Abstract
Pluronic block copolymers have been shown to sensitize cancer cells resulting in an increased activity of antineoplastic agents. In the current study we examined a new application of Pluronic bioactivity in potentiating hyperthermia-induced cancer cell injury. DHD/K12/TRb rat adenocarcinoma cells were exposed to low-grade hyperthermia at 43°C with or without Pluronic P85 or Pluronic L61. A range of Pluronic doses, pre-exposure and heat exposure durations were investigated, and the test conditions were optimized. Treatment efficacy was assessed by measurement of intracellular ATP and mitochondrial dehydrogenase activity. Both P85 and L61 in synergy with heat reduced cell viability appreciably compared to either heat or Pluronic alone. Under optimal conditions, P85 (10 mg/ml, 240 mins) combined with 15 mins heat reduced intracellular ATP to 60.1 ± 3.5% of control, while heat alone and P85 without heat caused a negligible decrease in ATP of 1.2% and 3.8%, respectively. Similarly, cells receiving 120 mins pre-exposure of L61 (0.3 mg/ml) showed reduction in intracellular ATP to 14.1 ± 2.1% of control. Again, heat or L61 pre-exposure alone caused a minor decrease in levels of intracellular ATP (1.5% and 4.4%, respectively). Comparable results were observed when viability was assessed by mitochondrial enzyme activity. Survival studies confirmed that the loss of viability translates to a long-term reduction in proliferative activity, particularly for L61 treated cells. Based on these results, we conclude that Pluronic is effective in improving hyperthermic cancer treatment in vitro by potentiating heat-induced cytotoxicity in a concentration and time dependent manner.
Keywords: hyperthermia, Pluronic P85, Pluronic L61, thermosensitizer, tumor thermal ablation
Introduction
Hyperthermia (also referred to as thermotherapy) has been widely investigated as a cancer treatment approach (1–9). While high-temperature hyperthermia (using temperatures in excess of 50°C) can be a viable stand-alone treatment, hyperthermia typically serves as an adjunct technique that utilizes sublethal heat (40–43°C) to enhance established cancer treatment modalities such as radiotherapy and chemotherapy (2–6). The cellular and molecular basis of hyperthermia has been thoroughly reviewed in the literature (1, 9). It is clear that hyperthermia induces both necrotic and apoptotic cell death and leads to a slew of physiologic, cellular and molecular changes, but a consensus on the exact mechanism of hyperthermia-induced cytotoxicity has not been reached (10). Although significant enhancement in local control and patient survival has been observed when in combination with other techniques (5, 11, 12), major challenges exist for hyperthermia as a single-modality treatment approach.
One fairly successful application of focused hyperthermia is percutaneous tumor ablation (13–15), which utilizes radiofrequency (RF) current to heat tumor tissue to cytotoxic temperatures (nearing 100°C) by means of a needle electrode. This approach leads to protein denaturation and loss of cytosolic and mitochondrial enzyme activity that results in adequate control of tumors less than 3 cm in diameter in patients for whom surgical resection is not a viable option (16, 17). While RF ablation illustrates the potential benefits of hyperthermia in cancer treatment, it also calls attention to a well documented limitation. Ablation of larger lesions (>3 cm in diameter) in highly perfused tissues, such as the kidney or liver, is often inadequate and results in tumor recurrence particularly at the tumor periphery, where sublethal heat was administered (18–20). This limitation is a result of several factors. First, cells resume normal function upon sublethal heat removal which entails recovery of normal cellular metabolism. Second, these cells potentially acquire thermal tolerance due to heat shock protein expression upon exposure to heat stress, making repeated heat application less effective (21).
To address these limitations, approaches for enlarging treatment volumes include altering the thermal (22) or electrical tissue conductivities (23), increasing heat deposition in the treatment region by reducing blood flow either physically (24, 25) or pharmacologically (26, 27), and modifying the ablation hardware, i.e., using multi-tined expandable electrode (28, 29), have shown improved results. Studies combining RF ablation with chemotherapeutic agents administered either intravenously or directly into the tumors have also been carried out in an attempt to improve treatment outcome and have shown increased tissue coagulation and tumor shrinkage compared to RF ablation alone (30–32). However, these drugs are often nonspecific, and undesirable side effects may result from collateral damage to surrounding normal cells. An ideal agent for coadministration with RF ablation would facilitate the effects of tumor ablation with minimal damage to surrounding normal tissue. In contrast to the invasive or toxic combination strategies, we have developed a new scheme that involves the use of relatively nontoxic sensitizing agents, Pluronic P85 and L61, to render the cancerous cells more susceptible to injury under sublethal heat, thereby enhancing local hyperthermia treatment.
Pluronic, or poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) or (EOx-POy-EOx), a family of triblock copolymers, has shown the ability to sensitize cancer cells to chemotherapy (32–35) by changing the fluidity of the cell membrane, modulating membrane G-glycoprotein pumps (36), and depleting intracellular ATP, (adenosine triphosphate) (37, 38). The numerous applications of Pluronic were recently reviewed by Batrakova et al. (38). Because many of the same pathways are connected to the cellular heat shock response (39–42), we speculated that the Pluronic would also be active in hyperthermia-induced cell injury. As an initial demonstration of this activity, we recently reported that Pluronic P85 (EO26-PO40-EO26) was successful in increasing cell susceptibility to hyperthermia both in in vitro and in vivo administered intravenously prior to RF ablation (43). The purpose of the current study was to explore in depth how Pluronic dose and exposure time influence the thermosensitizing effect of Pluronic P85 and L61 in an experimental colorectal adenocarcinoma cell line in vitro.
Materials and Methods
Pluronic P85 and L61 were donated by BASF (Shreveport, LA). Cell culture supplies including trypsin-EDTA, Dulbecco’s phosphate buffered saline, RPMI 1640 (with L-glutamine), and penicillin-streptomycin, were purchased from GIBCO (Grand Island, NY). Fetal bovine serum (characterized) was purchased from Hyclone (Logan, UT). The Rapid Cell Proliferation Kit was obtained from Oncogene™ (La Jolla, CA). Sterile 0.22-μm syringe-driven filter units (Millex-GP) and CellTiter-Glo luminescent ATP assays was purchased from Promega (Madison, WI). Costar 96-well, flat bottom, tissue culture treated, opaque walled plates were purchased from Fisher Scientific (Pittsburgh, PA). May-Grünwald and Giemsa stains were obtained from Sigma-Aldrich (St. Louis, MO).
Formulation of Test Solution
Pluronic P85, in the paste, and L61 in liquid form were added to RPMI as stock solutions and placed under refrigeration (4°C) until dissolved (about 24 hrs). Serial dilutions were made to obtain Pluronic concentrations between 0–70 mg/ml. Each test solution was filtered with a sterile 0.22 μm syringe filter (Millex TM-GP, Millipore, Billerica, MA) and stored at 4°C until use.
