Abstract
Bernard–Soulier syndrome (BSS) is an inherited bleeding disorder caused by a defect in the platelet glycoprotein (GP) Ib-IX-V complex. The main treatment for BSS is platelet transfusion but it is often limited to severe bleeding episodes or surgical interventions due to the risk of alloimmunization. We have previously reported successful expression of human GPIbα (hGPIbα) in human megakaryocytes using a lentiviral vector (LV) encoding human GP1BA under control of the platelet-specific integrin αIIb promoter (2bIbα). In this study, we examined the efficacy of this strategy for the gene therapy of BSS using GPIbαnull as a murine model of BSS. GPIbαnull hematopoietic stem cells (HSC) transduced with 2bIbα LV were transplanted into lethally irradiated GPIbαnull littermates. Therapeutic levels of hGPIbα expression were achieved that corrected the tail bleeding time and improved the macrothrombocytopenia. Sequential bone marrow (BM) transplants showed sustained expression of hGPIbα with similar phenotypic correction. Antibody response to hGPIbα was documented in 1 of 17 total recipient mice but was tolerated without any further treatment. These results demonstrate that lentivirus-mediated gene transfer can provide sustained phenotypic correction of murine BSS, indicating that this approach may be a promising strategy for gene therapy of BSS patients.
Introduction
The Bernard–Soulier syndrome (BSS) is an autosomal recessive disease characterized by thrombocytopenia, enlarged platelets, and bleeding symptoms.1,2 BSS is caused by mutations in one of the three genes encoding the glycoprotein (GP) Ib-IX-V complex—GP1BA, GP1BB, and GP9.3,4,5 GPIb-IX-V complex is expressed on the platelet plasma membrane and serves as a receptor for von Willebrand factor (VWF), thereby contributing to platelet adhesion and aggregation. Clinical manifestations of BSS often include epistaxis, gingival bleeding, and menorrhagia. Platelet transfusion is the primary treatment for hemorrhage but is often limited because of the potential for provoking alloimmunization and refractoriness.6,7 Recently, treatment with recombinant factor VIIa has been demonstrated to be hemostatically effective in patients with platelet disorder such as BSS or Glanzmann's thrombasthenia.7,8,9 However, there remain major concerns with recombinant factor VII treatment regarding adverse reactions, cost, and short half-life.10 To overcome these issues, a gene therapy approach that expresses FVIIa using adeno-associated virus 8 has been tested with BSS mice and improved hemostasis was observed.11 This strategy may also be applied to other bleeding disorders such as Glanzmann's thrombasthenia complicated by alloimmunization. Currently, allogenic hematopoietic stem cell (HSC) transplantation is the only therapy available to cure the disease, but in most cases, the risk of the procedure such as graft-versus-host disease is still higher than the ongoing bleeding tendency.6,12,13 Thus, gene therapy using autologous stem cells might be an attractive alternative that may provide an ultimate cure without a risk of graft-versus-host disease.14,15,16 We have previously applied this strategy to gene therapy of murine hemophilia A, utilizing transplantation of syngeneic HSCs transduced with lentivirus vectors (LV) to introduce a corrected gene copy. Factor VIII (FVIII) expression was targeted to transplant-derived platelets using the platelet-specific integrin αIIb gene promoter (2bIbα), resulting in improvement of the hemophilic bleeding phenotype.17,18
With the aim of gene therapy for BSS, we previously described a LV vector-encoding human GP1BA under transcriptional control of the integrin αIIb promoter that expressed hGPIbα efficiently in a lineage-specific manner.19 Ware and colleagues have developed a murine model of BSS by disrupting the Gp1ba gene (GPIbαnull), and have shown that the BSS phenotype was rescued by transgenic expression of hGPIbα.20 In the present study, we examined the efficacy of 2bIbα LV-mediated bone marrow (BM) transduction and syngeneic transplantation for the gene therapy of BSS using a GPIbαnull murine model of BSS.
Results
Expression of hGPIbα in GPIbαnull mice
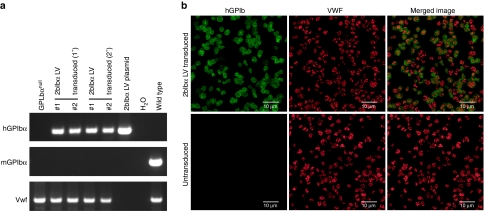
We had previously constructed a 2bIbα LV vector that expresses hGPIbα under the control of the integrin αIIb promoter and confirmed efficient expression in a megakaryocytic cell line (Dami) and human CD34+ cells.19 To assess the use of our 2bIbα LV for gene therapy of BSS, HSC isolated from GPIbαnull mice were transduced and transplanted into lethally irradiated GPIbαnull littermates. Recipients were analyzed after BM reconstitution and the presence of 2bIbα transgene in recipients was confirmed by PCR amplification of peripheral white blood cell-derived genomic DNA (Figure 1a). All GPIbαnull mice that received LV-transduced HSC were positive for 2bIbα transgene. The average copy number of 2bIbα proviral DNA was 0.42 ± 0.31 copies per white blood cell in transduced recipients. Expression of the hGPIbα transgene protein in platelets was confirmed by immunofluorescent confocal microscopy. Most of the platelets were positively stained for hGPIbα in 2bIbα LV-transduced HSC recipients (Figure 1b). The merged image shows that the hGPIbα protein did not colocalize with the endogenous α-granule protein, VWF, but was expressed on the plasma membrane of transduced platelets.
Figure 1.
Genetic and expression analysis of 2bIbαLV-transduced bone marrow transplantation (BMT) recipients. (a) PCR analysis of BMT recipients shows the presence of transgene in recipients. Genomic DNA was prepared from primary (1°) and secondary (2°) 2bIbα lentiviral vector (LV) transduced hematopoietic stem cells (HSC) recipients. GPIbαnull and C57BL/6J wild-type mice were used as controls. 2bIbα LV plasmid DNA was used as a positive control for human GPIbα (hGPIbα). Absence of mGPIbα PCR product confirmed the GPIbαnull background. The Vwf gene was used as an internal control. PCR product sizes; hGPIbα (458 bp), mGPIbα (486 bp), and Vwf (727 bp). (b) Immunofluorescent staining of mouse platelets. Platelets were isolated from GPIbαnull mice that received 2bF8 LV-transduced HSC (upper panel) and untransduced GPIbαnull control mice (lower panel) and stained for hGPIbα (green) and murine VWF (red). Nonspecific isotype-matched primary antibodies were used to assess background staining (data not shown). Bar = 10 µm.
