Abstract
Diagnosis of acute cellular rejection (ACR) requires a liver biopsy with attendant expense, and risk. The first aim was to prospectively determine, in an exploratory analysis, whether there is a serum proteome signature associated with histologically confirmed ACR. The second aim was to use simpler and faster ELISA-based assays for proteins identified as differentially abundant in the proteomic analysis to identify patients with ACR in a separate validation cohort. We used sequential high abundance protein depletion and iTRAQ LC/MS/MS mass spectrometry to characterize the serum proteome in serum samples of patients with ACR and without ACR. Of the 41 proteins identified as differentially abundant, 7 (serum amyloid A (SAA), complement 4 (C4), fibrinogen, complement 1q (C1q), complement 3 (C3), Heat Shock Protein 60 (HSP60), and Heat Shock Protein 70 (HSP70)) could be measured using ELISA-based assays in a validation cohort of patients with ACR (n=25) and without ACR (n=21). Mean ALT levels in patients with and without ACR were (mean +/− SD) 198+/−27 and 153+/−34 U/L respectively. Among the seven proteins for which ELISA assays were available, C4 and C1q were both independent predictors for ACR. C4 had the greatest predictivity in differentiating patients with/without ACR. C4 of ≤0.31gm/L had a 97% sensitivity, 62% specificity, 74% positive predictive value and 94% negative predictive value. The combination C4 ≤0.31 gm/L and ALT ≥70 IU/ml had 96% sensitivity, 81% specificity, 86% positive predictive value and 94% negative predictive value. In summary, in this exploratory analysis, serum complement C4 and ALT levels are highly predictive of ACR in liver transplant recipients. Confirmation in a prospective, larger, diverse population is needed.
Introduction
Survival one year after liver transplantation has increased from approximately 30% in the 1970s to greater than 80% today.(1–3). Acute cellular rejection (ACR) most commonly occurs in the early posttransplant period, usually within 6 weeks.(4) The majority of these episodes (~85%) resolve with anti-rejection treatment, typically pulses of systemic corticosteroids.(4) Late acute cellular rejection (occurring >6 months posttransplantation) occurs in a further 11–16% of liver transplant recipients.(4) Early acute cellular rejection is strongly predictive of subsequent death for recipients with hepatitis C infection (>40% of liver transplant recipients in the United States).(4, 5) However, late graft survival (graft survival beyond 1 year post-transplantation) is significantly lower, regardless of original liver disease, among patients who experience late acute rejection.(4) Thus, acute cellular rejection (ACR) is still a common and important complication of liver transplantation, occurring in about 30% of recipients.(6, 7)
In contrast to many aspects of clinical transplantation, the algorithm for diagnosing ACR has not changed since the advent of clinical transplantation in the 1960s. The diagnosis of ACR requires evidence of graft dysfunction (e.g. elevated transaminases), typically followed by confirmation with an allograft biopsy. Liver biopsy, which is been considered to be the best means of diagnosing ACR,(8) entails expense, risk, time and inconvenience. In addition, the interpretation of liver biopsy can be challenging. The classic triad of mixed portal tract inflammatory infiltrate, endotheliitis and bile duct injury, is not always present.(9, 10) Further more, endotheliitis has been reported in up to 60% of patients with hepatitis C infection.(11) Sampling error is another consideration in interpreting liver biopsies.(12, 13)
The need for a noninvasive method for the diagnosis of ACR has been highlighted by major societies and the National Institutes of Health. Noninvasive approaches have been made difficult by the lack of serum (or urinary) protein signature to ACR. Attempts to describe the serum (or urinary) proteome have been limited by the inability to accurately quantify low abundance proteins that comprise the great majority of the serum protein. Recent advances in protein separation, including depletion of highly abundant proteins(14) and high-throughput mass spectrometry(14)have facilitated detailed characterization of complex biological samples, including serum.(15, 16) We explored the possibility of a serum signature for acute cellular rejection through a prospective study conducted in two phases. In the first phase, we used a combination of high abundance protein depletion and iTRAQ mass spectrometry to characterize the serum proteomic signature of ACR. In the second phase, proteins identified in the first phase as differentially abundant in the serum proteome were validated on a separate set of patients using the more clinically applicable ELISA method of protein quantification.
Patients and Methods
First phase: Proteomic analysis
Study Participants
Two groups of subjects were studied:
ACR group (n=8): This group comprised eight liver transplant recipients with HCV infection who had Banff criteria for acute cellular rejection (ACR) on protocol day 7 liver biopsies (patients with HCV were chosen to provide a potential source of necroinflammation other than ACR in both groups)
Control group (No-ACR, n=8): This group comprised eight liver transplant recipients with HCV infection who had similar biochemical profiles as the ACR group, but who did not have cholangitis or endotheliitis histologically. Recipients in the control group did not receive treatment for acute cellular rejection at any point.
Both the ACR and control (ACR negative) groups were matched for age and gender. All subjects in the ACR group resolved ACR histologically and biochemically with a single course of methyl prednisolone therapy. Subjects in the control group did not require treatment for acute cellular rejection at any time and had subsequent allograft histology demonstrating persistent and progressive histological changes consistent with recurrence of HCV infection. 10 mL whole blood samples were collected in glass tubes without additive (10 mL BD VacutainerTM, Franklin Lakes, NJ) and allowed to clot at room temperature for 40 min. Serum was separated by centrifugation at 1500 rpm for 15 min. 1 mL aliquots of serum were taken and stored at −80 C until ready for use. The time from collection to frozen storage was no more than 30 min. Samples were collected blind to the investigators participating in the study and contained no identifying features that would make it possible to identify the subjects. The study was approved by the Institutional Review Board of the Mayo Foundation.
Depletion of Serum high abundant proteins
Serum samples were processed using a 4.6 × 50 mm Multiple Affinity Removal Column (Agilent Technologies, Palo Alto, CA), which selectively removes albumin, IgG, IgA, anti-trypsin, transferrin, and haptoglobin from the serum sample, attached to an EZChrome Elite HPLC (Hitachi High Technologies America, San Jose, CA). Samples were processed according to manufacturer's instructions.
Sample preparation for Multidimensional Liquid Chromatography Tandem mass Spectrometry (LC-MS/MS) analysis
The cleaned up iTRAQ labeled sample was fractionated into 10 fractions on a strong cation exchange column, Biox SCX 300μm x 5cm (Dionex, Sunnyvale, CA) using an off-line Agilent 1100 series capillary liquid chromatography system (Wilmington, DE). LC/MS/MS analysis of the peptides in each fraction was performed on an Applied Biosystems API Qstar XL quadrupole time of flight mass spectrometer configured with a Protana nano spray ion source (Proxeon, Denmark) and with an Ultimate nano liquid chromatography system (Dionex, Sunnyvale, CA). The peptides were separated on a Zorbax C18 100μm x 150mm microbore column (Agilent, Wilmington, DE) with a gradient from 5% to 60% buffer B over 120 minutes, where buffer A is 0.1% formic acid / 98% water / 2% acetonitrile and buffer B is 0.1% formic acid / 2% water / 98% acetonitrile. The ms/ms data was obtained via information-dependent acquisition (IDA) mode in the Analyst QS software. This consists of a 1.5 second survey scan from 350-1600 m/z and switching to 2.0 second fragmentation scans on three most intense ions from the survey. These ions were then excluded from repeating for 45 seconds. Collision energy applied was varied automatically depending on the precursor m/z and charge state.
