Abstract
Nitrogen monoxide (NO) markedly affects intracellular iron metabolism, and recent studies have shown that molecules traditionally involved in drug resistance, namely GST and MRP1 (multidrug resistance-associated protein 1), are critical molecular players in this process. This is mediated by interaction of these proteins with dinitrosyl-dithiol-iron complexes (Watts, R. N., Hawkins, C., Ponka, P., and Richardson, D. R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7670–7675; Lok, H. C., Suryo Rahmanto, Y., Hawkins, C. L., Kalinowski, D. S., Morrow, C. S., Townsend, A. J., Ponka, P., and Richardson, D. R. (2012) J. Biol. Chem. 287, 607–618). These complexes are bioavailable, have a markedly longer half-life compared with free NO, and form in cells after an interaction between iron, NO, and glutathione. The generation of dinitrosyl-dithiol-iron complexes acts as a common currency for NO transport and storage by MRP1 and GST P1-1, respectively. Understanding the biological trafficking mechanisms involved in the metabolism of NO is vital for elucidating its many roles in cellular signaling and cytotoxicity and for development of new therapeutic targets.
Keywords: Iron, Iron Metabolism, Metals, Protein-Metal Ion Interaction, Transport Metals
Nitric Oxide Mediates Many of Its Effects by Binding to Iron
Virtually all fields of biochemistry and physiology have been influenced by the discovery of nitrogen monoxide (NO) (1). NO is a small, unstable, potentially toxic, diatomic molecule that is produced by many mammalian cells (2, 3). It is well known that NO has a physiological role as a short-lived messenger and has two major functions in cells: regulation and cytotoxicity (2, 3). Some of the signaling roles of NO are mediated by its avid binding to iron in the heme group of soluble guanylate cyclase (sGC)2 (2, 3). In fact, the interaction of NO with iron forms a well known branch of coordination chemistry (1).
The importance of iron in mediating the functions of NO is also apparent when examining its cytotoxic effects. The cytotoxic functions of NO are observed when it is produced in large amounts by activated macrophages (Mφs) against pathogens and tumor cells (2, 4). These effects are mediated by the reactivity of NO with iron and can be explained, at least in part, by its binding to [Fe-S] clusters and heme centers in the mitochondrial electron transport chain, e.g. cytochrome c oxidase (5, 6). The high affinity of NO for Fe2+ results in the removal of iron from [Fe-S] centers and the formation of dinitrosyl-iron species within [Fe-S] proteins (7). Co-cultivation of tumor cells with activated Mφs results in the inhibition of target cell DNA synthesis and a loss of 64% of cellular iron in 24 h (4). All of these effects of NO severely impair ATP and DNA synthesis (4, 7).
NO is generated from l-arginine and O2 by the NOS family of proteins (1). The activity of this group of enzymes can be inhibited by l-arginine analogs, e.g. NG-monomethyl-l-arginine, which provides a very useful experimental tool (8). Inducible NOS (iNOS) can be induced with LPS and cytokines (e.g. IFN-γ) (9) in murine Mφs and mediates high NO output. The role of cytokine-inducible NO-generating activity in humans has also been described (10). Neuronal NOS and endothelial NOS (eNOS) are constitutively expressed and are generally thought to generate NO at lower levels.
Iron, an Obligate Requirement for Life
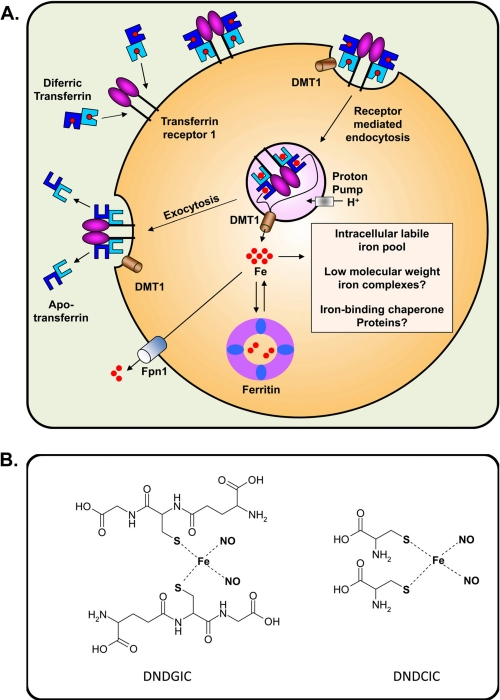
To understand the interaction of NO with iron, a brief overview of the basic mechanisms involved is important. Iron is crucial for the activity of many enzymes (2, 3). Extracellular iron is transported by transferrin (Tf) and is internalized after binding to Tf receptor 1 (TfR1) by receptor-mediated endocytosis (Fig. 1A) (2, 3). Ferric iron is released from Tf within the endosome after its acidification and is then reduced by an endosomal ferrireductase, e.g. STEAP3 (six-transmembrane epithelial antigen of the prostate 3) (11–13). This is then followed by transport of Fe2+ across the endosomal membrane by DMT1 (divalent metal transporter 1) (3). This nascent cytosolic iron then becomes part of the labile iron pool (LIP) that is utilized for metabolism or stored in ferritin (Fig. 1A) (2, 3). The nature of the LIP remains unclear and was thought to be composed of low-Mr complexes (14). However, work using reticulocytes demonstrated that this low-Mr iron was not an intermediate but instead had kinetic characteristics of an end product (15). These studies suggest that iron exiting the endosome may be bound to chaperone proteins and/or is transported by direct interactions between organelles (15). Other studies suggested a specific iron-delivering interaction of the Tf-containing endosome with the mitochondrion (16). More recent investigations have demonstrated a chaperone role for human PCBP1 (poly(rC)-binding protein 1) and PCBP2 in the transport of iron to ferritin (17) and also to hypoxia-inducible factor-1α prolyl hydroxylases (18).
FIGURE 1.
A, schematic showing the basic processes of cellular iron metabolism. Iron is transported in the blood bound to Tf. Tf binds to TfR1 on the cell surface and is internalized by receptor-mediated endocytosis. Iron is released from Tf by a decrease in pH and transported across the endosomal membrane by DMT1. Once in the cytosol, iron becomes part of the poorly characterized intracellular iron pool, which has been suggested to be bound by low-Mr ligands, e.g. citrate or chaperone molecules such as PCBP1 and PCBP2. Iron can be stored within the protein ferritin. In the absence of NO, iron can be released from cells by the trans-plasma membrane protein Fpn1. B, line drawings of the structures of physiologically relevant DNICs. These include DNDGIC and DNDCIC. Coordinating water molecules have been omitted for clarity in each case.
Cellular iron metabolism is controlled, at least in part, by IRP1 (iron regulatory protein 1) and IRP2 (2, 12). The iron regulatory proteins are RNA-binding factors that regulate iron homeostasis by controlling TfR1 and ferritin expression. They also regulate other molecules involved in iron metabolism, including Fpn1 (ferroportin 1), which functions in iron mobilization from cells (Fig. 1A) (19–21). For a more in-depth description of iron metabolism, the reader is directed to comprehensive reviews (11–13).
Effect of NO on Cellular Iron Metabolism
Because of its high affinity for iron and rich coordination chemistry, NO has been shown to form complexes with a variety of important iron-containing proteins such as ribonucleotide reductase (22), [Fe-S]-containing proteins such as ferrochelatase (23), heme-containing proteins (24, 25), and ferritin (26). In fact, ferritin has been suggested to act as a store of NO (26).
