Background: HLA-DM plays an essential role in MHC class II antigen presentation.
Results: MARCH family E3 ligases regulate HLA-DM trafficking by direct ubiquitination of DMα and indirectly through a tyrosine-based targeting signal.
Conclusion: Ubiquitination is a common mechanism regulating both classical and nonclassical MHC molecules.
Significance: HLA-DM activity can be controlled post-translationally allowing the immune system to adapt to different conditions.
Keywords: Immunology, Membrane Proteins, Protein Turnover, Ubiquitination, Western Blotting, HLA-DM
Abstract
HLA-DM plays an essential role in the peptide loading of classical class II molecules and is present both at the cell surface and in late endosomal peptide-loading compartments. Trafficking of DM within antigen-presenting cells is complex and is, in part, controlled by a tyrosine-based targeting signal present in the cytoplasmic tail of DMβ. Here, we show that DM also undergoes post-translational modification through ubiquitination of a single lysine residue present in the cytoplasmic tail of the α chain, DMα. Ubiquitination of DM by MARCH1 and MARCH9 induced loss of DM molecules from the cell surface by a mechanism that cumulatively involved both direct attachment of ubiquitin chains to DMα and a functional tyrosine-based signal on DMβ. In contrast, MARCH8-induced loss of surface DM was entirely dependent upon the tyrosine signal on DMβ. In the absence of this tyrosine residue, levels of DM remained unchanged irrespective of whether DMα was ubiquitinated by MARCH8. The influence of MARCH8 was indirect and may have resulted from modification of components of the endocytic machinery by ubiquitination.
Introduction
Antigen-presenting cells present exogenously and endogenously derived peptides bound to MHC class II heterodimers (pMHC-II) to CD4+ T helper cells. The synthesis and intracellular trafficking of MHC-II are tightly regulated, and multiple accessory molecules control antigen loading and presentation at the cell surface.
In the endoplasmic reticulum, newly synthesized MHC-II forms nonamers composed of MHC-II αβ dimers and the chaperone protein invariant chain (Ii)2 (1, 2). Dileucine-based endocytosis motifs in the Ii cytoplasmic tail guide the intracellular trafficking of the complexes to endosomes (3, 4) where Ii is degraded and the Ii-derived CLIP peptide is exchanged for peptide antigens by the class II-related molecule, HLA-DM (4–7). DM is a nonclassical MHC-II dimer that acts as a catalyst for CLIP exchange, edits the peptide-repertoire bound to MHC-II at acidic pH, and contributes to MHC-II stability (8–13). Trafficking of DM is independent of Ii; it is efficiently targeted to endosomes by a YxxΦ tyrosine-based endocytosis motif in the β chain cytoplasmic tail (14, 15). After loading, pMHC-II is transported to the cell surface for recognition by T cells. Surface pMHC-II is constantly endocytosed (a process that is partially controlled by a dileucine motif in the β chain cytoplasmic tail) and rapidly recycled back to the plasma membrane (16, 17).
The density of specific pMHC-II on the cell surface is crucial for CD4 T cell activation (18, 19). Ubiquitination has recently emerged as a key regulator of pMHC-II stability and availability to T cells. In immature dendritic cells (DCs), levels of MHC-II ubiquitination are high, leading to sequestration of MHC-II in endosomes, ubiquitination-dependent sorting into the luminal vesicles of multivesicular bodies, and lysosomal degradation. Therefore pMHC-II turnover in immature DCs is relatively high and surface levels relatively low. Upon activation of DCs by TLR ligands such as LPS, ubiquitination of MHC-II is reduced, and pMHC-II is redistributed to the cell surface (20–26). This regulation provides a “snap-shot” of antigens that are present at the time of DC activation.
The known ubiquitin E3 ligases responsible for MHC-II ubiquitination are members of the membrane-associated RING-CH (MARCH) family. In particular, MARCH1 is important for the dynamics of MHC-II ubiquitination in human (HLA-DR) (22, 24) and mouse DCs (23, 24, 26, 27). DC maturation is accompanied by down-regulation of MARCH1 mRNA, and immature DCs from MARCH1 knock-out mice have elevated MHC-II levels but do not up-regulate expression upon activation. MARCH1 also regulates MHC-II in mouse B cells (27) and human monocytes (28). MARCH8, the E3 ligase most closely related to MARCH1, regulates MHC-II in a number of cell types (22, 28–30) but is more widely expressed (31) and shows little down-regulation upon DC activation (22, 26). Although MARCH1 and 8 have been shown to down-regulate all human MHC-II isotypes (28), a third ligase, MARCH9, only affects HLA-DQ in human B cells (32).
The principal function of MHC-II ubiquitination appears to be the elimination of irrelevant antigens through rapid turnover. With exogenous expression of MARCH8, pMHC-II levels are reduced and DCs lose their ability to activate T cells (29). MARCH1 knock-out mice, which display elevated pMHC-II on immature DCs, also have DC defects (27), and it is suggested that MHC-II turnover is required to preserve DC activity in vivo (33). A defining property of plasmocytoid DCs is their ability to sample endogenous peptides even after maturation. To achieve this, they only partially down-regulate MARCH1 mRNA compared with conventional DCs, thus preserving their ability to present late viral antigens (23).
Classical MHC-II is ubiquitinated at conserved lysine residues on the β (20, 21, 29) and α chains (34). The MHC-II related molecule, DM, also contains a lysine in the α chain cytoplasmic tail. In this paper, we investigated the ubiquitination of DM by MARCH1, 8, and 9 and show that although all three E3 ligases cause redistribution of cell surface DM they do so by different mechanisms.
MATERIALS AND METHODS
Cell Culture and Transfection
Human embryonic kidney (HEK 293T) cells were maintained in DMEM and B lymphoblastoid Raji cells in RPMI 1640 medium. All media were supplemented with 10% FCS, 10 mm HEPES, 10 mm sodium pyruvate, 10 mm nonessential amino acids, and 2 mm glutamine. 293T cells were transiently transfected using 25-kDa linear polyethyleneimine (Sigma-Aldrich) as described by Ehrhardt et al. (35). For lentivirus production, 293T cells were transfected with pCMV8.91, pMD-G, and pHR'SIN-cPPT-SGW carrying EGFP-MARCH1, 8, 8mut, or 9. After 48 h, supernatants containing lentiviral particles were used directly to transduce Raji cells.
Antibodies
Antibodies were from ATCC (anti-CD8; clone OKT8), Thermo Scientific (rabbit anti-mouse IgG-Fc RPE), ImmunoTools (IgG isotype controls), Santa Cruz Biotechnology (rabbit anti-CD8α; polyclonal H-160), anti-ubiquitin-HRP (P4D1), Invitrogen (rabbit anti-GFP), eBioscience (anti-mouse TrueBlot-HRP; eB144/7A7), Dako (swine anti-rabbit and rabbit anti-mouse Igs), Cancer Research Technology (anti-human DMα; 5C1), Clontech (anti-EGFP; JL-8), and Roche Applied Science (anti-HA beads; 3F10). Anti-DM dimer antibody (MaP.DM1) (36) was a kind gift from Peter Cresswell; anti-DR dimer antibody was from clone L243.
