Background: Essential divisome protein FtsA polymers may be formed by a head-to-tail mechanism.
Results: Deletion of domain 1C or region S12–13, located at opposing ends, synergistically blocks polymerization.
Conclusion: FtsA polymerization proceeds head to tail and is bidirectional.
Significance: These domains, essential for FtsA polymerization, are among those that differ most from other members of the actin-MreB-ParM family.
Keywords: Bacterial Genetics, Cell Division, Protein Assembly, Protein Domains, Streptococcus, FtsA, Streptococcus pneumoniae, Polymerization
Abstract
The effect of two different truncations involving either the 1C domain or the simultaneous absence of the S12–13 β-strands of the FtsA protein from Streptococcus pneumoniae, located at opposite terminal sides in the molecular structure, suggests that they are essential for ATP-dependent polymerization. These two truncated proteins are not able to polymerize themselves but can be incorporated to some extent into the FtsA+ polymers during the assembling process. Consequently, they block the growth of the FtsA+ polymers and slow down the polymerization rate. The combined action of the two truncated proteins produces an additive effect on the inhibition of FtsA+ polymerization, indicating that each truncation affects a different interaction site within the FtsA molecule.
Introduction
We have investigated the ATP-dependent polymerization of FtsA from Streptococcus pneumoniae, and in particular, the role of two regions of the molecule that differ most from the structures of actin, MreB, and ParM, members on the same structural family (1, 2). FtsA is a phylogenetically conserved essential protein that assembles as part of the cell division machinery, the divisome, a ring-like multiprotein structure localized at midcell.
It is widely accepted that FtsA participates in the early stages of ring assembly by connecting FtsZ, the main ring component, to the membrane. Although its ability to bind ATP has been demonstrated for the protein obtained from several organisms (2–6), its ATP hydrolytic activity has not been evidenced in some species (7). Associated to the ability to bind ATP, the purified FtsA of S. pneumoniae forms long, corkscrew-like helixes in the presence of ATP and Mg2+ (4). This ability to polymerize is shared with other prokaryotic members of the ATP-binding superfamily, including the MreB and ParM proteins (8, 9). These two proteins form two-stranded filaments and depolymerize following a treadmilling dynamic dependent on ATP hydrolysis (10). In contrast, FtsA polymers may aggregate, forming large bundles that are stable during at least 48 h, which would indicate a negligible ATP hydrolytic activity, if any (4). Although in the absence of ATP the Bacillus subtilis FtsA may dimerize (3),4 the streptococcal FtsA is mostly a monomer (4).
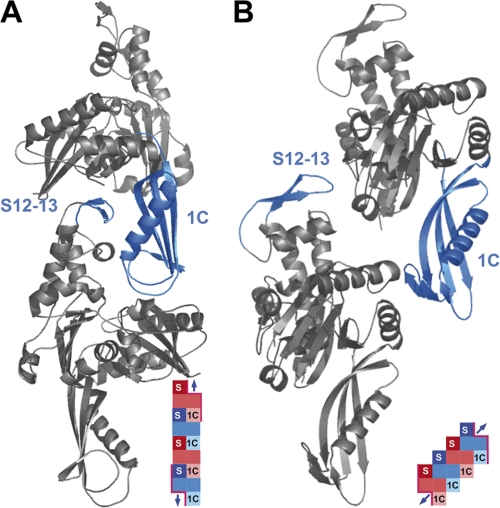
A predictive model to describe the dimerization of Escherichia coli FtsA (see Fig. 1) (11) suggests that two regions, located at opposite sites of the molecule and far from the ATP-binding pocket, may be crucial to establish the interactions involved in dimerization. A crystallographic model4 largely coincides with the predictive model, although the relative position of the interacting regions between the two monomers involves longer distances and the dimer is formed by a translation along a slanted axis relative to the long axis of the molecule. In contrast, in the predictive model, both a double translation, forward and partly backwards, along the vertical axis and a rotation around the same axis would produce a more compacted structure.
FIGURE 1.
Three-dimensional representations of structural models for FtsA dimer. A, the bioinformatics model as proposed in Carettoni et al. (11) and fitted to the sequence of the FtsA from S. pneumoniae. B, the crystallographic model of the FtsA dimer obtained from the crystal structure of T. maritima FtsA bound to a FtsZ protein peptide (not shown).4 The orientation of both models has been selected to show the best view of the interacting regions. Insets show graphics of the polymers. Monomers are differentiated using red or blue hues, and the interacting domains are abbreviated as S for the S12-S13 β strands or 1C for domain 1C. Surfaces available for interaction at the ends of the polymer are indicated by a pink border. Blue arrows indicate the potential to integrate one monomer.
Analysis of the effect of mutations in the E. coli FtsA molecule shows that homointeraction is essential for cell division (6, 12–14). FtsA oligomerization may be involved in some stages of proto-ring assembly at the membrane, including the binding of FtsA and the attachment and localization of the cytoplasmic FtsZ protein (14–17). It may also regulate the assembly of FtsZ rings in vivo (13).
The role of domain 1C or the S12 and S13 β-strands has been well documented in E. coli using constructions able to express each of the two truncated proteins in vivo. It seems that these truncations result in partially active FtsA proteins that do not allow the normal progression of septation but are able to localize together with FtsZ into division rings (17). The biochemical characterization of the E. coli FtsA, either the wild type or the truncated forms, is nevertheless hampered by its tendency to quickly aggregate after purification. In this study, we have constructed truncated variants of the FtsA of the S. pneumoniae lacking the domain 1C or the β-strands S12 and S13 that are equivalent to those already studied in vivo in E. coli (17). As the S. pneumoniae FtsA remains stable in solution after purification for much longer time, it has been possible to analyze in vitro their effects on the dynamics of polymerization and on the morphology and composition of the polymers.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
E. coli DH5α strain (18) was used as a host for gene cloning, and E. coli C41 strain (19) was employed for streptococcal protein overproduction and their subsequent purification. E. coli strains were routinely grown at 37 °C in Luria-Bertani (LB) agar or broth supplemented with ampicillin (100 μg ml−1) or kanamycin (50 μg ml−1) when necessary.
