Background: The pathogenic role of phosphorylation of α-synuclein in Parkinson disease remains unclear.
Results: Ser-129-substituted α-synuclein was expressed in transgenic C. elegans. Nonphosphorylatable (S129A) α-synuclein showed severe defects. The level of membrane-bound α-synuclein was increased in S129A-α-synuclein.
Conclusion: Ser-129 phosphorylation of α-synuclein attenuates neuronal dysfunction and downstream stress response by lowering the membrane binding property.
Significance: Phosphorylation of α-synuclein is protective against neurotoxicity.
Keywords: C. elegans, Neurodegeneration, Neurological Diseases, Parkinson Disease, Synuclein
Abstract
α-Synuclein is causative for autosomal dominant familial Parkinson disease and dementia with Lewy bodies, and the phosphorylation of α-synuclein at residue Ser-129 is a key posttranslational modification detected in Parkinson disease/dementia with Lewy bodies lesions. However, the role of Ser-129 phosphorylation on the pathogenesis of Parkinson disease/dementia with Lewy bodies remains unclear. Here we investigated the neurotoxicity of Ser-129-substituted α-synuclein in the transgenic Caenorhabditis elegans (Tg worm) model of synucleinopathy. Tg worms pan-neuronally overexpressing nonphosphorylatable (S129A) α-synuclein showed severe defects including motor dysfunction, growth retardation, and synaptic abnormalities. In contrast, Tg worms expressing phosphorylation mimic (S129D) α-synuclein exhibited nearly normal phenotypes. Biochemical fractionation revealed that the level of membrane-bound α-synuclein was significantly increased in S129A-α-synuclein Tg worms, whereas S129D- as well as A30P-α-synuclein displayed lower membrane binding properties. Furthermore, A30P/S129A double mutant α-synuclein did not cause neuronal dysfunction and displayed low membrane binding property. In human neuroblastoma SH-SY5Y cells, localization of S129A-α-synuclein to membranes was significantly increased. Finally, gene expression profiling of S129A-Tg worms revealed a dramatic up-regulation of Daf-16/FOXO pathway genes, which likely act against the dysfunction caused by S129A-α-synuclein. These results imply a role of Ser-129 phosphorylation of α-synuclein in the attenuation of α-synuclein-induced neuronal dysfunction and downstream stress response by lowering the membrane binding property.
Introduction
Mutations or multiplications of α-synuclein gene have been identified as a cause of rare autosomal dominant forms of familial Parkinson disease and dementia with Lewy bodies. To date, three missense mutations (A53T, A30P, E46K), triplication, and duplication were reported (1–6). In addition, polymorphisms in the promoter region (7, 8) and untranslated region (9) of α-synuclein gene are also reported as a risk of Parkinson disease or dementia with Lewy bodies, and recent genome-wide association studies also identified α-synuclein gene as one of the major risk loci for sporadic Parkinson disease (10, 11). The formation of Lewy bodies, which contain α-synuclein as a major component (12, 13), is the hallmark of neuropathological change in Parkinson disease and dementia with Lewy bodies, leading to the collective nomenclature of α-synucleinopathy. α-Synuclein deposited in α-synucleinopathy lesions is characteristically phosphorylated at Ser-129 (14). Thus, the understanding of the significance of the deposition and phosphorylation of α-synuclein in neurons may open up a way to the development of novel therapeutics for α-synucleinopathy.
α-Synuclein is a largely cytosolic protein, whereas a portion of α-synuclein is thought to be associated with cellular membranes through the N-terminal KTKEGV repeats (15–18); this association has been reported to be disrupted by the A30P mutation (16, 19, 20). Although the physiological significance of the interaction of α-synuclein to membranes remains unclear, α-synuclein knock-out mice exhibited defects in paired pulse inhibition of dopaminergic nerve terminals (21), which is accompanied by an overall reduction in size of the distal reserve pool of synaptic vesicles (22), and an antisense RNA knockdown of α-synuclein in hippocampal slice cultures similarly yields a reduction in the distal reserve synaptic vesicle pool (23). These studies implicate α-synuclein in the regulation of mobilization or maintenance of synaptic vesicles at the presynaptic terminals. Moreover, an in vitro study suggested that the binding of α-synuclein to artificial liposomes was significantly decreased by Ser-129 phosphorylation by G protein-coupled receptor kinase 5 (24), suggesting a role of Ser-129 phosphorylation on the regulation of membrane/vesicle binding of α-synuclein. However, it remains unknown how the membrane binding and phosphorylation of α-synuclein in vivo impact the function and neurotoxicity of α-synuclein.
To investigate the effect of Ser-129 phosphorylation on α-synuclein neurotoxicity in vivo, a series of transgenic (Tg)2 animals that overexpress mutant α-synuclein replaced at Ser-129 with alanine (S129A), a nonphosphorylatable form, or aspartate (S129D), a phosphorylation mimic form, have been generated. Two groups have reported that overexpression of recombinant adeno-associated virus-mediated S129A-α-synuclein in rat substantia nigra caused prominent loss of dopaminergic neurons, whereas that of S129D-α-synuclein displayed much milder toxicity (25, 26). Conversely, another rat model exhibited no differences in toxicities among wild-type (WT) and Ser-129 mutant α-synuclein expressed by recombinant adeno-associated virus (27), and S129D-α-synuclein showed more severe toxicity when compared with S129A-α-synuclein in Tg Drosophila (28). Thus, there remain discrepancies in the neurotoxicity of Ser-129 mutant α-synuclein among animal models, and the mechanisms underlying the toxicity are still unclear. Previously, we have generated Tg Caenorhabditis elegans overexpressing human WT and pathogenic mutant (A53T, A30P) α-synuclein in a pan-neuronal fashion (29). Although simple overexpression of WT or mutant α-synuclein alone was not sufficient to cause neurotoxicity, we invariably detected Ser-129 phosphorylation of α-synuclein in these Tg worms. Thus, the role of phosphorylation at Ser-129 on α-synuclein neurotoxicity also remained elusive in the C. elegans model.
Here we report the effect of Ser-129 substitution of α-synuclein in C. elegans neurons. S129A-α-synuclein caused severe phenotypic defects and increased binding to membranes, whereas S129D as well as A30P/S129A-α-synuclein showed few defects and decreased binding to membranes. Overexpression of S129A-α-synuclein resulted in the up-regulation of DAF-16/Forkhead box O (FOXO) target genes involved in stress response, and genetic deletion of daf-16 dramatically aggravated the phenotypes of S129A-Tg worms. These results suggest a novel link among α-synuclein phosphorylation, membrane association, and stress response in the mechanism of α-synuclein-mediated neuronal dysfunction.
EXPERIMENTAL PROCEDURES
Plasmid Constructs
Punc-51::α-synuclein constructs including A53T and A30P mutants were previously described (29). cDNAs of S129A, S129D, and A30P/S129A mutant α-synuclein were generated by the overlapping PCR mutagenesis strategy. All constructs were sequenced using Thermo SequenaseTM (Amersham Biosciences) on an automated sequencer (LI-COR, Lincoln, NE).
C. elegans Protocols
Nematodes were handled by standard methods (30). N2 is the wild-type strain. Strains carrying daf-16(mu86) and juIs1(Punc-25::snb-1::gfp) were obtained from the Caenorhabditis Genetics Center (University of Minnesota, St. Paul, MN). Worms were grown on nematode growth medium agar plates inoculated with Escherichia coli strain OP50 at 20 °C. For the generation of Tg worms pan-neuronally overexpressing Ser-129 mutant α-synuclein, plasmid DNAs encoding each mutant-α-synuclein were mixed with a marker plasmid Punc-51::EGFP (enhanced green fluorescent protein), and the mixtures (200 μg/ml each) were co-injected into the gonads of young adult N2 worms. Transgenic progeny carrying α-synuclein as extrachromosomal arrays were selected on the basis of the expression of markers. Transgenes were integrated into chromosomes by ultraviolet irradiation, and each isolated animal was outcrossed eight times. Worms carrying both S129A-α-synuclein transgene and daf-16(mu86) were generated by mating α-synuclein Tg worm with daf-16(mu86) mutant, and the genotyping was performed by PCR using following set of primers: 5′-TTCTTCACTCGCCTTCATCA-3′ and 5′-GGCGAGATTTTTCGAGTTGA-3′ for detecting deletion and 5′-TTCCCCTCACATTCACTTCC-3′ and 5′-CCCCATGGTGTTCTCACTCT-3′ for detecting the absence of deletion. Worms carrying α-synuclein and Punc-25::snb-1::gfp were generated by injecting plasmid encoding α-synuclein into juIs1(Punc-25::snb-1::gfp). Synchronized culture of worms was generated by isolation of eggs via the hypochlorite method as described (31).