Plasmid Constructs
HLA-DMα and DMβ cDNAs have been described (37). The CD8-DMα reporter constructs were based on the human CD8α luminal domain (amino acids 1–176) and DMα transmembrane domain (ENVLCGVAFGLGVLGIIVGIVLIIYF) and cytoplasmic tails (Table 1) analogous to previous studies (34). Constructs were synthesized by PCR using KOD HiFi polymerase (Calbiochem) and sequence alterations generated using overlapping primer pairs. PCR products were digested, ligated into pcDNA3.1/Zeo+ or/Neo− (Invitrogen), and verified by DNA sequencing. The protein sequence of relevant portions of the constructs is shown in Table 1. pEGFPc1-MARCH1 and 9, as well as pCMV8.91 (38), pMD-G, and pHR'SIN-cPPT-SGW were kindly provided by Paul Lehner (Cambridge, UK). pEGFP-C2-MARCH1mut was created by mutating cysteine residues 63, 66, 106, and 109 to serine. pEGFPc2-MARCH8 wt and MARCH8 mutant were created by PCR cloning human c-MIR-V5-HIS wt and mut from pTracer-GFP constructs (kind gifts from Satoshi Ishido, Yokohama, Japan) in-frame into pEGFP-c2. For subcloning the EGFP-MARCH constructs into pHR'SIN, the cloning sites of pHR'SIN-cPPT-SGW were first modified by inserting a linker oligonucleotide (forward, GATCTGCGGCCGCCTCGAGGGATCCAT and reverse, GGCCATGGATCCCTCGAGGCGGCCGCA) into the BamHI and NotI sites. This destroyed the original sites and provided three new sites: 5′-NotI-XhoI-BamHI-3′, creating pHR'SIN(−). EGFP-MARCH1, 8, 8mut, and 9 were excised from the pEGFP plasmids using NheI and BamHI and inserted into the pHR'SIN(−) NotI-BamHI sites using a NotI-NheI oligonucleotide linker (forward, GGCCATACTCGAGG and reverse, CTAGCCTCGAGTAT). This created pHR'SIN(−)-EGFP-MARCH1, -MARCH8, -MARCH8mut, and -MARCH9.
TABLE 1.
Amino acid composition of constructs used in this study
Numbering of residues is derived from mature proteins after signal peptide cleavage. Substituted residues are in boldface type.
| Construct | Extracellular domain | Trans-membrane | Cytoplasmic tail |
|---|---|---|---|
| CD8-DMα | CD8α | DMα | RKPCSGD |
| CD8-DMα-K230R | CD8α | DMα | RRPCSGD |
| DMα | DMα | DMα | RKPCSGD |
| DMα-K230R | DMα | DMα | RRPCSGD |
| DMα-K230R/C2132G/S233A (KCS-RGA) | DMα | DMα | RRPGAGD |
| DMβ | DMβ | DMβ | RRAGHSSYTPLPGSNYSEGWHIS |
| DMβ-Y230A | DMβ | DMβ | RRAGHSSATPLPGSNYSEGWHIS |
| DMβ-Δ224 | DMβ | DMβ | RR |
| DMβ-Y230F | DMβ | DMβ | RRAGHSSFTPLPGSNYSEGWHIS |
| DMβ-ST-A | DMβ | DMβ | RRAGHAAYAPLPGANYAEGWHIA |
| DMβ-HA | DMβ | DMβ | RRAGHSSYTPLPGSNYSEGWHISYPYDVPDYA |
Flow Cytometry
After transfection (24–36 h), 293T cells were harvested and stained on ice with primary and secondary antibodies for 30 min in FACS buffer (4% FCS, 2 mm EDTA in PBS) using standard procedures, then fixed in 3% formaldehyde in PBS for acquisition. Raji cells were transduced and grown for 48 h in RPMI 1640 medium in which glutamine had been replaced by 2 mm alanine-glutamine dipeptide to ensure normal class II processing (39). 36 h into the transduction, lysosome inhibitor chloroquine (60 μm) was added, and cells were incubated for the remaining 12 h before analysis. For intracellular staining, cells were fixed in 3% formaldehyde in PBS for 15 min, washed, permeabilized with 0.2% saponin in FACS buffer, FcR-blocked with 40% human AB serum (Sigma) for 10 min, and stained as above in FACS buffer with 0.2% saponin. Data acquisition was performed on a FACScan flow cytometer (BD Bioscience) and analyzed using Summit v4.3 software. Expression of surface antigens was calculated as the proportion of mean fluorescent intensity (MFI) in the presence of catalytically active E3 ligase (GFP expression was used as a marker for E3 ligase expression) compared with MFI in the presence of GFP alone: Surface expression (%) = (MFI cells transfected with E3 ligase × 100)/(MFI cells transfected with GFP vector alone). Statistical analysis was performed using GNUMERIC 1.10 software (one-tailed t test: unpaired samples, unequal variances, α = 0.05). Differences of means were classed as not significant (n.s.), marginally significant (p < 0.07), significant (*, p < 0.05) and highly significant (**, p < 0,01).
Immunoprecipitation and Western Blotting
293T transfectants were washed with cold PBS (5 mm EDTA) and lysed in lysis buffer (150 mm NaCl, 1% Nonidet P-40 substitute (Fluka), 50 mm Tris (pH 7.5), 5 mm EDTA, protease inhibitor mixture (Roche Applied Science), and 5 mm iodoacetamide) for 20 min at 4 °C. For isolation of endogenously expressed DM in Raji cells, membrane material was isolated as follows: 108 cells were pelleted in membrane preparation buffer (10 mm Tris (pH 7.5), 1 mm MgCl2) in a 1.5-ml microcentrifuge tube, frozen on dry ice, and thawed in a heating block (20 °C), and the freeze-thaw was repeated once more. Membranes were pelleted at 14,000 rpm for 15 min at 4 °C, and proteins were extracted with lysis buffer as above. Debris and nuclei were removed by centrifugation at 14,000 rpm, and proteins were precipitated using antibody and protein A- (Sigma) or G- (GE Healthcare) agarose, or anti-HA beads, according to standard protocols. For precipitation of endogenous DM, MaP.DM1 antibodies were first cross-linked to protein G using suberic acid bis(3-sulfo-N-hydroxysuccinimide ester) (BS3, Sigma) to reduce background. Precipitates were separated by SDS-PAGE, and ubiquitination was analyzed by Western blotting on Immobilon-P membranes (Millipore), using rapid immunodetection according to the manufacturer's instructions and chemiluminescence using ECL+ reagent (GE Healthcare) or ECL (40). Membranes were then blocked overnight (PBS, 5% dry milk, 0.05% Tween 20) and used for multiple detections.
