Abstract
Current gene therapy approaches for Parkinson's disease (PD) deliver neurotrophic factors like glial cell line-derived neurotrophic factor (GDNF) or neurturin via neuronal transgene expression. Since these potent signaling-inducing neurotrophic factors can be distributed through long-distance neuronal projections to unaffected brain sites, this mode of delivery may eventually cause side effects. To explore a localized and thus potentially safer alternative for gene therapy of PD, we expressed GDNF exclusively in astrocytes and evaluated the efficacy of this approach in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and rat 6-hydroxy-dopamine (6-OHDA) models of PD. In terms of protection of dopaminergic cell bodies and projections, dopamine (DA) synthesis and behaviour, astrocyte-derived GDNF demonstrated the same efficacy as neuron-derived GDNF. In terms of safety, unilateral striatal GDNF expression in astrocytes did not result in delivery of bio-active GDNF to the contralateral hemispheres (potential off-target sites) as happened when GDNF was expressed in neurons. Thus, astrocytic GDNF expression represents a localized but efficient alternative to current gene therapeutic strategies for the treatment of PD, especially if viral vectors with enhanced tissue penetration are considered. Astrocytic neurotrophic factor expression may open new venues for neurotrophic factor-based gene therapy targeting severe diseases of the brain.
Introduction
Parkinson's disease (PD) is the most common neurodegenerative movement disorder, affecting more than 2% of all individuals over 60 years of age.1 Depletion of the neurotransmitter dopamine (DA) through degeneration of the nigro-striatal projection represents the major pathological hallmark of the disease. Preclinical rodent and primate models demonstrated a strong protective and partially regenerative effect on the nigro-striatal dopaminergic projection by the glial cell line-derived neurotrophic factor (GDNF) family of ligands (GFLs).2,3,4 However, intrathecal infusion of GDNF protein or viral vector-mediated expression of neurturin in the striatum of late stage PD patients showed no significant clinical benefit over placebo controls.5,6 Deduced from what is known about the mode of action of GFLs,7,8 these failures may be attributed to several causes: first, GFLs may be generally not as effective in humans as in animal models, so that dosages tested successfully in 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine (MPTP) or 6-hydroxy-dopamine (6-OHDA) lesioned primates were not conferrable to humans; second, GFLs could not exert neuroprotective effects in late stage patients due to a lack of sufficient remaining dopaminergic innervation, which may not allow for any regeneration or may prevent appropriate transport of the GFL9; third, the penetration of delivered protein or viral vector may not have been effective in inducing a functional recovery in sufficiently large striatal areas; and/or fourth, placebo and side effects may have masked therapeutical benefits. Thus, successful gene therapy by GFLs may require either higher dosages of the neurotrophic factor to be expressed or that it be applied to younger patients when the disease is in a less advanced stage.8 Furthermore, more efficient vector systems may be needed to gain improved GDNF delivery. Given that gene therapy in its current stage is an irreversible process and that GDNF applications showed serious side effects in several studies,10,11,12 all these options require new safety evaluations.
Current gene therapeutic trials expressing neurotrophic factors in the brain7 predominantly use the adeno-associated virus serotype 2 (AAV-2) due to its proven safety record. In the animal and human central nervous system, AAV-2 predominately transduces neurons. However, the expression of neurotrophic factors in neurons may impose a serious safety issue, if indeed higher dosages and longer expression times are required to gain the desired therapeutic effect, since the factors can be secreted from the soma, unmyelinated projections, or synaptic sites of transduced neurons, thereby delivering a complex signaling-inducing molecule to potential off-target sites.
An alternative strategy with enhanced safety profile would be to express GFLs in striatal astrocytes rather than in neurons. Astrocytes are the major source of GDNF upon brain injury13,14 and may be responsible for maintenance of GDNF levels in the substantia nigra of PD patients' brains.15 Astrocytic protrusions rarely extend more than 20 µm from the cell body. Thus, unlike neurons which might project axons over distances of several centimeters, their radius of action is limited, preventing off-target secretion of neurotrophins.
Here, we demonstrate that GDNF expression in astrocytes is equally neuroprotective as neuronal GDNF in preventing MPTP-induced lesions of the nigro-striatal projection. Importantly, although neuron-expressed GDNF demonstrated robust influences on neurotransmitter synthesis in the contralateral hemisphere, exemplifying the noncontrollable distribution of bio-active GDNF to remote sites, astrocyte-derived GDNF exerted biological effects only in the viral vector-injected hemisphere, resulting in efficient GDNF delivery only to the target structures. In a clinically relevant paradigm, we applied AAV-5-GFAP-GDNF after a 6-OHDA lesion of the nigro-striatal projection and achieved a robust restoration of motor behavior. These studies suggest that astrocytic neurotrophic factor expression achieved by a viral vector with enhanced tissue spread can be considered an efficient alternative to current gene therapy strategies.
Results
AAV-5 vector for high efficacy and mutually exclusive expression in neurons or astrocytes
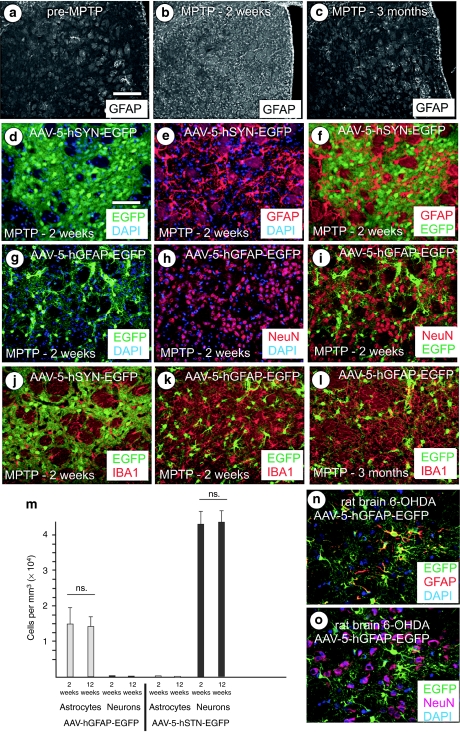
An AAV-5 serotype vector was engineered for efficient and mutually exclusive neuronal or astrocytic transgene expression through the use of appropriate transcriptional control elements. As inflammatory processes are common implications in neurodegenerative diseases, vector tropism was assessed not only in healthy rodent brains but under conditions of pronounced gliosis in the MPTP model of PD. As shown in Figure 1a–c, strong striatal astroglial activation is evident at 2 weeks after toxin application, which has disappeared 3 months after lesion. Despite this gliosis, expression of the reporter enhanced green fluorescent protein (EGFP) was absolutely restricted to neurons if expressed from the AAV-5-hSYN-EGFP vector, which drives EGFP expression by the 0.4 kbp human synapsin 1 gene (hSYN) promoter (Figure 1d–f,j, Supplementary Figure S1k). In contrast, EGFP expression was absolutely restricted to astrocytes if expressed from the AAV-5-hGFAP-EGFP vector, which drives EGFP expression from the 2.2 kbp human glial fibrillary acidic protein gene (hGFAP) promoter (Figure 1g–i,k,l, Supplementary Figure S1l). Stereological quantification of transduced cells revealed that indeed no neurons or microglial cells expressed EGFP after transduction with the AAV-5-hGFAP-EGFP vector and that numbers of EGFP-expressing astrocytes were stable over the course of the experiment, indicating an absence of significant astroglial proliferation and the subsequent loss of episomal vector genomes. These studies were performed after injection of high-titre vectors (6 × 109 vector genomes (vg)), and confirmed for the AAV-5-hGFAP-EGFP vector in the 6-OHDA lesioned rat brain (Figure 1n,o). Thus, an AAV-5 vector expressing transgenes from the hSYN or the hGFAP promoter can be considered as an absolutely specific expression system for the two brain cell types. As shown in Supplementary Figures S1 and S2, injection of either AAV-5-hSYN or AAV-5-hGFAP vectors resulted in vector distribution and EGFP or GDNF protein delivery throughout almost the entire injected striatum at both high (6 × 109 vg) and low (6 × 108 vg) titre. EGFP fluorescence and GDNF immunoreactivity were area-matched, indicating no gross diffusion of GDNF through the brain parenchyma away from the vector-transduced area. Of note, vector diffusion extended to the globus pallidus, and after striatal AAV-5-hGFAP-EGFP injection astrocytes within the globus pallidus were transduced with higher efficacy than were striatal astrocytes, indicated by higher levels of EGFP fluorescence in the latter cells (Supplementary Figure S1m–p).