Protein Identification, Quantification and Data Analysis
Protein identification was performed by searching MS/MS spectra against the CDS fasta database (Applied Biosystems, Framingham, MA) and quantified using ProQuant software (Applied Biosystems, Framingham, MA). The data was further analyzed by ProGroup (Applied Biosystems, Framingham, MA), a functionality which provides an important second stage of protein identification analysis. The results of the quantification were normalized using the overall ratio obtained for all tagged peptide pairs in the sample.(17) A difference of 2 SDs, i.e., about a 1.2-fold difference in abundance, was considered to be significant, with a confidence limit of >90% using a simple Gaussian approximation.(17, 18) This approximation would, therefore, apply to normalized expression levels >1.2 or, in reciprocal form, <0.8.
Second phase: ELISA Validation
For the validation of the results obtained in the proteomic analysis, sera were collected from two groups of patients who underwent liver transplantation at the same center who were not included in the first phase of experiments:
ACR group (n=25): This group comprised liver transplant recipients who had Banff criteria for acute cellular rejection (ACR) on protocol day 7 liver biopsies
Control group (No-ACR, n=21): This group comprised transplant recipients who had similar biochemical profiles as the ACR group, but who did not have histological evidence of rejection. Recipients in the control group did not receive treatment for acute cellular rejection at any point.
Patients with equivocal diagnosis or more than one diagnosis on liver biopsy e.g. ACR and perfusion injury, unavailable day 7 liver biopsy and multiple organs transplantation were excluded.
Enzyme-linked Immunosorbent Assay (ELISA)
Forty one proteins were identified as differentially abundant in the serum of patients with ACR in the proteomic analysis. Commercial ELISA assays were available for seven of these proteins: serum amyloid A (SAA), complement 4 (C4), fibrinogen, complement 1q (C1q), complement 3 (C3), Heat Shock Protein 60 (HSP60), and Heat Shock Protein 70 (HSP70). These seven proteins were then measured in the serum of patients with ACR (n=25) and patients with no ACR (n=21). Abundance of each of the proteins measured by ELISA was calculated using spline-algorithm. Optical density was measured within 30 minutes at 450 nm.
Serum SAA levels were determined using a two-step ELISA from Abazyme (Abazyme LLC, Needham, MA), that utilizes a monoclonal antibody specific for human SAA. The Complement C4 ELISA kit was from Assaypro (Assaypro, St. Charles, MO). In this assay, the C4 in samples was competed by a biotinylated complement C4 sandwiched by immobilized antibody and streptavidin-peroxidase conjugate. Fibrinogen concentration was determined by a two-step ELISA from ALPCO (ALPCO Diagnostics, Salem, NH). Complement 1q (C1q) (RnD Systems, Minneapolis, MN). Complement C3 ELISA kit from Abnova was used (Abnova, Taiwan). Twenty five μl of standard or sample was added to each well in duplicate. Hsp60 ELISA Kit from Assay Designs, Inc., Ann Arbor, MI was used. One hundred μl of the standard, samples and control were added in duplicate to the respective wells. Hsp70 ELISA Kit from Assay Designs, Inc., Ann Arbor, MI was used. One hundred μl of the standard, samples and control were added in duplicate to the respective wells. All assays were performed according to manufacturers’ suggested protocols.
Statistical analysis
Wilcoxon rank-sum test was used to compare the means of protein concentration in the two groups (ACR and non-ACR). Simple logistic regression was used to test individual variables that may predict ACR. Variables which were significant predictors for ACR on simple logistic regression were entered into multiple logistic regression model. P<0.05 was considered significant. Statistical analysis was performed using JMPR 8.0.1 Software (SAS Institute Inc, NC).
Results
Proteomic analysis results
The demographic and clinical characteristics of patients in acute cellular rejection (ACR) and no acute cellular rejection (No-ACR) groups were summarized in Table 1.
Table 1.
Demographic and clinical characteristics of proteomic analysis patients*:
| ACR | No-ACR | |
|---|---|---|
| Age (mean +/− SD) | 44.0 +/− 1.5 | 47.8 +/− 2.8 |
| Male/female | 4/4 | 4/4 |
| Total bilirubin (mean +/− SD) | 6.3 +/− 1.9 | 4.2 +/− 1.2 |
| Alkaline phosphatase (mean +/− SD) | 771 +/− 150 | 328 +/− 56 |
| AST (mean +/− SD) | 181 +/− 58 | 183 +/− 118 |
| ALT (mean +/− SD) | 316 +/− 66 | 260 +/− 74 |
None of the differences was statistically significant.
Out of a total of 2801 proteins that were analyzed across all serum samples, 41 proteins were found to be significantly differentially abundant in the sera of all patients with ACR. Of these 41 proteins, 28 were up-regulated, and 13 were down-regulated in the ACR patients when compared to the No-ACR patients (Table 2 and 3). Standardized ELISA assays were available for seven of the 41 differentially abundant proteins namely serum α-amyloid (SAA), complement 4 (C4), fibrinogen, complement 1q (C1q), complement 3 (C3), Heat Shock Protein 60 (HSP60), and Heat Shock Protein 70 (HSP70).
Table 2.
Proteins which are increased in patients with ACR in comparison with No-ACR patients.
| Protein | Fold increase |
|---|---|
| Ubiquitin-conjugating enzyme E2 | 6.28 ± 0.35 |
| Heat shock protein HSP60 | 5.83 ± 0.27 |
| Nuclear factor of activated T-cells (T cell transcription factor NFAT1) | 4.87 ± 0.26 |
| Ubiquitin | 4.19 ± 0.34 |
| Heat shock protein HSP70 | 3.42 ± 0.12 |
| Zinc finger protein 135 | 2.88 ± 0.18 |
| Complement component 1q | 2.54 ± 0.23 |
| Nuclear factor of activated T-cells 2 isoform B | 2.51 ± 0.16 |
| FK506 binding protein 10 precursor | 2.35 ± 0.11 |
| HSP-C078 | 2.21 ± 0.23 |
| UDP-glucose pyrophosphorylase 2 | 2.17 ± 0.22 |
| Complement C3 | 2.04 ± 0.48 |
| Alpha-fibrinogen precursor | 2.04 ± 0.48 |
| Sulfated glycoprotein-2 | 2.00 ± 0.32 |
| Serum amyloid A1 | 1.99 ± 0.21 |
| Glyceraldehyde 3-phosphate dehydrogenase | 1.94 ± 0.14 |
| Complement component 4A | 1.90 ± 0.32 |
| Complement component 4B | 1.87 ± 0.32 |
| Proapo-A-I protein | 1.85 ± 0.11 |
| Retinol Binding Protein | 1.72 ± 0.26 |
| A Chain A, Crystal Structure Of A Serpin:protease Complex | 1.69 ± 0.11 |
| Leucine-rich alpha-2-glycoprotein | 1.55 ± 0.21 |
| Zinc-alpha-2-glycoprotein precursor | 1.55 ± 0.21 |
| RBP4 gene product | 1.52 ± 0.26 |
| Myeloid cell surface antigen CD33 precursor | 1.51 ± 0.05 |
| Alpha-2-glycoprotein 1, zinc | 1.51 ± 0.05 |
| FK506 binding protein 10 | 1.48 ± 0.07 |
| AMBP protein precursor | 1.45 ± 0.36 |
Table 3.
