Abstract
Neurotrophic factors are integrally involved in the development of the nigrostriatal system and in combination with gene therapy, possess great therapeutic potential for Parkinson's disease (PD). Pleiotrophin (PTN) is involved in the development, maintenance, and repair of the nigrostriatal dopamine (DA) system. The present study examined the ability of striatal PTN overexpression, delivered via psueudotyped recombinant adeno-associated virus type 2/1 (rAAV2/1), to provide neuroprotection and functional restoration from 6-hydroxydopamine (6-OHDA). Striatal PTN overexpression led to significant neuroprotection of tyrosine hydroxylase immunoreactive (THir) neurons in the substantia nigra pars compacta (SNpc) and THir neurite density in the striatum, with long-term PTN overexpression producing recovery from 6-OHDA-induced deficits in contralateral forelimb use. Transduced striatal PTN levels were increased threefold compared to adult striatal PTN expression and approximated peak endogenous developmental levels (P1). rAAV2/1 vector exclusively transduced neurons within the striatum and SNpc with approximately half the total striatal volume routinely transduced using our injection parameters. Our results indicate that striatal PTN overexpression can provide neuroprotection for the 6-OHDA lesioned nigrostriatal system based upon morphological and functional measures and that striatal PTN levels similar in magnitude to those expressed in the striatum during development are sufficient to provide neuroprotection from Parkinsonian insult.
Introduction
Parkinson's disease (PD) is a progressive, aging-related neurodegenerative movement disorder that currently impacts ~4.6 million world wide with prevalence of the disease anticipated to climb considerably within the next few decades as life expectancy increases.1 The motor symptoms of PD primarily are the result of degenerating dopamine (DA) neurons of the substantia nigra pars compacta (SNpc) leading to loss of dopaminergic neurotransmission in the striatum. Due to the progressive nature of PD and a lack of therapies that can modify disease progression, neuroprotective agents have been investigated in an attempt to rescue vulnerable nigral neurons before they succumb to the disease. Neurotrophic factors are intrinsically involved in the development and maintenance of the nigrostriatal system and therefore possess incredible potential to repair it. Most notably, delivery of the trophic factors glial cell line-derived neurotrophic factor (GDNF) and neurturin (NTN) can protect and restore the nigrostriatal system in parkinsonian animal models.2,3,4,5,6,7,8,9 Despite setbacks in the GDNF and NTN clinical trials,10,11,12 perhaps due to selection of PD subjects in the advanced stages of disease, the promise of trophic factors as a therapeutic strategy remains to be fully realized. The evaluation of the neuroprotective potential of trophic factors that do not signal via the GFR1α/Ret receptor complex, as do GDNF and NTN, may offer distinct therapeutic avenues.
Pleiotrophin (PTN) (also commonly referred to as heparin-binding growth-associated molecule) is an 18 kDa secretory cell surface and extracellular associated protein that is associated with neurite outgrowth during development.13 PTN expression peaks during early postnatal development, including within the midbrain and striatum, with limited expression in the adult brain.14 PTN expression levels in the striatum peak during early postnatal nigrostriatal synaptogenesis, and decrease to low levels in adulthood.15,16 The PTN receptors, syndecan-3 and receptor protein tyrosine phosphatase β/ζ (RPTPβ/ζ) are expressed by SNpc DA neurons and in the striatum.16,17,18 PTN protein, mRNA, and receptor expression in the striatum is upregulated in response to 6-hydroxydopamine (6-OHDA)-induced denervation and -dopa administration.15,16,18,19 The addition of PTN to embryonic mesencephalic cultures specifically promotes tyrosine hydroxylase immunoreactive (THir) neuronal survival and neurite outgrowth.15,16,20 Furthermore, endogenous PTN has been implicated in the differentiation of mesencephalic DA neurons.15 Lastly, our laboratory has previously demonstrated that PTN protein is upregulated in surviving nigral DA neurons of PD patients.16
Collectively these findings suggest that exogenous supplementation of PTN holds great potential to provide neuroprotection and promote reconstruction of nigrostriatal circuitry following insult. The present study examines PTN's potential as an exogenous therapeutic to provide neuroprotection and promote functional recovery in the damaged nigrostriatal system. We report that the overexpression of PTN in the adult rat striatum using recombinant adeno-associated virus 2/1 (rAAV2/1) vector protects both SNpc DA neurons and striatal dopaminergic neurites from intrastriatal 6-OHDA with long-term PTN overexpression facilitating functional recovery. Additionally, we report that the levels of PTN protein expression achieved via rAAV2/1 injection approximate peak developmental levels of PTN in the striatum.
Results
Experiment 1: Transduction efficiency after intrastriatal rAAV2/1-PTN/GFP or rAAV2/1-GFP injection
To determine whether efficient transduction occurred following vector injection, unlesioned rats received bilateral intrastriatal vector injections (2 µl × 1 site/hemisphere). Immunolabeling with GFP at 4 weeks following injection revealed that a single injection of either rAAV2/1-PTN/green fluorescent protein (GFP) or rAAV2/1-GFP vector [vector schematics (Figure 1c–d)] transduced a large number of cells within the striatum, almost the entire dorsolateral parenchyma (Figure 2a,b,e). Additionally, a number of cells within the SNpc were also GFPir, indicating that the intrastriatal vector injection resulted in retrograde transport of the vector and subsequent transduction of cells within the SNpc (Figure 2c,d). Furthermore, dense GFP immunoreactivity also was observed in the SN pars reticulata (SNpr) indicating that striatal vector infusion produced considerable anterograde transport via the direct pathway (Figure 2c). PTN immunoreactivity confirmed overexpression of PTN within the striatum with the pattern of immunostaining colocalizing with cellular structures (Figure 2f). In order to determine which cells specifically were transduced, triple label confocal microscopy for PTN, GFAP, and NeuN was performed on sections through the striatum and SN. This analysis confirmed that rAAV2/1-PTN/GFP exclusively transduced neurons as has been reported previously for the rAAV2/1 vector construct21,22 (Figure 3a–h).
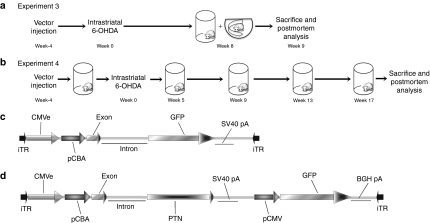
Figure 1.
Experimental design and vector schematics. (a–b) Experimental timeline of pleiotrophin (PTN) neuroprotection experiments 3 and 4. All rats were injected with rAAV2/1-PTN/GFP, rAAV2/1-GFP, or phosphate-buffered saline (PBS) at time 0. Four weeks later rats were unilaterally injected in the striatum with 6-hydroxydopamine (6-OHDA). (a) In experiment 3, rats were tested once for forelimb akinesia in the cylinder test, then performed amphetamine induced rotations before sacrifice and postmortem analysis at 9 weeks post-6-OHDA. (b) In experiment 4, rats were repeatedly cylinder tested before 6-OHDA injection, and at 5, 9, 13, and 17 weeks afterward before sacrifice and postmortem analysis at 18 weeks post-6-OHDA. (c–d) Recombinant adeno-associated virus (rAAV) genomic maps of rAAV2/1-GFP (c) and rAAV2/1-PTN/GFP (d) vectors used in all experiments.
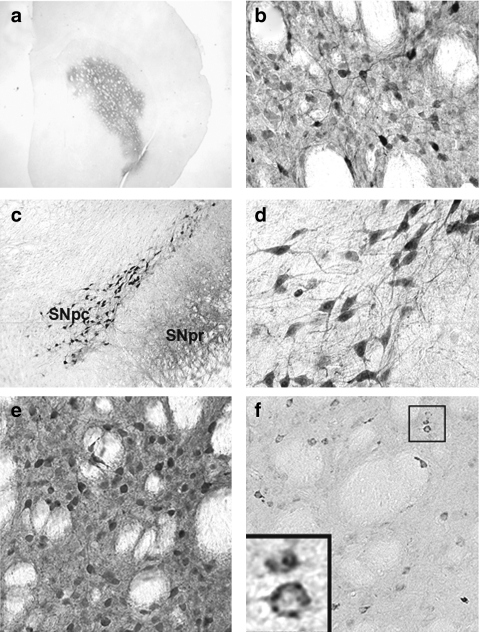
Figure 2.
rAAV2/1-PTN/GFP injection yields efficient transduction of the striatum accompanied by both retrograde and anterograde transport to the SN. (a–b). GFPir cells within the striatum of a rat 4 weeks after receiving a single 2 µl injection of rAAV2/1-PTN/GFP. (c). GFPir cells within the substantia nigra pars compacta (SNpc) of same rat, indicating retrograde transport of rAAV2/1-PTN/GFP. GFPir neurites within the SNpr reveals anterograde transport. (d) GFPir cells within the SNpc at higher magnification. (e) GFPir cells within the striatum of a rat 12 weeks after receiving rAAV2/1-GFP. (f) PTNir cells within the striatum of a rat 12 weeks after rAAV2/1-PTN/GFP. PTN, pleiotrophin; rAAV, recombinant adeno-associated virus.