Cell Culture Maintenance
The DHD/K12/TRb cell line (European Collection of Cell Cultures) originates from a 1,2-dimethylhydrazine-induced colon adenocarcinoma in BDIX rats (33). Cells were maintained in completed RPMI medium, with 10% FBS and 1% penicillin/streptomycin. Upon reaching 70% confluence, the cells were propagated. One day before treatment, cells were detached with trypsin-EDTA, resuspended in RPMI, and plated into flat bottom, tissue culture treated, transparent (or opaque) walled, 96-well plates at 105 cells/ml (200 μl/well). Plates were kept in the incubator to allow cell adhesion. After 24 hrs, cells were exposed to appropriate test solutions.
Treatments
After the 24 hrs incubation period, the supernatant was aspirated and cells were exposed to P85 (0, 0.3, 1, 10, 30, 50 and 70 mg/ml) or L61 (0, 0.005, 0.01, 0.05, 0.1, 0.3 and 1 mg/ml) test solutions (50 μl) for 0–360 mins at 37°C. Select plates received additional exposure to the test solutions for 0 to 45 mins at 43 ± 0.05°C. At the endpoints, treatment solutions were removed from the wells, and cells were washed twice with completed RPMI. When applicable, treatment solutions were replaced with completed RPMI, and cells were returned to a 37°C humidified incubator for 24–72 hrs. ATP and mitochondrial enzyme activity assays were performed either immediately (t = 0), 24, 48 or 72 hrs after treatment.
Intracellular ATP Measurement
Intracellular ATP was measured with the CellTiter Glo® luminescent assay. This assay measures ATP through the energy-dependent luciferase/luciferin reaction and provides information on cell viability. Briefly, plates were removed from the incubator and left to equilibrate to room temperature for 30 mins. Then, an appropriate amount (100 μl) of the assay reagent was added to each well. Cell lysis was promoted by shaking the plate for 2 mins. Finally, luminescence was recorded with a plate reader (TECAN US Infinite 240) with the software iControl (Durham, NC) and an integration time of 500 msecs.
Mitochondrial Succinate Dehydrogenase Activity
In addition to intracellular ATP measurement, cell viability was assessed using a mitochondrial succinate dehydrogenase activity assay (Rapid Cell Proliferation assay, EMD Biosciences). This assay not only serves as a confirmation to results from CellTiter Glo® luminescent assay, but it also allows fixation, staining and visualization of cells in the same plates. Briefly, mitochondrial dehydrogenase cleaves the tetrazolium salt, WST-1, and releases formazan, and the activity of mitochondrial dehydrogenase is directly proportional to cell viability. The WST-1 assay was performed immediately, 24, 48 and 72 hrs after treatment. The assay was performed following manufacturer directions. Briefly, supernatant was aspirated while reagents provided by the manufacturer were diluted with the completed RPMI medium. Then 100 μl of the diluted reagents were added to each well. The plates were then kept for 1 hr at room temperature, and the optical density was determined at λ = 450 nm using a micro plate reader (ELx808, Bio-Tek, Winooski, VT). The assay was performed on selected wells; remaining wells were reserved for fixation and staining as described below.
Cell Staining
At each endpoint, supernatant was aspirated. Subsequently, cells were fixed in methanol for 3–5 mins and air dried before staining. The double staining was carried out by first incubating cells with the May Grünwald reagent for 5 mins, washing with PBS, and then incubating with the Giemsa stain for 10 mins. After washing with ddH2O twice, cells were air dried and stored for analysis.
Statistical Analysis
For all studies, data was normalized to the untreated controls. A two-tailed, unpaired Student’s t test was performed for comparisons of all treatment groups. For multiple comparisons, significance levels were corrected using a Bonferroni adjustment. Unless otherwise noted, all data is reported as mean ± SEM (standard error of mean). A P value ≤ 0.05 was considered statistically significant before Bonferroni correction.
Results
The following phrases are used interchangeably in the following text: “acute” refers to 15 mins exposure to Pluronic (either P85 or L61) at 37°C; “heat” indicates treatment receiving 15 mins heat at 43°C without the presence of Pluronic; “P85 (or L61) + heat” specifies treatment receiving 15 mins Pluronic exposure at 43°C; “P85 (or L61) pre-exposure” denotes extended Pluronic exposure at 37°C; and “P85 (or L61) pre-exposure + heat” implies treatment with Pluronic pre-exposure at 37°C with an additional exposure to 43°C for 15 mins. IC50 indicates the Pluronic concentration required to reduce the cell viability to 50% of untreated control; and PT50 indicates the Pluronic pre-exposure time required to decrease the cell viability to 50% of the untreated control.
Effect of Low-Grade Hyperthermia on Cell Viability (Assay at t = 24 Hours)
To mimic the sublethal low grade hyperthermic environment around the peripheral region of an ablated tumor, 43°C was employed in the present study as the primary treatment. When cells were exposed to heat alone for 15 mins, intracellular ATP levels decreased to 90.4 ± 4.5% of the untreated control. After 30 mins of exposure, ATP decreased to 81.4 ± 2.1% of control. Similar results in viability were observed when assessed with mitochondrial enzyme activity.
Effect of P85 Dose and Pre-Exposure Time on Levels of Intracellular ATP (Assay at t = 24 Hours)
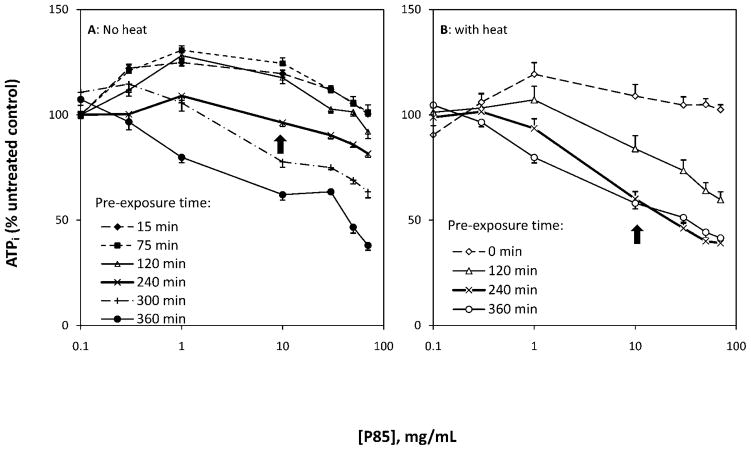
Measurement of ATP was utilized to acquire the optimal P85 dose and pre-exposure time for enhancing hyperthermia-induced cell destruction. Results suggested that the therapeutic effect of P85 was dependent on its concentration and duration of cell pre-exposure at 37°C prior to hyperthermia (Fig. 1). P85 alone (0.3 to 70 mg/ml) at shorter pre-exposure times (up to 120 mins) led to an initial 30% elevation in intracellular ATP levels above baseline followed by a decrease to the initial level. At longer pre-exposure times, P85 led to a dose-dependent decrease in cell viability. A P85 dose of 10 mg/ml and pre-exposure time of 240 mins were selected as the optimal treatment conditions for maximum thermosensitizing effect without lethal damage to the cells by Pluronic alone. Under these conditions, P85 alone displayed a trivial effect on cell viability (decrease to 96.2 ± 1.8% of untreated control). In contrast, when combined with heat, P85 decreased cell viability to 60.1 ± 3.5%. Also, under these optimal conditions, the combination of P85 pre-exposure and heat reduced the IC50 of P85 from 166 mg/ml to 22 mg/ml and reduced the PT50 from 493 mins to 419 mins, respectively (Table 1). After 360 mins of pre-exposure to P85 alone, ATP levels were markedly reduced by 61.9% for a dose of 70 mg/ml suggesting time and dose dependent toxicity of P85 at extremely high doses (Fig. 1).