Proteins which are decreased in patients with ACR in comparison with NO-ACR patients.
| Protein | Fold decrease |
|---|---|
| Human Apolipoprotein C-I | 0.80 ± 0.02 |
| Nuclear protein | 0.72 ± 0.11 |
| Zn-alpha2-glycoprotein | 0.63 ± 0.12 |
| Apolipoprotein B-100 | 0.46 ± 0.09 |
| Apolipoprotein-H | 0.46 ± 0.03 |
| Serine (or cysteine) proteinase inhibitor | 0.36 ± 0.21 |
| Ribosomal protein L15 | 0.31 ± 0.02 |
| Apolipoprotein D | 0.26 ± 0.32 |
| Adenylate kinase 7 | 0.26 ± 0.02 |
| Plasma protease C1 inhibitor precursor (C1 Inh) | 0.23 ± 0.11 |
| Beta-2-glycoprotein I precursor | 0.21 ± 0.04 |
| Insulin-like growth factor binding protein | 0.17 ± 0.06 |
| Ribonucleoprotein autoantigen 60 kd subunit | 0.12 ± 0.04 |
Validation results
The demographic and clinical characteristics of the validation group were summarized in Table 4. Quantitative ELISA results for C4 and C1q are shown in Figures 1 and 2. The means of both C4 and C1q were significantly different between ACR and No-ACR groups (p=0.0001 and p=0.0183 respectively). Using simple logistic regression model for the individual proteins, C4 (p= 0.0024) and C1q (p= 0.0341) were both found to be predictive for ACR. When these two proteins were entered into a multiple logistic regression model, both were independent predictors for ACR (p=0.0015 for C4 and p=0.0092 for C1q) (Table 5). None of the other measured proteins improved the predictivity.
Table 4.
Demographic and clinical characteristics of patients in the validation groups.
| ACR (n= 25) | No-ACR (n=21) | |
|---|---|---|
| Age (median (range))* | 52 (19–72) | 57 (40–70) |
| Male/female | 13/12 | 13/8 |
| Liver disease** | ||
| HCV | 3 | 2 |
| Alcohol | 4 | 4 |
| NASH | 0 | 3 |
| PSC | 5 | 3 |
| PBC | 2 | 2 |
| AIH | 2 | 0 |
| Cholangiocarcinoma | 2 | 6 |
| Hepatocellular carcinoma | 2 | 3 |
| Acute fulminant hepatitis | 3 | 0 |
| Wilson’s disease | 1 | 0 |
| Amyloidosis | 2 | 0 |
| Oxalosis | 1 | 0 |
| Hepatic artery thrombosis | 2 | 1 |
| A-1 Antitrypsin deficiency | 0 | 1 |
| Cryptogenic | 0 | 1 |
P= 0.143
Some patients have more than one diagnosis
Figure 1. C4 serum level in Acute Cellular Rejection and Non-rejection groups.
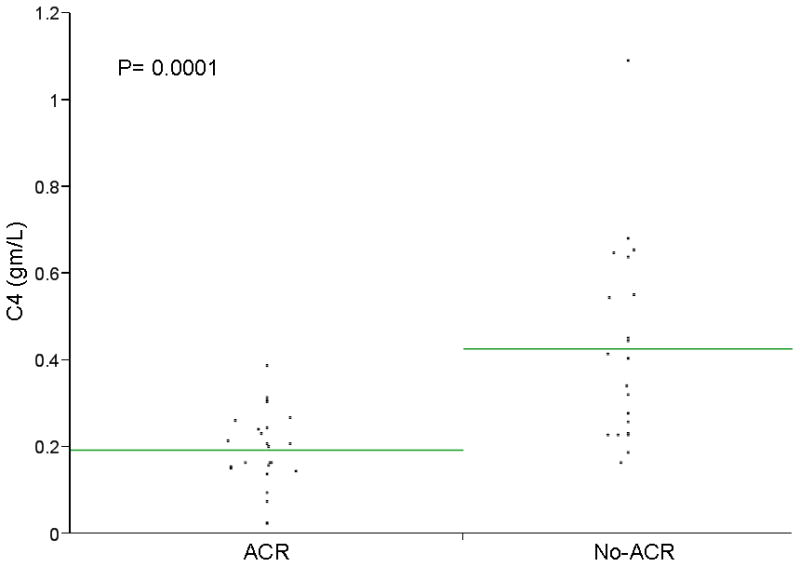
A scatter plot of individual values for C4 and C1q are shown. Mean C4 levels were significantly lower among patients with ACR (p=0.0001). Using a cut off value for C4 of ≤0.31gm/L, C4 had a sensitivity of 97%, a specificity of 62%, a positive predictive value of 74% and a negative predictive value of 94% in identifying patients with ACR. Mean C1q was also significantly lower among patients with ACR (p=0.0183).
Figure 2. Serum C1q level in acute cellular rejection and non-acute cellular rejection groups.
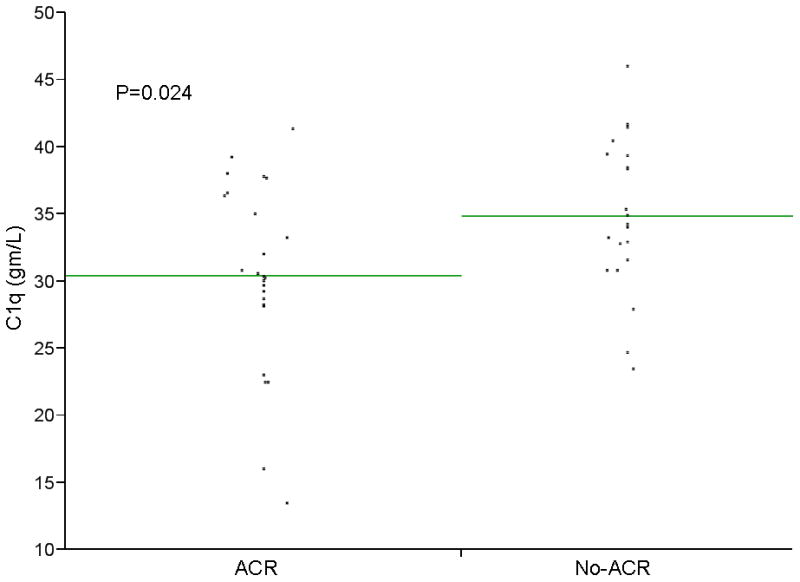
A scatter plot of individual values for C4 and C1q are shown. Mean C4 levels were significantly lower among patients with ACR (p=0.0001). Using a cut off value for C4 of ≤0.31gm/L, C4 had a sensitivity of 97%, a specificity of 62%, a positive predictive value of 74% and a negative predictive value of 94% in identifying patients with ACR. Mean C1q was also significantly lower among patients with ACR (p=0.0183).