Plasmid Construction and DNA Manipulation
Standard protocols for molecular cloning, transformation, and DNA analysis were performed as described by Sambrook et al. (20). Oligonucleotides were ordered from Sigma-Aldrich. Restriction enzymes and DNA polymerase were purchased from Roche Diagnostics.
Plasmids encoding the truncated FtsA proteins for their overproduction and purification were obtained by the polymerase chain reaction (PCR)-based method of site-directed mutagenesis as in Rico et al. (17). Primers AR37 (5′-AGGCAAGCCGACATTCACTG-3′) and AR38 (5′-ACAGGACCTCGTACTATCTTGC-3′), phosphorylated by T4 polynucleotide kinase from Promega, were used to amplify the sequence of pRSETA_ftsA (4), except the sequence encoding the domain 1C of FtsA from Gly-81 to Tyr-159. Plasmid pARV48 contains this deletion to produce FtsAΔ1C, the truncated FtsA lacking the entire domain 1C. In the same way, the phosphorylated primers AR39 (5′-TTCTTTGCTTGCAAGAGGCGG-3′) and AR40 (5′-GAAGCCTACTTGTCAGAAATTA-3′) were used to amplify the sequence of pRSETA-ftsA, except the sequence encoding the FtsA region S12–13, comprising the β-chains S12 and S13 and the connecting loop between them, from Thr-271 to Thr-286. The resulting plasmid pARV49 encodes FtsAΔS12–13. The presence of all the mutations was confirmed by DNA sequencing.
For bacterial two-hybrid constructions, primers MK17(+1) (5′-CGGGATCCTATGGCTAGAGAAGGCTT-3′) and MK18 (5′-CGGAATTCTTATTCGTCAAACATGC-3′) were used to amplify the ftsA, ftsAΔ1C, and ftsAΔS12–13 genes using pRSETA-ftsA, pARV48, and pARV49, respectively, as templates. The corresponding PCR products were subcloned into the pUT18C and pKT25, which were kindly provided by Gouzel Karimova (Institut Pasteur, Paris, France). Plasmids and primers used in this study are listed in the Table 1.
TABLE 1.
Plasmids used in this study
| Plasmid | Relevant characteristics | Source/reference |
|---|---|---|
| pRSETA | Cloning and expression vector for His tag fusion proteins | Invitrogen |
| pRSETA_ftsA | ftsA cloned into pRSETA | Lara et al. (4) |
| pARV48 | ftsAΔ1C cloned into pRSETA | This study |
| pARV49 | ftsAΔS12–13 cloned into pRSETA | This study |
| pUT18C | T18 fragment of B. pertussis CyaA in pUC19 | Karimova et al. (27) |
| pKT25 | T25 fragment of B. pertussis CyaA in pSU40 | Karimova et al. (27) |
| pMKV19 | T18-ftsA in pUT18C | This study |
| pMKV20 | T18-ftsAΔ1C in pUT18C | This study |
| pMKV21 | T18-ftsAΔS12–13 in pUT18C | This study |
| pMKV24 | T25-ftsA in pKT25 | This study |
| pMKV25 | T25-ftsAΔ1C in pKT25 | This study |
| pMKV26 | T25-ftsAΔS12–13 in pKT25 | This study |
Overproduction and Purification of Streptococcal His-tagged Proteins
The FtsA protein and its variants were purified from inclusion bodies resulting from their overproduction.5 A 2-liter culture of E. coli C41 cells transformed with the plasmids pRSETA_ftsA, pARV48, or pARV49 was grown at 37 °C in LB medium supplemented with ampicillin until reaching 0.25 A600 units. The overexpression of the corresponding genes was induced with 1 mm isopropyl-1-thio-β-d-galactopyranoside during 3 h. Cells were then pelleted, suspended in 30 ml of buffer A (20 mm Tris-HCl, pH 7.5, 500 mm NaCl), and stored at −80 °C until use. The thawed cell suspension was supplemented with 1 mm PMSF, 0.1 mg ml−1 DNase, and 0.1 mg ml−1 lysozyme to be broken by sonication. After centrifugation for 15 min at 11,000 g at 4 °C, the pellet was suspended in 20 ml of buffer B (buffer A with 5 m guanidine chloride (Thermo Scientific)). The proteins were then immobilized in the affinity column HisTrap FF (GE Healthcare, Uppsala, Sweden), and the purified fraction was eluted with buffer B containing 500 mm imidazole (Merck, Darmstadt, Germany). The purified proteins were stabilized in 10% glycerol, renaturalized by sequential dialysis in the polymerization buffer (20 mm Tris-HCl, pH 7.5, 50 mm NaCl, 5 mm MgCl2 and 10% glycerol) supplemented with decreasing concentrations of guanidine chloride (2.5, 1.25, and 0.5 m), and finally stored at −80 °C. The correct protein renaturalization was confirmed using the circular dichroism technique using a JASCO J-810 spectropolarimeter (Tokyo, Japan).
FtsA Polymerization Assays and Data Analysis
For standard polymerization assays, purified proteins were centrifuged at 13,000 × g during 15 min to remove protein aggregates and diluted to the final concentration of 5 μm in the polymerization buffer. After equilibrating the purified protein at room temperature for 5 min in the reaction mix, 2 mm ATP (buffered with 1 m Tris-HCl, pH 8) was added to start the polymerization. It was followed by light-scattering measurements and/or pulldown analysis.
To follow the polymerization process of the FtsA protein in the presence of their variants along the time after the addition of ATP, 90° light-scattering experiments were performed using a Hitachi F-2500 fluorescence spectrophotometer. Excitation and emission wavelengths were set to 320 nm with slid widths of 5 nm. All the experiments were done at 25 °C as described in Mingorance et al. (21). The empirical time-dependent progress of scattering (S) was well described by the empirical Hill function, as in Hatters et al. (22),
 |
where S320(t) is the scattering (apparent fluorescence) in the long time limit, t50 is the elapsed time at which S320 is equal to one-half of S320(∞), and n is a cooperativity parameter. Although it does not provide a detailed description of the kinetics of FtsA polymerization, this analysis allows us to compare the overall rate of polymer formation of the truncated and the native proteins.
