Abstract
The cell cycle is a temporal program that regulates DNA synthesis and cell division. When we compared the codon usage of cell cycle-regulated genes with that of other genes, we discovered that there is a significant preference for non-optimal codons. Moreover, genes encoding proteins that cycle at the protein level exhibit non-optimal codon preferences. Remarkably, cell cycle-regulated genes expressed in different phases display different codon preferences. Here, we show empirically that transfer RNA (tRNA) expression is indeed highest in the G2 phase of the cell cycle, consistent with the non-optimal codon usage of genes expressed at this time, and lowest toward the end of G1, reflecting the optimal codon usage of G1 genes. Accordingly, protein levels of human glycyl-, threonyl-, and glutamyl-prolyl tRNA synthetases were found to oscillate, peaking in G2/M phase. In light of our findings, we propose that non-optimal (wobbly) matching codons influence protein synthesis during the cell cycle. We describe a new mathematical model that shows how codon usage can give rise to cell-cycle regulation. In summary, our data indicate that cells exploit wobbling to generate cell cycle-dependent dynamics of proteins.
Keywords: cell cycle, non-optimal codons, translation regulation, tRNA expression during cell cycle, wobbling
Introduction
The cell cycle is a fundamental cellular process that allows cells to multiply and faithfully transfer their genetic information to their offspring (Csikász-Nagy, 2009). The full complexity of this process became apparent a decade ago with the first genome-wide microarray studies of the mitotic cell cycle of budding yeast (Cho et al, 1998; Spellman et al, 1998). During the eukaryotic cell cycle, gene expression is regulated at different levels, including through the translation of mRNAs into proteins (Sonenberg and Hinnebusch, 2009). Accurate translation is a complex event coordinated by essential components of the cell, such as the ribosome, messenger RNAs, aminoacylated (charged) transfer RNAs (tRNAs), and a host of additional protein and RNA factors (Francklyn et al, 2002; Lackner and Bähler, 2008).
The tRNAs have a central role in translation as they are adaptor molecules that link the nucleotide sequence of the mRNA and the amino-acid sequence of a protein (Lowe and Eddy, 1997; Percudani et al, 1997; Schattner et al, 2005; Goodenbour and Pan, 2006). The expression of tRNAs is tissue specific and it varies in distinct cellular conditions (Dittmar et al, 2006). Recent studies demonstrate that the redundancy of the genetic code allows a choice to be made between ‘synonymous’ codons for the same amino acid, which may have dramatic effects on the rate of translation due to the tRNA recycling and channeling into the ribosome (Cannarozzi et al, 2010; Weygand-Durasevic and Ibba, 2010; Brackley et al, 2011; Gingold and Pilpel, 2011; Plotkin and Kudla, 2011). Moreover, mRNAs usually start by using the codons corresponding to rarer tRNAs, undergoing a slower phase of elongation, which is then followed by a faster phase (Tuller et al, 2010).
The ‘redundancy’ in the genetic code implies that 61 codons are translated requiring fewer than 61 tRNAs according to the ‘wobble’ base-pairing rules (isoaccepting codons; Crick, 1966). This is especially true when the base at the 5′ end of the anticodon is inosine (abbreviated as I), which deviates from the standard base-pairing rules. The four main wobble base pairs are guanine-uracil, inosine-uracil, inosine-adenine, and inosine-cytosine (G:U, I:U, I:A, I:C; Lander et al, 2001). Finally, the Percudani rules state that tRNAs only wobble with a synonymous codon if there is no better tRNA for that codon (Percudani et al, 1997).