Figure 1.
Astroglial and neuronal targeting of AAV-5 vectors in the lesioned rodent parkinsonian striatum. (a) MPTP-induced striatal gliosis in mouse brain as demonstrated by GFAP immunohistochemistry (GFAP-IHC) is shown before lesion, (b) 2 weeks after lesion, and (c) 3 months after lesion. (d) EGFP expression by the AAV-5-hSYN-EGFP vector, (e) GFAP-IHC of the same section, and (f) absence of any astrocytic EGFP expression by the EGFP/GFAP overlay. (g) EGFP expression by the AAV-5-hGFAP-EGFP vector, (h) NeuN-IHC of the same section, and (i) absence of any neuronal transgene expression by EGFP/NeuN overlay. (j) Absence of transgene expression in microglia by EGFP/IBA1-IHC overlay is shown for the AAV-5-hSYN-EGFP vector, (k) for the AAV-5-hGFAP-EGFP vector for 2 weeks after MPTP and in (l) for 3 months after MPTP. (m) Stereological quantification of transgene-expressing astrocytes and neurons after AAV-5-hSYN-EGFP or AAV-5-hGFAP-EGFP transduction, gray bars showing astrocyte numbers × 104/mm3 striatal tissue, black bars showing neuron numbers × 104/mm3 striatal tissue. (n) Specific targeting of AAV-5-hGFAP-EGFP to astrocytes in the 6-OHDA lesioned rat brain as overlay of EGFP fluorescence with GFAP-IHC and (o) absence of neuronal transgene expression from this vector as overlay of EGFP fluorescence with NeuN-IHC. Representative pictures from n = 3 mice per group. Bar = 400 µm in a–c; 100 µm in d–o. ns, not significant. 6-OHDA, 6-hydroxy-dopamine; DAPI, 4′,6-diamidino-2-phenylindole; EGFP, enhanced green fluorescent protein; hGFAP, human glial fibrillary acidic protein; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
Differential delivery of GDNF from striatum to ipsilateral nigra after expression in striatal neurons or striatal astrocytes
In order to compare both efficacy and off-target delivery of neuronal versus astrocytic GDNF expression, we used the MPTP lesion of the nigro-striatal system in mice. Systemic application of MPTP induces bilateral loss of about 50% of nigral dopaminergic neurons and their striatal terminals, which can be prevented by neurotrophic factors like GDNF. Injection of the AAV-5-hSYN-GDNF or AAV-5-hGFAP-GDNF vector was performed unilaterally into the left striatum, 2 weeks before MPTP application. This experimental setup allowed for a functional assessment of the distribution of bio-active GDNF: a delivery restricted to the target structures (i.e., ipsilateral striatum and ipsilateral nigra) would result in protection of nigral dopaminergic neurons, striatal fibres, and striatal DA levels only in this hemisphere, while leakage of GDNF to remote sites through neuronal projections would be traceable by the assessment of the contralateral dopaminergic projection.
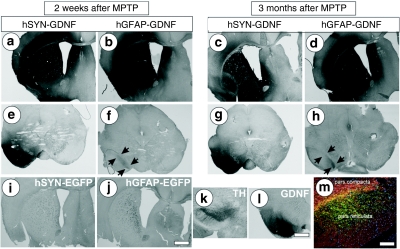
Both neuronal and astrocytic GDNF expression resulted in strong striatal staining for GDNF (Figure 2a–d) lasting over the time course of the experiment (3 months after MPTP application). Striatal astrocyte-expressed GDNF was found in the nigra strictly confined to the pars compacta which harbors the dopaminergic neurons projecting towards the striatum (Figure 2f,h, marked by arrows), consistent with a mechanism of retrograde delivery of striatal GDNF to nigral DA neurons.16
Figure 2.
Differences in delivery of GDNF to the nigra after neuronal versus astrocytic striatal expression. (a,c,e,g,l) GDNF immunoreactivity after unilateral striatal vector applications of high-titres of AAV-5-hSYN-GDNF or (b,d,f,h) AAV-5-hGFAP-GDNF, detected at 2 weeks in a,b,e,f,l and 3 months after MPTP treatment in c,d,g,h. (i,j) Absence of GDNF immunoreactivity after transduction with EGFP encoding vectors. Representative pictures from n = 4 mice per group. (m) Projections of striatal and/or pallidal neurons into the substantia nigra pars reticulata are demonstrated by fibrous EGFP fluorescence, (for more details see Supplementary Figure S3). (k) Nigral dopaminergic neurons are shown by TH immunoreactivity in (k), while GDNF immunoreactivity ventral to the pars compacta is shown in l. EGFP, enhanced green fluorescent protein; GDNF, glial cell line-derived neurotrophic factor; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
In pronounced contrast, striatal neuron-expressed GDNF was detected to a strong extent in areas outside the pars compacta, e.g., the pars reticulata (Figure 2e,g). A comparison of spatial localization of tyrosine hydroxylase and GDNF immunoreactivity (Figure 2k,l) confirmed the substantial delivery of GDNF to extra-nigral midbrain areas. This finding was in agreement with a substantial anterograde delivery of GDNF to the midbrain after neuronal striatal expression17,18 and substantiated by the finding of EGFP fluorescent fibres in the pars reticulata after striatal injection of AAV-5-hSYN-EGFP (Figure 2m). Nigral GDNF delivery was not due to retrogradely transported viral vector particles, indicated by the absence of EGFP-labeled nigral cell bodies (Supplementary Figure S3a–f).