Figure 1.
Effect of P85 concentration (0, 0.3, 1, 10, 30, 50 and 70 mg/ml), pre-exposure time (0–360 mins) and hyperthermia on intracellular ATP. A: ATP changes in response to Pluronic P85 in the absence of heat; and B: ATP changes in response to Pluronic P85 and hyperthermia (43°C). Arrows indicate cell response at optimal P85 dose and pre-exposure time.
Table 1.
IC50 and PT50 of Pluronic P85 and L61
| IC50 (mg/ml)a | PT50 (min)b | |
|---|---|---|
| P85 pre-exposure | 166 | 493 |
| P85 pre-exposure + heat | 22 | 419 |
| L61 pre-exposure | 0.81 | 248 |
| L61 pre-exposure + heat | 0.16 | 0 |
The Pluronic concentration required to reduce the cell viability to 50% of the untreated control.
The Pluronic pre-exposure time required to decrease the cell viability to 50% of the untreated control.
Effect of L61 Dose, Pre-Exposure Time on Levels of Intracellular ATP (Assay at t = 24 Hours)
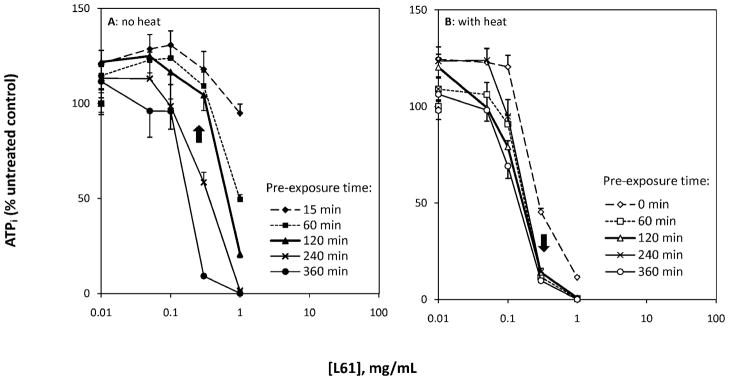
Similar to the effects of P85, L61 alone reduced cell viability in a dose and exposure time dependent manner at 37°C. At lower concentrations (up to 0.1 mg/ml), intracellular ATP was not influenced by L61 alone (maximum decrease of 4.1%, n = 8). Likewise, shorter exposure times, i.e., acute L61 (15 mins) at 1 mg/ml (the highest tested concentration), caused negligible reduction in intracellular ATP levels (maximum decrease of 5.1%, n = 8). In contrast, at higher concentrations and longer exposure times, L61 appeared to be toxic to the DHD/K12/TRb cells and caused complete destruction of the cells at 1 mg/ml after 360 mins (n = 8; Fig. 2A).
Figure 2.
Effect of L61 dose (0, 0.005, 0.01, 0.05, 0.1, 0.3 and 1 mg/ml), pre-exposure time (0–360 mins) and hyperthermia on intracellular ATP. A: ATP changes in response to Pluronic L61 in the absence of heat; and B: ATP changes in response to Pluronic L61 and hyperthermia (43°C). Arrows indicate cell response at optimal L61 dose and pre-exposure time.
Our data also suggests that there exists a threshold of L61 concentration (0.3 mg/ml), above which L61 pre-exposure for 60 mins was sufficient to enhance the hyperthermia induced ATP depletion from 98.6 ± 4.8% (heat only, n = 8) to 11.6 ± 2.5% (n = 8). Below 0.3 mg/ml, L61 pre-exposure had little effect. The optimal outcome was achieved when cells received 120 min pre-exposure of L61 at 0.3 mg/ml. Under these conditions, L61 alone did not show direct influence on cell viability (104.4 ± 8.1% viable), but did show significant enhancement in heat injury (14.1 ± 2.1% viable; Fig. 2B).
The role of L61 in enhancing heat injury was also assessed by comparing the IC50 in cells exposed to L61 alone to those exposed to L61 pre-exposure + heat. Here, the IC50 was reduced from 0.81 to 0.16 mg/ml. In terms of PT50, a 248 mins exposure time was required for L61 alone at 0.3 mg/ml to reduce the cell viability to 50%; however, once combined with heat, no additional pre-exposure time was required (Table 1).
Effects of Pre-Exposure Time on Intracellular ATP
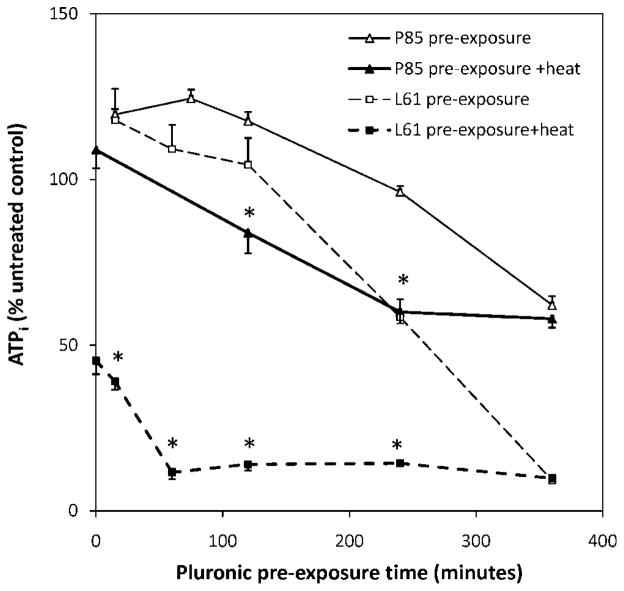
The characteristic behavior of P85 and L61 at their optimal concentrations is illustrated in Figure 3. Results show that at shorter pre-exposure times, P85 increased intracellular ATP. The peak increase in ATP was detected after a 75 mins pre-exposure (124.4 ± 2.7%). At longer pre-exposure times, synergistic effects were observed when P85 was combined with heat. After a 240 mins pre-exposure to P85, the addition of heat depleted ATP from 96.2 ± 1.7% to 60.1± 3.5% (P < 0.0001). However, after a 360 mins pre-exposure, the addition of heat showed little benefit.
Figure 3.
Effects of Pluronic pre-exposure time on intracellular ATP at the optimal P85 (10 mg/ml) and L61 (0.3 mg/ml) concentrations. *P < 0.05.