Due to the degeneracy of the genetic code, all amino acids except methionine and tryptophan are encoded by multiple, synonymous codons. The usage of synonymous codons is far from uniform and there is a strong preference toward certain codons in highly expressed genes when compared with other genes (Sharp et al, 1986; Lavner and Kotlar, 2005; Goodenbour and Pan, 2006). Indeed, codon usage preferences are closely correlated with the abundance of corresponding tRNAs in bacteria and yeast (Grantham et al, 1981; Ikemura, 1981, 1982; Futcher et al, 1999), which maximizes the speed and accuracy of protein translation (Gouy and Gautier, 1982; Ikemura, 1985; Akashi and Eyre-Walker, 1998; Duret and Mouchiroud, 1999; Coghlan and Wolfe, 2000; Duret, 2000; Wright et al, 2004; Drummond et al, 2006). However, charging level of some tRNAs matches some anomalous codon usage patterns for different groups of genes in bacteria (Liljenström et al, 1985; Dittmar et al, 2005). Moreover, the correspondence between codon adaptation and gene expression makes translation efficient at a global level rather than at the level of specific genes (Kudla et al, 2009). More specifically, the first 30–50 codons of most mRNA sequences are less efficiently translated than the following part of their sequences (Tuller et al, 2010). The optimal correlation between tRNA levels and their corresponding codon frequencies are dependent on the total amount of tRNAs, ribosomes (Kudla et al, 2009), and the aminoacyl-tRNA synthetases (aaRSs) that charge tRNAs through a two-step aminoacylation reaction using ATP (Orfanoudakis et al, 1987). Finally, changes in the ATP availability in cells influence the concentration of charged tRNAs during a cell cycle (Ibba and Söll, 2004).
Non-optimal codons adapt wobble codon–anticodon base pairing with a low binding affinity. Recent studies revealed that synonymous changes for non-optimal codons can alter the expression of human genes (Kimchi-Sarfaty et al, 2007). Moreover, the codons with the least amount of tRNAs and, thus, the lowest rate of translation, do not necessarily have the lowest genome frequency (Parmley and Huynen, 2009), and they may fulfill a role in translation ‘pausing’ between protein domains (Makhoul and Trifonov, 2002). However, the function of non-optimal codons, in general, and of wobble codon–anticodon base pairing, in particular, in regulating the temporal aspects of protein translation remains unclear in eukaryotes.
We have studied translation regulation of cell cycle-dependent genes through comparative analyses of codon preferences, dynamic quantitative proteomics (Sigal et al, 2006a; Cohen et al, 2008) and mathematical modeling. We discovered that in four distant eukaryotes, proteins encoded by cell cycle-regulated mRNAs have similar preferences in terms of non-optimal codon usage and wobble codon–anticodon base pairing. The dynamics of the charged tRNA pool is expected to vary during the cell cycle as a result of the variations in the ATP availability (Orfanoudakis et al, 1987). In addition, we found experimentally that the levels of glycyl-, threonyl-, and glutamyl-prolyl-aminoacyl-tRNA synthetases oscillate during the human cell cycle, and that tRNA expression levels increase in the G2/M phase of the yeast cell cycle. Moreover, tRNAs are most weakly expressed toward the end of G1 phase. Similarly, we found that genes expressed in different phases of the cell cycle adopt different codon preferences. We show that about 15% of the cell cycle-regulated genes expressed in the G1 phase adopt relatively optimal codon usage, even at the beginning of their coding sequences. All other cell cycle-regulated genes prefer non-optimal codons for their coding sequences. Finally, we developed a mathematical model based on a competitive mechanism in which the cycling of charged tRNAs leads to oscillations in the rate of translation for mRNAs containing non-optimal codons.
Results
Codon preferences of cell cycle-regulated genes
In unicellular prokaryotes and eukaryotes, the abundance of certain tRNAs correlates with the codon preferences of genes encoding highly expressed proteins, for example, ribosomal proteins (Percudani et al, 1997; Kanaya et al, 1999; Bernstein et al, 2002; Lavner and Kotlar, 2005; Kotlar and Lavner, 2006). Thus, codons that perfectly match the anticodons of the tRNAs are preferentially used in highly expressed genes (Grosjean and Fiers, 1982). The mRNAs coding for rare proteins also have selective codon usage, albeit much weaker than the mRNAs coding abundant proteins (Liljenström and von Heijne, 1987).
We hypothesized that cell cycle-regulated genes should also exhibit a preference for certain codons and thus, we analyzed the codon usage preferences for synonymous codons in three sets of human cell cycle-regulated genes, B1, B2, top-600, from an earlier study (Jensen et al, 2006; see Materials and methods). Although the B1 set of genes is the most reliable group of cycling genes, it includes highly expressed genes that are strongly biased in terms of their codon usage, a situation which is undesirable for our purposes. By contrast, highly expressed genes are not so abundant in the B2 and top-600, although they are of somewhat less reliable.