NO strongly activates the RNA-binding activity of IRP1 and IRP2 and has a marked effect on iron metabolism (2, 3, 27, 28). In fact, the effect of NO on IRP1 RNA-binding activity occurs via two possible mechanisms: 1) a direct effect on the [4Fe-4S] cluster of the aconitase form of IRP1, leading to its disassembly, and/or 2) iron mobilization from cells, leading to iron depletion (27–30). The effect of NO on iron regulatory protein RNA-binding activity increases TfR1 expression and results in a slight increase in iron uptake from Tf (27). The discordance between the ability of NO to markedly increase TfR1 expression but only slightly increase iron uptake from Tf could be related to the ability of NO to reduce ATP synthesis, which is vital for iron uptake (31, 32).
The importance of cellular iron depletion by NO has recently been confirmed by Hickok et al. (33), who showed that NO suppresses tumor cell migration via up-regulation of the iron-regulated metastasis suppressor molecule, NDRG1 (N-Myc downstream regulated gene-1). NDRG1 is a cytosolic protein (34, 35) that is strongly up-regulated by cellular iron depletion via hypoxia-inducible factor-1α-dependent and -independent pathways (34–36). The effect of NO on the up-regulation of NDRG1 probably occurs through its ability to deplete the LIP with the subsequent mobilization of iron (28, 37–39). The importance of this iron pool in NO activity has been confirmed by studies showing that it provides iron for dinitrosyl-dithiol-iron complex (DNIC) generation (40).
Nitric Oxide-Iron Interactions: A Possible Mechanism by Which Activated Mφs Inhibit Tumor Target Cell Proliferation
The nonspecific effector component mediating resistance to tumor cells is activated Mφs (41). Mφ activation occurs after infection with mycobacteria and stimuli such as LPS and cytokines (42), which result in the synthesis of tumor necrosis factor-α (43) and NO (8).
Many of the effects of NO result from its tenacious binding to iron (3, 27, 28). Intracellular iron release via NO generated by Mφs mediates, at least in part, their cytotoxic effector activity against tumor cells (4). NO induces a loss of aconitase activity, complexes I and II in Mφs and co-cultured tumor target cells, and the formation of DNICs in both cell types (44). These species can be readily detected by EPR spectroscopy with a signal of g = 2.04 (45). Vanin (46) showed that they have the formula Fe(RS)2(NO)2, i.e. a DNIC. Physiologically relevant examples of DNICs include the dinitrosyl-diglutathionyl-iron complex (DNDGIC) and the dinitrosyl-dicysteinyl-iron complex (DNDCIC) (Fig. 1B). However, these mononuclear DNICs may not be the only species present and responsible for physiological/pathological reactions. In fact, a recent report showed that NO reacts with [2Fe-2S] clusters, forming dinuclear complexes (47).
Identification of DNIC formation in tumor cells links Mφ-induced inhibition of iron-containing enzymes with NO biosynthesis. As NO mediates tumor killing via its interaction with iron (2–4), it is important to reveal the nature of these interactions.
DNICs Are the Major Cellular Form of NO
DNICs are formed rapidly in cells upon exposure to NO, which binds iron from the intracellular LIP (40, 48). Later during this process, NO mobilizes iron from other sources, and this coincides with increasing cytotoxicity (48). It has been shown that DNICs are the largest NO-derived adduct in cells, greatly exceeding the production of S-nitrosothiols (48), which are another NO-stabilizing and signaling species (1).
The similarity of EPR spectra obtained at 77 K and at ambient temperature indicates that most cellular DNICs have a high Mr, suggesting that they are bound to macromolecules (38, 40, 49). Nevertheless, low-Mr DNICs such as DNDGICs and DNDCICs are likely to exist in cells in equilibrium with protein-bound complexes. In fact, low-Mr DNICs could be a mobile NO-transporting component between proteins (50, 51). The role of DNICs in the storage and transport of NO is discussed below.
Role of DNICs in Cytotoxicity
Although the cytotoxic effects of NO are well known, the role of DNICs in this process is unclear. DNICs induce apoptosis in Jurkat cells even when the anti-apoptotic molecule Bcl-2 is overexpressed (52). However, recent studies by Vanin and co-workers (53) demonstrated that DNICs (derived from GSH or cysteine) did not show any apoptotic activity in HeLa cells, in contrast to the pro-apoptotic effects of S-nitrosoglutathione (GSNO). These authors concluded that DNICs were bound to membrane proteins, as protein-bound DNICs had been shown to limit the reaction of NO with superoxide and the formation of toxic peroxynitrite (54).
Other studies also do not agree regarding the toxicity of DNICs, where the pro-apoptotic effect of NO on Mφs could be eliminated via its incorporation into DNICs (55). To add to the controversy, Pedersen et al. (56) showed that GSTs sequester toxic DNICs in cells. This property is important, as DNICs may cause irreversible inhibition of glutathione reductase (57, 58) and other biomolecules.
Collectively, it can be suggested that, at lower concentrations, DNICs may function as NO carriers and release it (59, 60), which can occur by spontaneous decomposition of DNICs in vitro (61), to acceptors with higher affinity for NO (e.g. the heme center of sGC). In fact, for DNICs to be a storage form of NO would imply that NO is liberated and/or transferred at some point (49, 60, 62). Although yet to be directly demonstrated, this could assist NO-dependent signaling in vivo. However, the cytotoxic effects of DNICs may occur at higher concentrations, as may be expected under conditions of chronic inflammation, where the mechanisms involved in their storage (e.g. GSTs) could potentially be overwhelmed.
Model of d-Glucose-dependent NO-mediated Iron Mobilization
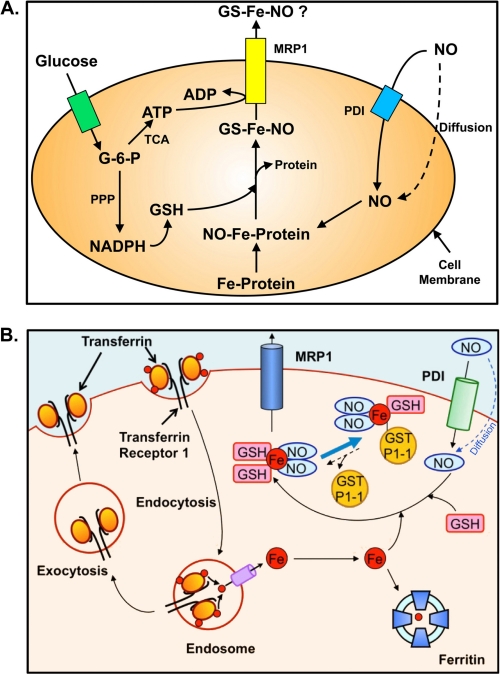
A number of studies have examined the effect of NO on cellular iron mobilization (3, 32, 38, 39, 63). From these investigations, a model of d-glucose-dependent NO-mediated iron mobilization from cells was proposed (Fig. 2A) (3). These experiments demonstrated that d-glucose must be transported into cells and then metabolized to enable NO-mediated iron mobilization (39). In fact, only metabolizable sugars, and not those that cannot be metabolized or transported across the cell membrane, could promote iron mobilization (39).
FIGURE 2.
A, hypothetical model of d-glucose-dependent NO-mediated iron mobilization from cells. Studies using a variety of metabolizable and non-metabolizable sugars demonstrated that NO-mediated iron mobilization from cells is dependent on the transport of d-glucose into cells and its metabolism (39). Glucose is used by the tricarboxylic acid cycle for the production of ATP and by the pentose phosphate pathway (PPP) for the generation of NADPH, which is involved in the synthesis of GSH (39). NO either diffuses or is transported into cells by protein-disulfide isomerase (PDI) (100). Once within cells, NO intercepts and binds iron bound to proteins or iron that is en route to ferritin. The high affinity of NO for iron results in the formation of DNICs (represented here as GS-Fe-NO). GSH may either be involved as a reductant to remove iron from endogenous ligands and/or complete the iron coordination shell along with NO in the DNIC. This complex as an intact entity or its separate components can be released from the cell by an active process requiring MRP1. This model has been modified from Ref. 3. G-6-P, glucose 6-phosphate. B, schematic illustrating the respective roles of GST P1-1 and MRP1 in NO storage and transport. NO can diffuse through the membrane or may be actively transported into cells by protein-disulfide isomerase (100). Because of the ability of NO to act as a ligand, it can bind iron transported into cells and released from Tf. GSH completes the coordination sphere of the NO-iron complex to form a DNIC. DNICs can be bound by GST P1-1 or effluxed out of cells via MRP1 (37, 38).
