Abstract
Using a mouse model we show that self-complementary (sc) adeno-associated virus (AAV) vectors pseudotyped with capsids of serotypes 2, 7 or 8 induce more potent transgene product-specific CD8+ T cell and antibody responses compared to corresponding single-stranded (ss)AAV vectors. These data suggest that the higher and more rapidly appearing amounts of transgene product achieved with scAAV vectors may increase detrimental immune responses in gene transfer recipients.
Introduction
Adeno-associated viruses (AAV) are members of the parvovirus family. They are dependoviruses and require a helper virus such as an adenovirus (Ad) for replication. Upon entry into the nucleus of their target cells, the AAV's single-stranded (ss)DNA genome is converted into a double-stranded (ds)DNA, which is then transcribed.1 Different AAV serotypes have been isolated from human and nonhuman primates2,3 and none of them have been associated with diseases.
AAV vectors, deleted of viral sequences encoding regulatory sequences and the capsid proteins, but still containing the long terminal repeats, have been developed for in vivo gene transfer and different serotypes have been used to target specific tissues, such as the serotype 1 for muscle,4,5,6 serotype 8 for liver,7 or serotypes 5 and 7 for brain.8 First generation AAV vectors carrying a ssDNA genome also termed ssAAV vectors have been tested preclinically and clinically for the treatment of numerous genetic diseases9,10,11,12 and clinical trials have shown some efficacy in patients with inherited blindness receiving ssAAV serotype 2 vectors.13
Studies have shown that conversion of the ssDNA into a dsDNA is rate-limiting for the onset and levels of transgene expression14 in ssAAV vectors. Second generation AAV vectors with a ds genome also called self-complementary AAV (scAAV) vectors have been developed. ScAAV vectors produce higher levels of transgene products compared to ssAAV vectors.15,16 In addition, onset of transgene product expression is accelerated. Early clinical trials using scAAV8 vectors expressing human factor IX have achieved efficacy in the partial correction of hemophilia B.17
The accelerated expression kinetics and the higher level of transgene product expression in target tissues could potentially induce more potent transgene product-specific immune responses, which could be detrimental for sustained correction of diseases. Here, we investigated T and B cell responses to a transgene product expressed by different serotypes of scAAV and ssAAV vectors. We purposely tested responses to a highly immunogenic viral antigen, i.e., a truncated and hence secreted form of gag of the human immunodeficiency virus (HIV)-1, to create a worst-case scenario for gene transfer using a completely foreign protein, as would be encountered during correction of a defect caused by gene deletion or an early stop codon rather than function-ablating point mutations. The former is known to pose greater risk for adverse immune responses as has been demonstrated for the association between genotype of the protein VIII or IX mutations and the likelihood of inhibitor formation upon clotting factor replacement therapy.18 In addition, a foreign antigen was chosen to drive sufficiently high immune responses to allow for an in-depth analysis of the functionality of the transgene product-specific CD8+ T cell responses. Our results show that scAAV vectors of serotypes 7 and 8 induce higher CD8+ T and B cell responses than ssAAV vectors of the same serotypes. ScAAV2 vectors are only poorly immunogenic but still elicit stronger gag-specific CD8+ T cell responses than ssAAV2 vectors. In addition, CD8+ T cell responses to the transgene product of scAAV7 or 8 vectors are functionally superior to those induced by ssAAV7 or 8 vectors. AAV7 and AAV8 vectors also stimulate transgene product-specific antibody responses dominated upon intramuscular (i.m.) injection of high-vector doses by antibodies of the IgG2a and IgG1 isotypes, while ssAAV7 or 8 vectors induce lower antibody titers.