Table 5.
Multiple logistic regression for C4 and C1q
| Term | Estimate | Std Error | ChiSquare | Prob>ChiSq |
|---|---|---|---|---|
| Intercept | 14.6306791 | 4.7010899 | 9.69 | 0.0019* |
| C4 gm/L | −18.242127 | 5.730138 | 10.13 | 0.0015* |
| C1q | −0.2810648 | 0.1078881 | 6.79 | 0.0092* |
Statistically significant.
Using a cut off value of ≤0.31gm/L, C4 had a sensitivity of 97%, a specificity of 62%, positive predictive value of 74% and a negative predictive value of 94%. We found that adding elevated ALT would improve the specificity of C4 without significant change in the sensitivity. A combination of C4 ≤ 0.31 gm/L and alanine aminotransferase (ALT) ≥70 IU/ml (two times the upper limit of normal) had a sensitivity of 96%, a specificity of 81%, a positive predictive value of 86% and a negative predictive value of 94%. The area under the receiver operating characteristic curve was 0.88. 0.88. C1q was also significantly predictive of ACR. C1q levels were, however, less sensitive than C4 (56% vs. 97%) and more specific (86% vs 62%). Combining the two markers, C4 and C1q, significantly lowers the sensitivity of C4.
Discussion
The first important finding of this exploratory study is that ACR is associated with the differential abundance of a distinct set of proteins that can be measured in the serum proteome at or before the time ACR is suspected or apparent clinically. Only 41 (0.14%) of identified serum proteins were differentially abundant in patients with ACR when compared to controls in the first (proteomic) phase of experiments. Because we removed high abundance serum proteins, which are typically proteins whose function depends on their presence in serum, our analysis was able to detect proteins that were present in serum on a transient basis, (e.g. due to cell destruction, or secreted proteins) such as cytokines, receptor ligands and hormones, that rely on serum for transportation to cells at anatomically remote sites. The proteins that we found to be differentially abundant in ACR are diverse in known functions, including transcription factors, stress response, protein degradation, lipid metabolism, complement activation and immune function, cell adhesion, growth factors and signal transduction. The identification of a distinct serum proteomic signature for ACR, which includes proteins not previously associated with ACR, raises the possibility of new diagnostic and therapeutic approaches to the management of immunosuppression in LT and, perhaps, other organ transplant recipients. The second important finding of our experiments was that, in our exploratory analysis of a validation cohort, patients with ACR could be identified using a rapid turnaround and inexpensive ELISA measurement of C4 component of complement.
Although there are, so far as we are aware, no previously published proteomic analyses of serum or tissue in ACR in liver transplant recipients, urine proteomic analyses of acute rejection in renal transplant recipients,(19–21) identified urinary beta 2-microglobulin as potential biomarkers for ACR.(22) The abundance of alpha B-crystallin and tropomyosin are increased in serum during ACR in heart transplant recipients.(23) Direct comparison of our results is thus difficult, probably reflecting the difficulty in measuring the abundance of low concentration serum proteins. A single mass spectrum is limited to a dynamic range of <104–5. Serum protein, however, has a dynamic range of >1010, ranging from albumin at >4.5 g/dL to the cytokines at 1–10 pg/mL. As LC-MS analysis has absolute detection limits in the attomolar-zeptomolar range(24, 25)the sensitivity of serum proteomic analysis is limited not by the lower limits of detection of the instrument but by the presence of the high abundance proteins. We applied a high abundance protein depletion method(13) to circumvent this limitation. We selectively removed high abundance serum proteins using high-performance liquid chromatography, removing ~94% of total serum proteins. All of the differentially abundant proteins in our analysis were thus present only in small amounts in serum. In contrast to previous proteomic analyses of serum, we employed the iTRAQ peptide label, eliminating the dependence on relatively non-abundant cysteine containing peptides intrinsic to ICAT-based methods,(26) and yields labeled peptides which are identical in mass and which also produce strong, diagnostic, low-mass MS/MS signature ions.(27, 28) This difference in labeling strategy allows the tagging of most tryptic peptides, simplifying analysis and increasing analytical precision.(17) While we are certain that there are still many ultra-low abundance proteins that we were not able to differentially quantify, our protein depletion/iTRAQ approach yielded considerable novel information.
The diverse function of the proteins found to be differentially abundant in ACR suggests a complex basis for the serum protein signature. A comprehensive review of the biological function of the 28 proteins that were differentially abundant in ACR is beyond the scope of this manuscript. The overall pattern of differentially abundant proteins suggests, however, that patients with ACR have increased immune activation when compared to the controls who had HCV infection in the absence of ACR. Reported peripheral blood markers of immune activation include IL-2,(29, 30) soluble IL-2R,(29, 31) IL-6,(31, 32) IL- 7,(33) IL-8,(32) IFN-gamma,(29) soluble ICAM-1,(34) and soluble major histocompatibility complex antigens.(34) None of these were identified as differentially abundant in our analysis. This does not imply that levels of these immune activation markers are not elevated in ACR, but rather that their levels were not measurably different from those seen in the serum of our control group - liver transplant recipients with HCV infection. In contrast, the mediators of immune activation that we found to be differentially overabundant in the ACR group were: T-cell transcription factor NFAT-1, heat shock proteins (HSP) 60 and 70, complement components 1q, 3, and 4 and CD33. The differential abundance of these proteins merits individual consideration.