The three sets of cell cycle-regulated genes gave consistent results, either all showing positive or negative preferences for a given codon (Table I). To evaluate the statistical significance of this result, P-values were calculated from 10 000 bootstrap samples with the same codon adaptation index (CAI) distribution as cell cycle-regulated genes (see Materials and methods and Table I). The codon preference was considered as significant when P-value <0.01 for at least two of the three sets of cycling genes (Table I). In fact, the codon usage is confounded by the local GC content (Drummond and Wilke, 2008) and thus, we produced an additional bootstrap procedure preserving the GC content instead of the CAI distribution of the cell cycle-regulated genes. The P-values obtained by this procedure did not alter the final conclusions (see Supplementary information).
Table 1. The codon preferences for the sets of human cell cycle-regulated genes: B1, B2 and top-600 sets (Jensen et al, 2006).
| Aa | Codon 5′ → 3′ | Preferences human |
P-values human |
Anticodon 3′ → 5′ | Binding at third position | Affinity | Organisma |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 | B2 | Top-600 | B1 | B2 | Top-600 | S.p. | S.c. | A.t. | |||||
| P-values were calculated using bootstrapping procedure over 10 000 random samples. The binding affinity was found from the study of Watkins and SantaLucia (2005). Non-optimal codons characterized by low codon–anticodon affinities are presented in bold. Human cell cycle-regulated genes usually have high preferences for these codons. Most of the preferences in human are supported by that of Schizosaccharomyces pombe (S.p.), Saccharomyces cerevisiae (S.c.) or Arabidopsis thaliana (A.t.) for their corresponding sets of the cell cycle-regulated genes. | |||||||||||||
| aS.p. is Schizosaccharomyces pombe, S.c. is Saccharomyces cerevisiae, A.t. is Arabidopsis thaliana. | |||||||||||||
| Ala | GCA | 0.04 | 0.05 | 0.03 | 0.05 | 0.09 | 0.14 | CGI | I:A | Low | • | ||
| Ala | GCC | −0.1 | −0.07 | −0.04 | 0.0001 | 0.01 | 0.16 | CGI | I:C | High | • | ||
| Ala | GCG | −0.01 | −0.03 | −0.02 | 0.58 | 0.01 | 0.03 | CGC | C:G | High | • | • | • |
| Ala | GCT | 0.07 | 0.05 | 0.03 | 0.0001 | 0.0001 | 0.05 | CGI | I:T | Low | • | • | • |
| Arg | AGA | 0.07 | 0.05 | 0.04 | 0.02 | 0.14 | 0.13 | UCU | U:A | Low | • | ||
| Arg | AGG | −0.02 | −0.02 | −0.01 | 0.17 | 0.0001 | 0.02 | UCC | C:G | High | • | • | |
| Arg | CGA | 0 | 0.03 | 0.02 | 0.19 | 0.0001 | 0.0001 | GCI | I:A | Low | |||
| Arg | CGC | −0.01 | −0.04 | −0.03 | 0.75 | 0.09 | 0.06 | GCI | I:C | High | |||
| Arg | CGG | −0.06 | −0.04 | −0.03 | 0.0001 | 0.2 | 0.19 | GCC | C:G | High | • | • | • |
| Arg | CGT | 0.02 | 0.02 | 0.01 | 0.07 | 0.0001 | 0.04 | GCI | I:T | Low | • | • | |
| Asn | AAC | −0.13 | −0.11 | −0.08 | 0.0001 | 0.0001 | 0.0001 | UUG | G:C | High | • | ||
| Asn | AAT | 0.13 | 0.11 | 0.08 | 0.0001 | 0.0001 | 0.0001 | UUG | G:T | Low | • | ||
| Asp | GAC | −0.1 | −0.1 | −0.07 | 0.01 | 0.0001 | 0.01 | CUG | G:C | High | • | ||