Immunohistochemistry
Preparation of paraffin-embedded worm sections and immunostaining were performed as described (29). α-Synuclein was detected with a mouse monoclonal antibody Syn211 (32), and α-synuclein phosphorylated at serine 129 was detected with a mouse monoclonal antibody pSyn#64 (33).
Western Blot
Worms were washed three times with M9 buffer (22 mm KH2PO4, 22 mm Na2HPO4, 85 mm NaCl, 1 mm MgSO4), and worm pellets were frozen at −80 °C. Extracts were prepared by resuspending frozen pellets in SDS sample buffer and sonicating. The sonicates were centrifuged at 10,000 × g for 5 min, and the supernatants were collected. 30 μg of protein per each sample was separated by SDS-PAGE followed by immunoblotting as described previously (29). α-Synuclein was detected with an antibody LB509 (13), and α-tubulin was detected with an antibody DM1A (Sigma).
Detergent Fractionation Analysis
Worms were washed three times with M9 buffer and collected as an ∼100-μl pellet, and the pellet was frozen and stored at −80 °C. The pellets were homogenized in 50 mm Tris-HCl buffer at pH 7.5 with Complete protease inhibitor mixture (Roche Applied Science) by brief sonication, and the sonicates were centrifuged two times at 1000 × g for 5 min to remove debris of worm tissue. The supernatant was then ultracentrifuged at 350,000 × g for 15 min, and the supernatant was collected as a Tris-HCl soluble fraction. The resulting pellet was subsequently extracted by sonication in Triton X-100 (Tris-HCl buffer with 1% Triton X-100), Sarkosyl (Tris-HCl with 1% Sarkosyl), and SDS (Tris-HCl with SDS sample buffer containing 2% SDS) followed by centrifugation at 350,000 × g for 15 min. The Tris-HCl fraction containing 20 μg of total proteins, along with equal volumes of Triton X-100, Sarkosyl, and SDS fractions to the Tris-HCl fraction, were loaded onto the acrylamide gel and separated by SDS-PAGE.
Phenotypic Analysis
A liquid thrashing assay was performed by placing individual young adult worms into the isotonic M9 buffer, and after 30 s, counting the frequency of body bends per 1 min. Growth rate was examined by measuring the body length of the slide-mounted worms at each stage under a microscope.
Fluorescence Microscopy
Worms were anesthetized by placing them in a drop of 50 mm sodium azide in M9 buffer, which was put on the solidified pads of 4% agarose laid on the slides. After the addition of a coverslip, worms were examined with an Axio Observer.Z1 microscope equipped with the AxioVision 4.6 software (Carl Zeiss).
Immunocytochemistry Using SH-SY5Y Cells
Human neuroblastoma SH-SY5Y cell line was maintained in a mixture of F12 and Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and penicillin/streptomycin at 37 °C in a 5% CO2 atmosphere. Transfection was performed with Lipofectamine 2000 reagent (Invitrogen) following the manufacturer's instructions. Digitonin permeabilization of cells was carried out essentially as described previously (34). Twenty-four hours after transfection, the cells were permeabilized for 3 min in an ice-cold buffer (12.5 mm HEPES−KOH, pH 7.4, 50 mm PIPES−KOH, pH 6.9, 1 mm MgSO4, and 4 mm EGTA−KOH) containing 40 μm digitonin (Sigma). After permeabilization, cells were washed four times briefly in PBS. The cells were fixed in the fixation buffer (4% paraformaldehyde, 4% sucrose in PBS) and blocked and permeabilized in the blocking buffer (3% bovine serum albumin, 0.1% Triton X-100 in PBS). Immunolabeling with anti-α-synuclein LB509 or anti-phosphorylated α-synuclein pSer129 antibodies was visualized by an indirect immunofluorescence method using Alexa Fluor-conjugated secondary antibodies (Invitrogen), and the nuclei were stained with SYTOX Orange (Invitrogen). Fluorescence images were acquired on a confocal laser-scanning microscope (LSM510; Zeiss) using a fixed laser power and detector gain for all samples and quantified using the ImageJ software.
DNA Microarray
Synchronized young adult worms of N2, WT-α-synuclein-Tg (synWT-Tg), A53T-Tg, and S129A-Tg genotypes were washed three times with M9 buffer and collected as a 100-μl worm pellet, and total RNAs were isolated using the ISOGEN reagent (Nippon Gene). Subsequent reverse transcription and cRNA synthesis were performed using One-Cycle target labeling and control reagents (Affymetrix), and the fragmented biotinylated cRNAs were hybridized to the Affymetrix C. elegans genome array, which represents 22,500 transcripts from C. elegans. Signal intensity of each strain was normalized by the median value of the transcripts, and genes whose expression levels were up-regulated or down-regulated more than 1.5-fold when compared with N2 were selected.
Real-time RT-PCR Analysis
Total RNA was isolated from 30 μl of worm pellet using the ISOGEN reagent (Nippon Gene), and cDNA synthesis was performed from 1 μg of total RNA using SuperScript II reverse transcriptase (Invitrogen). Quantitative real-time RT-PCR analysis was performed using the LightCycler 480 system (Roche Applied Science). Primer sequences for real-time PCR are as follows: 5′-AAACGTGCTGTAGCGGTTCT-3′ and 5′-TCCATGAAGTCCTGGTGACA-3′ for sod-5, 5′-GGCTGTTTCGAAAGGGAATC-3′ and 5′-CCTTTGAAGGTTCTCCACCA-3′ for sod-3, 5′-TGCAAGTGTGACTGCAAAAAC-3′ and 5′-TGCAGTCTCCCTTACATCCA-3′ for mtl-1, 5′-GGAGTCCTGCTCTCAGATGAA-3′ and 5′-ACATGAACACCGGCTCATTC-3′ for dod-3, and 5′-CCAATCCAAGAGAGGTATCCTT-3′ and 5′-ACATGGCTGGGGTATTGAAG-3′ for act-2. Expression levels of genes were normalized by the level of act-2 in each strain.
Statistics
All quantitative data presented are expressed as the mean ± S.E. The results were statistically evaluated for significance by using one-way analysis of variance with Fisher's protected least significant difference test. Differences were considered significant when p < 0.01 unless otherwise noted.
RESULTS
Generation of Tg C. elegans Overexpressing Ser-129 Mutant α-Synuclein
We have previously reported transgenic worms overexpressing human α-synuclein (WT, A53T, A30P) in a pan-neuronal fashion under the control of unc-51 promoter (29). Although these Tg worms did not show any severe neurological defects, phosphorylation of α-synuclein at Ser-129, a characteristic posttranslational modification in synucleinopathy lesions, was invariably detected. To examine the role of Ser-129 phosphorylation in α-synuclein neurotoxicity, we generated Tg worms pan-neuronally overexpressing S129A- or S129D-α-synuclein, representing nonphosphorylatable or phosphorylation mimic α-synuclein, respectively. The plasmid harboring S129A- or S129D-α-synuclein downstream of the unc-51 promoter (Fig. 1A) was microinjected into wild-type N2 worms together with Punc-51::EGFP (enhanced green fluorescent protein) as a transgenic marker, and chromosomally integrated strains were obtained. Western blot analysis revealed that both S129A-α-synuclein and S129D-α-synuclein were abundantly expressed, at similar levels to previously generated Tg worms expressing WT α-synuclein (Fig. 1B). Furthermore, immunohistochemical analysis of paraffin-embedded sections of Tg worms revealed that both S129A-α-synuclein and S129D-α-synuclein were mainly expressed in neurons including nerve ring and ventral nerve cord (Fig. 1C). We also confirmed that these Tg worms do not react with the antibody against Ser-129-phosphorylated α-synuclein (Fig. 1D).
