Abstract
SERCA2a gene therapy improves contractile and energetic function of failing hearts and has been shown to be associated with benefits in clinical outcomes, symptoms, functional status, biomarkers, and cardiac structure in a phase 2 clinical trial. In an effort to enhance the efficiency and homogeneity of gene uptake in cardiac tissue, we examined the effects of nitroglycerin (NTG) in a porcine model following AAV1.SERCA2a gene delivery. Three groups of Göttingen minipigs were assessed: (i) group A: control intracoronary (IC) AAV1.SERCA2a (n = 6); (ii) group B: a single bolus IC injection of NTG (50 µg) immediately before administration of intravenous (IV) AAV1.SERCA2a (n = 6); and (iii) group C: continuous IV NTG (1 µg/kg/minute) during the 10 minutes of AAV1.SERCA2a infusion (n = 6). We found that simultaneous IV infusion of NTG and AAV1.SERCA2a resulted in increased viral transduction efficiency, both in terms of messenger RNA (mRNA) as well as SERCA2a protein levels in the whole left ventricle (LV) compared to control animals. On the other hand, IC NTG pretreatment did not result in enhanced gene transfer efficiency, mRNA or protein levels when compared to control animals. Importantly, the transgene expression was restricted to the heart tissue. In conclusion, we have demonstrated that IV infusion of NTG significantly improves cardiac gene transfer efficiency in porcine hearts.
Introduction
The sarcoplasmic reticulum Ca2+ ATPase (SERCA2a) plays a pivotal role in intracellular Ca2+ handling in cardiac myocytes1 and its expression is significantly decreased in both experimental animal models and human heart failure, which leads to abnormal Ca2+ handling and a deficient contractile state.2,3 Enhancing sarcoplasmic reticulum Ca2+ uptake is an important therapeutic target for the treatment of various cardiovascular diseases.4,5,6,7,8,9 Correction of the enzyme deficiency via SERCA2a gene transfer restores Ca2+ homeostasis, which results in significant improvement in cardiac function, energetics, and survival. The overall beneficial profile of SERCA2a targeting has led to the initiation of a First-in-Man gene therapy trial (CUPID) to investigate the effects of overexpressing SERCA2a via an adeno-associated viral (AAV) vector type 1 in patients with heart failure.10,11,12
The efficiency and homogeneity of delivery to the myocardium is an important aspect of gene therapy for cardiovascular diseases. A variety of inflammatory mediators and growth factors including histamine, bradykinin, serotonin and combinations of sildenafil, vascular endothelial growth factor, and nitroglycerin (NTG) have been used in order to increase the efficiency of cardiac gene transfer.13,14,15,16,17,18,19,20
Nitrates, such as NTG act through the nitric oxide pathway to produce vasodilatation of the coronary vasculature and are widely used in the management of coronary artery disease and symptoms of congestion with few side effects.21 NTG represents a potent vasodilator that stimulates the guanylate cyclase with a consecutive rise in cyclic guanoside monophosphate in the smooth muscle cells, inducing coronary vasodilatation and improvement in myocardial blood flow.22,23
The purpose of the current study was to evaluate the effects of intracoronary (IC) and intravenous (IV) administration of NTG on the efficiency of myocardial transduction by AAV1.SERCA2a. The overall effect was assessed 30 days after administration in swine model by measuring vector-delivered AAV1.SERCA2a genome copies, messenger RNA (mRNA), and SERCA2a protein in separate walls of heart tissue.
Results
Transduction of myocardium with AAV1-SERCA2a and NTG infusion in vivo
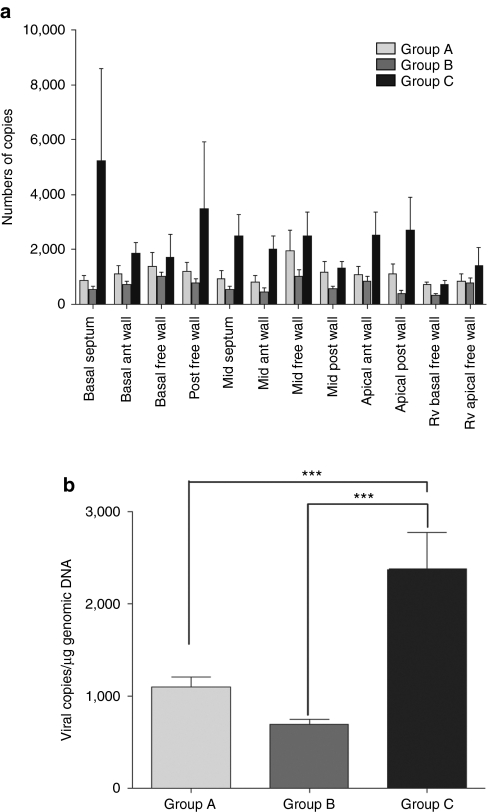
Thirty days following the administration of AAV1.SERCA2a (1012 DNAse resistant particles (DRP)), quantitative PCR analysis was performed to detect and quantify the vector DNA in 10 different areas of the left ventricle (LV) and 2 areas in the right ventricle (RV). The results showed detectable and quantifiable vector genome presence in all animals (n = 6) of the three treatment groups, with vector DNA homogeneously distributed in the LV (Figure 1a). Presence of vector genome copies in the LV was similar in animals receiving AAV1.SERCA2a infusion alone (group A) and in animals receiving a single bolus IC NTG pretreatment at 50 µg before the AAV1.SERCA2a infusion (group B) (P = nonsignificant). In contrast, the simultaneous IV administration of NTG at 1 µg/kg/minute for the entire 10 minutes of IC AAV1.SERCA2a infusion (group C) resulted in a significant increase in the viral genome copies transferred in the heart compared to group A and group B (P < 0.001) (Figure 1b).