| Asp | GAT | 0.1 | 0.1 | 0.07 | 0.01 | 0.0001 | 0.01 | CUG | G:T | Low | • | ||
| Cys | TGC | −0.15 | −0.12 | −0.04 | 0.0001 | 0.0001 | 0.37 | UCG | G:C | High | • | • | • |
| Cys | TGT | 0.15 | 0.12 | 0.04 | 0.0001 | 0.0001 | 0.37 | UCG | G:T | Low | • | • | • |
| Gln | CAA | 0.1 | 0.06 | 0.05 | 0.0001 | 0.42 | 0.09 | GUU | U:A | Low | |||
| Gln | CAG | −0.1 | −0.06 | −0.05 | 0.0001 | 0.43 | 0.1 | GUC | C:G | High | |||
| Glu | GAA | 0.13 | 0.1 | 0.08 | 0.0001 | 0.03 | 0.04 | CUU | U:A | Low | • | • | |
| Glu | GAG | −0.13 | −0.1 | −0.08 | 0.0001 | 0.04 | 0.04 | CUC | C:G | High | • | • | |
| Gly | GGA | 0.04 | 0.05 | 0.04 | 0.35 | 0.15 | 0.29 | CCU | U:A | Low | • | ||
| Gly | GGC | −0.04 | −0.06 | −0.04 | 0.17 | 0.01 | 0.21 | CCG | G:C | High | • | ||
| Gly | GGG | −0.05 | −0.03 | −0.03 | 0.02 | 0.09 | 0.0001 | CCC | C:G | High | • | ||
| Gly | GGT | 0.05 | 0.04 | 0.03 | 0.01 | 0.0001 | 0.0001 | CCG | G:T | Low | • | • | |
| His | CAC | −0.14 | −0.13 | −0.07 | 0.0001 | 0.0001 | 0.05 | GUG | G:C | High | • | • | |
| His | CAT | 0.14 | 0.13 | 0.07 | 0.0001 | 0.0001 | 0.05 | GUG | G:T | Low | • | • | |
| Ile | ATA | 0.05 | 0.05 | 0.04 | 0.03 | 0.12 | 0.08 | UAI | I:A | Low | |||
| Ile | ATC | −0.12 | −0.12 | −0.08 | 0.01 | 0.0001 | 0.02 | UAI | I:C | High | • | ||
| Ile | ATT | 0.07 | 0.07 | 0.04 | 0.02 | 0.0001 | 0.06 | UAI | I:T | Low | • | • | • |
| Leu | CTA | 0.02 | 0.01 | 0.01 | 0.0001 | 0.03 | 0.02 | GUI | I:A | Low | • | ||
| Leu | CTC | −0.05 | −0.04 | −0.03 | 0.0001 | 0.0001 | 0.0001 | GUI | I:C | High | • | • | |
| Leu | CTG | −0.1 | −0.08 | −0.06 | 0.0001 | 0.04 | 0.02 | GUC | C:G | High | |||
| Leu | CTT | 0.03 | 0.04 | 0.03 | 0.06 | 0.01 | 0.05 | GUI | I:T | Low | • | • | |
| Leu | TTA | 0.06 | 0.04 | 0.03 | 0.0001 | 0.03 | 0.0001 | AAU | U:A | Low | • | ||
| Leu | TTG | 0.04 | 0.03 | 0.02 | 0.0001 | 0.0001 | 0.0001 | AAC | C:G | High | • | • | |
| Lys | AAA | 0.04 | 0.09 | 0.06 | 0.43 | 0.01 | 0.11 | UUU | U:A | Low | |||
| Lys | AAG | −0.04 | −0.09 | −0.06 | 0.44 | 0.01 | 0.11 | UUC | C:G | High | |||
| Met | ATG | 0 | 0 | 0 | 1 | 1 | 1 | UAC | C:G | High | • | • | • |
| Phe | TTC | −0.13 | −0.1 | −0.07 | 0.0001 | 0.0001 | 0.0001 | AAG | G:C | High | • | ||
| Phe | TTT | 0.13 | 0.1 | 0.07 | 0.0001 | 0.0001 | 0.0001 | AAG | G:T | Low | • | ||
| Pro | CCA | 0.07 | 0.04 | 0.04 | 0.01 | 0.06 | 0.02 | GGI | I:A | Low | • | • | • |
| Pro | CCC | −0.1 | −0.06 | −0.06 | 0.0001 | 0.02 | 0.0001 | GGI | I:C | High | • | • | |
| Pro | CCG | −0.02 | −0.03 | −0.02 | 0.19 | 0.02 | 0.07 | GGC | C:G | High | • | • | • |
| Pro | CCT | 0.05 | 0.05 | 0.04 | 0.01 | 0.0001 | 0.0001 | GGI | I:T | Low | |||
| Ser | AGC | −0.05 | −0.05 | −0.03 | 0.0001 | 0.0001 | 0.02 | UCG | G:C | High | • | • | |
| Ser | AGT | 0.03 | 0.04 | 0.03 | 0.03 | 0.0001 | 0.0001 | UCG | G:T | Low | |||
| Ser | TCA | 0.03 | 0.03 | 0.02 | 0.1 | 0.02 | 0.34 | AGI | I:A | Low | • | ||
| Ser | TCC | −0.05 | −0.04 | −0.03 | 0.0001 | 0.0001 | 0.0001 | AGI | I:C | High | • | ||
| Ser | TCG | −0.02 | −0.02 | −0.02 | 0.0001 | 0.01 | 0.57 | AGC | C:G | High | • | • | |
| Ser | TCT | 0.06 | 0.04 | 0.03 | 0.0001 | 0.0001 | 0.01 | AGI | I:T | Low | • | • | • |
