Background: ERα36 is present in ERα-negative breast cancer and mediates rapid responses.
Results: Estrogen promoted cell survival and increased metastatic factors in breast cancer through membrane ERα36.
Conclusion: ERα36 plays a major role in estrogen responses of ERα-negative breast cancers.
Significance: Examining the role of ERα36 in ERα-negative breast cancer is essential for understanding the negative effects of estrogen in breast cancer.
Keywords: Apoptosis, Breast Cancer, Estrogen, Estrogen Receptor, Metastasis, Plasma Membrane, Proliferation, Protein Kinase C (PKC), ER-alpha36, ER-negative
Abstract
Protein kinase C (PKC) signaling can be activated rapidly by 17β-estradiol (E2) via nontraditional signaling in ERα-positive MCF7 and ERα-negative HCC38 breast cancer cells and is associated with tumorigenicity. Additionally, E2 has been shown to elicit anti-apoptotic effects in cancer cells counteracting pro-apoptotic effects of chemotherapeutics. Supporting evidence suggests the existence of a membrane-associated ER that differs from the traditional receptors, ERα and ERβ. Our aim was to identify the ER responsible for rapid PKC activation and to evaluate downstream effects, such as proliferation, apoptosis, and metastasis. RT-PCR, Western blot, and immunofluorescence were used to determine the presence of ER splice variants in multiple cell lines. E2 effects on PKC activity were measured with and without ER-blocking antibodies. Cell proliferation was determined by [3H]thymidine incorporation, and cell viability was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, (MTT) whereas apoptosis was determined by DNA fragmentation and TUNEL. Quantitative RT-PCR and sandwich ELISA were used to determine the effects on metastatic factors. The role of membrane-dependent signaling in cancer cell invasiveness was examined using an in vitro assay. The results indicate the presence of an ERα splice variant, ERα36, in ERα-positive MCF7 and ERα-negative HCC38 breast cancer cells, which localized to plasma membranes and rapidly activated PKC in response to E2, leading to deleterious effects such as enhancement of proliferation, protection against apoptosis, and enhancement of metastatic factors. These findings propose ERα36 as a novel target for the development of therapies that can prevent progression of breast cancer in the primary tumor as well as during metastasis.
Introduction
The complexities of breast cancer growth and metastasis present several problems in development of treatments for patients. The main screening process in determining the treatment and prognosis of breast cancer patients is receptor status. Growth of estrogen receptor (ER)2-positive breast cancers is typically enhanced by estrogen, but ER interactions with DNA are not necessary for this growth to occur (1, 2), suggesting that non-nuclear actions of ERs may play a role. Triple negative breast cancers, which are ER-negative, progesterone receptor-negative, and human epidermal growth factor receptor 2 (HER2)-negative, are typically characterized as more aggressive and less responsive to hormone treatments (3). These tumors are also less responsive to treatments such as tamoxifen, a commonly used estrogen antagonist, which reduces tumor aggressiveness in ER-positive breast cancer and prevents recurrence of cancer after chemotherapy or radiotherapy (4). However, estrogen may have some effects in ER-negative breast cancers that are not fully understood, and with the discovery of novel splice variants of traditional ERs (5, 6), patients with ER-negative breast cancer should not be assumed as nonresponsive to treatments typical for ER-positive breast cancer patients.
Steroid hormone receptors traditionally function as transcription factors upon ligand binding, but many studies have identified them in plasma membranes and have shown that they can rapidly activate signal transduction pathways, leading to events such as increased proliferation or attenuated apoptosis (7). Estrogens, in particular, elicit many of their effects on cells through classical steroid hormone receptor mechanisms involving two primary receptor classes, ERα and ERβ.
It is now understood that 17β-estradiol (E2) exerts some of its effects via signaling mechanisms other than traditional nuclear receptor-mediated pathways (1, 8–10). Several studies have reported the presence of both ERα and ERβ in plasma membranes (1, 7), including truncated forms of the receptors, suggesting that they may be involved in membrane-mediated effects of the hormone. This is supported by studies using E2 conjugated to bovine serum albumin (E2-BSA), which cannot pass through the plasma membrane and reach the nuclear receptor, yet it elicits many of the same effects as E2 (11–13). E2 and E2-BSA rapidly increase protein kinase C (PKC)-specific activity without new gene expression or protein synthesis, and antibodies to nuclear and cytosolic ERα and ERβ as well as activators and inhibitors of classical ERs do not block this effect (13). However, which one of the ER isoforms is responsible is not known. Moreover, rapid responses to E2 and E2-BSA are seen in ERα-negative HCC38 breast cancer cells as well as in ERα-positive MCF7 cells (13), suggesting that either ERβ is responsible or that another ERα isoform mediates the effects of E2.
There are multiple isoforms of ERα as follows: the traditional ERα66 (66 kDa) and a lower molecular weight variant ERα46. Both ERα66 and ERα46 are localized to the nucleus upon ligand binding (14). Recently, a novel ERα variant was discovered with a molecular mass of ∼36 kDa (ERα36) (14–16). It differs from ERα66 by lacking both transcriptional activation domains (AF1 and AF2) (exons 7 and 8) but retains the DNA-binding domain and partial dimerization and ligand-binding domains (14). It also contains a novel exon 9, which encodes 27 amino acids of unknown function. ERα36 was shown to be involved in estrogen-stimulated MAPK (ERK) activation in HEK293 cells in which the receptor was overexpressed, as well as in testosterone-stimulated ERK and Akt activation in endometrial cancer cells (16), raising the possibility that this ERα isoform is involved.
The G-protein-coupled receptor GPR30 has also been shown to mediate non-nuclear responses of estrogen and has been reported as an alternative membrane receptor for E2 (17). Kang et al. (18) reported that GPR30 is not responsible for nongenomic signaling of estrogen in the context of rapid enzyme activation such as ERK1 and ERK2. In addition, we show in this study that GPR30 does not play a role in membrane-associated E2-dependent cell proliferation, but it is not known if GPR30 mediates other responses related to apoptosis or metastasis, and further examination is needed.
The purpose of this study was to evaluate the role of ERα36 in membrane-associated estrogen signaling in breast cancer. We hypothesized that ERα36-associated E2 membrane signaling in breast cancer cells leads to enhanced cancer cell survival by promoting proliferation, protecting against apoptosis, and stimulating downstream gene expression associated with enhanced tumorigenicity and metastasis. The main goal of this investigation was to help us gain a greater understanding of the underlying mechanisms of breast cancer tumor aggression and invasion, providing us with new knowledge vital in the development of novel treatments to control breast cancer growth and metastasis.
EXPERIMENTAL PROCEDURES
Reagents
ERα-positive MCF7 and ERα-negative HCC38 human breast cancer cells as well as SkBr3, COS7, and HeLa cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). The PKC assay kit was purchased from GE Healthcare. Minimal essential media (MEM) and Roswell Park Memorial Institute 1640 media (RPMI 1640) were purchased from Invitrogen. Charcoal/dextran-filtered fetal bovine serum was purchased from HyClone (Logan, UT). E2, E2-BSA, and taxol (paclitaxel) were purchased from Sigma. Chelerythrine, a PKC inhibitor, was purchased from EMD Chemicals (Gibbstown, NJ). Protein content of samples was measured using the Macro BCA reagent kit from Pierce/Thermo Scientific (Rockford, IL). Primers were purchased from Eurofins (Des Moines, IA). Reverse transcription and PCR reagents were purchased from Bio-Rad. Quantitative RT-PCR reagents were purchased from Applied Biosystems (Carlsbad, CA). [32P]ATP and [3H]thymidine were obtained from PerkinElmer Life Sciences. Polyclonal ERα66 and ERα36 antibodies were purchased from Chi Scientific (Maynard, MA). Polyclonal ERβ and monoclonal glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies were obtained from Millipore (Billerica, MA). Polyclonal antibodies to caveolin-1 and GPR30 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal antibodies to cytochrome C oxidase (COX) IV were from Abcam (Cambridge, MA). Goat anti-rabbit horseradish peroxidase (HRP) and goat anti-mouse HRP-conjugated secondary antibodies were obtained from Bio-Rad. Goat anti-rabbit Alexa 488, goat anti-rabbit Alexa 594, Hoechst 4322 (nuclear stain), and Select FX 488 endoplasmic reticulum stain were purchased from Molecular Probes (Carlsbad, CA).
