-amyloidogenic APP Processing
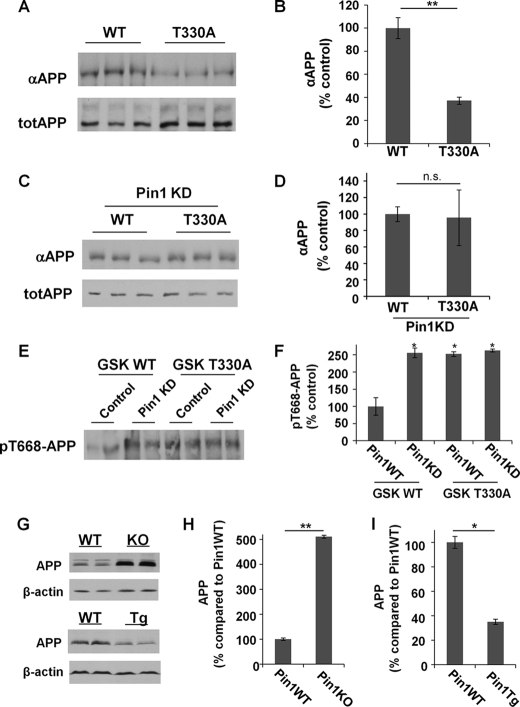
Our results showed that Pin1 knockdown or GSK3β overexpression significantly reduced the level of αAPPs (supplemental Fig. 1, B–H). Moreover, βAPPs production was increased when Pin1 was knocked down (supplemental Fig. 1, I and J). These results confirm our previous findings that Pin1 increases non-amyloidogenic but decreases amyloidogenic APP processing (7). To examine whether Pin1 binding to GSK3β affects αAPP production, GSK3β knockdown cells were transfected with GSK3β WT or T330A constructs, and the level of αAPPs was assayed. Mutation T330A of GSK3β reduced the level of αAPPs (Fig. 3, A and B), but there was no difference in αAPPs secretion between GSK3β WT and its T330A mutant when Pin1 was knocked down (Fig. 3, C and D), suggesting that GSK3β Thr-330 is critical for Pin1 to promote non-amyloidogenic APP processing.
FIGURE 3.
GSK3β Thr-330 is critical for Pin1 to promote non-amyloidogenic APP processing. A and B, mutation of the Pin1-binding site (T330A) reduced αAPPs production. totAPP, total APP. C and D, when Pin1 was knocked down (Pin1 KD), there was no difference between GSK3β WT and T330A on the level of αAPPs production. E and F, the Thr-668 phosphorylation level was reduced in the presence of Pin1 when cells were transfected with GSK3β WT, but not GSK3β T330A. G–I, higher and lower endogenous APP levels were found in Pin1-KO and Pin1-Tg mouse brain lysates, respectively, when compared with Pin1-WT controls. *, p < 0.05; **, p < 0.005; n.s., p > 0.05 (not significant). Error bars in panels B, D, F, H, and I indicate S.D.
Given that GSK3β has been shown to phosphorylate the pT668-P motif in APP (20), it is possible that Pin1 might regulate APP phosphorylation through GSK3β. To examine this possibility, H4 cells with control or Pin1 siRNA treatment were transfected with GSK3β WT or T330A constructs, and Thr-668 phosphorylation levels were compared. Our results showed that Pin1 reduced pT668-APP in cells transfected with GSK3β WT, but not GSK3β T330A (Fig. 3, E and F), suggesting that Thr-330 of GSK3β is a critical site for Pin1 to regulate APP phosphorylation.
Pin1 Inhibits GSK3β to Promote APP Turnover
The APP level is a major determining factor in the pathogenesis of AD. However, so far, little is known about the regulation of APP protein stability.
To address this question, we first compared the endogenous levels of APP in brain lysates from Pin1-KO mice and Pin1-Tg mice and their respective littermates. When compared with Pin1-WT controls, significant higher levels of APP were observed in Pin1-KO mice, similar to those observed previously (7), but reduced levels of APP were found in Pin1-Tg mice (Fig. 3, G–I), suggesting a role for Pin1 in regulating APP stability.
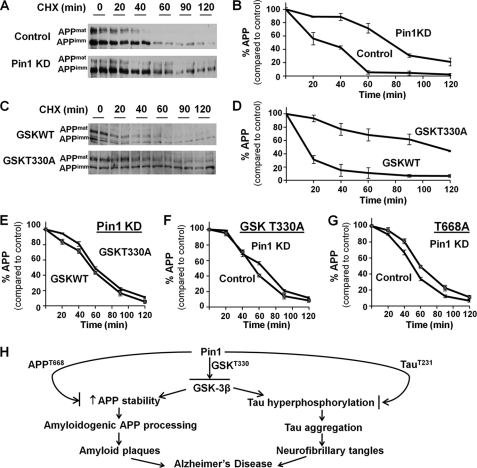
To examine this possibility, H4 cells with control or Pin1 siRNA treatment were incubated with cycloheximide to inhibit de novo APP synthesis, and the steady-state levels of APP were determined at the indicated times. Our results showed that Pin1 knockdown significantly increased the stability of APP when compared with the vector control (Fig. 4, A and B). Similar results were also obtained by determining APP protein stability using [35S]methionine pulse-chase experiments (supplemental Fig. 2, A and B). Thus, Pin1 is important for regulating APP protein turnover.