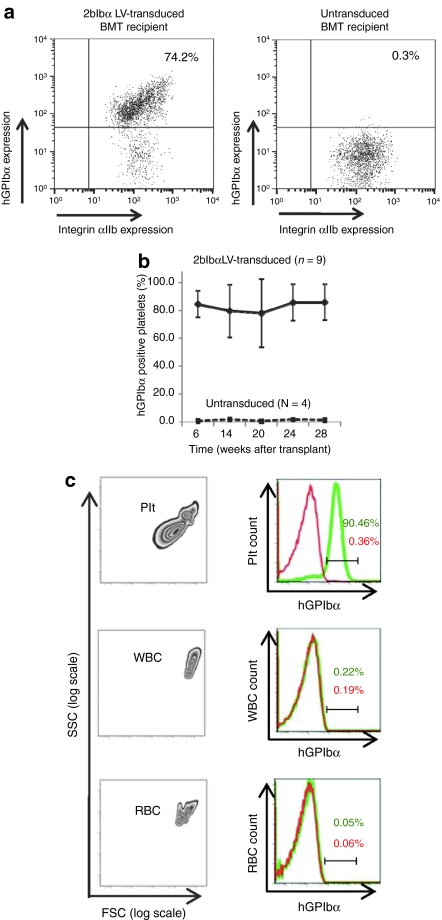
The percentage of platelets that expressed hGPIbα was analyzed by flow cytometry and ranged from ~70 to 90% (Figure 2a). On average, 84.5 ± 9.5% (n = 9) of total platelets were expressing hGPIbα at 6 weeks after transplantation in 2bIbα LV-transduced HSC recipients and stable expression was maintained through the entire observation period of 7 months (Figure 2b). The integrin αIIb gene promoter that we used in our LV vector has previously been characterized and shown to induce platelet-specific expression in vitro and in vivo.18,21,22,23 To confirm that the expression of hGPIbα driven by the integrin αIIb gene promoter is confined to platelets, flow cytometric analysis was performed on whole blood of 2bIbα LV-transduced HSC recipient mice (Figure 2c). As expected, blood cells with the forward- and side-scattering properties of white blood cells and red blood cells did not express hGPIbα, confirming platelet-specific expression of 2bIbα in blood cells.
Figure 2.
Flow cytometric analysis of human GPIbα (hGPIbα) expression in bone marrow transplantation (BMT) recipients. (a) Expression of hGPIbα in the platelets of 2bIbα lentiviral vector (LV) transduced (upper panel) and untransduced (lower panel) hematopoietic stem cells (HSC) recipients were analyzed by flow cytometry. The platelet population was gated with anti-mouse CD41/integin αIIb mAb and hGPIbα expression was analyzed using AlexaFluor 647 labeled anti-hGPIbα mAb (AP1). (b) Expression of hGPIbα was monitored for 28 weeks after BMT and average expression (percentage of hGPIbα-positive platelets) in 2bIbα LV-transduced HSC recipients (n = 9) was plotted at each time point. Untransduced controls (n = 4) were analyzed in parallel each time. Data is expressed as the mean ± SD. (c) Platelet-specific expression of hGPIbα. Entities exhibiting the forward (FSC) and side (SSC) scattering properties of platelets (Plt), white blood cells (WBC), and red blood cells (RBC) from whole blood of 2bIbα LV-transduced HSC recipients (left columns) were gated to analyze hGPIbα expression on the various blood cell populations. Right columns show histograms of hGPIbα expression in transduced (green) and untransduced (red) HSC recipients. Only platelets from transduced recipient display hGPIbα on their surface.
Analysis of platelets in 2bIbα LV-transduced HSC recipients
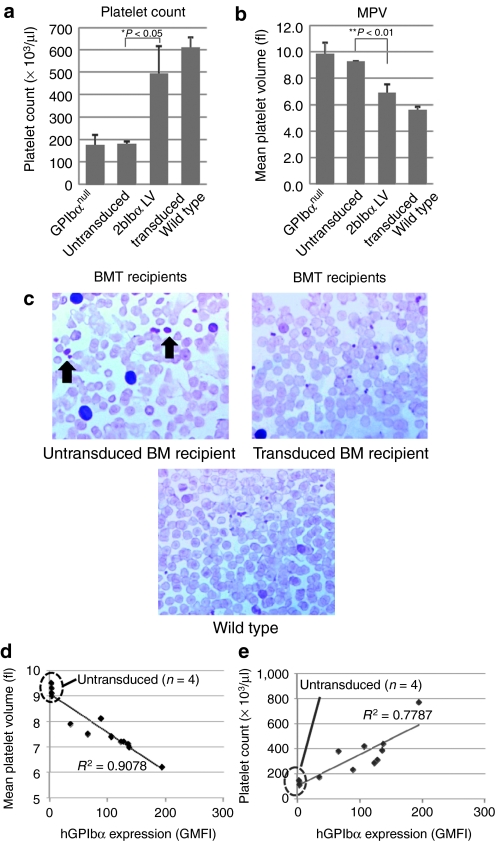
Since macrothrombocytopenia is a characteristic phenotype of BSS, platelet size, and counts were analyzed in 2bIbα LV-transduced HSC recipients. As shown in Figure 3a, the number of platelets in untransduced BM recipients were similar to those of GPIbαnull controls (181 ± 11 × 103/µl, n = 4 versus 176 ± 45 × 103/µl, n = 6). In 2bIbα LV-transduced HSC recipients, on the other hand, platelet counts were significantly increased and were close to wild-type mice (492 ± 126 × 103/µl, n = 9 versus 611 ± 47 × 103/µl, n = 6). Figure 3b shows that mean platelet volumes (MPV) in untransduced BM recipients were similar to GPIbαnull (9.3 ± 0.1 fL, n = 4 versus 9.8 ± 0.9 fL, n = 6, P = 0.24) but were significantly reduced in 2bIbα LV-transduced HSC recipients, with MPVs close to wild-type mice (6.9 ± 0.7 fL, n = 9 versus 5.6 ± 0.2 fL, n = 6, P < 0.01).