Cell Culture
All cells (SkBr3, MDA-MB-231, HEK293, and COS7) were cultured in appropriate media as specified by the ATCC containing 10% charcoal/dextran-filtered FBS and lacking phenol red, which can mimic the effects of E2 at low levels. MCF7 cells were maintained in minimum Eagle's-based media, and HCC38 cells were maintained in RPMI 1640-based media.
Presence of ER Isoforms
To determine whether ERα36 is expressed in both ERα-positive MCF7 and ERα-negative HCC38 breast cancer cells, we designed sequence-specific primers that would selectively identify the three known alternative splicing variants of ERα: ERα66, ERα46, and ERα36. Primers used are shown in Table 1. Because of the sequence homology of ERα66 and ERα46, we could not identify ERα46 mRNA independent of ERα66. However, because of the existence of exon 9, which is not expressed in ERα66 or ERα46 (14), we successfully designed primers that spanned this exon to analyze expression of ERα36.
TABLE 1.
RT-PCR primer sequences for ERα splice variants
Vector NTI software was used to determine alignment of the three alternatively spliced variants for ERαas follows: ERα66, ERα46, and ERα36. We determined that it is not possible to distinguish ERα46 from ERα66; however, it is possible to recognize ERα66 from both smaller isoforms. Primers were designed for ERα66 that spanned exon 1 in its mRNA. Primers for ERα46 and ERα66, designated for ERα46/66, spanned exons 7 and 8, which are both found in ERα46 and ERα66. Finally, primers for ERα36 were designed to span exon 9.
| Target | Primer direction | Sequence (5′ to 3′) |
|---|---|---|
| ERα66 | Sense | TGCCTGGAGTGATGTTTAAGC |
| Antisense | ACGGGAGCAAGTGCAGTC | |
| ERα46/66 | Sense | CCACACGGTTCAGATAATCC |
| Antisense | ATCCCTTTGGCTGTTCCC | |
| ERα36 | Sense | GTGGTTTCCTCGTGTCTAAAGC |
| Antisense | GGTGTTGAGTGTTGGTTGCC |
RNA was extracted from cells using the TRIzol method. Reverse transcription was performed to produce cDNA for various ERs from both MCF7 and HCC38 cells as well as several other cell lines reported as lacking ERα, including SkBr3, MDA-MB-231, HEK293, and COS7 (19–21). PCR was then performed to determine whether mRNAs for the various alternative splice variants of ERα were expressed in these cells.
Western blots of isolated membrane fractions were used to determine the subcellular location of ERα36. For Western blots of whole cell lysates, cells were lysed with RIPA buffer containing 5 mm Nonidet P-40 (Sigma). We first performed Western blots using unfiltered lysates from MCF7, HCC38, and COS7 cells, which are derived from a non-human primate embryonic kidney cell line and reported to not contain ERs (21). Whole cell lysates were filtered with a molecular mass cutoff of 100 kDa prior to separation on 4–20% SDS-polyacrylamide gels. In addition, cell lysates were fractionated according to the method previously described by Smart et al. (22) to obtain crude nuclear, plasma membrane, and pure caveolae fractions.
Following SDS-PAGE of the isolated membrane fractions, protein was transferred onto nitrocellulose. Membranes were blocked with 2% bovine serum albumin in 1× phosphate-buffered saline (PBS) containing 0.05% Tween 20 (Sigma) and probed with primary antibodies. Secondary HRP-conjugated antibodies were used for detection by chemiluminescence using the West Pico chemiluminescence substrate kit (Pierce).
Immunofluorescence microscopy was used to identify the subcellular localization of ERα66 and ERα36 in MCF7 cells. Cells were cultured on 4-well chamber slides. At the time of harvest, cells were fixed with 4% paraformaldehyde. Some samples were then permeabilized with 0.01% Triton X-100 for 10 min and subsequently stained for ERs using specific antibodies. These samples were imaged with a Leica DMLB fluorescent microscope using a Hamamatsu Orca camera. To determine whether ERα36 colocalizes to lipid rafts and caveolae, cells fixed in chamber slides were either pre-stained for lipid rafts (Vybrant lipid raft labeling kit, Invitrogen) and/or probed with antibodies against ERα36 and protein-disulfide isomerase (endoplasmic reticulum) followed by incubation with fluorescent-tagged secondary antibodies (488 and 594 nm). All samples were treated with Hoechst dye for nuclear staining (333 nm). These samples were then imaged using a Nikon LSM510 confocal laser-scanning microscope, and multiple Z-slices were obtained to obtain representative images.
PKC Activity
To determine whether ERα36 mediated the stimulatory effect of E2 on PKC activity, MCF7 and HCC38 cells were cultured to confluence in 24-well tissue culture-treated plates. Cells were pretreated with antibodies against ERα36 followed by treatment with E2 for 9 min. Cells were washed two times in cold 1× PBS and lysed using RIPA buffer at time of harvest. Samples were then aliquoted and assayed for protein content by Macro BCA protein assay (Pierce) and measurement of PKC activation using the Biotrak protein kinase C assay kit (GE Healthcare).
HCC38 cultures were also treated with 1 mm methyl β-cyclodextrin for 30 min to deplete the membranes of cholesterol, thereby disrupting the caveolae and lipid rafts (23). The cells were then treated with E2 for 9 min, and protein content and PKC activity were determined as described.
Cell Proliferation, Viability, and Apoptosis
To determine the consequences of rapid activation of PKC in the context of breast cancer cell survival, we performed assays to determine the effect of inhibiting PKC on apoptosis and proliferation. PKC activity was inhibited using chelerythrine as described previously (13). Confluent cultures of HCC38 cells were cultured in the presence of 0.1, 1.0, and 10 μm chelerythrine for 24 h, and MTT was measured. In addition, HCC38 cells were treated with 5 and 7.5 mm phosphate to induce apoptosis, as we have previously shown that phosphate induces apoptosis in other cell types (24). We previously showed that tamoxifen, an ER agonist that inhibits PKC, blocks the stimulatory effects of E2 on proliferation. Finally, to determine whether ERα36 mediates the anti-apoptotic effect of E2, we took advantage of the observation that E2 inhibits taxol-induced apoptosis via membrane-associated signaling (25). Subconfluent cultures of HCC38 cells were pretreated with E2 for 90 min followed by taxol treatment for 4 h, after which cells were assays for caspase-3 activity and TUNEL.
Cell proliferation was assessed as a function of [3H]thymidine incorporation, as described previously (13). Subconfluent cultures of HCC38 cells were made quiescent by starvation for 48 h prior to treatment in starvation media containing 0.1% charcoal/dextran-filtered FBS. Cells were then treated with varying concentrations of E2 or E2-BSA for 24 h. In addition, cells were treated with antibodies to ERα36 and GPR30. At 20 h after the start of treatment time, 1.0 μCi/ml of [3H]thymidine was added to all samples for the remainder of the treatment. At 24 h, media were removed, and cells were washed two times with 1× PBS. Cells were then fixed by washing three times with cold 5% trichloroacetic acid (TCA). During the third wash, the TCA was left on the cells for 30 min at 4 °C. After fixation, the TCA was removed, and the cell layers were allowed to dry at which point 100 μl of 1% SDS was added. The cell layers were then scraped and transferred to glass scintillation vials for measurement of radioactive decay (dpm) to determine relative incorporation of [3H]thymidine.