Biologically active GDNF is delivered to the contralateral hemisphere after unilateral expression in striatal neurons
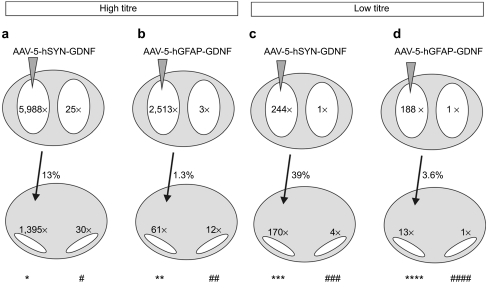
After either neuronal or astrocytic expression in the left striatum, we followed the delivery of GDNF to remote areas quantitatively by enzyme-linked immunosorbent assay as schematically shown in Figure 3 (for details see Supplementary Table S1). Biological effects of GDNF were determined by stereological quantification of surviving DA neurons in the substantia nigra (Figure 4 and Supplementary Figure S4), density of striatal DA fibres (Supplementary Figure S5), and levels of striatal DA (Figure 4) and its metabolites 3,4-dihydroxyphenylacetic acid (DOPAC) and homovanillic acid (HVA) (Supplementary Figure S6). These data were collected in both hemispheres at 2 weeks and 3 months after MPTP lesion, and after application of high-titre and low-titre vectors.
Figure 3.
Schematical representation of GDNF delivery to ipsilateral and contralateral structures after expression in neurons versus astrocytes of the left mouse striatum. Injection of (a,b) high titre vectors and (c,d) low titre vectors. GDNF levels as determined by ELISA at 2 weeks after MPTP (for absolute values see Supplementary Table S1) are given within the respective structure (striatum, top row; nigra, bottom row), as n-fold GDNF level as compared to AAV-EGFP transduced controls. The percentage of striatal GDNF detected in the ipsilateral nigra is given besides the connecting arrows. Conditions *, #, **, ***, and **** demonstrated protection of DA neurons, fibres, and striatal DA at 2 weeks and 3 months after MPTP; condition ### demonstrated restoration of striatal DA levels at 3 months after MPTP; conditions ## and #### did not show any neuroprotection. N = 4–5 mice for each condition. DA, dopamine; EGFP, enhanced green fluorescent protein; ELISA, enzyme-linked immunosorbent assay; GDNF, glial cell line-derived neurotrophic factor; MPTP, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.
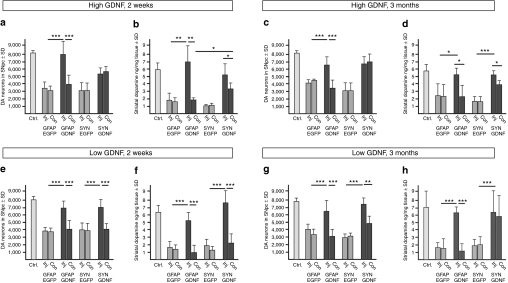
Figure 4.
Short and long-term follow-up of numbers of nigral DA neurons and levels of striatal dopamine after unilateral neuronal or astrocytic GDNF expression. (a,c,e,g) Stereological assessment of nigral DA neuron numbers and (b,d,f,h) HPLC-determined striatal dopamine levels after unilateral striatal vector application of high-titre in a–d and low-titre in e–h. AAV-5-hSYN-EGFP, AAV-5-hGFAP-EGFP, AAV-5-hSYN-GDNF, and AAV-5-hGFAP-GDNF vectors for the ipsilateral (“inj”) and contralateral (“con”) hemisphere at 2 weeks in a,b,e,f and 3 months after MPTP lesion in c,d,g,h. Values given as mean ± SD, n = 5–6 mice for each vector type, vector dosage, and time point. DA, dopamine; EGFP, enhanced green fluorescent protein; HPLC, high-performance liquid chromatography; GDNF, glial cell line-derived neurotrophic factor.
We detected significant contralateral GDNF delivery and biological effects after neuronal GDNF expression under all conditions. In the high-titre group, unilateral neuronal GDNF expression resulted in an almost complete prevention of loss of contralateral nigral DA neurons (Figure 4a,c). Levels of DA in the contralateral striatum were partially elevated as compared to controls (Figure 4b,d), while levels of DOPAC and HVA were almost entirely at control level (Supplementary Figure S6). In the low-titre group, effects of neuron-expressed GDNF were restricted to the ipsilateral hemisphere at 2 weeks after MPTP (Figure 4e,f). However, while 3 months after the lesion contralateral nigral DA neuron numbers showed only a tendency to re-express tyrosine hydroxylase, contralateral striatal DA levels were restored to pre-lesion levels, indicating a powerful long-term effect of GDNF, even at only fourfold levels over baseline (nigra) and almost control levels in striatum in the contralateral hemisphere (Figure 3c; Figure 4g,h).
After AAV-5-hSYN-GDNF injection into the ipsilateral striatum GDNF may be delivered to contralateral nigra by retrograde transport through the crossed nigro-striatal projection, which consists of about 5% of nigral neurons with axon collaterals projecting to the respective contralateral striatum.19 However, careful examination revealed that EGFP expression in striatal neurons resulted in clearly detectable fluorescent fibres in both contralateral striatum (Supplementary Figure S3j) and contralateral midbrain (Supplementary Figure S3g), suggesting that EGFP and GDNF can be delivered anterogradely to the contralateral hemisphere through long-distance projections.
GDNF acts only in the target structures after astrocytic striatal expression
In sharp contrast to neuronal expression, we did not detect any influence on contralateral nigral DA neuron numbers and contralateral striatal DA levels when GDNF was expressed unilaterally in striatal astrocytes by the AAV-5-hGFAP-GDNF vector (Figure 4). In the high-titre group this could theoretically be due to the fact that ipsilateral striatal levels of neuron-expressed GDNF were twofold higher than those obtained after expression of GDNF in astrocytes (Figure 3a,b; Supplementary Table S1). However, neuronal and astrocytic ipsilateral striatal GDNF levels were equal in the low-titre group (Figure 3c,d; Supplementary Table S1), suggesting that it is indeed the neuronal expression mode that was responsible for the remote distribution of GDNF. In both the high- and low-titre groups striatal astrocytes-derived GDNF was highly effective in completely preventing loss of ipsilateral DA neurons, fibres, and striatal DA (Figure 4, Supplementary Figures S4, S5 and S6).