Figure 3.
Analysis of platelet count and size. (a) Platelet count and (b) size of GPIbαnull (n = 6), untransduced bone marrow (BM) recipients (n = 4), 2bIbα lentiviral vector (LV)-transduced BM recipients (n = 9), and C57BL/6J wild-type mice (n = 6) are depicted. Both parameters were monitored every 4 weeks after bone marrow transplantation (BMT) and representative data (20 weeks after BMT) are shown. Data is expressed as the mean ± SD. (c) Blood smears were stained with hematoxylin and eosin. Arrows show giant platelets that were observed in untransduced BMT recipients. (d) Expression of human GPIbα (hGPIbα) inversely correlates with platelet size. hGPIbα expression level determined by geometric mean fluorescence intensity (GMFI) of platelet-bound AlexaFluor 647 labeled anti-hGPIbα mAb (AP1) signal was plotted against platelet size (mean platelet volume). (e) Expression of hGPIbα correlates with platelet count. AP1GMFI was plotted against platelet count. Untransduced BM recipients (n = 4) and 2bIbα LV-transduced BM recipients (n = 9) are plotted in (d) and (e), and untransduced recipients are marked with a dotted oval.
In accordance with these findings, giant platelets were observed in the peripheral blood smears of untransduced HSC recipients but not in transduced HSC recipients (Figure 3c). Thus, macrothrombocytopenia was significantly corrected in 2bIbα LV-transduced animals.
It has been shown in the past that transgenic expression of hGPIbα on the GPIbαnull background corrected platelet size as well as platelet count and bleeding time.20,24 However, it is not known whether there is a threshold of hGPIbα expression or if there exists a dose-dependent effect of hGPIbα expression on platelet size/count correction. To analyze the relationship of hGPIbα expression and platelet size in more detail, platelet count and size in relation to hGPIbα expression were analyzed among individual recipients. Figure 3d shows that platelet size inversely correlates with hGPIbα expression level (R2 = 0.9078). This result shows that the expression level of hGPIbα has a dose-dependent effect on GPIbαnull platelet size reduction. Similarly, platelet counts are shown to be increased with the expression of hGPIbα (R2 = 0.7767, Figure 3e). These results suggest that platelet size and number are regulated by the expression level of hGPIbα.
Association of hGPIbα with murine GPIbβ and GPIX
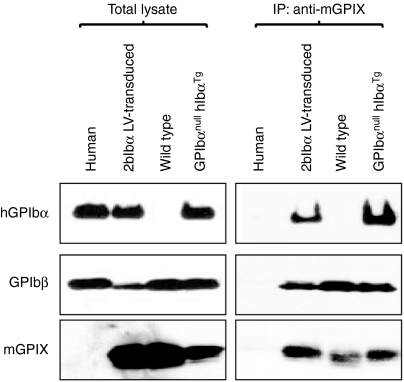
To investigate whether transgene protein hGPIbα associates with endogenous murine GPIbβ and GPIX, facilitating expression of GPIb complex on the platelet surface, platelets were lysed and immunoprecipitated with anti-mGPIX monoclonal antibody (mAb), then analyzed by western blotting. As shown in Figure 4, hGPIbα and mGPIbβ coprecipitated with mGPIX in 2bIbα LV-transduced BM recipients (right panel). This demonstrates that hGPIbα expressed on GPIbαnull mouse platelets associates with endogenous mGPIbβ and mGPIX. No immunoprecipitated GPIb components were detected in human platelet lysate because the anti-mGPIX antibody specifically recognizes mouse but not human GPIX. Also the mAb used for western blot detection of hGPIbα specifically recognizes human but not mouse, so mGPIbα which is associated with mGPIbβ and mGPIX does not show up in the wild-type sample even though it is present on the gel in both the lysate and immunoprecipitate lanes.
Figure 4.
Complex formation of human GPIbα (hGPIbα) with mGPIbβ and mGPIX. Platelet lysates were immunoprecipitated with anti-mGPIX mAb (Xia.B4), followed by western blotting using anti-hGPIbα mAb (142.11), anti-GPIbβ mAb (MBC257.4), and anti-mGPIX mAb (Xia.B4) (right panels). MBC257.4 was raised against human GPIbβ and crossreacts with mouse GPIbβ. Total platelet lysates are shown on the left panels. GPIbαnull hIbαTg represents transgenic mice that stably express hGPIbα on the GPIbαnull background.20 hGPIbα expressed on GPIbαnull mouse platelets is complexed with endogenous mGPIbβ and mGPIX.
Correction of the bleeding phenotype
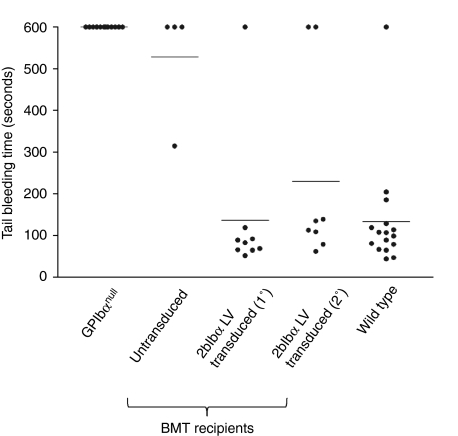
Next, we assessed whether 2bIbα LV-mediated BM transduction and syngeneic transplantation could rescue the bleeding phenotype of BSS mice. As shown in Figure 5, tail bleeding times were corrected to normal levels (134.5 ± 131.6 seconds, n = 16) in the GPIbαnull recipients who received 2bIbα LV-transduced HSCs (138.1 ± 174.3 seconds, n = 9). On the other hand, recipients who received untransduced GPIbαnull HSC exhibited prolonged bleeding times (528.8 ± 142.5 seconds, n = 4) that were similar to GPIbαnull mice. These results demonstrate the bleeding phenotype of GPIbαnull was rescued by transplantation of 2bIbα LV-transduced GPIbαnull HSC.