These investigations indicated that the metabolism of glucose to generate NADPH via the pentose phosphate pathway was required to generate GSH (l-γ-glutamyl-l-cysteinylglycine), which was critical for NO-mediated iron release (Fig. 2A) (3, 32, 38, 39, 63). GSH is a major antioxidant in cells (64) and exists in reduced (GSH) and oxidized (GSSG) forms (65).
Interestingly, a specific inhibitor of GSH synthesis, l-buthionine (SR)-sulfoximine (BSO) (66), prevented NO-mediated iron release, an effect reversed by reconstitution of GSH (39). Moreover, the process of NO-mediated iron mobilization was shown to be dependent on ATP generation (39). However, the dependence on GSH for NO-mediated iron release was not observed when assessing the mobilization of iron via synthetic chelators (39). Hence, these two processes may be mediated by different mechanisms.
Considering these results in the context of the form of iron released by NO, it is known that NO enters cells, where it interacts with protein-bound iron or iron en route to ferritin (Fig. 2A) (3, 39). The high affinity of NO for iron results in a NO-iron complex, and GSH could act as a ligand to complete the coordination shell, forming a DNIC (Fig. 2A) (3). Studies were initiated to examine the mechanism responsible (38).
Ubiquitous GSH Transporter MRP1 Mediates NO-mediated Iron and GSH Release
Considering that NO-mediated iron efflux from cells requires GSH (39), it was hypothesized that a DNIC composed of iron, GSH, and NO was released (3, 63). An active process was indicated due to the requirement for glucose metabolism, ATP, GSH, and an intact cell membrane (39, 63). Previous studies have described the role of MRP1 (multidrug resistance-associated protein 1; ABCC1) in the transport of GSH conjugates (67) and particularly complexes of antimony and arsenic (67, 68). Considering this together with the fact that MRP1 is ubiquitously expressed (69) and requires ATP (70), this transporter was a candidate for exporting DNDGICs.
MRP1 is a member of the ATP-binding cassette transporter superfamily (71, 72). It uses ATP hydrolysis to efflux various anticancer drugs and other organic anions, often as conjugates of GSH (67, 73).
Initial studies (38) examining the role of MRP1 in NO-mediated iron release from cells investigated the MRP1-hyperexpressing human breast cancer cell line MCF7-VP and its relevant parental counterpart, MCF7-WT, which expresses only very low levels of MRP1 (74). The MCF7-VP cell line demonstrated a 3–4-fold increase in NO-mediated 59Fe and GSH efflux compared with WT cells. Furthermore, the NO-mediated 59Fe and GSH efflux was prevented by the GSH-depleting agent BSO, which inhibits GSH synthesis (66). Classical MRP1 inhibitors such as MK571, probenecid, and difloxacin inhibited NO-mediated 59Fe release (38). Moreover, EPR spectroscopy demonstrated that MRP1 inhibitors increased the DNIC signal in cells after NO treatment, indicating inhibition of release of the complex from the cell via MRP1 (38). More recent studies have shown that NO generated by several different sources, including transfection with iNOS or exposure to two exogenous NO generators (i.e. GSNO or spermine NONOate), leads to iron release from wild-type murine embryonic fibroblasts, but not from murine embryonic fibroblasts from MRP1 knock-out mice (37).
Considering the NO-mediated release of both iron and GSH from cells (38, 39), it is notable that GSH efflux is a key signal mediating apoptosis (75), and it is well known that iron efflux from cells using chelators results in antitumor activity (76). Hence, the dual action of NO resulting in iron and GSH mobilization could play a role in Mφ-mediated cytotoxicity against tumor cells.
It is notable that MRP1 is responsible only for NO-mediated iron mobilization and does not play a role in iron release in the absence of NO. This was shown by studies in control cells (incubated without NO) in which BSO had no marked effect on iron mobilization (39, 77). In fact, in the absence of NO, Fpn1 mediates iron mobilization (19–21).
Potential Intermediary or Storage Role of DNICs by GST Enzymes
The GST enzymes form an integrated detoxification mechanism with MRP1 that eliminates toxic exogenous (e.g. anticancer drugs) and endogenous agents as GSH conjugates (78). These enzymes catalyze the attack of GSH on compounds to form GSH conjugates. Human cytosolic GSTs are sorted into seven classes (α, μ, π, σ, θ, ω, and ξ) (78).
Considering the coordinated role of GSTs and MRP1 in detoxification (79–81) and that MRP1 transports iron and GSH in a form consistent with DNICs (37, 38), it is of interest that the most abundant GST isoenzymes, α (GST A1-1), μ (GST M1-1), and π (GST P1-1), bind DNDGICs with high affinity (Kd = 10−9 to 10−10 m) (82, 83). A crystal structure of the DNDGIC-GST P1-1 complex revealed that Tyr-7 in the active site of the enzyme coordinates via its phenolate group to iron, displacing one GSH ligand (82). Using EPR and bacterial cells, it was shown that binding of DNDGIC to human GST P1-1 was reversible, with the loss of the EPR signal occurring within 1 h (82). In tissue homogenates, the half-life (t½) of the complex was markedly longer: 4.5 h for α and μ class GSTs and 8 h for GST π (84).
Most studies examining DNIC-GST complexes have been performed on purified proteins or these proteins heterologously expressed in bacterial cells. In hepatocytes, DNDGIC binds to GST α (56), and it was hypothesized that NO removes iron from ferritin and Tf to form DNICs (56). However, NO does not directly remove Fe3+ from Tf (31) or ferritin (63), and other sites of iron acquisition are likely (31, 63).
The interaction of DNDGICs with GSTs raises the question of their function and if there could be an interaction with MRP1 considering the strongly associated role of these proteins in detoxification (79–81, 85). Of importance, studies in vitro showed that DNDGICs lead to inactivation of glutathione reductase (56). Thus, GSTs may act as a protective mechanism against high levels of DNICs. Alternatively or in combination with this latter function, GSTs may act as a NO store and regulate the release of DNICs via MRP1.
Recent studies (37) have focused on the more abundant GST classes and have examined MCF7-VP cells (hyperexpressing MRP1) and MCF7-WT cells (expressing very low MRP1 levels) stably transfected with vector alone or with GSTA1 (encoding GST A1-1), GSTM1 (encoding GST M1-1), or GSTP1 (encoding GST P1-1). MCF7-VP or MCF7-WT cells transfected with the empty vector alone (without a GST insert) do not highly express GST A1-1, GST M1-1, or GST P1-1. In contrast, cells transfected with GSTA1, GSTM1, and GSTP1 express very high levels of these proteins and thus provide appropriate models for experimentation (37).