RESULTS
HLA-DM Is Subject to Ubiquitination on DMα Cytoplasmic Tail
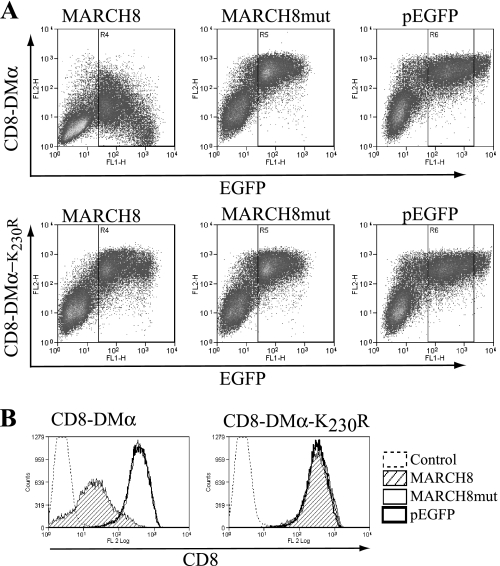
To investigate the ubiquitination of DM, we generated a reporter construct containing the CD8α extracellular domain and the DMα transmembrane and cytoplasmic tail regions, an approach used previously for the analysis of HLA-DR ubiquitination (34). The amino acid composition of this and the other constructs are given in Table 1. We determined whether this reporter molecule was subject to regulation by MARCH8 because members of this family of E3 ligases are known to ubiquitinate classical class II molecules (22, 28, 29). Upon transient transfection of 293T cells, catalytically active MARCH8 was able to down-regulate CD8-DMα, whereas a catalytically inactive MARCH8 mutant did not (Fig. 1).
FIGURE 1.
Down-regulation of CD8-DMα reporter molecules by MARCH8. CD8-DM reporters were transiently expressed in HEK-293T cells together with EGFP-MARCH8, EGFP-MARCH8mut, or pEGFP. Cell surface expression of CD8 was measured by FACS using anti-CD8 mAb OKT8 and PE anti-mouse mAb (FL2). A, dot plots showing levels of CD8-DMα (upper) and CD8-DMα-K230R (lower) in cells co-expressing EGFP-MARCH8, EGFP-MARCH8 mutant, or pEGFP. B, histograms showing surface levels of CD8-DMα (left) and CD8-DMα-K230R (right) in cells expressing EGFP-MARCH8, as determined by EGFP expression using gates R4–6 of A. Data are representative of at least three independent experiments.
DMα contains a single lysine residue that could act as a potential ubiquitination target. Following substitution of this residue for arginine (K230R), CD8-DMα was no longer down-regulated (Fig. 1), indicating that residue Lys230 is modified by the E3 ligase. The related enzymes MARCH1 and 9 also down-regulated CD8-DMα in a Lys230-dependent manner (data not shown).
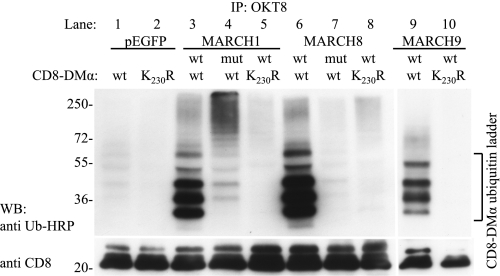
To confirm that MARCH E3 ligases target lysine 230, we used immunoprecipitation and anti-ubiquitin Western blotting to analyze CD8-DMα reporters from cells transfected with MARCH1, 8, and 9. All three enzymes led to the polyubiquitination of the CD8-DMα wild-type reporter (Fig. 2, lanes 3, 6, and 9). No ubiquitinated products were present in a CD8-DMα construct lacking Lys230 (CD8-DMα K230R; lanes 5, 8, and 10). CD8-DMα was also ubiquitinated at a low level by endogenously expressed E3 ligases, as seen in cells transfected with the pEGFP control plasmid (lane 1).
FIGURE 2.
MARCH E3 ligases polyubiquitinate lysine 230 of CD8-DMα. CD8-DMα and CD8-DMα-K230R reporter molecules were co-transfected into HEK-293T cells together with wild type (wt) or mutant (mut) constructs of EGFP-MARCH1, EGFP-MARCH8, EGFP-MARCH9, or pEGFP. After 24 h, CD8-DMα was immunoprecipitated with nondepleting amounts of mAb OKT8 and analyzed by SDS-PAGE and Western blotting (WB). Ubiquitinated products were detected with P4D1-HRP (upper). The lower panel was probed with anti-CD8 as a loading control. Lanes 1–8 were run on a single gel and lanes 9 and 10 on a separate gel. Bands migrating as ubiquitinated CD8-DMα products are indicated. Data are representative of three independent experiments.
We investigated the fate of the cell surface CD8-DMα reporter molecule by comparing the relative distribution of cell surface and internalized CD8-DMα. In cells expressing wild-type MARCH8, 38% of surface exposed CD8-DMα was internalized within 30 min compared with 16% in cells expressing the catalytically inactive mutant (supplemental Fig. 1). Additionally, the total level of CD8-DMα expressed in MARCH8-transfected cells was only 9% of that seen in cells expressing mutant MARCH8. From this we conclude that MARCH8 induces the internalization and degradation of CD8-DMα.
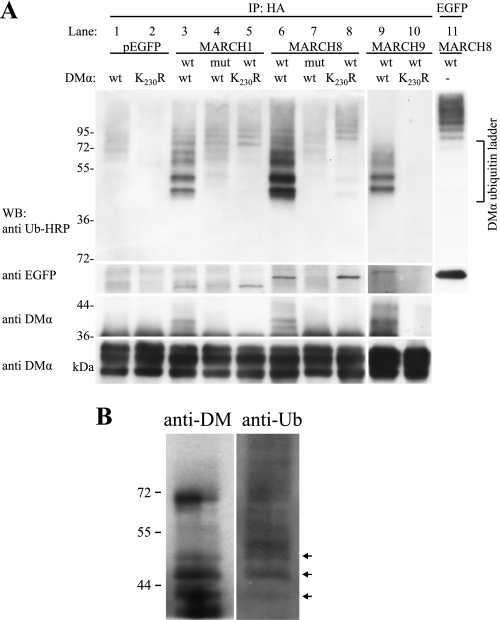
We next analyzed the ubiquitination of DMαβ dimers. HLA-DM was immunoprecipitated from cells co-transfected with DMαβ and MARCH8 (or MARCH1 or 9) and Western blots probed with the anti-ubiquitin antibody P4D1 (Fig. 3A, upper). The resulting ubiquitination ladders were complex and represented ubiquitinated DMα together with ubiquitinated MARCH proteins that co-precipitated with HLA-DM (Fig. 3A, upper). Ubiquitinated products below the 72-kDa marker (lanes 3, 6, and 9) represent ubiquitinated DMα because they were not present in lysates from cells transfected with the DMα-K230R mutant (lane 8) or catalytically inactive MARCH8 (lane 7). Ubiquitinated products above the 72-kDa marker most likely represent ubiquitinated MARCH8 (Fig. 3A, lanes 6–8). These bands correspond in size to ubiquitinated MARCH8-EGFP (lane 11). Similarly, DMα is ubiquitinated by MARCH1, and ubiquitinated MARCH1 is co-precipitated together with DM (Fig. 3A, upper, lanes 3–5). Ubiquitinated MARCH1 migrated faster than ubiquitinated MARCH8, as would be expected from the difference in molecular mass of the two MARCH proteins (Fig. 3A, WB:anti EGFP, lanes 3–5 (MARCH1) and lanes 6–8 (MARCH8)). MARCH9 was also able to ubiquitinate DMαβ on lysine residue 230, and the ubiquitin ladders generated by all three MARCH proteins were similar (Fig. 3A, upper, lanes 3, 6, and 9). MARCH9 was also co-precipitated with DMαβ (Fig. 3A, WB:anti EGFP, lane 9), but in this case ubiquitinated MARCH9 was not detected.
