Abstract
Inhibition of the inhibitor of kappa B kinase (IKK)/nuclear factor-kappa B (NF-κB) pathway enhances muscle regeneration in injured and diseased skeletal muscle, but it is unclear exactly how this pathway contributes to the regeneration process. In this study, we examined the role of NF-κB in regulating the proliferation and differentiation of muscle-derived stem cells (MDSCs). MDSCs isolated from the skeletal muscles of p65+/– mice (haploinsufficient for the p65 subunit of NF-κB) had enhanced proliferation and myogenic differentiation compared to MDSCs isolated from wild-type (wt) littermates. In addition, selective pharmacological inhibition of IKKβ, an upstream activator of NF-κB, enhanced wt MDSC differentiation into myotubes in vitro. The p65+/– MDSCs also displayed a higher muscle regeneration index than wt MDSCs following implantation into adult mice with muscular dystrophy. Additionally, using a muscle injury model, we observed that p65+/– MDSC engraftments were associated with reduced inflammation and necrosis. These results suggest that inhibition of the IKK/NF-κB pathway represents an effective approach to improve the myogenic regenerative potential of MDSCs and possibly other adult stem cell populations. Moreover, our results suggest that the improved muscle regeneration observed following inhibition of IKK/NF-κB, is mediated, at least in part, through enhanced stem cell proliferation and myogenic potential.
Introduction
Nuclear factor-kappa B (NF-κB) is a ubiquitously expressed nuclear transcription factor that is evolutionarily conserved. In mammals, the NF-κB family consists of five subunits, p65 (RelA), c-Rel, RelB, p50, and p52.1 Transcriptionally active NF-κB exists as a dimer, with the most common form being a p50–p65 heterodimer. Under nonstress conditions, the heterodimer is maintained in an inactive state in the cytoplasm via its interaction with inhibitor of kappa B (IkB) proteins. Classic NF-κB activation is mediated by IkB kinase (IKK), a large, 700–900 kDa complex consisting of two catalytic subunits, IKKα and IKKβ, and a regulatory subunit named IKKγ or NEMO (NF-κB essential modulator). In response to a variety of stimuli, including proinflammatory cytokines, bacterial products, viruses, growth factors, and oxidative stress, the complex is activated. Activated IKKβ phosphorylates IkB, leading to its polyubiquitylation and subsequent degradation by the 26S proteasome. IkB degradation allows NF-κB to translocate to the nucleus where it binds to its cognate DNA site, as well as coactivators such as CBP/p300, to induce gene expression.2,3,4,5 Dysregulation of this pathway can result in chronic activation of IKK or NF-κB, and is seen in several pathophysiological states including cancer, rheumatoid arthritis, sepsis, muscular dystrophy, heart disease, inflammatory bowel disease, bone resorption, and both type I and II diabetes.6,7
The NF-κB pathway, long recognized as an important component of innate and adaptive immunity, has also more recently emerged as a key player in the regulation of skeletal muscle homeostasis.8 Furthermore, activation of NF-κB in skeletal muscle has been linked to cachexia, muscular dystrophies, and inflammatory myopathies.9,10,11,12,13 Conversely, knockout of p65, but not other subunits of NF-κB, enhances myogenic activity in MyoD-expressing mouse embryonic fibroblasts.14 Although it is known that genetic depletion of p65 enhances muscle regeneration in both mdx and wild-type (wt) murine skeletal muscle,13 the mechanism through which reduced of NF-κB activity positively impacts skeletal muscle remains unclear.
Given that the repair of damaged tissues is mediated by adult stem cell populations, we hypothesized that NF-κB activity negatively regulates muscle stem cell function. In this study, we specifically focus on the role of p65 in regulating muscle-derived stem cell (MDSC) growth and differentiation. This population of adult stem cells is capable of restoring muscle function.15,16 As complete knockout of p65 (p65–/–) results in embryonic lethality, we isolated MDSCs from the skeletal muscles (SKM) of p65+/– mice and wt littermates.17 We observed that, in vitro, p65 haploinsuffiency was associated with increased cell proliferation and myogenic differentiation. Pharmacologic inhibition of IKK/NF-κB also enhanced myogenic differentiation. We also demonstrated that p65+/– MDSCs have a higher capacity for muscle regeneration after implantation into dystrophic, mdx mouse SKM. Furthermore, we show that muscle inflammation and necrosis post-injury is decreased following p65+/– MDSC implantation into cardiotoxin (CTX) injured SKM. These results suggest that reducing the activity of the IKK/NF-κB pathway is an effective approach to improve the myogenic potential of MDSCs and possibly other adult stem cell populations. Our results provide a novel mechanistic insight as to why the inhibition of this pathway promotes SKM healing.
Results
Isolation and phenotypic characterization of MDSCs from p65+/– and wt mice
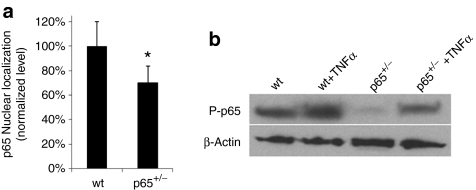
To examine the effect of NF-κB activity on MDSC function, we purified populations of muscle stem cells from the SKM of mice heterozygous for the p65 subunit of NF-κB (p65+/–) and wt littermates. Using a modified preplate technique,18 we isolated independent populations of MDSCs from three mice of each genotype. To confirm that p65 haploinsufficiency reduced basal levels of NF-κB activity, nuclear p65 was measured via ArrayScan. Nuclear, or active, p65 was found to be 30% lower in p65+/– than the wt MDSCs (Figure 1a). Upon activation, NF-κB subunits undergo post-translational modifications, such as phosphorylation, to enhance their activity.19 Immunoblot analysis revealed that the level of phosphorylated p65 (P-p65) was also reduced; however, stimulation with tumor necrosis factor-α (TNFα) led to an increased level of P-p65 in both wt and p65+/– MDSCs (Figure 1b), demonstrating that basal, but not induced, NF-κB activity is affected by knocking-out one allele of p65.