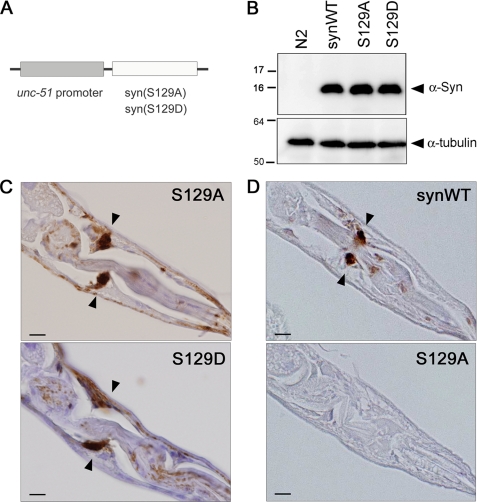
FIGURE 1.
Generation of Tg worms overexpressing S129A- and S129D-α-synuclein in pan-neuronal manner. A, construct of the transgene. S129A- or S129D-α-synuclein was expressed downstream of unc-51 promoter. B, immunoblot analysis of 3-day-old young adult Tg worms expressing WT or mutant α-synuclein (α-Syn). Whole lysate of each strain was separated by SDS-PAGE and reacted with anti-α-synuclein (upper panel) or anti-α-tubulin (lower panel) antibodies. Expression levels of α-synuclein were comparable among these strains. C, formalin-fixed, paraffin-embedded sections of S129A- or S129D-α-synuclein Tg worms were immunostained with anti-α-synuclein antibody Syn211. α-Synuclein was abundantly expressed in neurons and mainly localized to the nerve ring (arrows). D, WT- or S129A-α-synuclein Tg worms were immunostained with an anti-Ser-129-phosphorylated α-synuclein antibody. Immunopositive staining was detected at the nerve ring of WT-α-synuclein Tg worms (upper, arrows), which was not present in S129A-Tg worms (lower). Scale bar in C and D = 20 μm.
S129A-α-Synuclein Tg Worms Showed Severe Motor Dysfunction, Growth Retardation, and Synaptic Abnormality
We found that Tg worms expressing S129A-α-synuclein (S129A-Tg) were obviously defective in growth rate and motor function, whereas Tg worms expressing S129D-α-synuclein (S129D-Tg) appeared nearly normal (Fig. 2A). A characteristic feature of S129A-Tg worms was the coiler phenotype, representing motor dysfunction (Fig. 2A, inset). To quantitatively evaluate the growth rate, we measured the length of individual worms over time after hatch and found that two independent lines of S129A-Tg worms were significantly defective in growth rate when compared with other strains including synWT-Tg and S129D-Tg worms (Fig. 2B). Furthermore, we assessed the motor function of each strain at day 1 (L2 larva), day 3 (young adult), and day 8 by counting the frequency of body bends in the isotonic liquid. We found that two independent S129A-Tg strains showed strikingly severe motor defects throughout development and aging, suggesting constant neuronal dysfunction (Fig. 2C). Overall morphology of S129A-Tg worms, which grew up to adulthood, was also abnormal, and a majority these worms showed a severe egg-laying defect (Fig. 2D). In addition, we examined the superficial morphologies of S129A-Tg worms by differential interference microscope and found that abnormal swellings of intestine and defective morphogenesis of gonad, associated with tissue vacuolation in the hypodermis of the head, were observed (supplemental Fig. 1, and data not shown). We also assessed the possible occurrence of morphological changes of neurons expressing S129A-α-synuclein by observing the fluorescence of EGFP, which was co-expressed as a marker. However, no apparent abnormalities, e.g. disappearance of neurites or nerve cell body, were observed, and TUNEL staining was negative in S129A-Tg worms (data not shown). We next assessed the synaptic morphology by observation of GFP fluorescence in worms expressing both S129A-α-synuclein and SNB-1::GFP (GFP-tagged synaptobrevin); these worms were generated by microinjection of S129A-α-synuclein plasmid into the strain juIs1, which expresses SNB-1::GFP in a subset of ventral nerve cord neurons. GFP fluorescence of SNB-1::GFP was broadly diminished or extremely weak in the middle of the nerve cord in S129A-Tg worms (Fig. 2E), whereas the SNB-1::GFP fluorescence was preserved in S129D-Tg worms as in juIs1. Taken together, S129A-α-synuclein, a nonphosphorylatable form, caused severe dysfunction in neurons of worms, suggesting that phosphorylation of α-synuclein at Ser-129 protects against neurotoxicity of α-synuclein in C. elegans neurons.
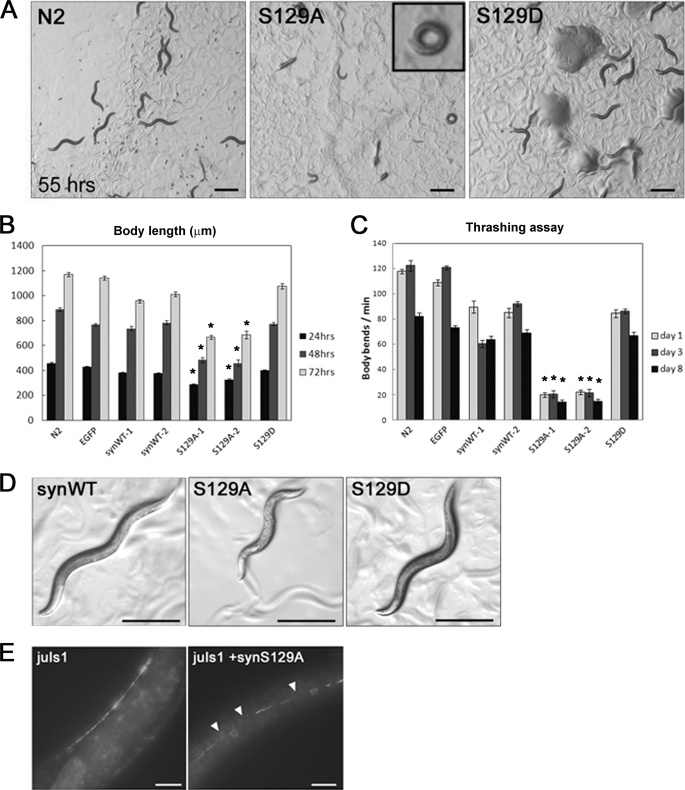
FIGURE 2.
Overexpression of S129A-α-synuclein in C. elegans caused severe phenotypic defects. A, representative pictures of synchronized cultures of N2, S129A-Tg, and S129D-Tg worms 55 h after hatching. The inset in the S129A-Tg panel shows the coiler phenotype. Scale bar = 500 μm. B, growth rate of each strain assessed by measuring the body length. The growth of S129A-Tg was significantly delayed. Data represent mean ± S.E., n = 20, *, p < 0.0001 when compared with N2. C, motor function of each strain at the age of day 1 (L2 larva), day 3 (young adult), and day 8 was assessed by thrashing assay. Each worm was soaked in M9 buffer, and the frequency of body bends was counted. Data represent mean ± S.E., n = 15, *, p < 0.0001 when compared with N2. D, overall appearances of representative synWT-Tg, S129A-Tg, and S129D-Tg worms (left, middle, and right, respectively). A majority of S129A-Tg worms exhibited an egg-laying defect. Bar = 500 μm. E, synaptic abnormalities in L2 stage worms expressing SNB-1::GFP as detected by the GFP fluorescence. S129A-α-synuclein was overexpressed in the strain juIs1(Punc-25::snb-1::gfp). Arrowheads indicate the sites at which fluorescence is broadly diminished. Bar = 10 μm.
S129A-α-Synuclein Showed High Affinity to Membranes
Next, we examined the biochemical properties of S129A or S129D versus WT α-synuclein expressed in C. elegans. Worm protein lysates were sequentially extracted by Tris-HCl buffer, then by Tris-HCl buffer containing Triton X-100, then by Sarkosyl, and finally by SDS (Fig. 3A). The amount of α-synuclein extracted in each fraction was assessed by immunoblotting. The most insoluble α-synuclein species, which were expected to be extracted in the SDS fraction, were hardly detected in both synWT-Tg and S129A-Tg worms. On the other hand, α-synuclein in the Triton X-100 fraction, in which membrane-bound proteins were highly concentrated, was significantly increased in S129A-Tg worms (Fig. 3B). We also examined Tg worms expressing A30P-α-synuclein (A30P-Tg), which is known to have lower membrane binding properties (16, 19, 20), and confirmed that the amount of A30P-α-synuclein in the Triton X-100 fraction was low (Fig. 3B). From these data, we hypothesized that the increased membrane binding of α-synuclein is related to the dysfunction caused by S129A-α-synuclein.