Results
Magnitude and kinetics of transgene product-specific CD8+ T cell responses to sc and ssAAV vectors
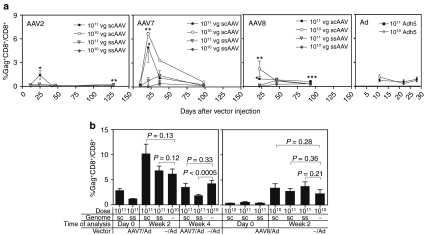
To assess AAV-induced CD8+ T cell responses, BALB/c mice were injected i.m. with 1010 or 1011 genome copies (gc) of sc and ssAAV vectors of serotypes 2, 7 or 8 expressing truncated gag (p37) of HIV-1. For comparison additional mice were injected with an Ad vector of human serotype 5 expressing the same transgene product at 1010 or 1011 virus particles (vp). Frequencies of gag-specific CD8+ T cells were measured from blood at various times after the injection by staining with a specific tetramer (Figure 1a). Peak responses to gag expressed by scAAV2, 7, and 8 vectors were observed ~3 weeks after immunization while peak responses to ssAAV7 and AAV8 vectors were delayed. At both doses of vectors regardless of serotype, early responses were significantly higher in mice injected with scAAV vectors. At later time points after contraction of responses the differences in ssAAV versus scAAV-induced frequencies of gag-specific CD8+ T cells remained significant for AAV8 and 2 vectors. The overall magnitude of responses was influenced by the serotype of the capsid. AAV2 vectors were poorly immunogenic and frequencies of specific CD8+ T cells exceeding 1% of all CD8+ T cells in blood could only be observed transiently at the highest dose of the scAAV2 vector. AAV7 vectors induced the most potent CD8+ T cell responses; there was a clear shift in kinetics with ssAAV7 vectors inducing delayed responses and again responses contracted down to very low levels when tested 14 weeks after immunization. Peak responses to the immunodominant epitope of gag as expressed by AAV8 vectors were higher than those induced by AAV2 vectors. There was also a marked delay in peak responses to ssAAV8 vectors. Injections of scAAV7 and to a lesser degree scAAV8 vectors caused a paradox dose–response curve and responses were higher at the lower vector dose of 1010 vg than at 1011 vg. The underlying causes for the lower response at the highest vector dose were not investigated further and could be a reflection of several mechanisms such as increased innate immunity which through production of interferons may dampen the activity of the cytomegalovirus promoter controlling transgene expression in the AAV vectors. Also, transduction capacity at the inoculation site may have been saturated already at the 1010 vg dose. Comparing AAV-induced peak responses to those stimulated by Ad vectors expressing the same transgene showed that responses induced by the scAAV7 vector were markedly higher than those induced by the Ad vector.
Figure 1.
AAV vector-induced gag-specific CD8+ T cell responses. Groups of 5–10 BALB/c mice were injected intramuscularly with the indicated doses of AAV or Ad vectors expressing gag. (a) Frequencies of gag-specific CD8+ T cells were assessed from blood at different times after injection of vector by staining with an antibody to CD8 and the gag-tetramer. Graphs show average frequencies of tetramer+ CD8+ cells over all CD8+ cells ± SE. Student's t-tests were used to determine differences between T cell responses to ss and scAAV vectors at each individual time point. *,** indicate significant differences (*P < 0.05, **P < 0.01). (b) Frequencies of gag-specific CD8+ T cells measured as described in a before (d0) and at different times (week 2 and 4) after a booster immunization with an Ad vector expressing gag. Differences between responses in AAV-primed versus unprimed mice following Ad boost were not significant (P > 0.05) but for the comparison between ssAAV7 prime + Ad boost and Ad boost only at week 4 (P < 0.01). AAV, adeno-associated virus; Ad, adenovirus; scAAV, self-complementary AAV; ssAAV; single-stranded AAV; vg, vector genome.
To assess if AAV vector-induced CD8+ T cells could expand after re-exposure to their cognate antigen, mice injected with ss or scAAV7 or 8 vectors were boosted 1 or 2 months later respectively by injection of a single dose of an Ad vector at 1010 vp expressing the same transgene product. Control mice only received the Ad vector. Frequencies of gag-specific CD8+ T cells were tested from blood immediately before immunization and then for the AAV7-injected group at 2 and 4 weeks after the boost and for AAV8 vector-primed mice at 2 weeks after the boost. Gag-specific CD8+ T cell responses increased in AAV vector-primed mice after the booster injection. Nevertheless, after the Ad immunization frequencies of gag-specific CD8+ T cells were not significantly higher in AAV-primed mice compared to control mice, which had only received the Ad vector (Figure 1b), suggesting that the proliferative capacity of AAV-induced gag-specific CD8+ T cells is suboptimal.