Bio-activity of GDNF depends on route of delivery
In the high-titre group, GDNF expressed in neurons of the ipsilateral striatum was delivered to the contralateral substantia nigra in an amount sufficient to fully protect DA neurons from MPTP toxicity and to partially restore DA levels in the contralateral striatum (Supplementary Table S1, Figure 3a, Figure 4a–d; Supplementary Figures S4, S5 and S6). After astrocytic striatal expression, delivery of GDNF to the contralateral nigra was in the same order of magnitude (12 × control levels as compared to 30 × after neuronal expression; Figure 3b) but did not exert any protective effects in the contralateral hemisphere (Figure 4a–d; Supplementary Figures S4, S5 and S6). In the low-titre group, we found that a 13-fold increase of GDNF levels over controls in the ipsilateral nigra was sufficient to fully protect DA neurons and striatal DA levels after astrocytic expression (Figure 3d; Figure 4e–h). Furthermore, a mere fourfold increase of GDNF levels over controls in the contralateral nigra was sufficient to fully restore DA levels in the contralateral striatum after neuronal expression (Figure 3c; Figure 4e–h). Thus, after high-titre AAV-5-hGFAP-GDNF injection, GDNF levels in the contralateral nigra were in a range demonstrated to be sufficient for effective neuroprotection, but showed no biological effect. We conclude from these results that it is not only the amount of GDNF delivered to a remote area but also the route of delivery that determines its effects. As after astrocytic striatal expression, GDNF can reach the contralateral nigra only by retrograde transport through the crossed nigro-striatal projection,19 our results suggest that this retrogradely transported GDNF can not exert a protective or restorative function.
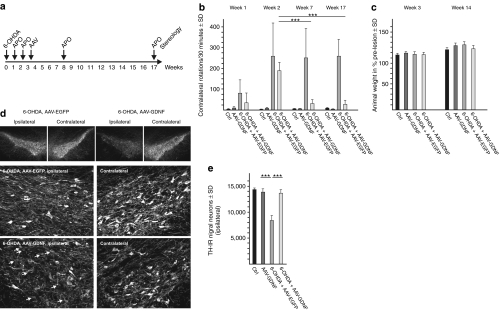
Astrocyte-expressed GDNF provides restoration of motor performance in a clinically relevant lesion paradigm
MPTP lesions in mice do not normally cause a level of DA depletion necessary to induce robust behavioral motor impairments in experimental animals.20 Furthermore, application of viral vectors before lesioning the nigro-striatal projection could only be used to test the neuroprotective effects of GDNF. This paradigm is sufficient to demonstrate that the action of astrocyte-expressed GDNF is restricted to the vector-injected hemisphere, but it is not sufficient to demonstrate that astrocyte-expressed GDNF could be useful in a clinically relevant setting, i.e., if expressed after lesion of the nigro-striatal projection as it would be the case in a gene therapeutic application. We thus used the unilateral infusion of 6-OHDA into the striatum of rats to induce robust apomorphine-induced rotation behavior. At 2 weeks after 6-OHDA injection the rats showed about 200 contraversive rotations per 30 minutes, while rats which received an injection of phosphate-buffered saline (PBS) or were injected with the AAV-5-hGFAP-GDNF virus only did not show any rotation behavior (Figure 5b). At 3 weeks after the confirmed 6-OHDA lesion, we injected either AAV-5-hGFAP-EGFP or AAV-5-hGFAP-GDNF into the lesioned striatum. The animals were then behaviorally assessed at 4 and 14 weeks after vector injections. While rats injected with the EGFP-expressing control virus demonstrated stable rotation behavior over time, rats injected with the vector-expressing GDNF within astrocytes dramatically improved in terms of rotation behavior, in that rotations were reduced to almost unlesioned control levels at 4 weeks after vector expression. This restorative effect proved to be stable in the long-term, as at 14 weeks after vector application GDNF-expressing animals also demonstrated an absence of apomorphine-induced rotations, while EGFP-expressing animals still rotated as at 2 weeks after lesion (Figure 5b). Weight gain of animals in all groups was comparable, indicating absence of weight loss as a prominent side effect of GDNF expression (Figure 5c).
Figure 5.
Astrocyte-derived GDNF functionally restores motor performance and protects from DA neuron degeneration in the partial 6-OHDA lesion paradigm of the rat brain. (a) The time scale of the experiment. 6-OHDA = unilateral striatal injection of 6-hydroxy dopamine; APO = apomorphine application to induce rotation behaviour; AAV = unilateral striatal injection of AAV vectors; time given in weeks. (b) Rotations counted within 30 minutes ± SD at 1, 2, 7, and 17 weeks after 6-OHDA injections. Ctrl = PBS injected rats; AAV-5-hGFAP-GDNF = vector-injected rats without 6-OHDA lesion; 6-OHDA + AAV-EGFP = rats injected with AAV-5-GFAP-EGFP control virus after 6-OHDA lesion; 6-OHDA + AAV-GDNF = rats injected with the AAV-5-GFAP-GDNF vector after 6-OHDA lesion. (c) Weight gain of animal groups is exemplarily shown for 3 and 14 weeks after 6-OHDA lesion. (d) Immunohistochemical staining for tyrosine hydroxylase (TH). Upper panel: low maginification view of the ipsilateral and contralateral nigra of animals injected with AAV-5-hGFAP-EGFP or AAV-5-hGFAP-GDNF after 6-OHDA lesion. Middle panel: high magnification of the nigra ipsilaterally (left) and contralaterally (right) after 6-OHDA and AAV-5-hGFAP-EGFP injection. Lower panel: high magnification of the nigra ipsilaterally (left) and contralaterally (right) after 6-OHDA and AAV-5-hGFAP-GDNF injection. Arrows point to DA neurons with reduced TH immunoreactivity. (e) Stereological quantification of dopaminergic neurons. 6-OHDA, 6-hydroxy-dopamine; AAV, adeno-associated virus; EGFP, enhanced green fluorescent protein; GDNF, glial cell line-derived neurotrophic factor; hGFAP, human glial fibrillary acidic protein; PBS, phosphate-buffered saline.
Histological analysis (Figure 5d) and stereological counting of nigral DA neurons (Figure 5e) at 17 weeks after the unilateral 6-OHDA lesion (corresponding to 14 weeks after AAV vector injection) revealed a loss of about 40% of nigral DA neurons in the ipsilateral hemisphere due to 6-OHDA infusion, which was completely prevented by astrocytic GDNF expression while expression of EGFP had no effect (Figure 5e). Of note, the long-term expression of GDNF in striatal astrocytes resulted in a reduction of TH immunoreactivity in nigral DA neurons (Figure 5d), although nigral GDNF levels as determined by enzyme-linked immunosorbent assay were enhanced only twofold over EGFP-expressing controls. This effect was not detectable in vesicular monoamine transporter-2 immunohistochemistry; stereological assessment showed the number of DA neuron to be identical after TH or vesicular monoamine transporter-2 immunohistochemistry (data not shown). Thus, the negative impact of continuous GDNF expression on nigral DA neurons in terms of downregulation of TH immunoreactivity appeared to be similar to that reported after lentiviral-mediated GDNF expression in striatal neurons within the healthy nigro-striatal system.21 Nonetheless, under clinically relevant conditions of slowly progressive degeneration of the nigro-striatal system, astrocytic GDNF expression demonstrated highly efficient restoration of motor performance, and protection from DA neuron degeneration following loss of their terminals. The motor recovery and protection of DA neurons obtained by our AAV-5-hGFAP-GDNF vector was similar to a previous study using an AAV-2 vector with predominant neuronal transgene expression in a similarly scheduled partial 6-OHDA lesion paradigm,22 exemplifying that no attenuation of therapeutic efficacy has to be expected from targeted astrocytic GDNF delivery.