NFATs 1, 2 and 4 are induced by calcineurin and transactivate cytokine genes that regulate proliferative responses of T cells.(35) The major immunosuppressive action of calcineurin inhibitors is prevention of nuclear translocation NFAT.(36) The relative increased abundance of NFAT-1 in our ACR group may have conferred resistance to calcineurin inhibition. HSPs are a ubiquitously expressed family of molecules. Immune reactivity to HSPs has been implicated in the pathogenesis of ACR. Anti-HSP immune reactivity is thought to be important in transplant rejection responses. Of particular interest is that proliferation to Hsp60 and Hsp70, both relatively overabundant in ACR in our analysis, has been significantly associated with rejection.(37, 38) HSP 60 and 70 gene expression has been reported to be increased in cardiac allografts during ACR.(23) Complement components 1q, 3, 4A and 4B were all overexpressed in ACR in our ACR group. T-cells, B-cells and antigen-presenting cells (APCs) express complement receptors that respond to stimulation by split complement products, including C4a and C4b. T-cell and APC cell surfaces also bear several complement control proteins that recognise covalently bound fragments of C4 that are capable of signal transduction with subsequent T-cell and APC activation in ACR.(39–41) CD33 was also overexpressed in ACR in our experiments. CD33 is a 67-kd glycoprotein that, although found predominantly on myeloid cells,(42, 43) has also been reported on dendritic cells, natural killer (NK) cells, and in vitro-expanded T cells.(44–47) Although there are no reports on the role of CD33+ cells in ACR in liver transplantation, following bone marrow stem cell transplantation allo-responses against hemopoietic progenitor cells (HPC) bearing CD33, cause graft rejection.(48) Whether the overexpression of CD33 contributed to alloimmunity or was a marker of T-cell induction in patients with ACR in our study cannot be ascertained. Ubiquitin was also greatly overexpressed in the ACR group in our experiments. Ubiquitin is important in regulating signal transduction and gene expression of a variety of proteins, including TGF[beta]/SMAD, STAT, Jun, and p53.(49–52) The impact of lower circulating levels of ubiquitin is hard to predict as the actions of ubiquitin are so diverse and occur intracellularly. We can find no reports of differential abundance of circulating ubiquitin levels in ACR. The zinc finger transcription factor Kruppel-like factor 4 (KLF4), a potent negative regulator of cell proliferation, however, is inhibited by extracellular ubiquitin.(53) Taken together, the relative overabundance of these proteins in ACR in our experiments may provide a novel index of immune activation.
Interpretation of the relative overabundance of zinc finger protein 135 is similarly difficult. Although zinc fingers have diverse effects, including stimulation of interleukin-2 independent growth of T-cells,(54–56) a role of zinc finger protein 135 in alloimmunity has not been described.
FKBP65 (65-kDa FK506-binding protein), which, along with its precursor protein was relatively overabundant in ACR, belongs to the immunophilin family of intracellular proteins.(57) FKBP65, assists in cis-trans isomerization of X-proline bonds in newly synthesized proteins and is upregulated in response to tissue injury.(58) Upregulation of FKBP65 may have been on the basis of increased non-hepatic tissue injury in ACR.
Several proteins that were relatively underabundant in the ACR group in our experiments are also of particular interest: IGFBP-1, alpha-fibrinogen, and adenylate kinase 7. Hepatic IGFBP-1 synthesis is under the regulation of mTOR, an important regulator of T-cell signaling cascades and T-cell responsiveness.(59, 60) Lower IGFBP-1 levels suggests lower mTOR levels. If lower serum IGFBP-1 are indicative of lower intracellular mTOR levels, lower serum IGFBP-1 levels may be a surrogate of relatively greater baseline immune activation in the ACR group.
It would be reasonable to consider whether the differential abundance of proteins that we observed in the proteomic phase of experiments was simply on the basis of inflammation, rather than ACR. We had considered this possibility prior to conducting the study and chose controls, age and gender matched patients with HCV infection also undergoing protocol liver day 7 post-LT liver biopsies, that were matched for biochemical profiles and degree of necroinflammation. Both the ACR and no-ACR groups had HCV infection. Levels of immunosuppression were also similar between study groups by design. Although parameters of immunosuppression were similar between groups it is possible, indeed likely, that the physiological levels of immunosuppression were, in fact, different between groups as evidenced by the development of ACR. This raises the possibility that indices of immune activation may be more predictive of ACR than twelve hour tacrolimus troughs, which do not take into account differences in baseline innate or adaptive immunity between patients. We did not compare the serum proteome of patients who had biochemical abnormalities or biopsy abnormalities due etiologies other than HCV +/− ACR, such as biliary or vascular abnormalities, and our results may should not be extrapolated to those conditions. A further limitation of our analysis is that we were only able to study the serum proteome and perform ELISA-based quantitation of differentially abundant proteins at single time point. A longitudinal analysis may yield different/new information.
Mass spectrometry proteomic analysis is technically challenging and quite slow. Measurement of protein abundance by ELISA, in contrast, is simple, inexpensive, fast and thus potentially clinically applicable. We selected 7 of the 41 differentially abundant proteins (the ones for which ELISA kits are currently available) to validate using ELISA-based protein quantification in a validation cohort of patients. These proteins were serum α-amyloid (SAA), complement 4 (C4), fibrinogen, complement 1q (C1q), complement 3 (C3), Heat Shock Protein 60 (HSP60), and Heat Shock Protein 70 (HSP70). Based on our proteomic analysis, we believe that other proteins may also be of value in distinguishing ACR from non-ACR patients. Our validation study showed that C4 levels distinguished between ACR and non-ACR with high sensitivity (97%) but modest specificity (62%). Adding ALT substantially improved the specificity (from 62% to 81%) without changing the sensitivity (from 97% to 96%). Interestingly, the C4 was low in all but one of our 25 ACR patients, although it was one of the 28 up-regulated proteins in our proteomic analysis. The explanation of this finding is uncertain. However, the forms of C4 that were found to be high in serum in our proteomic analysis are not the same as those which we measured in the serum by ELISA. In the proteomic analysis we measured C4 split products C4a and C4b, while in the ELISA we measured intact C4. When the complement cascade is activated intact C4 is cleaved, generating increased C4 split products and subsequent decrease in the level of intact C4. C4d, the activated component of C4, is a central factor of the classical complement pathway. Since it was first described by Feuch(61), it has been established as a marker of acute humoral as well as chronic rejection in kidney transplant patients.(62, 63) In liver, C4d deposition in association with B lymphocyte and plasma cells has been described in acute allograft rejection.(64–67) This suggests that the humoral immune response may play a role in acute allograft rejection in liver.(64) Both C4d(68) and fibrinogen(69) have been described as potential markers for acute rejection after heart transplantation. The high sensitivity of serum C4 in differentiating between ACR and No-ACR patients, with a sensitivity of 97%, positive predictive value of 74% and a negative predictive value of 94%, suggests that C4 levels may have value as a screening tool for identifying patients who may have ACR. Obtaining liver biopsies in patients with C4 levels >0.31gm/L is highly unlikely to identify ACR. The concentration of C1q was also significantly lower in ACR group compared with no-ACR group, although much less sensitive than C4. Both C4 and C1q were independent predictor of ACR in the multiple logistic regression model. Adding elevated ALT improves the specificity of C4 without significant change in the sensitivity. A combination of C4 ≤ 0.31 gm/L and alanine aminotransferase (ALT) ≥70 IU/ml (two times the upper limit of normal) had a sensitivity of 96%, a specificity of 81%, a positive predictive value of 86% and a negative predictive value of 94%. The area under the receiver operating characteristic curve was 0.88. The relatively small sample size, single center nature and the limitation of study subjects, by design, to recipients with HCV infection all limit the applicability of our results. Our goal was to stimulate further, more comprehensive studies in this important field–noninvasive diagnosis of rejection.