For the pulldown assays, after 20 min of incubation at room temperature, the reaction mixture was centrifuged for 20 min at 13,000 rpm in a microcentrifuge. The supernatant was separated from the pellet, which was resuspended in the same volume of polymerization buffer. Equivalent volumes of the supernatant and pellet contents were analyzed by SDS-PAGE, both qualitatively and quantitatively by densitometry of the corresponding bands.
For sedimentation velocity assays, samples of purified proteins, adjusted to a concentration of 0.4 mg ml−1, were centrifuged at 20 °C at 40,000 rpm in a Beckman Optima XL-I analytical ultracentrifuge (Beckman-Coulter Inc.), equipped with UV-visible and Rayleigh interference optics detection systems, using an An50-Ti rotor and 12-mm double-sector centerpieces. Differential sedimentation coefficient distributions, c(s), were calculated by least squares boundary modeling of sedimentation velocity data using the program SEDFIT (23, 24). The experimental sedimentation coefficients were corrected for buffer composition using the program SEDNTERP (25) to calculate the corresponding standard s20,w values (20 °C and water).
Electron Microscopy
Polymers containing FtsA+ or its variants were observed using transmission electron microscopy. After the addition of ATP, the reaction mixture was incubated at room temperature during 20 min as was described above for pulldown experiments. Samples were applied to a 400-mesh collodion-coated, glow-discharged copper grid and negatively stained with 2% uranyl acetate. They were inspected with a Jeol JEM1011 transmission electron microscope and photographed with an 11-megapixel charge-coupled device Gatan Erlangshen ES1000W camera. The software used for image capture was Gatan Digital Micrograph 1.8.0, and Adobe Photoshop CS5 was used for processing.
Bacterial Two-hybrid Assays
The interactions among FtsA+ and its variants were analyzed by the bacterial two-hybrid method based on the reconstitution of the adenylate cyclase to lead to the cAMP synthesis (26). Plasmids pMKV19, pMKV20, and pMKV21 (pUT18C carrying the ftsA+, ftsAΔ1C, and ftsAΔS12–13 genes, respectively) and pMKV24, pMKV25, and pMKV26 (pKT25 carrying the same genes) were used to co-transform the E. coli cya strain DHM1 (27). Co-transformants were grown at 30 °C on M63 agar minimum medium (100 mm KH2PO4, 15 mm (NH4)2SO4, 1.7 μm FeSO4, 1 mm MgSO4, 0.2% maltose, and 0.0001% vitamin B1; pH = 7) supplemented with ampicillin (50 μg ml−1) and kanamycin (25 μg ml−1), X-gal (40 μg ml−1), and 0.5 mm isopropyl-1-thio-β-d-galactopyranoside. Plates were incubated at 30 °C and inspected for growth and color after 4 days.
RESULTS
Deletion Proteins FtsAΔ1C and FtsAΔS12–13 Do Not Polymerize in Vitro
Deletions of the whole domain 1C or the β-strands S12 and S13 in domain 2B of E. coli FtsA resulted in partially active proteins in vivo (17). As these two domains are formed by continuous peptide chains and protrude from the ATP-binding core of the molecule, their removal is predicted to cause limited alterations in the structure of the remaining protein fragment. Both regions were proposed to be involved in the interactions leading to the dimerization of FtsA (11), as evidenced by yeast two-hybrid experiments (17). As purified FtsA+ and their variants from E. coli are prone to aggregate in solution, precluding a reliable biochemical analysis, we have used FtsA proteins of S. pneumoniae produced by expressing suitable constructions transformed into E. coli.
Two variants of this protein lacking each of these regions were constructed following the same criteria used for the equivalent constructs produced in Rico et al. (17) for the E. coli FtsA. To allow the continuity of the peptide chain without altering the three-dimensional structure of the remainder of the molecule, the first and last residues of the deletions were chosen as being in close proximity and located inside a flexible loop. Using the three-dimensional model of the S. pneumoniae FtsA derived from the crystal structure of the Thermotoga maritima FtsA (2) as a blueprint (Fig. 1), the Gly-81 to Tyr-159 residues were chosen for the FtsAΔ1C variant, and those from Thr-271 to Thr-286 were chosen for the FtsAΔS12–13 variants.
The streptococcal FtsA+ produced in E. coli has been shown to polymerize in vitro (4). Moreover in nonconcentrated solutions, both the wild type and the truncated streptococcal proteins obtained from heterologous expression in E. coli are more stable than the native E. coli protein.
In addition, to circumvent the tendency of streptococcal FtsA+ and its variants to aggregate in vivo when overproduced in E. coli, they were purified from inclusion bodies and renatured as described under “Experimental Procedures.” This method improved the yield of the purified protein in comparison with the previous reported method of FtsA purification from the soluble fraction (4) (Fig. 2A). For FtsA+, the procedure yields a mainly monomeric protein, as confirmed by sedimentation velocity (data not shown), that is correctly refolded as confirmed by circular dichroism. Similar data for the truncated proteins suggest that minimum changes are introduced as a consequence of the short deletion in FtsAΔS12–13, whereas more conspicuous variations are observed for the extensive deletion in FtsAΔ1C (see supplemental Fig. S2). In addition, this purified FtsA+ protein retains its ability to polymerize upon the addition of ATP as measured by light scattering (Fig. 2B) and as evidenced in electron microscopy images (see Fig. 4, upper right panel).
FIGURE 2.