Figure 1.
Transduction of myocardium with AAV1-SERCA2a and NTG infusion in vivo. (a) Mean (±SEM) AAV1.SERCA2a genome copies in myocardium following infusion of AAV1.SERCA2a and administration of NTG, detected in 10 separate LV and 2 RV walls in the three treatment groups. (b) Mean (±SEM) AAV1.SERCA2a genome in the myocardium (LV and RV) of the three groups (n = 6). ***P < 0.001. ant, anterior; LV, left ventricle; NTG, nitroglycerin; RV, right ventricle.
SERCA2a mRNA expression after delivery of AAV1.SERCA2a and administration of NTG
SERCA2a mRNA expression levels were also quantified 30 days after treatment in the 10 LV samples of the heart and two areas of the RV (Figure 2a). The concomitant IV administration of NTG and IC infusion AAV1.SERCA2a (group C) significantly increased SERCA2a mRNA expression levels by 2.5—in comparison to the animals receiving AAV1.SERCA2a alone (group A) (Figure 2b). In contrast, IC administration of NTG before AAV1.SERCA2a infusion produced no change in the SERCA2a mRNA expression (Figure 2b).
Figure 2.
SERCA2a mRNA expression after delivery of AAV1.SERCA2a and administration of NTG. Real-time RT-PCR analysis of SERCA2a mRNA expression in myocardium 1 month after infusion of AAV1.SERCA2a. (a) Fold change in SERCA2a mRNA expression in 10 separate LV and 2 RV walls among the three animal groups. Fold changes were calculated relative to group A as a control. (b) Results are expressed as average fold change in gene expression, as measured by RT-PCR in the myocardium (LV and RV) of the three groups, ***P < 0.001. ant, anterior; LV, left ventricle; mRNA, messenger RNA; RT-PCR, reverse transcription-PCR; RV, right ventricle.
SERCA2a protein expression after delivery of AAV1.SERCA2a and infusion of NTG
Immunoblotting was used to confirm SERCA2a protein expression 30 days after gene transfer. Figure 3a shows a representative immunoblot from the LV middle-anterior wall tissue samples (Figure 3a). Protein expression of SERCA2a was analyzed and quantified in 10 sections of the LV tissue from the three experimental animal groups and the data are summarized in Figure 3b. A significant increase (P < 0.05) in protein expression was observed in the animals after IV administration of NTG infusion (group C) compared to control group A (Figure 3b). To evaluate whether SERCA2a was expressed in other tissues after gene transfer, we examined samples of liver and lungs of all animals from each group infected with rAAV1-SERCA2a with and without NTG. A representative western blot of one animal from each group shows that SERCA2a protein expression is not observed in the lung and liver tissues in the three treated groups compared to middle-anterior and LV samples (Figure 3c).
Figure 3.
SERCA2a protein expression after delivery of AAV1.SERCA2a and infusion of NTG. (a) Representative immunoblot from the LV middle-anterior wall samples from six animals per group is shown. GAPDH was used as a loading control. (b) Quantification of SERCA2a protein expression in the whole LV in the three groups. GAPDH was used as a loading control to normalize the data *P < 0.05. (c) A representative western blot of SERCA2a is shown in LV, middle-anterior, lungs, and liver samples from one animal in each of the three groups. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; LV, left ventricle.
SERCA2a mRNA expression in cardiomyocytes after infection of AAV1.SERCA2a and treatment of NTG
To determine whether the improvement of NTG on in vivo gene transfer to the heart was due to a direct effect on viral transduction of cells, the effect of NTG on transduction of cardiac myocytes by AAV1.SERCA2a was measured in vitro. Adult rat ventricular myocytes were treated with NTG before treatment with AAV1.SERCA2a at 5 × 104 DRP/cell. Five days after AAV1.SERCA2a treatment, real-time PCR showed that NTG incubation of adult cardiac myocytes at various concentrations did not have any effect on viral transduction (Figure 4).
Figure 4.