FIGURE 3.
HLA-DM is modified by polyubiquitination. DMα/DMβHA and DMα-K230R/DMβHA constructs were transfected into HEK-293T cells together with wt or mutant (mut) constructs of EGFP-MARCH1, EGFP-MARCH8, EGFP-MARCH9, or pEGFP. After 24 h, DMαβHA was immunoprecipitated (IP) with nondepleting quantities of anti-HA mAb and products analyzed by SDS-PAGE and Western blotting (WB). A, ubiquitinated molecules associated with immunoprecipitated DMαβHA were detected using anti-ubiquitin antibody P4D1-HRP (upper, lanes 1–10). Products corresponding in size to ubiquitinated DMα are labeled (DMα ubiquitin ladder). Ubiquitinated EGFP-MARCH8 is shown in lane 11. Products above the DMα ubiquitin ladder likely represent ubiquitinated EGFP-MARCH proteins. Co-immunoprecipitating EGFP-MARCH proteins detected with anti-GFP antibody are shown in panel WB:anti EGFP. Anti-DMα mAb 5C1 was used to detect both ubiquitinated (panel WB:anti DMα upper, lanes 3, 6, and 9, arrowheads) and nonubiquitinated (panel WB:anti DMα lower) DMα products. Long and short exposure times were required for panels WB:anti DMα upper and lower, respectively. Data are representative of three independent experiments. Lanes 9 and 10 were run on a separate gel. B, endogenously expressed DMαβ was precipitated from membrane fractions of Raji cells with anti-DMαβ mAb immobilized to protein G beads and analyzed by SDS-PAGE and Western blotting. The blot was first probed with anti-ubiquitin-HRP and then with anti-DMα antibody. Arrows denote bands with the same migration properties. Data are representative of three independent experiments.
Western blotting with the DMα-specific antibody 5C1 confirmed the presence of products with a higher molecular mass than native DM only in lysates from cells transfected with wild-type DM and catalytically active MARCH proteins (Fig. 3A, lanes 3, 6, and 9, arrowheads). Together, the data demonstrate that MARCH1, 8, and 9 are all able to polyubiquitinate DMα on lysine 230. In the absence of co-transfected MARCH constructs, some ubiquitination of DM was also observed (lane 1), demonstrating that ubiquitin ligases at endogenous levels were also able to ubiquitinate HLA-DM.
Finally, we attempted to investigate ubiquitination in cells expressing DM and E3 ligases at endogenous levels, even though steady-state levels of ubiquitinated HLA-DM would be expected to be extremely low and difficult to capture experimentally. We precipitated DMαβ from the B cell line Raji and detected a ladder of products with the anti-ubiquitin antibody P4D1 (Fig. 3B). Using anti-DMα antibody on the same blot, we detected bands of the same mobility as those detected with the anti-ubiquitin antibody (Fig. 3B, arrows). The relative intensity of bands detected with the two antibodies is not directly comparable because detection with the anti-ubiquitin antibody is influenced by the number of ubiquitin molecules attached. As expected from the relative expression of class II and DM, overall levels of ubiquitinated DM were very low. The presence of DM ubiquitination in Raji cells demonstrates that at physiological expression levels DM is ubiquitinated.
Degradation of HLA-DR and DM Is Regulated by the Same MARCH E3 Ligases
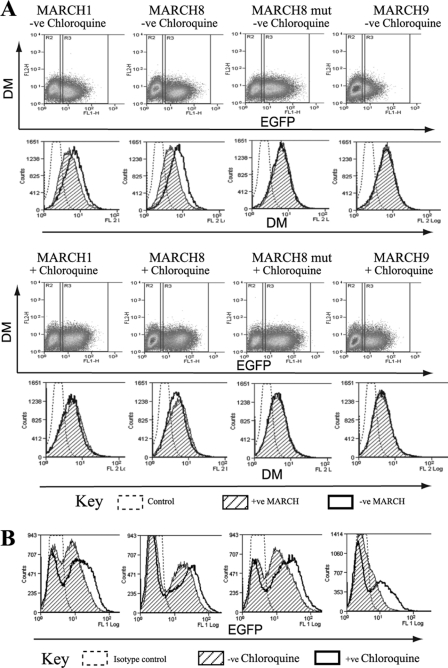
Ubiquitination of HLA-DR by MARCH1 and 8, as well as HLA-DQ by MARCH9, has been shown to reduce DR/DQ recycling to the plasma membrane and to promote lysosomal degradation (27, 29, 32, 41). To investigate the effect of MARCH expression on cellular DM levels, we transduced Raji cells with MARCH1, 8, and 9 and analyzed DM by intracellular flow cytometry. Expression of MARCH1 and 8 reduced DM staining in transduced compared with untransduced cells (Fig. 4A, upper panels), whereas MARCH9 led to little or no down-regulation. Parallel surface staining against HLA-DQ confirmed that all three MARCH constructs were catalytically active in Raji cells and down-regulated DQ (data not shown). Catalytically inactive MARCH8 was transduced as a control and had no effect on levels of DM (Fig. 4A, upper panels) or DQ (not shown). These results suggested that MARCH1 and 8 promoted the degradation of DM. To determine the mechanism of degradation we incubated MARCH-transduced cells with chloroquine, an established inhibitor of lysosomal acidification. Chloroquine treatment severely reduced the degradation of DM in MARCH1- and 8-expressing cells (Fig. 4A, lower panels). This suggested that MARCH1 and 8 promote the lysosomal degradation of DM in Raji cells. DM levels did not appear to be influenced by MARCH9, but MARCH9 levels were substantially increased in chloroquine-treated cells, indicating that the E3 ligase was itself subject to lysosomal degradation (Fig. 4, A, lower panels, and B). MARCH1 and 8 levels were also increased in chloroquine-treated cells, but to a lesser extent (Fig. 4, A and B).
FIGURE 4.