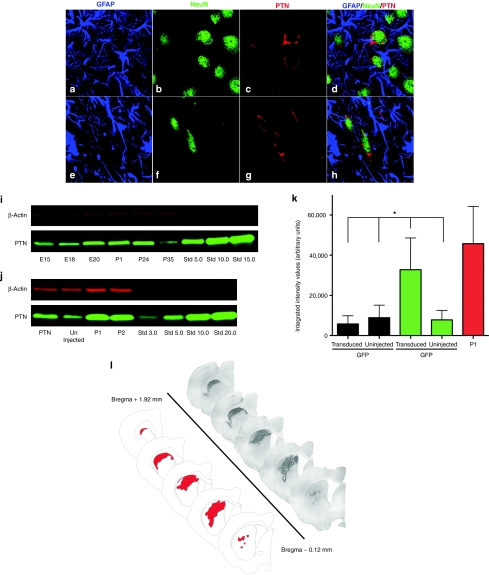
Figure 3.
Characterization of pleiotrophin (PTN) expression after intrastriatal rAAV2/1-PTN/GFP injection. (a–h) Triple labeled immunofluorescence confocal images of (a–d) striatal and (e–h) nigral neurons in rats injected with rAAV2/1-PTN/GFP (2 µl × 1 sites). Merged GFAP, NeuN, and PTN images (d, h) indicate that rAAV2/1-PTN/GFP transduction is localized to neurons. (i) Western blot PTN immunodetection in naive striatal samples from rats across developmental ages with highest expression between ages E20 and P1. (j) Western blot immunodetection from striatal samples of naive P1 and P2 rats or 6-hydroxydopamine (6-OHDA) lesioned adult rats transduced with rAAV2/1-PTN/GFP (2 µl × 2 sites) or contralateral uninjected striatum. (k) Western blot quantification of striatal PTN levels in naive P1 rats or 6-OHDA lesioned adult rats transduced with either rAAV2/1-PTN/GFP or rAAV2/1-GFP (2 µl × 2 sites) and the corresponding contralateral uninjected striatum. PTN expression in the adult rAAV2/1-PTN/GFP striatum approximately tripled that of rAAV2/1-GFP-injected animals and naive controls, and was not significantly different from P1 levels (*P > 0.05). Values expressed as the mean percent of control ± SEM for each group. (l) An example of GFP immunoreactivity in striatum of rAAV2/1-PTN/GFP transduced adult rat 20 weeks after vector injection (2 µl × 2 sites). The average striatal volume was 1.32 × 1010 ± 9.65 × 108 µm3 and average GFPir volume within the striatum was 7.58 × 109 ± 2.31 × 109 µm3, ~57% of total striatal volume. Values are expressed as mean ± SEM. rAAV, recombinant adeno-associated virus.
Experiment 2: Characterization of developmental and transduced PTN expression
Developmental PTN expression. Before the quantification of developmental peak striatal PTN levels, western blot analyses were performed to look for gender differences in striatal PTN expression at postnatal ages P1, P14, and P35. Results revealed no significant gender differences in PTN expression at any age (P ≥ 0.05). Western blot analyses were performed to examine peak striatal PTN expression across development ages E15, E18, E20, P1, P2, P14, and P35. Densitometry measurements confirmed that PTN expression peaked between E20 and P14 and decreased dramatically by P35. These results are depicted in Figure 3i.
PTN expression in adult 6-OHDA lesioned rats transduced with either rAAV2/1-PTN/GFP or rAAV2/1-GFP. Four or ten weeks following vector injection (2 µl × 2 sites), rats were sacrificed and western blots were performed to determine PTN levels in both PTN/GFP and GFP transduced striatum as well as in contralateral control striatum. No significant differences in PTN expression were detected between 4- and 10-week time points (P ≥ 0.05) and these time points were combined for subsequent statistical analysis. Overall there was a significant difference in PTN expression between treatment groups (F(1, 6) = 18.245, P = 0.002). Integrated intensity values for PTN expression were significantly greater in the PTN/GFP transduced striatum compared to the contralateral intact striatum (P ≤ 0.001), the rAAV2/1-GFP injected striatum (P = 0.002), and the GFP contralateral intact striatum (P = 0.048). Specifically, rAAV2/1-PTN/GFP injection resulted in an approximately threefold increase in PTN protein compared to all other treatment groups. The level of striatal PTN expression achieved by rAAV2/1-PTN/GFP injection was statistically identical to the peak developmental PTN levels (P1, P ≥ 0.05). No significant differences were detected between rAAV2/1-GFP transduced striatum or contralateral intact samples from either PTN or GFP groups (P > 0.05). These results are depicted in Figure 3j–k.
rAAV2/1-PTN/GFP striatal volume in transduced, 6-OHDA lesioned rats. A second cohort of rats was injected with rAAV2/1-PTN/GFP (2 µl × 2 sites), and was sacrificed 20 weeks after vector injection. Brain tissue was immunostained for GFP. The total striatal area and areas of GFPir staining were outlined using the Neurolucida program (Microbrightfield Bioscience, Williston, VT) in order to determine the volume of the striatum transduced by injecting 4 µl of vector. The average striatal volume was 1.32 × 1010 ± 9.65 × 108 µm3 and average GFPir volume within the striatum was 7.58 × 109 ± 2.31 × 109 µm3. GFPir transduction volume was ~57% of the average striatal volume. An example of GFP staining and transduction volume from a rat injected with rAAV2/1-PTN/GFP is depicted in Figure 3l.
Experiment 3: Neuroprotection from intrastriatal 6-OHDA
We next examined the impact of PTN gene transfer (2 µl × 2 sites) on 6-OHDA-induced degeneration of SNpc THir neurons and density of THir neurites in the striatum (Figure 1a). A two-way repeated measures ANOVA revealed that significantly more THir neurons were spared in the ipsilateral rAAV2/1-PTN/GFP SNpc compared to the ipsilateral rAAV2/1-GFP SNpc (F(1,10) = 23.4, P = 0.001). Additionally, the contralateral, intact SNpc possessed significantly more THir neurons than the ipsilateral, lesioned SNpc in both groups (F(1,10) = 154.4 (17.67), P ≤ 0.001). GFP transduced animals maintained ~32% of the intact THir neurons in the ipsilateral SNpc, whereas PTN/GFP transduction increased THir neuron survival in the ipsilateral SNpc to 69% of the intact control. These results are depicted in Figure 4a–d and g.
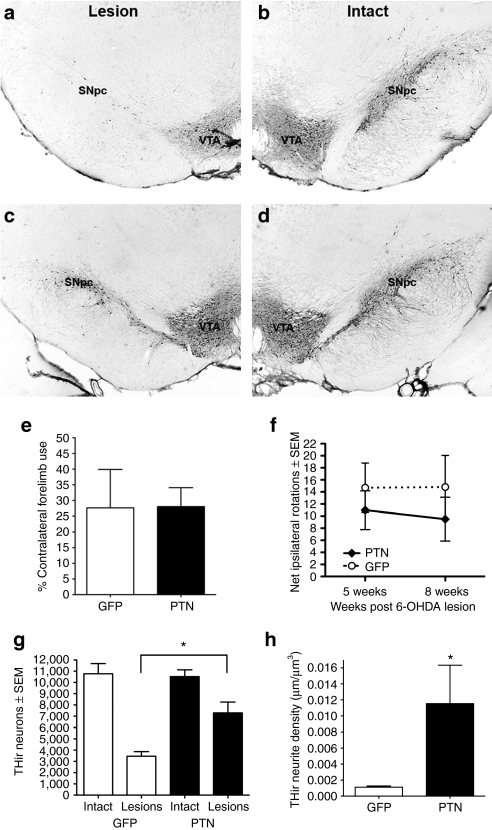
Figure 4.
Intrastriatal rAAV2/1-PTN/GFP injection (2 µl × 2 sites) provides neuroprotection against 6-hydroxydopamine (6-OHDA). (a–d) THir neurons within the SNc of rats ipsilateral to striatal 6-OHDA (a, c, Lesion) or no toxin (b, d, Intact). (a) Reporter vector rAAV2/1-GFP does not protect THir neurons from degeneration. (c) Increased THir neuron survival in the SNc is associated with injections of rAAV2/1-PTN/GFP to the striatum. (e) Forelimb akinesia in the cylinder task. No differences were observed in contralateral forelimb use for lesioned rats 8 weeks after rAAV2/1-PTN-GFP or rAAV2/1-GFP injection. (f) Amphetamine-induced rotational behavior. No differences were observed in number of ipsilateral rotations at 5 or 8 weeks in rats injected with either rAAV2/1-PTN/GFP or rAAV2/1-GFP. (g) Striatal injection of rAAV2/1-PTN/GFP provides significant neuroprotection from 6-OHDA for THir nigral neurons (*P < 0.05). (h) pleiotrophin (PTN) transduction is associated with a significant increase in THir neurites in the striatum compared to GFP transduction alone (*P < 0.05). Values expressed as the mean percent of control ± SEM for each group. rAAV, recombinant adeno-associated virus.