FIGURE 4.
Pin1 inhibits GSK3β to promote APP turnover. A and B, cells were treated with cycloheximide (CHX)for the indicated time and then subjected to Western blot for APP. When Pin1 was knocked down (Pin1 KD), APP stability was increased, and thus the rate of APP degradation slowed down. APPmat, mature form of APP; APPimm, immature form of APP. C and D, GSK3β T330A reduced the rate of APP degradation when compared with GSK3β WT. E, when Pin1 was knocked down, there was no difference in APP degradation with GSK3β WT or T330A. F, when cells were transfected with GSK3β T330A, Pin1 knockdown did not affect APP degradation. G, Pin1 did not affect the stability of the APP T668A mutant, which abolished the binding site for Pin1 or GSK3β on APP. H, model showing the role of Pin1 on the regulation of GSK3β, which regulates the downstream pathway on APP and Tau, modulating the pathogenesis of AD.
To examine the importance of GSK3β for Pin1 to regulate APP stability, we examined whether the abolishment of the Pin1-binding site in GSK3β would mimic the effects of Pin1 knockdown. GSK3β knockdown cells were transfected with GSK3β WT or T330A constructs followed by the cycloheximide chase. APP was more stable in GSK3β T330A-overexpressing cells (Fig. 4, C and D), similar to Pin1 knockdown cells (Fig. 4, A and B). However, in Pin1 knockdown cells, no difference in APP stability was observed among GSK3β WT or T330A transfected cells (Fig. 4E, supplemental Fig. 2C). In GSK3β T330A transfected cells, when the binding site for Pin1 was abolished, the presence or absence of Pin1 showed no effect on APP stability (Fig. 4F, supplemental Fig. 2D). All these results suggested that GSK3β Thr-330 is important not only for Pin1 to act on GSK3β, but also for GSK3β to regulate APP degradation. To examine whether Pin1 might regulate APP degradation through Thr-668 phosphorylation, H4 cells with control or Pin1 siRNA treatment were transfected with T668A mutant followed by the cycloheximide chase. No difference in the stability of the APP mutant was observed in the presence or absence of Pin1 (Fig. 4G, supplemental Fig. 2F). Taken together, these results indicate that Pin1 increases APP turnover through the regulation of GSK3β activity.
Decreased Pin1 (14) and increased GSK3β (10) levels were reported in brains of AD patients. There were also studies suggesting the independent roles of Pin1 and GSK3β on AD, including the conformational changes on Tau (14, 16) and APP (7) by Pin1, hyperphosphorylation of Tau (11), and increased production of Aβ by GSK3β; however, the link between Pin1 and GSK3β on the pathogenesis of AD is unclear. Our current study identified GSK3β as the substrate of Pin1 and the regulation of GSK3β by Pin1 through the binding of Pin1 and GSK3β at the T330-P motif. In the absence of Pin1 or abolishment of the Pin1-binding site in GSK3β, we found that there was an increase in GSK3β activity and increased stability of APP favoring the production of toxic Aβ, suggesting the important role of Pin1 in the regulation of GSK3β and the downstream pathway. Different studies have already shown that Pin1 has a direct effect on Tau phosphorylation (14, 16) and APP processing (7). Our current study suggested that Pin1 can regulate APP turnover directly and indirectly through the regulation of GSK3β. We propose a model in which Pin1 binds GSK3β Thr-330 and inhibits its activity, thus regulating APP processing and stability as well as Tau phosphorylation, stability, and aggregation. Pin1 can also regulate APP and Tau directly through APP Thr-668 and Tau Thr-231 after they are phosphorylated by GSK3β or other kinases (Fig. 4H). These findings are highly significant as they demonstrate a novel mechanism for Pin1 to protect against AD.
Acknowledgments
We are grateful to Prof. Nelson L. S. Tang and Holly Y. Chen for the experimental assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants R01AG017870 and R01AG039405 (to K. P. L).

This article contains supplemental Figs. 1 and 2.
- AD
- Alzheimer disease
- APP
- amyloid precursor protein
- APPs
- secreted APP
- Aβ
- amyloid-β
- GSK3
- glycogen synthase kinase-3
- Pin1-WT
- Pin1 wild-type mice
- Pin1-KO
- Pin1 knockout mice
- Pin1-Tg
- Pin1 transgenic mice
- CIP
- calf intestinal phosphatase
- Co-IP
- co-immunoprecipitation
- p-GSK-3β (S9)
- phosphorylated GSK-3β at Ser-9.