Only GST P1-1 Inhibits Cellular Iron Release from Cells Hyperexpressing MRP1
The GST-transfected cells and their relative vector-transfected controls described above were examined in terms of cellular 59Fe mobilization in the presence and absence of NO (37). Intracellular iron pools were labeled by incubation with the physiological iron donor radiolabeled diferric Tf (viz. [59Fe2]Tf) (Fig. 1A). Reincubation of labeled cells with control medium alone did not result in any alteration in iron release when cells transfected with GST A1-1, GST M1-1, or GST P1-1 were compared with cells transfected with the vector alone (37). Reincubation of cells with GSNO led to a marked increase in iron release relative to those incubated with control medium. Importantly, only the GST P1-1-transfected cells showed a significant decrease in NO-mediated 59Fe efflux relative to cells transfected with the empty vector control. A similar ability of GST P1-1 to reduce iron release was also observed in cells that were transfected with iNOS. Hence, NO provided extracellularly (e.g. as spermine NONOate) or intracellularly from iNOS led to similar results, with cells expressing GST P1-1 decreasing the release of cellular iron via MRP1 (37).
Assessment of the form of intracellular iron in NO-treated GST-transfected cell types using native (nondenaturing) fast pressure liquid chromatography demonstrated that an accumulation of iron occurred in fractions containing GST P1-1, but not GST A1-1 or GST M1-1 (37). Further studies using EPR spectroscopy demonstrated that, in cells expressing GST P1-1, but not GST A1-1 or GST M1-1, the DNIC signal was significantly more pronounced and was a different shape than in their vector-transfected counterparts (37). These observations are consistent with x-ray crystallography data demonstrating the different coordination sphere of the DNIC once bound to GST P1-1 (82). Thus, GST P1-1, but neither GST A1-1 nor GST M1-1, decreases iron release by MRP1 by binding DNICs and preventing their release from cells (Fig. 2B).
These latter results do not agree with those obtained by Pedersen et al. (56) using rat hepatocytes and liver homogenates, which suggested that DNICs bind to α class GSTs. The difference in results may be explained by the metabolic disparities between rat and human cells and/or the cell types studied (37, 56). It is notable that MCF7 cells naturally express low levels of GST P1-1, but not GST A1-1 or M1-1 (86). Thus, the biochemistry needed for the interaction between GST P1-1, MRP1, and DNICs may exist in these cells, whereas that for GST A1-1 or GST M1-1 may be absent.
Considering that the affinity of GST α for DNICs is very high and that it is present in hepatocytes at considerable concentrations (0.3 mm) (56), it could be that different cell types have different GST isoenzymes to protect against NO cytotoxicity. Hence, their roles in DNIC metabolism and interaction with MRP1 are important to investigate.
It is intriguing that although GST P1-1 is found in the cytosol, nucleus, and mitochondrion (87), it was recently identified to be associated with the intracellular surface of the plasma membrane (88). If this is validated, it could enable the DNIC bound to GST P1-1 to be brought into proximity to MRP1, allowing its efficient transport out of the cell.
Implications of MRP1-GST P1-1 Interaction in Understanding NO Biology
MRP1 is involved in the NO-mediated efflux of iron and GSH from cells in a form consistent with DNICs (Fig. 2B) (37, 38). Furthermore, GST P1-1 that binds DNICs could act as an intermediate form of NO for regulating biological processes, e.g. vascular tone (82). Considering this, it is of interest that GST polymorphisms correlate with preeclampsia, i.e. high blood pressure during pregnancy (89).
In addition to the significant role of diffusion, the ability of the cell to actively transport, store, and traffic NO augments the random process of diffusion-mediated NO delivery that is inefficient and non-targeted. Because GST enzymes and MRP1 form a well integrated system for removing a variety of toxic agents (79–81), these molecules could coordinately regulate NO levels by binding and transporting DNICs (Fig. 2B) (37, 38). This has important consequences for NO signaling, NO-mediated apoptosis, and Mφ-mediated cytotoxicity that is due, in part, to iron release from tumor targets.
Vital to the role of GST P1-1 and MRP1 in NO metabolism is the concept of DNICs as a useful currency for NO storage and transport. This is crucial due to the greater t½ of NO when found complexed as a DNIC (33, 84, 90, 91). DNICs have been identified in tissues, sera, Mφs, and many cell types (44–46, 92). In addition, DNICs bind with GSTs to stabilize NO for hours (t½ = 4.5–8 h) (84), which exceeds the t½ of “free NO,” i.e. 2 ms to 2 s (93). Moreover, DNICs appear to be transported across membranes to donate iron to cellular pools (91) and can nitrosylate targets (94, 95), illustrating their bioavailability.
Additional studies are needed to elucidate the regulation of three steps involved in NO metabolism and DNIC formation: 1) NO generation and the formation of DNICs (via iNOS), 2) NO storage and regulation (via GST P1-1), and 3) NO transport as DNICs (via MRP1). As increased intracellular iron reduces iNOS expression (96), we postulate a model of NO generation, storage, and transport that may be regulated by DNIC levels, and this requires validation.
Apart from DNICs, which form a bioavailable currency for NO, it is well known that S-nitrosylation also mediates stabilization and transport of NO. There is evidence that DNICs and S-nitrosylated molecules act to mediate the effects of NO and may coexist in equilibrium. In fact, it has been suggested that cellular DNICs also serve as a NO reservoir for protein S-nitrosothiol formation (97).
Possible Physiological Roles of DNICs and New Questions
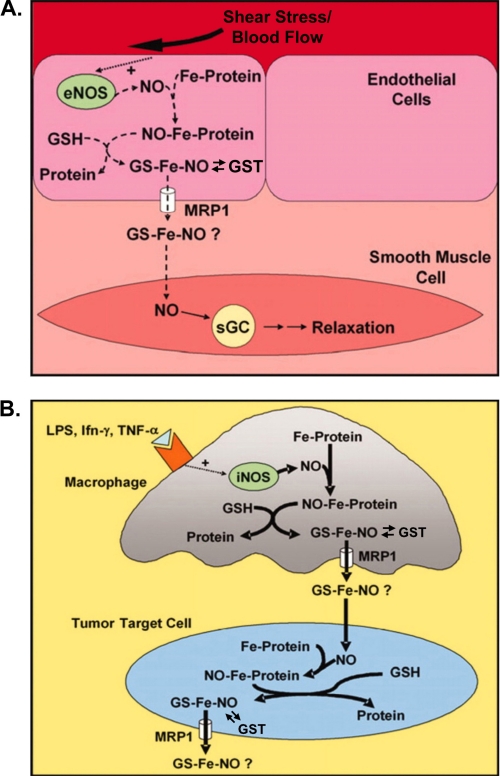
The interaction between proteins that transport and store NO leads to crucial questions. For example, given DNICs are involved in the storage and transport of NO (37, 38), it would be worthy to investigate if other protein ligands bind DNICs. Moreover, it is relevant to discuss the potential biological functions of protein-bound DNICs. Indeed, although the role of NO in vasodilation is well established (98), there is evidence that DNICs also have an effect on blood pressure. Initially, the formation and release of DNICs were observed in endothelial cells in which eNOS was induced, implicating their role in endothelium-dependent relaxation (99). Indeed, by incubating aortic segments with DNICs, Mülsch et al. (90) reported relaxation of pre-contracted femoral artery segments. Similar observations were also made by Alencar et al. (59).
The generation of NO by eNOS and the formation of DNICs must now be assessed in endothelial cells, with emphasis on the role of GSTs and MRP1 in NO storage and transport. This could have implications for understanding the response of smooth muscle cells to NO via its interaction with sGC (Fig. 3A). Similarly, it remains to be examined how the cooperative mechanism of DNIC metabolism by GSTs and MRP1 is involved in the cytotoxic effector mechanism of Mφs against tumor cells (Fig. 3B).
FIGURE 3.
Schematic illustration of the interdependence of iron, NO, GSH, and MRP1 and the hypothetical consequences of DNIC efflux. A, endothelial cells. The efficient efflux of DNICs (represented here as GS-Fe-NO) by active transport by MRP1 may be crucial where NO is produced in small quantities, e.g. in blood vessels, in which eNOS generates NO to effect smooth muscle relaxation. B, activated macrophages targeting tumor cells. When NO is generated in large amounts, e.g. by iNOS of Mφs, it could lead to substantial iron and GSH efflux from tumor cells as DNICs and induce cytotoxicity. The schemes have been modified from Ref. 38.