To determine whether PTN gene transfer could protect THir neurites in the striatum, the density of THir neuritic profiles within the PTN/GFP transduced and GFP transduced areas of the striatum were directly compared. Rats in both groups exhibited severe loss of ipsilateral striatal THir neurite density as a result of intrastriatal 6-OHDA as has been reported previously.23 Nine weeks following vector injection, THir neurite density within the rAAV2/1-PTN/GFP transduced striatum was significantly higher then that of rAAV2/1-GFP-injected animals (t(5) = 2.591, P = 0.049). The PTN/GFP transduced area of the striatum contained an average THir neurite density of 0.01153 ± 0.00480 µm/µm3 whereas the GFP transduced striatal area possessed an average striatal THir neurite density of 0.00112 ± 0.00015 µm/µm3. These results are depicted in Figure 4h.
PTN/GFP and GFP transduced rats were also examined for the contralateral forelimb use in the cylinder task and amphetamine-induced ipsilateral rotations 8 weeks after 6-OHDA. No significant differences were observed between the two treatment groups in either behavioral measure (P > 0.05, Figure 4e,f).
Experiment 4: Functional neuroprotection after long-term PTN overexpression
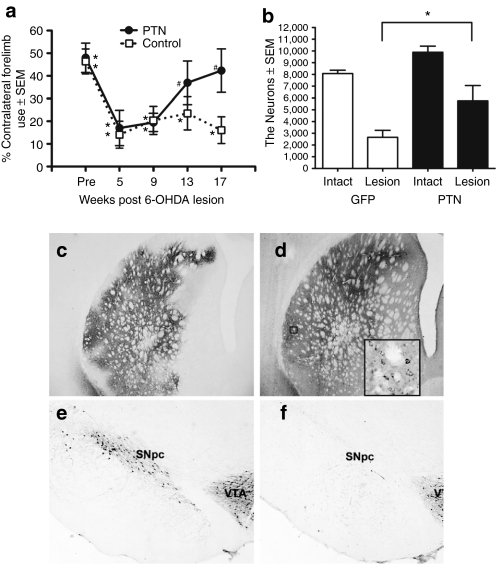
We next examined the impact of PTN gene transfer (3 µl × 1 site), on functional restoration and protection of SNpc THir neurons in unilaterally 6-OHDA lesioned rats over a period of 17 weeks (Figure 1b). PTN/GFP and GFP transduced rats and phosphate-buffered saline (PBS) sham-injected rats were examined for contralateral limb use in the cylinder task before 6-OHDA injection, and 5, 9, 13, and 17 weeks afterward. An overall decrease in contralateral forepaw use was observed in all groups at 5 weeks after 6-OHDA as compared to their pre-lesion baseline, likely due to the loss of dopaminergic terminals in the striatum after 6-OHDA.23 There were no significant differences between GFP and PBS-injected rats at any of the five time points (P > 0.05) and these two control groups were merged for subsequent statistical analysis (control). One-way RMANOVAs revealed significant changes in cylinder task performance over time, post-lesion for both control and PTN/GFP-treated groups (F(4,9) = 7.058, P < 0.001 control group, F(4,5) = 3.651, P = 0.027 PTN/GFP group). However, rats injected with rAAV2/1-PTN/GFP showed functional recovery in contralateral forepaw use similar to prelesion performance by 13 weeks (P = n.s. compared to baseline), which persisted through the 17-week measure (P = n.s.), whereas control (rAAV2/1-GFP or sham injected) rats never recovered contralateral forepaw use compared to pre-lesion levels and continued to demonstrate significant impairment throughout the 17-week evaluation period (P = 0.016 at 13 weeks, P < 0.001 at 17 weeks). These results are depicted in Figure 5a.
Figure 5.
Long-term pleiotrophin (PTN) overexpression facilitates functional restoration of 6-hydroxydopamine (6-OHDA) induced deficits in contralateral forelimb use. (a) Forelimb akinesia in the cylinder task. Rats received unilateral intrastriatal injection of rAAV2/1-GFP, phosphate-buffered saline (PBS) or rAAV2/1-PTN/GFP (3 µl × 1 site) 4 weeks before intrastriatal 6-OHDA. 6-OHDA produced significant deficits in contralateral forelimb use in control rats (rAAV2/1 GFP or PBS, open square) at all post-6-OHDA time points evaluated and in rats receiving PTN vector at 5 and 9 weeks after 6-OHDA (filled circle, *P < 0.05). However, rats receiving PTN vector exhibited recovery of contralateral forepaw use to near pre-6-OHDA levels at 13 and 17 weeks postlesion (#P = n.s.). (b) Intrastriatal rAAV2/1-PTN/GFP injection yielded significant neuroprotection for substantia nigra pars compacta (SNpc) THir nigral neurons 18 weeks after 6-OHDA (*P = 0.009). (c) GFP and (d) PTN immunoreactivity 22 weeks following striatal injection of rAAV2/1-PTN/GFP. Inset in d. At higher magnification PTN expression appears to colocalize with cellular structures in the striatum. (e–f) Representative photomicrographs of THir SNpc neurons in lesioned SNpc of rAAV2/1-GFP (f) and rAAV2/1-PTN/GFP (e) injected rats. rAAV2/1-PTN/GFP transduction spared more THir neurons in the lesioned SNpc (e) compared to rAAV2/1-GFP transduction (f). Values expressed as the mean percent of control ± SEM for each group. rAAV, recombinant adeno-associated virus.
Eighteen weeks following 6-OHDA, the same cohort of rats was perfused and surviving THir SNpc neurons were quantitated. A two-way RMAVOVA found main effects of the group factor (PTN vs. GFP) (F(1,9) = 9.719, P = 0.017) and the treatment factor (6-OHDA or naive) (F(1,9) = 56.356, P ≤ 0.001), confirming that remaining populations of SNpc THir neurons differed between rAAV2/1-PTN/GFP and rAAV2/1-GFP transduced rats or lesion status. Significantly more THir neurons were spared in the SNpc ipsilateral to the 6-OHDA and rAAV2/1-PTN/GFP-injected striatum compared to the SNpc ipsilateral to the 6-OHDA and rAAV2/1-GFP-injected striatum (main effect: P = 0.009). The contralateral, intact SNpc possessed significantly more THir neurons then ipsilateral lesioned SN in both groups (PTN, P = 0.003, GFP, P ≤ 0.001). GFP transduced animals maintained ~33% of the control THir neurons in the ipsilateral SNpc, whereas PTN/GFP transduction increased THir neuron survival in the SNpc ipsilateral to striatal vector injection to 58% of the intact control. These results are depicted in Figure 5b,e–f.
Discussion
Numerous studies have demonstrated that a variety of neurotrophic molecules elicit positive effects on SN DA neuron survival and striatal innervation (for review, see refs. 24,25). To date, GDNF and NTN have been the most extensively characterized neurotrophic factors for gene therapy and are the only neurotrophic factors to progress to clinical trials in PD patients. Arguably, GDNF can be considered the best preclinically characterized gold standard of gene transfer approaches for PD, followed closely by NTN. Therefore, any novel neurotrophic therapy, such as our PTN gene transfer approach, must compare favorably to the efficacy of GDNF gene transfer. Intrastriatal injection of rAAV-GDNF yields neuroprotection of THir striatal neurites and nigral neurons as well as functional recovery from lesion with expression lasting at least 6 months.4 AAV2-NTN (commercially known as CERE-120) administered to the striatum also provides THir fiber and nigral neuron preservation, functional recovery, and 12-month long-term expression in NTN transduced animals.6 Retrograde transport of the vector was observed in some animals. Volumes of NTN or GDNF expression after transduction were 58% or 83% of total rat striatal volume.6 Comparatively, we report in this study that PTN provides neuroprotection in the striatum and nigra resembling that of GDNF and NTN and facilitates functional recovery of forelimb use. rAAV2/1-PTN/GFP vector injection yields significant PTN expression for as long as 5 months after vector injection, with a transduction area covering 57% of total rat striatal volume. Retrograde and anterograde transport of the vector was also observed in PTN/GFP-transduced animals.