The number of viable cells was determined by the MTT assay, which measures the reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (Sigma) to purple formazan by mitochondrial reductase of living cells. To determine the role of PKC signaling in breast cancer cell viability, confluent cultures of HCC38 cells were treated with the PKC inhibitor chelerythrine (10−7, 10−6, and 10−5 m) for 24 h, and MTT activity and DNA fragmentation were measured. To determine whether signaling via ERα36 could protect breast cancer cells against apoptosis, HCC38 cells were treated with E2 and E2-BSA in conjunction with taxol, which induces cell death in breast cancer cells (26). Apoptosis was assessed using DNA fragmentation as described previously (27). TUNEL assay was used to determine the protective effect of E2 against taxol-induced cell death. In addition, caspase-3 activity was measured using the CaspAce assay kit from Promega (Madison, WI).
E2 Effects on Factors That Promote Bone Metastasis
Breast cancer has a particular affinity for metastasizing to bone (28, 29). Therefore, we measured the effects of E2 and E2-BSA on expression of factors associated with bone metastasis at 12 h. Quantitative RT-PCR was used to determine the quantitative effect of E2 on expression of receptor activator of nuclear factor-κB ligand (RANKL), Snail1, and e-cadherin (CDH1) in HCC38 cells. In addition, we assessed expression of CXC chemokine receptor type 4 (CXCR4), which is a receptor for the chemotactic ligand, stromal cell-derived factor-1 (SDF1) that is present in high levels in bone (30), syndecan-4, which is involved cell interaction with matrix (31), and matrix metalloproteinase-9 (MMP9), which has been shown to be regulated through signaling of ERα (31). We also examined the effects of E2-BSA on secretion of factors related to osteoclast activation and inhibition. Sandwich ELISAs were used to measure levels of osteoprotegerin (OPG) and interleukin-6 (IL6) (R&D Systems, Minneapolis, MN) in the conditioned media 24 h after treatment with E2 or E2-BSA.
Scratch-wound Assay to Measure in Vitro Breast Cancer Cell Invasion
A 10-μl micropipette tip was used to create scratch-wounds in confluent cultures of HCC38 cells. After washing with media to remove detached cells and debris, the cultures were treated with E2-BSA, anti-ERα36 antibody, or both. Phase-contrast images were obtained at the beginning of the assay as well as at 6- and 12-h increments until all wounds were healed. MatLab was used to quantify the mean diameter of the wounds over time, and data are presented as percent wound closure.
Statistical Analyses
For all experiments, statistical analyses were performed by analysis of variance with Bonferroni's correction for multiple comparisons at a significance level of 0.05. Identification of symbols to signify statistical significance is found in the respective figure legends.
RESULTS
ERα36 Is Expressed in Both ERα-positive and ERα-negative Cells
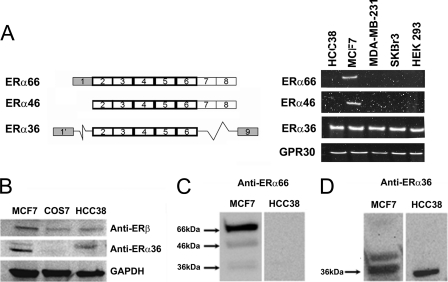
Fig. 1A shows the different exons expressed in the mRNA of the three known splice variants of ERα. RT-PCR analysis showed expression of all ERα variants in MCF7 cells. HCC38 cells expressed only ERα36; neither ERα46 nor ERα66 was detected by RT-PCR. We performed a screen of more than 20 human cell lines for the expression of ERα36 and did not find a suitable negative control for ERα36 expression (data not shown). MDA-MB-231, SkBr3, and HEK293 cells exhibited expression of only ERα36 but not the other variants of ERα (Fig. 1, A and B). COS7 African green monkey embryonic kidney cells, although they do not express ERα36, are not a relevant negative control for our studies as they are not human cells, and they are of embryonic origin. All cells expressed GPR30.
FIGURE 1.
mRNA expression and protein presence of ERα splice variants in MCF7 and HCC38 cells. A, different cell types show variable expression of ERα splicing variants. MCF7 express all variants, although ERα-negative HCC38, MDA-MB-231, SkBr3, and HEK293 cells only express ERα36. B, Western blots of unfiltered cell lysates verify protein presence of ERβ and ERα36 in MCF7 and HCC38 cells but no ERα36 in COS7 cells. Filtered lysates of MCF7 and HCC38 cells clearly show presence of ERα66 (C) and ERα36 (D) by Western blot, although HCC38 cells only show the presence of ERα36.
Western blot analysis confirmed the presence of ERα36 in both MCF7 and HCC38 cell lines but not in COS7 cells, and ERβ was detected in lysates from MCF7, COS7, and HCC38 cells (Fig. 1B). Because we are limited to using polyclonal antibodies to identify ERα66 separately from ERα36 in this study, and we noticed nonspecific bands above 100 kDa in blots of our unfiltered lysates, we performed Western analysis on filtered lysates to verify the specificity of the ERα36 antibody. This allowed us to look more closely at the recognition bands from 36 to 66 kDa. The antibodies to ERα66 recognized three bands of varying intensity on blots of MCF7 cells but did not recognize a single band in HCC38 cells (Fig. 1C). Polyclonal antibodies raised against the unique C-terminal end of ERα36 recognized two bands in MCF7 cells (Fig. 1C), but they only recognized a single band at ∼37 kDa in HCC38 cells (Fig. 1D). The presence of a second band in the MCF7 cell lysate does not appear to be ERα46 but more likely may be representative of a post-translationally modified form of ERα36.
Rapid E2-induced PKC Activation in MCF7 and HCC38 Cells Occurs via Membrane Signaling through ERα36
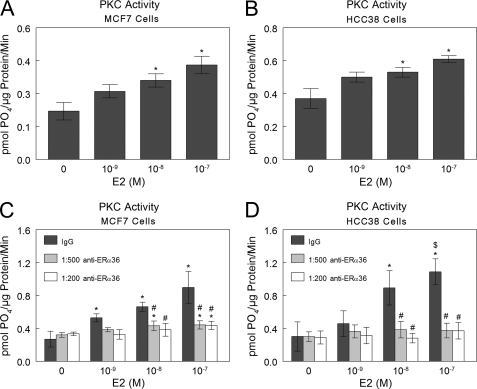
E2 caused a dose-dependent increase in PKC activity in MCF7 cells (Fig. 2A) and in HCC38 cells (Fig. 2B) at 9 min. Antibodies to ERα36 completely abolished this effect in both cell types, and a nonspecific IgG had no effect (Fig. 2, C and D).
FIGURE 2.
E2-induced rapid activation of PKC in MCF7 and HCC38 cells occurs through ERα36. E2 rapidly induces PKC activity within 9 min in both MCF7 and HCC38 cells (A and B), and antibody blocking of ERα36 on the cell membranes abolishes this effect (C and D). *, p < 0.05 compared with 0 m E2; $, p < 0.05 compared with 10−9 m E2; #, p < 0.05 compared with IgG.
PKC Maintains Cell Survival in HCC38 Cells
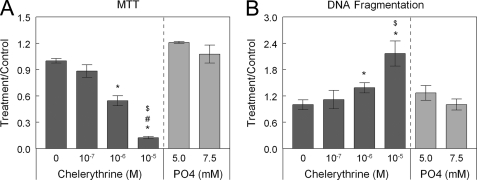
Chelerythrine caused a dose-dependent decrease in MTT activity in HCC38 cells (Fig. 3A), indicating decreased cell viability. In addition, chelerythrine caused a dose-dependent increase in DNA fragmentation (Fig. 3B), indicating that inhibition of PKC promotes apoptosis of HCC38 cells.