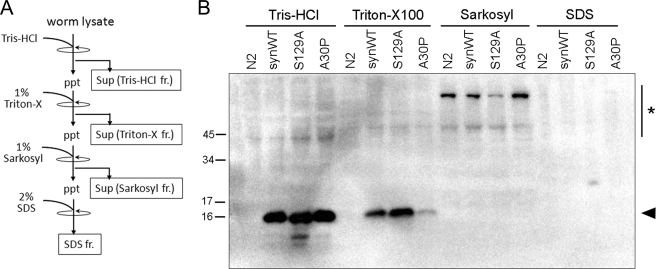
FIGURE 3.
S129A-α-synuclein harbors high membrane binding property, without forming detergent-insoluble species. A, a schematic chart of the procedure of protein fractionation. α-Synuclein was differentially extracted with Tris-HCl, Triton X-100, Sarkosyl, and SDS in this order. Sup, supernatant; ppt, precipitate; fr., fraction. B, the extracted proteins from N2, synWT-Tg, S129A-Tg, and A30P-Tg were analyzed by immunoblotting with an anti-α-synuclein antibody LB509. The amount of α-synuclein in the Triton X-100 fraction was significantly higher in S129A-Tg and lower in A30P-Tg when compared with synWT-Tg worm. The arrowhead indicates α-synuclein, and the asterisk indicates nonspecific bands.
Phosphorylation of α-Synuclein Protects against Neuronal Dysfunction along with Reduction in Its Membrane Binding Property
To ensure the correlation among phosphorylation, membrane binding property, and neurotoxicity of α-synuclein, we next generated Tg worms overexpressing A30P/S129A double-mutant α-synuclein, which is expected to retain low membrane binding property with a lack of phosphorylation. We generated three independent A30P/S129A-Tg lines and found that all three lines showed nearly normal motor activities (Fig. 4A). In addition, the overall morphologies (Fig. 4B) as well as the growth rates of A30P/S129A-Tg worms (data not shown) were also normal. The levels of α-synuclein in A30P/S129A-Tg lines were confirmed to be comparable with those in other α-synuclein Tg lines (Fig. 4C). We then tested the membrane binding property of A30P/S129A-α-synuclein by detergent fractionation analysis and found that the amount of A30P/S129A-α-synuclein extracted in the Triton X-100 fraction was significantly lower than those in S129A-Tg and synWT-Tg worms (Fig. 4, D and E). We also found that the membrane binding property of S129D-α-synuclein was lower than that of WT-α-synuclein (Fig. 4, D and E). These results suggest that the dysfunction might have been caused by nonphosphorylatable α-synuclein exhibiting high membrane binding property and that phosphorylation at Ser-129 is sufficient to reduce α-synuclein-mediated impairment possibly by lowering its membrane binding property.
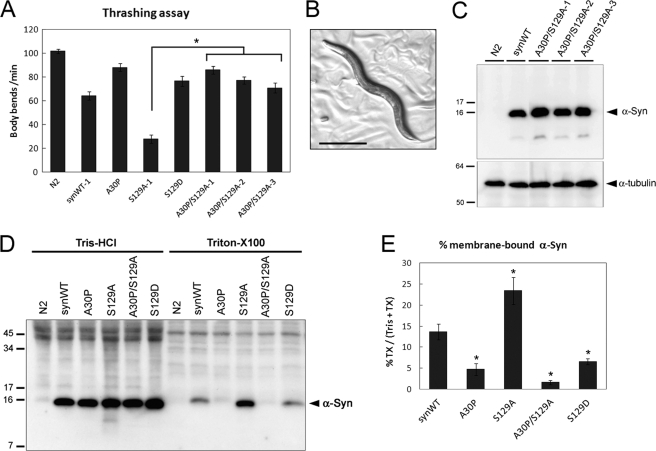
FIGURE 4.
A30P/S129A- and S129D-α-synuclein cause little dysfunction and have lower membrane binding property. A, liquid thrashing assay. Three independent A30P/S129A-Tg worms were normal, in contrast to severe motor dysfunction in two S129A-Tg lines. Data represent mean ± S.E. n = 15. *, p < 0.0001. B, overall appearance of representative A30P/S129A-Tg worm, showing normal morphology. Bar = 500 μm. C, immunoblot analysis showing the expression levels of α-synuclein (α-Syn) in the indicated lines at the young adult stage. D, detergent fractionation analysis. The extracted proteins were analyzed by immunoblotting with an anti-α-synuclein antibody LB509. Positive α-synuclein immunoreactivities were detected only in the Tris-HCl and Triton X-100 fractions. E, the percentages of the membrane-bound α-synuclein (Triton X-100 fraction) that composed the total α-synuclein (sum of α-synuclein in the Tris-HCl and Triton X-100 fractions) were quantified by the densitometric measurement of the bands. Data represent mean ± S.E. from four independent tests. *, p < 0.05 when compared with synWT-Tg.
S129A-α-Synuclein Exhibited Increased Targeting to Membranes in SH-SY5Y Cells
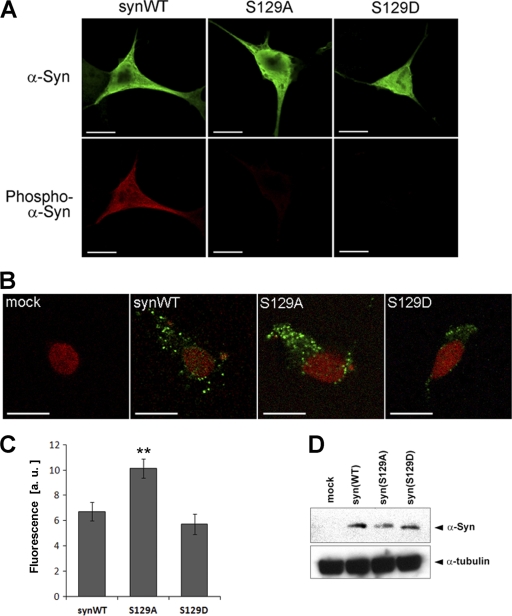
We next sought to obtain the visible evidence that phosphorylation regulates the membrane localization of α-synuclein. As C. elegans neuronal cell bodies are too small to examine the subcellular localization of expressed proteins by immunostaining, we used human neuroblastoma SH-SY5Y cells for examining the distribution pattern of WT, S129A, and S129D-α-synuclein. A prior study suggested that a membrane-bound fraction of α-synuclein can be visually seen by treatment with digitonin, which selectively permeabilizes the plasma membrane, releasing soluble and cytoplasmic α-synuclein (34). In the absence of digitonin treatment, all α-synuclein species similarly exhibited diffuse distribution patterns in the cytoplasm, masking a small fraction associated with membrane structures (Fig. 5A, upper panels). Phosphorylated α-synuclein at Ser-129 was also detected in cells expressing WT-α-synuclein but not in those expressing S129A or S129D-α-synuclein (Fig. 5A, lower panels). When cells were permeabilized with digitonin, residual α-synuclein associated with membranes was detected as a granular staining pattern (Fig. 5B). We found that the cells expressing WT and S129D-α-synuclein showed relatively moderate residual staining of α-synuclein, whereas those expressing S129A-α-synuclein showed much stronger staining (Fig. 5B). This increase of residual granular staining was quantitatively verified by the measurement of fluorescence intensity (Fig. 5C). We also confirmed that the expression levels of α-synuclein were comparable among the cells expressing these α-synuclein species (Fig. 5D). We also performed the same experiment using HEK293 cells and found that S129D-α-synuclein exhibited decreased localization to membranes when compared with WT and S129A-α-synuclein (supplemental Fig. 2), suggesting that mimicking phosphorylation reduces the membrane localization in non-neuronal cells. As the residual membrane structure after digitonin treatment is thought to be mainly composed of lipid rafts (34), we double-stained for flotillin, a known marker for lipid rafts, and we could confirm clear co-localization of α-synuclein with flotillin after digitonin treatment (supplemental Fig. 2). Together, these results suggest that α-synuclein phosphorylation reduces its localization to membranes that include lipid rafts.