Figure 1.
Muscle-derived stem cells (MDSCs) obtained from the skeletal muscles (SKM) of p65+/– mice have a lower level of activated p65 compared to wild-type (wt) MDSCs. (a) ArrayScan analysis of nuclear p65 in MDSCs isolated from p65+/– and wt mice. Error bars indicate “mean + SD.” (b) Immunoblotting for phosphorylated p65 in whole cell lysates of MDSCs before and after tumor necrosis factor-α (TNFα) stimulation for 30 minutes.
To confirm the MDSC phenotype of p65+/– and wt cells, each population was analyzed for the expression of stem (CD34, Sca-1), myogenic (MyoD, desmin), and endothelial (CD144, CD31) cell markers by reverse transcriptase-PCR. For each of the markers, there was variability in expression between cell populations of a single genotype. However upon quantification, no significant differences were found between the different genotypes, with the exception of CD144, which was elevated in p65+/– MDSCs. (Figure 2a,c, P < 0.05). Such variability in marker expression has been previously reported and interpreted as evidence that these cell populations contain a mixture of stem and committed progenitor cells.20,21 We next examined the expression of Pax7 and MyoD protein by immmunostaining, and also found no significant difference between p65+/– and wt cells (Figure 2b,d). These results suggest that genetic reduction of p65 does not dramatically alter the phenotype of MDSCs.
Figure 2.
p65+/– and wild-type (wt) muscle-derived stem cells (MDSCs) exhibit a similar molecular marker profile. (a) RNA was isolated from three independent cell populations of each genotype. Reverse transcriptase-PCR (RT-PCR) was performed to characterize the MDSC populations for the expression of stem (CD34 and Sca-1), endothelial (CD31 and CD144) and myogenic (MyoD and desmin) cell markers. (b) Immunostaining for the muscle stem cell markers Pax7 and MyoD was also performed (bar = 25 µm). (c) Quantification of RT-PCR results. Error bars indicate “mean + SD” (n = 3 independent experiments). (d) Quantification of Immunostaining of Pax7 and MyoD.
p65+/– MDSCs proliferate faster than wt MDSCs
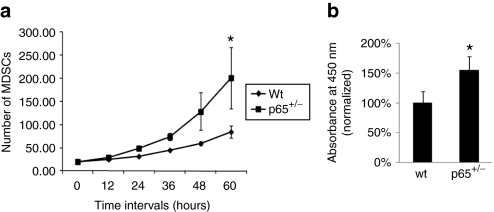
NF-κB is known to regulate cell division, so we investigated whether p65 reduction would alter MDSC proliferation. The three populations of p65+/– and wt MDSCs were plated in collagen-coated flasks and expanded in growth medium for 10–12 passages. Cells were then transferred to 24-well plates and proliferation measured using a previously described Live Cell Imaging system.22 We observed that p65+/– MDSCs proliferated significantly faster than wt cells (Figure 3a and Supplementary Videos S1–S2). Equal numbers of cells were also plated on a 96-well plate and grown for three days at which point the differences in cell number were determined using an MTS assay. This assay demonstrated a similar significant increase in cell proliferation in p65+/– MDSCs (Figure 3b) suggesting that NF-κB, and in particular p65, limits the proliferation of MDSCs.
Figure 3.
Muscle-derived stem cells (MDSCs) isolated from p65+/– mouse skeletal muscles (SKM) have a higher rate of proliferation than wild-type (wt) cells. (a) Cell proliferation rate was measured by Live Cell Imaging and (b) by an MTS assay (P < 0.05).
p65+/– MDSCs have enhanced myogenic differentiation compared to wt cells
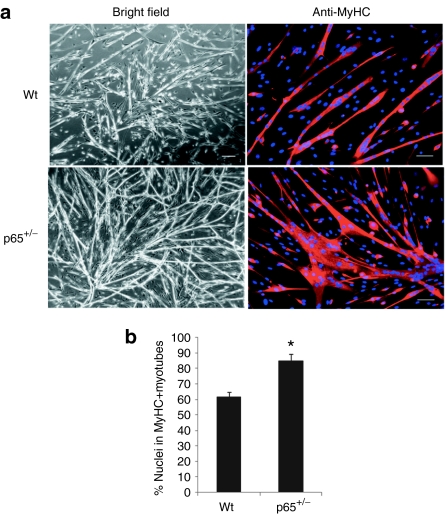
We next measured the ability of the p65+/– and wt MDSCs to undergo myogenic differentiation in vitro. Equal numbers of cells were plated in a 24-well plate and switched to differentiation medium once the cells adhered. After 3 days the majority (80%) of the p65+/– cells had differentiated into myotubes, as determined by immunodetection of myosin heavy chain (Figure 4a). The differentiation potential of the p65+/– MDSCs was significantly greater than the wt MDSCs (60%; P < 0.01; Figure 4b). The difference was also demonstrated using the live cell imaging system described above (Supplementary Videos S3–S4). These results demonstrate that NF-κB, and in particular p65, represses MDSC differentiation in vitro.
Figure 4.