Figure 5.
Correction of the GPIbαnull bleeding phenotype by 2bIbα lentiviral vector (LV)-transduced bone marrow transplantation (BMT). 2bIbα LV-transduced and untransduced hematopoietic stem cells (HSC) recipients were analyzed by tail bleeding time assays 10 weeks after transplantation. 2bIbα (1°) represents primary recipients that were transplanted with 2bIbα LV-transduced BM (n = 9), and 2bIbα (2°) represents secondary recipients that that received BMT from primary recipients. When bleeding did not cease within 10 minutes, the tail was cauterized and bleeding time was recorded as 600 seconds. Wild-type C57BL/6J and GPIbαnull mice were used as controls. Prolonged bleeding time of GPIbαnull is rescued in both 2bIbα LV-transduced (1°) and 2bIbα LV-transduced (2°) recipients.
Sustained expression of hGPIbα and phenotypic correction in secondary GPIbαnull BMT recipients
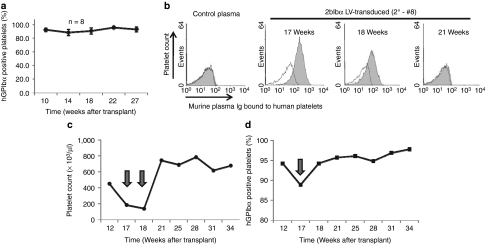
To confirm gene transfer in long-term repopulating HSCs, we performed secondary transplantation using BM cells from some of the primary recipients (1°) collected at 7 months after transplantation. PCR detection of 2bIbα transgene showed that viable engraftment was achieved in the secondary recipients (2°) (see Figure 1a). Flow cytometric analysis showed that 91.9% ± 2.8% (n = 8) of platelets in the secondary GPIbαnull recipients were expressing hGPIbα at 10 weeks after BM transplantation (BMT) and similar expression levels were maintained for 27 weeks (Figure 6a). Platelet count and size (MPV) were similar to those of the primary recipients (566.0 ± 102.1 × 103/µl, and 7.1 ± 0.4 fL, n = 8). Tail bleeding time was also corrected in the secondary BMT recipients [see Figure 5, 2bIbα (2°)].
Figure 6.
Human GPIbα (hGPIbα) expression after secondary transplantation and antibody response. (a) BM mononuclear cells prepared from primary recipients that had been given 2bIbα lentiviral vector (LV)-transduced hematopoietic stem cells (HSC) were transplanted into lethally irradiated GPIbαnull mice (n = 8). Average percentage of hGPIbα expressing platelets is plotted at each time point. Data is expressed as the mean ± SD. (b) Plasma samples from primary and secondary recipients were tested for the presence of antibody. Normal human washed platelets were incubated with mouse plasma, then with phycoerythrin (PE)-labeled anti-mouse IgG and were analyzed by flow cytometry. The shaded histograms show one of the secondary recipients (2°-#8) that presented with an antibody reaction at 17 and 18 weeks after transplantation. Each histogram is overlaid with a negative control in which platelets were incubated with buffer instead of mouse plasma. Control plasma is derived from C57BL/6J wild-type mouse. (c) Platelet count and (d) hGPIbα expression in recipient 2°-#8. Platelet number was decreased to GPIbαnull levels at 17 and 18 weeks after transplantation, then recovered to a wild-type level after 21 weeks. The percentage of platelets expressing hGPIbα was decreased at 17 weeks after transplantation but had recovered at 18 weeks. Over 90% of platelets maintained hGPIbα expression through the remaining observation period.
Immune response and immune-mediated thrombocytopenia in a 2bIbα LV-transduced BM recipient
To test whether there was an immune response against newly expressed hGPIbα, plasma samples from 2bIbα LV-transduced HSC primary and secondary recipients were incubated with human platelets, probed with phycoerythrin-labeled anti-mouse immunoglobulin G (IgG), and analyzed by flow cytometry at various time points. As shown in Figure 6b, at 17 weeks after transplantation, one of the secondary recipients produced antibody that reacted with human platelets and this antibody was still detected the following week (18 weeks after transplantation). Platelet number was decreased by ~60% at the 17-week time point and by ~70% at week 18, to the levels comparable to GPIbαnull mice (Figure 6c). The percentage of hGPIbα positive platelets was not significantly decreased but a small decrease of ~6% was noted at the 17-week time point (Figure 6d). This mouse was carefully observed and the antibody had disappeared from plasma by 21 weeks post-transplantation (see Figure 6b). Platelet count also quickly recovered to wild-type levels and was stably maintained through the remaining observation period. Thus, an immune reaction was documented in one of the secondary recipients but tolerance was induced without treatment.
Discussion
We have previously shown targeted expression of FVIII in platelets using a LV vector-encoding F8 under control of the integrin αIIb promoter.18 In this study, we attempted the treatment of BSS using a LV vector-encoding human GP1BA under control of the same integrin αIIb promoter via BM transduction and transplantation. This study shows that the animals receiving 2bIbα LV-transduced cells were able to synthesize transgene protein hGPIbα which associates with endogenous mouse proteins GPIbβ and IX, and rescues the macrothrombocytopenia and bleeding phenotype in BSS mice.
One important issue in our gene therapy approach is whether LV vector-mediated gene transfer can achieve therapeutic levels of hGPIbα expression that correct the BSS phenotype. In our experiments, over 70% of platelets expressed hGPIbα in the animals that receiving 2bIbα LV-transduced HSC and the overall expression level (determined by geometric mean fluorescence intensity) was comparable to that observed in hGPIbα transgenic mice which has been reported to rescue murine BSS.20 Sequential BMT showed that sustained hGPIbα expression was maintained in secondary recipients, indicating that long-term repopulating HSC were successfully modified by 2bIbα LV. Our studies demonstrate that the transduction efficiency using 2bIbα LV in the BSS mouse model is much higher than we achieved with 2bF8 LV in the hemophilia A mouse.18 One potential explanation for the increased transduction efficiency could be the much smaller size of the hGPIbα construct (1.1 kb) compared to the hFVIII construct (4.5 kb). A second factor could be the use of Sca-1+-selected HSC for transduction in this study, whereas unselected whole BM mononuclear cells were used as the target in our previous study. In this study, we showed that macrothrombocytopenia and tail bleeding times were normalized in both primary and secondary recipients. Thus expression levels of hGPIbα mediated by 2bIbα LV vector transduction and transplantation was adequate to correct the BSS phenotype.