We observed functional restoration in animals injected with rAAV2/1-PTN/GFP after long-term (13 and 17 weeks after 6-OHDA) but not short-term (9 weeks after 6-OHDA) PTN overexpression. In both experiments the magnitude of nigral DA neuron neuroprotection was approximately the same. It is possible that the functional recovery observed after 13 weeks is related to additional sprouting of THir neurites in the striatum, beyond the level observed in rAAV2/1-PTN/GFP injected rats at 9 weeks compared to controls. Further studies will need to directly examine this issue. Prior GDNF and NTN studies support the concept that extended periods of time are required to detect neurotrophic factor-induced compensatory sprouting and resulting recovery of motor function after a 6-OHDA lesion of the magnitude employed here. Two such studies used continuous direct protein infusion of GDNF or NTN into the striatum following 6-OHDA lesions. In the short-term study (4 weeks after 6-OHDA), no evidence of striatal neurite regeneration or functional recovery was observed in GDNF- or NTN-treated animals compared to controls.26 In a similar, long-term study, although sprouting of new THir neurites in the lesioned striatum was observed 8 weeks after lesion, no improvement in forelimb akinesia was observed.27 In contrast, two studies report significant AAV-GDNF-mediated recovery of striatal neurites with accompanying improvements in motor function 23 and 24 weeks after 6-OHDA.4,28 These results illustrate the ability of neurotrophic factor therapies to induce compensatory sprouting in the damaged nigrostriatal system, and that in the intrastriatal 6-OHDA rodent model, this reinnervation occurs over a protracted time course. Not surprisingly, behavioral deficits are corrected following striatal reinnervation and restoration of striatal DA. Similarly to GDNF and NTN studies, PTN gene therapy in our study has the ability to increase striatal THir innervation and produce functional recovery in animals with long-term PTN overexpression. These data together suggest that PTN gene therapy, like GDNF and NTN, has the potential to be a potent therapeutic for PD.
The mechanism by which PTN elicits neurite outgrowth and neuroprotection remains to be elucidated. PTN is known to have two receptors expressed by nigral DA neurons: syndecan-3 and receptor protein tyrosine phosphatase β/ζ (RPTPβ/ζ).15,16,17 PTN receptor RPTPβ/ζ, mRNA, and protein are upregulated following 6-OHDA lesion.15,18 The best described PTN-receptor interaction is that with RPTPβ/ζ. Briefly, in the absence of PTN, RPTPβ/ζ is found in the monomeric form, where it exerts phosphatase activity on its substrates. In the presence of PTN, PTN causes dimerization of RPTPβ/ζ, inactivating its phosphatase activity, therefore phosphorylating substrates such as β-catenin,29 Fyn,30 and β-adducin.31 These substrates are linked to changes in actin cytoskeleton stability.32 Furthermore, the binding of syndecan-3 to PTN is believed to elicit neuritogenic activities in the brain during development, although this relationship is less understood.33,34 It is unclear if cytoskeletal destabilization influences neuroprotection, but neuritic structural changes and elimination of cell-cell or cell-matrix contacts may provide a permissive environment for neuroplasticity in the injured nigrostriatal system. The present study did not examine the mechanism by which PTN elicits neuroprotection, but experiments in which RPTPβ/ζ function is blocked suggest that PTN-RPTPβ/ζ interactions may be key to generating neuroprotective effects (for review, see ref. 35). Inhibition of protein tyrosine phosphatases in the SN preserves striatal dopaminergic projections and SN dopaminergic neurons following nigral 6-OHDA lesioning.36 From a broader perspective, whether PTN resulting from rAAV2/1-PTN/GFP transduction is secreted from transduced neurons to induce neuroprotection and restoration, like the secretion of GDNF,37 has yet to be determined, but studies are currently underway in our lab to differentiate between autocrine or paracrine mechanisms of action. Combined with our present results, this suggests that PTN and its receptors play a role in neuroprotection in the nigrostriatal system via different signaling mechanisms than either GDNF or NTN.
Although GDNF gene therapy in animal models has been successful, it is not without unwanted side effects. In particular, long-term GDNF overexpression mediated by viral vectors can result in the downregulation of TH, a decrease in DA production, and aberrant sprouting in downstream target nuclei in parkinsonian rodent models.38,39,40 Rarely are levels of GDNF expression quantitated in these studies, although in some cases supraphysiological levels of expression have been documented. Georgievska et al.38 reported a downregulation of striatal TH and aberrant sprouting in the globus pallidus, entopeduncular nucleus, and SN in rats in which GDNF expression was increased as much as 200-fold compared to endogenous GDNF expression measured from the contralateral nontransduced striatum. More recently, an 800-fold increase in GDNF expression was reported in transduced striatum compared to expression in control lentiviral LacZ-injected striatum of aged parkinsonian monkeys treated with lentiviral GDNF.41 Our findings indicate that PTN overexpression that recapitulates peak striatal PTN levels observed during development, and therefore within physiological limits, can provide neuroprotection. Future studies will be required to confirm whether avoiding supraphysiological levels of PTN expression will circumvent any potential negative side effects associated with PTN overexpression.
To best approximate the clinical circumstances of PD, PTN gene therapy must impact nigral DA neuron survival and striatal DA levels after substantial nigrostriatal degeneration has occurred. A recent study by Taravini and colleagues17 reported that adenovirus-mediated PTN overexpression in mesencephalic astrocytes 1 week after intrastriatal 6-OHDA elicits modest but significant protective effects on nigral DA neurons and striatal terminals, suggesting that PTN may also possess neurorestorative potential after nigrostriatal injury. Our present results similarly indicate that long-term striatal PTN expression can facilitate neurorestoration to a level that can reverse 6-OHDA-induced deficits in contralateral forelimb use. Ideally, neurotrophic factor gene therapies should be implemented after degeneration has begun and at a time when a subpopulation of nigrostriatal DA neurons and neurites remain. Testing in late stage PD patients with severe nigrostriatal degeneration has been a suggested pitfall of prior gene therapy clinical trials that failed to meet primary endpoints.42 Studies underway in our laboratory are examining the morphological and behavioral effects of PTN overexpression by rAAV following 6-OHDA induced nigrostriatal degeneration. The ability of rAAV2/1-PTN/GFP striatal injection to deliver PTN to the SN through anterograde transport via striatal-nigral circuitry may prove particularly useful in treating PD patients, as has been suggested previously.43 Results from such neurorestorative studies will provide valuable insight to inform our ultimate goal of testing the efficacy of PTN overexpression in PD patients.
This study demonstrates that expression levels of PTN in the adult rat striatum that parallel peak developmental levels can provide marked morphological and functional neuroprotection in the rat nigrostriatal system from 6-OHDA insult. Our ultimate goal is to determine whether PTN gene transfer warrants testing as a PD therapeutic. Future studies will examine the optimal sites for PTN transduction as striatal transduction alone may not produce maximal neuroprotective results. Lastly, we recognize that transfer of a single trophic factor may not be the optimal strategy, rather overexpression of multiple neurotrophic factors may be required to more successfully restore the damaged nigrostriatal system. Along these lines it is of note that a combination treatment of PTN and GDNF protein leads to augmented DA neuron survival in vitro, as well as after transplantation, when compared to each trophic factor individually.15,44 Furthermore, intrastriatal injection of PTN and GDNF protein following 6-OHDA decreases amphetamine-induced rotational asymmetry more significantly then PTN or GDNF alone.45 A thorough examination of the optimal PTN transduction site and possible co-trophic factor treatments will be required in order to fully assess the potential of PTN gene therapy to provide a clinically effective strategy for the treatment of PD.
Methods and Materials
Animals. Male, Sprague-Dawley rats (Harlan, Indianapolis, IN; 200–225 g) were used in the rAAV2/1 PTN transduction efficiency and neuroprotection studies. Embryos and postnatal pups (E15, E18, E20, P1, P2, P14, P35) from timed pregnant Sprague-Dawley dams and male and female Sprague-Dawley rats (~5 months of age) were used for striatal PTN expression studies. All animals were given food and water ad libitum, and housed in reversed light-dark cycle conditions in the University of Cincinnati Medical Science Building vivarium, which is fully AAALAC approved.
Experimental overview
Experiment 1: Transduction efficiency after intrastriatal rAAV2/1-PTN/GFP injection: Rats (n = 13) received either unilateral or bilateral injections of rAAV2/1-PTN/GFP in the striatum (2 µl × 1 site/hemisphere). Four weeks following vector injection, rats were sacrificed and efficiency of gene transfer was examined by immunohistochemical examination of the striatum and SN.
Experiment 2: Characterization of developmental and transduced PTN expression. Levels of PTN were examined using western blot analysis of microdissected striatal tissue at E15, E18, E20 (minimum n = 9 pups from three litters of each age), P1, P2, P14, and P35 (minimum n = 4 pups from two litters each for each age). These PTN expression levels were compared to striatal PTN levels of lesioned adult rats 4 or 10 weeks following unilateral intrastriatal injection of either rAAV2/1-PTN/GFP or rAAV2/1-GFP in the left hemisphere (n = 8, 2 µl × 2 sites). A second cohort of rats (n = 3) also was examined at 20 weeks after rAAV2/1-PTN/GFP injection using GFP immunohistochemistry in order to determine transduction volume in the striatum.