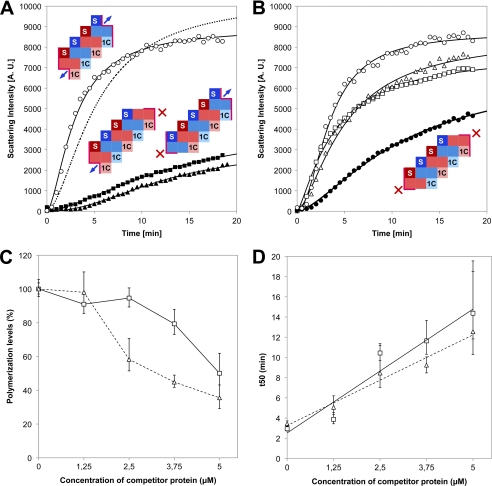
Nucleotide-dependent polymerization of FtsA+ and its variants. A, Coomassie Blue-stained 12% SDS-PAGE gel of 4 μg of the purified FtsA+ (lane A+) and its variants FtsAΔ1C (lane 1C) and FtsAΔS12–13 (lane S12–13). Molecular weight standards are shown in the left lane (MW). A faint lower band in lane A+ is a degradation product of FtsA+ that was already described in Lara et al. (4). B, analysis of the polymerization process followed by 90° light scattering. Purified proteins FtsA+ (○), FtsAΔ1C (□), and FtsAΔS12–13 (△) at the concentration of 5 μm were equilibrated separately for 5 min in a spectrofluorometer cuvette before the addition of 2 mm ATP. The polymerization reaction at 25 °C was followed during 20 min. The light-scattering intensity was measured as arbitrary units (A. U.). The insets show graphics of the polymer formed by FtsA+ as in Fig. 1, and the monomers of the two truncated forms. Surfaces available for monomer addition are outlined in pink, arrows indicate the possibility to add one monomer, whereas crosses indicate that the missing interaction prevents polymerization. C, sedimentation velocity analysis of the purified FtsA variants in the absence (upper panel) and in the presence of ATP (bottom panel). FtsAΔ1C (red line), FtsAΔS12–13 (blue line), and a mixture of equimolar concentrations of FtsAΔ1C and FtsAΔS12–13 (green line) are represented. For comparison, the sedimentation coefficient of FtsA+ derived from Lara et al. (4) is 3.2 S.
FIGURE 4.
Electron microscopy of polymers formed by FtsA. Polymerization of mixtures containing the proteins indicated in each frame was initiated by the addition of 2 mm ATP and left to proceed for 20 min. Samples from each mixture were applied to copper grids and negatively stained as described under “Experimental Procedures.” Numbers between brackets indicate the ratio of the protein components in the reaction mixtures, where 4 corresponds to 5 μm. The insets show graphics of the polymer formed by FtsA+ as in Fig. 1B and the polymer blocked at either end or at both ends by the incorporation of one monomer of a truncated protein or one of each truncated protein, respectively, with the two different truncations occupying opposite ends. Monomers are differentiated using red or blue hues, and the interacting domains are abbreviated as S for the S12-S13 β strands or 1C for domain 1C. Surfaces available for interaction at the ends of the polymer are indicated by a pink border. Blue arrows indicate the potential to integrate one monomer.
Sedimentation velocity analysis of the truncated FtsA proteins showed major peaks of FtsAΔ1C (92.1%) and FtsAΔS12–13 (92.9%) with sedimentation coefficients of 2.5 S and 3.0 S, respectively (Fig. 2C), in agreement with the size of the truncated proteins relative to the wild type protein (3.2 S) (4). Contrary to FtsA+, the purified FtsAΔ1C or FtsAΔS12–13 proteins were not able to increase either the light-scattering signal (Fig. 2B) or their sedimentation coefficients upon the addition of ATP (Fig. 2C), indicating that they were not able to polymerize in the presence of ATP. Under these conditions, their sedimentation coefficients were maintained at 2.5 S (90.4%) for FtsAΔ1C and at 3.4 S (87.8%) for FtsAΔS12–13. Although the FtsAΔS12–13 monomer may adopt a more compact shape in the presence of ATP, these values are compatible with the behavior of globular protein monomers. To detect whether the truncated proteins, FtsAΔ1C and FtsAΔS12–13, were able to cross-interact with each other as heterodimers, they were mixed at equimolar quantities in the presence of ATP. A main peak of 2.8 S (92%) was observed that coincides with the expected s-value of a noninteracting mixture of FtsAΔ1C and FtsAΔS12–13. We conclude that the two deletion proteins, either in isolation or combined, do not appear to assemble into homodimers or heterodimers, both in the presence and in the absence of ATP.
The ability of the three proteins to interact in vivo was assayed using the bacterial two-hybrid assay. For this purpose, all the potential interacting constructions, pMKV19, pMKV20, pMKV21, pMKV24, pMKV25, and pMKV26, were obtained (Table 1). They were transformed in pairs into DHM1, a recipient E. coli cya strain, the transformants were allowed to grow on X-gal indicator plates, and the ability to interact was assessed by visual inspection of the colonies as indicated under “Experimental Procedures.” The results confirm the positive interaction of the FtsA+-FtsA+ pair, as already published using yeast two-hybrid assays (4). All the pairs involving one of the two truncated variants with itself or in combination with the other truncated variant proved negative in this assay (supplemental Fig. S1). We conclude that the FtsA-deleted variants are not able to interact in vivo, which agrees with their inability to form polymers in the presence of ATP. These results are similar to those obtained for the equivalent forms of E. coli FtsA using the yeast two-hybrid assay (17) and support a role for the domain 1C and the S12–S13 β-strands in the self-interaction between FtsA molecules in both bacteria.
FtsAΔ1C or FtsAΔS12–13 Decreases Polymerization Rate of FtsA+, Diminishes Amount of Polymers, and Modifies Their Shape
To find whether the FtsAΔ1C and FtsAΔS12–13 inhibit the polymerization of FtsA+, the formation of polymers was followed by light-scattering measurements after the addition of 2 mm ATP to mixtures containing equimolar concentrations (5 μm each) of FtsA+ and one of the mutated proteins FtsAΔ1C or FtsAΔS12–13. Both truncated proteins inhibited extensively the polymerization of FtsA+, whereas bovine serum albumin used as an unspecific control at the same concentration did not (Fig. 3A).
FIGURE 3.