The immune response to transgene protein is a concern in any gene therapy approach.25 Ectopically expressed FVIII in platelets introduced by LV-mediated gene transfer was stored in platelet α-granules, released at the site of vascular injury, and corrected the bleeding phenotype of hemophilia A mice with neither inhibitory nor noninhibitory antibody development.18 However, unlike FVIII which was stored in the α-granules of platelets, transduced hGPIbα is constitutively expressed on the surface of the transduced platelets, which provides greater exposure to immune surveillance and may lead to an increased probability of provoking an immune reaction. Thus both hGPIbα expression and platelet count were monitored for ~7 months after BMT in both primary and secondary recipients. One of the secondary recipients exhibited antiplatelet antibody production and immune-mediated thrombocytopenia with 60–70% reduction at two consecutive time points. This immune response was diminished without any treatment within a month and both platelet count and the proportion of hGPIbα-expressing platelets were stably maintained for ~6 months thereafter. The induced antibodies were able to bind to human platelets but not to GPIbαnull or wild-type mouse platelets, suggesting that the antibody recognized hGPIbα (data not shown). Despite constitutive expression on the surface of platelets, an immune reaction was observed in only one of 17 total primary and secondary recipients. It is reported by Wilcox and colleagues that when human integrin β3 was expressed on β3−/− mouse platelets following transplantation of transduced BM cells, some of the recipients developed humoral immunity but expression of integrin αIIbβIII improved 9 weeks after treatment with intravenous Ig.22 The low incidence of antibody response and induction of tolerance without treatment in our study suggests that hGPIbα might be more easily tolerated than integrin β3.26
BSS can be caused by mutations in any one of the GP1BA, GP1BB, or GP9 genes.27 Causative mutations in GP1BA have been identified more often than in GP1BB or GP9.5 Since the entire coding sequence of GP1BA is contained in one exon, the presence of nonsense or missense mutations that introduce a premature termination codon may not result in nonsense-mediated mRNA decay, enabling the affected gene to produce a truncated form of the protein. Indeed, mutations such as a frame shift that introduces a premature stop codon in the transmembrane domain, and a nonsense mutation that produces a truncated form of GPIbα, have been characterized with significant amounts of the soluble, extracellular portion of GPIbα free in plasma.28,29,30,31,32 It is reasonable to expect that BSS patients having truncated forms of GPIbα in their plasma might be better candidates for gene therapy since their propensity of developing an immune reaction would be lower compared to patients totally lacking GPIbα protein.
One factor that needs to be considered for virus-mediated gene transfer is insertional mutagenesis caused by transgene integration that may lead to endogenous gene disruption or transactivation. In our study, a stable hGPIbα expression profile in both primary and secondary recipients during our observation period of 7 months strongly suggests that lentivirally transduced HSCs have no competitive repopulation advantages or disadvantages over untransduced HSCs. None of the primary or secondary recipients exhibited obvious tumor development during the observation period and blood cell counts remained normal, although autopsy and histologic analyses of recipient mice have not been performed. As has been suggested, LV vectors might have a lower oncogenic potential compared to other retroviral vectors.33,34,35,36 However, it is crucial to analyze integration sites to confirm the safety of LV-mediated gene therapy and thus, further assessment of 2bIb LV integration sites and post-transplant repopulation clonality by techniques such as linear amplification-mediated (LAM) PCR will be required in the future.37
In summary, we have demonstrated that lentivirus-mediated gene transfer can provide sustained phenotypic correction of murine BSS, indicating that this approach may be a promising strategy for gene therapy of BSS in human patients.
Materials and Methods
Vector construction, production, and purification. The 2bIbα LV vector-encoding human GP1BA under control of the integrin αIIb promoter was constructed as described previously.19 A fragment of integrin αIIb gene promoter was kindly provided by Dr David A. Wilcox at the Medical College of Wisconsin. LV production and purification were performed as described in our previous reports.17,18,23
HSC transduction and transplantation. Animal studies were performed according to a protocol approved by the Animal Care and Use Committee of the Medical College of Wisconsin. All mice used in this study were on the C57BL/6J background, and isoflurane (Phoenix Pharmaceuticals, St Joseph, MO) or 2.5% tribromoethanol (Sigma-Aldrich, St Louis, MO) were used for anesthesia when necessary.
BM cells were collected from femurs and tibia of GPIbαnull mice as described previously.15 Murine HSCs were isolated using the anti-Sca-1 MicroBead Kit (Miltenyl Biotec, Auburn, CA) following kit instructions. The Sca-1+ cells were transduced with 2bIbα LV using a similar procedure to those described in our previous reports.17,18 Briefly, the Sca-1+ cells were cultured in Iscove's modified Dulbecco medium (Gibco-Invitrogen, Carlsbad, CA) containing 10% fetal bovine serum and cytokine cocktail including 20 ng/ml of recombinant murine interleukin (IL)-3, 100 ng/ml rmIL-6, 100 ng/ml stem cell factor, 100 ng/ml thrombopoietin, and 100 ng/ml rmflt3 ligand (PeproTech, Rocky Hill, NJ) for 48 hours at 37 ºC in 5% CO2. The cells were transduced on RetroNectin-coated plates with 2bIbα LV at a multiplicity of infection of ~10 in the presence of cytokines, and 2 hours after the final transduction the cells were harvested, washed, and resuspended in Iscove's modified Dulbecco medium media with cytokines for transplantation. Six- to eight-week-old GPIbαnull mice (recipients) were conditioned with a lethal dose of 1,100 cGy total body irradiation using a Gammacell 40 Exactor cesium irradiator (Best Theratronics, Ottawa, Ontario, Canada). Twenty-four hours after irradiation, a dose of 1 × 106 transduced Sca-1+ cells in a volume of 400 µl of Iscove's modified Dulbecco medium per mouse were infused by retro-orbital vein injection. The same dose of untransduced cells were transplanted into lethally irradiated GPIbαnull mice as controls. Recipients were analyzed beginning at 3 weeks after BMT. Primary recipients were sacrificed at 7 months after transplantation and BM cells from five recipients who exhibited higher than 85% of platelets expressing hGPIbα were subsequently transplanted into secondary recipients. For secondary transplantation, BM cells from each donor were transplanted into two lethally irradiated GPIbαnull secondary recipients.