Experiment 3: Neuroprotection from intrastriatal 6-OHDA. Rats (n = 14) received unilateral intrastriatal injections of either rAAV2/1-PTN/GFP or rAAV2/1-GFP (2 µl × 2 sites) (Figure 1a). Four weeks following striatal vector injections, all rats were unilaterally injected in the ipsilateral striatum with 6-OHDA. At 5 and 7 weeks after 6-OHDA rats were assessed for amphetamine-induced rotations, at 8 weeks after transduction rats were assessed for contralateral forelimb use in the cylinder task. Nine weeks following 6-OHDA, rats were perfused and brains were processed for immunohistochemistry. Unbiased stereology was conducted to quantify tyrosine THir neurons in the SNpc and THir neurites in rats with verified striatal transduction (n = 5 rAAV2/1-PTN/GFP; n = 5 rAAV2/1-GFP).
Experiment 4: Functional neuroprotection after long-term PTN overexpression. Rats (n = 14) received unilateral intrastriatal injections of either rAAV2/1-PTN/GFP, rAAV2/1-GFP or an equal volume of PBS (PBS Sham, 3 µl × 1 site) (Figure 1b). Four weeks following vector injections all rats were unilaterally injected in the ipsilateral striatum with 6-OHDA. Before 6-OHDA and at 5, 9, 13, and 17 weeks after 6-OHDA rats were assessed for contralateral forelimb use in the cylinder task. Eighteen weeks following 6-OHDA, rats were perfused and brains were processed for immunohistochemistry. Unbiased stereology was conducted to quantify THir neurons in the SNpc in rats with verified striatal transduction (n = 5 rAAV2/1-PTN/GFP; n = 5 rAAV2/1-GFP).
Plasmid construction and rAAV2/1 vector production. Human PTN was cloned from human brain complementary DNA (Biochain Institute, Hayward, CA) and inserted into an AAV plasmid backbone downstream of a synthetic chicken β-actin promoter/cytomegalovirus enhancer promoter hybrid.46 In addition, the same vector genome contained humanized GFP under the control of the full cytomegalovirus promoter (Figure 1d). The GFP control virus contained the humanized GFP behind the chicken β-actin/cytomegalovirus promoter hybrid (Figure 1c). All vectors contained AAV2 terminal repeats and were packaged into AAV1 capsids by cotransfection of a plasmid containing the rAAV rep and cap genes as well as adenovirus helper functions and purified using iodixanol gradients and q-sepharose chromatography as previously described.47 Vector titers were determined using dot-blot assay as previously described.47 The rAAV2/1-GFP titer was 2.75 × 1012 genome copies/ml for all experiments. For experiments 1–3, the rAAV2/1-PTN/GFP virus titer was 3.45 × 1012 genome copies/ml, for experiment 4 the rAAV2/1-PTN/GFP virus titer 9.06 × 1012 genome copies/ml.
rAAV2/1-PTN/GFP, rAAV2/1-GFP, and PBS injection to the striatum. Fifteen minutes before vector injection, rats received 0.3 ml/100 g of 25% Mannitol solution intraperitoneally (i.p.) as described previously to increase transduction area by increasing hyperosmolality.48 Rats were anesthetized with Equi-Thesin (0.3 ml/100 g body weight i.p.; chloral hydrate 42.5 mg/ml + sodium pentobarbital 9.72 mg/ml) and placed into a stereotaxic frame. In experiment 1: (transduction efficiency) a 2 µl bilateral intrastriatal injection of either rAAV2/1-PTN/GFP or rAAV2/1-GFP was made at coordinates AP +1.3 mm, ML +2.6 mm, DV −5.0 mm. In experiments 2 and 3: a total of 4 µl of either rAAV2/1-PTN/GFP or rAAV2/1-GFP was injected in two separate sites in the striatum, 2 µl per site (AP +1.6 mm, ML +2.4 mm, DV −4.2 mm and AP −0.2 mm, ML +2.6 mm, DV −7.0 mm). In experiment 4, the total volume injected was reduced to 3 µl in order to compensate for the higher titer of the rAAV2/1-PTN/GFP vector. Therefore, in experiment 4, a total of 3 µl of rAAV2/1-PTN/GFP, rAAV2/1-GFP or PBS was injected to one site in the striatum (AP +0.7 mm, ML +3.0 mm, DV −5.5 mm). For each injection the needle was lowered to the site, 1 minute passed before injection began, and vector was infused at a rate of 0.5 µl/minute using convection enhanced delivery (Hamilton Gas Tight syringe 80,000, 26s/2′′ needle; Hamilton, Reno, NV) coated in SigmaCote; Sigma-Aldrich, St. Louis, MO 050M4340). At the end of the injection the needle remained in place for an additional 4 minutes before retraction.
Intrastriatal 6-OHDA. Four weeks following vector injections, rats in experiment 3 were anesthetized with Equi-Thesin as above. Each rat received four intrastriatal injections of 6-OHDA [MP Biomedicals, Solon, OH; 5 µg/µl (calculated as free base) 6-OHDA in 0.2% ascorbic acid, 0.9% saline solution] at 2 µl per site as previously described.49 Injection coordinates were (i) AP +1.3 mm, ML −2.6 mm, DV −5.0, (ii) MP +0.4 mm, ML −3.0 mm, DV −5.0, 3) MP −0.4 mm, ML −4.2 mm, DV −5.0, 4) MP −1.3 mm, ML −4.5 mm, DV −5.0. In experiment 3, rats received two 2 µl injections of 6-OHDA as described previously23 at injection coordinates AP +1.6 mm, ML +2.4 mm, DV −4.2 mm and AP −0.2 mm, ML +2.6 mm, DV −7.0 mm. In experiment 4, a total of 3 µl of 6-OHDA was injected to one site in the striatum (AP +0.7 mm, ML +3.0 mm, DV −5.5 mm). For all rats in all groups, the needle was zeroed at the skull directly above the injection site in order to target the DV coordinate. For each injection, the needle was lowered slowly to the injection site and 1 minute elapsed before injection commenced, 6-OHDA was injected at 0.5 µl/minute and at the end of the injection the needle remained in place for an additional 4 minutes before retraction.
Cylinder testing. In experiments 3 and 4, nondrugged, spontaneous use of the forepaws was measured 8 (experiment 3) or 5, 9, 13, and 17 weeks (experiment 4, Figure 5a) after 6-OHDA rats as originally described by Schallert and colleagues.50 Briefly, during the dark cycle rats were placed in a clear Plexiglas cylinder and behavior was videotaped until the animal produced 20 weight bearing paw placements on the side of the cylinder, or for 5 minutes, which ever occurred first. Videotapes were analyzed by a rater blinded to treatment. The number of times the rat used its left, right, or both paws for weight bearing in a given trial was determined and noted. Data was reported as the percentage of contralateral (to 6-OHDA), impaired forelimb use: [(contralateral + 1/2 both)/(ipsilateral + contralateral + both)] × 100.
Amphetamine-induced rotational asymmetry. In experiment 3, rats were assessed for ipsilateral rotations at 5 and 7 weeks after 6-OHDA as described previously.23 Rats received an i.p. injection of 5.0 mg/kg amphetamine in 0.9% saline and were placed in cylindrical bowls within which rotational behavior was quantified using the TSE LabMaster (Chesterfield, MO) rotometer program. Recording began 5 minutes after amphetamine injections and continued for the following 90 minutes. Data is expressed as the average number of ipsilateral rotations per minute.
Sacrifice. Experiment 1 rats were sacrificed 4 weeks after vector injection. Rats in experiment 2 were sacrificed at their appropriate developmental age or 4, 10 or 20 weeks after vector injection. All rats of age P1 or older were deeply anesthetized (60 mg/kg, pentobarbital, i.p.) and perfused intracardially with 0.9% saline containing 1 ml/l 10,000 USP heparin. E15, E18, and E20 rat embryos were collected from anesthetized timed pregnant dams as described in the following section. Experiment 3 rats were sacrificed 12 weeks after vector injection (8 weeks following 6-OHDA). Experiment 4 rats were sacrificed 22 weeks after vector injection (18 weeks following 6-OHDA). For immunohistochemistry, brains were removed, postfixed for 7 days in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield PA) in 0.1 mol/l PO4 buffer, and transferred to 30% sucrose in 0.1 mol/l PO4 buffer. For western blot analysis of striatal PTN levels the lateral ganglionic eminence (E15, E18, E20) or the adult striatum was immediately removed and flash frozen in 2-methylbutane on dry ice. Following saline perfusion, postnatal rats (P1, P2, P14, P35) for western blot analysis of striatal PTN levels were perfused intracardially with heparinized saline at 37 °C and brains were flash frozen in 2-methylbutane. Tissue used for western blot was stored at −80 °C until dissected.