| Thr | ACA | 0.01 | 0.03 | 0.02 | 0.63 | 0.29 | 0.72 | UGI | I:A | Low | • | ||
| Thr | ACC | −0.05 | −0.07 | −0.05 | 0.09 | 0.0001 | 0.1 | UGI | I:C | High | • | ||
| Thr | ACG | −0.05 | −0.03 | −0.02 | 0.0001 | 0.0001 | 0.11 | UGC | C:G | High | • | • | • |
| Thr | ACT | 0.09 | 0.07 | 0.05 | 0.0001 | 0.0001 | 0.0001 | UGI | I:T | Low | • | • | • |
| Trp | TGG | 0 | 0 | 0 | 1 | 1 | 1 | ACC | G:C | High | • | • | • |
| Tyr | TAC | −0.08 | −0.1 | −0.06 | 0.03 | 0.0001 | 0.05 | AUG | G:C | High | • | ||
| Tyr | TAT | 0.08 | 0.1 | 0.06 | 0.04 | 0.0001 | 0.05 | AUG | G:T | Low | • | ||
| Val | GTA | 0.07 | 0.05 | 0.03 | 0.0001 | 0.0001 | 0.0001 | CUI | I:A | Low | |||
| Val | GTC | −0.05 | −0.05 | −0.03 | 0.0001 | 0.0001 | 0.0001 | CUI | I:C | High | • | ||
| Val | GTG | −0.09 | −0.06 | −0.05 | 0.01 | 0.15 | 0.03 | CUC | C:G | High | • | ||
| Val | GTT | 0.07 | 0.06 | 0.05 | 0.01 | 0.01 | 0.01 | CUI | I:T | Low | • | • | |
We found that cell cycle-regulated genes prefer non-optimal codons, which are recognized by wobble base pairing, and thus have a low codon–anticodon binding affinity (Table I). For instance, TTT was overrepresented among cycling genes when we consider the TTT and TTC codons of phenylalanine (Table I). While no tRNA genes exist for the corresponding AAA anticodon, a tRNA gene does exists with the GAA anticodon. In addition, asparagine, aspartic acid, cysteine, histidine, and tyrosine were similarly seen to display a preference for the non-optimal codons (Table I). Using accurate thermodynamic data for binding affinities of all possible wobble base-pairing cases (I:C, I:A, I:T, G:T, G:C, C:G, U:A) (Watkins and SantaLucia, 2005), we found that for all amino acids cell cycle-regulated genes have a strong, significant (P<0.01) preference for codons with a low codon–anticodon binding affinity (Table I).
To assess the biological importance of the codon preferences observed, we tested whether they are evolutionarily conserved. To this end, we analyzed sets of cell cycle-regulated genes in Schizosaccharomyces pombe, Saccharomyces cerevisiae, and Arabidopsis thaliana (Jensen et al, 2006). For both yeasts species, these genes show significant and consistent preferences for non-optimal codons of amino acids, which use the inosine modification at the wobble position. There are eight such amino acids in Schizosaccharomyces pombe (as in higher eukaryotes) and seven in S. cerevisiae (Supplementary Tables 1 and 2). For Arabidopsis thaliana, a significant preference for non-optimal codons was found for amino acids encoded by two or more codons, also consistent with the trend in humans (Supplementary Table 3). Although the GC content of genes appears to influence the codon preferences of cell cycle-regulated genes in yeast (Supplementary Tables 4–7), the trends are nonetheless consistent with that observed for human genes. Together, these results show that the preference for using non-optimal codons to encode cell cycle-regulated proteins is conserved across distantly related eukaryotes (see Table I).