FIGURE 5.
S129A-α-synuclein harbors higher membrane binding property in human neuroblastoma SH-SY5Y cells. A, representative pictures of immunocytochemical analysis of SH-SY5Y cells transfected with WT or mutant α-synuclein (α-Syn). Cells were immunostained with anti-α-synuclein antibody LB509 (green) and anti-phosphorylated α-synuclein pSer129 (red). B, representative pictures of the cells treated with 40 μm digitonin followed by immunostaining for α-synuclein (green) and nuclei (red). Granular structures corresponding to membrane-bound α-synuclein were evident after digitonin permeabilization. Scale bar = 10 μm. C, fluorescence intensity of granular structures was quantified using the ImageJ software. S129A-α-synuclein exhibited significantly higher fluorescence intensity after digitonin permeabilization. Data represent mean ± S.E., n = 10, **, p < 0.005 when compared with WT-α-synuclein. a. u., arbitrary units. D, immunoblot analysis of SH-SY5Y cells transfected with WT or mutant α-synuclein. Whole lysate of transfected cells was separated by SDS-PAGE and reacted with anti-α-synuclein (upper panel) or anti-α-tubulin (lower panel) antibodies. Expression levels of α-synuclein were comparable among these cells.
Overexpression of S129A-α-Synuclein in C. elegans Neurons Caused Up-regulation of Daf-16/FOXO Signaling Pathway
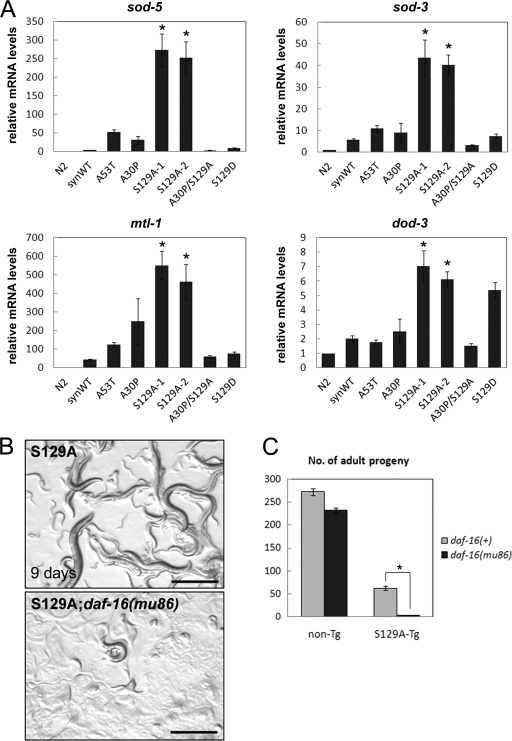
To identify the downstream molecular pathway of the overexpression of nonphosphorylatable and membrane-associated α-synuclein in C. elegans neurons, we performed genome-wide expression analysis of S129A-Tg worms and compared the results with those of N2, synWT-Tg, and A53T-Tg worms, using the Affymetrix C. elegans genome array. Gene expression profiles of ∼22,500 transcripts were compared among the strains, and the genes whose expression was significantly up-regulated or down-regulated more than 1.5-fold in S129A-Tg worms when compared with all other strains were selected. As a result of data mining and confirmation by real-time PCR analysis, we found that the expression levels of a set of representative DAF-16/FOXO target genes, i.e. sod-5, sod-3, mtl-1, and dod-3, were dramatically up-regulated in S129A-Tg worms (Fig. 6A). DAF-16/FOXO is a well known forkhead transcription factor responsible for various important physiological functions, e.g. lifespan extension and stress response. To gain insights into the significance of the up-regulation of DAF-16 targets, S129A-Tg worms were crossed with daf-16(mu86) mutant, which lacks functional DAF-16 protein expression but shows nearly normal development (35, 36). We found that the S129A-Tg;daf-16(mu86) worms were dramatically sick and almost nonviable, whereas the daf-16(mu86) mutant grew up without major defects (Fig. 6, B and C). These results suggest that the up-regulation of the daf-16/FOXO signaling pathway detected in S129A-Tg worms may represent a neuroprotective response against α-synuclein-induced neuronal dysfunction.
FIGURE 6.
Up-regulation of expression of Daf-16/FOXO signaling pathway genes caused by pan-neuronal overexpression of S129A-α-synuclein. A, real-time RT-PCR analysis of mRNA levels of DAF-16 targets sod-5, sod-3, mtl-1, and dod-3. Relative expression levels when compared with those in wild-type N2 are shown. Data represent mean ± S.E., n = 4, *, p < 0.0001 when compared with N2 and synWT-Tg. B, stereoscopic views of S129A-Tg worms with or without daf-16(mu86) allele, which are the progenies of a single adult placed 9 days before. Many progenies of S129A-Tg grew up after 9 days, whereas those of S129A-Tg;daf-16(mu86) hardly grew up. Bar = 500 μm. C, the average number of adults of indicated strains that are the progenies of a single adult placed 5 days before. Data represent mean ± S.E., n = 8, *, p < 0.0001.
DISCUSSION
In this study, we examined the role of Ser-129 phosphorylation of α-synuclein in its neurotoxicity using a transgenic C. elegans model and suggested a possible molecular mechanism of phosphorylation-mediated neuroprotection against α-synuclein-induced dysfunction. Although previous studies have reported the toxic effects of S129A or S129D-α-synuclein in neurons of transgenic rat or Drosophila models (25–28), detailed molecular mechanisms underlying the toxicity remained elusive. Here we demonstrated a strong correlation among α-synuclein binding to membrane, phosphorylation at Ser-129, and neuronal dysfunction. Although we cannot fully exclude the possibility that membrane binding of α-synuclein is independent of the dysfunction, our present data imply a mechanism whereby phosphorylation of α-synuclein reduces neuronal impairment through a reduction in the amount of membrane-bound α-synuclein. We also found that the inhibition of the Daf-16/FOXO signaling pathway dramatically exacerbated the neuronal dysfunction caused by S129A-α-synuclein.
In Tg rats that overexpress human α-synuclein by adeno-associated virus-mediated gene transfer, S129A-α-synuclein displayed higher neurotoxicity when compared with WT- and S129D-α-synuclein (25, 26), which was supplemented by an immunoelectron microscopic analysis showing a close localization of S129A-α-synuclein to cellular membranes (26). In addition, S129D-α-synuclein expressed in budding yeast exhibited decreased plasma membrane association (37). These observations are consistent with our results, suggesting the conserved mechanism of regulation of neurotoxicity and membrane binding by Ser-129 phosphorylation.
Our finding that introduction of A30P mutation reduced the neuronal dysfunction caused by S129A-α-synuclein seems a little paradoxical, given the effect of A30P as a disease-causing mutation (2). Although the reason for this discrepancy remains unknown, A30P-α-synuclein may have a somewhat different nature of toxicity, which has been implied by previous studies reporting that A30P-α-synuclein Tg mice did not show overt phenotype or pathology (38, 39). One possible pathogenic mechanism of A30P-α-synuclein would be the formation of neurotoxic prefibrillar oligomers (40, 41), which may not be working in some animal models including our Tg worms.
A portion of α-synuclein molecules is thought to be directly associated with membrane lipids (15–17), and importantly, it has been shown that α-synuclein harbors a high affinity for negatively charged, acidic phospholipids, e.g. phosphatidylserine or phosphatidylinositol (15, 18, 19, 42). Thus, it is reasonable to assume that Ser-129 phosphorylation provides α-synuclein with negative charges, which in turn lowers the electrostatic interaction of α-synuclein with acidic phospholipids, leading to the dissociation of α-synuclein from cellular membranes. Indeed, a prior study demonstrated that phosphorylation at Ser-129 reduced the affinity of α-synuclein to membranes in vitro (24). However, we should be careful about this notion as another in vitro study failed to show the perturbing effect of phosphorylation on its membrane-bound conformation (43), and a biochemical approach using synaptosomal membranes showed a lack of modifying effect of phosphorylation on membrane binding of WT α-synuclein (44).