FIGURE 3.
Inhibition of PKC with chelerythrine causes dose-dependent decreases in MTT (A) and dose-dependent increases in DNA fragmentation (B). Phosphate, which induces apoptosis in other cell types such as chondrocytes, does not induce apoptosis in breast cancer cells. *, p < 0.05 compared with 0 m chelerythrine; $, p < 0.05 compared with 10−7 m chelerythrine; #, p < 0.05 compared with 10−6 m chelerythrine.
E2-dependent Activation of PKC Requires Intact Caveolae
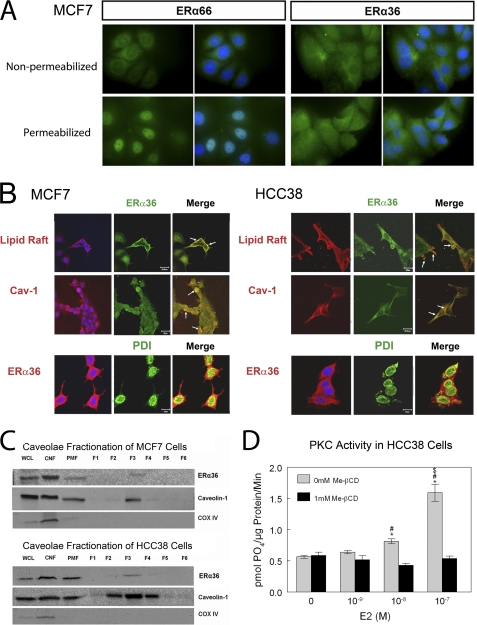
Immunofluorescence of ERα-positive MCF7 cells showed that ERα66 and ERα36 were differentially distributed. In MCF7 cells that were not permeabilized by detergent, ERα66 and ERα36 were both present on the plasma membrane (Fig. 4A). When cells were permeabilized by Triton X-100, ERα66 was mainly localized peri-nuclearly. ERα36 was primarily non-nuclear, but it was also distributed throughout the cells, although not to the same extent peri-nuclearly as ERα66.
FIGURE 4.
Immunofluorescence shows that ERα66 primarily exhibits peri-nuclear localization in MCF7 cells, although ERα36 exhibits nonspecific localization throughout the cells. Qualitatively, it appears that ERα36 exhibits greater cell surface expression than ERα66 (A). ERα36 colocalizes with lipid rafts and caveolin-1 in both MCF7 and HCC38 cells and does not appear to localize to endoplasmic reticulum in either cell (B). Caveolae fractions of both MCF7 and HCC38 cells show the existence of ERα36 in caveolae (F3) (C), and digestion of cholesterol and removal of lipid raft and caveolae from the cell membranes of HCC38 cells by β-cyclodextrin (Me-βCD) signify the requirement of caveolae for rapid signaling of E2 from the membrane (D). WCL, whole cell lysate; CNF, crude nuclear fraction; PMF, plasma membrane fraction. *, p < 0.05 compared with 0 m E2; #, p < 0.05 compared with 10−9 m E2; $, p < 0.05 compared with 10−8 m E2.
Confocal microscopy indicated that ERα36 co-localized with lipid rafts and caveolin-1 protein in both MCF7 and HCC38 cells (Fig. 4B). Staining with antibodies to protein-disulfide isomerase, which is primarily found in the endoplasmic reticulum, showed that ERα36 did not co-localize to endoplasmic reticulum. Western blots detected ERα36 and caveolin-1 in pure caveolae fractions (F3) from both cell lines (Fig. 4C). COX-IV was not found, demonstrating that the plasma membrane fractions were not contaminated with mitochondrial membranes. E2 did not stimulate PKC activity in HCC38 cells after treatment with methyl β-cyclodextrin, which destroys caveolae (Fig. 4D), indicating the importance of the specialized membrane compartment for E2-dependent PKC signaling.
Rapid E2 Signaling in HCC38 Breast Cancer Cells Protects Cells from Taxol-induced Apoptosis through ERα36 while Enhancing Proliferation
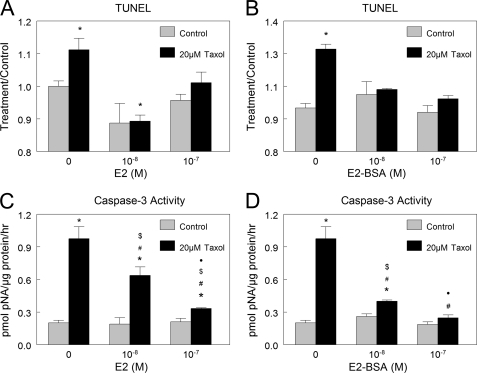
Treatment of HCC38 cells with taxol increased apoptosis (Fig. 5, A–D). When cells were pretreated with E2, the apoptotic effects of taxol were reduced, based on decreases in TUNEL (Fig. 5A) and caspase-3 activity (Fig. 4B). The fact that E2-BSA also exhibited this effect (Fig. 5, C and D) indicates that reduction of taxol-induced apoptosis by E2 was through a membrane-dependent mechanism.
FIGURE 5.
Effect of estrogen on taxol-induced apoptosis. E2 and E2-BSA protected HCC38 cells against taxol-induced apoptosis as measured by TUNEL (A and B) and caspase-3 activity (C and D). *, p < 0.05 compared with control + 0 m E2; #, p < 0.05 compared with 20 μm taxol + 0 m E2; $, p < 0.05 compared with control + 10−8 m E2; ●, p < 0.05 compared with control + 10−7 m E2.
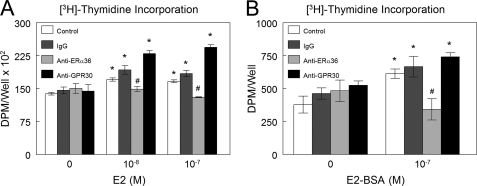
The proliferative effect of E2 also involved a membrane-associated mechanism. Both E2 and E2-BSA enhanced [3H]thymidine incorporation in HCC38 cells (Fig. 6, A and B). Treatment of the cultures with antibodies to ERα36 blocked this effect. In contrast, neither nonspecific IgG nor antibody against GPR30 reduced the effect of E2 or E2-BSA.
FIGURE 6.
Effect of estrogen through ERα36 on cell proliferation. E2 (A) and E2-BSA (B) caused an increase in DNA synthesis as measured by [3H]thymidine incorporation, and when cells were pretreated with antibody against ERα36, E2-BSA-induced cell proliferation was inhibited. *, p < 0.05 compared with control + 0 m E2; #, p < 0.05 compared with control + 10−8 m E2 or 10−7 m E2 or 10−7 m E2-BSA.