Functions of AAV vector-induced gag-specific CD8+ T cells
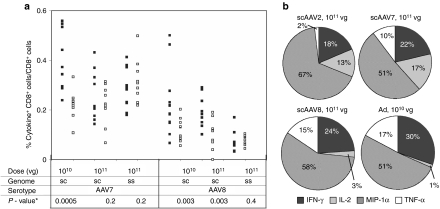
To test if responses were functional, CD8+ T cells from blood were assessed for production of cytokines, i.e., interferon-γ (IFN-γ), interleukin-2 (IL-2), tumor necrosis factor-α (TNF-α), and macrophage inflammatory protein-1α (MIP-1α) after in vitro stimulation with the peptide expressing the immunodominant epitope of gag. Frequencies of responding CD8+ T cells assessed at 3 weeks after vector application were markedly lower than those observed upon tetramer staining. Frequencies were highest for gag-specific CD8+ T cells induced by the 1010 vg dose of scAAV vectors (Figure 2a). As shown for the 1011 vg doses, most CD8+ T cells produced MIP-1α followed by IFN-γ (Figure 2b). SsAAV vectors failed to induce cytokine-producing CD8+ T cells with the exception of AAV2 vectors, which induced low levels of IL-2 producing CD8+ T cells. Most T cells exhibited only one function (data not shown).
Figure 2.
Cytokine/chemokine production by AAV vector-induced gag-specific CD8+ T cells. Groups of 10 BALB/c mice were immunized with the indicated vectors. Three weeks later PBMCs were tested by ICS for cytokine or chemokine production in response to the gag peptide AMQMLKETI. Cells in control wells were stimulated with an unrelated peptide. Frequencies of cytokine-producing CD8+ T cells were low and plots were therefore concatenated to allow for more accurate gating. (a) Shows frequencies of CD8+ T cells with individual mice producing any of the four cytokines/chemokines and combinations thereof tested. Filled squares indicate responses to gag, unfilled squares for control wells. (b) Shows the percentages of cytokine/chemokine positive cells producing either of the four cytokines/chemokines. AAV, adeno-associated virus; ICS, intracellular cytokine staining; IFN, interferon; IL, interleukin; MIP, macrophage inflammatory protein; PBMC, peripheral blood mononuclear cell; TNF, tumor necrosis factor; vg, vector genome.
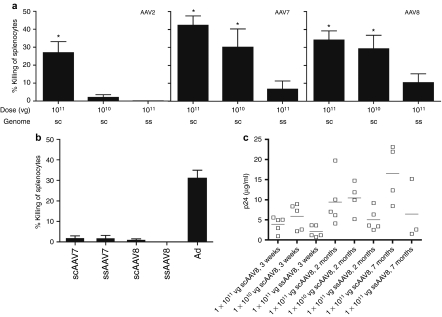
The very low frequencies of cytokine-producing gag-specific CD8+ T cells, which contrasted the by far more robust frequencies obtained by tetramer staining, suggest that AAV-induced gag-specific CD8+ T cells might be functionally impaired as we had reported previously.19 To further investigate the functionality of AAV vector-induced transgene product-specific CD8+ T cells, we used an assay, which measures whether T cells can specifically lyse antigen-expressing target cells in vivo, the function that is presumably most detrimental to sustained AAV-mediated gene transfer. Specific lysis tested at 3 weeks after injection of mice was observed with all three serotypes of scAAV vectors. At the 1011 vg dose of vector percent lysis was comparable for the three serotypes, while at the 1010 vg dose specific lysis was only observed in mice injected with scAAV7 or 8 vectors (Figure 3a). Mice injected with ssAAV vectors showed markedly lower lysis, and those injected with ssAAV2 vectors failed to kill their specific targets. Mice treated with the sc or ssAAV7 or AAV8 vectors at 1011 were retested at 5 months after vector injection. At this time specific lysis of gag peptide-pulsed target cells was not above background in any of the AAV vector-injected mice but remained readily detectable in Ad vector-treated mice again suggesting that over time AAV vector-induced CD8+ T cells show loss of function (Figure 3b). Functional impairment of CD8+ T cells was further suggested by sustained secretion of gag, which remained detectable, as was tested in mice that received scAAV8 vectors (Figure 3c).
Figure 3.
In vivo lysis by AAV vector-induced CD8+ T cells. (a) Groups of five mice were immunized with AAV vectors at the indicated doses. Three weeks later immunized as well as naive mice were injected with a mixture of splenocytes pulsed with gag peptide AMQMLKETI and labeled with a high concentration of CFSE or with a rabies peptide and labeled with a low concentration of CFSE. Splenocytes were isolated 24 hours later and analyzed for CFSE expression. Percentage-specific lysis was calculated as described in the Materials and Methods section. (b) T-cell-mediated lysis was tested at 5 months after immunizations. (c) HIV gag p24 in serum of immunized mice was measured by ELISA at the indicated time points. Graph shows results for individual mice (squares), the short lines show averages. AAV, adeno-associated virus; CFSE, carboxyfluorescein succinimidyl ester; ELISA, enzyme-linked immunosorbent assay; vg, vector genome.