From the immunocytochemical point of view, however, our studies using human cell lines support the notion that Ser-129 phosphorylation renders α-synuclein to dissociate from membrane structures. As for the difference in the degree of membrane targeting of WT α-synuclein between SH-SY5Y and HEK293 cells, this might be explained by the difference between neuronal versus non-neuronal cells or by the difference in the amount of phosphorylated α-synuclein between these cells. It was hard to examine the latter possibility as the quantitative approach by immunoblotting failed to detect phosphorylated α-synuclein in both cell lines, although phosphorylation was cytochemically detected. We should also carefully argue about the effect of S129D mutation as another study showed a difference in the structural property between in vitro purified S129D mutant and α-synuclein phosphorylated by CK1 (43). However, the molecular dynamics of α-synuclein in vivo are expected to be different from the in vitro state, considering the situation such that α-synuclein physiologically occurs in oligomer formations such as tetramers (45).
The notion that the membrane-bound form of α-synuclein causes neuronal dysfunction or toxicity is supported by previous studies using animal models, in which overexpression of α-synuclein caused defects in vesicle trafficking (46). In yeast, the earliest defect by α-synuclein overexpression was the block of endoplasmic reticulum-Golgi trafficking, and the toxicity was rescued by overexpression of Rab1, Rab3A, or Rab8A, all of which are involved in different steps in vesicle trafficking (47, 48). In addition, a recent study using α-synuclein Tg C. elegans showed that α-synuclein toxicity was rescued by Vps41, a protein involved in lysosomal trafficking (49). We have also shown that Tg C. elegans pan-neuronally overexpressing α-synuclein displayed defects in synaptic vesicle endocytosis (29); in this worm, the amount of membrane-bound α-synuclein was dramatically increased by the inhibition of AP-2, an adaptor molecule involved in the early steps of clathrin-mediated endocytosis.3 Taken together, it is conceivable that overexpression of α-synuclein interferes with the diverse steps of vesicle trafficking by directly associating with the vesicles.
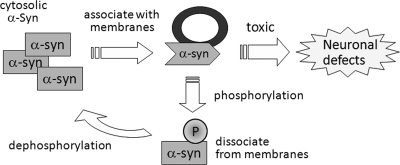
The percentage of α-synuclein phosphorylated at Ser-129 accounts only for ∼4% of total endogenous α-synuclein in normal mammalian brains (14), and the low percentage of phosphorylation is also likely true in Tg worms, given the result that Ser-129 phosphorylation is hardly detected on immunoblot analysis (data not shown). These facts suggest that phosphorylated α-synuclein is a minor species in neurons. Our observations that phosphorylation occurring in a small fraction of α-synuclein can rescue the dysfunction suggest that a limited fraction of α-synuclein might cause the neuronal impairment, which can be ameliorated by Ser-129 phosphorylation. Considering the increased membrane binding of S129A-α-synuclein, it is reasonable that membrane-bound S129A-α-synuclein interferes with neuronal function. Thus, the most likely scenario would be as follows. When the membrane-bound α-synuclein is phosphorylated, the α-synuclein species may then be dissociated from membranes and detoxified. Cytosolic, free α-synuclein species may subsequently be dephosphorylated, resulting in the low percentage of phosphorylated α-synuclein at the steady state (Fig. 7).
FIGURE 7.
Schematic depiction of regulatory role of α-synuclein phosphorylation on its neurotoxicity. The membrane-attached portion of α-synuclein (α-Syn), which causes neuronal dysfunction, is subsequently phosphorylated (P) at Ser-129 and then dissociated from membranes, resulting in detoxification. Dissociated α-synuclein in cytoplasm is subsequently dephosphorylated by phosphatases.
Then, how should we interpret the extensive phosphorylation of α-synuclein insolubilized in human α-synucleinopathy brains? Previous studies have shown that membrane-bound α-synuclein species promote α-synuclein aggregation, forming the core of aggregates (42, 50, 51). Phosphorylated α-synuclein that includes the membrane-bound form upon aggregation could no longer be dephosphorylated because of the decreased accessibility of phosphatases caused by the structural changes. In this regard, formation of fibrous aggregates such as Lewy bodies could be considered as a protective reaction to sequester the toxic α-synuclein species. Although no Lewy body-like inclusions that contain phosphorylated and aggregated α-synuclein were detected in the Tg C. elegans model, our finding that the neuronal dysfunction was most marked in S129A-α-synuclein Tg worms supports the notion that the preaggregated form of α-synuclein, especially the membrane-bound state, represents the detrimental species leading to dysfunction and neurodegeneration.
We also revealed an involvement of the Daf-16/FOXO signaling pathway downstream of the overexpression of nonphosphorylatable α-synuclein. It is well known that Daf-16/FOXO pathway is activated by insulin signaling or cellular stresses and involved in a broad range of bioregulation from metabolism to longevity (35, 52, 53). Although little is known about the involvement of FOXO signaling in Parkinson disease, a recent study showed that LRRK2, another gene causative to the autosomal dominant form of familial Parkinson disease, causes neurotoxicity via up-regulation of FOXO1 pathway in Drosophila (54). Among a number of target genes of Daf-16 thus far identified, up-regulation of antioxidative stress genes, i.e. sod-5, sod-3, and mtl-1, was especially prominent in our Tg worms overexpressing S129A-α-synuclein, implicating oxidative stress in the neuronal defects by S129A-α-synuclein. This view is supported by our finding that the genetic deletion of daf-16 dramatically enhanced the defects in S129A-Tg worms, although it still remains possible that this phenotypic aggravation is rather caused by an epistatic effect of daf-16. It also remains possible that other Daf-16/FOXO target genes or pathways in addition to antioxidative stress genes may play critical roles in response to the dysfunction caused by S129A-α-synuclein. Thus, it would further be required to examine the changes in various FOXO target genes in worms as well as other models or human pathology of α-synucleinopathies.
Based on the data derived from this study, three strategies to reduce the neurotoxicity of α-synuclein would be considered: (i) inhibition of association of α-synuclein with cellular membranes, (ii) promotion of α-synuclein phosphorylation or inhibition of dephosphorylation of α-synuclein at Ser-129, and (iii) activation of FOXO signaling pathway. Gaining the ability to assess these strategies in other models involving higher-order organisms may pave the way to the establishment of novel therapeutic strategies for α-synucleinopathies.
Supplementary Material
Acknowledgments
We thank the Caenorhabditis Genetics Center, funded by the National Institutes of Health National Center for Research Resources, for providing worms carrying daf-16(mu86) and juIs1. We thank Dr. N. Nukina and Dr. F. Oyama for DNA microarray analysis. We also thank members of the Iwatsubo laboratory and Dr. D. MacLeod for helpful suggestions and discussions.
This work was supported by a grant-in-aid for scientific research on priority areas from the Ministry of Education, Culture, Sports, Science and Technology of Japan.

This article contains supplemental Figs. 1 and 2.
T. Kuwahara and T. Iwatsubo, unpublished observations.
- Tg
- transgenic
- EGFP
- enhanced green fluorescent protein
- SynWT-TG
- WT-α-synuclein-Tg
- FOXO
- Forkhead box O.