MARCH1 and 8 induce lysosome-dependent degradation of HLA-DM. Raji B cells were transduced with EGFP fusion proteins of MARCH1, 8, and 9 or MARCH8 mutant. After 36 h, 60 μm chloroquine was added to half of the cells and incubation continued for 12 h. Cells were permeabilized and stained with anti HLA-DM mAb (MaP.DM1) and PE-anti-mouse Ab (FL2) and analyzed by flow cytometry. A, dot plots show DM expression (FL2) against EGFP expression (FL1). Left gates (R2) represent untransfected MARCH-EGFP−ve cells, right gates (R3) MARCH-EGFP+ve cells. Histograms show DM expression in MARCH-EGFP−ve (open histogram) and MARCH-EGFP+ve (hatched histogram) cells. Upper and lower dot plot/histograms show DM expression in the absence or presence of chloroquine. Data are representative of at least three independent experiments. B, histograms show EGFP expression in cells transfected with EGFP-MARCH1, EGFP-MARCH8, EGFP-MARCH9, or MARCH8 mutant with (open) or without (hatched) chloroquine. Data are representative of at least three independent experiments.
Cell Surface Down-regulation of HLA-DM by MARCH8 Is Independent of Ubiquitination but Requires Functional Tyrosine-based Endocytosis Motif
DM is subject to rapid endocytosis via a tyrosine-based targeting motif YTPL present in the cytoplasmic tail of DMβ (14, 15). Expression of DM in 293T cells allowed us to examine the influence of MARCH E3 ligases on surface DM levels. Surface down-regulation is used widely as a proxy of ubiquitination of transmembrane proteins (31). Surprisingly, we found that MARCH8 down-regulated DM containing the K230R substitution almost as efficiently as wild-type DM (Fig. 5A, lanes 1 and 2). The DMα cytoplasmic tail contains cysteine and serine residues, potential targets for unconventional ubiquitination (42). Substitution of all possible ubiquitinatable residues in DMα (KCS→RGA) still allowed efficient down-regulation (Fig. 5A, lane 3). Thus, although MARCH8 induced down-regulation of DM, it was independent of DMα ubiquitination. We considered two hypotheses that could explain the observed effects on DM. First, residues in the DMβ tail could be ubiquitinated or have an influence on down-regulation. Second, the effects could be indirect, possibly via influences on the endocytosis machinery.
FIGURE 5.
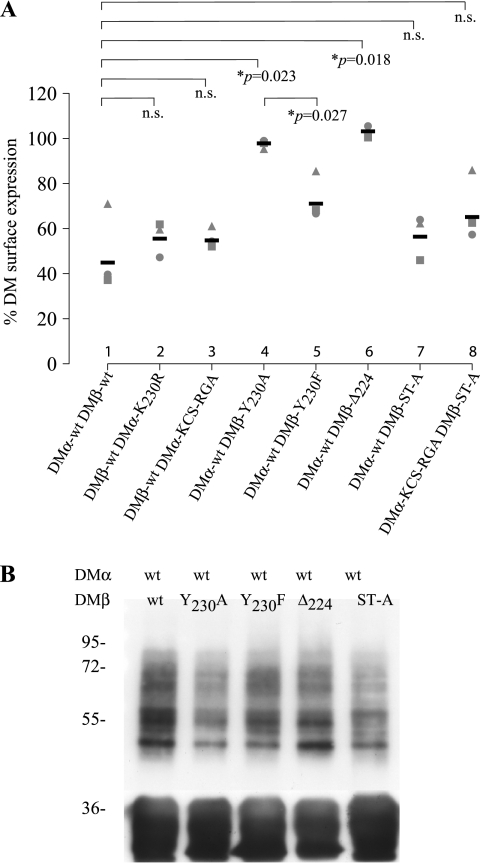
MARCH8 induced DM down-regulation is independent of direct DMα ubiquitination but requires a tyrosine-based endocytosis motif in the DMβ cytoplasmic tail. HEK-293T cells were transfected with EGFP-MARCH8, together with combinations of wt and mutant DMα/DMβ. Cell surface HLA-DM expression was determined by FACS analysis after staining with anti-DM antibody MaP.DM1 and PE anti-mouse mAb. A, percentage of DM construct present at the cell surface in the presence of EGFP-MARCH8. This was calculated as: % DM surface expression = (MFI of cells transfected with EGFP-MARCH8 × 100)/(MFI of cells transfected with EGFP vector alone). Data from independent experiments are shown by different shapes; means are represented by black bars. The corresponding FACS plots are shown in supplemental Fig. 2. B, immunoprecipitates of HEK-293T cells transfected with DMα, different DMβ constructs, and EGFP-MARCH8. Upper panel was probed with anti-ubiquitin antibody P4D1-HRP and the lower panel anti-DMα antibody 5C1. Data are representative of at least three independent experiments.
We first investigated whether the DMβ cytoplasmic tail could affect down-regulation by MARCH8. Truncation of the entire DMβ tail (DMβ-Δ224) abrogated down-regulation (Fig. 5A, lane 6). However, substitution of all DMβ (and DMα) residues that could be subject to conventional or unconventional ubiquitination (DMβ-ST-A) did not prevent down-regulation (Fig. 5A, lanes 7 and 8, and supplemental Fig. 2). We concluded that MARCH8-induced down-regulation of DM was independent of direct ubiquitination of DM but required sequences present in the DMβ cytoplasmic tail.
We next examined the effects of the DMβ YxxΦ motif upon MARCH-induced down-regulation. As expected, when we substituted the key tyrosine residue for alanine (DMβ Y230A), surface expression of DM was increased. However, no down-regulation of DM was observed in the presence of MARCH8 (Fig. 5A, lane 4, and supplemental Fig. 2). Substitution of Tyr230 with phenylalanine, a residue that can partially substitute for tyrosine with respect to interaction with the endocytic machinery, significantly restored MARCH8-induced DM down-regulation (Fig. 5A, lane 5, and supplemental Fig. 2) (43–46). In summary, we show in Figs. 1 and 2 that CD8-DMα absolutely requires residue Lys230 for MARCH8-induced down-regulation and that this residue is ubiquitinated by MARCH8. However, in the context of a DMα/β dimer, residue Lys230 is not essential for MARCH8-induced down-regulation. In the absence of residue Lys230, MARCH8 still induces down-regulation of surface DM through a mechanism that requires a functional DMβ encoded YxxΦ endocytosis motif.
We also investigated how the YxxΦ motif influenced the ubiquitination of DM. Immunoprecipitation and anti-ubiquitin Western blotting were performed on lysates from cells transfected with DMα wild type and different DMβ mutants in the presence of MARCH8 (Fig. 5B). Mutation or deletion of the tyrosine motif (DMβ-Y230A, DMβ-Δ224) reduced ubiquitination of DM compared with wild-type (Fig. 5B), but ubiquitination of DM was still observed in the presence or absence of the wild-type YxxΦ motif. In particular, deletion of the DMβ tail completely prevented MARCH8 induced down-regulation (Fig. 5A, lane 6) although ubiquitination of DM was still evident (Fig. 5B, fourth lane). We conclude that the down-regulation of DM by MARCH8 does not correlate directly with DM ubiquitination but instead requires a functional tyrosine-based endocytosis motif.