Transgene product-specific CD8+ T cell phenotypes
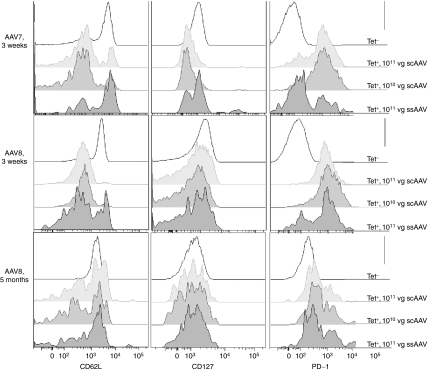
To assess differentiation of transgene product-specific AAV vector-induced CD8+ T cells, peripheral blood mononuclear cells were stained for CD62L (L-selectin) and CD127 (IL-7 receptor) expression. This analysis was restricted to cells from AAV7- and AAV8-injected mice. Those from AAV2-treated mice were not tested as their paucity of vector-induced CD8+ T cells precluded a reliable analysis. At ~3 weeks after i.m. injections, both doses of scAAV7 vectors induced predominantly gag-specific CD8+ T cells that expressed lower levels of CD62L and CD127 than naive CD8+ T cells indicating that most of the vector-induced cells were at the effector cell stage (Figure 4). In contrast, the ssAAV7 vector induced a mixture of CD62Llow/high and CD127low/high gag-specific CD8+ T cells suggesting either incomplete differentiation into effector T cells or differentiation of some of the cells into memory. This was further supported by PD-1 expression, which was lower on a subset of gag-specific CD8+ T cells in ssAAV7 vectors-injected mice than in scAAV7 vectors-induced CD8+ T cells. Phenotypes of gag-specific CD8+ T cells stimulated by AAV8 vectors were similar, although those induced by the scAAV vector showed a more pronounced transition into effector cells. Phenotypes of CD8+ T cells from AAV8-injected mice were retested 5 months after the injection. At this time ssAAV8 and scAAV vector-induced gag-specific CD8+ T cells showed comparable phenotypes with mixtures of effector (CD62Llow, CD127low) and memory cells (CD62Lhigh, CD127high). Of note, levels of PD-1 were largely higher on gag-specific CD8+ T cells than on naive CD8+ T cells at this time point in scAAV- and ssAAV- injected mice, suggesting their chronic activation and potential differentiation towards exhaustion.
Figure 4.
Phenotypes of AAV vector-induced CD8+ T cells. Groups of mice were injected with the indicated vectors. PBMC were harvested at different time points and stained with fluorochrome-conjugated antibodies to CD8, CD44, CD62L, CD127, and PD-1, and a tetramer for gag-specific CD8+ T cells. Cells were analyzed by flow cytometry. Events were gated onto CD8+CD44−tet− cells (naive) or onto CD8+CD44+tet+ (antigen-experienced cells from the different immunization groups). The histograms are based on concatenated samples from mice belonging to the same group. AAV, adeno-associated virus; PBMC, peripheral blood mononuclear cell; vg, vector genome.
Induction of transgene product-specific antibodies
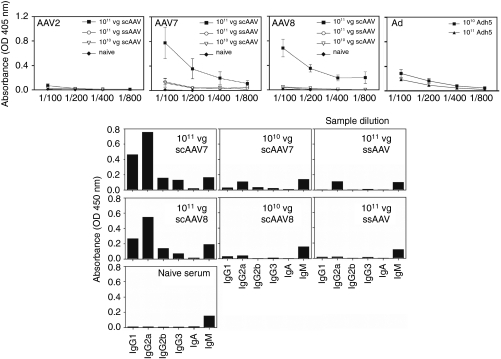
Sera of mice injected 6 weeks earlier with different ss or scAAV or for comparison Ad vectors were tested for transgene product-specific antibody responses (Figure 5). AAV2 vectors only induced very low antibody titers, which were slightly higher upon treatment with scAAV2 vectors. Gag-specific antibody responses to scAAV7 vectors were robust with a clear dosing effect. Antibody responses to ssAAV7 vectors were markedly lower than those to scAAV7 vectors at the equal dose. ScAAV8 vectors only induced high antibody titers at the dose of 1011 vg. The lower dose of 1010vg of scAAV8 vector and the 1011 dose of ssAAV8 vectors resulted in barely detectable antibody responses. Titers obtained with the highest dose of scAAV7 and AAV8 vector were above those induced by an Ad vector expressing the same transgene product (Figure 5a). Responses to the higher dose of scAAV7 and AAV8 vectors were dominated by antibodies of the IgG2a and IgG1 isotypes, suggesting a mixed Th1/Th2 response (Figure 5b).