Myogenic differentiation is enhanced in muscle-derived stem cells (MDSCs) isolated from p65+/– mouse skeletal muscles (SKM) compared to wild-type (wt) MDSCs in vitro. (a) MDSCs were cultured in myogenic differentiation medium for 3 days, during which cell fusion into multinucleated myotubes was monitored using bright field microscopy and then confirmed by immunostaining for myosin heavy chain (MyHC-f). (b) Quantitation of MyHC-f positive myotubes. The percentage of differentiated myotubes was quantified as the number of nuclei in MyHC-f positive myotubes relative to the total number of nuclei. A total of three populations of p65+/– and wild-type (wt) MDSCs were tested (P < 0.05). In panel “a” all bars = 200 µm on bright field and all bars = 50 µm on MyHC immunostaining.
Pharmacologic inhibition of IKKβ increases myogenic differentiation in vitro
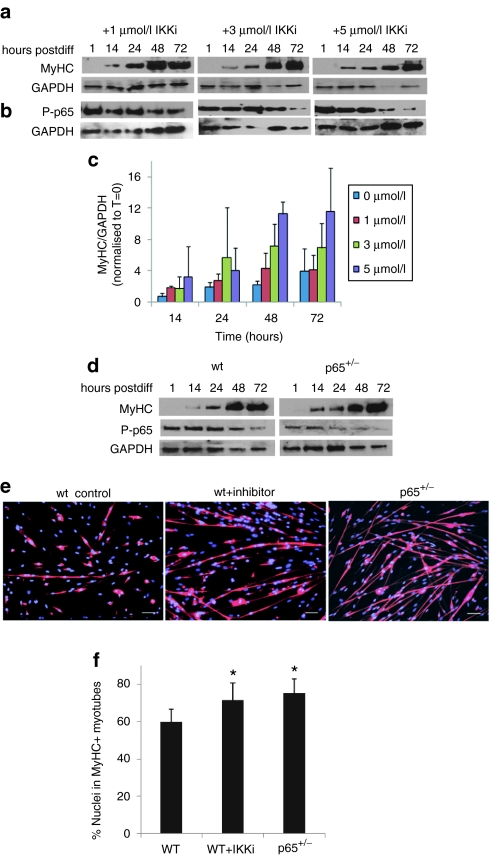
To confirm this finding implicating NF-kB as negatively impacting MDSC differentiation, we tested whether a pharmacologic inhibitor of NF-κB could enhance MDSC myogenic potential in vitro. wt MDSCs were exposed to differentiation medium containing various doses of IKK-2 inhibitor IV (IKKi), a specific, reversible inhibitor of IKKβ. Cell lysates were collected at 0, 1, 14, 24, 48, and 72 hours following treatment. Accordingly, myosin heavy chain (MyHC) levels dramatically increased, beginning at 14 hours (Figure 5a,c). As expected, we observed a robust time-dependent decrease in P-p65 that was dose-dependent (greater at 3 µmol/l than 1 µmol/l; Figure 5b). We next examined NF-κB activity in wt and p65+/– MDSCs at various time points during myogenic differentiation by immunodetection of P-p65 and MyHC. In wt cells, beginning at 48 hours post-transition to differentiation medium, the levels of p-p65 were detectably reduced (Figure 5d). This occurred more rapidly (by 24 hours) in p65+/– cells. Similarly, accumulation of MyHC was greater at earlier time points (14 hours) in p65+/– cells than wt. This timeframe for MyHC accumulation is similar to that observed in wt cells treated with IKKi (Figure 5a). In order to verify that increased MyHC expression was concomitant with increased myotube formation, we treated wt MDSCs with 5 µmol/l IKKi. After 3 days, differentiation was assessed by immunofluorescence detection of MyHC. As shown in Figure 5e, compared to nontreated controls, the inhibitor caused a significant increase in myotube formation. The level of myogenic differentiation was comparable to that of p65+/– MDSCs (P < 0.01; Figure 5f). These results provide strong support that MDSC myogenic potential can be improved using NF-κB inhibition ex vivo.
Figure 5.
Muscle-derived stem cells (MDSCs) myogenic differentiation is enhanced by pharmacological inhibition of inhibitor of kappa B kinase β (IKKβ). (a) Western blot for myosin heavy chain (MyHC) over 72 hours of wild-type (wt) MDSCs treated with 1, 3, or 5 µmol/l IKKi during differentiation. (b) Western blot for P-p65 over 72 hours of wt MDSCs treated with 1, 3, or 5 µmol/l IKK inhibitor (IKKi) during differentiation. (c) Quantification of b, myosin heavy chain (MyHC-f) levels during differentiation (n = 3 independent experiments). (d) In parallel, wt and p65+/– MDSCs were cultured in differentiation medium and lysates were collected at the various time points indicated. Lysates were used for western blot for MyHC and P-p65 levels. (e) wt MDSCs, p65+/– MDSCs, and wt MDSCs treated with IKKi (5 µmol/l), were grown under fusion conditons for 72 hours and immunostained for MyHC-f expression (f) Quantification of MyHC-f staining (P < 0.05). In panel “e” all bars = 100 µm.
p65+/– MDSCs have greater muscle regenerative capacity in vivo
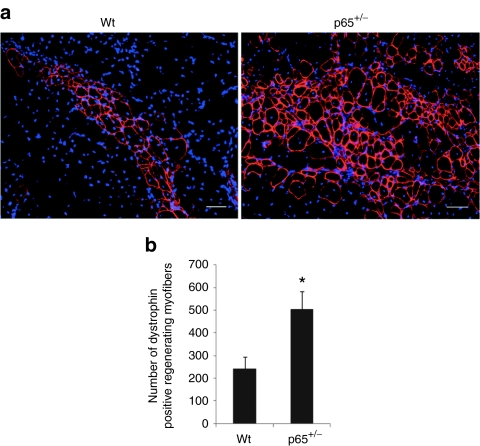
To determine whether genetic depletion of p65 increases the engraftment and muscle regenerative capacity of MDSCs in vivo, we examined the ability of p65+/– and wt MDSCs to regenerate muscle fibers following their intramuscular implantation into an immunocompromised model of Duchenne muscular dystrophy. For these experiments, 3 × 105 p65+/– and wt MDSCs were injected into the gastrocnemius muscles of 8-week-old dystrophin-deficient SCID (mdx/SCID) mice. Fourteen days after implantation, significantly more dystrophin-positive myofibers were detected in the muscle injected with p65+/– MDSCs than in muscle injected with wt MDSCs (P < 0.01; Figure 6a,b). These results confirm our in vitro observations and may provide a novel mechanism as to why IKK inhibitors have been reported to improve muscle regeneration.23
Figure 6.