Estrogen Signaling through ERα36 Increases Expression of Factors That Can Enhance Cancer Cell Metastasis and Bone Resorption
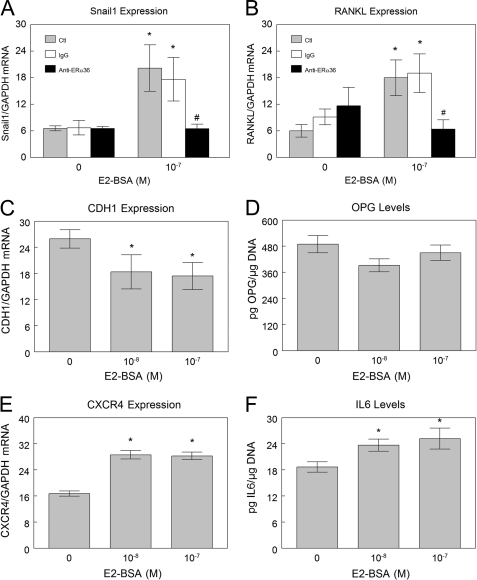
E2-BSA treatment of HCC38 cells caused increased expression of the metastatic factor Snail1, although pretreatment with antibody against ERα36 blocked this effect (Fig. 7A). At the same time, E2-BSA down-regulated e-cadherin (CDH1) (Fig. 7C) indicating that expression of factors associated with epithelial to mesenchymal transition may also be mediated by ERα36-dependent signaling. E2-BSA also had a stimulatory effect on CXCR4 expression (Fig. 7E). We also saw an inhibitory effect of E2 and E2-BSA on syndecan-4 expression when normalized to GAPDH, but this was not blocked by antibodies to ERα36 (supplemental Fig. 1). We did not see any effect of estrogen on MMP9 expression under these experimental conditions (data not shown). E2-BSA increased expression of the osteoclast activator RANKL by an ERα36-dependent mechanism, based on inhibition of the effect by anti-ERα36 antibodies (Fig. 7B). E2-BSA had no effect on osteoprotegerin production (Fig. 7D). Levels of this RANKL decoy receptor remained unchanged. Interestingly, E2 treatment led to a slight decrease in osteoprotegerin levels (data not shown), potentially via a mechanism not mediated by ERα36. Finally, E2-BSA increased production of interleukin-6 (Fig. 7F).
FIGURE 7.
Membrane estrogen signaling via ERα36 enhances expression of factors that can enhance metastasis of breast cancer cells. Quantitative RT-PCR analysis indicates that E2 signaling in HCC38 cells increases expression of Snail1 (A), and a correlating down-regulation of E-cadherin expression is also observed (C). E2-BSA also up-regulates expression of CXCR4, the chemokine receptor for SDF-1 (E). E2-BSA signaling via ERα36 enhances expression of osteoclastogenic factors. Quantitative RT-PCR analysis indicates that E2 signaling in HCC38 cells increases expression of RANKL through ERα36 at the membrane (B) with no observed change in osteoprotegerin (OPG) production (D), although IL-6 production is enhanced (F). Ctl, control. *, p < 0.05 compared with control + 0 m E2-BSA; #, p < 0.05 compared with control + 10−7 m E2-BSA.
Estrogen Signaling through Membrane-associated ERα36 Promotes in Vitro Invasiveness of HCC38 Breast Cancer Cells
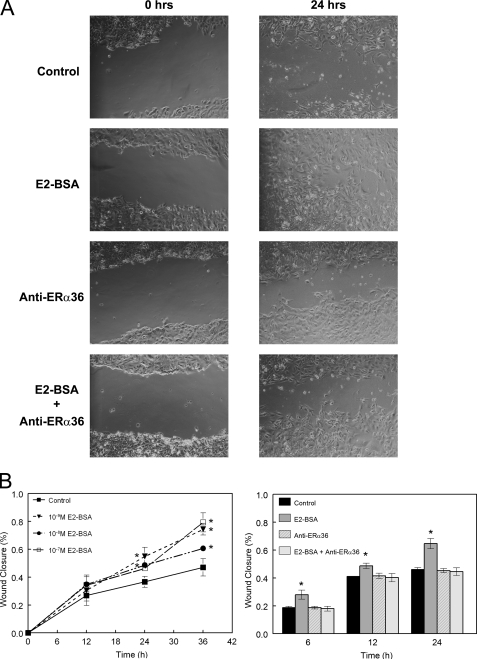
Using the scratch-wound method, we observed that E2-BSA treatment of HCC38 cells caused more rapid closure of the wound created in a two-dimensional culture (Fig. 8A). Furthermore, we found that treatment with antibody against ERα36 inhibited this effect (Fig. 8B).
FIGURE 8.
Membrane estrogen signaling via ERα36 promotes invasiveness of breast cancer cells in vitro. Results of the scratch-wound assay indicate that E2 signaling in HCC38 cells through membrane-associated ERα36 can lead to more rapid closure of the wound created in the culture. A shows select phase-contrast images of representative samples from control samples, E2-BSA-treated samples, anti-ERα36-treated samples, and samples treated with both. B shows time course data of samples treated with increasing concentrations of E2-BSA and samples treated as described in A. *, p < 0.05 compared with control.
DISCUSSION
This study exhibits that ERα-negative HCC38 breast cancer cells do in fact express ERα36 and that rapid activation of PKC in these cells in response to E2 occurs through membrane-associated ERα36. It is unknown if ERα36 is expressed in all cancers; however, the findings presented in this study open the possibility that many ERα-negative breast cancers may actually express ERα36. In fact, the HCC38 cell line used in this study is triple-negative (13, 32) and therefore does not express progesterone receptor or HER-2, suggesting that triple-negative breast cancers may also express this specific splice variant of ER. Although MDA-MB-231 and SkBr3 breast cancer cells have been cited in the literature as ERα-negative (33), our results show that even these cells express ERα36, which further supports the claim that ERα36 has a function in ER-negative breast cancers. We did however see that the COS7 cell line did not contain protein for ERα36, but this may be due to the fact that this cell line is derived from a non-human primate, and we can account for this based on inter-species differences as well as the fact that the cell line is of embryonic origin.
GPR30 is a very well studied alternative receptor for estrogen that remains controversial as far as its subcellular localization is concerned (18, 34, 36, 37). We saw that all ER-negative cells, including HCC38, expressed GPR30 mRNA. This may suggest a role of GPR30 in ER-negative breast cancer, but our results here do not indicate a role for GPR30 in breast cancer cell proliferation. The fact that the antibody against GPR30 did not block the effect of E2 nor E2-BSA on [3H]thymidine incorporation is evidence that GPR30 does not mediate the membrane-associated response of ERα66-negative cells to E2, at least where cell proliferation is concerned. However, ERα36 is responsible for mediating breast cancer cell proliferation. We did observe that when cells were treated with estrogen and pretreated with antibodies against GPR30, [3H]thymidine incorporation increased above base-line estrogen treatment. We can speculate that by blocking GPR30, we may be altering the kinetics of E2 signaling by allowing more E2 to be available for signaling through ERα36, but this requires more in depth analysis to come to this conclusion.
This study focused on nontraditional mechanisms of ERα36, as we were highly interested in the role that this protein plays in rapid membrane-initiated signaling. Our findings suggest that ERα66 plays a greater role in nuclear receptor signaling than it does in the cytosol or membrane, although ERα36 may have functions more prevalent outside of the nucleus. Localization of ERα36 to caveolae within the cell membrane suggests that ERα36 plays a major role in rapid membrane-initiated signaling. Caveolae typically house several proteins that are involved in many rapidly activated membrane-associated pathways (22, 23), such as those associated with vitamin D3 metabolites (38, 39), testosterone, and especially estrogen (39). The loss of caveolae by β-cyclodextrin treatment indicates the requirement of caveolae for these membrane-specific responses of estrogen. Because of the complex nature of caveolae and the many proteins that may be found within caveolae, it is quite possible that ERα36 does not work alone in these pathways.
As observed previously, we found that E2 rapidly activated PKC within minutes in both MCF7 and HCC38 cells. When we pretreated cells with antibodies that specifically target ERα36, the effects of E2 were completely abolished. When we account for the fact that in our previous study (13), we did not see inhibition of this PKC effect when we used antibodies to block ERα66, we can conclude that ERα36 is responsible for this effect, and this response is initiated from the membrane due to the fact that the antibodies cannot cross the membrane and are therefore targeting membrane-associated receptors.
We have found that these rapid membrane-mediated effects are associated with enhanced breast cancer cell survival. The PKC pathway appears to be crucial to the anti-apoptotic effects of estrogen in these cells as evidenced by the effect of chelerythrine on MTT and DNA fragmentation. Because we did not observe cell death in response to phosphate treatment, yet we did when we inhibited PKC with chelerythrine, we can conclude that PKC plays a major role in breast cancer cell survival and tumorigenesis.