Figure 5.
AAV vector-induced antibody response. (a) Groups of mice were injected with the indicated vectors. Four to six weeks later, sera of individual mice were collected and analyzed for antibodies to HIV gag p24 by an ELISA. The graphs show average values ± SE. (b) Isotypes of antibodies generated to HIV gag p24 in sera were determined by ELISA from pooled sera. AAV, adeno-associated virus; ELISA, enzyme-linked immunosorbent assay; OD, optical density; vg, vector genome.
Discussion
SsAAV vectors although they were found to be highly effective as gene delivery vehicles in preclinical trials, where they achieved long-term corrections of genetic diseases such as hemophilia15 or muscular dystrophy,20 failed in clinical trials,9 unless they were applied to immunoprivileged sites, such as the subretinal space for treatment of Leber's congenital amaurosis21 or the brain for treatment of Parkinson's disease.11 Transfer of ssAAVs to liver or muscle at the high doses needed to achieve therapeutic levels of the transgene product elicited CD8+ T cell responses to the vectors' capsid proteins,9,22 which presumably led to the destruction of the transduced cells. The gene encoding the capsid proteins is deleted in AAV vectors; epitopes derived from AAV capsids are thus only available for recognition by T cells in limited quantities and for a finite time till the capsid is degraded.23 Indeed T cell responses to capsid proteins that were elicited in a clinical trial for correction of lipoprotein lipase deficiency by i.m. delivery of an AAV1 vector became detectable with a dose-dependent kinetics.22 The dose-dependency of the anticapsid response suggests that lowering of AAV vector doses might circumvent the destructive capsid-specific CD8+ T cell responses, as could be attained with scAAV vectors, which achieve comparable loads of transgene product at significantly lower vector doses than ssAAV vectors.24 This was confirmed in a clinical trial with hepatic transfer of a scAAV8 vector expressing human factor IX for correction of hemophilia B, where, unlike in a previous trial based on a ssAAV2 vector expressing the same transgene product,9 patients developed sustained levels of human factor IX without evidence of liver cell damage,17 suggesting that scAAV vectors at least when applied at a low dose may indeed be able to dodge the immune system.
Nevertheless, three features of scAAV vectors may increase their ability to induce immune responses to their transgene product, which have thus far not been observed in clinical trials with ssAAV vectors. One of the main advantages of ssAAV vectors is that they only induce low and impaired transgene product-specific T cell responses.19 First, this may relate to their relative inability to elicit strong innate responses,25 which are needed to facilitate adaptive responses. The dsDNA genome of scAAV vectors is likely to be recognized by a different set of pathogen recognition receptors such as toll-like receptor 9 (TLR9) or other cytoplasmic DNA sensors, e.g., DAI (DNA-dependent activator of IFN-regulatory factors) and indeed recent studies suggest heightened activation of innate responses by scAAV as compared to ssAAV vectors.26,27 Second, the slow kinetics of onset of transgene expression by ssAAV vectors may also reduce immune responses as any inflammatory responses induced by vector components or injuries associated with their injections are temporarily dissociated from expression of potentially immunogenic transgene product; this is not the case with scAAV vectors, which transcribe their transgene more rapidly. Third, ssAAV vectors express, per vg and thus per transduced cell, lower amounts of transgene product than scAAV vectors. Tolerance to self proteins can readily be broken by its expression at unphysiologically high levels as this may allow for the association of low avidity T cell epitopes to major histocompatibility complex molecules and the expression of the resulting complexes on the cell surface, as has been demonstrated by vaccine-induced stimulation of T cell responses to ubiquitous self proteins such as p53.28
Results presented here show that scAAV vectors carrying the capsid proteins of three different serotypes drive markedly higher transgene product-specific CD8+ T and B cell responses compared to the corresponding ssAAV vectors, even if used at tenfold lower doses. At optimal doses they induced higher CD8+ T and B cell responses than Ad vectors of the human serotype 5 expressing the same transgene product. Not unexpectedly the capsid used for pseudotyping of the AAV vector influences the magnitude of the immune response, with vectors carrying an AAV2 capsid being poorly immunogenic while those pseudotyped with an AAV7 capsid are highly immunogenic presumably reflecting differences in the capsids' tropism for muscle cells. Although gag-specific CD8+ T cell responses can readily be detected by tetramer staining, which measures frequencies of antigen-specific T cells based on expression of the T cell receptor, frequencies were markedly lower when assessed functionally by intracellular cytokine staining, strongly suggesting that the scAAV vector-induced gag-specific CD8+ T cells are impaired, as was reported previously for T cell responses to transgene products of ssAAV vectors.19 Although scAAV vector-induced gag-specific CD8+ T cells are initially able to lyse target cells pulsed with a peptide carrying the immunodominant epitope of gag, this function is lost over time before all of the vector-transduced cells are eliminated. Furthermore, gag-specific CD8+ T cells expand poorly upon re-encounter of their cognate antigen and when tested several months after vector injection continue to express activation markers suggesting exhaustion due to prolonged exposure to antigen.29 Although T cell responses to AAV vector capsid components or vector expressed transgene products can be detrimental for sustained gene transfer, the low quality of AAV vector-induced gag-specific CD8+ T cells, which in our mouse model failed to abrogate expression of the viral antigen, suggest that they may be of minor concern in a clinical setting.