REFERENCES
- 1. Polymeropoulos M. H., Lavedan C., Leroy E., Ide S. E., Dehejia A., Dutra A., Pike B., Root H., Rubenstein J., Boyer R., Stenroos E. S., Chandrasekharappa S., Athanassiadou A., Papapetropoulos T., Johnson W. G., Lazzarini A. M., Duvoisin R. C., Di Iorio G., Golbe L. I., Nussbaum R. L. (1997) Mutation in the α-synuclein gene identified in families with Parkinson disease. Science 276, 2045–2047 [DOI] [PubMed] [Google Scholar]
- 2. Krüger R., Kuhn W., Müller T., Woitalla D., Graeber M., Kösel S., Przuntek H., Epplen J. T., Schöls L., Riess O. (1998) A30P mutation in the gene encoding α-synuclein in Parkinson disease. Nat. Genet. 18, 106–108 [DOI] [PubMed] [Google Scholar]
- 3. Zarranz J. J., Alegre J., Gómez-Esteban J. C., Lezcano E., Ros R., Ampuero I., Vidal L., Hoenicka J., Rodriguez O., Atarés B., Llorens V., Gomez Tortosa E., del Ser T., Muñoz D. G., de Yebenes J. G. (2004) The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 55, 164–173 [DOI] [PubMed] [Google Scholar]
- 4. Singleton A. B., Farrer M., Johnson J., Singleton A., Hague S., Kachergus J., Hulihan M., Peuralinna T., Dutra A., Nussbaum R., Lincoln S., Crawley A., Hanson M., Maraganore D., Adler C., Cookson M. R., Muenter M., Baptista M., Miller D., Blancato J., Hardy J., Gwinn-Hardy K. (2003) α-Synuclein locus triplication causes Parkinson disease. Science 302, 841. [DOI] [PubMed] [Google Scholar]
- 5. Chartier-Harlin M. C., Kachergus J., Roumier C., Mouroux V., Douay X., Lincoln S., Levecque C., Larvor L., Andrieux J., Hulihan M., Waucquier N., Defebvre L., Amouyel P., Farrer M., Destée A. (2004) α-Synuclein locus duplication as a cause of familial Parkinson disease. Lancet 364, 1167–1169 [DOI] [PubMed] [Google Scholar]
- 6. Ibáñez P., Bonnet A. M., Débarges B., Lohmann E., Tison F., Pollak P., Agid Y., Dürr A., Brice A. (2004) Causal relation between α-synuclein gene duplication and familial Parkinson disease. Lancet 364, 1169–1171 [DOI] [PubMed] [Google Scholar]
- 7. Pals P., Lincoln S., Manning J., Heckman M., Skipper L., Hulihan M., Van den Broeck M., De Pooter T., Cras P., Crook J., Van Broeckhoven C., Farrer M. J. (2004) α-Synuclein promoter confers susceptibility to Parkinson disease. Ann. Neurol. 56, 591–595 [DOI] [PubMed] [Google Scholar]
- 8. Maraganore D. M., de Andrade M., Elbaz A., Farrer M. J., Ioannidis J. P., Krüger R., Rocca W. A., Schneider N. K., Lesnick T. G., Lincoln S. J., Hulihan M. M., Aasly J. O., Ashizawa T., Chartier-Harlin M. C., Checkoway H., Ferrarese C., Hadjigeorgiou G., Hattori N., Kawakami H., Lambert J. C., Lynch T., Mellick G. D., Papapetropoulos S., Parsian A., Quattrone A., Riess O., Tan E. K., Van Broeckhoven C. (2006) Collaborative analysis of α-synuclein gene promoter variability and Parkinson disease. JAMA 296, 661–670 [DOI] [PubMed] [Google Scholar]
- 9. Mueller J. C., Fuchs J., Hofer A., Zimprich A., Lichtner P., Illig T., Berg D., Wüllner U., Meitinger T., Gasser T. (2005) Multiple regions of α-synuclein are associated with Parkinson disease. Ann. Neurol. 57, 535–541 [DOI] [PubMed] [Google Scholar]
- 10. Satake W., Nakabayashi Y., Mizuta I., Hirota Y., Ito C., Kubo M., Kawaguchi T., Tsunoda T., Watanabe M., Takeda A., Tomiyama H., Nakashima K., Hasegawa K., Obata F., Yoshikawa T., Kawakami H., Sakoda S., Yamamoto M., Hattori N., Murata M., Nakamura Y., Toda T. (2009) Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson disease. Nat. Genet. 41, 1303–1307 [DOI] [PubMed] [Google Scholar]
- 11. Simón-Sánchez J., Schulte C., Bras J. M., Sharma M., Gibbs J. R., Berg D., Paisan-Ruiz C., Lichtner P., Scholz S. W., Hernandez D. G., Krüger R., Federoff M., Klein C., Goate A., Perlmutter J., Bonin M., Nalls M. A., Illig T., Gieger C., Houlden H., Steffens M., Okun M. S., Racette B. A., Cookson M. R., Foote K. D., Fernandez H. H., Traynor B. J., Schreiber S., Arepalli S., Zonozi R., Gwinn K., van der Brug M., Lopez G., Chanock S. J., Schatzkin A., Park Y., Hollenbeck A., Gao J., Huang X., Wood N. W., Lorenz D., Deuschl G., Chen H., Riess O., Hardy J. A., Singleton A. B., Gasser T. (2009) Genome-wide association study reveals genetic risk underlying Parkinson disease. Nat. Genet. 41, 1308–1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spillantini M. G., Schmidt M. L., Lee V. M., Trojanowski J. Q., Jakes R., Goedert M. (1997) α-Synuclein in Lewy bodies. Nature 388, 839–840 [DOI] [PubMed] [Google Scholar]
- 13. Baba M., Nakajo S., Tu P. H., Tomita T., Nakaya K., Lee V. M., Trojanowski J. Q., Iwatsubo T. (1998) Aggregation of α-synuclein in Lewy bodies of sporadic Parkinson disease and dementia with Lewy bodies. Am. J. Pathol. 152, 879–884 [PMC free article] [PubMed] [Google Scholar]
- 14. Fujiwara H., Hasegawa M., Dohmae N., Kawashima A., Masliah E., Goldberg M. S., Shen J., Takio K., Iwatsubo T. (2002) α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 4, 160–164 [DOI] [PubMed] [Google Scholar]
- 15. Davidson W. S., Jonas A., Clayton D. F., George J. M. (1998) Stabilization of α-synuclein secondary structure upon binding to synthetic membranes. J. Biol. Chem. 273, 9443–9449 [DOI] [PubMed] [Google Scholar]
- 16. Jensen P. H., Nielsen M. S., Jakes R., Dotti C. G., Goedert M. (1998) Binding of α-synuclein to brain vesicles is abolished by familial Parkinson disease mutation. J. Biol. Chem. 273, 26292–26294 [DOI] [PubMed] [Google Scholar]
- 17. McLean P. J., Kawamata H., Ribich S., Hyman B. T. (2000) Membrane association and protein conformation of α-synuclein in intact neurons: effect of Parkinson disease-linked mutations. J. Biol. Chem. 275, 8812–8816 [DOI] [PubMed] [Google Scholar]
- 18. Perrin R. J., Woods W. S., Clayton D. F., George J. M. (2000) Interaction of human α-synuclein and Parkinson disease variants with phospholipids: structural analysis using site-directed mutagenesis. J. Biol. Chem. 275, 34393–34398 [DOI] [PubMed] [Google Scholar]
- 19. Stöckl M., Fischer P., Wanker E., Herrmann A. (2008) α-Synuclein selectively binds to anionic phospholipids embedded in liquid-disordered domains. J. Mol. Biol. 375, 1394–1404 [DOI] [PubMed] [Google Scholar]
- 20. Bodner C. R., Maltsev A. S., Dobson C. M., Bax A. (2010) Differential phospholipid binding of α-synuclein variants implicated in Parkinson disease revealed by solution NMR spectroscopy. Biochemistry 49, 862–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abeliovich A., Schmitz Y., Fariñas I., Choi-Lundberg D., Ho W. H., Castillo P. E., Shinsky N., Verdugo J. M., Armanini M., Ryan A., Hynes M., Phillips H., Sulzer D., Rosenthal A. (2000) Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25, 239–252 [DOI] [PubMed] [Google Scholar]
- 22. Cabin D. E., Shimazu K., Murphy D., Cole N. B., Gottschalk W., McIlwain K. L., Orrison B., Chen A., Ellis C. E., Paylor R., Lu B., Nussbaum R. L. (2002) Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking α-synuclein. J. Neurosci. 22, 8797–8807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murphy D. D., Rueter S. M., Trojanowski J. Q., Lee V. M. (2000) Synucleins are developmentally expressed, and α-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 20, 3214–3220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pronin A. N., Morris A. J., Surguchov A., Benovic J. L. (2000) Synucleins are a novel class of substrates for G protein-coupled receptor kinases. J. Biol. Chem. 275, 26515–26522 [DOI] [PubMed] [Google Scholar]