Heterozygous deletion of p65 promotes the regeneration capacity of muscle-derived stem cells (MDSCs). (a) Gastrocnemius cryosections from 8-week-old mdx/SCID mice in which p65+/– and wild-type (wt) MDSCs were implanted. Engraftment was determined by immunostaining for dystrophin (red). Three populations of p65+/– and wt MDSCs were transplanted into 12 mice in two independent experiments. (b) Quantitation of regenerated dystrophin-positive myofibers (P < 0.05). In panel “a” all bars = 50 µm. Error bars indicate “mean + SD” (n = 12).
Transplantation of p65+/– MDSCs postinjury reduces SKM inflammation and necrosis
The results above suggest that lowering basal levels of NF-κB activity increased the ability of MDSCs to engraft and differentiate following intramuscular injection (Figure 6). However, while it is possible this is mediated through enhanced proliferation and differentiation, the exact mechanism as to why more dystrophin-positive myofibers were found within the p65+/– MDSC engraftment sites remains unclear. Surrounding the engraftments of the wt MDSCs, we observed numerous cells positive for the macrophage marker CD14 as detected by immunofluorescent staining (data not shown), whereas the p65+/– MDSC engraftment sites were surrounded by fewer numbers of CD14+ cells (data not shown). As the mdx/SCID is an immunocompromised mouse model with a high level of background inflammation, we decided to further investigate this phenomenon using the well-established CTX muscle injury model in immunocompetent wt mice. In order to confirm that transplanted p65+/– MDSCs are able to reduce inflammation in host skeletal muscle, we injected p65+/– and wt MDSCs into the gastrocnemius muscles of 8-week-old C57BL/6J mice 24 hours post-CTX injury. Six days post-transplantation, the wt MDSC engraftment area demonstrated a greater number of inflammatory cells surrounding the wt donor MDSCs than the p65+/– MDSCs. Furthermore, numerous centrally located nuclei, characteristic of regenerating muscle fibers, were found within the p65+/– MDSC injection sites. Consistent with observations made in mdx/SCID mice, the p65+/– MDSC engraftment area was associated with significantly fewer CD14+ cells than the wt MDSC engraftment area (P < 0.01; Figure 7a,b). There was also a significant (42%) reduction in tissue necrosis, as determined by quantification of mouse immunoglobulin G (IgG) staining (P < 0.01; Figure 7a,c). These results suggest that the improved engraftment and differentiation of p65+/– MDSCs is potentially due to their ability to attenuate the inflammation and necrosis that typically occurs after muscle injury.
Figure 7.
p65+/– muscle-derived stem cells (MDSCs) attenuate muscle inflammation and necrosis. (a) Gastrocnemius cryosections from 8-week-old C57BL/6J mice, which were injected with p65+/– or wild-type (wt) MDSCs 24 hours post-CTX injury. LacZ and eosin staining identified the injection area and immunostaining for CD14 (red) and mouse immunoglobulin G (IgG) (red) identified macrophages and necrotic tissue, respectively. In immunological stains, fluorescent green beads in C57BL/6J muscle sections confirmed the location of injection sites. (b) Quantitation of CD14+ cells (the data represent six muscles per group). (c) Necrotic area in the gastrocnemius muscles was identified by IgG staining and quantified based on the total positive area per image (the data represent six muscles per group). In panel “a” all bars = 100 µm on LacZ+eosin and mouse IgG staining. All bars = 50 µm on CD14 staining.
Discussion
NF-κB signaling has been implicated in the regulation of muscle degeneration and regeneration. The five mammalian NF-κB transcription factors are all expressed in skeletal muscle to modulate a variety of processes, including apoptosis, inflammation, and myoblast differentiation. Although there have been conflicting results reported as to whether NF-κB acts as a repressor or promoter of myogenesis,8,24,25,26,27,28,29 recent results suggest that the classical NF-κB signaling pathway functions as a negative regulator of myogenesis.14 In addition, NF-κB activation is associated with the degeneration and/or lack of regeneration of dystrophic muscle in mdx mice.13 Thus, in this study, we examined the effect of NF-κB reduction on the proliferation and differentiation of MDSCs isolated from wt mice and mice heterozygous for p65. Although p65+/– MDSCs had more than a 30% reduction in p65/NF-kB levels compared to the wt MDSCs, the two genotypes expressed similar stem (CD34, Sca-1), myogenic (desmin, MyoD) and endothelial (CD144, CD31) cell markers. This result suggests that the reduction in NF-kB did not affect overall expression of MDSC markers, albeit there is some variability in stem cell marker expression between populations of the same genotype.