Apart from DNICs, it is currently unclear if complexes of iron, NO, and amino acids containing oxygen donors (e.g. glutamic or aspartic acid) form in cells or if they are substrates of MRP1. Although the formation of such complexes is theoretically possible, their existence and physiological relevance will require experimental validation.
Conclusions
NO is generally thought to be freely diffusible in cells. However, NOS, GST P1-1, and MRP1 have been shown to be three crucial players in NO metabolism. Elucidating their interactions is essential in formulating an integrated model of NO metabolism and its link to the metabolism of iron. This is vital in understanding NO and its effector functions.
This work was supported by Discovery Grant DP1092734 from the Australian Research Council (to D. R. R.); Cancer Institute NSW Early Career Fellowships 08/ECF/1-36 (to Y. S. R.), 08/ECF/1-30 (to D. S. K.), and 10/ECF/2-18 (to D. J. R. L.); and a Peter Doherty postdoctoral fellowship (to D. J. R. L.) and Senior Principal Research Fellowship 571123 and Project Grants 632778 and 632698 (to D. R. R.) from the National Health and Medical Research Council of Australia.
- sGC
- soluble guanylate cyclase
- Mφ
- activated macrophage
- iNOS
- inducible NOS
- eNOS
- endothelial NOS
- Tf
- transferrin
- TfR1
- transferrin receptor 1
- LIP
- labile iron pool
- DNIC
- dinitrosyl-dithiol-iron complex
- DNDGIC
- dinitrosyl-diglutathionyl-iron complex
- DNDCIC
- dinitrosyl-dicysteinyl-iron complex
- GSNO
- S-nitrosoglutathione
- BSO
- l-buthionine (SR)-sulfoximine.
REFERENCES
- 1. Stamler J. S., Singel D. J., Loscalzo J. (1992) Biochemistry of nitric oxide and its redox-activated forms. Science 258, 1898–1902 [DOI] [PubMed] [Google Scholar]
- 2. Richardson D. R., Ponka P. (1997) The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim. Biophys. Acta 1331, 1–40 [DOI] [PubMed] [Google Scholar]
- 3. Watts R. N., Ponka P., Richardson D. R. (2003) Effects of nitrogen monoxide and carbon monoxide on molecular and cellular iron metabolism: mirror-image effector molecules that target iron. Biochem. J. 369, 429–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hibbs J. B., Jr., Taintor R. R., Vavrin Z. (1984) Iron depletion: possible cause of tumor cell cytotoxicity induced by activated macrophages. Biochem. Biophys. Res. Commun. 123, 716–723 [DOI] [PubMed] [Google Scholar]
- 5. Henry Y., Ducrocq C., Drapier J. C., Servent D., Pellat C., Guissani A. (1991) Nitric oxide, a biological effector. Electron paramagnetic resonance detection of nitrosyl-iron-protein complexes in whole cells. Eur. Biophys. J. 20, 1–15 [DOI] [PubMed] [Google Scholar]
- 6. Cleeter M. W., Cooper J. M., Darley-Usmar V. M., Moncada S., Schapira A. H. (1994) Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 345, 50–54 [DOI] [PubMed] [Google Scholar]
- 7. Drapier J. C., Hibbs J. B., Jr. (1986) Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J. Clin. Invest. 78, 790–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hibbs J. B., Jr., Taintor R. R., Vavrin Z. (1987) Macrophage cytotoxicity: role for l-arginine deiminase and imino nitrogen oxidation to nitrite. Science 235, 473–476 [DOI] [PubMed] [Google Scholar]
- 9. Nathan C., Xie Q. W. (1994) Regulation of biosynthesis of nitric oxide. J. Biol. Chem. 269, 13725–13728 [PubMed] [Google Scholar]
- 10. Hibbs J. B., Jr., Westenfelder C., Taintor R., Vavrin Z., Kablitz C., Baranowski R. L., Ward J. H., Menlove R. L., McMurry M. P., Kushner J. P. (1992) Evidence for cytokine-inducible nitric oxide synthesis from l-arginine in patients receiving interleukin-2 therapy. J. Clin. Invest. 89, 867–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dunn L. L., Suryo Rahmanto Y., Richardson D. R. (2007) Iron uptake and metabolism in the new millennium. Trends Cell Biol. 17, 93–100 [DOI] [PubMed] [Google Scholar]
- 12. Hentze M. W., Muckenthaler M. U., Andrews N. C. (2004) Balancing acts: molecular control of mammalian iron metabolism. Cell 117, 285–297 [DOI] [PubMed] [Google Scholar]
- 13. Wang J., Pantopoulos K. (2011) Regulation of cellular iron metabolism. Biochem. J. 434, 365–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jacobs A. (1977) Low molecular weight intracellular iron transport compounds. Blood 50, 433–439 [PubMed] [Google Scholar]
- 15. Richardson D. R., Ponka P., Vyoral D. (1996) Distribution of iron in reticulocytes after inhibition of heme synthesis with succinylacetone: examination of the intermediates involved in iron metabolism. Blood 87, 3477–3488 [PubMed] [Google Scholar]
- 16. Sheftel A. D., Zhang A. S., Brown C., Shirihai O. S., Ponka P. (2007) Direct interorganellar transfer of iron from endosome to mitochondrion. Blood 110, 125–132 [DOI] [PubMed] [Google Scholar]
- 17. Shi H., Bencze K. Z., Stemmler T. L., Philpott C. C. (2008) A cytosolic iron chaperone that delivers iron to ferritin. Science 320, 1207–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nandal A., Ruiz J. C., Subramanian P., Ghimire-Rijal S., Sinnamon R. A., Stemmler T. L., Bruick R. K., Philpott C. C. (2011) Activation of the HIF prolyl hydroxylase by the iron chaperones PCBP1 and PCBP2. Cell Metab. 14, 647–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Abboud S., Haile D. J. (2000) A novel mammalian iron-regulated protein involved in intracellular iron metabolism. J. Biol. Chem. 275, 19906–19912 [DOI] [PubMed] [Google Scholar]
- 20. Donovan A., Brownlie A., Zhou Y., Shepard J., Pratt S. J., Moynihan J., Paw B. H., Drejer A., Barut B., Zapata A., Law T. C., Brugnara C., Lux S. E., Pinkus G. S., Pinkus J. L., Kingsley P. D., Palis J., Fleming M. D., Andrews N. C., Zon L. I. (2000) Positional cloning of zebrafish ferroportin 1 identifies a conserved vertebrate iron exporter. Nature 403, 776–781 [DOI] [PubMed] [Google Scholar]
- 21. McKie A. T., Marciani P., Rolfs A., Brennan K., Wehr K., Barrow D., Miret S., Bomford A., Peters T. J., Farzaneh F., Hediger M. A., Hentze M. W., Simpson R. J. (2000) A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol. Cell 5, 299–309 [DOI] [PubMed] [Google Scholar]
- 22. Lepoivre M., Fieschi F., Coves J., Thelander L., Fontecave M. (1991) Inactivation of ribonucleotide reductase by nitric oxide. Biochem. Biophys. Res. Commun. 179, 442–448 [DOI] [PubMed] [Google Scholar]
- 23. Kim Y. M., Bergonia H. A., Müller C., Pitt B. R., Watkins W. D., Lancaster J. R., Jr. (1995) Loss and degradation of enzyme-bound heme induced by cellular nitric oxide synthesis. J. Biol. Chem. 270, 5710–5713 [DOI] [PubMed] [Google Scholar]