- 25. Gorbatyuk O. S., Li S., Sullivan L. F., Chen W., Kondrikova G., Manfredsson F. P., Mandel R. J., Muzyczka N. (2008) The phosphorylation state of Ser-129 in human α-synuclein determines neurodegeneration in a rat model of Parkinson disease. Proc. Natl. Acad. Sci. U.S.A. 105, 763–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Azeredo da Silveira S., Schneider B. L., Cifuentes-Diaz C., Sage D., Abbas-Terki T., Iwatsubo T., Unser M., Aebischer P. (2009) Phosphorylation does not prompt, nor prevent, the formation of α-synuclein toxic species in a rat model of Parkinson disease. Hum. Mol. Genet. 18, 872–887 [DOI] [PubMed] [Google Scholar]
- 27. McFarland N. R., Fan Z., Xu K., Schwarzschild M. A., Feany M. B., Hyman B. T., McLean P. J. (2009) α-Synuclein S129 phosphorylation mutants do not alter nigrostriatal toxicity in a rat model of Parkinson disease. J. Neuropathol. Exp. Neurol. 68, 515–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen L., Feany M. B. (2005) α-Synuclein phosphorylation controls neurotoxicity and inclusion formation in a Drosophila model of Parkinson disease. Nat. Neurosci. 8, 657–663 [DOI] [PubMed] [Google Scholar]
- 29. Kuwahara T., Koyama A., Koyama S., Yoshina S., Ren C. H., Kato T., Mitani S., Iwatsubo T. (2008) A systematic RNAi screen reveals involvement of endocytic pathway in neuronal dysfunction in α-synuclein transgenic C. elegans. Hum. Mol. Genet. 17, 2997–3009 [DOI] [PubMed] [Google Scholar]
- 30. Brenner S. (1974) The genetics of Caenorhabditis elegans. Genetics 77, 71–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lewis J. A., Fleming J. T. (1995) Basic culture methods. Methods Cell Biol. 48, 3–29 [PubMed] [Google Scholar]
- 32. Giasson B. I., Jakes R., Goedert M., Duda J. E., Leight S., Trojanowski J. Q., Lee V. M. (2000) A panel of epitope-specific antibodies detects protein domains distributed throughout human α-synuclein in Lewy bodies of Parkinson disease. J. Neurosci. Res. 59, 528–533 [DOI] [PubMed] [Google Scholar]
- 33. Saito Y., Kawashima A., Ruberu N. N., Fujiwara H., Koyama S., Sawabe M., Arai T., Nagura H., Yamanouchi H., Hasegawa M., Iwatsubo T., Murayama S. (2003) Accumulation of phosphorylated α-synuclein in aging human brain. J. Neuropathol. Exp. Neurol. 62, 644–654 [DOI] [PubMed] [Google Scholar]
- 34. Fortin D. L., Troyer M. D., Nakamura K., Kubo S., Anthony M. D., Edwards R. H. (2004) Lipid rafts mediate the synaptic localization of α-synuclein. J. Neurosci. 24, 6715–6723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lin K., Dorman J. B., Rodan A., Kenyon C. (1997) daf-16: an HNF-3/forkhead family member that can function to double the lifespan of Caenorhabditis elegans. Science 278, 1319–1322 [DOI] [PubMed] [Google Scholar]
- 36. Lin K., Hsin H., Libina N., Kenyon C. (2001) Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat. Genet. 28, 139–145 [DOI] [PubMed] [Google Scholar]
- 37. Fiske M., Valtierra S., Solvang K., Zorniak M., White M., Herrera S., Konnikova A., Brezinsky R., Debburman S. (2011) Contribution of alanine-76 and serine phosphorylation in α-synuclein membrane association and aggregation in yeasts. Parkinsons Dis. 2011, 392180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lee M. K., Stirling W., Xu Y., Xu X., Qui D., Mandir A. S., Dawson T. M., Copeland N. G., Jenkins N. A., Price D. L. (2002) Human α-synuclein-harboring familial Parkinson disease-linked Ala-53 → Thr mutation causes neurodegenerative disease with α-synuclein aggregation in transgenic mice. Proc. Natl. Acad. Sci. U.S.A. 99, 8968–8973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Unger E. L., Eve D. J., Perez X. A., Reichenbach D. K., Xu Y., Lee M. K., Andrews A. M. (2006) Locomotor hyperactivity and alterations in dopamine neurotransmission are associated with overexpression of A53T mutant human α-synuclein in mice. Neurobiol. Dis. 21, 431–443 [DOI] [PubMed] [Google Scholar]
- 40. Conway K. A., Lee S. J., Rochet J. C., Ding T. T., Williamson R. E., Lansbury P. T., Jr. (2000) Acceleration of oligomerization, not fibrillization, is a shared property of both α-synuclein mutations linked to early-onset Parkinson disease: implications for pathogenesis and therapy. Proc. Natl. Acad. Sci. U.S.A. 97, 571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Volles M. J., Lansbury P. T., Jr. (2002) Vesicle permeabilization by protofibrillar α-synuclein is sensitive to Parkinson disease-linked mutations and occurs by a pore-like mechanism. Biochemistry 41, 4595–4602 [DOI] [PubMed] [Google Scholar]
- 42. Jo E., McLaurin J., Yip C. M., St George-Hyslop P., Fraser P. E. (2000) α-Synuclein membrane interactions and lipid specificity. J. Biol. Chem. 275, 34328–34334 [DOI] [PubMed] [Google Scholar]
- 43. Paleologou K. E., Schmid A. W., Rospigliosi C. C., Kim H. Y., Lamberto G. R., Fredenburg R. A., Lansbury P. T., Jr., Fernandez C. O., Eliezer D., Zweckstetter M., Lashuel H. A. (2008) Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of α-synuclein. J. Biol. Chem. 283, 16895–16905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Visanji N. P., Wislet-Gendebien S., Oschipok L. W., Zhang G., Aubert I., Fraser P. E., Tandon A. (2011) Effect of Ser-129 phosphorylation on interaction of α-synuclein with synaptic and cellular membranes. J. Biol. Chem. 286, 35863–35873 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45. Bartels T., Choi J. G., Selkoe D. J. (2011) α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477, 107–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Auluck P. K., Caraveo G., Lindquist S. (2010) α-Synuclein: membrane interactions and toxicity in Parkinson disease. Annu. Rev. Cell Dev. Biol. 26, 211–233 [DOI] [PubMed] [Google Scholar]
- 47. Cooper A. A., Gitler A. D., Cashikar A., Haynes C. M., Hill K. J., Bhullar B., Liu K., Xu K., Strathearn K. E., Liu F., Cao S., Caldwell K. A., Caldwell G. A., Marsischky G., Kolodner R. D., Labaer J., Rochet J. C., Bonini N. M., Lindquist S. (2006) α-Synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson models. Science 313, 324–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gitler A. D., Bevis B. J., Shorter J., Strathearn K. E., Hamamichi S., Su L. J., Caldwell K. A., Caldwell G. A., Rochet J. C., McCaffery J. M., Barlowe C., Lindquist S. (2008) The Parkinson disease protein α-synuclein disrupts cellular Rab homeostasis. Proc. Natl. Acad. Sci. U.S.A. 105, 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ruan Q., Harrington A. J., Caldwell K. A., Caldwell G. A., Standaert D. G. (2010) VPS41, a protein involved in lysosomal trafficking, is protective in Caenorhabditis elegans and mammalian cellular models of Parkinson disease. Neurobiol. Dis. 37, 330–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lee H. J., Choi C., Lee S. J. (2002) Membrane-bound α-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J. Biol. Chem. 277, 671–678 [DOI] [PubMed] [Google Scholar]
- 51. Perrin R. J., Woods W. S., Clayton D. F., George J. M. (2001) Exposure to long chain polyunsaturated fatty acids triggers rapid multimerization of synucleins. J. Biol. Chem. 276, 41958–41962 [DOI] [PubMed] [Google Scholar]
- 52. Ogg S., Paradis S., Gottlieb S., Patterson G. I., Lee L., Tissenbaum H. A., Ruvkun G. (1997) The Forkhead transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature 389, 994–999 [DOI] [PubMed] [Google Scholar]
- 53. Kenyon C. J. (2010) The genetics of ageing. Nature 464, 504–512 [DOI] [PubMed] [Google Scholar]
- 54. Kanao T., Venderova K., Park D. S., Unterman T., Lu B., Imai Y. (2010) Activation of FoxO by LRRK2 induces expression of proapoptotic proteins and alters survival of postmitotic dopaminergic neuron in Drosophila. Hum. Mol. Genet. 19, 3747–3758 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.