Distinctively, L61 was able to boost the hyperthermia-induced cell death in a highly synergistic manner. Once combined with heat, the L61 sensitizing effect was less dependent on pre-exposure time. Results showed that the addition of L61 during the 15 mins hyperthermia treatment was able to deplete ATP to 45.3 ± 1.9% of control when compared to 15 mins L61 pre-exposure alone (117.9 ± 9.4%, P < 0.0001). After a 120 mins L61 pre-exposure and heat, ATP decreased to 14.1 ± 2.1% compared to 104.4 ± 8.1% after L61 pre-exposure alone (P < 0.0001). Similar to P85, when cells were pre-exposed to L61 for 360 mins, the presence of heat did not show additional effects (9.2 ± 0.8% without heat, 9.8 ± 0.9% with heat), Figure 3.
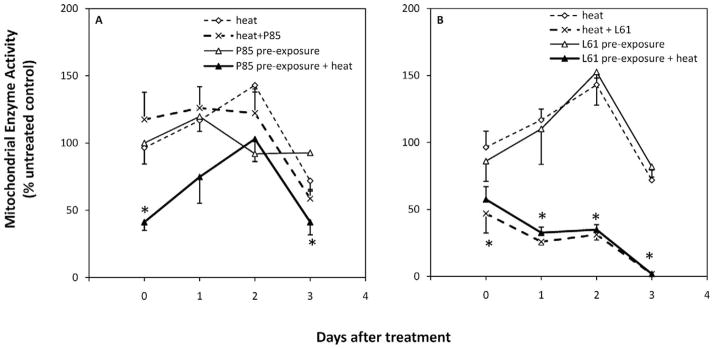
Effects of P85 and L61 on Mitochondrial Enzyme Activity
In order to determine whether the cell damage induced by the combination of P85 or L61 and hyperthermia is permanent, cell viability was assessed immediately, 1, 2 and 3 days after treatment. Figure 4 demonstrates the relative cell viabilities after P85 and L61 exposure (n = 4). Results show that immediately after treatment, activity of mitochondrial dehydrogenase in cells receiving P85 pre-exposure + heat was significantly lower than treatment with heat alone (41.2 ± 6.1% versus 96.4 ± 12.0%, P < 0.01). However, no significant difference was observed between cells receiving heat only and cells receiving P85 + heat (117.5 ± 20.3%, P = 0.4). At day 3, viability of cells exposed to heat decreased to 71.9 ± 7.5% and that of cells exposed to P85 +heat decreased to 58.7 ± 6.8%. Compared to the heat alone, P85 pre-exposure +heat caused the most significant decrease in cell viability (P < 0.05) to 41.2 ± 9.4% of untreated control.
Figure 4.
Levels of mitochondrial dehydrogenase immediately (day 0) and 1, 2 and 3 days after treatment. A: P85 effect on cell survival with and without heat; B: L61 effect on cell survival with and without heat under optimal concentrations ([P85]:10 mg/ml; [L61]: 0.3 mg/ml) and pre-exposure time (P85: 240 mins; L61: 120 mins). * P < 0.05.
Immediately after treatment, cells receiving L61 pre-exposure + heat showed a significant decrease in the enzyme activity (57.5 ± 9.7%) compared to heat only (P < 0.05). Yet, unlike P85 treated cells, L61 + heat decreased viability to 46.9 ± 14.6%, which was also significantly different from heat alone (P < 0.05). Little change was detected in enzyme activity for cells receiving L61 pre-exposure (86.1 ± 15.0%). At day 3, no mitochondrial enzyme activity was detected for cells receiving L61 pre-exposure + heat and cells receiving L61 + heat. Statistically significant differences were detected between cells receiving L61 pre-exposure + heat and cells receiving heat only (P < 0.05), L61 pre-exposure only (P < 0.01), and between cells receiving L61 + heat and heat only (P < 0.05).
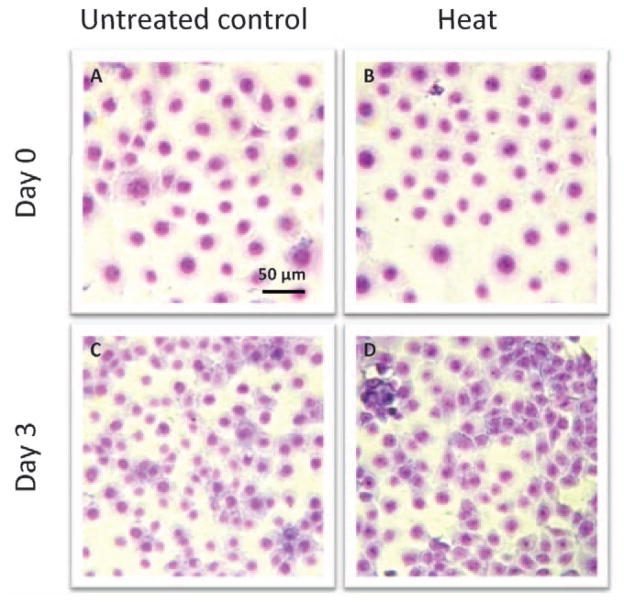
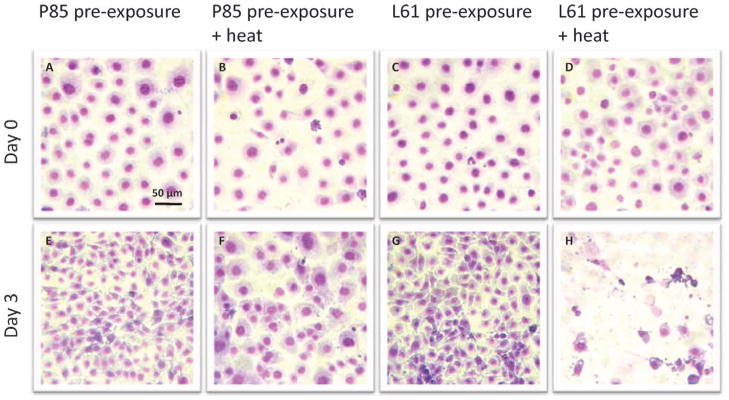
Cellular Morphology
Figure 5 illustrates that cells exposed to low grade hyperthermia not only survived the heat stress (Fig. 5B) but also were able to proliferate continuously. This was demonstrated by drastically increased cell density on day 3 after treatment (Fig. 5D). Both the cell density and cell morphology at this stage were comparable to untreated cells (Fig. 5C). Immediately after treatment, neither P85 pre-exposure (Fig. 6A) or L6 pre-exposure (Fig. 6C) nor combination of P85 pre-exposure (Fig. 6B) or L61 pre-exposure (Fig. 6D) + heat caused direct physical destruction of cells, shown by the unaltered cell density and intact cell morphology. However, at day 3 after treatment, while both P85 (Fig. 6E) and L61 (Fig. 6G) pre-exposure showed significant increase in cell density compared to day 0, lower cell density was observed for P85 pre-exposure + heat (Fig. 6F) when compared to the P85 only control. In addition, cells from this condition appeared unhealthy compared to the controls with less defined nuclear, cytoplasm, and faint nuclear chromatin. Most significantly, cells treated with L61 pre-exposure + heat lost both their ability to propagate as well as their structural integrity (Fig. 6H).