More worrisome is the ability of scAAV vectors derived from serotypes with high tropism to muscle to elicit potent antibody responses. ScAAV vectors at the higher dose induce both gag-specific IgG2a and IgG1 indicative of germinal center formation and T helper-dependant immunoglobulin isotype switching. For gene transfer-mediated correction of diseases that are caused by a lack of secreted proteins, such as hemophilia A or B, neutralizing antibody responses against the therapeutic protein can be even more detrimental than CD8+ T cell-mediated destruction of transduced cells as they not only negate any beneficial effects of gene transfer but, in addition, rob the patients of traditional treatment options. Thus, the effect of dosing with scAAV vectors on B cell responses to the therapeutic transgene product needs to be examined carefully in clinical trials.
Materials and Methods
Viral vectors. Recombinant AAV vectors were produced as previously described.9,22 Briefly, DNA plasmids containing the scAAV or ssAAV HIV-1 gag transgene cassette were transfected into HEK 293 cells along with helper DNA plasmids and AAV capsid DNA plasmids. Three days after transfection, virions were isolated, purified, and titrated as described.9,22 A truncated codon-optimized HIV-1 gag encoding a secreted 37 kD protein was used as the transgene. Ad vectors were produced, purified, titrated, and quality controlled as described.30
Mice. Female BALB/c mice were purchased from the National Cancer Institute (Bethesda, MA). They were kept at the Animal Facility of the Wistar Institute and treated according to approved protocols. Mice were injected i.m. into the hind legs with various doses of AAV vectors. The vectors were diluted with phosphate-buffered saline (PBS) to 100 µl, and 50 µl were applied to each upper leg muscle.
Analysis of T cell frequencies and phenotypes. Peripheral blood mononuclear cells were isolated from mice injected with vectors. Cells were stained with fluorochrome-labeled antibodies against CD62L and CD127 (BD Biosciences, San Jose, CA) and a HIV gag-specific CD8 tetramer (NIAID Tetramer Facility, Atlanta, GA) recognizing the AMQMLKETI sequence of gag in BALB/c mice.30 The stained cells were fixed and analyzed in a BD LSRII flow cytometer (BD Biosciences). Post-acquisition analysis was conducted with FlowJo 7.6 software (TreeStar, Ashland, OR). Histograms showing the phenotypes of T cells are based on concatenated data for which FlowJo was used to combine the events from individual samples of the same groups.
Intracellular cytokine staining. Peripheral blood mononuclear cells from mice injected with vectors were isolated. Cells were incubated with the AMQMLKETI peptide or an irrelevant peptide from rabies virus in Dulbecco's Modified Eagle's medium supplemented with 10% fetal bovine serum (FBS) for 6 hours at 37 °C at 10% CO2. Cells were stained with fluorochrome-conjugated antibodies against CD8 (BD Biosciences) and permeablized with Cytofix/Cytoperm (BD Biosciences). Intracellular cytokines were detected upon staining with fluorochrome-labeled antibodies, fixed and analyzed in a BD LSRII flow cytometer. Post-acquisition analysis was conducted with FlowJo 7.6 software. Boolean gates were established for all of the possible combinations of factors. Graphs show a sum of all possible functions.