Striatal dissections. Rat pups ages E15, E18, and E20 were taken from a terminally anesthetized (pentobaribitol 50 mg/kg, i.p.) dam via cesarean section. Uterine horns were removed and placed into ice-cold sterile saline for subsequent dissection. The skull and meninges were removed from the embryonic brains and the lateral ventricle was opened to expose the lateral ganglionic eminence. Whole lateral ganglionic eminence, developmental precursor to the striatum, was dissected, rinsed in sterile saline, and immediately frozen on dry ice. Three embryonic lateral ganglionic eminences were pooled in a single vial for western blot analysis. Postnatal tissue (P1, P2, P14, P35) was collected from saline perfused pups and was dissected similarly to adult tissue (see below). Postnatal (P1, P2, P14, P35) striatal sample vials contained both left and right striatal hemispheres from a single animal. Frozen brains from naive postnatal brains and unilaterally transduced adults were held at −18 °C for at least 1 hour before dissection. 1–2 mm coronal slabs were blocked from each brain utilizing a brain blocker (Zivic, Pittsburg, PA) and striatal tissue from both hemispheres was microdissected while being held at a constant −12 °C on a cold plate (Teca, Chicago, IL). Frozen dissected structures were stored at –80 °C until analysis.
Immunohistochemistry. Brains were frozen on dry ice and sectioned at a 40-µm thickness using a sliding microtome. Every sixth section was processed for labeling with antisera using the free-floating method. Following blocking in 10% normal goat serum tissue was incubated in primary antisera directed against TH (Chemicon MAB318, mouse anti-TH 1:4,000) with 5 mg/100 ml sodium azide overnight at room temperature. Triton-X (0.5%) was added to the Tris buffer during incubations and rinses to permeabilize cell membranes. Following primary incubation, sections were incubated in biotinylated secondary antisera against mouse IgG (Vector; BA-2001, 1:200) followed by the Vector ABC detection kit employing horseradish peroxidase (Vector Laboratories, Burlingame, CA). Antibody labeling was visualized by exposure to 0.5 mg/ml 3,3′ diaminobenzidine and 0.03% H2O2 in Tris buffer. Sections were mounted on subbed slides, dehydrated to xylene and coverslipped with Cytoseal (Richard-Allan Scientific, Waltham, MA).
PTN and GFP immunohistochemistry. Following blocking in 10% normal goat serum (GFP), 10% normal horse serum (PTN) tissue sections were incubated in primary antisera directed against PTN (Biotinylated R&D Systems AF252PB, goat antihuman PTN, 1:25) or GFP (Abcam ab290, Rabbit anti-GFP, 1:2,000) in 0.1 mol/l Tris–HCl containing 5 mg/100 ml sodium azide and 0.5% Triton-X overnight at room temperature. Following primary incubation, sections for GFP labeling were incubated in a biotinylated secondary antisera against rabbit IgG (Abcam ab6720, 1:400). Sections were then processed utilizing the Vector ABC detection kit employing horseradish peroxidase (Vector Laboratories). Antibody labeling was visualized by exposure to 0.5 mg/ml 3,3′ diaminobenzidine and 0.03% H2O2 in 0.1 mol/l Tris buffer. Sections were mounted on subbed slides, dehydrated to xylene and coverslipped with Cytoseal.
Fluorescence PTN, GFAP, NeuN immunohistochemistry for confocal analysis. Every sixth striatal section was processed for labeling with antisera against PTN, GFAP, and NeuN using the free floating method. Following blocking with 10% donkey serum, tissue was incubated in primary antisera directed against PTN (R&D Systems AF252PB, biotinylated goat antihuman PTN, 1:25), GFAP (Chemicon AB5804, rabbit anti-GFAP, 1:500), and NeuN (Chemicon MAB377, mouse anti-NeuN, 1:200) for 24 hours at room temperature. Triton-X (0.5 %) was added to the Tris buffer during incubations and rinses to permeabilize cell membranes. Following primary incubation, sections were incubated in appropriate secondary antisera against PTN (Invitrogen S11226, Alexa Fluor 568 Streptavidin conjugate, 1:200), GFAP (Invitrogen A31573, Alexa Flour 647 donkey anti-rabbit, 1:200) and NeuN (Invitrogen A21202, Alexa Fluor 488 donkey anti-mouse IgG, 1:200). Sections were mounted on subbed slides, coverslipped with Fluoromount-G (Southern Biotech 010001, Birmingham, AL), and stored in the dark until examined. Antibody labeling was visualized using confocal imaging.
Confocal imaging. Fluorescently immunolabeled sections were analyzed on a Zeiss LSM510 confocal laser scanning microscope and images were acquired and reconstructed using the Zeiss LSM software. Confocal imaging and Z sectioning were performed at 1 µm intervals for verification of colocalization of GFAP, NeuN, and PTN immunostaining. Figures were produced in Photoshop 7.0 (San Jose, CA). Brightness, saturation, and sharpness were adjusted only as necessary to best replicate the immunostaining as viewed directly under the microscope.
Stereology. Stereology was performed using a BX52 Olympus microscope (Olympus America, Center Valley, PA) equipped with Stereo Investigator stereological software (Microbrightfield Bioscience) and a Microfire CCD camera (Optronics, Goleta, CA) as described previously.23 Using the optical fractionator principle, the SNs of rAAV2/1-GFP and rAAV2/1-PTN/GFP rats were individually outlined on each section under a low magnification (×1.25) and cell counts of THir neurons were made according to stereological principles while focusing down through the z-axis at ×60. An entire series of nigral brain sections cut 1:6 was analyzed. The total number of stained neurons (N) in the regions of interest was calculated using the following formula: N = NV · VROI where NV is the numerical density and VROI is the volume of the region of interest. The volume was calculated according to the procedure of Cavalieri and variability within groups was assessed via the coefficient of error. Using a ×60 magnification lens with a 1.4 N/A the section thickness was empirically determined by first bringing the top of the section into focus and then using the Stereo Investigator (Microbrightfield Bioscience) program to step through the z-axis in 1-µm increments until the very bottom of the section was in focus. Once the top of the section was in focus, the z-plane was lowered at 1–2 µm intervals and cell counts were made according to stereological principles while focusing down through the z-axis. PBS Sham injected rats in experiment 4 were qualitatively examined for number of THir neurons to confirm 6-OHDA lesion.
Subsets of rats transduced with either rAAV2/1-GFP (n = 4) or rAAV2/1-PTN/GFP (n = 3) were chosen for analysis of THir neurite densities. Rats in which the majority of the transduction was evident in the dorsal lateral striatum were selected in order to examine density of THir neurites in identical striatal regions. Computer generated virtual slices of striatal sections immunolabeled for GFP were superimposed over adjacent striatal sections labeled with antisera against TH in order to delineate the region of transduction of either rAAV2/1-PTN/GFP or rAAV2/1-GFP within the 6-OHDA lesioned striatum. Analysis of THir neurite density was performed used the Space Balls probe in Stereo Investigator. This hemispheric probe was used to obtain an unbiased estimate of THir neurite length in the striatum using previously reported methods.23 Every sixth coronal section through the entire rostral-caudal span of the striatum was analyzed. The dorsolateral portion of the striatum was outlined starting at the lateral striatal border at a depth even with the ventral border of the lateral ventricle, continuing around the lateral border of the striatum to its dorsal border and then a line was drawn through the striatum to connect this dorsal most point with the starting point. All tracings were made at ×1.25. Counts of the neurites that crossed the borders of the Space Balls probe were made while focusing down through the z-axis at ×60. The volume was calculated according to the procedure of Cavalieri and variability within groups was assessed via the coefficient of error. Neurite density was calculated using the following formula: Density = Neurite length/volume. All samples were analyzed from coded slides by one investigator. CEs for all analyses were ≤0.10.