REFERENCES
- 1. Sleegers K., Brouwers N., Gijselinck I., Theuns J., Goossens D., Wauters J., Del-Favero J., Cruts M., van Duijn C. M., Van Broeckhoven C. (2006) APP duplication is sufficient to cause early onset Alzheimer dementia with cerebral amyloid angiopathy. Brain 129, 2977–2983 [DOI] [PubMed] [Google Scholar]
- 2. Theuns J., Brouwers N., Engelborghs S., Sleegers K., Bogaerts V., Corsmit E., De Pooter T., van Duijn C. M., De Deyn P. P., Van Broeckhoven C. (2006) Promoter mutations that increase amyloid precursor-protein expression are associated with Alzheimer disease. Am. J. Hum. Genet. 78, 936–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mann D. M. (1988) Alzheimer disease and Down syndrome. Histopathology 13, 125–137 [DOI] [PubMed] [Google Scholar]
- 4. Prasher V. P., Farrer M. J., Kessling A. M., Fisher E. M., West R. J., Barber P. C., Butler A. C. (1998) Molecular mapping of Alzheimer-type dementia in Down syndrome. Ann. Neurol. 43, 380–383 [DOI] [PubMed] [Google Scholar]
- 5. Hsiao K., Chapman P., Nilsen S., Eckman C., Harigaya Y., Younkin S., Yang F., Cole G. (1996) Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274, 99–102 [DOI] [PubMed] [Google Scholar]
- 6. Thinakaran G., Koo E. H. (2008) Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 283, 29615–29619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pastorino L., Sun A., Lu P. J., Zhou X. Z., Balastik M., Finn G., Wulf G., Lim J., Li S. H., Li X., Xia W., Nicholson L. K., Lu K. P. (2006) The prolyl isomerase Pin1 regulates amyloid precursor protein processing and amyloid-β production. Nature 440, 528–534 [DOI] [PubMed] [Google Scholar]
- 8. Rayasam G. V., Tulasi V. K., Sodhi R., Davis J. A., Ray A. (2009) Glycogen synthase kinase-3: more than a namesake. Br. J. Pharmacol. 156, 885–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Castaño Z., Gordon-Weeks P. R., Kypta R. M. (2010) The neuron-specific isoform of glycogen synthase kinase-3β is required for axon growth. J. Neurochem. 113, 117–130 [DOI] [PubMed] [Google Scholar]
- 10. Pei J. J., Tanaka T., Tung Y. C., Braak E., Iqbal K., Grundke-Iqbal I. (1997) Distribution, levels, and activity of glycogen synthase kinase-3 in the Alzheimer disease brain. J. Neuropathol. Exp. Neurol. 56, 70–78 [DOI] [PubMed] [Google Scholar]
- 11. Flaherty D. B., Soria J. P., Tomasiewicz H. G., Wood J. G. (2000) Phosphorylation of human Tau protein by microtubule-associated kinases: GSK3β and cdk5 are key participants. J. Neurosci. Res. 62, 463–472 [DOI] [PubMed] [Google Scholar]
- 12. Engel T., Hernández F., Avila J., Lucas J. J. (2006) Full reversal of Alzheimer disease-like phenotype in a mouse model with conditional overexpression of glycogen synthase kinase-3. J. Neurosci 26, 5083–5090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thornton T. M., Pedraza-Alva G., Deng B., Wood C. D., Aronshtam A., Clements J. L., Sabio G., Davis R. J., Matthews D. E., Doble B., Rincon M. (2008) Phosphorylation by p38 MAPK as an alternative pathway for GSK3β inactivation. Science 320, 667–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lu P. J., Wulf G., Zhou X. Z., Davies P., Lu K. P. (1999) The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated Tau protein. Nature 399, 784–788 [DOI] [PubMed] [Google Scholar]
- 15. Lu K. P., Zhou X. Z. (2007) The prolyl isomerase PIN1: a pivotal new twist in phosphorylation signaling and disease. Nat. Rev. Mol. Cell Biol. 8, 904–916 [DOI] [PubMed] [Google Scholar]
- 16. Lim J., Balastik M., Lee T. H., Nakamura K., Liou Y. C., Sun A., Finn G., Pastorino L., Lee V. M., Lu K. P. (2008) Pin1 has opposite effects on wild-type and P301L Tau stability and tauopathy. J. Clin. Invest. 118, 1877–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakamura K., Greenwood A., Binder L., Bigio E. H., Denial S. J., Nicholson L., Zhou X. Z., Lu K. P. (2012) Cell, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liou Y. C., Sun A., Ryo A., Zhou X. Z., Yu Z. X., Huang H. K., Uchida T., Bronson R., Bing G., Li X., Hunter T., Lu K. P. (2003) Role of the prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature 424, 556–561 [DOI] [PubMed] [Google Scholar]
- 19. Ma S. L., Tang N. L., Tam C. W., Cheong Lui V. W., Lam L. C., Chiu H. F., Driver J. A., Pastorino L., Lu K. P. (2012) A PIN1 polymorphism that prevents its suppression by AP4 associates with delayed onset of Alzheimer's disease. Neurobiol. Aging, in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Aplin A. E., Gibb G. M., Jacobsen J. S., Gallo J. M., Anderton B. H. (1996) In vitro phosphorylation of the cytoplasmic domain of the amyloid precursor protein by glycogen synthase kinase-3β. J. Neurochem. 67, 699–707 [DOI] [PubMed] [Google Scholar]