- 24. Ignarro L. J. (1991) Heme-dependent activation of guanylate cyclase by nitric oxide: a novel signal transduction mechanism. Blood Vessels 28, 67–73 [DOI] [PubMed] [Google Scholar]
- 25. Khatsenko O. G., Gross S. S., Rifkind A. B., Vane J. R. (1993) Nitric oxide is a mediator of the decrease in cytochrome P450-dependent metabolism caused by immunostimulants. Proc. Natl. Acad. Sci. U.S.A. 90, 11147–11151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee M., Arosio P., Cozzi A., Chasteen N. D. (1994) Identification of the EPR-active iron-nitrosyl complexes in mammalian ferritins. Biochemistry 33, 3679–3687 [DOI] [PubMed] [Google Scholar]
- 27. Richardson D. R., Neumannova V., Nagy E., Ponka P. (1995) The effect of redox-related species of nitrogen monoxide on transferrin and iron uptake and cellular proliferation of erythroleukemia (K562) cells. Blood 86, 3211–3219 [PubMed] [Google Scholar]
- 28. Wardrop S. L., Watts R. N., Richardson D. R. (2000) Nitrogen monoxide activates iron regulatory protein 1 RNA-binding activity by two possible mechanisms: effect on the [4Fe-4S] cluster and iron mobilization from cells. Biochemistry 39, 2748–2758 [DOI] [PubMed] [Google Scholar]
- 29. Drapier J. C., Hirling H., Wietzerbin J., Kaldy P., Kühn L. C. (1993) Biosynthesis of nitric oxide activates iron regulatory factor in macrophages. EMBO J. 12, 3643–3649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Weiss G., Goossen B., Doppler W., Fuchs D., Pantopoulos K., Werner-Felmayer G., Wachter H., Hentze M. W. (1993) Translational regulation via iron-responsive elements by the nitric oxide/NO synthase pathway. EMBO J. 12, 3651–3657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Watts R. N., Richardson D. R. (2000) Examination of the mechanism of action of nitrogen monoxide on iron uptake from transferrin. J. Lab. Clin. Med. 136, 149–156 [DOI] [PubMed] [Google Scholar]
- 32. Watts R. N., Richardson D. R. (2004) Differential effects on cellular iron metabolism of the physiologically relevant diatomic effector molecules, NO and CO, that bind iron. Biochim. Biophys. Acta 1692, 1–15 [DOI] [PubMed] [Google Scholar]
- 33. Hickok J. R., Sahni S., Mikhed Y., Bonini M. G., Thomas D. D. (2011) Nitric oxide suppresses tumor cell migration through N-Myc downstream regulated gene-1 (NDRG1) expression: role of chelatable iron. J. Biol. Chem. 286, 41413–41424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kovacevic Z., Richardson D. R. (2006) The metastasis suppressor, Ndrg-1: a new ally in the fight against cancer. Carcinogenesis 27, 2355–2366 [DOI] [PubMed] [Google Scholar]
- 35. Le N. T., Richardson D. R. (2004) Iron chelators with high antiproliferative activity up-regulate the expression of a growth inhibitory and metastasis suppressor gene: a link between iron metabolism and proliferation. Blood 104, 2967–2975 [DOI] [PubMed] [Google Scholar]
- 36. Ellen T. P., Ke Q., Zhang P., Costa M. (2008) NDRG1, a growth- and cancer-related gene: regulation of gene expression and function in normal and disease states. Carcinogenesis 29, 2–8 [DOI] [PubMed] [Google Scholar]
- 37. Lok H. C., Suryo Rahmanto Y., Hawkins C. L., Kalinowski D. S., Morrow C. S., Townsend A. J., Ponka P., Richardson D. R. (2012) Nitric oxide storage and transport in cells are mediated by glutathione S-transferase P1-1 and multidrug resistance protein 1 via dinitrosyl-iron complexes. J. Biol. Chem. 287, 607–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Watts R. N., Hawkins C., Ponka P., Richardson D. R. (2006) Nitrogen monoxide (NO)-mediated iron release from cells is linked to NO-induced glutathione efflux via multidrug resistance-associated protein 1. Proc. Natl. Acad. Sci. U.S.A. 103, 7670–7675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Watts R. N., Richardson D. R. (2001) Nitrogen monoxide (NO) and glucose: unexpected links between energy metabolism and NO-mediated iron mobilization from cells. J. Biol. Chem. 276, 4724–4732 [DOI] [PubMed] [Google Scholar]
- 40. Toledo J. C., Jr., Bosworth C. A., Hennon S. W., Mahtani H. A., Bergonia H. A., Lancaster J. R., Jr. (2008) Nitric oxide-induced conversion of cellular chelatable iron into macromolecule-bound paramagnetic dinitrosyl-iron complexes. J. Biol. Chem. 283, 28926–28933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hibbs J. B., Jr., Taintor R. R., Chapman H. A., Jr., Weinberg J. B. (1977) Macrophage tumor killing: influence of the local environment. Science 197, 279–282 [DOI] [PubMed] [Google Scholar]
- 42. Klimp A. H., de Vries E. G., Scherphof G. L., Daemen T. (2002) A potential role of macrophage activation in the treatment of cancer. Crit. Rev. Oncol. Hematol. 44, 143–161 [DOI] [PubMed] [Google Scholar]
- 43. Gifford G. E., Lohmann-Matthes M. L. (1987) γ-Interferon priming of mouse and human macrophages for induction of tumor necrosis factor production by bacterial lipopolysaccharide. J. Natl. Cancer Inst. 78, 121–124 [DOI] [PubMed] [Google Scholar]
- 44. Lancaster J. R., Jr., Hibbs J. B., Jr. (1990) EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc. Natl. Acad. Sci. U.S.A. 87, 1223–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pellat C., Henry Y., Drapier J. C. (1990) IFN-γ-activated macrophages: detection by electron paramagnetic resonance of complexes between l-arginine-derived nitric oxide and non-heme iron proteins. Biochem. Biophys. Res. Commun. 166, 119–125 [DOI] [PubMed] [Google Scholar]
- 46. Vanin A. F. (1991) Endothelium-derived relaxing factor is a nitrosyl-iron complex with thiol ligands. FEBS Lett. 289, 1–3 [DOI] [PubMed] [Google Scholar]
- 47. Tinberg C. E., Tonzetich Z. J., Wang H., Do L. H., Yoda Y., Cramer S. P., Lippard S. J. (2010) Characterization of iron-dinitrosyl species formed in the reaction of nitric oxide with a biological Rieske center. J. Am. Chem. Soc. 132, 18168–18176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hickok J. R., Sahni S., Shen H., Arvind A., Antoniou C., Fung L. W., Thomas D. D. (2011) Dinitrosyl-iron complexes are the most abundant nitric oxide-derived cellular adduct: biological parameters of assembly and disappearance. Free Radic. Biol. Med. 51, 1558–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Vanin A. F. (2009) Dinitrosyl-iron complexes with thiolate ligands: physicochemistry, biochemistry, and physiology. Nitric Oxide 21, 1–13 [DOI] [PubMed] [Google Scholar]
- 50. Ueno T., Yoshimura T. (2000) The physiological activity and in vivo distribution of dinitrosyl-dithiolato-iron complex. Jpn. J. Pharmacol. 82, 95–101 [DOI] [PubMed] [Google Scholar]