Kinetics of inhibition of FtsA polymerization. A, light-scattering measurements upon the addition of 2 mm ATP to 5 μm FtsA+ alone (○) or in combination with an equimolar amount of one of the truncated proteins, FtsAΔ1C (■) or FtsAΔS12–13 (▴). The discontinuous line shows the best-fit trend line obtained from measurements of the ATP-dependent polymerization of 5 μm FtsA in the presence of 5 μm BSA used as a control. Insets show graphics of the polymers formed by each mixture. Monomers are differentiated using red or blue hues, and the interacting domains are abbreviated as S for the S12-S13 β strands or 1C for domain 1C. Surfaces available for interaction at the ends of the polymer are indicated by a pink border. Blue arrows indicate the potential to integrate one monomer. A. U., arbitrary units. B, light-scattering measurements upon the addition of 2 mm ATP to 5 μm FtsA+ alone, as in A (○), or to mixtures containing 5 μm FtsA+ plus 1.25 μm FtsAΔ1C (□), 1.25 μm FtsAΔS12–13 (△), or 1.25 μm FtsAΔ1C plus 1.25 μm FtsAΔS12–13 (●). Measurements were taken every 2 s; points are plotted for 30-s intervals. Plotted curves are tendency lines calculated according to the Hill function using the best-fit parameter values given in supplemental Table S1. The inset shows the graphic of one polymer blocked at both ends by the incorporation of one monomer of a truncated protein with the two different truncations occupying opposite ends. Graphic conventions are as for panel A. C, maximum polymerization levels of 5 μm wild type FtsA+ in the presence of 2 mm ATP and increasing concentrations, as indicated in the graph, of the truncated proteins FtsAΔ1C (□) or FtsAΔS12–13 (△). The data were calculated from the empirical light-scattering measurements according to the Hill function (see “Experimental Procedures”) and normalized to the results obtained in the absence of any competitor protein (100%). D, the time required to reach 50% of the maximum polymerization level (t50) at increasing concentrations of the competitor proteins is represented for the data shown in panel C (continuous line for FtsAΔ1C and discontinuous line for FtsAΔS12–13). The error bars correspond to 2 ± S.D. for a 95% confidence limit as calculated from the Hill equation.
To further analyze the inhibition of FtsA+ polymerization by the truncated proteins, a constant concentration of FtsA+ (5 μm) was preincubated in the polymerization buffer at 25 °C for 5 min with increasing amounts of each truncated form (1.25, 2.5, 3.75, and 5 μm to obtain ratios of FtsA:truncated protein of 4:0, 4:1, 4:2, 4:3, and 4:4, respectively). A brief inspection of the kinetics of polymerization indicated that both the maximum polymerization levels and the polymerization rate diminish in the presence of any of the two truncated FtsA variants. To quantify the inhibitory effects of the truncated forms on the FtsA+ polymerization, the experimental values obtained for each reaction were fitted using the Hill equation. The results show that inhibition of both the maximum polymerization levels and the polymerization rate is directly proportional to the amounts of FtsAΔ1C or FtsAΔS12–13 present in the reactions (supplemental Table S1). Both forms of the protein affect the rate of polymerization to the same extent (Fig. 3D). However, FtsAΔS12–13 proved a more effective inhibitor when considering the 50% of the maximum polymerization level parameter, attained at 3 μm for this protein in comparison with 5 μm needed for FtsAΔ1C (Fig. 3C). In accordance to this result, the interaction of FtsAΔS12–13 and FtsA+, measured in a bacterial two-hybrid assay, although weaker than the one exhibited by the FtsA+-FtsA+ pair, was still detected. On the other hand, there was no detectable interaction between FtsAΔ1C and FtsA+ (supplemental Fig. S1). These results are similar to those already published for the E. coli FtsA proteins using the yeast two-hybrid system assay (17).
To find whether the inhibition caused by truncated proteins has any effect on the shape of the FtsA+ polymers, 5 μm FtsA+ was polymerized in the presence of 2.5 μm FtsAΔS12–13 or 5 μm FtsAΔ1C, chosen as the concentrations that reduced the polymerization levels by half (Fig. 3C). These heteropolymers were photographed using transmission electron microscopy after negative staining. They appeared as shorter, less abundant, less compact, and less defined than the FtsA+ polymers observed in the absence of any truncated protein (Fig. 4).
Consistent with the results obtained from light-scattering measurements (Fig. 3C), the FtsA+ polymers formed compact bundles of helical filaments in a background free of short filaments or residual nonpolymerized proteins (Fig. 4, upper left panel), whereas the presence of truncated proteins showed polymers with shorter and less abundant bundles with a large amount of residual material appearing in the background. Representative images (Fig. 4A) show that even if the individual filaments formed in the presence of the truncated proteins still exhibit a helical shape, they form frayed and less compact bundles with a significant number of protruding filaments. We conclude that the presence of any of the two truncated proteins alters the shape of the FtsA+ polymers, negatively affecting the relative amount of polymerized material and its association into bundles.
FtsAΔS12–13 and FtsAΔ1C May Be Incorporated into the FtsA+ Polymers
To find whether the inhibitory effect of the truncated proteins is exerted during the process of polymer formation or whether they can even affect the already formed polymers, a sample of FtsA+ at 5 μm concentration was allowed to polymerize after the addition of ATP for 20 min. Each of the two truncated proteins was added at a final concentration of 5 μm in polymerization buffer to the reaction mixtures containing the already assembled FtsA+ polymers. The effect of each truncated protein on the polymers was then measured by light scattering. Once the values obtained from the addition of an equivalent volume of the polymerization buffer were subtracted to exclude any possible effect caused by dilution, no significant differences were detected after the addition of any of the two truncated proteins to the FtsA+ polymers (data not shown). Therefore, the presence of mutated proteins does not appear to alter the stability of the already assembled polymers of FtsA+. We then conclude that the truncated proteins may affect the polymerization process itself, acting upon their incorporation to the polymers during the polymerization reaction.
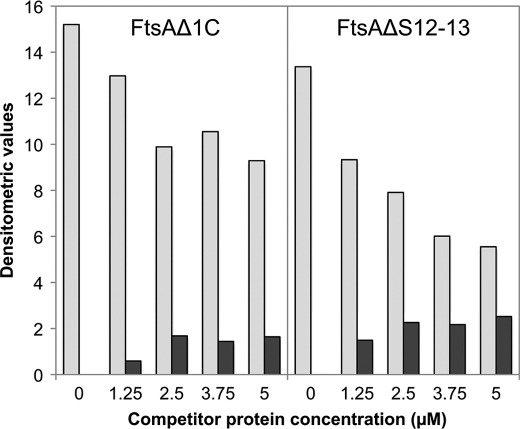
To further investigate whether the truncated variants were actually integrated into the FtsA+ polymers, mixtures of FtsA+ and increasing concentrations of each truncated protein were allowed to interact for 20 min. High molecular weight material was spun down and electrophoretically resolved to quantify the amounts of each component (supplemental Fig. S3). The results (Fig. 5) show that the contents of FtsA+ in the pellets decreased as the concentration of the truncated proteins in the mixtures increased. In addition, the relative amounts of the truncated and wild type proteins contained in the pellet remained constant once the amount of the truncated proteins in the polymerization mixture was above 2.5 μm. This result indicates that the truncated proteins FtsAΔS12–13 and FtsAΔ1C may block FtsA+ polymerization by being incorporated into polymers at the time of polymerization. As the domains missing in FtsAΔ1C and FtsAΔS12–13 deletions are different and located at opposite sites of the molecule, it may be inferred that FtsA polymerization involves at least two distinct regions of the protein.