SERCA2a mRNA expression in cardiomyocytes after infection of AAV1.SERCA2a and treatment of NTG. Mean fold change (±SEM) in SERCA2a expression compared to non-infected cells used as control. Bars depict the mean fold change in mRNA expression in adult rat ventricular myocytes in either NTG treated or untreated, and infected with AAV1.SERCA2a. Fold changes in gene expression were determined using the ΔΔCt method with normalization to β-actin endogenous control and reported to the endogenous SERCA2a expression levels in control non-infected and nontreated cells. mRNA, messenger RNA; NS, nonsignificant; NTG, nitroglycerin.
Changes in regional myocardial blood flow induced by NTG in vivo
Since myocardial viral uptake has been shown to be closely related to perfusion pressure, the effects of IC or IV injection of NTG on coronary pressure were examined. We measured systolic blood pressure, systemic vascular resistance indexed to body surface area, and pulmonary vascular resistance in four pigs before and after nicardipine, and before and after NTG. As shown in Table 1, there was a trend for systolic blood pressure to decrease with NTG but not significantly. Systemic vascular resistance and pulmonary vascular resistance indexed to body surface area were not affected by NTG.
Table 1. Nicarpidine administration effects on systemic and pulmonary vascular resistance and systolic blood pressure.

Changes in heart rate were minimal for all treated groups (data not shown). In order to quantify the changes in regional blood flow due to NTG, we injected colored microspheres into the LV (Figure 5). The microspheres were administered under baseline conditions and after the injection of IV and IC NTG (n = 3). Figure 5 shows a significant increase in regional blood flow of the anterior epicardium compared to control (0.86 ± 0.1 versus 0.52 ± 0.13, P < 0.05) as well as the epicardium of the inferior left ventricular wall (0.92 ± 0.14 versus 0.54 ± 0.16, P < 0.05) compared to baseline conditions.
Figure 5.
Changes in regional myocardial blood flow induced by NTG in vivo. The coronary flow under intravenous infusion of NTG was measured at a constant rate of 1 µg/kg/minute using coral-high colored microspheres in anterior and inferior LV. The black circle represents the baseline group without NTG; the red square represents IV-injected NTG group and blue triangle represents IC-injected NTG group, *P < 0.05. CF, coronary flow; Endo, endocardium; Epi, epicardium; IC, intracoronary; IV, intravenous; LV, left ventricle; NTG, nitroglycerin.
SERCA2a mRNA expression after delivery of AAV1.SERCA2a and administration of nicardipine
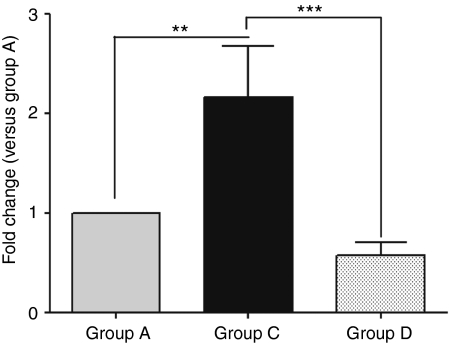
In group D in which animals received IV nicardipine during the IC AAV1.SERCA2a infusion, systemic vascular resistance decreased significantly as shown in Table 1 but there were no significant changes in systemic blood pressure. SERCA2a mRNA expression levels were quantified 30 days after treatment in the LV mid anterior and compared to LV mid-anterior of groups A and C. The concomitant IV administration of nicardipine and IC infusion AAV1.SERCA2a did not result in increased level of SERCA2a mRNA compared to group A (Figure 6).
Figure 6.
SERCA2a mRNA expression after delivery of AAV1.SERCA2a and administration of nicarpidine. Real-time RT-PCR analysis of SERCA2a mRNA expression in LV middle-anterior of groups A, C, and D, 1 month after infusion of AAV1.SERCA2a. Fold changes were calculated relative to group A as control, **P < 0.01, ***P < 0.001. LV, left ventricle; mRNA, messenger RNA; RT-PCR, reverse transcription-PCR.