Figure 5.
Cellular morphology of untreated cells and cells treated with heat alone (15 mins at 43°C) immediately (A, B) and 3 days (C, D) after treatment (May Grünwald/Giemsa stain ×400). A color version of the figure is available in the online journal.
Figure 6.
Cellular morphology of cells pre-treated with Pluronic P85 (240 mins) or L61 (120 mins) alone or combined with heat (15 mins at 43°C) immediately (A–D) and 3 days (E–H) after treatment (May Grünwald/Giemsa stain, ×400). A color version of the figure is available in the online journal.
Discussion
This study examined the distinctive thermal sensitizing effects of Pluronic P85 and L61 and the dependence of these effects on Pluronic dose and exposure time in an in vitro model of rat colorectal adenocarcinoma. The optimal thermal sensitizing dose and pre-exposure times of P85 and L61 were defined as those where Pluronic alone caused minor alteration in cell viability but combined with sublethal heat was able to deplete intracellular ATP to fatal levels. To mimic the low grade hyperthermic environment around the periphery of ablated tumors, 43°C was selected as the hyperthermic stress temperature in these studies.
Results indicate that 43°C low grade hyperthermia for up to 45 mins was insufficient to cause irreparable cell damage, stressing the need for a superior means of eliminating the possibility of peripheral tumor residue or recurrence after radiofrequency ablation (18–20). While ATP depletion is a critical indicator of cell viability, our data showed little or no change in intracellular ATP levels measured 24 hrs after the heat exposure. In addition, when evaluated with a secondary assay that examined the effects of our treatment on the mitochondrial enzyme activity, results revealed that the relative activity of mitochondrial succinate dehydrogenase increased until day 2 after the treatment followed by a modest decrease to 71.9 ± 7.5% of control. The initial increase and the eventual decrease in the ATP levels suggest that 43°C heat induced cellular stress, but this stress was not potent enough to lead to physical destruction or cell cycle arrest. This was further supported by the undisturbed cell structure and increased cell density seen 3 days after treatment.
More importantly, our current results indicate that both P85 and L61 were effective in sensitizing DHD/K12/TRb cells to heat stress. Previous studies have reported that Pluronic P85 was significantly more effective in sensitizing multi drug resistant (MDR) tumor cells to chemotherapy when compared to non-MDR cells. After correlating drug effects and levels of ATP depletion, the study concluded that the chemosensitizing effect of P85 was due to its ability to deplete levels of intracellular ATP (44). Our results also demonstrated that both P85 and especially L61 combined with sublethal heat were able to diminish intracellular ATP, but on their own fail to do so unless administered at high concentrations. These results may suggest that ATP depletion is not the primary cause of Pluronic thermal sensitization in our cell line, but may be a downstream effect of other altered metabolic pathways, such as a heat shock response (45). Furthermore, in contrast to the modest chemosensitizing effect of Pluronic on non-MDR cells (44), our observations show significant therapeutic benefits of Pluronic in combination with heat.
In the extended survival study, strong correlation in mitochondrial enzyme activity was observed between cells receiving heat only and cells receiving L61 pre-exposure. This may allude to a similar mechanism between low grade hyperthermia and L61 in provoking cell stress. When mammalian cells are exposed to stresses such as elevated/depressed temperatures, deprivation of nutrients or exposure to toxins, heat shock response will be elicited (9). Heat shock response is characterized by i) induction of heat shock protein synthesis, and ii) inhibition of many metabolic pathways in prevention of uncontrollable and unpredictable production of toxic proteins (46–48). Upon removal of stress, the heat shock response downregulates (49, 50). Studies have shown that Pluronic P85 upregulates heat shock protein (hsp68) genes in a murine cell model (45) and heat shock caused a transient elevation of intracellular ATP similar to the one observed in our study (51). Pluronic in our study may have agitated cells in a similar manner as heat, which instigated heat shock response in an attempt for self-protection (52). While a modest activation of heat shock response protects cells from external stress, Pluronic in addition to heat may overstimulate this response and, consequently, lead to irreparable cell damage and death. This may help to explain the increased intracellular ATP in cells receiving P85 or L61 alone at shorter exposure times and lower concentrations. Conversely, Pluronic may down-regulate the heat shock response, decreasing the protective activities of chaperone proteins and increasing protein coagulation and subsequent cell death.
Finally, our results show that L61 is considerably more potent than P85 in sensitizing cells to low grade hyperthermia. L61 at a dose of 0.3 mg/ml is sufficient to produce a desirable sensitizing effect, while a much higher dose of P85 (10 mg/ml) is required to produce similar outcome. Above 0.3 mg/ml, L61 becomes lethal to the cells; however, below this concentration, cells appear unaffected suggesting 0.3 mg/ml as a critical concentration of L61. Of note is that 0.3 mg/ml (0.15 mM) is above the critical micelle concentration (CMC) of L61 at body temperature (Table 2). This is contrary to previous studies which have concluded that Pluronic unimers exhibit more biological activity than Pluronic micelles (53). Besides the lower L61 concentration that was required to produce similar thermal sensitizing effect as P85, cells receiving L61 +heat showed no signs of recovery, suggesting that L61 is more potent than P85. Cells receiving the combination of P85 pre-exposure + heat showed substantial lower cell viability immediately after treatment compare to control. However, these cells were able to revitalize temporally. In contrast, L61 + heat produced similar results as L61 pre-exposure + heat, and under these conditions, cells viability was immediately reduced and no recovery was detected. More noticeably, L61 + heat are able to eradicate the cell population by day 3 after the treatment without immediate alteration in cell density or morphology.
Table 2.
Properties of Pluronic P85 and L61
| MW (Da)a | HLBb | CMC (M)c | EOd | POe | EO (nm)f | PO (nm)g |
|---|---|---|---|---|---|---|
| P85 4,600 | 16 | 6.5E-05 | 52 | 40 | 7.8 | 11.8 |
| L61 2,000 | 3 | 1.1E-04 | 4 | 30 | 0.7 | 9.2 |
Molecular weight in Daltons.
Hydrophilic and lipophilic balance.
Critical micelle concentration (adapted from Kabanov et al.) (55).
Number of ethylene oxide blocks.
Number of propylene oxide blocks.
Length of one continuous EO block.
Length of PO blocks in the polymer.