Discussion
Although on first sight, our finding of GDNF delivery and bio-activity to the contralateral hemisphere after unilateral vector application and neuronal expression suggested an approach with high therapeutic efficacy for the nigro-striatal projection, it revealed serious concerns in terms of potential side effects. GDNF has been identified as an essential factor for differentiation and survival of dopaminergic neurons, with highest expression levels during development and greatly reduced expression in the adult brain.23 However, catecholaminergic neurons crucially depend on GDNF signaling in the adult brain,24 and the prototype receptors GDNF family receptor α-1 (GFRα) and Ret are expressed throughout the adult brain.25,26 Furthermore, GDNF has been proposed to be able to induce signaling through additional receptor systems like neural cell adhesion molecule and integrins.27,28 Thus, in the current setting of gene therapy being an irreversible process and the putative need for higher dosages of GFLs, increased vector spread and younger patients to become a successful strategy for Parkinson's patients, dissemination of a potent neurotrophic factor to areas outside the affected nigro-striatal system may impose serious side effects after long-term exposure.8 The detailed wiring of the human brain has not been fully elucidated,29 and it is impossible to predict into which distant central nervous system nuclei neurons may project and deliver GDNF after being transduced by a neuronal gene therapy vector, especially in case of vectors highly efficient in distribution through the target tissue like AAV-5. The long-term presence of a neurotrophic factor could result in the activation of low affinity receptors that in turn could induce unforeseeable signaling events.
For example, in mouse, cat, and monkey the nigro-collicular projection originating in the substantia nigra pars reticulata links the basal ganglia to the sensorimotor layers of the superior colliculus and is crucially involved in promotion of orienting behavior.30,31,32 Long-term exposure of these neurons to a potent neurotrophic factor as seen after neuronal GDNF expression in the striatum may eventually modify their functional capabilities and impact on essential motor control mechanisms. This issue becomes even more important if delivery of a neurotrophin-expressing vector directly into the nigra is considered,9 a scenario in which astrocytic expression will prevent off-target delivery through neuronal projections as well. Furthermore, nigral astrocytes may be less affected from loss of DA neuron activity and resulting impaired GDNF secretion.
Secretion of a neurotrophic factor from terminal neuronal sites into disease-affected circuits may be a beneficial option under certain conditions. However, secretion of neuron-expressed GDNF from unmyelinated axons in the medial forebrain bundle and activation of hypothalamic corticotrophin releasing hormone secreting neurons has recently been suggested to cause weight loss,12 a common side effect in a number of preclinical and clinical trials33,34,35,36 and currently the best-characterized off-target effect of GDNF. As an important safety measure, we found that in no case striatal astrocytic-derived GDNF exerted measurable biological effects on the dopaminergic system outside the target structures (ipsilateral striatum and nigra), not even in the case of injection of high-titre of virus. Nonetheless, in the ipsilateral hemisphere astrocyte-derived GDNF was as efficient as neuron-derived GDNF in preventing the degeneration of nigral DA neurons, their striatal terminals and their striatal DA content from MPTP toxicity, and provided robust improvement in motor control under clinically relevant conditions after 6-OHDA lesion. Thus, without any restriction on neuroprotective efficacy in the ipsilateral nigro-striatal projection the astrocytic expression of GDNF provided an additional safety criterion by avoiding remote and potential off-target biological effects.
Although lowering vector titre improved the remote delivery profile of neuronal GDNF expression, even at very low levels it still resulted in a pronounced impact on contralateral neurotransmitter synthesis. A fourfold increase of GDNF over control levels in the contralateral nigra was sufficient to fully restore striatal DA levels after long-term (3-month) expression, exemplifying that some biological effects of GDNF may take considerable time to build up. In contrast, after astrocytic GDNF expression, a 13-fold increase of GDNF in the ipsilateral nigra was fully protective (low-titre group), but the same increase in GDNF levels did not show measurable biological effect in the contralateral nigra (in the high-titre group), demonstrating that it is not only the absolute level of GDNF which might exert biological effects but also the route of delivery or the differential availability to receptors.
Mechanistically, these findings imply that GDNF delivered at least partially by anterograde transport through contralateral projections (see Supplementary Figure S3g,j) can exert neuroprotective signaling, while GDNF delivered retrogradely through the crossed nigro-striatal projection19 cannot. From this it would follow that it is not internalized GDNF but rather extracellular GDNF that can mediate neuroprotection and neurorestoration. Likewise, the neuroprotective and neurorestorative effect of astrocytic striatal GDNF may not depend on GDNF retrogradely transported towards the dopaminergic cell bodies in the nigra but rather on GDNF-induced signaling at synaptic and/or axonal sites. This view would strongly support the hypothesis that indeed sufficient functional dopaminergic innervation must be present in the caudate/putamen of PD patients to allow for beneficial effects of neurotrophic factor gene therapy.8,9
Efficient transduction of astrocytes to express GDNF has been demonstrated earlier by adenoviral and lentiviral vectors in vitro and in vivo.37,38,39 AAV vectors have been used for astrocytic transgene expression only very recently but were still hampered by considerable coexpression in neurons,40 the need of very high vector dosages41 or the need of systemic vector application in neonates.42 In contrast, the AAV-5 based strategy presented here demonstrated very low vector dosages to be still highly efficient in astrocytic transgene expression, while high-titres were still completely astrocyte-specific. A potential drawback of astrocytes as hosts for neurotrophic factor gene therapy may be the fact that these cells are capable of dividing upon stimulations such as the inflammatory processes present in PD brains. As AAV genomes persist episomally such cell division would dilute the vector, making it unlikely to become a risk factor but rather resulting in a self-limitation of transduction. This situation was mimicked in the MPTP model demonstrating severe astrogliosis at 2 weeks after lesion, which has faded at 3 months after lesion. GDNF was detected to the same extent by immunohistochemistry in striatum and nigra and EGFP-expressing astrocytes were equal in number at both time points, indicating that the pronounced temporal astrogliosis provoked no major changes in either astroglial GDNF production or the number of transgene-expressing cells.