Discussion
Effective and homogeneous viral gene delivery to the myocardium has been the ultimate goal of gene therapy delivery systems targeting the heart.10,24 A number of factors modulate cardiac gene transfer including perfusion, vector size and concentration, virus contact time, and vascular permeability.25
We have developed a simple method for cardiac gene delivery that optimizes to a certain degree of many of these parameters while being easily clinically applicable. The antegrade virus injection, using a slow 10-minute flow, down the coronary artery without any blockage, has yielded significant gene transfer in the myocardium even at low doses.10,11,12 In addition, this has been effectively used in the clinical catheterization laboratory, and so far, 51 patients with advanced heart failure have received this long time infusion without any complications.10,11,12
Chemical approaches which include the use of vasodilatory and permeabilizing agents have been used to facilitate transfer of vectors from the vascular lumen to the myocardium.17,25,26 In fact a number of agents that increase the permeability of the vascular bed have been used in small and large animals including NTG, nitroprusside, serotonin, bradykinin, histamine, substance P, and vascular endothelial growth factor.25,27,28 Clinically and in the setting of heart failure, these agents must be used with caution so as not to decrease systemic blood pressure. In our study, we found that IV NTG induced an increase in viral uptake within all the walls of the ventricles. The significant improvement in gene transfer efficiency noted with NTG in comparison to nicarpidine treatment may be due to a combination of enhanced coronary flow (CF) and increased vascular permeability.27 Nitric oxide donor, NTG produces coronary vasodilation29 and therefore augments virus-mediated gene transfer to the heart in vivo.30 Sasano et al. showed in a swine model, that NTG increased efficiency of AAV-overexpressing β-galactosidase gene transfer in antegrade IC vector delivery and NTG exclusion reduced gene transfer compared to baseline treatment with NTG.27
To examine the mechanisms by which the enhanced gene transfer occurred in the setting of IV NTG, we measured the effects of NTG on CF and on the uptake of AAV1 into the cardiac myocytes. In cardiac myocytes, NTG did not affect AAV1 entry or transport to the cell nucleus. Therefore, NTG had no effect on the thermodynamic interaction of AAV1 and cardiac myocyte receptors. In contrast, regional coronary blood flow increased significantly in the presence of IV NTG group. Interestingly, regional CF increased only slightly in the IC NTG group, most likely due to the short effect of NTG in the circulation. From our data, the enhanced uptake of AAV1.SERCA2a in the setting of IV NTG seems to be related in part to a combination of increased CF and enhanced permeability. This is in agreement with previously described data, indicating that IV injection of NTG resulted in a significant increase in coronary blood flow in dogs recovering from heart failure.23 In our study, we showed that nicardipine, did not influence SERCA2a mRNA expression after AAV.SERCA gene transfer, suggesting that decreasing afterload alone (nicardipine decreased systemic vascular resistance) does not influence gene delivery. Moreover, several studies showed that gene transfer efficiently correlated with coronary blood flow rate in small and large animals.26,31,32 Gene transfer was minimal when the coronary and myocardial pressures equalized and improved when the coronary pressure remained above the myocardial pressure. Taken together these results demonstrate that minimum levels of CF and pressure are needed to fill the entire microvasculature with virus-containing solution.27
The advantages of using NTG as opposed to other agents are: its safety in the setting of severe heart failure and the fact that it is distributed fast since its half time is very short in the blood stream. In addition, the effects of NTG can be quickly reversed by discontinuing the infusion. Furthermore NTG does not require invasive hemodynamic assessment when administered, as opposed to nitroprusside.13
Overall, our study shows that IV NTG is a safe and effective method to enhance AAV1 vector uptake using antegrade IC gene transfer. The use of NTG can be therefore of major importance in enhancing AAV gene transfer to the heart in current and future clinical trials.
Material and Methods
The investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Animals. Göttingen minipigs were 4 months old at the time of dosing and the weight of the animals ranged from ~8–12 kg. Animals were housed at the Skirball Center of Cardiovascular Research, Cardiovascular Research Foundation (Orangeburg, NY). Serum samples were obtained from animals before the start of the study for prescreening for anti-AAV1 neutralizing antibodies. All animals that were selected for the study had AAV1 neutralizing antibody titers of <1:2.
Experimental groups. Four of the groups studied (six animals/group): group A received IC AAV1.SERCA2a without NTG; group B received 50 µg of NTG via direct IC infusion as a single bolus injection immediately before administration of AAV1.SERCA2a using the same catheter; and group C received 1 µg/kg/minute NTG simultaneously as an IV infusion during the IC administration of AAV1.SERCA2a. Group D (n = 4) received IV nicardipine infusion (0.1 mg/kg/hour), instead of NTG during the IC administration of AAV1.SERCA2a gene transfer. Vital signs and hemodynamic monitoring (blood pressure, heart rate and rhythm, respiratory rate, O2 by pulse oximeter, and end-tidal carbon dioxide) were performed during the procedure.
Viral vector. The AAV1.SERCA2a vector was produced under cyclic guanoside monophosphate conditions at Targeted Genetics (Seattle, WA). The AAV2/1 viral particle was constructed as a hybrid or pseudotype vector in which the vector genome incorporates the capsid sequence from AAV1 and the inverted terminal repeat from native AAV2. In conjunction, the vector incorporates the human SERCA2a expression cassette under the control of the cytomegalovirus enhancer/promoter.