In conclusion, serum C4 is a relatively non-invasive, inexpensive, easy to measure and quick marker that, in these preliminary studies, accurately identifies patients with histological ACR after liver transplantation. These results need to be prospectively validated on a larger and more diverse group of recipients before any recommendation can be made for clinical use. Whether C4 levels have other potential uses, e.g. to confirm a diagnosis of ACR when liver biopsy is equivocal or to monitor the immune activation, should be the subject of further research.
Acknowledgments
This work has been supported by Public Health Service grant NIDDK RO1 DK069757-01 and GCRC RR00585.
References
- 1.Adam R, McMaster P, O'Grady JG, Castaing D, Klempnauer JL, Jamieson N, Neuhaus P, et al. Evolution of liver transplantation in Europe: report of the European Liver Transplant Registry. Liver Transpl. 2003;9:1231–1243. doi: 10.1016/j.lts.2003.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Roberts MS, Angus DC, Bryce CL, Valenta Z, Weissfeld L. Survival after liver transplantation in the United States: a disease-specific analysis of the UNOS database. Liver Transpl. 2004;10:886–897. doi: 10.1002/lt.20137. [DOI] [PubMed] [Google Scholar]
- 3.Lee KW, Cameron AM, Maley WR, Segev DL, Montgomery RA. Factors affecting graft survival after adult/child split-liver transplantation: analysis of the UNOS/OPTN data base. Am J Transplant. 2008;8:1186–1196. doi: 10.1111/j.1600-6143.2008.02211.x. [DOI] [PubMed] [Google Scholar]
- 4.Wiesner RH, Demetris AJ, Belle S, Seaberg EC, Lake JR, Zetterman RK, Everhart JE, et al. Acute hepatic allograft rejection: Incidence, risk factors and impact on outcome. Hepatology. 1998;28:638–645. doi: 10.1002/hep.510280306. [DOI] [PubMed] [Google Scholar]
- 5.Charlton M, Seaberg E. Impact of immunosuppression and acute rejection on recurrence of hepatitis C: results of the National Institute of Diabetes and Digestive and Kidney Diseases Liver Transplantation Database. Liver Transplantation & Surgery. 1999;5:107–114. doi: 10.1053/JTLS005s00107. [DOI] [PubMed] [Google Scholar]
- 6.Shaked A, Ghobrial RM, Merion RM, Shearon TH, Emond JC, Fair JH, Fisher RA, et al. Incidence and severity of acute cellular rejection in recipients undergoing adult living donor or deceased donor liver transplantation. Am J Transplant. 2009;9:301–308. doi: 10.1111/j.1600-6143.2008.02487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klintmalm GB, Washburn WK, Rudich SM, Heffron TG, Teperman LW, Fasola C, Eckhoff DE, et al. Corticosteroid-free immunosuppression with daclizumab in HCV(+) liver transplant recipients: 1-year interim results of the HCV-3 study. Liver Transpl. 2007;13:1521–1531. doi: 10.1002/lt.21182. [DOI] [PubMed] [Google Scholar]
- 8.Demetris A, Adams D, Bellamy C, Blakolmer K, Clouston A, Dhillon AP, Fung J, et al. Update of the International Banff Schema for Liver Allograft Rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An International Panel. 2000;31:792–799. doi: 10.1002/hep.510310337. [DOI] [PubMed] [Google Scholar]
- 9.Regev A, Molina E, Moura R, Bejarano PA, Khaled A, Ruiz P, Arheart K, et al. Reliability of histopathologic assessment for the differentiation of recurrent hepatitis C from acute rejection after liver transplantation. Liver Transpl. 2004;10:1233–1239. doi: 10.1002/lt.20245. [DOI] [PubMed] [Google Scholar]
- 10.Sawada T, Shimizu A, Kubota K, Fuchinoue S, Teraoka S. Lobular damage caused by cellular and humoral immunity in liver allograft rejection. Clin Transplant. 2005;19:110–114. doi: 10.1111/j.1399-0012.2004.00310.x. [DOI] [PubMed] [Google Scholar]
- 11.Thuluvath PJ, Krok KL. Noninvasive markers of fibrosis for longitudinal assessment of fibrosis in chronic liver disease: are they ready for prime time? Am J Gastroenterol. 2006;101:1497–1499. doi: 10.1111/j.1572-0241.2005.00304.x. [DOI] [PubMed] [Google Scholar]
- 12.Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, Pudifin DJ. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523–525. doi: 10.1016/s0140-6736(86)90883-4. [DOI] [PubMed] [Google Scholar]
- 13.Bjorhall K, Miliotis T, Davidsson P. Comparison of different depletion strategies for improved resolution in proteomic analysis of human serum samples. Proteomics. 2005;5:307–317. doi: 10.1002/pmic.200400900. [DOI] [PubMed] [Google Scholar]
- 14.Washburn MP, Wolters D, Yates JR., 3rd Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 15.Petricoin EF, Ardekani AM, Hitt BA, Levine PJ, Fusaro VA, Steinberg SM, Mills GB, et al. Use of proteomic patterns in serum to identify ovarian cancer. 2002. [see comment] [DOI] [PubMed] [Google Scholar]
- 16.Jacobs JM, Adkins JN, Qian WJ, Liu T, Shen Y, Camp DG, 2nd, Smith RD. Utilizing human blood plasma for proteomic biomarker discovery. J Proteome Res. 2005;4:1073–1085. doi: 10.1021/pr0500657. [DOI] [PubMed] [Google Scholar]
- 17.DeSouza L, Diehl G, Rodrigues MJ, Guo J, Romaschin AD, Colgan TJ, Siu KW. Search for cancer markers from endometrial tissues using differentially labeled tags iTRAQ and cICAT with multidimensional liquid chromatography and tandem mass spectrometry. Journal of Proteome Research. 2005;4:377–386. doi: 10.1021/pr049821j. [DOI] [PubMed] [Google Scholar]
- 18.DeSouza L, Diehl G, Yang EC, Guo J, Rodrigues MJ, Romaschin AD, Colgan TJ, et al. Proteomic analysis of the proliferative and secretory phases of the human endometrium: protein identification and differential protein expression. 2005;5:270–281. doi: 10.1002/pmic.200400920. [DOI] [PubMed] [Google Scholar]
- 19.O'Riordan E, Orlova TN, Mei JJ, Butt K, Chander PM, Rahman S, Mya M, et al. Bioinformatic analysis of the urine proteome of acute allograft rejection. 2004. [DOI] [PubMed] [Google Scholar]
- 20.Clarke W, Silverman BC, Zhang Z, Chan DW, Klein AS, Molmenti EP. Characterization of renal allograft rejection by urinary proteomic analysis. 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaub S, Rush D, Wilkins J, Gibson IW, Weiler T, Sangster K, Nicolle L, et al. Proteomic-based detection of urine proteins associated with acute renal allograft rejection. Journal of the American Society of Nephrology. 2004;15:219–227. doi: 10.1097/01.asn.0000101031.52826.be. [DOI] [PubMed] [Google Scholar]