One mechanism by which E2-dependent activation of PKC may function in cell survival is by promoting cell proliferation. We previously demonstrated that the addition of tamoxifen, an antagonist of the estrogen receptor, which actually inhibits PKC in breast cancer, caused inhibition of DNA synthesis in MCF7 and HCC38 breast cancer cells (13). Together with data from Marino et al. (40) showing that estradiol increases DNA synthesis through PKC activation, our observations support this hypothesis.
In addition, numerous studies (41–45), including a previous study by our group (13), have implicated PKC signaling as a major contributor to tumor progression. We found a strong correlation among PKC activity and tumor size and recurrence, which contained well over 100 patients. This observation indicated that patients diagnosed as ER-negative according to the current screening method may in fact respond to tamoxifen.
Because we observed that tamoxifen inhibits PKC through this specific pathway of E2, we decided to observe the effects of E2 on different pathways of other chemotherapeutics, such as taxol. Our results indicate that not only does E2 enhance cell survival by increasing cell proliferation through this membrane-mediated mechanism, as evidenced by effects of E2-BSA, but E2 also elicits anti-apoptotic effects on these cells that can oppose the effects of very commonly used chemotherapeutic agents, such as taxol. Enhancement of cell proliferation in conjunction with the anti-apoptotic effects of E2 can be implicated in primary aggressive tumor growth. The ability of estrogen to counteract the effects of taxol in these breast cancer cells poses a great problem in the treatment of the disease.
The progression of a tumor and development of metastasis is a very complex process. As the tumor continues to grow, the intracellular signaling of local factors associated with angiogenesis alleviates the requirement of a neo-vasculature. Expression of several factors such as Snail1 and Snail2 leads to a down-regulation of cell-cell interaction proteins such as cadherins, leading to what is known as an epithelial to mesenchymal transition (46–48). This alteration in cancer cell phenotype imparts the ability of the cells to detach from the primary tumor and migrate to distant sites of the body. Breast as well as prostate cancer cells appear to have a particular affinity for metastasizing to osseous tissue (28), which indicates poor prognosis for the patient. The effect of estrogen on RANK ligand in our system indicates the possibility that estrogen signaling within the primary breast tumor can enhance secretion of factors by the tumor inducing osteoclastogenesis, thereby promoting bone remodeling. This can create a “fertile” soil for the attachment of migrating cancer cells, and because bone is so highly vascularized (28), the probability of migrating cancer cells to find a location in bone on which to attach and form metastases is extremely high. Increases in chemotactic receptors such as CXCR4, as we observed, also promote migration to sites where ligands for this receptor, such as SDF-1, reside in high levels. In addition, effects of estrogen on extracellular matrix interacting components, such as membrane syndecan-4, can also promote detachment of cancer cells from the primary tumor.
Our results suggest that many breast cancer cell responses to estrogen include up-regulation of many of these factors, and the fact that ERα36 directly mediates these responses, as well as promoting in vitro invasiveness, suggests that this receptor may play a major role in breast cancer metastasis. ERα36 has even been shown to have a high association with lymph node metastasis in patients with gastric cancer (49).
Because ERα36 does retain the DNA-binding domain found in traditional ERα, it is still possible that this receptor may play some role in directly regulating gene expression; however, the fact that it does not retain either transcriptional activation domain (AF1/AF2) suggests that it may only function as a cofactor for transcription or even as a transcriptional repressor. This suggests that ERα36 may play multiple roles in regulating cell differentiation and survivability. Taking this into account with data showing different effects of estrogen signaling through ERα36 in rat costochondral resting zone chondrocytes (35), the possibility remains that ERα36 also plays different roles depending on the cell type, especially whether the cells are cancerous or not.
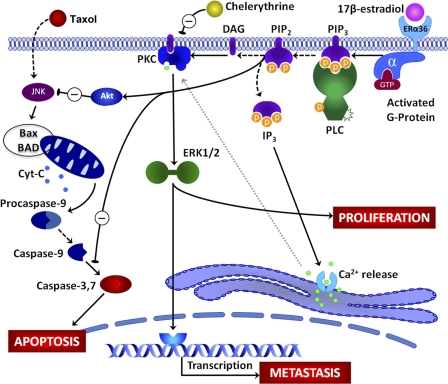
From our previous data on estrogen signaling in MCF7 and HCC38 breast cancer cells and with our current findings, we hypothesize that E2 interacts with ERα36 at the cell membrane to rapidly activate PKC through a phosphatidylinositol-specific phospholipase C-dependent mechanism that cleaves PIP2 to IP3 and DAG. IP3 promotes calcium influx from the smooth endoplasmic reticulum, and DAG helps to anchor PKC to the cell membrane, thereby promoting activation of PKC. Because PKC can activate ERK1/2 (12), which can rapidly enhance phosphorylation of proteins that promote proliferation, as well as factors that may enhance downstream gene expression, this pathway may lead to indirect effects on gene expression. This can be inhibited by chelerythrine, which directly inhibits PKC. Genes that may be affected, such as RANKL and Snail1, can promote metastatic activity of the breast cancer cells. These responses that can rapidly activate ERK1/2 can lead to diverging pathways by which PIP2 cleavage can also enhance anti-apoptotic effects of E2 possibly through activation of Akt. Taxol has been shown to activate apoptosis of breast cancer cells by enhancing activity of c-Jun kinase (JNK), which alters the ratio of the Bcl-2 family of proteins such as Bax (pro-apoptotic) and BAD (anti-apoptotic), which in turn leads to enhanced permeability of the mitochondrial membrane and release of cytochrome c (25). This eventually leads to activation of the apoptotic caspase cascade, eventually leading to activation of caspase-3 and apoptosis. This study has specifically shown that membrane-associated estrogen signaling can inhibit taxol-induced caspase-3 activity. A schematic representation of this proposed pathway is seen in Fig. 9.
FIGURE 9.
Proposed model of rapid membrane-initiated signaling of estrogen via ERα36. E2 interacts with ERα36 at the cell membrane initiating a signaling cascade consisting of phosphatidylinositol-specific phospholipase C (PLC)-dependent cleavage of PIP2 to IP3 and DAG. IP3 signals Ca2+ release from the smooth endoplasmic reticulum, although DAG anchors PKC to the membrane. The membrane localization along with Ca2+ association with PKC leads to its activation, which can be inhibited by chelerythrine, with downstream activation of ERK1/2 mitogen-activated protein kinase (MAPK). Activation of ERK1/2 can affect cell proliferation as well as phosphorylation of transcription factors that can activate or repress gene transcription, possibly leading to expression of factors associated with metastasis and tumor aggressiveness. Taxol induces apoptosis through a mechanism involving JNK MAPK and the Bcl2-associated proteins Bax and BAD, leading to release of cytochrome c from the mitochondria. This initiates the caspase cascade involving caspases-9 and -7, eventually leading to activation of caspase-3, which causes apoptosis. E2 signaling through membrane-associated receptor prevents taxol-induced apoptosis through a mechanism that may activate Akt and cause inhibition of caspase-3.
The findings of this study present a novel target for development of new therapies such as pharmaceutical intervention against progression of breast cancer primary tumor growth and prevention of metastasis. The mechanism of the membrane receptor for estrogen in breast cancer tumorigenesis and metastasis has not been previously reported. This study identifies a membrane receptor for estrogen in breast cancer cells that activates an array of pathways. We show that estrogen signaling via ERα36 initiated at the cell membrane activates cross-talk among multiple pathways important for breast cancer aggressiveness. Fig. 9 proposes a model for the mechanism by which membrane estrogen signaling through ERα36 enhances cell proliferation, anti-apoptotic effects, and metastatic effects. A drug that specifically targets the membrane-associated estrogen receptor, ERα36, may not pose harmful effects on normal estrogen processes that occur within the cells, as ERα36 cannot activate expression of genes containing traditional estrogen response elements. A multifaceted approach to treatment remains necessary as cancer, especially breast cancer, exemplifies a class of diseases rather than a single disease. Every patient is unique, but further investigation and understanding of membrane-associated signaling of estrogen in breast cancer can possibly lead to new routes of treatment for this devastating disease.