Blood cell analysis. Blood samples were collected by tail bleeding into tubes containing 0.1 volume of 0.1 mol/l sodium citrate or from the retro-orbital venous plexus using heparin-coated capillary tubes. Blood cell parameters including platelet number and the MPV were analyzed by the Scil Vet ABC Counter (Scil, Viernheim, Germany). Blood smears were stained with hematoxylin and eosin by the Histology Laboratory at the Medical College of Wisconsin and were visualized using a Nikon ECLIPSE E600 microscope (Nikon, Tokyo, Japan).
PCR and quantitative real-time PCR. Genomic DNA was purified from peripheral white blood cells using QIAamp DNA blood mini kit (Qiagen, Hilden, Germany) and analyzed by PCR using primers specific for hGPIbα: 5′-CCCTGGGGTCTATTCTACTCA-3′ and 5′-AGTGATAC GGGTTTTGTGGTA-3′, or for mGPIbα: 5′-GGGT GGAAGGAAAGTGAGAAT-3′ and 5′-CAGCAGGAAGAGCAAGATG AG-3′. PCR of murine Vwf was used as an internal control to confirm DNA integrity. The primers used to amplify Vwf were 5′-GATGACCCTGCACACTGTCAGA-3′ and 5′-ACACTGAGGATGGA ATAGCAC-3′. Amplification was performed using GoTaq Green Master Mix (Promega, Madison, WI). DNA from 2bIbα plasmid DNA was used as a positive control. DNA from a GPIbαnull mouse and a reaction without DNA were used as negative controls. For quantitative real-time PCR, peripheral blood-derived genomic DNA was analyzed for the LV LTR sequence, with normalization to the Apo B sequence using iQ Supermix (Bio-Rad Laboratories, Hercules, CA) as previously described.18
Immunoprecipitation. Washed platelets were prepared from citrated whole blood as previously described.38 Briefly, whole blood was diluted 1:2 with modified Tyrode's buffer (20 mmol/l HEPES pH 7.4, 137 mmol/l NaCl, 2.5 mmol/l KCl, 5.5 mmol/l glucose, 0.25% bovine serum albumin) containing 50 ng/ml of Prostaglandin E1, centrifuged at 150g for 5 minutes at room temperature, and platelet-rich plasma was collected. Platelets were washed by sedimentation at 800 g for 5 minutes in modified Tyrode's buffer-containing Prostaglandin E1. Washed platelets were lysed in phosphate-buffered saline (PBS) containing 5 mmol/l EDTA and 1% Triton X-100 supplemented with Complete Mini-protease Inhibitors cocktail (Roche Diagnostic, Mannheim, Germany). Platelet lysates were precleared with protein G-Sepharose beads (GE Healthcare, Piscataway, NJ) for 1 hour at 4 °C and then incubated with rat anti-mGPIX mAb (Xia.B4; Emfret Analytics, Eibelstadt, Germany) coupled protein G-sepharose for 2 hours at 4 °C. Sepharose beads were collected by centrifugation, washed, and eluted in 2% sodium dodecyl sulfate at 100 °C for 5 minutes. Reduced or unreduced samples were analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis on 4–20% Tris-Glycine Gels (Invitrogen, Carlsbad, CA) and electroblotted onto polyvinylidine difluoride membrane. Membranes were blocked with PBS-containing 5% powdered milk and 0.05% Tween-20, and incubated overnight with anti-hGPIbα mAb 142.11, anti-mGPIbβ mAb MBC257.4, or anti-mGPIX mAb Xia.B4 at 5 µg/ml. Antibodies 142.11 and MBC257.4 were generated in our laboratory. The membranes were washed three times with PBS-containing 0.05% Tween-20, then incubated for 1 hour at room temperature with horseradish peroxidase-conjugated goat anti-mouse IgG (1:5,000) or horseradish peroxidase-conjugated goat anti-rat IgG (Pierce-Thermo Scientific, Rockford, IL) (1:5,000). The membranes were washed again three times and were developed with SuperSignal West Pico Chemiluminescent Substrate (Pierce-Thermo Scientific).
Flow cytometry analysis. Blood was collected by tail bleed and stained with anti-mouse CD41 mAb directly conjugated with phycoerythrin (MWreg30; Santa Cruz Biotechnology, Santa Cruz, CA) and antihuman GPIbα mAb, AP1 directly conjugated with AlexaFluor 647 (Invitrogen). Three microliters of mouse whole blood was incubated in a total volume of 50 µl PBS with 8 µg/ml of AP1 and 4 µg/ml of anti-mouse CD41 mAb at room temperature for 30 minutes. Cells were analyzed using a LSRII flow cytometer (BD Biosciences, San Jose, CA). Isotype IgG controls were stained in parallel.
For the detection of antibodies against human GPIbα (hGPIbα) in mouse plasma samples, washed human platelets were prepared using the same procedure described above for washed mouse platelets and resuspended in PBS at 2 × 105/µl.
Five microliters of murine plasma samples were incubated with 5 µl of washed platelets (1 × 106 platelets in total) at room temperature for 30 minutes. Phycoerythrin-labeled donkey anti-mouse IgG (Jackson Immuno Research, West Grove, PA) diluted in PBS was then added at a final dilution of 1:100 in a volume of 25 µl. The samples were incubated for an additional 20 minutes, then examined using the LSRII flow cytometer. C57BL/6J wild-type mice plasma was used as a negative control. Human platelet studies complied with institutional guidelines approved by the Human Research Review Committee of the Medical College of Wisconsin.