The distinctive thermal sensitizing potencies between P85 (EO26-PO40-EO26) and L61 (EO2-PO30-EO2) can be related to the differences in structure of the two polymers (Table 2). Previous studies have shown that an increased P-glycoprotein (P-gp) inhibiting ability corresponded to increased Pluronic PO block length (54). In addition, Pluronic with an intermediate PO block length (between 30–40) and shorter EO block length (between 2–25) had the most profound effects in sensitizing MDR cells to chemotherapy (36, 55). Another study showed that with increasing hydrophilic EO block, Pluronic’s ability to accelerate doxorubicin permeation decreased (56). These observations may also be explained by the distinctive mechanisms of Pluronic intracellular uptake, which have been shown to be structure, temperature and time dependent (57, 58). For example, reports by Rapoport et al. have suggested that structural differences in different Pluronics can lead to intracellular uptake through either simple diffusion or through fluid phase endocytosis of micelles when the Pluronic dose is above the critical micelle concentration (CMC). This, in turn can drastically affect the availability of Pluronic at the target site, leading to variable end effects (57–59). Furthermore, a prolonged exposure time has also been shown to affect intracellular uptake and release of the Pluronic from endocytotic vesicles (58). This is in agreement with our data showing that cells receiving 360 mins Pluronic L61 or P85 alone was able to deplete ATP to a level that at which the addition of 15 mins heat showed no added effects. In the current study, L61, a highly hydrophobic polymer, has a PO block length (approximately 9.3 nm) comparable to the thickness of the cell membrane which is about 7–10 nm (60) and a very short hydrophilic block (0.7 nm) length. This may facilitate its interaction with the lipid bilayer, such as increased lipid “flip-flop” action of the membrane (61), hence reaching the site of action with more ease, and leading to a more potent thermal sensitizing effect (54, 62). On the other hand, while the PO block of P85 (11.8 nm) promotes the interaction of this polymer with the cell membrane, the relatively longer EO (7.8 nm) blocks grant some degree of hindrance which reduces the probability of P85 interact with the cell membrane in a given time. This may explain why the thermosensitizing effect of P85 show more time-dependence than that of L61.
Conclusion
We have demonstrated that both Pluronic P85 and L61 are able to sensitize DHD/K12/TRb carcinoma cells to low-grade heat shock in a dose and time dependent manner. The Pluronic thermosensitization should ensure that the cells that may otherwise receive sublethal heat levels and recover from their injury will succumb to the heat shock and reduce the onset of tumor recurrence following focused tumor hyperthermia treatment. While ATP depletion has been a widely cited mechanism for Pluronic chemosensitization, our results indicate that other mechanisms may be at play in the Pluronic thermal sensitization. Distinctive thermal sensitizing effects were observed between P85 and L61 with L61 being more potent under the tested conditions, an effect potentially related to the differences in the Pluronic structure. The results presented in this study broaden the impact of Pluronic beyond its well-characterized chemo-sensitization and drug carrier attributes and provide a new potential insight into the mechanism of action of its bioactivity.
Acknowledgments
This work was supported by the NIH (R01CA1118399, PI: AE).
We thank Irene Panagopoulos and the Basilion Lab for their assistance.
References
- 1.Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 2.Chen B, Roskams T, de Witte PA. Enhancing the antitumoral effect of hypericin-mediated photodynamic therapy by hyperthermia. Lasers Surg Med. 2002;31:158–163. doi: 10.1002/lsm.10089. [DOI] [PubMed] [Google Scholar]
- 3.Mayhew JF. Intraoperative hyperthermia in a child with neuroblastoma. Paediatr Anaesth. 2006;16:890–891. doi: 10.1111/j.1460-9592.2006.01878.x. [DOI] [PubMed] [Google Scholar]
- 4.Schlemmer M, Lindner LH, Abdel-Rahman S, Issels RD. Principles, technology and indication of hyperthermia and part body hyperthermia. Radiologe. 2004;44:301–309. doi: 10.1007/s00117-004-1044-6. [DOI] [PubMed] [Google Scholar]
- 5.Mambrini A, Del Freo A, Pacetti P, Orlandi M, Torri T, Fiorentini G, Cantore M. Intra-arterial and systemic chemotherapy plus external hyperthermia in unresectable biliary cancer. Clin Oncol (R Coll Radiol) 2007;19:805–806. doi: 10.1016/j.clon.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 6.Jones EL, Marks LB, Prosnitz LR. Point: hyperthermia with radiation for chest wall recurrences. J Natl Compr Canc Netw. 2007;5:339–344. doi: 10.6004/jnccn.2007.0029. [DOI] [PubMed] [Google Scholar]
- 7.Wakisaka O, Takahashi N, Shinohara T, Ooie T, Nakagawa M, Yonemochi H, Hara M, Shimada T, Saikawa T, Yoshimatsu H. Hyperthermia treatment prevents angiotensin II-mediated atrial fibrosis and fibrillation via induction of heat-shock protein 72. J Mol Cell Cardiol. 2007;43:616–626. doi: 10.1016/j.yjmcc.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Deger S, Bohmer D, Roigas J, Turk I, Budach V, Loening SA. Interstitial hyperthermia using thermoseeds in combination with conformal radiotherapy for localized prostate cancer. Front Radiat Ther Oncol. 2002;36:171–176. doi: 10.1159/000061342. [DOI] [PubMed] [Google Scholar]
- 9.Horsman MR, Overgaard J. Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol) 2007;19:418–426. doi: 10.1016/j.clon.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 10.Nakanoma T, Ueno M, Ohigashi T, Nonaka S, Iida M, Hirata R, Suzuki M, Murai M, Deguchi N. Anti-proliferative effects of heating on the human prostatic carcinoma cells in culture. Hum Cell. 1998;11:167–174. [PubMed] [Google Scholar]
- 11.van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, van Dijk JD, van Putten WL, Hart AA. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet. 2000;355:1119–1125. doi: 10.1016/s0140-6736(00)02059-6. [DOI] [PubMed] [Google Scholar]
- 12.Harima Y, Nagata K, Harima K, Ostapenko VV, Tanaka Y, Sawada S. A randomized clinical trial of radiation therapy versus thermoradiotherapy in stage IIIB cervical carcinoma. Int J Hyperthermia. 2001;17:97–105. doi: 10.1080/02656730010001333. [DOI] [PubMed] [Google Scholar]