- 22.Schaub S, Wilkins JA, Antonovici M, Krokhin O, Weiler T, Rush D, Nickerson P. Proteomic-based identification of cleaved urinary beta2-microglobulin as a potential marker for acute tubular injury in renal allografts. 2005. [DOI] [PubMed] [Google Scholar]
- 23.Borozdenkova S, Westbrook JA, Patel V, Wait R, Bolad I, Burke MM, Bell AD, et al. Use of proteomics to discover novel markers of cardiac allograft rejection. 2004. Apr, [DOI] [PubMed] [Google Scholar]
- 24.Martin SE, Shabanowitz J, Hunt DF, Marto JA. Subfemtomole MS and MS/MS peptide sequence analysis using nano-HPLC micro-ESI fourier transform ion cyclotron resonance mass spectrometry. Analytical Chemistry. 2000;72:4266–4274. doi: 10.1021/ac000497v. [DOI] [PubMed] [Google Scholar]
- 25.Belov ME, Gorshkov MV, Udseth HR, Anderson GA, Smith RD. Zeptomole-sensitivity electrospray ionization--Fourier transform ion cyclotron resonance mass spectrometry of proteins. Analytical Chemistry. 2000;72:2271–2279. doi: 10.1021/ac991360b. [DOI] [PubMed] [Google Scholar]
- 26.Gygi SP, Rist B, Gerber SA, Turecek F, Gelb MH, Aebersold R. Quantitative analysis of complex protein mixtures using isotope-coded affinity tags. 1999;17:994–999. doi: 10.1038/13690. [DOI] [PubMed] [Google Scholar]
- 27.Chong PK, Gan CS, Pham TK, Wright PC. Isobaric tags for relative and absolute quantitation (iTRAQ) reproducibility: Implication of multiple injections. Journal of Proteome Research. 2006;5:1232–1240. doi: 10.1021/pr060018u. [DOI] [PubMed] [Google Scholar]
- 28.Keshamouni VG, Michailidis G, Grasso CS, Anthwal S, Strahler JR, Walker A, Arenberg DA, et al. Differential protein expression profiling by iTRAQ-2DLC-MS/MS of lung cancer cells undergoing epithelial-mesenchymal transition reveals a migratory/invasive phenotype. Journal of Proteome Research. 2006;5:1143–1154. doi: 10.1021/pr050455t. [DOI] [PubMed] [Google Scholar]
- 29.Simpson MA, Madras PN, Monaco AP. Immunologic heterogeneity among potential transplant recipients. Prospects for predicting immune responses to allografts with in vitro tests. [Review] [105 refs] Clinics in Laboratory Medicine. 1991;11:733–762. [PubMed] [Google Scholar]
- 30.Toyoda M, Galfayan K, Wachs K, Czer L, Jordan SC. Immunologic monitoring of OKT3 induction therapy in cardiac allograft recipients. Clinical Transplantation. 1995;9:472–480. [PubMed] [Google Scholar]
- 31.Deng MC, Erren M, Kammerling L, Gunther F, Kerber S, Fahrenkamp A, Assmann G, et al. The relation of interleukin-6, tumor necrosis factor-alpha, IL-2, and IL-2 receptor levels to cellular rejection, allograft dysfunction, and clinical events early after cardiac transplantation. 1995;60:1118–1124. doi: 10.1097/00007890-199511270-00011. [DOI] [PubMed] [Google Scholar]
- 32.Kimball PM, Radovancevic B, Isom T, Spickard A, Frazier OH. The paradox of cytokine monitoring-predictor of immunologic activity as well as immunologic silence following cardiac transplantation. 1996;61:909–915. doi: 10.1097/00007890-199603270-00012. [DOI] [PubMed] [Google Scholar]
- 33.Wu CJ, Kurbegov D, Lattin B, Burchard E, Finkle C, Valantine H, Billingham ME, et al. Cytokine gene expression in human cardiac allograft recipients. Transplant Immunology. 1994;2:199–207. doi: 10.1016/0966-3274(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 34.Satoh S, Thomson AW, Nakamura K, Warty V, Todo S. Circulating ICAM-1, E-selectin, IL-2 receptor, and HLA class I in human small bowel, liver, and small bowel-plus-liver transplant recipients. 1995;60:558–562. doi: 10.1097/00007890-199509270-00007. [DOI] [PubMed] [Google Scholar]
- 35.Venkatesh N, Feng Y, Decker B, Yacono P, Golan D, Mitchison T, McKeon F. Chemical genetics to identify NFAT inhibitors: potential of targeting calcium mobilization in immunosuppression. 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy LL, Hughes CC. Endothelial cells stimulate T cell NFAT nuclear translocation in the presence of cyclosporin A: involvement of the wnt/glycogen synthase kinase-3 beta pathway. 2002. [DOI] [PubMed] [Google Scholar]
- 37.Granja C, Moliterno RA, Ferreira MS, Fonseca JA, Kalil J, Coelho V. T-cell autoreactivity to Hsp in human transplantation may involve both proinflammatory and regulatory functions. 2004. [DOI] [PubMed] [Google Scholar]
- 38.Birk OS, Gur SL, Elias D, Margalit R, Mor F, Carmi P, Bockova J, et al. The 60-kDa heat shock protein modulates allograft rejection. 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lakkis FG. Transplant rejection: mind your T-cell language. [comment] 2002;8:1043–1044. doi: 10.1038/nm1002-1043a. [DOI] [PubMed] [Google Scholar]
- 40.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. [see comment] 2002;8:582–587. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 41.Marsh JE, Farmer CK, Jurcevic S, Wang Y, Carroll MC, Sacks SH. The allogeneic T and B cell response is strongly dependent on complement components C3 and C4. 1915;72:1310–1318. doi: 10.1097/00007890-200110150-00022. [DOI] [PubMed] [Google Scholar]
- 42.Griffin JD, Linch D, Sabbath K, Larcom P, Schlossman SF. A monoclonal antibody reactive with normal and leukemic human myeloid progenitor cells. Leukemia Research. 1984;8:521–534. doi: 10.1016/0145-2126(84)90001-8. [DOI] [PubMed] [Google Scholar]
- 43.Andrews RG, Torok-Storb B, Bernstein ID. Myeloid-associated differentiation antigens on stem cells and their progeny identified by monoclonal antibodies. Blood. 1983;62:124–132. [PubMed] [Google Scholar]
- 44.Nakamura Y, Noma M, Kidokoro M, Kobayashi N, Takei M, Kurashima S, Mukaiyama T, et al. Expression of CD33 antigen on normal human activated T lymphocytes. Blood. 1994;83:1442–1443. [PubMed] [Google Scholar]
- 45.Thomas R, Davis LS, Lipsky PE. Comparative accessory cell function of human peripheral blood dendritic cells and monocytes. 1993;151:6840–6852. [PubMed] [Google Scholar]