Western blot. For experiment 2, all samples were homogenized on ice in 2% SDS. Total protein concentration was determined by Bradford protein assay. Adult samples were prepared as 25 ng total protein samples, and embryonic samples were prepared as 15 ng or 25 ng total protein samples using whole tissue homogenates. PTN standards (R&D Systems AF-252-PL, recombinant human PTN, 100 µl/ml) of 3, 5, 10, 15, or 20 ng were run on each blot. Western blot protocol was completed as previously described.45 Samples were run on SDS/PAGE and transferred to Immobilon-FL membranes (Millipore, Bedford, MA). Membranes were incubated in in primary PTN antisera (R&D Systems AF252PB, antihuman PTN goat IgG, 1:2,500) and β-actin antisera (Santa Cruz Biotechnology, Santa Cruz, CA; mouse monoclonal IgG, SC-47778, 1:2,000) overnight. IRDye800 conjugated donkey anti-goat (LI-COR Biosciences, Lincoln, NE, 926–32214, 1:15,000) and IRDye680 conjugated donkey anti-mouse (LI-COR Biosciences; 926–32222, 1:20,000) were used as secondary antibodies. All antibody dilutions were made in LI-COR specific blocking buffer (LI-COR Biosciences, 927–40000) Multiplexed signal intensities were imaged with both 700 and 800 nm channels in a single scan with a resolution of 84 µm using the Odyssey infrared image system (LI-COR Biosciences). Reported integrated intensity measurements of PTN expression was standardized accordingly to corresponding β-actin densitometry measurements.
Vector-transduced striatal volume. Neurolucida v.7, Neurolucida Explorer (MicroBrightField), a BX52 Olympus microscope (Olympus America), and a Microfire CCD camera (Optronics) were used to calculate GFPir transduced striatal areas in rats 20 weeks following rAAV2/1-PTN/GFP injection. Volumes were determined from manual tracings of the striatum and GFPir staining within the striatum approximately every 6th serial section along the anterior–posterior (coronally sectioned) axis. Striatal tracings began at ~2 mm anterior to bregma and were terminated at ~0.2 mm posterior to bregma. The 40-µm section thickness and the distance between each section (~240 µm) allowed Neurolucida to construct a 3D model of the striatum and the transduced area within the striatum from 2D tracings. Using these models, characteristic volumes of the striatum and the transduced area within the striatum were determined by Neurolucida Explorer.
Statistical analysis. For experiment 2, a Student's t-test was performed to look for differences in PTN expression between male and female P1, P2, P14, and P35 striatal samples and to look at differences in PTN expression between P1 and P2 striatal samples. Normalized integrated intensity values (arbitrary units) reported by LI-COR Odyssey software v3.0 (LI-COR Biosciences) were used for all statistical analyses. Data were transformed to ensure normality and equal variances before statistical tests were performed. A one-way ANOVA was performed to look at differences in PTN expression between striatal tissue samples from rats of ages E15, E18, E20, P1, P2, P14, P35. In adult transduced and lesioned rats, a two-way repeated measures ANOVA was performed to look at significant differences in PTN expression between rats receiving rAAV2/1-PTN/GFP or rAAV2/1-GFP and lesioned versus intact sides. Significant differences in main effects were determined by Holm–Sidak post hoc analysis.
For experiments 3 and 4, the mean ± SEM was calculated for each group of rats for each parameter and is reported in the results section. A Student's t-test was performed to look at significant differences in THir striatal terminal densities between rats receiving rAAV2/1-PTN/GFP and rAAV2/1-GFP. A two-way RMANOVA was performed to look at significant differences in THir SNpc neuron numbers between rats receiving rAAV2/1-PTN/GFP or rAAV2/1-GFP (treatment factor) and between lesioned versus intact sides (group factor). For this test, the group factor, defining whether the striatum was lesioned or intact, was the RM because the intact ipsilateral internal controls were from the same parent animals. Additionally, one-way RMANOVAs were performed to determine significant differences in contralateral forepaw use behaviors between pre- and post-6-OHDA time points. Significant differences in main effects were determined by Holm–Sidak post hoc analysis. SigmaPlot 11.0 (Systat Software, San Jose, CA) was used for all statistical analyses and level of statistical significance was set at P ≥ 0.05.
Acknowledgments
This work was supported by NS058682 (C.E.S.), the Michael J. Fox Foundation for Parkinson's Research and the Morris K. Udall Center of Excellence for Parkinson's Disease Research at Michigan State University NS058830 (T.J.C.).
References
- Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K.et al. (2007Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030 Neurology 68384–386. [DOI] [PubMed] [Google Scholar]
- Tomac A, Lindqvist E, Lin LF, Ogren SO, Young D, Hoffer BJ.et al. (1995Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo Nature 373335–339. [DOI] [PubMed] [Google Scholar]
- Björklund A, Kirik D, Rosenblad C, Georgievska B, Lundberg C., and, Mandel RJ. Towards a neuroprotective gene therapy for Parkinson's disease: use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res. 2000;886:82–98. doi: 10.1016/s0006-8993(00)02915-2. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A., and, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson's model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik D, Georgievska B, Rosenblad C., and, Björklund A. Delayed infusion of GDNF promotes recovery of motor function in the partial lesion model of Parkinson's disease. Eur J Neurosci. 2001;13:1589–1599. doi: 10.1046/j.0953-816x.2001.01534.x. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Herzog CD, Dass B, Bakay RA, Stansell J, 3rd, Gasmi M.et al. (2006Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys Ann Neurol 60706–715. [DOI] [PubMed] [Google Scholar]
- Fjord-Larsen L, Johansen JL, Kusk P, Tornøe J, Grønborg M, Rosenblad C.et al. (2005Efficient in vivo protection of nigral dopaminergic neurons by lentiviral gene transfer of a modified Neurturin construct Exp Neurol 19549–60. [DOI] [PubMed] [Google Scholar]
- Mandel RJ, Spratt SK, Snyder RO., and, Leff SE. Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson's disease in rats. Proc Natl Acad Sci USA. 1997;94:14083–14088. doi: 10.1073/pnas.94.25.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kells AP, Eberling J, Su X, Pivirotto P, Bringas J, Hadaczek P.et al. (2010Regeneration of the MPTP-lesioned dopaminergic system after convection-enhanced delivery of AAV2-GDNF J Neurosci 309567–9577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr, ICV GDNF Study Group. Implanted intracerebroventricular. Glial cell line-derived neurotrophic factor et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R.et al. (2006Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease Ann Neurol 59459–466. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N.et al. (2010Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial Lancet Neurol 91164–1172. [DOI] [PubMed] [Google Scholar]
- Rauvala H., and, Pihlaskari R. Isolation and some characteristics of an adhesive factor of brain that enhances neurite outgrowth in central neurons. J Biol Chem. 1987;262:16625–16635. [PubMed] [Google Scholar]
- Vanderwinden JM, Mailleux P, Schiffmann SN., and, Vanderhaeghen JJ. Cellular distribution of the new growth factor pleiotrophin (HB-GAM) mRNA in developing and adult rat tissues. Anat Embryol. 1992;186:387–406. doi: 10.1007/BF00185989. [DOI] [PubMed] [Google Scholar]
- Hida H, Jung CG, Wu CZ, Kim HJ, Kodama Y, Masuda T.et al. (2003Pleiotrophin exhibits a trophic effect on survival of dopaminergic neurons in vitro Eur J Neurosci 172127–2134. [DOI] [PubMed] [Google Scholar]
- Marchionini DM, Lehrmann E, Chu Y, He B, Sortwell CE, Becker KG.et al. (2007Role of heparin binding growth factors in nigrostriatal dopamine system development and Parkinson's disease Brain Res 114777–88. [DOI] [PubMed] [Google Scholar]
- Taravini IR, Chertoff M, Cafferata EG, Courty J, Murer MG, Pitossi FJ.et al. (2011Pleiotrophin over-expression provides trophic support to dopaminergic neurons in parkinsonian rats Mol Neurodegener 640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario JE, Rojas-Mayorquín AE, Saldaña-Ortega M, Salum C, Gomes MZ, Hunot S.et al. (2008Pleiotrophin receptor RPTP-ζ/β expression is up-regulated by L-DOPA in striatal medium spiny neurons of parkinsonian rats J Neurochem 107443–452. [DOI] [PubMed] [Google Scholar]