Immunofluorescent confocal microscopy. Intracellular location of transgene protein was determined by immunofluorescent confocal microscopy as described in our previous report.17,19 Briefly, platelets isolated from BMT recipients were cytospun and fixed on glass slides. The slides were stained with a mAb against hGPIbα (AP1) and a rabbit antihuman VWF polyclonal antibody that crossreacts with mouse VWF (DAKO, Carpinteria, CA) and detected by AlexaFluor-488- or AlexaFluor-568-labeled anti-mouse and anti-rabbit secondary antibodies (Invitrogen), respectively. Untransduced platelets from GPIbαnull mice were processed in parallel for each immunofluorescence-staining assay using identical conditions. Nonspecific isotype control antibodies served as negative controls. Immunofluorescent detection was performed by confocal microscopy using an Olympus FV1000-MPE Multiphoton Microscope (Olympus, Tokyo, Japan).
Tail bleeding time assay. Mouse tail bleeding times were determined as previously described.20 Briefly, mice were anesthetized using isoflurane in a precision vaporizer at an inducing dose of 3%, and a maintenance dose of 1%. A 3 mm portion of distal tail tip was removed using a sterile scalpel blade and the wounded tail was immersed in isotonic saline maintained at 37 °C. The point at which complete cessation of blood flow occurred was defined as the bleeding time. If bleeding had not yet ceased after 600 seconds, tails were cauterized and bleeding time was recorded as 600 seconds.
Statistical analysis. Data are presented as mean ± SD. Two groups were compared by the unpaired Student's t-test (Prism; GraphPad Software, San Diego, CA). Probability values >0.05 were considered statistically significant.
Acknowledgments
This work was supported by National Blood Foundation (Q.S.), Children's Hospital Foundation (Children's Hospital of Wisconsin) (Q.S.), American Heart Association National Center Scientific Development Award (0730183N) (Q.S.), National Hemophilia Foundation Career Development Award (Q.S.), Hemophilia Association of New York (Q.S.), National Institutes of Health grants HL-102035 (Q.S.), HL-56027 (R.R.M.), HL-44612 (R.R.M.), HL-33721 (R.R.M.), and American Heart Association Postdoctoral Fellowship (10POST261016) (S.K.). The authors declared no conflict of interest.
REFERENCES
- Bernard J., and, Soulier JP.1948[Not Available] Sem Hop 24Spec. No.3217–3223.18116504 [Google Scholar]
- Nurden P., and, Nurden AT. Congenital disorders associated with platelet dysfunctions. Thromb Haemost. 2008;99:253–263. doi: 10.1160/TH07-09-0568. [DOI] [PubMed] [Google Scholar]
- Clemetson KJ, McGregor JL, James E, Dechavanne M., and, Lüscher EF. Characterization of the platelet membrane glycoprotein abnormalities in Bernard-Soulier syndrome and comparison with normal by surface-labeling techniques and high-resolution two-dimensional gel electrophoresis. J Clin Invest. 1982;70:304–311. doi: 10.1172/JCI110618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen I, Nurden A, Bjerrum OJ, Solum NO., and, Caen J. Immunochemical evidence for protein abnormalities in platelets from patients with Glanzmann's thrombasthenia and Bernard-Soulier syndrome. J Clin Invest. 1980;65:722–731. doi: 10.1172/JCI109719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López JA, Andrews RK, Afshar-Kharghan V., and, Berndt MC. Bernard-Soulier syndrome. Blood. 1998;91:4397–4418. [PubMed] [Google Scholar]
- Locatelli F, Rossi G., and, Balduini C. Hematopoietic stem-cell transplantation for the Bernard-Soulier syndrome. Ann Intern Med. 2003;138:79. doi: 10.7326/0003-4819-138-1-200301070-00028. [DOI] [PubMed] [Google Scholar]
- Ozelo MC, Svirin P., and, Larina L. Use of recombinant factor VIIa in the management of severe bleeding episodes in patients with Bernard-Soulier syndrome. Ann Hematol. 2005;84:816–822. doi: 10.1007/s00277-005-1080-y. [DOI] [PubMed] [Google Scholar]
- Peters M., and, Heijboer H. Treatment of a patient with Bernard-Soulier syndrome and recurrent nosebleeds with recombinant factor VIIa. Thromb Haemost. 1998;80:352. [PubMed] [Google Scholar]
- White GC.2nd (2006Congenital and acquired platelet disorders: current dilemmas and treatment strategies Semin Hematol 431 Suppl 1S37–S41. [DOI] [PubMed] [Google Scholar]
- Lopez-Vilchez I, Hedner U, Altisent C, Diaz-Ricart M, Escolar G., and, Galan AM. Redistribution and hemostatic action of recombinant activated factor VII associated with platelets. Am J Pathol. 2011;178:2938–2948. doi: 10.1016/j.ajpath.2011.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljamali MN, Margaritis P, Arruda VR, Schlachterman A, Dunn D, Ware J.et al. (2006Gene transfer-mediated expression of murine factor VIIa improves clot formation in a mouse model for Bernard-Soulier syndrome Mol Ther 13S28 [Google Scholar]
- Rieger C, Rank A, Fiegl M, Tischer J, Schiel X, Ostermann H.et al. (2006Allogeneic stem cell transplantation as a new treatment option for patients with severe Bernard-Soulier Syndrome Thromb Haemost 95190–191. [PubMed] [Google Scholar]
- Balduini CL, Iolascon A., and, Savoia A. Inherited thrombocytopenias: from genes to therapy. Haematologica. 2002;87:860–880. [PubMed] [Google Scholar]
- Wilcox DA, Olsen JC, Ishizawa L, Bray PF, French DL, Steeber DA.et al. (2000Megakaryocyte-targeted synthesis of the integrin β(3)-subunit results in the phenotypic correction of Glanzmann thrombasthenia Blood 953645–3651. [PubMed] [Google Scholar]
- Wilcox DA, Shi Q, Nurden P, Haberichter SL, Rosenberg JB, Johnson BD.et al. (2003Induction of megakaryocytes to synthesize and store a releasable pool of human factor VIII J Thromb Haemost 12477–2489. [DOI] [PubMed] [Google Scholar]