- 46.Handgretinger R, Schafer HJ, Baur F, Frank D, Ottenlinger C, Buhring HJ, Niethammer D. Expression of an early myelopoietic antigen (CD33) on a subset of human umbilical cord blood-derived natural killer cells. Immunology Letters. 1993;37:223–228. doi: 10.1016/0165-2478(93)90034-y. [DOI] [PubMed] [Google Scholar]
- 47.Davey FR, Mick R, Nelson DA, MacCallum J, Sobol RE, Royston I, Cuttner J, et al. Morphologic and cytochemical characterization of adult lymphoid leukemias which express myeloid antigen. Leukemia. 1988;2:420–426. [PubMed] [Google Scholar]
- 48.Raptis A, Clave E, Mavroudis D, Molldrem J, Van RF, Barrett AJ. Polymorphism in CD33 and CD34 genes: a source of minor histocompatibility antigens on haemopoietic progenitor cells? 1998. [DOI] [PubMed] [Google Scholar]
- 49.Wicks SJ, Haros K, Maillard M, Song L, Cohen RE, Dijke PT, Chantry A. The deubiquitinating enzyme UCH37 interacts with Smads and regulates TGF-beta signalling. 2001;24:8080–8084. doi: 10.1038/sj.onc.1208944. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka T, Soriano MA, Grusby MJ. SLIM is a nuclear ubiquitin E3 ligase that negatively regulates STAT signaling. Immunity. 2005;22:729–736. doi: 10.1016/j.immuni.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 51.Gao M, Labuda T, Xia Y, Gallagher E, Fang D, Liu YC, Karin M. Jun turnover is controlled through JNK-dependent phosphorylation of the E3 ligase Itch. 1908;306:271–275. doi: 10.1126/science.1099414. [DOI] [PubMed] [Google Scholar]
- 52.Rajendra R, Malegaonkar D, Pungaliya P, Marshall H, Rasheed Z, Brownell J, Liu LF, et al. Topors functions as an E3 ubiquitin ligase with specific E2 enzymes and ubiquitinates p53. 1927;279:36440–36444. doi: 10.1074/jbc.C400300200. [DOI] [PubMed] [Google Scholar]
- 53.Chen ZY, Wang X, Zhou Y, Offner G, Tseng CC. Destabilization of Kruppel-like factor 4 protein in response to serum stimulation involves the ubiquitin-proteasome pathway. 2005. [DOI] [PubMed] [Google Scholar]
- 54.Zornig M, Schmidt T, Karsunky H, Grzeschiczek A, Moroy T. Zinc finger protein GFI-1 cooperates with myc and pim-1 in T-cell lymphomagenesis by reducing the requirements for IL-2. 1996;12:1789–1801. [PubMed] [Google Scholar]
- 55.Gilks CB, Bear SE, Grimes HL, Tsichlis PN. Progression of interleukin-2 (IL-2)-dependent rat T cell lymphoma lines to IL-2-independent growth following activation of a gene (Gfi-1) encoding a novel zinc finger protein. 1993;13:1759–1768. doi: 10.1128/mcb.13.3.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van L, Verbeek S, Scheijen B, Wientjens E, van d, Berns A. Identification of cooperating oncogenes in E mu-myc transgenic mice by provirus tagging. [see comment] 1991;65:737–752. doi: 10.1016/0092-8674(91)90382-9. [DOI] [PubMed] [Google Scholar]
- 57.Schreiber SL. Chemistry and biology of the immunophilins and their immunosuppressive ligands. [Review] [57 refs] 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 58.Patterson CE, Abrams WR, Wolter NE, Rosenbloom J, Davis EC. Developmental regulation and coordinate reexpression of FKBP65 with extracellular matrix proteins after lung injury suggest a specialized function for this endoplasmic reticulum immunophilin. 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. 2001. [DOI] [PubMed] [Google Scholar]
- 60.Sarbassov dD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. 2005. [Review] [86 refs] [DOI] [PubMed] [Google Scholar]
- 61.Feucht HE, Felber E, Gokel MJ, Hillebrand G, Nattermann U, Brockmeyer C, Held E, et al. Vascular deposition of complement-split products in kidney allografts with cell-mediated rejection. Clin Exp Immunol. 1991;86:464–470. doi: 10.1111/j.1365-2249.1991.tb02954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Crespo M, Pascual M, Tolkoff-Rubin N, Mauiyyedi S, Collins AB, Fitzpatrick D, Farrell ML, et al. Acute humoral rejection in renal allograft recipients: I. Incidence, serology and clinical characteristics. Transplantation. 2001;71:652–658. doi: 10.1097/00007890-200103150-00013. [DOI] [PubMed] [Google Scholar]
- 63.Crespo M, Lozano M, Sole M, Mila J, Esforzado N, Martorell J, Oppenheimer F. Diagnosis and treatment of acute humoral rejection after kidney transplantation: preliminary experience. Transplant Proc. 2003;35:1677–1678. doi: 10.1016/s0041-1345(03)00620-1. [DOI] [PubMed] [Google Scholar]
- 64.Krukemeyer MG, Moeller J, Morawietz L, Rudolph B, Neumann U, Theruvath T, Neuhaus P, et al. Description of B lymphocytes and plasma cells, complement, and chemokines/receptors in acute liver allograft rejection. Transplantation. 2004;78:65–70. doi: 10.1097/01.tp.0000132324.14207.8b. [DOI] [PubMed] [Google Scholar]
- 65.Lorho R, Turlin B, Aqodad N, Triki N, de Lajarte-Thirouard AS, Camus C, Lakehal M, et al. C4d: a marker for hepatic transplant rejection. Transplant Proc. 2006;38:2333–2334. doi: 10.1016/j.transproceed.2006.06.120. [DOI] [PubMed] [Google Scholar]
- 66.Bu X, Zheng Z, Wang C, Yu Y. Significance of C4d deposition in the follicular lymphoma and MALT lymphoma and their relationship with follicular dendritic cells. Pathol Res Pract. 2007;203:163–167. doi: 10.1016/j.prp.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 67.Jain A, Ryan C, Mohanka R, Orloff M, Abt P, Romano J, Bryan L, et al. Characterization of CD4, CD8, CD56 positive lymphocytes and C4d deposits to distinguish acute cellular rejection from recurrent hepatitis C in post-liver transplant biopsies. Clin Transplant. 2006;20:624–633. doi: 10.1111/j.1399-0012.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 68.Lee KC, Chang CY, Chuang YC, Sue SH, Chu TW, Chen RJ, Chen SH, et al. Measurement of human erythrocyte C4d to erythrocyte complement receptor 1 ratio in cardiac transplant recipients with acute symptomatic allograft failure. Transplant Proc. 2008;40:2638–2642. doi: 10.1016/j.transproceed.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 69.Sanchez-Soriano RM, Almenar L, Martinez-Dolz L, Reganon E, Martinez-Sales V, Chamorro CI, Vila V, et al. Diagnostic usefulness of inflammatory markers in acute cellular rejection after heart transplantation. Transplant Proc. 2006;38:2569–2571. doi: 10.1016/j.transproceed.2006.09.002. [DOI] [PubMed] [Google Scholar]