FIGURE 5.
Composition of FtsA polymers formed by FtsA+ with increasing concentrations of FtsAΔ1C or FtsAΔS12–13 as in Fig. 3. Polymerization was allowed to proceed for 20 min, and high molecular weight material was then pelleted at 13,000 rpm for 20 min in a tabletop microcentrifuge. The material contained in the pellets was resuspended in an equivalent volume of polymerization buffer (see “Experimental Procedures”). Equal volumes of each sample were run on an SDS-PAGE and stained with Coomassie Blue (supplemental Fig. S3). The densitometric values of the bands corresponding to the amount of FtsA+ (gray bars) and each variant (black bars) present in the pellets are shown. The total protein present in each supernatant was quantified and used to check that the recovery of the protein present in the two fractions added up to the total amount contained in each initial mixture.
Synergy of Inhibition of FtsA+ Polymerization by FtsAΔ1C and FtsAΔS12–13
If FtsA+ polymerization can proceed by adding monomers at both ends of the growing polymer, the two truncated forms of FtsA used in this study could have an additive effect by blocking simultaneously elongation at both ends of the oligomers. To test this possibility, the polymerization of FtsA+ was measured by light scattering upon the addition of 2 mm ATP to a reaction mixture containing 5 μm FtsA+, 1.25 μm FtsAΔ1C, and 1.25 μm FtsAΔS12–13. The results show that at these relatively low concentrations, the addition of either FtsAΔ1C or FtsAΔS12–13 to the polymerization reaction produced a modest inhibition of the maximum polymerization levels, 9 and 2%, respectively. On the other hand, the simultaneous addition of both truncated forms produced an additive effect, causing a 25% decrease in the maximum polymerization level of FtsA+ (Fig. 3B and supplemental Table S1). This additive effect is also observed when measuring the polymerization rate. Although the wild type protein takes 2.94 min to achieve 50% polymerization, the reaction is slowed down by the presence of any of the two truncated proteins: 3.87 min for FtsAΔ1C or 5.06 min for FtsAΔS12–13. Polymerization proceeds at even a slower rate when both inhibiting proteins are present (11.20 min to achieve 50% polymerization) (supplemental Table S1). Electron microscopy images of polymers formed in the mixtures of FtsA+ together with FtsAΔ1C or FtsAΔS12–13 indicated that these polymers form bundles that are less frequent and more disorganized than those formed by the mixtures containing FtsA+ and any of the two truncated forms (Fig. 4). These results are compatible with a model in which domain 1C and region S12–13 are two separate regions involved in establishing important interactions between FtsA+ molecules leading to polymer formation in the test tube.
DISCUSSION
In many bacteria, FtsA is an important component of the divisome, the machinery responsible for cytokinesis (7). When compared with the relatively well known and more phylogenetically conserved FtsZ protein, its study has progressed slowly. This is due in part to the technical difficulties involved in the purification and stabilization of the FtsA protein from E. coli, whereas on the other hand, a good deal of genetic and in vivo data pertaining to its role in division and its interactions with other components of the divisome, notably with the other proto-ring elements FtsZ and ZipA, are available. In this work, we have used FtsA from S. pneumoniae produced heterologously in E. coli because it is more amenable to purification, retaining its stability and other biochemical properties as ATP binding for a longer time in solution, and moreover because it has an ATP-dependent polymerization activity (4). We have studied the polymerization process of the S. pneumoniae protein, analyzing the behavior of the wild type and of two truncated forms affecting two different regions located at opposite ends of the FtsA molecule that are known to play a crucial role in the interaction and activity of the E. coli protein in vivo (17).
In summary, we find that the polymerization of S. pneumoniae FtsA is sensitive to the addition of truncated forms in which either the domain 1C or the β-strands S12 and S13 are absent. These truncated forms are themselves unable to polymerize, and they seem to exert their inhibitory effect during the polymerization process but not on preformed FtsA+ polymers. The extent of the inhibition, affecting the polymerization rate and the amount of molecules present in the polymers, is directly proportional to the concentration of the truncated proteins, with the FtsAΔS12–13 form being slightly more potent. A lower concentration of FtsAΔS12–13 (3 μm) than of FtsAΔ1C (5 μm) is required to reduce the polymerization levels by half (Fig. 3C), which agrees with the strength of their interaction when measured using a bacterial two-hybrid system. These results agree with our previous description of the interaction behavior of similar truncations in the E. coli FtsA protein (17), recently corroborated by Pichoff et al. (28) using a microscopy detection assay. On the other hand, we cannot decide from these results whether the differences are due to the different structures of the deleted proteins or whether the incorporation of the monomers into the growing polymer proceeds at a faster rate in one direction than in the opposite direction. We can nevertheless propose that the truncated proteins, unable themselves even to dimerize, may compete with FtsA+ at the growing ends of the polymer, displacing the reaction toward smaller sized oligomers and monomers. As previously shown by Lara et al. (4), the polymers formed by FtsA+ are very stable when in the presence of magnesium and ATP and do not dissociate unless treated with EDTA. This indicates that FtsA polymerization is not readily reversible. Therefore, it is unlikely that the binding of a mutated form to one end of the oligomer can be reverted; on the contrary, binding of a blocking monomer to one end of an oligomer should block the further addition of monomers, which agrees with our experimental observation (Fig. 3).