Gene transfer. The direct infusion system is composed of standard (commercially available) components including a conventional guide sheath, 0.014” guide wire, a 5F infusion catheter, and two programmable syringe pumps. Animals were anesthetized using standard procedures and the direct IC infusion procedure started with introduction of the conventional guide sheath using a femoral arterial approach. The Coronary Infusion Catheter (Cordis Vista Brite Tip Guiding Catheter; Cordis, Bridgewater, NJ) was then placed in the left main coronary artery under fluoroscopic guidance. Once the catheter was in place, it was connected to the first programmable syringe pump (NE-l 000 Programmable Syringe Pump; New Era Pump Systems, Farmingdale, NY) using standard tubing and purging techniques. Each animal received a single direct IC infusion of 1 × 1012 DRP AAV1.SERCA2a at a constant rate of 1.2 ml/minute over a 10-minute period, followed by a 3.0 ml of sterile saline flush (0.9% sodium chloride injection) of the catheter dead volume with the second programmable syringe pump.
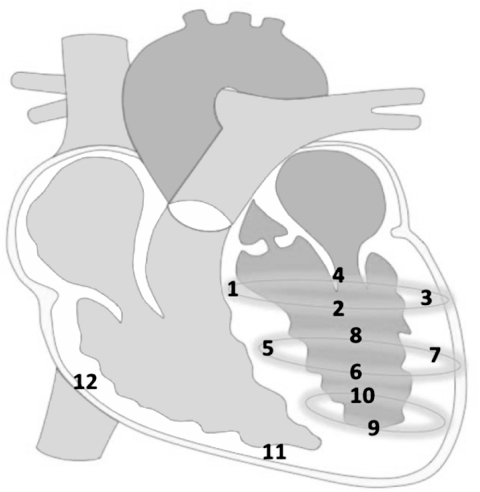
Tissue collection. Animals were sacrificed 30 days after AAV1.SERCA2a administration with the IV administration of euthasol (euthanasia solution; 390 mg/ml pentobarbital sodium and 50 mg/ml phenytoin). The tissues from 12 separate heart regions (Figure 7) were immediately placed in ice-cold 0.9% NaCl, washed three times to remove residual blood, snap frozen in liquid nitrogen and stored at −80 °C.
Figure 7.
Schema of the heart in which the left ventricle (LV) was divided in 10 separate walls and right ventricle (RV) in 2 walls. 1) Basal septum; 2) basal anterior wall; 3) basal free wall; 4) posterior free wall; 5) middle septum; 6) middle anterior wall; 7) middle free wall; 8) middle posterior wall; 9) apical anterior wall; 10) apical posterior wall; 11) RV apical free wall; 12) RV basal free wall.
CF measurement. Regional perfusion was quantified using colored microspheres. Briefly, 2 × 107 polystyrene fluorescent microspheres (15 µm) (Interactive Medical technologies, Irvine, CA) were injected into the LV. Reference blood was withdrawn for 2 minutes at a rate of 2.9 ml/minute from a femoral artery sheath using a precision pump (Harvard Apparatus, Holliston, MA).
First, purple-low colored microspheres were injected under baseline conditions. After IC injection of 50 µg NTG (American Regent, Shirley, NY), a second microsphere measurement was performed using coral-med colored microspheres. Thereafter, the CF under IV infusion of NTG was measured at a constant rate of 1 µg/kg/minute using coral-high colored microspheres. Distribution of fluorescent microspheres in the region of the anterior and inferior wall of the LV was quantified by flow cytometric analysis (Interactive Medical technologies) among all three layers of the myocardium. Regional CF was calculated using the formula: CF (ml/minute/g) = (R × lt)/(Ibr × Wt), where R = blood reference withdrawal rate (2.9 ml/minute), lt and Ibr are fluorescent counts in the tissue and the blood reference sample, respectively. Wt is the weight of the tissue sample (g).
Viral genome copies quantification. A quantitative PCR was used to detect and quantify AAV1.SERCA2a vector DNA in both the LV and RV (conducted by Althea Technologies, San Diego, CA). The quantitative PCR assay detects a 107 bp sequence unique to AAV1.SERCA2a using the ABI Prism 7500 Sequence Detection System (ABI, Foster City, CA). The number of copies of AAV1.SERCA2a detected in 1 µg of genomic DNA extracted from each tissue was quantified using serial dilutions of a plasmid containing the target sequences as standards. The lower limit detection of the assay was 20 copies/µg DNA and the lower limit of quantification was 200 copies/µg DNA.
Real-time PCR (quantitative reverse transcription-PCR). Relative gene expression was determined using two-step quantitative reverse transcription-PCR. Total RNA was isolated with TRIzol reagent (Invitrogen, Carlsbad, CA) followed by a cleanup step as described in the RNeasy Isolation kit (Qiagen, Valencia, CA) with on-column DNase I treatment to eliminate contaminating genomic DNA with RNase-Free DNase Set (Qiagen, Valencia, CA). About 1 µg of total RNA from each sample was reverse transcribed using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA) according to the manufacturer's protocol. Primers for SERCA2a were designed with the Primer Express software (forward: CTGTCCATGTCACTCCACTTCC, reverse: AGCGGTTACTCCAGTAT TGCAG). Quantitative PCR reactions were performed with Power SYBR Green Master Mix (Applied Biosystems) on an ABI Prism 7500 Real Time PCR System (ABI). The PCR protocol consisted of one cycle at 95 °C (10 minutes) followed by 40 cycles of 95 °C (15 seconds) and 60 °C (1 minute). Fold changes in gene expression were determined using the ΔΔCt method with normalization to glyceraldehyde 3-phosphate dehydrogenase endogenous control.