- Makris M, Colvin B, Gupta V, Shields ML., and, Smith MP. Venous thrombosis following the use of intermediate purity FVIII concentrate to treat patients with von Willebrand's disease. Thromb Haemost. 2002;88:387–388. [PubMed] [Google Scholar]
- Shi Q, Wilcox DA, Fahs SA, Weiler H, Wells CW, Cooley BC.et al. (2006Factor VIII ectopically targeted to platelets is therapeutic in hemophilia A with high-titer inhibitory antibodies J Clin Invest 1161974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Wilcox DA, Fahs SA, Fang J, Johnson BD, DU LM.et al. (2007Lentivirus-mediated platelet-derived factor VIII gene therapy in murine haemophilia A J Thromb Haemost 5352–361. [DOI] [PubMed] [Google Scholar]
- Shi Q, Wilcox DA, Morateck PA, Fahs SA, Kenny D., and, Montgomery RR. Targeting platelet GPIbα transgene expression to human megakaryocytes and forming a complete complex with endogenous GPIbβ and GPIX. J Thromb Haemost. 2004;2:1989–1997. doi: 10.1111/j.1538-7836.2004.00961.x. [DOI] [PubMed] [Google Scholar]
- Ware J, Russell S., and, Ruggeri ZM. Generation and rescue of a murine model of platelet dysfunction: the Bernard-Soulier syndrome. Proc Natl Acad Sci USA. 2000;97:2803–2808. doi: 10.1073/pnas.050582097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox DA, Olsen JC, Ishizawa L, Griffith M., and, White GC.2nd (1999Integrin αIIb promoter-targeted expression of gene products in megakaryocytes derived from retrovirus-transduced human hematopoietic cells Proc Natl Acad Sci USA 969654–9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Hodivala-Dilke K, Johnson BD, Du LM, Hynes RO, White GC.2ndet al. (2005Therapeutic expression of the platelet-specific integrin, αIIbβ3, in a murine model for Glanzmann thrombasthenia Blood 1062671–2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q, Wilcox DA, Fahs SA, Kroner PA., and, Montgomery RR. Expression of human factor VIII under control of the platelet-specific αIIb promoter in megakaryocytic cell line as well as storage together with VWF. Mol Genet Metab. 2003;79:25–33. doi: 10.1016/s1096-7192(03)00049-0. [DOI] [PubMed] [Google Scholar]
- Kanaji T, Russell S., and, Ware J. Amelioration of the macrothrombocytopenia associated with the murine Bernard-Soulier syndrome. Blood. 2002;100:2102–2107. doi: 10.1182/blood-2002-03-0997. [DOI] [PubMed] [Google Scholar]
- Shi Q., and, Montgomery RR. Platelets as delivery systems for disease treatments. Adv Drug Deliv Rev. 2010;62:1196–1203. doi: 10.1016/j.addr.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward CP, Rao AK., and, Cattaneo M. Congenital platelet disorders: overview of their mechanisms, diagnostic evaluation and treatment. Haemophilia. 2006;12 Suppl 3:128–136. doi: 10.1111/j.1365-2516.2006.01270.x. [DOI] [PubMed] [Google Scholar]
- Alamelu J., and, Liesner R. Modern management of severe platelet function disorders. Br J Haematol. 2010;149:813–823. doi: 10.1111/j.1365-2141.2010.08191.x. [DOI] [PubMed] [Google Scholar]
- Kanaji T, Okamura T, Kurolwa M, Noda M, Fujimura K, Kuramoto A.et al. (1997Molecular and genetic analysis of two patients with Bernard-Soulier syndrome–identification of new mutations in glycoprotein Ibα gene Thromb Haemost 771055–1061. [PubMed] [Google Scholar]
- Kenny D, Newman PJ, Morateck PA., and, Montgomery RR. A dinucleotide deletion results in defective membrane anchoring and circulating soluble glycoprotein Ibα in a novel form of Bernard-Soulier syndrome. Blood. 1997;90:2626–2633. [PubMed] [Google Scholar]
- Kunishima S, Miura H, Fukutani H, Yoshida H, Osumi K, Kobayashi S.et al. (1994Bernard-Soulier syndrome Kagoshima: Ser 444–>stop mutation of glycoprotein (GP) Ibα resulting in circulating truncated GPIbα and surface expression of GPIbβ and GPIX Blood 843356–3362. [PubMed] [Google Scholar]
- Afshar-Kharghan V., and, López JA. Bernard-Soulier syndrome caused by a dinucleotide deletion and reading frameshift in the region encoding the glycoprotein Ibα transmembrane domain. Blood. 1997;90:2634–2643. [PubMed] [Google Scholar]
- Kenny D, Jónsson OG, Morateck PA., and, Montgomery RR. Naturally occurring mutations in glycoprotein Ibα that result in defective ligand binding and synthesis of a truncated protein. Blood. 1998;92:175–183. [PubMed] [Google Scholar]
- Montini E, Cesana D, Schmidt M, Sanvito F, Ponzoni M, Bartholomae C.et al. (2006Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration Nat Biotechnol 24687–696. [DOI] [PubMed] [Google Scholar]
- De Palma M, Montini E, Santoni de Sio FR, Benedicenti F, Gentile A, Medico E.et al. (2005Promoter trapping reveals significant differences in integration site selection between MLV and HIV vectors in primary hematopoietic cells Blood 1052307–2315. [DOI] [PubMed] [Google Scholar]
- Manilla P, Rebello T, Afable C, Lu X, Slepushkin V, Humeau LM.et al. (2005Regulatory considerations for novel gene therapy products: a review of the process leading to the first clinical lentiviral vector Hum Gene Ther 1617–25. [DOI] [PubMed] [Google Scholar]
- Chang AH., and, Sadelain M. The genetic engineering of hematopoietic stem cells: the rise of lentiviral vectors, the conundrum of the ltr, and the promise of lineage-restricted vectors. Mol Ther. 2007;15:445–456. doi: 10.1038/sj.mt.6300060. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Murillo A, Lozano ML, Montini E, Bueren JA., and, Guenechea G. Unaltered repopulation properties of mouse hematopoietic stem cells transduced with lentiviral vectors. Blood. 2008;112:3138–3147. doi: 10.1182/blood-2008-03-142661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathore V, Stapleton MA, Hillery CA, Montgomery RR, Nichols TC, Merricks EP.et al. (2003PECAM-1 negatively regulates GPIb/V/IX signaling in murine platelets Blood 1023658–3664. [DOI] [PubMed] [Google Scholar]