In conclusion, given that neurotrophins are potent modulators of neuronal physiology and thus may potentially provoke unwanted side effects if present in brain areas unaffected by PD, it seems reasonable to restrict their impact to the immediate vicinity of the site of lesion. This was achieved by an astrocyte-specific AAV vector delivering bio-active GDNF only to the target sites in the ipsilateral striatum and nigra, thereby adding substantially to the appropriate targeting of forthcoming gene therapeutic approaches in PD patients. Given the impact of long-term presence of GDNF on DA neurons' TH levels as shown here for expression in striatal astrocytes and earlier for expression in striatal neurons,21 we suggest that—preferentially in combination with regulated transgene expression43—a safe and efficient astrocyte-based GFL gene therapy for PD can be achieved.
Material and Methods
Recombinant vector production. Recombinant AAV-5 viruses were produced by transient cotransfection of the vector genome plasmid with the pDP5 helper plasmid,44 iodixanol step gradient ultracentrifugation followed by anion-exchange chromatography, further concentration on 150 kDa spun concentrators and final dialysis against PBS overnight. Genome titres were determined by quantitative PCR, purity >99% was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining. Promoters used were the 0.4 kbp fragment of the hSYN45 or the 2.2 kbp fragment of the hGFAP.46
Animal procedures. All experimental animal procedures were conducted according to approved experimental animal licenses issued by the responsible animal welfare authority (Niedersächsisches Landesamt für Verbraucherschutz und Lebensmittelsicherheit) and controlled by the local animal welfare committee of the University Medical Center Göttingen. To target the striatum, 2–3 month-old C57Bl/6J male mice were injected unilaterally with 3 × 108 or 3 × 109 vg of AAV-5-hGFAP-EGFP, AAV-5-hGFAP-GDNF, AAV-5-hSYN-EGFP or AAV-5-hSYN-GDNF, in a 2 µl volume each. Mice were anesthetized with ketamine/xylazine solution (100/5 mg/kg bodyweight) and placed in a stereotaxic frame (World Precision Instruments, Sarasota, FL). Two injections at the following anterior, lateral, and ventral coordinates (mm relative to bregma) 0.0, −2.2, 3.5 and 0.9, −1.5, 3.5 were performed using a Nanoliter2000 microinjector (World Precision Instruments). MPTP was administered as described47 according to published safety guidelines, intraperitoneally, 30 mg/kg free base in NaCl, for 5 days at 24 hours intervals. Animals were either sacrificed by cervical dislocation, the striata dissected, frozen, and stored at −80 °C or animals were sacrificed using CO2 and after transcardial perfusion (10 minutes, 0.1 mol/l PBS, pH 7.4 and 10 minutes, 4% paraformaldehyde in PBS) the whole brain was fixed and cryoprotected.
Unilateral 6-OHDA lesions were induced by stereotaxic injection of 5 µg 6-OHDA each at coordinates anteroposterior +0.5, midlateral +2.1; dorsoventral −5.0 and anteroposterior -0.5, midlateral +3.8, dorsoventral −5.0 into striata of adult Wistar rats. This lesion model results in acute degeneration of striatal DA termini, while a slowly progressive death of nigral DA neurons occurs over a period of several weeks48 Lesions were confirmed by apomorphine-induced (0.4 mg/kg) rotations at 1 and 2 weeks after 6-OHDA application. Three weeks after 6-OHDA lesion AAV-5-hGFAP-EGFP or AAV-5-hGFAP-GDNF were injected at the same coordinates as used for 6-OHDA injection at 1.5 × 109 vector genomes each deposit. Rotation behavior was recorded again at 7 and 17 weeks after 6-OHDA lesion.
Quantification of DA, DOPAC, and HVA. Catecholamine levels were determined by ion-pair high-performance liquid chromatography with electrochemical detection as described.49
Detection and quantification of GDNF. Immunohistochemistry was performed according to standard protocols using the polyclonal anti-GDNF #BAF212 from R&D Systems (Wiesbaden, Germany). The GDNF enzyme-linked immunosorbent assay was performed following the manufacturer's instructions (Promega, Madison, WI).
Stereology and quantification of striatal fibre density. Stereological counting of dopaminergic neurons was performed by the optical fractionation method using StereoInvestigator (MicroBrightField, Magdeburg, Germany) as described.47 To quantify TH fibre density in the striatum, five coronal sections between bregma +1.10 and −0.10 mm were stained essentially as described above. For every section, three pictures were acquired. To automatically delineate the fibres and to increase the signal-to-noise ratio, the images were first thresholded, binarized and the optical density of photographs evaluated with ImageJ software (NIH, Bethesda, MD; http://imagej.nih.gov/ij/, 1997–2011. Striatal values were normalized by subtracting the background staining, measured in the cortex of the same section.
Statistical analysis. Data are expressed as means ± SD. To compare different group means for independent samples, the statistical analysis was performed by analysis of variance, followed by Tukey's post hoc test (GraphPad Prism 4.0; GraphPad Software, La Jolla, CA). Significance levels were set at *P < 0.05, **P < 0.01, and ***P < 0.001.
SUPPLEMENTARY MATERIAL Figure S1. Large area transduction by AAV-5-hSYN and AAV-5-hGFAP vectors in mouse striatum demonstrated by EGFP fluorescence. Figure S2. Large area transduction by low-titre AAV-5-hSYN and AAV-5-hGFAP vectors in mouse striatum demonstrated by GDNF immunoreactivity. Figure S3. AAV-5 vector particles are not retrogradely transported to SNpC cell bodies. Figure S4. Unilateral GDNF expression in striatal neurons protects dopaminergic neurons in both hemispheres, while unilateral GDNF expression in astrocytes protects dopaminergic neurons only in the ipsilateral hemisphere. Figure S5. Unilateral GDNF expression in striatal neurons protects dopaminergic fibres in both hemispheres, while unilateral GDNF expression in astrocytes protects dopaminergic fibres only in the ipsilateral hemisphere. Figure S6. Assessment of striatal catecholamine levels. Table S1. Levels of GDNF as determined by ELISA in the different brain areas at 2 weeks after MPTP treatment (= 4 weeks after vector application).
Acknowledgments
We are grateful to Christiane Fahlbusch, Kirsten Fladung, Monika Zebski, and Ulrike Schöll for expert technical assistance and Cathy Ludwig for proofreading the manuscript. This work was supported by the European Community's Seventh Framework Programme FP7/2007–2013 under grant agreement n° HEALTH-F5-2008-222925, the BMBF-funded NGFNplus (PNP-01GS08137-5), and by the German Research Council-funded Center for Molecular Physiology of the Brain (CMPB).
Supplementary Material
Large area transduction by AAV-5-hSYN and AAV-5-hGFAP vectors in mouse striatum demonstrated by EGFP fluorescence.
Large area transduction by low-titre AAV-5-hSYN and AAV-5-hGFAP vectors in mouse striatum demonstrated by GDNF immunoreactivity.
AAV-5 vector particles are not retrogradely transported to SNpC cell bodies.