We observed that MDSCs with reduced p65 levels have improved proliferation compared to wt cells, suggesting that p65/NF-κB activity negatively controls MDSC expansion. More importantly, we also observed that both the rate and extent of myogenic differentiation was accelerated in MDSCs with reduced p65 and in wt MDSCs treated with an IKKβ inhibitor. Together, these data suggest that NF-κB inhibits muscle stem cell differentiation. Our results are in agreement with previous studies showing that regulation of myogenesis is dependent on p65 transcriptional activity, which is able to inhibit myogenesis.14 It has been suggested previously that the negative effect of NF-κB on differentiation is mediated through the transcriptional activation of cyclin D1 and YinYang1 (YY1).30,31 Interestingly, we have observed a reduction in the level of cyclin D1 in p65+/– MDSCs compared to wt cells, but found no difference in the level of YY1 expression (data not shown).
Recent genetic evidence supports the role of IKK/NF-κB in driving the pathogenesis of muscular dystrophy, identifying this signaling pathway a potential therapeutic target for the treatment of DMD.13 The activity of NF-κB in dystrophic muscle is associated with not only immune cells, but also regenerative muscle fibers. Thus, we investigated whether the p65+/– MDSCs have a higher muscle regeneration potential than wt MDSCs after their intramuscular injection into dystrophic mdx/SCID skeletal muscles. Our results demonstrated that p65+/– MDSCs are more efficient at regenerating dystrophin-positive myofibers compared to wt MDSCs, which is consistent with the enhanced ability of the p65+/– MDSCs to differentiate in culture.
We also assessed inflammation around the engrafted site by immunofluorescent staining for CD14, a macrophage marker. While we found very few CD14+ cells within the injection sites of the p65+/– cells, many CD14+ cells were detected within the wt MDSC engraftment areas. As a decreased number of macrophages in the p65+/– cell engraftment area correlated with a reduction in necrosis, it is possible that a reduction in p65 enhances the local anti-inflammatory properties of MDSCs via regulation of paracrine factors. Several cytokines under the control of NF-κB, such as TNFα and interleukin-6 (IL-6), are potent inhibitors of myogenic differentiation.8 Thus, taken together, these results suggest that inhibition of NF-κB/p65 may enhance myogenesis by reducing inflammation and necrosis.
During regeneration following injury, numerous paracrine factors such as myostatin, hepatocyte growth factor, and basic fibroblast growth factor play critical roles coordinating repair.32 Other groups have demonstrated the importance of non-NF-kB proteins in muscle development and pathology. For example, myostatin acts independently of the classical TNFα and NF-kB pathway to inhibit MyoD expression and signal cachexia by reversing the IGF-1/PI3K/AKT hypertrophy pathway.33 However, other factors, such as hepatocyte growth factor and IL-6, have been reported to activate NF-кB.34 Given the numerous growth factors and cytokines present in damaged and regenerating muscle, the NF-кB pathway likely directs inflammation or regeneration in response to more than one factor. In summary, here we described a negative role for the p65/NF-κB signaling pathway in MDSC growth and differentiation in vitro, as well as muscle regeneration in vivo. Similarly, pharmacological inhibition of IKKβ identifies the IKK/NF-κB signaling pathway as a potential therapeutic target to improve the myogenic potential of MDSCs and muscle regeneration after injury and diseases.
Materials and Methods
Animals. The C57BL/6J mice heterozygous for p65/RelA were originally described by Amer Beg (Cambridge, MA).17 The mdx/SCID (C57BL/10ScSn DMDmdx/J/CB17-Prkdcscid/J) and C57BL/6J mice were obtained from the Jackson Laboratory (Bar Harbor, ME). All animal protocols used for these experiments were approved by the Children's Hospital of Pittsburgh's Institutional Animal Care and Use Committee.
Isolation of MDSCs from p65+/– mice. The mice were sacrificed at 5 months of age and muscle stem cell isolation was performed as previously described via a modified preplate technique.18 Briefly, the SKM tissue was minced and processed through a series of enzymatic dissociations: 0.2% of collagenase type XI (Sigma-Aldrich, St Louis, MO) for 1 hour, 2.4 units/ml of dispase (Invitrogen, Carlsbad, CA) for 45 minutes, and 0.1% of trypsin-EDTA (Invitrogen) for 30 minutes at 37 °C. After enzymatic dissociation, the muscle cells were centrifuged and resuspended in proliferation medium (Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 10% horse serum, 0.5% chicken embryo extract, and 1% penicillin-streptomycin), and the resulting cell suspension from both p65+/– and wt muscle were plated in collagen type I coated flasks. Different populations of muscle-derived cells were isolated based on their adhesion characteristics. After 7 days, late preplate populations (slow-adhering cells) were obtained and cultured in proliferation medium. The slowly adhering fraction of muscle cells has been previously shown to contain MDSCs.18 For all experiments, congenic p65+/– and p65+/+ MDSCs of the same passage number were compared.
p65 staining and ArrayScan assay. Cells were fixed with 4% paraformaldehyde for 15 minutes at room temperature (RT), rinsed two times with phosphate-buffered saline (PBS), and the cells' membrane permeabilized for 10 minutes with 0.1% Triton X-100 in PBS. A 10% goat serum blocking solution was used for 1 hour and the cells were incubated with a 1:200 dilution of rabbit polyclonal anti-p65 (Abcam, Cambridge, MA) for 1 hour at RT. After washing three times, the cells were incubated for 30 minutes with Cy3-conjugated anti-rabbit IgG (1:500; Sigma-Aldrich). The nuclei were revealed by 4′,6-Diamidino-2-phenylindole (DAPI) staining. Nuclear localization of the NF-κB subunit p65 was measured via ArrayScan. This technique allows for the rapid, automated quantification of p65 and DAPI colocalization, as identified by immunocytochemistry in cells grown on a 96-well plate. Recordings were taken from multiple fields of view per well, generating data representative of each well.