As the inhibitory effect of the two truncated proteins on FtsA+ polymerization is additive, we reason that a possibility is that the addition of monomers may occur at both ends of the growing polymer. In the “head-to-tail” structural models proposed for the FtsA self-interaction (Fig. 1), the β-strands S12 and S13 and the domain 1C occupy opposite locations along the FtsA three-dimensional structure, on the top and on the bottom, respectively (8). According to both models, incorporation of the truncated proteins into the FtsA+ polymer would interrupt the incorporation of additional FtsA+ monomers at the top end in the case of FtsAΔS1213, or at the bottom in the case of FtsAΔ1C, and at both ends when the two truncated proteins are present. Therefore, polymerization would proceed bidirectionally.
Bidirectional FtsA polymerization, as we find for the streptococcal protein, differs from the unidirectional process described for the polymerization of eukaryotic actin and other prokaryotic members of the ATP-binding superfamily, such as MreB or ParM. Monomers of these proteins are incorporated to one end of two-stranded filaments and released from the other end, involving a treadmilling dynamic dependent on ATP hydrolysis (10). In contrast, the absence of a measurable ATP hydrolytic activity of the purified streptococcal FtsA+ (4) would fit with the growth of the polymers being bidirectional and with their stability during prolonged times.
PilM, a cytoplasmic component of the Type IV pilus biogenesis system from Thermus thermophilus belonging to the actin family, shows an almost similar structure as FtsA, including the presence and relative orientation of domain 1C (29), which is represented by the differently oriented domain 1B in the rest of the proteins. PilM is nevertheless devoid of the β-strands S12-S13 and the specific protruding loops in 1C involved in establishing contacts with the other monomer, as proposed in the crystallographic model. Both FtsA and PilM have an ATP binding ability with no significant hydrolysis activity. However, PilM cannot polymerize in an ATP-dependent way, indicating that the β-strands S12 and S13 and the loops located at the bottom of the domain 1C are needed for polymerization, as suggested by the crystallographic model4 and according to our results.
Although the amount of FtsA within a S. pneumoniae cell (some 2000 molecules) (4) should be high enough to support polymerization in the cytoplasm, which normally contains sufficient ATP levels, its biological significance is not known. Most of the data on the FtsA function in bacterial division have been obtained studying E. coli, in which FtsA is present in a number of nearly 700 molecules per cell (30), one-third the amount contained in the smaller sized S. pneumoniae cell. The extrapolation of data between the two species should therefore proceed cautiously. For example, our data on in vitro polymerization of the streptococcal protein do not fit with the need for a previous interaction between FtsA and FtsZ polymers to promote FtsA self-interaction (16). On the other hand, our results are compatible with evidence supporting the role of the FtsA β-strands S12 and S13 in some functions of FtsZ, namely its affinity for FtsA (12, 31), the regulation of FtsZ assembly (13), and the correct placement of the Z-ring (17), or even including a possible role for FtsA in ring constriction (32). Similarly, the role of domain 1C has been related to the recruitment of the late division proteins in E. coli (17, 33–35).
Our biochemical evidence regarding the roles of the two spatially independent domains would fit well into the crystallographic model in which the interaction between monomers resides at two separate sites. When comparing the bioinformatics (11) and the crystallographic models4 proposed for FtsA polymerization, the relative orientation of the monomers differs between the two. The bioinformatics model predicts a complex displacement involving both translation and rotation along the longitudinal axis of the molecules, whereas the crystallographic model would simply involve the displacement of FtsA dimeric units. As a consequence, in the more compact bioinformatics model, domain 1C and β-strands S12–13 are in contact, whereas both regions establish independent interactions at different sites of the molecule in the crystallographic model. Although the synergy of the inhibitory effects that we observe when the two truncated proteins are present in the FtsA polymerization mixtures may perhaps be accommodated more easily into a model involving two independent interaction sites, as occurs in the crystallographic model, rather than into one in which the interaction site is integrated into a unique structure, as would be the case in the bioinformatics model, our biochemical data are not sufficient to exclude any of them.
Supplementary Material
Acknowledgments
We thank Jan Löwe (Medical Research Council Laboratory of Molecular Biology) for the communication of an unpublished three-dimensional model of a polymer formed by the T. maritima FtsA. We also thank M. Jiménez and A. Martos (both at CIB-CSIC) for useful suggestions on the protocol for FtsA purification, C. Patiño, (CNB-CSIC) for help with electron microscopy, and C. Alfonso, (CIB-CSIC) for assistance with analytical centrifugation.
Footnotes
This work was supported by the Spanish Ministerio de Educación y Ciencia through Grant BIO2008-04478-C03 and by the European Commission through Contract HEALTH-F3-2009-223431 (DIVINOCELL) (both to M. V. and G. R.).

This article contains supplemental Figs. S1–S3 and Table S1.
P. Szwedziak, Q. Wang, S. M. V. Freund, and J. Löwe, manuscript in preparation.
A. Martos, B. Monterroso, S. Zorrilla, B. Reija, C. Alfonso, J. Mingorance, G. Rivas, and M. Jiménez, manuscript in preparation.