In vivo killing assay. Splenocytes from naive BALB/c mice were isolated and pulsed with HIV gag AMQMLKETI peptide or an irrelevant rabies virus peptide at 5 µg/ml for 2 hours at 37 °C in a 10% CO2 incubator. Cells were then labeled with carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen, Carlsbad, CA) at different concentrations for the two peptide-labeled splenocyte populations. Both peptide-labeled cell populations were mixed at a 1:1 ratio and transferred via tail vein into BALB/c mice previously injected with sc or ssAAV vectors. After 20 hours, splenocytes from injected mice were harvested and analyzed by flow cytometry for expression of CFSE. Loss of CFSE+ cells labeled with the AMQMLKETI was used as a measure for specific in vivo lysis. Lysis was calculated using the formula:
Enzyme-linked immunoabsorbant assay. To test for antibodies to gag, Nunc 96 well plates (Thermo Fisher Scientific, Rochester, NY) were coated with HIV gag p24 protein (ImmunoDiagnostics, Woburn, MA) in 0.1 mol/l carbonate coating buffer (pH 9.6) at 4 °C for 12–18 hours. The plates were washed with PBS and then blocked with 3% FBS/PBS at 4 °C for 12–18 hours. After washing, sera from mice injected with AAV vectors were serially diluted and incubated on the plates for 1 hour at 37 °C. The plates were washed with PBS and incubated with alkaline phosphatase-conjugated antibodies (ICN CAPPEL goat alkaline phosphatase for mouse immunoglobulin; MP Biomedicals, Solon, OH) for 1 hour. After washing with PBS, the substrate solution for alkaline phosphatase was added and the color change was read under 405 nm with a spectrometer. Isotyping for the antibodies was performed with the Calbiochem Hybridoma Subisotyping Kit (Calbiochem, Darmstadt, Germany).
To test for HIV gag protein, mouse serum was treated with acid (0.2 mol/l Glycine/HCl buffer, pH 2.5) for 3 minutes at room temperature and neutralized with base (1 mol/l Tris/HCl buffer, pH 9.0) and PBS to an end dilution of 1:100 similar to a previous described method31 to dissociate antibodies from gag. Nunc 96 well plates were incubated with acid/base-treated mouse serum for 2 hours at 37 °C. The plate was washed with PBS and blocked with 3% FBS/PBS at room temperature for 1 hour, and blocked with 10% FBS/PBS for an additional 1 hour. The plates were incubated with biotin-labeled goat polyclonal antibody to HIV p24 (Abcam, Cambridge, UK) diluted 1 to 1,000 in 10% FBS/PBS for 1 hour at room temperature. The plates were washed and incubated with streptavidin-peroxidase polymer (Sigma, St Louis, MO) diluted 1 to 1,000 in 10% FBS/PBS for 1 hour at room temperature. After PBS washing, the plates were incubated with TMB substrate (Calbiochem, Darmstadt, Germany). The reactions were stopped with 1N HCl and the color change was read at 450 nm.
Acknowledgments
This work was supported by a grant from NHLBL (P01 HL078810). We wish to thank Ms Cole for assistance in preparing the figures and the manuscript. The authors declared no conflict of interest.
REFERENCES
- Gonçalves MA. Adeno-associated virus: from defective virus to effective vector. Virol J. 2005;2:43. doi: 10.1186/1743-422X-2-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coura Rdos S., and, Nardi NB. The state of the art of adeno-associated virus-based vectors in gene therapy. Virol J. 2007;4:99. doi: 10.1186/1743-422X-4-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao G, Vandenberghe LH., and, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5:285–297. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT.et al. (2009Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy Proc Natl Acad Sci USA 10616363–16368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauck B., and, Xiao W. Characterization of tissue tropism determinants of adeno-associated virus type 1. J Virol. 2003;77:2768–2774. doi: 10.1128/JVI.77.4.2768-2774.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X.et al. (2002Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity J Virol 76791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Zhang H, Franco LM, Young SP, Schneider A, Bird A.et al. (2005Efficacy of an adeno-associated virus 8-pseudotyped vector in glycogen storage disease type II Mol Ther 1157–65. [DOI] [PubMed] [Google Scholar]
- Taymans JM, Vandenberghe LH, Haute CV, Thiry I, Deroose CM, Mortelmans L.et al. (2007Comparative analysis of adeno-associated viral vector serotypes 1, 2, 5, 7, and 8 in mouse brain Hum Gene Ther 18195–206. [DOI] [PubMed] [Google Scholar]
- Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ.et al. (2006Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response Nat Med 12342–347. [DOI] [PubMed] [Google Scholar]