Western blot assay. The cell populations isolated from p65+/– and wt mice were cultured in proliferation medium and stimulated with TNFα (10 ng/ml) for 30 minutes before harvesting. Cells were then lysed in Laemmli sample buffer (Bio-Rad, Hercules, CA), boiled for 5 minutes, and centrifuged at 4,000 r.p.m. for 5 minutes. Each sample was loaded on a 10% SDS-polyacrylamide gel, which was run for 2 hours and then transferred for 1.5 hours at 100 V while stirring on ice. The membrane was blocked with 5% bovine serum albumin (Sigma-Aldrich, St Louis, MO) in PBS for 1 hour and then incubated with rabbit anti-phospho-NF-кB/p65 monoclonal antibody (1:1,000; Cell Signaling, Danvers, MA) overnight at 4 °C. After washing three times with Tris-buffered saline Tween-20, the membrane was incubated with goat anti-rabbit IgG (H+L) (1:5,000; Pierce, Rockford, IL) for 50 minutes at RT. Blots were developed by ECL solution (Pierce).
RT-PCR analysis. Total RNA was extracted from cells using Nucleo Spin RNA II column (Clontech, Mountain View, CA). Following isolation, complementary DNA was synthesized with SuperScript II reverse transcriptase (Invitrogen), according to the manufacturer's instructions. PCR was performed with Taq polymerase (Invitrogen) as per the manufacturer's instructions and PCR products were separated by electrophoresis with 1% agarose gels. The primers used for PCR are listed in Table 1. Each set of oligonucleotides was designed to span two different exons to avoid background amplification of genomic DNA. The data were quantified by densitometry using Adobe Photoshop 7.0.
Table 1. Primers used for RT–PCR.

Pax7 and MyoD staining. P65+/– and wt cells were fixed and permeabilized with 2% paraformaldehyde plus 1% Triton X-100 for 30 minutes at 4 °C and rinsed two times with PBS. Cells were blocked with 5% horse serum and then incubated with a 1:100 dilution of mouse monoclonal anti-Pax7 (DSHB, Iowa City, IA) or anti-MyoD (Santa Cruz Biotechnology, Santa Cruz, CA) over night at 4 °C. After washing three times, the cells were incubated for 1 hour with biotinylated anti-mouse IgG (1:300; Vector Lab, Burlingame, CA), which acted as a secondary antibody. Streptavidin 594 conjugate (1:500; Invitrogen) was added in the last step. The nuclei were revealed by DAPI staining. Negative control staining was performed by an identical procedure, with the exception that the primary antibody was omitted.
In vitro assessment of cell proliferation. In order to compare the proliferative potential of p65+/– MDSCs to wt MDSCs, we used a previously described Live Cell Imaging system (Kairos Instruments LLC, Pittsburgh, PA).22 Bright field images were taken at a ×100 magnification at 10 minutes intervals over a 72-hour period in three fields of view per well, with three wells per population. The images were combined to generate a video using ImageJ software (NIH). Proliferation was assessed by counting the number of cells per field of view, over 12 hours. All six populations were also plated in 96-well plates in quadruplicate (500 cells/well) and cultured under normal conditions for 72 hours. At this time, 20 µl of CellTiter96 AQueous One Reagent (Promega, Madison, WI) was added to each well and incubated in 5% CO2 at 37 °C. Following another 3-hour incubation, absorbance at 490 nm was read with a 96-well plate reader.
Myogenic differentiation assay and fast MyHC (MyHC-f) staining. After 15 passages, cells were plated on 24-well plates (30,000 cells/well) with Dulbecco's modified Eagle's medium supplemented with 2% fetal bovine serum to stimulate myotube formation. Three days later, immunocytochemical staining for fast skeletal MyHC was performed. After rinsing three times with PBS, cells were fixed for 2 minutes in cold methanol, blocked with 10% horse serum for 1 hour and then incubated with a mouse anti-MyHC-f (1:250; Sigma-Aldrich, clone MY-32) antibody for 2 hours at RT. The primary antibody was detected with a secondary anti-mouse IgG antibody conjugated with Cy3 (1:300; Sigma-Aldrich) for 15 minutes. The nuclei were revealed by DAPI staining. The percentage of differentiated myotubes was quantified as the number of nuclei in MyHC-f positive myotubes relative to the total number of nuclei. The myogenic differentiation was also monitored over a period of 5 days using Live Cell Imaging. The images were combined to create a video using ImageJ software (NIH).
Selective inhibition of IKKβ. To determine the effects of IKK/NF-κB inhibition on wt MDSCs during myogenic differentiation, we used IKK-2 inhibitor IV (IKKi), or 2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide (Calbiochem, San Diego, CA), a reversible competitive inhibitor of IKKβ ATP binding. Cells were plated at 105 cells/well in 6-well plates and exposed to IKK inhibitor in differentiation medium. Cells were treated with either 1, 3, or 5 µmol/l IKKi. Lysates were collected at 0 minute, 14, 24, 48, and 72 hours following treatment. NF-kB activity and myogenic differentation was assessed by western blot for phosphorylated NF-kB/p65 (1:1,000; Cell Signaling) and MyHC-f (1:500; Sigma-Aldrich, clone MY-32), as detailed above.