REFERENCES
- 1. Bork P., Sander C., Valencia A. (1992) An ATPase domain common to prokaryotic cell cycle proteins, sugar kinases, actin, and hsp70 heat shock proteins. Proc. Natl. Acad. Sci. U.S.A. 89, 7290–7294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van den Ent F., Löwe J. (2000) Crystal structure of the cell division protein FtsA from Thermotoga maritima. EMBO J. 19, 5300–5307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feucht A., Lucet I., Yudkin M. D., Errington J. (2001) Cytological and biochemical characterization of the FtsA cell division protein of Bacillus subtilis. Mol Microbiol 40, 115–125 [DOI] [PubMed] [Google Scholar]
- 4. Lara B., Rico A. I., Petruzzelli S., Santona A., Dumas J., Biton J., Vicente M., Mingorance J., Massidda O. (2005) Cell division in cocci: localization and properties of the Streptococcus pneumoniae FtsA protein. Mol. Microbiol. 55, 699–711 [DOI] [PubMed] [Google Scholar]
- 5. Sánchez M., Valencia A., Ferrándiz M. J., Sander C., Vicente M. (1994) Correlation between the structure and biochemical activities of FtsA, an essential cell division protein of the actin family. EMBO J. 13, 4919–4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yim L., Vandenbussche G., Mingorance J., Rueda S., Casanova M., Ruysschaert J. M., Vicente M. (2000) Role of the carboxyl terminus of Escherichia coli FtsA in self-interaction and cell division. J. Bacteriol. 182, 6366–6373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vicente M., Rico A. I. (2006) The order of the ring: assembly of Escherichia coli cell division components. Mol. Microbiol. 61, 5–8 [DOI] [PubMed] [Google Scholar]
- 8. van den Ent F., Amos L. A., Löwe J. (2001) Prokaryotic origin of the actin cytoskeleton. Nature 413, 39–44 [DOI] [PubMed] [Google Scholar]
- 9. van den Ent F., Møller-Jensen J., Amos L. A., Gerdes K., Löwe J. (2002) F-actin-like filaments formed by plasmid segregation protein ParM. EMBO J. 21, 6935–6943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Graumann P. (2007) Cytoskeletal elements in bacteria. Annu. Rev. Microbiol. 61, 589–618 [DOI] [PubMed] [Google Scholar]
- 11. Carettoni D., Gómez-Puertas P., Yim L., Mingorance J., Massidda O., Vicente M., Valencia A., Domenici E., Anderluzzi D. (2003) Phage display and correlated mutations identify an essential region of subdomain 1C involved in homodimerization of Escherichia coli FtsA. Proteins 50, 192–206 [DOI] [PubMed] [Google Scholar]
- 12. Geissler B., Elraheb D., Margolin W. (2003) A gain-of-function mutation in ftsA bypasses the requirement for the essential cell division gene zipA in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 100, 4197–4202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shiomi D., Margolin W. (2007) Dimerization or oligomerization of the actin-like FtsA protein enhances the integrity of the cytokinetic Z ring. Mol. Microbiol. 66, 1396–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shiomi D., Margolin W. (2008) Compensation for the loss of the conserved membrane targeting sequence of FtsA provides new insights into its function. Mol. Microbiol. 67, 558–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pichoff S., Lutkenhaus J. (2005) Tethering the Z ring to the membrane through a conserved membrane targeting sequence in FtsA. Mol. Microbiol. 55, 1722–1734 [DOI] [PubMed] [Google Scholar]
- 16. Pichoff S., Lutkenhaus J. (2007) Identification of a region of FtsA required for interaction with FtsZ. Mol. Microbiol. 64, 1129–1138 [DOI] [PubMed] [Google Scholar]
- 17. Rico A. I., García-Ovalle M., Mingorance J., Vicente M. (2004) Role of two essential domains of Escherichia coli FtsA in localization and progression of the division ring. Mol. Microbiol. 53, 1359–1371 [DOI] [PubMed] [Google Scholar]
- 18. Hanahan D. (1983) Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 166, 557–580 [DOI] [PubMed] [Google Scholar]
- 19. Miroux B., Walker J. E. (1996) Overproduction of proteins in Escherichia coli: mutant hosts that allow synthesis of some membrane proteins and globular proteins at high levels. J. Mol. Biol. 260, 289–298 [DOI] [PubMed] [Google Scholar]
- 20. Sambrook J., Fritsch E. F., Maniatis T. (1989) Molecular Cloning: A Laboratory Manual, 2nd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 21. Mingorance J., Rueda S., Gómez-Puertas P., Valencia A., Vicente M. (2001) Escherichia coli FtsZ polymers contain mostly GTP and have a high nucleotide turnover. Mol. Microbiol. 41, 83–91 [DOI] [PubMed] [Google Scholar]
- 22. Hatters D. M., Minton A. P., Howlett G. J. (2002) Macromolecular crowding accelerates amyloid formation by human apolipoprotein C-II. J. Biol. Chem. 277, 7824–7830 [DOI] [PubMed] [Google Scholar]
- 23. Schuck P. (2000) Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys. J. 78, 1606–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schuck P. (2003) On the analysis of protein self-association by sedimentation velocity analytical ultracentrifugation. Anal. Biochem. 320, 104–124 [DOI] [PubMed] [Google Scholar]
- 25. Laue T. M., Shah B. D., Ridgeway T. M., Pelletier S. L. (1992) in Analytical Ultracentrifugation in Biochemistry and Polymer Science (Harding S. E., ed) pp. 90–125, Royal Society of Chemistry, Cambridge, UK [Google Scholar]
- 26. Karimova G., Pidoux J., Ullmann A., Ladant D. (1998) A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. U.S.A. 95, 5752–5756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karimova G., Dautin N., Ladant D. (2005) Interaction network among Escherichia coli membrane proteins involved in cell division as revealed by bacterial two-hybrid analysis. J. Bacteriol. 187, 2233–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pichoff S., Shen B., Sullivan B., Lutkenhaus J. (2012) FtsA mutants impaired for self-interaction bypass ZipA suggesting a model in which FtsA's self-interaction competes with its ability to recruit downstream division proteins. Mol. Microbiol. 83, 151–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Karuppiah V., Derrick J. P. (2011) Structure of the PilM-PilN inner membrane type IV pilus biogenesis complex from Thermus thermophilus. J. Biol. Chem. 286, 24434–24442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rueda S., Vicente M., Mingorance J. (2003) Concentration and assembly of the division ring proteins FtsZ, FtsA, and ZipA during the Escherichia coli cell cycle. J. Bacteriol. 185, 3344–3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Geissler B., Shiomi D., Margolin W. (2007) The ftsA* gain-of-function allele of Escherichia coli and its effects on the stability and dynamics of the Z ring. Microbiology 153, 814–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Beuria T. K., Mullapudi S., Mileykovskaya E., Sadasivam M., Dowhan W., Margolin W. (2009) Adenine nucleotide-dependent regulation of assembly of bacterial tubulin-like FtsZ by a hypermorph of bacterial actin-like FtsA. J. Biol. Chem. 284, 14079–14086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bernard C. S., Sadasivam M., Shiomi D., Margolin W. (2007) An altered FtsA can compensate for the loss of essential cell division protein FtsN in Escherichia coli. Mol. Microbiol. 64, 1289–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Corbin B. D., Geissler B., Sadasivam M., Margolin W. (2004) Z-ring-independent interaction between a subdomain of FtsA and late septation proteins as revealed by a polar recruitment assay. J. Bacteriol. 186, 7736–7744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Goehring N. W., Robichon C., Beckwith J. (2007) Role for the nonessential N terminus of FtsN in divisome assembly. J. Bacteriol. 189, 646–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.