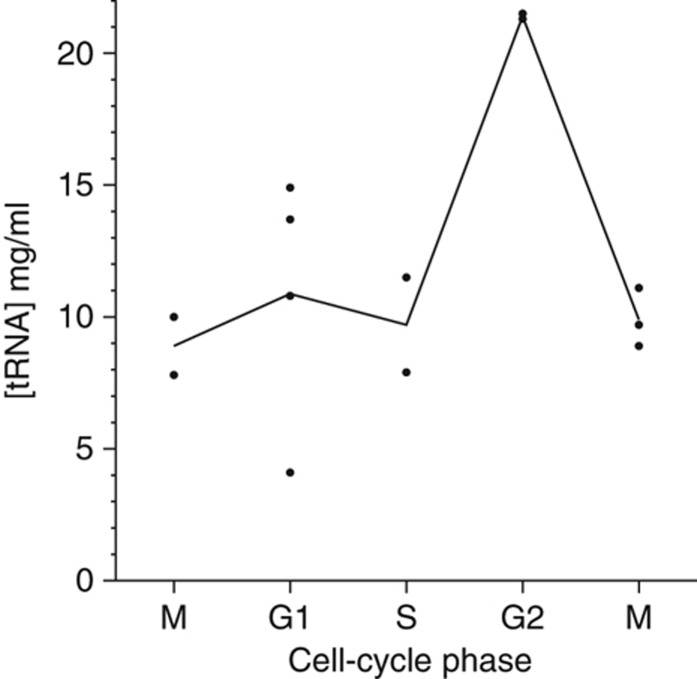
To study if the cell cycle-regulated genes expressed in different phases of the cell cycle adopt the same codon preferences, we used the top-600 sets of genes. Notably, non-optimal codon usage was observed for genes expressed in all phases except the G1 phase (see Supplementary information). In this phase of the cell cycle, both ATP and charged tRNA concentrations are likely to be low (Orfanoudakis et al, 1987), as is the total tRNA pool, which we found to be lowest toward the G1 phase in yeast S. cerevisiae (Table II; Figure 1). As a result, relatively optimal codon preferences were observed in human and yeast genes expressed in G1 phase (Supplementary Table 8). Finally, we found that the level of aaRSs is also likely to be low in the G1 phase, while augmented in the G2/M phase of the human cell cycle (Figure 2A; Supplementary Figure 1). Taken together, these findings indicate that genes may use synonymous codons to adjust their expression pattern during a cell cycle.
Table 2. The concentration of tRNA during the cell cycle in the yeast S. cerevisiae.
| Time points (min) | tRNA concentration (mg/ml) | Estimated cell-cycle phase |
|---|---|---|
| The measurements were performed using cells synchronized in M phase and then released. Over the 4-h time course the cells started in and subsequently returned to M phase. Accordingly, the tRNA concentrations measured at the beginning and end of the experiment are consistent. | ||
| 0 | 10.0 | Synchronized in M phase |
| 30 | 7.8 | M |
| 60 | 14.9 | G1 |
| 90 | 13.7 | G1 |
| 120 | 4.1 | G1 |
| 150 | 10.8 | G1 |
| 180 | 7.9 | S |
| 210 | 11.5 | S |
| 240 | 21.3 | G2 |
| 270 | 21.5 | G2 |
| 300 | 9.7 | M |
| 330 | 8.9 | M |
| 360 | 11.1 | M |
Figure 1.
The tRNA concentration during the cell cycle of S. cerevisiae. The concentration was calculated as an average of the different points in the same phases of the cell cycle according to Table II.