MARCH8 Down-regulation of HLA-DM Is More Dependent upon YxxΦ Endocytosis Motif than MARCH1 or 9
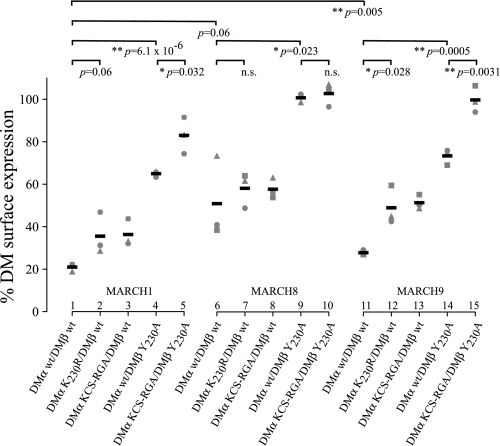
The molecular basis of MARCH E3 ligase target specificity is poorly understood (31, 32). To compare the interaction of MARCH1, 8, and 9 with DM, we monitored changes in DM expression in transiently transfected 293T cells (Fig. 6). MARCH1 down-regulated DM most efficiently (21% surface expression remaining in the presence of MARCH1 compared with EGFP control) followed by MARCH9 (28%) and then MARCH8 (49%).
FIGURE 6.
MARCH E3 ligase down-regulation of DM is influenced by a YxxΦ endocytosis motif and DMα ubiquitination. HEK-293T cells were transiently transfected with DMα and DMβ wild-type or mutant constructs and either EGFP-MARCH1, 8, or 9. Cell surface HLA-DM expression was determined by FACS analysis after staining with anti-DM antibody MaP.DM1 and PE anti-mouse mAb. Surface expression was calculated as: % DM surface expression = (MFI of cells transfected with EGFP-MARCH × 100)/(MFI of cells transfected with EGFP vector alone). Data from independent experiments are shown by different shapes; means are represented by black bars. The corresponding FACS plots are shown in supplemental Fig. 2.
Interestingly, MARCH8 down-regulated DM independently of ubiquitination of DMα-Lys230 but required a functional YxxΦ motif (Fig. 6, lanes 7 and 9). We examined whether MARCH1 and 9 behaved in a similar way and surprisingly found considerable differences. We observed that down-regulation of surface DM by MARCH1 and 9 was influenced by the presence of the YxxΦ motif (Fig. 6, MARCH1 lanes 1 and 4, MARCH9 lanes 11 and 14) and also the presence of the ubiquitinated residue DMα-Lys230 (MARCH1 lanes 1 and 2, MARCH9, lanes 11 and 12). These observations are summarized in supplemental Fig. 4. We conclude that HLA-DM down-regulation by MARCH8 is entirely dependent on the YxxΦ endocytosis motif, whereas MARCH1 and 9 down-regulate DM through the cumulative action of DMα-Lys230 and the YxxΦ endocytosis motif. A schematic figure summarizing these findings is presented in supplemental Fig. 4. This indirect, YxxΦ motif-dependent, regulation of molecules by MARCH1, 8, and 9 represents a previously unreported property of these E3 ligases.
DISCUSSION
In this study we investigated whether the nonclassical class II molecule HLA-DM was subject to post-translational regulation through ubiquitination. We observed that three E3 ligases, MARCH1, 8, and 9, which are known to target HLA-DP, -DR, and/or -DQ, are also capable of adding polyubiquitin chains to a single lysine residue present in the cytoplasmic tail of DM. Ubiquitination was associated with increased degradation of DM, presumably in lysosomal compartments, because degradation was blocked by the addition of chloroquine. We also found that the MARCH proteins co-immunoprecipitated with DM. This association was independent of the presence of the target lysine residue and did not require catalytic activity of the E3 ligase. Also, DM-associated MARCH proteins were themselves ubiquitinated and subject to lysosomal degradation. This did not require cis-E3 ligase catalytic activity, suggesting that these MARCH proteins are targets for endogenous E3 ligases.
During biosynthesis DM traffics from the ER to the cell surface and is then targeted into the late endocytic pathway by a tyrosine-based endocytic motif, YTPL. To investigate the influence of ubiquitination on the trafficking of DM we expressed DM in the absence of HLA-DP, -DQ, -DR, and -DO because association with ubiquitinated forms of these molecules could indirectly influence DM trafficking. We observed loss of DM surface expression in the presence of MARCH1, 8, or 9. In the case of MARCH1 and MARCH9, lysine 230 and the YTPL endocytic motif acted cumulatively to induce loss of surface DM expression. Intriguingly, in the absence of lysine 230, MARCH8 still induced loss of surface DM. MARCH8-induced DM down-regulation was entirely dependent upon the presence of the YTPL endocytosis motif. We considered a number of possible explanations for this. One possibility was that the tyrosine residue could be phosphorylated and then recruit E3 ligase activity. CD33, for example, uses this mechanism to recruit the ubiquitin ligases Cbl and Cbl-b (47). We do not think this is the case for DM as we were unable to demonstrate phosphorylation of the tyrosine residue (supplemental Fig. 3). In addition, loss of lysine 230 completely eliminated all detectable ubiquitination of DM, but MARCH8 still induced loss of surface expression. We consider it more likely that MARCH8 acts indirectly through ubiquitination of components of the endocytic machinery. In support of this hypothesis, substitution of YTPL for FTPL, which cannot be phosphorylated but can function as an endocytosis signal, maintained MARCH8-induced DM down-regulation. Our proposal is not without precedent. The human growth hormone receptor contains a “ubiquitin-dependent endocytosis motif” that directs internalization and sorting of growth hormone receptor to lysosomes, in the absence of direct ubiquitination of growth hormone receptor itself (48). In the case of DM, trafficking by clathrin-dependent endocytosis pathways involves a multitude of adaptor proteins (for review, see Ref. 49). A number of these adaptors such as epsin, Eps15, and ESCRT sorting components are regulated by ubiquitination (for review, see Ref. 50). We suggest that ubiquitination of components of the endocytic machinery by MARCH8 leads to internalization and degradation of DM, without a need for direct ubiquitination of DM.
In previous studies with HLA-DR we observed a direct correlation between ubiquitination of the class II molecule by MARCH8 and removal from the cell surface. For DM, the situation differed in that similar levels of surface expression were observed in the presence or absence of residues that could act as substrates for conventional or unconventional ubiquitination. Additionally, DM was ubiquitinated, even in the absence the YTPL motif, but surface levels did not appear to alter. In the absence of the YTPL motif, the level of DM ubiquitination was reduced. To explain these results we propose that the MARCH8-induced ubiquitination of DM is enhanced by internalization via the YTPL endocytic motif. This would be in agreement with the localization of MARCH8 to endocytic compartments, where ubiquitination of both DM and components of the endocytic machinery could occur. DM contains a well characterized endocytic motif so ubiquitination of the molecule would not be required for internalization from the cell surface. It appears more likely that ubiquitination is involved in trafficking of DM through later endocytic compartments.