- Ferrario JE, Taravini IR, Mourlevat S, Stefano A, Delfino MA, Raisman-Vozari R.et al. (2004Differential gene expression induced by chronic levodopa treatment in the striatum of rats with lesions of the nigrostriatal system J Neurochem 901348–1358. [DOI] [PubMed] [Google Scholar]
- Kinnunen T, Raulo E, Nolo R, Maccarana M, Lindahl U., and, Rauvala H. Neurite outgrowth in brain neurons induced by heparin-binding growth-associated molecule (HB-GAM) depends on the specific interaction of HB-GAM with heparan sulfate at the cell surface. J Biol Chem. 1996;271:2243–2248. doi: 10.1074/jbc.271.4.2243. [DOI] [PubMed] [Google Scholar]
- Reimsnider S, Manfredsson FP, Muzyczka N., and, Mandel RJ. Time course of transgene expression after intrastriatal pseudotyped rAAV2/1, rAAV2/2, rAAV2/5, and rAAV2/8 transduction in the rat. Mol Ther. 2007;15:1504–1511. doi: 10.1038/sj.mt.6300227. [DOI] [PubMed] [Google Scholar]
- Burger C, Gorbatyuk OS, Velardo MJ, Peden CS, Williams P, Zolotukhin S.et al. (2004Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system Mol Ther 10302–317. [DOI] [PubMed] [Google Scholar]
- Spieles-Engemann AL, Behbehani MM, Collier TJ, Wohlgenant SL, Steece-Collier K, Paumier K.et al. (2010Stimulation of the rat subthalamic nucleus is neuroprotective following significant nigral dopamine neuron loss Neurobiol Dis 39105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier TJ., and, Sortwell CE. Therapeutic potential of nerve growth factors in Parkinson's disease. Drugs Aging. 1999;14:261–287. doi: 10.2165/00002512-199914040-00003. [DOI] [PubMed] [Google Scholar]
- Kordower JH., and, Sortwell CE. Neuropathology of fetal nigra transplants for Parkinson's disease. Prog Brain Res. 2000;127:333–344. doi: 10.1016/s0079-6123(00)27016-7. [DOI] [PubMed] [Google Scholar]
- Rosenblad C, Kirik D, Devaux B, Moffat B, Phillips HS., and, Björklund A. Protection and regeneration of nigral dopaminergic neurons by neurturin or GDNF in a partial lesion model of Parkinson's disease after administration into the striatum or the lateral ventricle. Eur J Neurosci. 1999;11:1554–1566. doi: 10.1046/j.1460-9568.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- Rosenblad C, Kirik D., and, Björklund A. Sequential administration of GDNF into the substantia nigra and striatum promotes dopamine neuron survival and axonal sprouting but not striatal reinnervation or functional recovery in the partial 6-OHDA lesion model. Exp Neurol. 2000;161:503–516. doi: 10.1006/exnr.1999.7296. [DOI] [PubMed] [Google Scholar]
- Wang L, Muramatsu S, Lu Y, Ikeguchi K, Fujimoto K, Okada T.et al. (2002Delayed delivery of AAV-GDNF prevents nigral neurodegeneration and promotes functional recovery in a rat model of Parkinson's disease Gene Ther 9381–389. [DOI] [PubMed] [Google Scholar]
- Meng K, Rodriguez-Peña A, Dimitrov T, Chen W, Yamin M, Noda M.et al. (2000Pleiotrophin signals increased tyrosine phosphorylation of β β-catenin through inactivation of the intrinsic catalytic activity of the receptor-type protein tyrosine phosphatase β/ζ Proc Natl Acad Sci USA 972603–2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariser H, Ezquerra L, Herradon G, Perez-Pinera P., and, Deuel TF. Fyn is a downstream target of the pleiotrophin/receptor protein tyrosine phosphatase β/ζ-signaling pathway: regulation of tyrosine phosphorylation of Fyn by pleiotrophin. Biochem Biophys Res Commun. 2005;332:664–669. doi: 10.1016/j.bbrc.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Pariser H, Perez-Pinera P, Ezquerra L, Herradon G., and, Deuel TF. Pleiotrophin stimulates tyrosine phosphorylation of β-adducin through inactivation of the transmembrane receptor protein tyrosine phosphatase β/ζ. Biochem Biophys Res Commun. 2005;335:232–239. doi: 10.1016/j.bbrc.2005.07.060. [DOI] [PubMed] [Google Scholar]
- Perez-Pinera P, Alcantara S, Dimitrov T, Vega JA., and, Deuel TF. Pleiotrophin disrupts calcium-dependent homophilic cell-cell adhesion and initiates an epithelial-mesenchymal transition. Proc Natl Acad Sci USA. 2006;103:17795–17800. doi: 10.1073/pnas.0607299103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen A, Kinnunen T, Kaksonen M, Nolo R, Panula P., and, Rauvala H. N-syndecan and HB-GAM (heparin-binding growth-associated molecule) associate with early axonal tracts in the rat brain. Eur J Neurosci. 1998;10:635–648. doi: 10.1046/j.1460-9568.1998.00082.x. [DOI] [PubMed] [Google Scholar]
- Kinnunen T, Kaksonen M, Saarinen J, Kalkkinen N, Peng HB., and, Rauvala H. Cortactin-Src kinase signaling pathway is involved in N-syndecan-dependent neurite outgrowth. J Biol Chem. 1998;273:10702–10708. doi: 10.1074/jbc.273.17.10702. [DOI] [PubMed] [Google Scholar]
- Herradón G., and, Ezquerra L. Blocking receptor protein tyrosine phosphatase β/ζ: a potential therapeutic strategy for Parkinson's disease. Curr Med Chem. 2009;16:3322–3329. doi: 10.2174/092986709788803240. [DOI] [PubMed] [Google Scholar]
- Yang J, Teng Q, Garrity-Moses ME, McClelland S, 3rd, Federici T, Carlton E.et al. (2007Reversible unilateral nigrostriatal pathway inhibition induced through expression of adenovirus-mediated clostridial light chain gene in the substantia nigra Neuromolecular Med 9276–284. [DOI] [PubMed] [Google Scholar]
- Manfredsson FP, Tumer N, Erdos B, Landa T, Broxson CS, Sullivan LF.et al. (2009Nigrostriatal rAAV-mediated GDNF overexpression induces robust weight loss in a rat model of age-related obesity Mol Ther 17980–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgievska B, Kirik D., and, Björklund A. Aberrant sprouting and downregulation of tyrosine hydroxylase in lesioned nigrostriatal dopamine neurons induced by long-lasting overexpression of glial cell line derived neurotrophic factor in the striatum by lentiviral gene transfer. Exp Neurol. 2002;177:461–474. doi: 10.1006/exnr.2002.8006. [DOI] [PubMed] [Google Scholar]
- Rosenblad C, Georgievska B., and, Kirik D. Long-term striatal overexpression of GDNF selectively downregulates tyrosine hydroxylase in the intact nigrostriatal dopamine system. Eur J Neurosci. 2003;17:260–270. doi: 10.1046/j.1460-9568.2003.02456.x. [DOI] [PubMed] [Google Scholar]
- Sajadi A, Bauer M, Thöny B., and, Aebischer P. Long-term glial cell line-derived neurotrophic factor overexpression in the intact nigrostriatal system in rats leads to a decrease of dopamine and increase of tetrahydrobiopterin production. J Neurochem. 2005;93:1482–1486. doi: 10.1111/j.1471-4159.2005.03139.x. [DOI] [PubMed] [Google Scholar]
- Emborg ME, Moirano J, Raschke J, Bondarenko V, Zufferey R, Peng S.et al. (2009Response of aged parkinsonian monkeys to in vivo gene transfer of GDNF Neurobiol Dis 36303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredsson FP, Okun MS., and, Mandel RJ. Gene therapy for neurological disorders: challenges and future prospects for the use of growth factors for the treatment of Parkinson's disease. Curr Gene Ther. 2009;9:375–388. doi: 10.2174/156652309789753400. [DOI] [PubMed] [Google Scholar]
- Ciesielska A, Mittermeyer G, Hadaczek P, Kells AP, Forsayeth J., and, Bankiewicz KS. Anterograde axonal transport of AAV2-GDNF in rat basal ganglia. Mol Ther. 2011;19:922–927. doi: 10.1038/mt.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida H, Masuda T, Sato T, Kim TS, Misumi S., and, Nishino H. Pleiotrophin promotes functional recovery after neural transplantation in rats. Neuroreport. 2007;18:179–183. doi: 10.1097/WNR.0b013e328011398e. [DOI] [PubMed] [Google Scholar]
- Piltonen M, Bespalov MM, Ervasti D, Matilainen T, Sidorova YA, Rauvala H.et al. (2009Heparin-binding determinants of GDNF reduce its tissue distribution but are beneficial for the protection of nigral dopaminergic neurons Exp Neurol 219499–506. [DOI] [PubMed] [Google Scholar]
- Manfredsson FP, Burger C, Sullivan LF, Muzyczka N, Lewin AS., and, Mandel RJ. rAAV-mediated nigral human parkin over-expression partially ameliorates motor deficits via enhanced dopamine neurotransmission in a rat model of Parkinson's disease. Exp Neurol. 2007;207:289–301. doi: 10.1016/j.expneurol.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ., Jret al. (2002Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors Methods 28158–167. [DOI] [PubMed] [Google Scholar]
- Burger C, Nguyen FN, Deng J., and, Mandel RJ. Systemic mannitol-induced hyperosmolality amplifies rAAV2-mediated striatal transduction to a greater extent than local co-infusion. Mol Ther. 2005;11:327–331. doi: 10.1016/j.ymthe.2004.08.031. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C., and, Björklund A. Characterization of behavioral and neurodegenerative changes following partial lesions of the nigrostriatal dopamine system induced by intrastriatal 6-hydroxydopamine in the rat. Exp Neurol. 1998;152:259–277. doi: 10.1006/exnr.1998.6848. [DOI] [PubMed] [Google Scholar]
- Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]