- 51. Vanin A. F. (1998) Dinitrosyl-iron complexes and S-nitrosothiols are two possible forms for stabilization and transport of nitric oxide in biological systems. Biochemistry 63, 782–793 [PubMed] [Google Scholar]
- 52. Kleschyov A. L., Strand S., Schmitt S., Gottfried D., Skatchkov M., Sjakste N., Daiber A., Umansky V., Munzel T. (2006) Dinitrosyl-iron triggers apoptosis in Jurkat cells despite overexpression of Bcl-2. Free Radic. Biol. Med. 40, 1340–1348 [DOI] [PubMed] [Google Scholar]
- 53. Giliano N. Y., Konevega L. V., Noskin L. A., Serezhenkov V. A., Poltorakov A. P., Vanin A. F. (2011) Dinitrosyl-iron complexes with thiol-containing ligands and apoptosis: studies with HeLa cell cultures. Nitric Oxide 24, 151–159 [DOI] [PubMed] [Google Scholar]
- 54. Shumaev K. B., Gubkin A. A., Serezhenkov V. A., Lobysheva I. I., Kosmachevskaya O. V., Ruuge E. K., Lankin V. Z., Topunov A. F., Vanin A. F. (2008) Interaction of reactive oxygen and nitrogen species with albumin- and methemoglobin-bound dinitrosyl-iron complexes. Nitric Oxide 18, 37–46 [DOI] [PubMed] [Google Scholar]
- 55. Kim Y. M., Chung H. T., Simmons R. L., Billiar T. R. (2000) Cellular non-heme iron content is a determinant of nitric oxide-mediated apoptosis, necrosis, and caspase inhibition. J. Biol. Chem. 275, 10954–10961 [DOI] [PubMed] [Google Scholar]
- 56. Pedersen J. Z., De Maria F., Turella P., Federici G., Mattei M., Fabrini R., Dawood K. F., Massimi M., Caccuri A. M., Ricci G. (2007) Glutathione transferases sequester toxic dinitrosyl-iron complexes in cells. A protection mechanism against excess nitric oxide. J. Biol. Chem. 282, 6364–6371 [DOI] [PubMed] [Google Scholar]
- 57. Boese M., Keese M. A., Becker K., Busse R., Mülsch A. (1997) Inhibition of glutathione reductase by dinitrosyl-iron-dithiolate complex. J. Biol. Chem. 272, 21767–21773 [DOI] [PubMed] [Google Scholar]
- 58. Becker K., Savvides S. N., Keese M., Schirmer R. H., Karplus P. A. (1998) Enzyme inactivation through sulfhydryl oxidation by physiologic NO carriers. Nat. Struct. Biol. 5, 267–271 [DOI] [PubMed] [Google Scholar]
- 59. Alencar J. L., Chalupsky K., Sarr M., Schini-Kerth V., Vanin A. F., Stoclet J. C., Muller B. (2003) Inhibition of arterial contraction by dinitrosyl-iron complexes: critical role of the thiol ligand in determining rate of nitric oxide (NO) release and formation of releasable NO stores by S-nitrosation. Biochem. Pharmacol. 66, 2365–2374 [DOI] [PubMed] [Google Scholar]
- 60. Chen Y. J., Ku W. C., Feng L. T., Tsai M. L., Hsieh C. H., Hsu W. H., Liaw W. F., Hung C. H. (2008) Nitric oxide physiological responses and delivery mechanisms probed by water-soluble Roussin's red ester and (Fe(NO)2)10 DNIC. J. Am. Chem. Soc. 130, 10929–10938 [DOI] [PubMed] [Google Scholar]
- 61. Severina I. S., Bussygina O. G., Pyatakova N. V., Malenkova I. V., Vanin A. F. (2003) Activation of soluble guanylate cyclase by NO donors–S-nitrosothiols, and dinitrosyl-iron complexes with thiol-containing ligands. Nitric Oxide 8, 155–163 [DOI] [PubMed] [Google Scholar]
- 62. Remizova M. I., Kochetygov N. I., Gerbout K. A., Lakomkin V. L., Timoshin A. A., Burgova E. N., Vanin A. F. (2011) Effect of dinitrosyl-iron complexes with glutathione on hemorrhagic shock followed by saline treatment. Eur. J. Pharmacol. 662, 40–46 [DOI] [PubMed] [Google Scholar]
- 63. Watts R. N., Richardson D. R. (2002) The mechanism of nitrogen monoxide (NO)-mediated iron mobilization from cells. NO intercepts iron before incorporation into ferritin and indirectly mobilizes iron from ferritin in a glutathione-dependent manner. Eur. J. Biochem. 269, 3383–3392 [DOI] [PubMed] [Google Scholar]
- 64. Meister A. (1988) Glutathione metabolism and its selective modification. J. Biol. Chem. 263, 17205–17208 [PubMed] [Google Scholar]
- 65. Lomonosova E. E., Kirsch M., de Groot H. (1998) Calcium- versus iron-mediated processes in hydrogen peroxide toxicity to L929 cells: effects of glucose. Free Radic. Biol. Med. 25, 493–503 [DOI] [PubMed] [Google Scholar]
- 66. Griffith O. W., Meister A. (1979) Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J. Biol. Chem. 254, 7558–7560 [PubMed] [Google Scholar]
- 67. Ballatori N., Hammond C. L., Cunningham J. B., Krance S. M., Marchan R. (2005) Molecular mechanisms of reduced glutathione transport: role of the MRP/CFTR/ABCC and OATP/SLC21A families of membrane proteins. Toxicol. Appl. Pharmacol. 204, 238–255 [DOI] [PubMed] [Google Scholar]
- 68. Leslie E. M., Haimeur A., Waalkes M. P. (2004) Arsenic transport by the human multidrug resistance protein 1 (MRP1/ABCC1). Evidence that a tri-glutathione conjugate is required. J. Biol. Chem. 279, 32700–32708 [DOI] [PubMed] [Google Scholar]
- 69. Flens M. J., Zaman G. J., van der Valk P., Izquierdo M. A., Schroeijers A. B., Scheffer G. L., van der Groep P., de Haas M., Meijer C. J., Scheper R. J. (1996) Tissue distribution of the multidrug resistance protein. Am. J. Pathol. 148, 1237–1247 [PMC free article] [PubMed] [Google Scholar]
- 70. Hou Y. X., Cui L., Riordan J. R., Chang X. B. (2002) ATP binding to the first nucleotide-binding domain of multidrug resistance protein MRP1 increases binding and hydrolysis of ATP and trapping of ADP at the second domain. J. Biol. Chem. 277, 5110–5119 [DOI] [PubMed] [Google Scholar]
- 71. Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. (1992) Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258, 1650–1654 [DOI] [PubMed] [Google Scholar]
- 72. Mirski S. E., Gerlach J. H., Cole S. P. (1987) Multidrug resistance in a human small cell lung cancer cell line selected in adriamycin. Cancer Res. 47, 2594–2598 [PubMed] [Google Scholar]
- 73. Chang X. B. (2010) Molecular mechanism of ATP-dependent solute transport by multidrug resistance-associated protein 1. Methods Mol. Biol. 596, 223–249 [DOI] [PubMed] [Google Scholar]
- 74. Richardson D. R. (2007) in Radicals for Life: The Various Forms of Nitric Oxide (van Faasen E., Vanin A. F. eds) pp. 97–118, Elsevier, Amsterdam [Google Scholar]
- 75. He Y. Y., Huang J. L., Ramirez D. C., Chignell C. F. (2003) Role of reduced glutathione efflux in apoptosis of immortalized human keratinocytes induced by UVA. J. Biol. Chem. 278, 8058–8064 [DOI] [PubMed] [Google Scholar]
- 76. Richardson D. R., Tran E. H., Ponka P. (1995) The potential of iron chelators of the pyridoxal isonicotinoyl hydrazone class as effective antiproliferative agents. Blood 86, 4295–4306 [PubMed] [Google Scholar]