Supplementary Material
Acknowledgments
We thank Paul Lehner, Satoshi Ishido, and Yasuhiro Ikeda for plasmid constructs and Peter Cresswell for antibody reagents. Liam Price contributed to this study as a project student.
This work was supported by the Wellcome Trust, the Medical Research Council, and the National Institute of Health Research Cambridge Biomedical Research Centre.

This article contains supplemental Figs. 1–4.
- Ii
- invariant chain
- DC
- dendritic cell
- MARCH
- membrane-associated RING-CH
- MFI
- mean fluorescent intensity
- PE
- phycoerythrin.
REFERENCES
- 1. Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. (1982) Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell 29, 61–69 [DOI] [PubMed] [Google Scholar]
- 2. Roche P. A., Marks M. S., Cresswell P. (1991) Formation of a nine-subunit complex by HLA class II glycoproteins and the invariant chain. Nature 354, 392–394 [DOI] [PubMed] [Google Scholar]
- 3. Pieters J., Bakke O., Dobberstein B. (1993) The MHC class II-associated invariant chain contains two endosomal targeting signals within its cytoplasmic tail. J. Cell Sci. 106, 831–846 [DOI] [PubMed] [Google Scholar]
- 4. Bremnes B., Madsen T., Gedde-Dahl M., Bakke O. (1994) An LI and ML motif in the cytoplasmic tail of the MHC-associated invariant chain mediate rapid internalization. J. Cell Sci. 107, 2021–2032 [DOI] [PubMed] [Google Scholar]
- 5. Morris P., Shaman J., Attaya M., Amaya M., Goodman S., Bergman C., Monaco J. J., Mellins E. (1994) An essential role for HLA-DM in antigen presentation by class II major histocompatibility molecules. Nature 368, 551–554 [DOI] [PubMed] [Google Scholar]
- 6. Denzin L. K., Robbins N. F., Carboy-Newcomb C., Cresswell P. (1994) Assembly and intracellular transport of HLA-DM and correction of the class II antigen-processing defect in T2 cells. Immunity 1, 595–606 [DOI] [PubMed] [Google Scholar]
- 7. Avva R. R., Cresswell P. (1994) In vivo and in vitro formation and dissociation of HLA-DR complexes with invariant chain-derived peptides. Immunity 1, 763–774 [DOI] [PubMed] [Google Scholar]
- 8. Sloan V. S., Cameron P., Porter G., Gammon M., Amaya M., Mellins E., Zaller D. M. (1995) Mediation by HLA-DM of dissociation of peptides from HLA-DR. Nature 375, 802–806 [DOI] [PubMed] [Google Scholar]
- 9. Sherman M. A., Weber D. A., Jensen P. E. (1995) DM enhances peptide binding to class II MHC by release of invariant chain-derived peptide. Immunity 3, 197–205 [DOI] [PubMed] [Google Scholar]
- 10. Weber D. A., Evavold B. D., Jensen P. E. (1996) Enhanced dissociation of HLA-DR-bound peptides in the presence of HLA-DM. Science 274, 618–620 [DOI] [PubMed] [Google Scholar]
- 11. van Ham S. M., Grüneberg U., Malcherek G., Bröker I., Melms A., Trowsdale J. (1996) Human histocompatibility leukocyte antigen (HLA)-DM edits peptides presented by HLA-DR according to their ligand binding motifs. J. Exp. Med. 184, 2019–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kropshofer H., Vogt A. B., Moldenhauer G., Hammer J., Blum J. S., Hämmerling G. J. (1996) Editing of the HLA-DR-peptide repertoire by HLA-DM. EMBO J. 15, 6144–6154 [PMC free article] [PubMed] [Google Scholar]
- 13. Denzin L. K., Hammond C., Cresswell P. (1996) HLA-DM interactions with intermediates in HLA-DR maturation and a role for HLA-DM in stabilizing empty HLA-DR molecules. J. Exp. Med. 184, 2153–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marks M. S., Roche P. A., van Donselaar E., Woodruff L., Peters P. J., Bonifacino J. S. (1995) A lysosomal targeting signal in the cytoplasmic tail of the β chain directs HLA-DM to MHC class II compartments. J. Cell Biol. 131, 351–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Copier J., Kleijmeer M. J., Ponnambalam S., Oorschot V., Potter P., Trowsdale J., Kelly A. (1996) Targeting signal and subcellular compartments involved in the intracellular trafficking of HLA-DMB. J. Immunol. 157, 1017–1027 [PubMed] [Google Scholar]
- 16. Reid P. A., Watts C. (1990) Cycling of cell surface MHC glycoproteins through primaquine-sensitive intracellular compartments. Nature 346, 655–657 [DOI] [PubMed] [Google Scholar]
- 17. Zhong G., Romagnoli P., Germain R. N. (1997) Related leucine-based cytoplasmic targeting signals in invariant chain and major histocompatibility complex class II molecules control endocytic presentation of distinct determinants in a single protein. J. Exp. Med. 185, 429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Demotz S., Grey H. M., Sette A. (1990) The minimal number of class II MHC-antigen complexes needed for T cell activation. Science 249, 1028–1030 [DOI] [PubMed] [Google Scholar]
- 19. Harding C. V., Unanue E. R. (1990) Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature 346, 574–576 [DOI] [PubMed] [Google Scholar]
- 20. Shin J. S., Ebersold M., Pypaert M., Delamarre L., Hartley A., Mellman I. (2006) Surface expression of MHC class II in dendritic cells is controlled by regulated ubiquitination. Nature 444, 115–118 [DOI] [PubMed] [Google Scholar]
- 21. van Niel G., Wubbolts R., Ten Broeke T., Buschow S. I., Ossendorp F. A., Melief C. J., Raposo G., van Balkom B. W., Stoorvogel W. (2006) Dendritic cells regulate exposure of MHC class II at their plasma membrane by oligoubiquitination. Immunity 25, 885–894 [DOI] [PubMed] [Google Scholar]
- 22. De Gassart A., Camosseto V., Thibodeau J., Ceppi M., Catalan N., Pierre P., Gatti E. (2008) MHC class II stabilization at the surface of human dendritic cells is the result of maturation-dependent MARCH I down-regulation. Proc. Natl. Acad. Sci. U.S.A. 105, 3491–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Young L. J., Wilson N. S., Schnorrer P., Proietto A., ten Broeke T., Matsuki Y., Mount A. M., Belz G. T., O'Keeffe M., Ohmura-Hoshino M., Ishido S., Stoorvogel W., Heath W. R., Shortman K., Villadangos J. A. (2008) Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat. Immunol. 9, 1244–1252 [DOI] [PubMed] [Google Scholar]