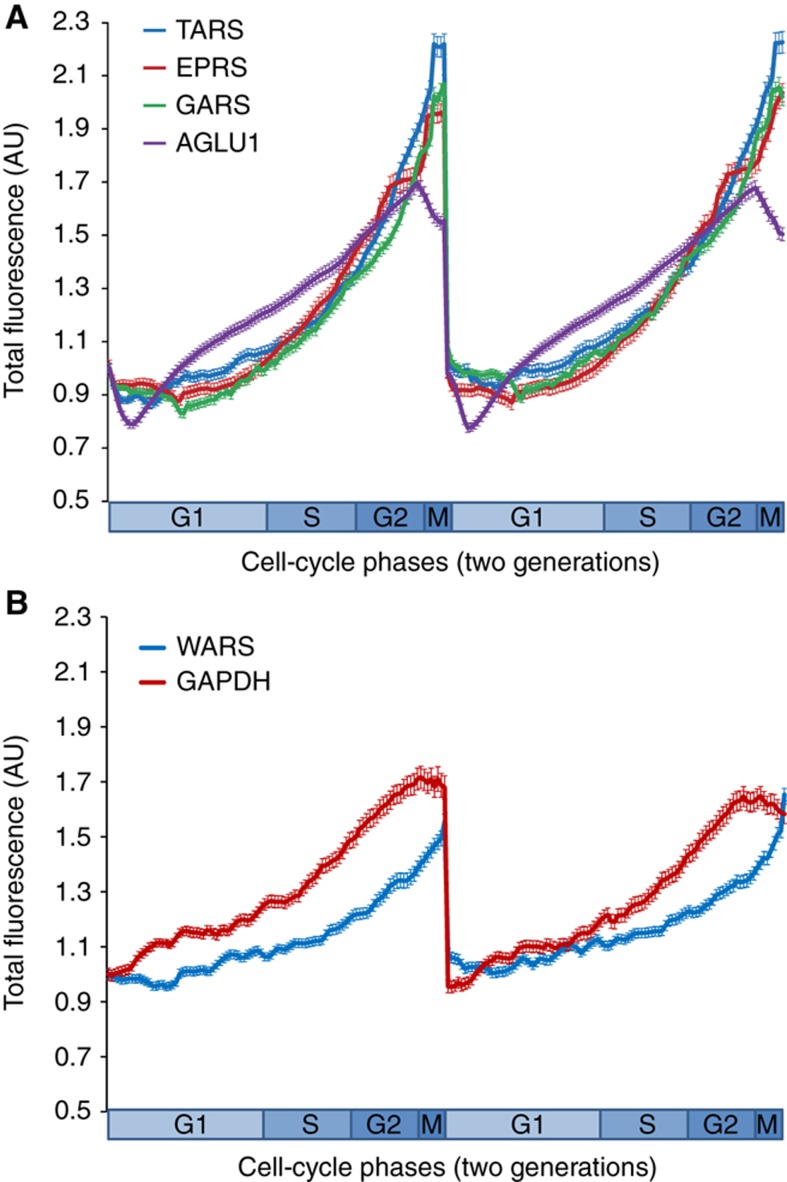
Figure 2.
Total fluorescence as a function of the time during two cell cycles for YFP-tagged proteins, glycyl-tRNA synthetase (GARS), threonyl-tRNA synthetase (TARS), tryptophanyl-tRNA synthetase (WARS), and glutamyl-prolyl-tRNA synthetase (EPRS), when compared with GAPDH and ARGLU1 . (A) The lines represent the average fluorescence (±standard error) from >15 individual cells during two generations for the synthetases that show significant cell cycle-dependent protein dynamics. ARGLU1 is used as a positive control. (B) The total fluorescence (±standard error) for WARS and GAPDH as a negative control. WARS and GAPDH do not show the cell cycle-dependent protein dynamics. Source data is available for this figure in the Supplementary Information.
Protein dynamics of aaRSs
aaRSs covalently attach amino acids to tRNAs and consequently, they have a fundamental role in controlling the amount of charged tRNAs available for protein synthesis (Ibba and Söll, 2004; Francklyn et al, 2008). Thus, we systematically measured the aaRSs available during the cell cycle of individual human cells. We used time-lapse microscopy to measure the dynamics of four aaRSs found in the LARC library (Sigal et al, 2006a, 2006b, 2007; Cohen et al, 2008; see Supplementary information), namely glycyl-tRNA synthetase (GARS), threonyl-tRNA synthetase (TARS), tryptophanyl-tRNA synthetase (WARS) and glutamyl-prolyl-tRNA synthetase (EPRS). In these studies, we also measured the dynamics of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) as a negative control and that of the arginine-glutamate-rich protein-1 (ARGLU1) as a positive control, the expression of which is regulated through the cell cycle at the protein and mRNA levels (Sigal et al, 2006a; Supplementary Figure 2). Each synthetase was tagged with the yellow fluorescent protein (eYFP) at its endogenous chromosomal location in the H1299 cell line (see Supplementary information), and the resulting videos (recorded over 72 h) were analyzed to quantify the accumulation of the proteins at each time point as described previously (Sigal et al, 2006a).