Immunobloting. Tissue samples (~50 mg) were homogenized in Radioimmunoprecipitation assay (RIPA) buffer (50 mmol/l Tris, pH 7.4, 150 mmol/l NaCl, 1% NP40, 0.5% sodium deoxycholate, 0.1% SDS) containing protease inhibitor cocktail. The lysates were centrifuged (12,000g, 10 minutes) and the protein concentration determined with the Bradford protein assay method. Protein lysates (10 µg) were separated on a 4–20% polyacrylamide and transferred to polyvinylidene fluoride membranes. After blocking with 5% milk in tris-buffered saline with Tween (T-TBS) buffer (50 mmol/l Tris–HCl, 150 mmol/l NaCl, 0.05% Tween-20, pH 7.4) for 1 hour at room temperature, the membrane was incubated with polyclonal rabbit anti-SERCA2a (1:3,000) at 4 °C overnight, washed three times in T-TBS and treated with anti-rabbit IRDye-680-conjugated secondary antibody for 1 hour at room temperature. The blots were washed in TBS-T and the signals were detected in the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
Adult rat ventricular myocyte isolation. All experiments were performed in compliance with the Animal Welfare Act regulations and Public Health Service policies and were approved by the Institutional Animal Care and Use Committee at Mount Sinai School of Medicine. Ventricular myocytes were isolated from adult male Sprague Dawley rats, ~300 g in weight, using a modified protocol from O'Connell et al.33 Briefly, the rat was anaesthetized using ketamine (2 mg/g), the heart excised, cannulated to a Langendorff perfusion system and perfused at a constant flow of 4 ml/minute first with perfusion buffer (in: 120.4 mmol/l NaCl, 14.7 mmol/l KCl, 0.6 mmol/l KH2PO4, 0.6 mmol/l Na2HPO4, 1.2 mmol/l MgSO4-7H2O, 10 mmol/l NaO4S, 4.6 mmol/l NaHCO3) for 3 minutes and then with collagenase type II (Worthington Biochemical, Lakewood, NJ) at a final concentration of 2 mg/ml. After 20 minutes of digestion, the heart appeared flaccid, at which point it was transferred to a petri dish and was further dissociated mechanically by trituration. From this point forward, all manipulations were performed under a cell culture hood. The calcium was reintroduced in five 4-minute steps (CaCl2 in µmol/l: 62.5, 125, 250, 500, 1,000). The cardiomyocytes were centrifuged at 50g for 1 minute and resuspended in culture medium (Medium 199 supplemented with 1× Insulin, Tranferrin, Selenium (ITS; Invitrogen), 10 mmol/l 2,3-Butanedione monoxime (BDM; Sigma Aldrich, St Louis, MO), 1% bovine serum albumin (Sigma Aldrich), 1% Penicillin-Streptomycin (Invitrogen)). At this point the viability was determined as percentage of rod-shaped cells. Only isolations with >80% rod-shaped cells were used for downstream applications. Cells were plated at 5 × 104 cells/well onto 12 well laminin-coated (5 µg/ml, 37 °C for 30 minutes) culture dishes. The cells were allowed to attach for 1 hour before treatment with NTG and infection.
Treatment with NTG. Adult rat ventricular myocytes in culture were treated with NTG (American Regent) using the following concentrations: 100, 10, 1, 0.1 µmol/l and infected with AAV1.SERCA2a at 105 vg/cell (5 × 109 DRP/well). Control cardiomyocytes were infected with AAV1.SERCA2a without NTG, or were left untreated. Five days later the cells were washed twice with phosphate-buffered saline and collected in RNAeasy Lysis (RLT) buffer (Qiagen).
Statistical analysis. The results are presented as means ± SEM. Statistical differences between groups were analyzed using one-way analysis of variance. Comparisons were determined using Bonferroni-procedure test with *P < 0.05, **P < 0.01, and ***P < 0.001.
Acknowledgments
This work is supported by the Leducq Foundation through the CAERUS network (R.J.H.), by NIH R01 HL093183, HL088434, HL071763, HL080498, HL083156, and P20HL100396 (R.J.H.). L.H. was supported by NIH K01 HL103176. D.L. was supported in part by an award from the German Research Foundation (DFG). R.J.H. and K.Z. are the scientific cofounder of Celladon, with plans to commercialize AAV1.SERCA2a for the treatment of heart failure. The other authors declared no conflict of interest.