- 24. Walseng E., Furuta K., Bosch B., Weih K. A., Matsuki Y., Bakke O., Ishido S., Roche P. A. (2010) Ubiquitination regulates MHC class II-peptide complex retention and degradation in dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 107, 20465–20470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walseng E., Furuta K., Goldszmid R. S., Weih K. A., Sher A., Roche P. A. (2010) Dendritic cell activation prevents MHC class II ubiquitination and promotes MHC class II survival regardless of the activation stimulus. J. Biol. Chem. 285, 41749–41754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tze L. E., Horikawa K., Domaschenz H., Howard D. R., Roots C. M., Rigby R. J., Way D. A., Ohmura-Hoshino M., Ishido S., Andoniou C. E., Degli-Esposti M. A., Goodnow C. C. (2011) CD83 increases MHC II and CD86 on dendritic cells by opposing IL-10-driven MARCH1-mediated ubiquitination and degradation. J. Exp. Med. 208, 149–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsuki Y., Ohmura-Hoshino M., Goto E., Aoki M., Mito-Yoshida M., Uematsu M., Hasegawa T., Koseki H., Ohara O., Nakayama M., Toyooka K., Matsuoka K., Hotta H., Yamamoto A., Ishido S. (2007) Novel regulation of MHC class II function in B cells. EMBO J. 26, 846–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thibodeau J., Bourgeois-Daigneault M. C., Huppé G., Tremblay J., Aumont A., Houde M., Bartee E., Brunet A., Gauvreau M. E., de Gassart A., Gatti E., Baril M., Cloutier M., Bontron S., Früh K., Lamarre D., Steimle V. (2008) Interleukin-10-induced MARCH1 mediates intracellular sequestration of MHC class II in monocytes. Eur. J. Immunol. 38, 1225–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ohmura-Hoshino M., Matsuki Y., Aoki M., Goto E., Mito M., Uematsu M., Kakiuchi T., Hotta H., Ishido S. (2006) Inhibition of MHC class II expression and immune responses by c-MIR. J. Immunol. 177, 341–354 [DOI] [PubMed] [Google Scholar]
- 30. Goto E., Mito-Yoshida M., Uematsu M., Aoki M., Matsuki Y., Ohmura-Hoshino M., Hotta H., Miyagishi M., Ishido S. (2008) An excellent monitoring system for surface ubiquitination-induced internalization in mammals. PLoS ONE 3, e1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bartee E., Mansouri M., Hovey Nerenberg B. T., Gouveia K., Früh K. (2004) Down-regulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins. J. Virol. 78, 1109–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hör S., Ziv T., Admon A., Lehner P. J. (2009) Stable isotope labeling by amino acids in cell culture and differential plasma membrane proteome quantitation identify new substrates for the MARCH9 transmembrane E3 ligase. Mol. Cell Proteomics 8, 1959–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ishido S., Matsuki Y., Goto E., Kajikawa M., Ohmura-Hoshino M. (2010) MARCH-I: a new regulator of dendritic cell function. Mol. Cells 29, 229–232 [DOI] [PubMed] [Google Scholar]
- 34. Lapaque N., Jahnke M., Trowsdale J., Kelly A. P. (2009) The HLA-DRα chain is modified by polyubiquitination. J. Biol. Chem. 284, 7007–7016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ehrhardt C., Schmolke M., Matzke A., Knoblauch A., Will C., Wixler V., Ludwig S. (2006) Polyethylenimine, a cost-effective transfection agent. Signal Transduction 6, 179–184 [Google Scholar]
- 36. Hammond C., Denzin L. K., Pan M., Griffith J. M., Geuze H. J., Cresswell P. (1998) The tetraspan protein CD82 is a resident of MHC class II compartments where it associates with HLA-DR, -DM, and -DO molecules. J. Immunol. 161, 3282–3291 [PubMed] [Google Scholar]
- 37. Kelly A. P., Monaco J. J., Cho S. G., Trowsdale J. (1991) A new human HLA class II-related locus, DM. Nature 353, 571–573 [DOI] [PubMed] [Google Scholar]
- 38. Zufferey R., Nagy D., Mandel R. J., Naldini L., Trono D. (1997) Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15, 871–875 [DOI] [PubMed] [Google Scholar]
- 39. ten Broeke T., de Graaff A., van't Veld E. M., Wauben M. H., Stoorvogel W., Wubbolts R. (2010) Trafficking of MHC class II in dendritic cells is dependent on but not regulated by degradation of its associated invariant chain. Traffic 11, 324–331 [DOI] [PubMed] [Google Scholar]
- 40. Haan C., Behrmann I. (2007) A cost effective noncommercial ECL solution for Western blot detections yielding strong signals and low background. J. Immunol. Methods 318, 11–19 [DOI] [PubMed] [Google Scholar]
- 41. Gauvreau M. E., Côté M. H., Bourgeois-Daigneault M. C., Rivard L. D., Xiu F., Brunet A., Shaw A., Steimle V., Thibodeau J. (2009) Sorting of MHC class II molecules into exosomes through a ubiquitin-independent pathway. Traffic 10, 1518–1527 [DOI] [PubMed] [Google Scholar]
- 42. Cadwell K., Coscoy L. (2005) Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science 309, 127–130 [DOI] [PubMed] [Google Scholar]
- 43. McGraw T. E., Maxfield F. R. (1990) Human transferrin receptor internalization is partially dependent upon an aromatic amino acid on the cytoplasmic domain. Cell Regul. 1, 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Canfield W. M., Johnson K. F., Ye R. D., Gregory W., Kornfeld S. (1991) Localization of the signal for rapid internalization of the bovine cation-independent mannose 6-phosphate/insulin-like growth factor-II receptor to amino acids 24–29 of the cytoplasmic tail. J. Biol. Chem. 266, 5682–5688 [PubMed] [Google Scholar]
- 45. Sosa M. A., Schmidt B., von Figura K., Hille-Rehfeld A. (1993) In vitro binding of plasma membrane-coated vesicle adaptors to the cytoplasmic domain of lysosomal acid phosphatase. J. Biol. Chem. 268, 12537–12543 [PubMed] [Google Scholar]
- 46. Bonifacino J. S., Dell'Angelica E. C. (1999) Molecular bases for the recognition of tyrosine-based sorting signals. J. Cell Biol. 145, 923–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Walter R. B., Häusermann P., Raden B. W., Teckchandani A. M., Kamikura D. M., Bernstein I. D., Cooper J. A. (2008) Phosphorylated ITIMs enable ubiquitylation of an inhibitory cell surface receptor. Traffic 9, 267–279 [DOI] [PubMed] [Google Scholar]
- 48. van Kerkhof P., Putters J., Strous G. J. (2007) The ubiquitin ligase SCF(βTrCP) regulates the degradation of the growth hormone receptor. J. Biol. Chem. 282, 20475–20483 [DOI] [PubMed] [Google Scholar]
- 49. Robinson M. S. (2004) Adaptable adaptors for coated vesicles. Trends Cell Biol. 14, 167–174 [DOI] [PubMed] [Google Scholar]
- 50. Raiborg C., Stenmark H. (2009) The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 458, 445–452 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.