- 13.Herrera LJ, Fernando HC, Perry Y, Gooding WE, Buenaventura PO, Christie NA, Luketich JD. Radiofrequency ablation of pulmonary malignant tumors in nonsurgical candidates. J Thorac Cardiovasc Surg. 2003;125:929–937. doi: 10.1067/mtc.2003.18. [DOI] [PubMed] [Google Scholar]
- 14.Kim BM, Cho JH, Won JH, Lee do Y, Lee JT, Kim HC, Park SI. Altered findings of hepatic arteriography after radiofrequency ablation of hepatocellular carcinoma: comparison of pre-ablation and post-ablation angiograms. Abdom Imaging. 2007;32:332–338. doi: 10.1007/s00261-006-9059-2. [DOI] [PubMed] [Google Scholar]
- 15.Shetty SK, Rosen MP, Raptopoulos V, Goldberg SN. Cost-effectiveness of percutaneous radiofrequency ablation for malignant hepatic neoplasms. J Vasc Interv Radiol. 2001;12:823–833. doi: 10.1016/s1051-0443(07)61507-3. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg SN. Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound. 2001;13:129–147. doi: 10.1016/s0929-8266(01)00126-4. [DOI] [PubMed] [Google Scholar]
- 17.Goldberg SN, Gazelle GS, Compton CC, Mueller PR, Tanabe KK. Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer. 2000;88:2452–2463. [PubMed] [Google Scholar]
- 18.Curley SA, Cusack JC, Jr, Tanabe KK, Stoelzing O, Ellis LM. Advances in the treatment of liver tumors. Curr Probl Surg. 2002;39:449–571. doi: 10.1067/msg.2002.122810. [DOI] [PubMed] [Google Scholar]
- 19.Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, Cova L, Halpern EF, Gazelle GS. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159–166. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- 20.Vogl TJ, Straub R, Eichler K, Sollner O, Mack MG. Colorectal carcinoma metastases in liver: laser-induced interstitial thermotherapy—local tumor control rate and survival data. Radiology. 2004;230:450–458. doi: 10.1148/radiol.2302020646. [DOI] [PubMed] [Google Scholar]
- 21.Honma T. Characteristics of hyperthermia-induced apoptotic cell death. Nippon Rinsho. 1996;54:1949–1954. [PubMed] [Google Scholar]
- 22.Merkle EM, Goldberg SN, Boll DT, Shankaranarayanan A, Boaz T, Jacobs GH, Wendt M, Lewin JS. Effects of superparamagnetic iron oxide on radio-frequency-induced temperature distribution: in vitro measurements in polyacrylamide phantoms and in vivo results in a rabbit liver model. Radiology. 1999;212:459–466. doi: 10.1148/radiology.212.2.r99au44459. [DOI] [PubMed] [Google Scholar]
- 23.Livraghi T, Goldberg SN, Monti F, Bizzini A, Lazzaroni S, Meloni F, Pellicano S, Solbiati L, Gazelle GS. Saline-enhanced radio-frequency tissue ablation in the treatment of liver metastases. Radiology. 1997;202:205–210. doi: 10.1148/radiology.202.1.8988212. [DOI] [PubMed] [Google Scholar]
- 24.Goldberg SN, Hahn PF, Tanabe KK, Mueller PR, Schima W, Athanasoulis CA, Compton CC, Solbiati L, Gazelle GS. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998;9:101–111. doi: 10.1016/s1051-0443(98)70491-9. [DOI] [PubMed] [Google Scholar]
- 25.Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998;227:559–565. doi: 10.1097/00000658-199804000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horkan C, Ahmed M, Liu Z, Gazelle GS, Solazzo SA, Kruskal JB, Goldberg SN. Radiofrequency ablation: effect of pharmacologic modulation of hepatic and renal blood flow on coagulation diameter in a VX2 tumor model. J Vasc Interv Radiol. 2004;15:269–274. doi: 10.1097/01.rvi.0000109396.74740.c4. [DOI] [PubMed] [Google Scholar]
- 27.Rossi S, Garbagnati F, Lencioni R, Allgaier HP, Marchiano A, Fornari F, Quaretti P, Tolla GD, Ambrosi C, Mazzaferro V, Blum HE, Bartolozzi C. Percutaneous radio-frequency thermal ablation of non-resectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology. 2000;217:119–126. doi: 10.1148/radiology.217.1.r00se02119. [DOI] [PubMed] [Google Scholar]
- 28.Bitsch RG, Dux M, Helmberger T, Lubienski A. Effects of vascular perfusion on coagulation size in radiofrequency ablation of ex vivo perfused bovine livers. Invest Radiol. 2006;41:422–427. doi: 10.1097/01.rli.0000201231.60420.a2. [DOI] [PubMed] [Google Scholar]
- 29.Rossi S, Buscarini E, Garbagnati F, Di Stasi M, Quaretti P, Rago M, Zangrandi A, Andreola S, Silverman D, Buscarini L. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998;170:1015–1022. doi: 10.2214/ajr.170.4.9530052. [DOI] [PubMed] [Google Scholar]
- 30.Goldberg SN, Saldinger PF, Gazelle GS, Huertas JC, Stuart KE, Jacobs T, Kruskal JB. Percutaneous tumor ablation: increased necrosis with combined radio-frequency ablation and intratumoral doxorubicin injection in a rat breast tumor model. Radiology. 2001;220:420–427. doi: 10.1148/radiology.220.2.r01au44420. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg SN, Girnan GD, Lukyanov AN, Ahmed M, Monsky WL, Gazelle GS, Huertas JC, Stuart KE, Jacobs T, Torchillin VP, Halpern EF, Kruskal JB. Percutaneous tumor ablation: increased necrosis with combined radio-frequency ablation and intravenous liposomal doxorubicin in a rat breast tumor model. Radiology. 2002;222:797–804. doi: 10.1148/radiol.2223010861. [DOI] [PubMed] [Google Scholar]
- 32.Krupka TM, Weinberg BD, Ziats NP, Haaga JR, Exner AA. Injectable polymer depot combined with radiofrequency ablation for treatment of experimental carcinoma in rat. Invest Radiol. 2006;41:890–897. doi: 10.1097/01.rli.0000246102.56801.2f. [DOI] [PubMed] [Google Scholar]
- 33.Exner AA, Krupka TM, Scherrer K, Teets JM. Enhancement of carboplatin toxicity by Pluronic block copolymers. J Control Release. 2005;106:188–197. doi: 10.1016/j.jconrel.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Krupka TM, Weinberg BD, Wu H, Ziats NP, Exner AA. Effect of intratumoral injection of carboplatin combined with pluronic P85 or L61 on experimental colorectal carcinoma in rats. Exp Biol Med (Maywood) 2007;232:950–957. [PubMed] [Google Scholar]
- 35.Alakhov V, Moskaleva E, Batrakova EV, Kabanov AV. Hypersensitization of multidrug resistant human ovarian carcinoma cells by pluronic P85 block copolymer. Bioconjug Chem. 1996;7:209–216. doi: 10.1021/bc950093n. [DOI] [PubMed] [Google Scholar]
- 36.Batrakova E, Lee S, Li S, Venne A, Alakhov V, Kabanov A. Fundamental relationships between the composition of pluronic block copolymers and their hypersensitization effect in MDR cancer cells. Pharm Res. 1999;16:1373–1379. doi: 10.1023/a:1018942823676. [DOI] [PubMed] [Google Scholar]
- 37.Batrakova EV, Li S, Vinogradov SV, Alakhov VY, Miller DW, Kabanov AV. Mechanism of pluronic effect on P-glycoprotein efflux system in blood-brain barrier: contributions of energy depletion and membrane fluidization. J Pharmacol Exp Ther. 2001;299:483–493. [PubMed] [Google Scholar]