Supplementary Material
Acknowledgments
We thank Monica Liou, Christopher Hermann, Sharon Hyzy, and Srilaxmi Vemula for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants UL1 RR025008 from USPHS and TL1 RR025010 (Clinical and Translational Science Award Program) from NCRR. This work was also supported by the Price Gilbert, Jr., Foundation.

This article contains supplemental Fig. 1.
- ER
- estrogen receptor
- E2
- 17β-estradiol
- E2-BSA
- 17β-estradiol conjugated to bovine serum albumin
- RANK
- receptor activator of nuclear factor-κB
- RANKL
- RANK ligand
- CDH1
- e-cadherin
- PIP2
- phosphatidylinositol bisphosphate
- IP3
- inositol trisphosphate
- DAG
- diacylglycerol
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide.
REFERENCES
- 1. Kampa M., Pelekanou V., Castanas E. (2008) Membrane-initiated steroid action in breast and prostate cancer. Steroids 73, 953–960 [DOI] [PubMed] [Google Scholar]
- 2. DeNardo D. G., Cuba V. L., Kim H., Wu K., Lee A. V., Brown P. H. (2007) Estrogen receptor DNA binding is not required for estrogen-induced breast cell growth. Mol. Cell. Endocrinol. 277, 13–25 [DOI] [PubMed] [Google Scholar]
- 3. Dent R., Trudeau M., Pritchard K. I., Hanna W. M., Kahn H. K., Sawka C. A., Lickley L. A., Rawlinson E., Sun P., Narod S. A. (2007) Triple-negative breast cancer. Clinical features and patterns of recurrence. Clin. Cancer Res. 13, 4429–4434 [DOI] [PubMed] [Google Scholar]
- 4. Fernö M., Baldetorp B., Bendahl P. O., Borg A., Ewers S. B., Olsson H., Rydén S., Sigurdsson H., Killander D. (1995) Recurrence-free survival in breast cancer improved by adjuvant tamoxifen. Especially for progesterone receptor-positive tumors with a high proliferation. Breast Cancer Res. Treat. 36, 23–34 [DOI] [PubMed] [Google Scholar]
- 5. Madsen M. W., Reiter B. E., Lykkesfeldt A. E. (1995) Differential expression of estrogen receptor mRNA splice variants in the tamoxifen-resistant human breast cancer cell line, MCF-7/TAMR-1 compared to the parental MCF-7 cell line. Mol. Cell. Endocrinol. 109, 197–207 [DOI] [PubMed] [Google Scholar]
- 6. Poola I., Koduri S., Chatra S., Clarke R. (2000) Identification of 20 alternatively spliced estrogen receptor α mRNAs in breast cancer cell lines and tumors using splice targeted primer approach. J. Steroid Biochem. Mol. Biol. 72, 249–258 [DOI] [PubMed] [Google Scholar]
- 7. Levin E. R. (1999) Cellular functions of the plasma membrane estrogen receptor. Trends Endocrinol. Metab. 10, 374–377 [DOI] [PubMed] [Google Scholar]
- 8. Sylvia V. L., Boyan B. D., Dean D. D., Schwartz Z. (2000) The membrane effects of 17β-estradiol on chondrocyte phenotypic expression are mediated by activation of protein kinase C through phospholipase C and G-proteins. J. Steroid. Biochem. Mol. Biol. 73, 211–224 [DOI] [PubMed] [Google Scholar]
- 9. Song R. X., Zhang Z., Santen R. J. (2005) Estrogen rapid action via protein complex formation involving ERα and Src. Trends Endocrinol. Metab. 16, 347–353 [DOI] [PubMed] [Google Scholar]
- 10. Kim K. H., Bender J. R. (2009) Membrane-initiated actions of estrogen on the endothelium. Mol. Cell. Endocrinol. 308, 3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schwartz Z., Sylvia V. L., Guinee T., Dean D. D., Boyan B. D. (2002) Tamoxifen elicits its anti-estrogen effects in growth plate chondrocytes by inhibiting protein kinase C. J. Steroid Biochem. Mol. Biol. 80, 401–410 [DOI] [PubMed] [Google Scholar]
- 12. McMillan J., Fatehi-Sedeh S., Sylvia V. L., Bingham V., Zhong M., Boyan B. D., Schwartz Z. (2006) Sex-specific regulation of growth plate chondrocytes by estrogen is via multiple MAP kinase signaling pathways. Biochim. Biophys. Acta 1763, 381–392 [DOI] [PubMed] [Google Scholar]
- 13. Boyan B. D., Sylvia V. L., Frambach T., Lohmann C. H., Dietl J., Dean D. D., Schwartz Z. (2003) Estrogen-dependent rapid activation of protein kinase C in estrogen receptor-positive MCF-7 breast cancer cells and estrogen receptor-negative HCC38 cells is membrane-mediated and inhibited by tamoxifen. Endocrinology 144, 1812–1824 [DOI] [PubMed] [Google Scholar]
- 14. Wang Z., Zhang X., Shen P., Loggie B. W., Chang Y., Deuel T. F. (2005) Identification, cloning, and expression of human estrogen receptor-α36, a novel variant of human estrogen receptor-α66. Biochem. Biophys. Res. Commun. 336, 1023–1027 [DOI] [PubMed] [Google Scholar]
- 15. Lin S. L., Yan L. Y., Liang X. W., Wang Z. B., Wang Z. Y., Qiao J., Schatten H., Sun Q. Y. (2009) A novel variant of ER-α, ER-α36 mediates testosterone-stimulated ERK and Akt activation in endometrial cancer Hec1A cells. Reprod. Biol. Endocrinol. 7, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Z., Zhang X., Shen P., Loggie B. W., Chang Y., Deuel T. F. (2006) A variant of estrogen receptor-α, hER-α36. Transduction of estrogen- and antiestrogen-dependent membrane-initiated mitogenic signaling. Proc. Natl. Acad. Sci. U.S.A. 103, 9063–9068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Revankar C. M., Cimino D. F., Sklar L. A., Arterburn J. B., Prossnitz E. R. (2005) A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 307, 1625–1630 [DOI] [PubMed] [Google Scholar]
- 18. Kang L., Zhang X., Xie Y., Tu Y., Wang D., Liu Z., Wang Z. Y. (2010) Involvement of estrogen receptor variant ER-α36, not GPR30, in nongenomic estrogen signaling. Mol. Endocrinol. 24, 709–721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hu P., Kinyamu H. K., Wang L., Martin J., Archer T. K., Teng C. (2008) Estrogen induces estrogen-related receptor α gene expression and chromatin structural changes in estrogen receptor (ER)-positive and ER-negative breast cancer cells. J. Biol. Chem. 283, 6752–6763 [DOI] [PubMed] [Google Scholar]
- 20. Touitou I., Mathieu M., Rochefort H. (1990) Stable transfection of the estrogen receptor cDNA into HeLa cells induces estrogen responsiveness of endogenous cathepsin D gene but not of cell growth. Biochem. Biophys. Res. Commun. 169, 109–115 [DOI] [PubMed] [Google Scholar]
- 21. Kahlert S., Nuedling S., van Eickels M., Vetter H., Meyer R., Grohe C. (2000) Estrogen receptor α rapidly activates the IGF-1 receptor pathway. J. Biol. Chem. 275, 18447–18453 [DOI] [PubMed] [Google Scholar]
- 22. Smart E. J., Ying Y. S., Mineo C., Anderson R. G. (1995) A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc. Natl. Acad. Sci. U.S.A. 92, 10104–10108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boyan B. D., Wang L., Wong K. L., Jo H., Schwartz Z. (2006) Plasma membrane requirements for 1α,25(OH)2D3-dependent PKC signaling in chondrocytes and osteoblasts. Steroids 71, 286–290 [DOI] [PubMed] [Google Scholar]