Cell implantation and dystrophin staining. MDSCs from p65+/– and wt muscle were grown in proliferation medium until the cell number was sufficient for injection. A total of 3 × 105 viable cells was suspended in 20 µl of Hank's balanced salts solution and injected into the gastrocnemius muscles of 8–12-week-old mdx/SCID mice using a Hamilton syringe. The same number of cells was injected into the gastrocnemius muscles of 8-week-old wt C57BL/6J mice that had been injured 1 day earlier by a 30 µl intramuscular injection of 2 µmol/l CTX (Sigma-Aldrich) in PBS. The cell suspension was mixed with green fluorescent-labeled beads before injection to detect the injection sites. Six or fourteen days after implantation, the mice were sacrificed and the gastrocnemius muscles were harvested and flash frozen in liquid nitrogen-cooled 2-methylbutane. Serial cryosections 10 µmol/l in thickness were obtained for immunohistochemical analyses. Cryosections were fixed with 5% formalin and blocked with 5% donkey serum in PBS for 1 hour, then incubated with rabbit anti-dystrophin (1:300; Abcam) for 2 hours at RT. The sections were exposed to secondary 594-conjugated anti-rabbit IgG (1:500; Invitrogen) in PBS for 30 minutes. The nuclei were revealed by DAPI staining. Immunostaining was visualized and images were taken by fluorescence microscopy (Nikon Eclipse E800; Nikon, Melville, NY). Northern Eclipse software was used for quantitative analysis of the regenerated dystrophin-positive myofibers. A series of pictures were taken, and Adobe Photoshop 7.0 was used to construct a composite picture of the dystrophin-positive myofibers, which were then manually counted.
Retroviral transduction of MDSCs. MDSCs were plated at an initial confluence of 30–40% and retrovirally transduced [in the presence of Polybrene (8 µg/ml)] to express the β-galactosidase gene (LacZ), as previously described.35
LacZ staining. The cryosections were fixed in 1% glutaraldehyde and incubated 3 hours with 5-bromo-4-chloro-3-indolyl b-𝒟-galactopyranoside (X-gal) substrate at 37 °C. Sections were counterstained with Eosin.
CD14 staining. Cryosections were fixed with 5% formalin and blocked with 5% donkey serum in PBS for 1 hour, then incubated with rat anti-CD14 (1:200; Biolegend, San Diego, CA) overnight at 4 °C. This was followed by a 1-hour incubation with biotinylated anti-rat IgG (1:300; Vector Lab). Streptavidin Cy3 conjugate (1:500; Sigma-Aldrich) was added in the last step followed by several rinses in PBS. Following CD14 staining, five random pictures per slide were taken and the number of CD14+ cells was counted manually.
Mouse IgG staining and quantification of necrosis. Muscle sections were fixed with 5% formalin and blocked with 10% horse serum in PBS for 1 hour, then incubated with biotinylated anti mouse IgG (1:300; Vector Lab) for 1 hour at RT. This was followed by a 15-minute incubation with streptavidin Cy3 conjugate (1:500; Sigma-Aldrich). The nuclei were revealed by DAPI staining. The IgG positive area was measured and quantified as the percentage of mouse IgG expressing area per total area using Northern Eclipse software (Cheektowaga, NY).
Statistical analysis. All results are given as the mean ± SD. Means from p65+/– and wt or treated and untreated were compared using Students' t-test. Differences were considered statistically significant when the P value was < 0.05.
SUPPLEMENTARY MATERIAL Video S1. p65+/– MDSC proliferation. Video S2. wt MDSC proliferation. Video S3. P65+/– MDSC differentiation. Video S4. wt MDSC differentiation.
Acknowledgments
The authors thank the members of the Huard Laboratory, especially Jenny Zhu and Bin Sun for discussions and technical advice. Special thanks go to Joseph Feduska and Bridget Deasy for live cell imaging advice. This work was supported in part by the Henry J. Mankin Endowed Chair for Orthopedic Research at the University of Pittsburgh, the William F. and Jean W. Donaldson Chair at Children's Hospital of Pittsburgh. L.J.N. is supported by NIEHS (ES016114) and NIA (AG033907). The authors do not have conflicts of interest to disclose other than the corresponding author who receives consulting fees from Cook MyoSite Inc.
Supplementary Material
p65+/– MDSC proliferation.
wt MDSC proliferation.
P65+/– MDSC differentiation.
wt MDSC differentiation.
REFERENCES
- Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D., and, Miyamoto S. Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- Baeuerle PA., and, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Li Z., and, Nabel GJ. A new member of the I kappaB protein family, I kappaB epsilon, inhibits RelA (p65)-mediated NF-kappaB transcription. Mol Cell Biol. 1997;17:6184–6190. doi: 10.1128/mcb.17.10.6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karin M. How NF-kappaB is activated: the role of the IkappaB kinase (IKK) complex. Oncogene. 1999;18:6867–6874. doi: 10.1038/sj.onc.1203219. [DOI] [PubMed] [Google Scholar]
- Hayden MS., and, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR., and, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Langen RC, Schols AM, Kelders MC, Wouters EF., and, Janssen-Heininger YM. Inflammatory cytokines inhibit myogenic differentiation through activation of nuclear factor-kappaB. FASEB J. 2001;15:1169–1180. doi: 10.1096/fj.00-0463. [DOI] [PubMed] [Google Scholar]
- Baghdiguian S, Martin M, Richard I, Pons F, Astier C, Bourg N.et al. (1999Calpain 3 deficiency is associated with myonuclear apoptosis and profound perturbation of the IkappaB alpha/NF-kappaB pathway in limb-girdle muscular dystrophy type 2A Nat Med 5503–511. [DOI] [PubMed] [Google Scholar]