REFERENCES
- Bers DM. Calcium fluxes involved in control of cardiac myocyte contraction. Circ Res. 2000;87:275–281. doi: 10.1161/01.res.87.4.275. [DOI] [PubMed] [Google Scholar]
- de la Bastie D, Levitsky D, Rappaport L, Mercadier JJ, Marotte F, Wisnewsky C.et al. (1990Function of the sarcoplasmic reticulum and expression of its Ca2(+)-ATPase gene in pressure overload-induced cardiac hypertrophy in the rat Circ Res 66554–564. [DOI] [PubMed] [Google Scholar]
- Mercadier JJ, Lompré AM, Duc P, Boheler KR, Fraysse JB, Wisnewsky C.et al. (1990Altered sarcoplasmic reticulum Ca2(+)-ATPase gene expression in the human ventricle during end-stage heart failure J Clin Invest 85305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Monte F., and, Hajjar RJ. Efficient viral gene transfer to rodent hearts in vivo. Methods Mol Biol. 2003;219:179–193. doi: 10.1385/1-59259-350-x:179. [DOI] [PubMed] [Google Scholar]
- del Monte F., and, Hajjar RJ. Targeting calcium cycling proteins in heart failure through gene transfer. J Physiol (Lond) 2003;546 Pt 1:49–61. doi: 10.1113/jphysiol.2002.026732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawase Y., and, Hajjar RJ. The cardiac sarcoplasmic/endoplasmic reticulum calcium ATPase: a potent target for cardiovascular diseases. Nat Clin Pract Cardiovasc Med. 2008;5:554–565. doi: 10.1038/ncpcardio1301. [DOI] [PubMed] [Google Scholar]
- Kawase Y, Ly HQ, Prunier F, Lebeche D, Shi Y, Jin H.et al. (2008Reversal of cardiac dysfunction after long-term expression of SERCA2a by gene transfer in a pre-clinical model of heart failure J Am Coll Cardiol 511112–1119. [DOI] [PubMed] [Google Scholar]
- Miyamoto MI, del Monte F, Schmidt U, DiSalvo TS, Kang ZB, Matsui T.et al. (2000Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure Proc Natl Acad Sci USA 97793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano FJ, He H, McDonough P, Meyer M, Sayen MR., and, Dillmann WH. Adenovirus-mediated gene transfer reconstitutes depressed sarcoplasmic reticulum Ca2+-ATPase levels and shortens prolonged cardiac myocyte Ca2+ transients. Circulation. 1997;96:400–403. doi: 10.1161/01.cir.96.2.400. [DOI] [PubMed] [Google Scholar]
- Hajjar RJ, Zsebo K, Deckelbaum L, Thompson C, Rudy J, Yaroshinsky A.et al. (2008Design of a phase ½ trial of intracoronary administration of AAV1/SERCA2a in patients with heart failure J Card Fail 14355–367. [DOI] [PubMed] [Google Scholar]
- Jaski BE, Jessup ML, Mancini DM, Cappola TP, Pauly DF, Greenberg B.et al. (2009Calcium upregulation by percutaneous administration of gene therapy in cardiac disease (CUPID Trial), a first-in-human phase ½ clinical trial J Card Fail 15171–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessup M, Greenberg B, Mancini D, Cappola T, Pauly DF, Jaski B.et al. (2011Calcium Upregulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID): a phase 2 trial of intracoronary gene therapy of sarcoplasmic reticulum Ca2+-ATPase in patients with advanced heart failure Circulation 124304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth DM, Lai NC, Gao MH, Fine S, McKirnan MD, Roth DA.et al. (2004Nitroprusside increases gene transfer associated with intracoronary delivery of adenovirus Hum Gene Ther 15989–994. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Gu Y, Iwanaga Y, Hoshijima M, Oh SS, Giordano FJ.et al. (2002Restoration of deficient membrane proteins in the cardiomyopathic hamster by in vivo cardiac gene transfer Circulation 105502–508. [DOI] [PubMed] [Google Scholar]
- Ding Z, Fach C, Sasse A, Gödecke A., and, Schrader J. A minimally invasive approach for efficient gene delivery to rodent hearts. Gene Ther. 2004;11:260–265. doi: 10.1038/sj.gt.3302167. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Wightman LM, Latchman DS., and, Marber MS. In vivo myocardial gene transfer: optimization and evaluation of intracoronary gene delivery in vivo. Gene Ther. 2001;8:1833–1839. doi: 10.1038/sj.gt.3301614. [DOI] [PubMed] [Google Scholar]
- Donahue JK, Kikkawa K, Thomas AD, Marban E., and, Lawrence JH. Acceleration of widespread adenoviral gene transfer to intact rabbit hearts by coronary perfusion with low calcium and serotonin. Gene Ther. 1998;5:630–634. doi: 10.1038/sj.gt.3300649. [DOI] [PubMed] [Google Scholar]