- 24. Denison T. A., Koch C. F., Shapiro I. M., Schwartz Z., Boyan B. D. (2009) Inorganic phosphate modulates responsiveness to 24,25(OH)2D3 in chondrogenic ATDC5 cells. J. Cell. Biochem. 107, 155–162 [DOI] [PubMed] [Google Scholar]
- 25. Razandi M., Pedram A., Levin E. R. (2000) Plasma membrane estrogen receptors signal to antiapoptosis in breast cancer. Mol. Endocrinol. 14, 1434–1447 [DOI] [PubMed] [Google Scholar]
- 26. Sui M., Huang Y., Park B. H., Davidson N. E., Fan W. (2007) Estrogen receptor α mediates breast cancer cell resistance to paclitaxel through inhibition of apoptotic cell death. Cancer Res. 67, 5337–5344 [DOI] [PubMed] [Google Scholar]
- 27. Zhong M., Carney D. H., Ryaby J. T., Schwartz Z., Boyan B. D. (2009) Inhibition of phosphate-induced apoptosis in resting zone chondrocytes by thrombin peptide 508. Cells Tissues Organs 189, 56–59 [DOI] [PubMed] [Google Scholar]
- 28. Akhtari M., Mansuri J., Newman K. A., Guise T. M., Seth P. (2008) Biology of breast cancer bone metastasis. Cancer Biol. Ther. 7, 3–9 [DOI] [PubMed] [Google Scholar]
- 29. Rose A. A., Siegel P. M. (2006) Breast cancer-derived factors facilitate osteolytic bone metastasis. Bull. Cancer 93, 931–943 [PubMed] [Google Scholar]
- 30. Nakayama T., Mutsuga N., Tosato G. (2007) FGF2 post-transcriptionally down-regulates expression of SDF1 in bone marrow stromal cells through FGFR1 IIIc. Blood 109, 1363–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kousidou O. Ch., Berdiaki A., Kletsas D., Zafiropoulos A., Theocharis A. D., Tzanakakis G. N., Karamanos N. K. (2008) Estradiol-estrogen receptor. A key interplay of the expression of syndecan-2 and metalloproteinase-9 in breast cancer cells. Mol. Oncol. 2, 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Merrell M., Suarez-Cuervo C., Harris K. W., Väänänen H. K., Selander K. S. (2003) Bisphosphonate induced growth inhibition of breast cancer cells is augmented by p38 inhibition. Breast Cancer Res. Treat. 81, 231–241 [DOI] [PubMed] [Google Scholar]
- 33. Peng J., Jordan V. C. (2010) Expression of estrogen receptor α with a Tet-Off adenoviral system induces G0/G1 cell cycle arrest in SKBr3 breast cancer cells. Int. J. Oncol. 36, 451–458 [PMC free article] [PubMed] [Google Scholar]
- 34. Filardo E., Quinn J., Pang Y., Graeber C., Shaw S., Dong J., Thomas P. (2007) Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 148, 3236–3245 [DOI] [PubMed] [Google Scholar]
- 35. Zhong M., Carney D. H., Boyan B. D., Schwartz Z. (2011) 17β-Estradiol regulates rat growth plate chondrocyte apoptosis through a mitochondrial pathway not involving nitric oxide or MAPKs. Endocrinology 152, 82–92 [DOI] [PubMed] [Google Scholar]
- 36. Revankar C. M., Mitchell H. D., Field A. S., Burai R., Corona C., Ramesh C., Sklar L. A., Arterburn J. B., Prossnitz E. R. (2007) Synthetic estrogen derivatives demonstrate the functionality of intracellular GPR30. ACS Chem. Biol. 2, 536–544 [DOI] [PubMed] [Google Scholar]
- 37. Olde B., Leeb-Lundberg L. M. (2009) GPR30/GPER1. Searching for a role in estrogen physiology. Trends Endocrinol. Metab. 20, 409–416 [DOI] [PubMed] [Google Scholar]
- 38. Buitrago C., Boland R. (2010) Caveolae and caveolin-1 are implicated in 1α,25(OH)2-vitamin D3-dependent modulation of Src, MAPK cascades and VDR localization in skeletal muscle cells. J. Steroid Biochem. Mol. Biol. 121, 169–175 [DOI] [PubMed] [Google Scholar]
- 39. Kim H. P., Lee J. Y., Jeong J. K., Bae S. W., Lee H. K., Jo I. (1999) Nongenomic stimulation of nitric oxide release by estrogen is mediated by estrogen receptor α localized in caveolae. Biochem. Biophys. Res. Commun. 263, 257–262 [DOI] [PubMed] [Google Scholar]
- 40. Marino M., Distefano E., Caporali S., Ceracchi G., Pallottini V., Trentalance A. (2001) β-Estradiol stimulation of DNA synthesis requires different PKC isoforms in HepG2 and MCF7 cells. J. Cell. Physiol. 188, 170–177 [DOI] [PubMed] [Google Scholar]
- 41. Carey I., Williams C. L., Ways D. K., Noti J. D. (1999) Overexpression of protein kinase C-α in MCF-7 breast cancer cells results in differential regulation and expression of αvβ3 and αvβ5. Int. J. Oncol. 15, 127–136 [DOI] [PubMed] [Google Scholar]
- 42. Weldon C. B., McKee A., Collins-Burow B. M., Melnik L. I., Scandurro A. B., McLachlan J. A., Burow M. E., Beckman B. S. (2005) PKC-mediated survival signaling in breast carcinoma cells. A role for MEK1-AP1 signaling. Int. J. Oncol. 26, 763–768 [PubMed] [Google Scholar]
- 43. Jain S., Chakraborty G., Kundu G. C. (2006) The crucial role of cyclooxygenase-2 in osteopontin-induced protein kinase C α/c-Src/IκB kinase α/β-dependent prostate tumor progression and angiogenesis. Cancer Res. 66, 6638–6648 [DOI] [PubMed] [Google Scholar]
- 44. Ways D. K., Kukoly C. A., deVente J., Hooker J. L., Bryant W. O., Posekany K. J., Fletcher D. J., Cook P. P., Parker P. J. (1995) MCF-7 breast cancer cells transfected with protein kinase C-α exhibit altered expression of other protein kinase C isoforms and display a more aggressive neoplastic phenotype. J. Clin. Invest. 95, 1906–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Connolly J., Rose D. (1997) Expression of the invasive phenotype by MCF-7 human breast cancer cells transfected to overexpress protein kinase C-α or the erbB2 proto-oncogene. Int. J. Oncol. 10, 71–76 [PubMed] [Google Scholar]
- 46. Tomaskovic-Crook E., Thompson E. W., Thiery J. P. (2009) Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res. 11, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Blick T., Widodo E., Hugo H., Waltham M., Lenburg M. E., Neve R. M., Thompson E. W. (2008) Epithelial mesenchymal transition traits in human breast cancer cell lines. Clin. Exp. Metastasis 25, 629–642 [DOI] [PubMed] [Google Scholar]
- 48. Vincent-Salomon A., Thiery J. P. (2003) Host microenvironment in breast cancer development. Epithelial-mesenchymal transition in breast cancer development. Breast Cancer Res. 5, 101–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Deng H., Huang X., Fan J., Wang L., Xia Q., Yang X., Wang Z., Liu L. (2010) A variant of estrogen receptor-α, ER-α36 is expressed in human gastric cancer and is highly correlated with lymph node metastasis. Oncol. Rep. 24, 171–176 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.