Unilateral GDNF expression in striatal neurons protects dopaminergic neurons in both hemispheres, while unilateral GDNF expression in astrocytes protects dopaminergic neurons only in the ipsilateral hemisphere.
Unilateral GDNF expression in striatal neurons protects dopaminergic fibres in both hemispheres, while unilateral GDNF expression in astrocytes protects dopaminergic fibres only in the ipsilateral hemisphere.
Assessment of striatal catecholamine levels.
Levels of GDNF as determined by ELISA in the different brain areas at 2 weeks after MPTP treatment (= 4 weeks after vector application).
REFERENCES
- Thomas B., and, Beal MF. Parkinson's disease. Hum Mol Genet. 2007;16 ( Spec No. 2:R183–R194. doi: 10.1093/hmg/ddm159. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A., and, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson's model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. J Neurosci. 2000;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ovadia A, Cass WA, Yi A, Simmerman L.et al. (1996Functional recovery in parkinsonian monkeys treated with GDNF Nature 380252–255. [DOI] [PubMed] [Google Scholar]
- Eslamboli A, Georgievska B, Ridley RM, Baker HF, Muzyczka N, Burger C.et al. (2005Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson's disease J Neurosci 25769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R.et al. (2006Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease Ann Neurol 59459–466. [DOI] [PubMed] [Google Scholar]
- Marks WJ, Jr, Bartus RT, Siffert J, Davis CS, Lozano A, Boulis N.et al. (2010Gene delivery of AAV2-neurturin for Parkinson's disease: a double-blind, randomised, controlled trial Lancet Neurol 91164–1172. [DOI] [PubMed] [Google Scholar]
- Björklund T., and, Kirik D. Scientific rationale for the development of gene therapy strategies for Parkinson's disease. Biochim Biophys Acta. 2009;1792:703–713. doi: 10.1016/j.bbadis.2009.02.009. [DOI] [PubMed] [Google Scholar]
- Manfredsson FP, Okun MS., and, Mandel RJ. Gene therapy for neurological disorders: challenges and future prospects for the use of growth factors for the treatment of Parkinson's disease. Curr Gene Ther. 2009;9:375–388. doi: 10.2174/156652309789753400. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Herzog CD, Chu Y, Wilson A, Brown L, Siffert J.et al. (2011Bioactivity of AAV2-neurturin gene therapy (CERE-120): differences between Parkinson's disease and nonhuman primate brains Mov Disord 2627–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Palfi S, Chen EY, Ma SY, Sendera T, Cochran EJ.et al. (1999Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson's disease Ann Neurol 46419–424. [DOI] [PubMed] [Google Scholar]
- Su X, Kells AP, Huang EJ, Lee HS, Hadaczek P, Beyer J.et al. (2009Safety evaluation of AAV2-GDNF gene transfer into the dopaminergic nigrostriatal pathway in aged and parkinsonian rhesus monkeys Hum Gene Ther 201627–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredsson FP, Tumer N, Erdos B, Landa T, Broxson CS, Sullivan LF.et al. (2009Nigrostriatal rAAV-mediated GDNF overexpression induces robust weight loss in a rat model of age-related obesity Mol Ther 17980–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T., and, Schwartz JP. Gene expression profiles of reactive astrocytes in dopamine-depleted striatum. Brain Pathol. 2004;14:275–280. doi: 10.1111/j.1750-3639.2004.tb00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresjanac M., and, Antauer G. Reactive astrocytes of the quinolinic acid-lesioned rat striatum express GFRalpha1 as well as GDNF in vivo. Exp Neurol. 2000;164:53–59. doi: 10.1006/exnr.2000.7416. [DOI] [PubMed] [Google Scholar]
- Mogi M, Togari A, Kondo T, Mizuno Y, Kogure O, Kuno S.et al. (2001Glial cell line-derived neurotrophic factor in the substantia nigra from control and parkinsonian brains Neurosci Lett 300179–181. [DOI] [PubMed] [Google Scholar]
- Leitner ML, Molliver DC, Osborne PA, Vejsada R, Golden JP, Lampe PA.et al. (1999Analysis of the retrograde transport of glial cell line-derived neurotrophic factor (GDNF), neurturin, and persephin suggests that in vivo signaling for the GDNF family is GFRalpha coreceptor-specific J Neurosci 199322–9331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rind HB., and, von Bartheld CS. Anterograde axonal transport of internalized GDNF in sensory and motor neurons. Neuroreport. 2002;13:659–664. doi: 10.1097/00001756-200204160-00025. [DOI] [PubMed] [Google Scholar]
- Ciesielska A, Mittermeyer G, Hadaczek P, Kells AP, Forsayeth J., and, Bankiewicz KS. Anterograde axonal transport of AAV2-GDNF in rat basal ganglia. Mol Ther. 2011;19:922–927. doi: 10.1038/mt.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas R, Kellaway L, Mintz M., and, van Wageningen G. The crossed nigrostriatal projection decussates in the ventral tegmental decussation. Brain Res. 1987;418:111–121. doi: 10.1016/0006-8993(87)90967-x. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Sonsalla PK., and, Chesselet MF. Animal models of Parkinson's disease progression. Acta Neuropathol. 2008;115:385–398. doi: 10.1007/s00401-008-0350-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajadi A, Bauer M, Thöny B., and, Aebischer P. Long-term glial cell line-derived neurotrophic factor overexpression in the intact nigrostriatal system in rats leads to a decrease of dopamine and increase of tetrahydrobiopterin production. J Neurochem. 2005;93:1482–1486. doi: 10.1111/j.1471-4159.2005.03139.x. [DOI] [PubMed] [Google Scholar]
- Wang L, Muramatsu S, Lu Y, Ikeguchi K, Fujimoto K, Okada T.et al. (2002Delayed delivery of AAV-GDNF prevents nigral neurodegeneration and promotes functional recovery in a rat model of Parkinson's disease Gene Ther 9381–389. [DOI] [PubMed] [Google Scholar]
- Saavedra A, Baltazar G., and, Duarte EP. Driving GDNF expression: the green and the red traffic lights. Prog Neurobiol. 2008;86:186–215. doi: 10.1016/j.pneurobio.2008.09.006. [DOI] [PubMed] [Google Scholar]
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gómez-Díaz R., and, López-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Glazner GW, Mu X., and, Springer JE. Localization of glial cell line-derived neurotrophic factor receptor alpha and c-ret mRNA in rat central nervous system. J Comp Neurol. 1998;391:42–49. doi: 10.1002/(sici)1096-9861(19980202)391:1<42::aid-cne4>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Sarabi A, Hoffer BJ, Olson L., and, Morales M. Glial cell line neurotrophic factor-family receptor alpha-1 is present in central neurons with distinct phenotypes. Neuroscience. 2003;116:261–273. doi: 10.1016/s0306-4522(02)00559-6. [DOI] [PubMed] [Google Scholar]