- 38.Batrakova EV, Kabanov AV. Pluronic block copolymers: evolution of drug delivery concept from inert nanocarriers to biological response modifiers. J Control Release. 2008;130:98–106. doi: 10.1016/j.jconrel.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogt S, Troitzsch D, Abdul-Khaliq H, Moosdorf R. Heat stress attenuates ATP-depletion and pH-decrease during cardioplegic arrest. J Surg Res. 2007;139:176–181. doi: 10.1016/j.jss.2006.07.041. [DOI] [PubMed] [Google Scholar]
- 40.Horvath I, Glatz A, Varvasovszki V, Torok Z, Pali T, Balogh G, Kovacs E, Nadasdi L, Benko S, Joo F, Vigh L. Membrane physical state controls the signaling mechanism of the heat shock response in Synechocystis PCC 6803: identification of hsp17 as a “fluidity gene”. Proc Natl Acad Sci U S A. 1998;95:3513–3518. doi: 10.1073/pnas.95.7.3513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murata N, Los DA. Membrane fluidity and temperature perception. Plant Physiol. 1997;115:875–879. doi: 10.1104/pp.115.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balogh G, Horvath I, Nagy E, Hoyk Z, Benko S, Bensaude O, Vigh L. The hyperfluidization of mammalian cell membranes acts as a signal to initiate the heat shock protein response. FEBS J. 2005;272:6077–6086. doi: 10.1111/j.1742-4658.2005.04999.x. [DOI] [PubMed] [Google Scholar]
- 43.Weinberg BD, Krupka TM, Haaga JR, Exner AA. Combination of sensitizing pretreatment and radiofrequency tumor ablation: evaluation in rat model. Radiology. 2008;246:796–803. doi: 10.1148/radiol.2463070228. [DOI] [PubMed] [Google Scholar]
- 44.Batrakova EV, Li S, Alakhov VY, Elmquist WF, Miller DW, Kabanov AV. Sensitization of cells overexpressing multidrug-resistant proteins by pluronic P85. Pharm Res. 2003;20:1581–1590. doi: 10.1023/a:1026179132599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sriadibhatla S, Yang Z, Gebhart C, Alakhov VY, Kabanov A. Transcriptional activation of gene expression by pluronic block copolymers in stably and transiently transfected cells. Mol Ther. 2006;13:804–813. doi: 10.1016/j.ymthe.2005.07.701. [DOI] [PubMed] [Google Scholar]
- 46.Lebret T, Watson RW, Fitzpatrick JM. Heat shock proteins: their role in urological tumors. J Urol. 2003;169:338–346. doi: 10.1016/S0022-5347(05)64123-7. [DOI] [PubMed] [Google Scholar]
- 47.Roigas J, Wallen ES, Loening SA, Moseley PL. Effects of combined treatment of chemotherapeutics and hyperthermia on survival and the regulation of heat shock proteins in Dunning R3327 prostate carcinoma cells. Prostate. 1998;34:195–202. doi: 10.1002/(sici)1097-0045(19980215)34:3<195::aid-pros7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 48.Sreedhar AS, Pardhasaradhi BV, Begum Z, Khar A, Srinivas UK. Lack of heat shock response triggers programmed cell death in a rat histiocytic cell line. FEBS Lett. 1999;456:339–342. doi: 10.1016/s0014-5793(99)00970-9. [DOI] [PubMed] [Google Scholar]
- 49.Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–1191. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- 50.Solomon JM, Rossi JM, Golic K, McGarry T, Lindquist S. Changes in hsp70 alter thermotolerance and heat-shock regulation in Drosophila. New Biol. 1991;3:1106–1120. [PubMed] [Google Scholar]
- 51.Soini J, Falschlehner C, Mayer C, Bohm D, Weinel S, Panula J, Vasala A, Neubauer P. Transient increase of ATP as a response to temperature up-shift in Escherichia coli. Microb Cell Fact. 2005;4:9. doi: 10.1186/1475-2859-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trieb K, Blahovec H, Kubista B. Effects of hyperthermia on heat shock protein expression, alkaline phosphatase activity and proliferation in human osteosarcoma cells. Cell Biochem Funct. 2007;25:669–672. doi: 10.1002/cbf.1371. [DOI] [PubMed] [Google Scholar]
- 53.Batrakova EV, Li S, Elmquist WF, Miller DW, Alakhov VY, Kabanov AV. Mechanism of sensitization of MDR cancer cells by Pluoronic block copolymers: selective energy depletion. Br J Cancer. 2001;85:1987–1997. doi: 10.1054/bjoc.2001.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Batrakova EV, Li S, Alakhov VY, Miller DW, Kabanov AV. Optimal structure requirements for pluronic block copolymers in modifying P-glycoprotein drug efflux transporter activity in bovine brain micro-vessel endothelial cells. J Pharmacol Exp Ther. 2003;304:845–854. doi: 10.1124/jpet.102.043307. [DOI] [PubMed] [Google Scholar]
- 55.Kabanov AV, Batrakova EV, Alakhov VY. An essential relationship between ATP depletion and chemosensitizing activity of Pluronic block copolymers. J Control Release. 2003;91:75–83. doi: 10.1016/s0168-3659(03)00211-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demina T, Grozdova I, Krylova O, Zhirnov A, Istratov V, Frey H, Kautz H, Melik-Nubarov N. Relationship between the structure of amphiphilic copolymers and their ability to disturb lipid bilayers. Biochemistry. 2005;44:4042–4054. doi: 10.1021/bi048373q. [DOI] [PubMed] [Google Scholar]
- 57.Melik-Nubarov NS, Pomaz OO, Dorodnych T, Badun GA, Kseno-fontov AL, Schemchukova OB, Arzhakov SA. Interaction of tumor and normal blood cells with ethylene oxide and propylene oxide block copolymers. FEBS Lett. 1999;446:194–198. doi: 10.1016/s0014-5793(99)00208-2. [DOI] [PubMed] [Google Scholar]
- 58.Muniruzzaman MD, Marin A, Luo Y, Prestwich GD, Pitt WG, Husseini G, Rapoport NY. Intracellular uptake uptake of Pluronic copolymer: effects of the aggregation state. Colloids Surf B Biointerfaces. 2002;25:233–241. [Google Scholar]
- 59.Rappaport N, Marin A, Luo Y, Prestwich GD, Muniruzzaman MD. Intracellular uptake and trafficking of Pluronic micelles in drug-sensitive and MDR cells: effect on the intracellular drug localization. J Pharm Sci. 2002;91:157–170. doi: 10.1002/jps.10006. [DOI] [PubMed] [Google Scholar]
- 60.Gartner L, Hiatt J. Color Textbook of Histology. 3. Philadelphia: Elsevier Inc; 2007. p. 11. [Google Scholar]
- 61.Krylova OO, Melik-Nubarov NS, Badun GA, Ksenofontov AL, Menger FM, Yaroslavov AA. Pluronic L61 accelerates flip-flop and transbilayer doxorubicin permeation. Chemistry. 2003;9:3930–3936. doi: 10.1002/chem.200204621. [DOI] [PubMed] [Google Scholar]
- 62.Chang LC, Chang YY, Gau CS. Interfacial properties of Pluronics and the interactions between Pluronics and cholesterol/DPPC mixed monolayers. J Colloid Interface Sci. 2008;322:263–273. doi: 10.1016/j.jcis.2008.02.051. [DOI] [PubMed] [Google Scholar]