- Mendell JR, Rodino-Klapac LR, Rosales XQ, Coley BD, Galloway G, Lewis S.et al. (2010Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D Ann Neurol 68629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu S, Fujimoto K, Kato S, Mizukami H, Asari S, Ikeguchi K.et al. (2010A phase I study of aromatic L-amino acid decarboxylase gene therapy for Parkinson's disease Mol Ther 181731–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroes ES, Nierman MC, Meulenberg JJ, Franssen R, Twisk J, Henny CP.et al. (2008Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients Arterioscler Thromb Vasc Biol 282303–2304. [DOI] [PubMed] [Google Scholar]
- Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL.et al. (2010Gene therapy for Leber's congenital amaurosis is safe and effective through 1.5 years after vector administration Mol Ther 18643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty DM, Monahan PE., and, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, McIntosh J, Ng CY, Zhou J, Spence Y.et al. (2007Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates Blood 1091414–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Sun J, Zhang T, Yin C, Yin F, Van Dyke T.et al. (2008Optimization of self-complementary AAV vectors for liver-directed expression results in sustained correction of hemophilia B at low vector dose Mol Ther 16280–289. [DOI] [PubMed] [Google Scholar]
- Nathwani AC.2010. Haemophilia B gene therapy study in the UK. Hemophilia 2010 World Congress: LB01.
- Coppola A, Santoro C, Tagliaferri A, Franchini M., and, Di Minno G. Understanding inhibitor development in haemophilia A: towards clinical prediction and prevention strategies. Haemophilia. 2010;16 suppl. 1:13–19. doi: 10.1111/j.1365-2516.2009.02175.x. [DOI] [PubMed] [Google Scholar]
- Lin SW, Hensley SE, Tatsis N, Lasaro MO., and, Ertl HC. Recombinant adeno-associated virus vectors induce functionally impaired transgene product-specific CD8+ T cells in mice. J Clin Invest. 2007;117:3958–3970. doi: 10.1172/JCI33138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Li J., and, Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc Natl Acad Sci USA. 2000;97:13714–13719. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F.et al. (2009Age-dependent effects of RPE65 gene therapy for Leber's congenital amaurosis: a phase 1 dose-escalation trial Lancet 3741597–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingozzi F, Meulenberg JJ, Hui DJ, Basner-Tschakarjan E, Hasbrouck NC, Edmonson SA.et al. (2009AAV-1-mediated gene transfer to skeletal muscle in humans results in dose-dependent activation of capsid-specific T cells Blood 1142077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Tuyishime S, Wu TL, Giles-Davis W, Zhou D, Xiao W.et al. (2011Adeno-associated virus vectors serotype 2 induce prolonged proliferation of capsid-specific CD8+ T cells in mice Mol Ther 19536–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathwani AC, Gray JT, Ng CY, Zhou J, Spence Y, Waddington SN.et al. (2006Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver Blood 1072653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS., and, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol. 2002;76:4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog RW.2010. Abstract self-complementary AAV vectors cause a substantially heightened TLR9-dependent innate immune response in the liver. ASH mtg. Orlando, FL [Google Scholar]
- Martino AT, Suzuki M, Markusic DM, Zolotukhin I, Ryals RC, Moghimi B.et al. (2011The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver Blood 1176459–6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaszczyk-Thurin M, Ertl IO., and, Ertl HC. An experimental vaccine expressing wild-type p53 induces protective immunity against glioblastoma cells with high levels of endogenous p53. Scand J Immunol. 2002;56:361–375. doi: 10.1046/j.1365-3083.2002.01119.x. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Blattman JN, Murali-Krishna K, van der Most R., and, Ahmed R. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J Virol. 2003;77:4911–4927. doi: 10.1128/JVI.77.8.4911-4927.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsis N, Lin SW, Harris-McCoy K, Garber DA, Feinberg MB., and, Ertl HC. Multiple immunizations with adenovirus and MVA vectors improve CD8+ T cell functionality and mucosal homing. Virology. 2007;367:156–167. doi: 10.1016/j.virol.2007.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishanian P, Huskins KR, Stehn S, Detels R., and, Fahey JL. A simple method for improved assay demonstrates that HIV p24 antigen is present as immune complexes in most sera from HIV-infected individuals. J Infect Dis. 1990;162:21–28. doi: 10.1093/infdis/162.1.21. [DOI] [PubMed] [Google Scholar]