- Logeart D, Hatem SN, Heimburger M, Le Roux A, Michel JB., and, Mercadier JJ. How to optimize in vivo gene transfer to cardiac myocytes: mechanical or pharmacological procedures. Hum Gene Ther. 2001;12:1601–1610. doi: 10.1089/10430340152528101. [DOI] [PubMed] [Google Scholar]
- Logeart D, Hatem SN, Rücker-Martin C, Chossat N, Névo N, Haddada H.et al. (2000Highly efficient adenovirus-mediated gene transfer to cardiac myocytes after single-pass coronary delivery Hum Gene Ther 111015–1022. [DOI] [PubMed] [Google Scholar]
- Wang Y, Boros P, Liu J, Qin L, Bai Y, Bielinska AU.et al. (2000DNA/dendrimer complexes mediate gene transfer into murine cardiac transplants ex vivo Mol Ther 2602–608. [DOI] [PubMed] [Google Scholar]
- Elkayam U, Janmohamed M, Habib M., and, Hatamizadeh P. Vasodilators in the management of acute heart failure. Crit Care Med. 2008;36 suppl. 1:S95–105. doi: 10.1097/01.CCM.0000297161.41559.93. [DOI] [PubMed] [Google Scholar]
- Dinerman JL, Lawson DL., and, Mehta JL. Interactions between nitroglycerin and endothelium in vascular smooth muscle relaxation. Am J Physiol. 1991;260 3 Pt 2:H698–H701. doi: 10.1152/ajpheart.1991.260.3.H698. [DOI] [PubMed] [Google Scholar]
- Gill RM, Braz JC, Jin N, Etgen GJ., and, Shen W. Restoration of impaired endothelium-dependent coronary vasodilation in failing heart: role of eNOS phosphorylation and CGMP/cGK-I signaling. Am J Physiol Heart Circ Physiol. 2007;292:H2782–H2790. doi: 10.1152/ajpheart.00831.2006. [DOI] [PubMed] [Google Scholar]
- Hajjar RJ, Schmidt U, Matsui T, Guerrero JL, Lee KH, Gwathmey JK.et al. (1998Modulation of ventricular function through gene transfer in vivo Proc Natl Acad Sci USA 955251–5256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Marbán E, Lawrence JH., and, Donahue JK. Phosphodiesterase inhibitor-mediated potentiation of adenovirus delivery to myocardium. J Mol Cell Cardiol. 2001;33:575–580. doi: 10.1006/jmcc.2000.1322. [DOI] [PubMed] [Google Scholar]
- Donahue JK, Kikkawa K, Johns DC, Marban E., and, Lawrence JH. Ultrarapid, highly efficient viral gene transfer to the heart. Proc Natl Acad Sci USA. 1997;94:4664–4668. doi: 10.1073/pnas.94.9.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasano T, Kikuchi K, McDonald AD, Lai S., and, Donahue JK. Targeted high-efficiency, homogeneous myocardial gene transfer. J Mol Cell Cardiol. 2007;42:954–961. doi: 10.1016/j.yjmcc.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwathmey JK, Yerevanian AI., and, Hajjar RJ. Cardiac gene therapy with SERCA2a: from bench to bedside. J Mol Cell Cardiol. 2011;50:803–812. doi: 10.1016/j.yjmcc.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman S, Moreno R, Rowe KA., and, Verrier RL. Persistent primary coronary dilation induced by transatrial delivery of nitroglycerin into the pericardial space: a novel approach for local cardiac drug delivery. J Am Coll Cardiol. 1999;33:2073–2077. doi: 10.1016/s0735-1097(99)00131-x. [DOI] [PubMed] [Google Scholar]
- Roth DM, Lai NC, Gao MH, Drumm JD, Jimenez J, Feramisco JR.et al. (2004Indirect intracoronary delivery of adenovirus encoding adenylyl cyclase increases left ventricular contractile function in mice Am J Physiol Heart Circ Physiol 287H172–H177. [DOI] [PubMed] [Google Scholar]
- Emani SM, Shah AS, Bowman MK, Emani S, Wilson K, Glower DD.et al. (2003Catheter-based intracoronary myocardial adenoviral gene delivery: importance of intraluminal seal and infusion flow rate Mol Ther 8306–313. [DOI] [PubMed] [Google Scholar]
- Champion HC, Georgakopoulos D, Haldar S, Wang L, Wang Y., and, Kass DA. Robust adenoviral and adeno-associated viral gene transfer to the in vivo murine heart: application to study of phospholamban physiology. Circulation. 2003;108:2790–2797. doi: 10.1161/01.CIR.0000096487.88897.9B. [DOI] [PubMed] [Google Scholar]
- O'Connell KM, Whitesell JD., and, Tamkun MM. Localization and mobility of the delayed-rectifer K+ channel Kv2.1 in adult cardiomyocytes. Am J Physiol Heart Circ Physiol. 2008;294:H229–H237. doi: 10.1152/ajpheart.01038.2007. [DOI] [PubMed] [Google Scholar]