- Sariola H., and, Saarma M. Novel functions and signalling pathways for GDNF. J Cell Sci. 2003;116 Pt 19:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- Cao JP, Yu JK, Li C, Sun Y, Yuan HH, Wang HJ.et al. (2008Integrin beta1 is involved in the signaling of glial cell line-derived neurotrophic factor J Comp Neurol 509203–210. [DOI] [PubMed] [Google Scholar]
- Guye M, Bartolomei F., and, Ranjeva JP. Imaging structural and functional connectivity: towards a unified definition of human brain organization. Curr Opin Neurol. 2008;21:393–403. doi: 10.1097/WCO.0b013e3283065cfb. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Isa K, Yanagawa Y., and, Isa T. Nigral inhibition of GABAergic neurons in mouse superior colliculus. J Neurosci. 2008;28:11071–11078. doi: 10.1523/JNEUROSCI.3263-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harting JK, Updyke BV., and, Van Lieshout DP. The visual-oculomotor striatum of the cat: functional relationship to the superior colliculus. Exp Brain Res. 2001;136:138–142. doi: 10.1007/s002210000606. [DOI] [PubMed] [Google Scholar]
- Huerta MF, Van Lieshout DP., and, Harting JK. Nigrotectal projections in the primate Galago crassicaudatus. Exp Brain Res. 1991;87:389–401. doi: 10.1007/BF00231856. [DOI] [PubMed] [Google Scholar]
- Tümer N, Scarpace PJ, Dogan MD, Broxson CS, Matheny M, Yurek DM.et al. (2006Hypothalamic rAAV-mediated GDNF gene delivery ameliorates age-related obesity Neurobiol Aging 27459–470. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER., Jret al. (2003Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD Neurology 6069–73. [DOI] [PubMed] [Google Scholar]
- Aoi M, Date I, Tomita S., and, Ohmoto T. The effect of intrastriatal single injection of GDNF on the nigrostriatal dopaminergic system in hemiparkinsonian rats: behavioral and histological studies using two different dosages. Neurosci Res. 2000;36:319–325. doi: 10.1016/s0168-0102(00)00097-3. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Zhang Z, Ovadia A, Lapchak PA, Collins F, Hilt D.et al. (1997Glial cell line-derived neurotrophic factor-levodopa interactions and reduction of side effects in parkinsonian monkeys Ann Neurol 42208–214. [DOI] [PubMed] [Google Scholar]
- Sandhu JK, Gardaneh M, Iwasiow R, Lanthier P, Gangaraju S, Ribecco-Lutkiewicz M.et al. (2009Astrocyte-secreted GDNF and glutathione antioxidant system protect neurons against 6OHDA cytotoxicity Neurobiol Dis 33405–414. [DOI] [PubMed] [Google Scholar]
- Pertusa M, García-Matas S, Mammeri H, Adell A, Rodrigo T, Mallet J.et al. (2008Expression of GDNF transgene in astrocytes improves cognitive deficits in aged rats Neurobiol Aging 291366–1379. [DOI] [PubMed] [Google Scholar]
- Do Thi NA, Saillour P, Ferrero L, Dedieu JF, Mallet J., and, Paunio T. Delivery of GDNF by an E1,E3/E4 deleted adenoviral vector and driven by a GFAP promoter prevents dopaminergic neuron degeneration in a rat model of Parkinson's disease. Gene Ther. 2004;11:746–756. doi: 10.1038/sj.gt.3302222. [DOI] [PubMed] [Google Scholar]
- Koerber JT, Klimczak R, Jang JH, Dalkara D, Flannery JG., and, Schaffer DV. Molecular evolution of adeno-associated virus for enhanced glial gene delivery. Mol Ther. 2009;17:2088–2095. doi: 10.1038/mt.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor PA, Bland RJ, Mouravlev A, Young D., and, During MJ. Efficient gene delivery and selective transduction of glial cells in the mammalian brain by AAV serotypes isolated from nonhuman primates. Mol Ther. 2009;17:1692–1702. doi: 10.1038/mt.2009.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust KD, Nurre E, Montgomery CL, Hernandez A, Chan CM., and, Kaspar BK. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manfredsson FP, Burger C, Rising AC, Zuobi-Hasona K, Sullivan LF, Lewin AS.et al. (2009Tight Long-term dynamic doxycycline responsive nigrostriatal GDNF using a single rAAV vector Mol Ther 171857–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Zhou S, Nakai H, Thomas CE, Storm TA, Fuess S.et al. (2003Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy Blood 1022412–2419. [DOI] [PubMed] [Google Scholar]
- Kügler S, Meyn L, Holzmüller H, Gerhardt E, Isenmann S, Schulz JB.et al. (2001Neuron-specific expression of therapeutic proteins: evaluation of different cellular promoters in recombinant adenoviral vectors Mol Cell Neurosci 1778–96. [DOI] [PubMed] [Google Scholar]
- Lee Y, Messing A, Su M., and, Brenner M. GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia. 2008;56:481–493. doi: 10.1002/glia.20622. [DOI] [PubMed] [Google Scholar]
- Kowsky S, Pöppelmeyer C, Kramer ER, Falkenburger BH, Kruse A, Klein R.et al. (2007RET signaling does not modulate MPTP toxicity but is required for regeneration of dopaminergic axon terminals Proc Natl Acad Sci USA 10420049–20054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer H., and, Oertel WH. Progressive degeneration of nigrostriatal dopamine neurons following intrastriatal terminal lesions with 6-hydroxydopamine: a combined retrograde tracing and immunocytochemical study in the rat. Neuroscience. 1994;59:401–415. doi: 10.1016/0306-4522(94)90605-x. [DOI] [PubMed] [Google Scholar]
- Eberhardt O, Coelln RV, Kugler S, Lindenau J, Rathke-Hartlieb S, Gerhardt E.et al. (2000Protection by synergistic effects of adenovirus-mediated X-chromosome-linked inhibitor of apoptosis and glial cell line-derived neurotrophic factor gene transfer in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease J Neurosci 209126–9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Large area transduction by AAV-5-hSYN and AAV-5-hGFAP vectors in mouse striatum demonstrated by EGFP fluorescence.
Large area transduction by low-titre AAV-5-hSYN and AAV-5-hGFAP vectors in mouse striatum demonstrated by GDNF immunoreactivity.
AAV-5 vector particles are not retrogradely transported to SNpC cell bodies.
Unilateral GDNF expression in striatal neurons protects dopaminergic neurons in both hemispheres, while unilateral GDNF expression in astrocytes protects dopaminergic neurons only in the ipsilateral hemisphere.
Unilateral GDNF expression in striatal neurons protects dopaminergic fibres in both hemispheres, while unilateral GDNF expression in astrocytes protects dopaminergic fibres only in the ipsilateral hemisphere.
Assessment of striatal catecholamine levels.
Levels of GDNF as determined by ELISA in the different brain areas at 2 weeks after MPTP treatment (= 4 weeks after vector application).