- 77. Schneider E., Horton J. K., Yang C. H., Nakagawa M., Cowan K. H. (1994) Multidrug resistance-associated protein gene overexpression and reduced drug sensitivity of topoisomerase II in a human breast carcinoma MCF7 cell line selected for etoposide resistance. Cancer Res. 54, 152–158 [PubMed] [Google Scholar]
- 78. Hayes J. D., Flanagan J. U., Jowsey I. R. (2005) Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 45, 51–88 [DOI] [PubMed] [Google Scholar]
- 79. Morrow C. S., Diah S., Smitherman P. K., Schneider E., Townsend A. J. (1998) Multidrug resistance protein and glutathione S-transferase P1-1 act in synergy to confer protection from 4-nitroquinoline 1-oxide toxicity. Carcinogenesis 19, 109–115 [DOI] [PubMed] [Google Scholar]
- 80. Morrow C. S., Smitherman P. K., Diah S. K., Schneider E., Townsend A. J. (1998) Coordinated action of glutathione S-transferases (GSTs) and multidrug resistance protein 1 (MRP1) in antineoplastic drug detoxification. Mechanism of GST A1-1- and MRP1-associated resistance to chlorambucil in MCF7 breast carcinoma cells. J. Biol. Chem. 273, 20114–20120 [DOI] [PubMed] [Google Scholar]
- 81. Morrow C. S., Smitherman P. K., Townsend A. J. (1998) Combined expression of multidrug resistance protein (MRP) and glutathione S-transferase P1-1 (GSTP1-1) in MCF7 cells and high-level resistance to the cytotoxicities of ethacrynic acid but not oxazaphosphorines or cisplatin. Biochem. Pharmacol. 56, 1013–1021 [DOI] [PubMed] [Google Scholar]
- 82. Cesareo E., Parker L. J., Pedersen J. Z., Nuccetelli M., Mazzetti A. P., Pastore A., Federici G., Caccuri A. M., Ricci G., Adams J. J., Parker M. W., Lo Bello M. (2005) Nitrosylation of human glutathione transferase P1-1 with dinitrosyl-diglutathionyl-iron complex in vitro and in vivo. J. Biol. Chem. 280, 42172–42180 [DOI] [PubMed] [Google Scholar]
- 83. De Maria F., Pedersen J. Z., Caccuri A. M., Antonini G., Turella P., Stella L., Lo Bello M., Federici G., Ricci G. (2003) The specific interaction of dinitrosyl-diglutathionyl-iron complex, a natural NO carrier, with the glutathione transferase superfamily. Suggestion for an evolutionary pressure in the direction of the storage of nitric oxide. J. Biol. Chem. 278, 42283–42293 [DOI] [PubMed] [Google Scholar]
- 84. Turella P., Pedersen J. Z., Caccuri A. M., De Maria F., Mastroberardino P., Lo Bello M., Federici G., Ricci G. (2003) Glutathione transferase superfamily behaves like storage proteins for dinitrosyl-diglutathionyl-iron complex in heterogeneous systems. J. Biol. Chem. 278, 42294–42299 [DOI] [PubMed] [Google Scholar]
- 85. Peklak-Scott C., Townsend A. J., Morrow C. S. (2005) Dynamics of glutathione conjugation and conjugate efflux in detoxification of the carcinogen, 4-nitroquinoline 1-oxide: contributions of glutathione, glutathione S-transferase, and MRP1. Biochemistry 44, 4426–4433 [DOI] [PubMed] [Google Scholar]
- 86. de Luca A., Moroni N., Serafino A., Primavera A., Pastore A., Pedersen J. Z., Petruzzelli R., Farrace M. G., Pierimarchi P., Moroni G., Federici G., Sinibaldi Vallebona P., Lo Bello M. (2011) Treatment of doxorubicin-resistant MCF7/Dx cells with nitric oxide causes histone glutathionylation and reversal of drug resistance. Biochem. J. 440, 175–183 [DOI] [PubMed] [Google Scholar]
- 87. Kawakatsu M., Goto S., Yoshida T., Urata Y., Li T. S. (2011) Nuclear translocation of glutathione S-transferase Pi is mediated by a non-classical localization signal. Biochem. Biophys. Res. Commun. 411, 745–750 [DOI] [PubMed] [Google Scholar]
- 88. Qazi S. S., Osoria Pérez A., Sam M., Leslie E. M. (2011) Glutathione transferase P1 interacts strongly with the inner leaflet of the plasma membrane. Drug Metab. Dispos. 39, 1122–1126 [DOI] [PubMed] [Google Scholar]
- 89. Canto P., Canto-Cetina T., Juárez-Velázquez R., Rosas-Vargas H., Rangel-Villalobos H., Canizales-Quinteros S., Velázquez-Wong A. C., Villarreal-Molina M. T., Fernández G., Coral-Vázquez R. (2008) Methylenetetrahydrofolate reductase C677T and glutathione S-transferase P1 A313G are associated with a reduced risk of preeclampsia in Maya-Mestizo women. Hypertens. Res. 31, 1015–1019 [DOI] [PubMed] [Google Scholar]
- 90. Mülsch A., Mordvintcev P., Vanin A. F., Busse R. (1991) The potent vasodilating and guanylyl cyclase-activating dinitrosyl-iron(II) complex is stored in a protein-bound form in vascular tissue and is released by thiols. FEBS Lett. 294, 252–256 [DOI] [PubMed] [Google Scholar]
- 91. Ueno T., Suzuki Y., Fujii S., Vanin A. F., Yoshimura T. (1999) In vivo distribution and behavior of paramagnetic dinitrosyl dithiolato-iron complex in the abdomen of mouse. Free Radic. Res. 31, 525–534 [DOI] [PubMed] [Google Scholar]
- 92. Drapier J. C., Pellat C., Henry Y. (1991) Generation of EPR-detectable nitrosyl-iron complexes in tumor target cells co-cultured with activated macrophages. J. Biol. Chem. 266, 10162–10167 [PubMed] [Google Scholar]
- 93. Thomas D. D., Liu X., Kantrow S. P., Lancaster J. R., Jr. (2001) The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2. Proc. Natl. Acad. Sci. U.S.A. 98, 355–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Boese M., Mordvintcev P. I., Vanin A. F., Busse R., Mülsch A. (1995) S-Nitrosation of serum albumin by dinitrosyl-iron complex. J. Biol. Chem. 270, 29244–29249 [DOI] [PubMed] [Google Scholar]
- 95. Vanin A. F., Malenkova I. V., Serezhenkov V. A. (1997) Iron catalyzes both decomposition and synthesis of S-nitrosothiols: optical and electron paramagnetic resonance studies. Nitric Oxide 1, 191–203 [DOI] [PubMed] [Google Scholar]
- 96. Weiss G., Werner-Felmayer G., Werner E. R., Grünewald K., Wachter H., Hentze M. W. (1994) Iron regulates nitric-oxide synthase activity by controlling nuclear transcription. J. Exp. Med. 180, 969–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Bosworth C. A., Toledo J. C., Jr., Zmijewski J. W., Li Q., Lancaster J. R., Jr. (2009) Dinitrosyliron complexes and the mechanism(s) of cellular protein nitrosothiol formation from nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 106, 4671–4676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ignarro L. J. (1989) Endothelium-derived nitric oxide: actions and properties. FASEB J. 3, 31–36 [DOI] [PubMed] [Google Scholar]
- 99. Mülsch A., Mordvintcev P. I., Vanin A. F., Busse R. (1993) Formation and release of dinitrosyl-iron complexes by endothelial cells. Biochem. Biophys. Res. Commun. 196, 1303–1308 [DOI] [PubMed] [Google Scholar]
- 100. Zai A., Rudd M. A., Scribner A. W., Loscalzo J. (1999) Cell-surface protein-disulfide isomerase catalyzes transnitrosation and regulates intracellular transfer of nitric oxide. J. Clin. Invest. 103, 393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]