- Kumar A, Lnu S, Malya R, Barron D, Moore J, Corry DB.et al. (2003Mechanical stretch activates nuclear factor-kappaB, activator protein-1, and mitogen-activated protein kinases in lung parenchyma: implications in asthma FASEB J 171800–1811. [DOI] [PubMed] [Google Scholar]
- Monici MC, Aguennouz M, Mazzeo A, Messina C., and, Vita G. Activation of nuclear factor-kappaB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology. 2003;60:993–997. doi: 10.1212/01.wnl.0000049913.27181.51. [DOI] [PubMed] [Google Scholar]
- Hunter RB., and, Kandarian SC. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J Clin Invest. 2004;114:1504–1511. doi: 10.1172/JCI21696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharyya S, Villalta SA, Bakkar N, Bupha-Intr T, Janssen PM, Carathers M.et al. (2007Interplay of IKK/NF-kappaB signaling in macrophages and myofibers promotes muscle degeneration in Duchenne muscular dystrophy J Clin Invest 117889–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakkar N, Wang J, Ladner KJ, Wang H, Dahlman JM, Carathers M.et al. (2008IKK/NF-kappaB regulates skeletal myogenesis via a signaling switch to inhibit differentiation and promote mitochondrial biogenesis J Cell Biol 180787–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne TR, Oshima H, Okada M, Momoi N, Tobita K, Keller BB.et al. (2007A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts J Am Coll Cardiol 501677–1684. [DOI] [PubMed] [Google Scholar]
- Ambrosio F, Ferrari RJ, Distefano G, Plassmeyer JM, Carvell GE, Deasy BM.et al. (2010The synergistic effect of treadmill running on stem-cell transplantation to heal injured skeletal muscle Tissue Eng Part A 16839–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg AA, Sha WC, Bronson RT, Ghosh S., and, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-kappa B. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M.et al. (2008Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique Nat Protoc 31501–1509. [DOI] [PubMed] [Google Scholar]
- Wan F., and, Lenardo MJ. Specification of DNA binding activity of NF-kappaB proteins. Cold Spring Harb Perspect Biol. 2009;1:a000067. doi: 10.1101/cshperspect.a000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco A, Doyonnas R, Kraft P, Vitorovic S., and, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski RJ, Haluszczak C, Trucco M., and, Huard J. Flow cytometric characterization of myogenic cell populations obtained via the preplate technique: potential for rapid isolation of muscle-derived stem cells. Hum Gene Ther. 2001;12:619–628. doi: 10.1089/104303401300057306. [DOI] [PubMed] [Google Scholar]
- Deasy BM, Jankowski RJ, Payne TR, Cao B, Goff JP, Greenberger JS.et al. (2003Modeling stem cell population growth: incorporating terms for proliferative heterogeneity Stem Cells 21536–545. [DOI] [PubMed] [Google Scholar]
- Tang Y, Reay DP, Salay MN, Mi MY, Clemens PR, Guttridge DC.et al. (2010Inhibition of the IKK/NF-?B pathway by AAV gene transfer improves muscle regeneration in older mdx mice Gene Ther 171476–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen SK, Rahkila P, Helenius M, Korhonen P., and, Salminen A. Down-regulation of transcription factors AP-1, Sp-1, and NF-kappa B precedes myocyte differentiation. Biochem Biophys Res Commun. 1996;229:36–43. doi: 10.1006/bbrc.1996.1754. [DOI] [PubMed] [Google Scholar]
- Guttridge DC, Albanese C, Reuther JY, Pestell RG., and, Baldwin AS., Jr NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliman PA., and, Barannik TV. Regulation of delta-aminolevulinate synthase activity during the development of oxidative stress. Biochemistry Mosc. 1999;64:699–704. [PubMed] [Google Scholar]
- Canicio J, Ruiz-Lozano P, Carrasco M, Palacin M, Chien K, Zorzano A.et al. (2001Nuclear factor kappa B-inducing kinase and Ikappa B kinase-alpha signal skeletal muscle cell differentiation J Biol Chem 27620228–20233. [DOI] [PubMed] [Google Scholar]
- Munz B, Hildt E, Springer ML., and, Blau HM. RIP2, a checkpoint in myogenic differentiation. Mol Cell Biol. 2002;22:5879–5886. doi: 10.1128/MCB.22.16.5879-5886.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeza-Raja B., and, Muñoz-Cánoves P. p38 MAPK-induced nuclear factor-kappaB activity is required for skeletal muscle differentiation: role of interleukin-6. Mol Biol Cell. 2004;15:2013–2026. doi: 10.1091/mbc.E03-08-0585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttridge DC, Mayo MW, Madrid LV, Wang CY., and, Baldwin AS., Jr NF-kappaB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science. 2000;289:2363–2366. doi: 10.1126/science.289.5488.2363. [DOI] [PubMed] [Google Scholar]
- Wang H, Hertlein E, Bakkar N, Sun H, Acharyya S, Wang J.et al. (2007NF-kappaB regulation of YY1 inhibits skeletal myogenesis through transcriptional silencing of myofibrillar genes Mol Cell Biol 274374–4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalaki M, Fili S, Philippou A., and, Koutsilieris M. Muscle regeneration: cellular and molecular events. In Vivo. 2009;23:779–796. [PubMed] [Google Scholar]
- McFarlane C, Plummer E, Thomas M, Hennebry A, Ashby M, Ling N.et al. (2006Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB-independent, FoxO1-dependent mechanism J Cell Physiol 209501–514. [DOI] [PubMed] [Google Scholar]
- Yao P, Zhan Y, Xu W, Li C, Yue P, Xu C.et al. (2004Hepatocyte growth factor-induced proliferation of hepatic stem-like cells depends on activation of NF-kappaB J Hepatol 40391–398. [DOI] [PubMed] [Google Scholar]
- Li Y., and, Huard J. Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol. 2002;161:895–907. doi: 10.1016/S0002-9440(10)64250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
p65+/– MDSC proliferation.
wt MDSC proliferation.
P65+/– MDSC differentiation.
wt MDSC differentiation.