Cell-cycle regulation was defined on the basis of a criterion of at least two-fold difference in the rate of accumulation over the cell cycle, and a difference of at least eight-fold standard errors between the highest and lowest protein accumulation rate (Sigal et al, 2006a). Based on these criteria, the protein dynamics of GARS, TARS, EPRS, and ARGLU1 were clearly cell cycle dependent, whereas WARS and GAPDH could not be considered to have cell cycle-dependent protein dynamics (Figure 2; Supplementary Figure S1). Interestingly, glycine, threonine, and proline are encoded by four different codons and glutamic acid is by two codons. Therefore, cell cycle-dependent protein levels of GARS, TARS, and EPRS may be a source for the cell cycle-regulated behavior of charged tRNAGly, tRNAThr, tRNAGlu, and tRNAPro, as evident in our mathematical model described below. Tryptophan is only encoded by one codon, which leaves no margin for gene-specific, cell cycle-dependent translation rates through the use of suboptimal codons, and which would explain why WARS does not exhibit cell cycle-dependent protein dynamics (Figure 2B). In general, changes in the concentration of aaRSs are not necessary for all the corresponding amino acids to be cell cycle dependent because the ATP pool oscillates during the human cell cycle (Orfanoudakis et al, 1987), and because tRNA levels also rise and fall during the cell cycle (Table II; Figure 1). Thus, in steady-state circumstance, the cycling of ATP and aaRSs levels together provides a mechanism to generate oscillating levels of charged tRNAs (aa-tRNAs) synthesized by steady-state levels of aaRSs. Taken together, these observations indicate that the availability of charged tRNAs during a cell cycle may regulate the expression of genes with regard to their codon usage preferences.
Codon usage of proteins with cell cycle-dependent protein dynamics
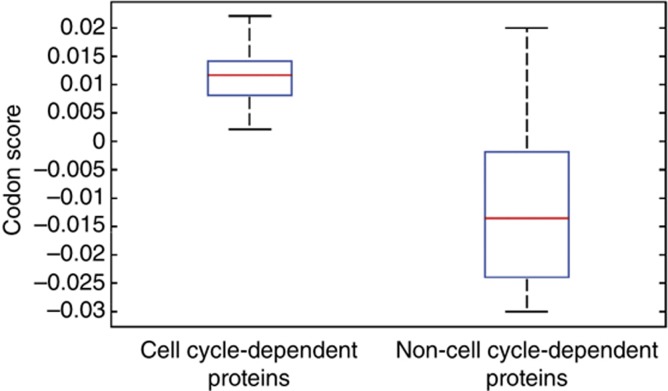
To evaluate the translational regulation of proteins that do not cycle at the mRNA, but do cycle at protein levels, we used the protein data set studied previously (Sigal et al, 2006a) but extended with the five additional proteins (Figure 2). Thus, 11 proteins were found to have cycling protein levels but non-cycling mRNA levels (Whitfield et al, 2002; Gauthier et al, 2008, 2010): DDX5, USP7, TOP1, ANP32B, H2AFV, GTF2F2, RBBP7, SFRS10, GARS, TARS, and EPRS, which were determined as cell cycle regulated in means of protein dynamics in human cells. ARGLU1 cycles at the mRNA level and was excluded from that analysis. As a negative set, we used the 11 proteins that were found to not cycle at the protein level despite the mRNA cycling (Whitfield et al, 2002): SAE1, SET, HMGA2, YPEL1, DDX46, LMNA, HMGA1, ZNF433, KIAA1937, GAPDH, and WARS. The cell-cycle codon scores (CCCS) (see Materials and methods) were calculated for all the proteins analyzed (Supplementary Table 9) and consistent with our hypothesis, we found a significant difference between median distributions of the two groups (Wilcoxon's test; P-value<1E−3) (Figure 3). All of the 11 cycling proteins had a positive CCCS, while the non-cycling proteins had both negative and positive scores (Figure 3). Taken together, these observations indicate that the presence of many non-optimal codons in a gene is not sufficient to cause large-amplitude oscillations at the protein level.
Figure 3.
Comparison of CCCS for proteins with cell cycle-dependent protein dynamics versus proteins with non-cell cycle-dependent protein dynamics. The CCCS evaluates the proportion of wobble codon–anticodon base pairing similar to that of the top-600 genes. A red line represents the distribution mean.
Mathematical model
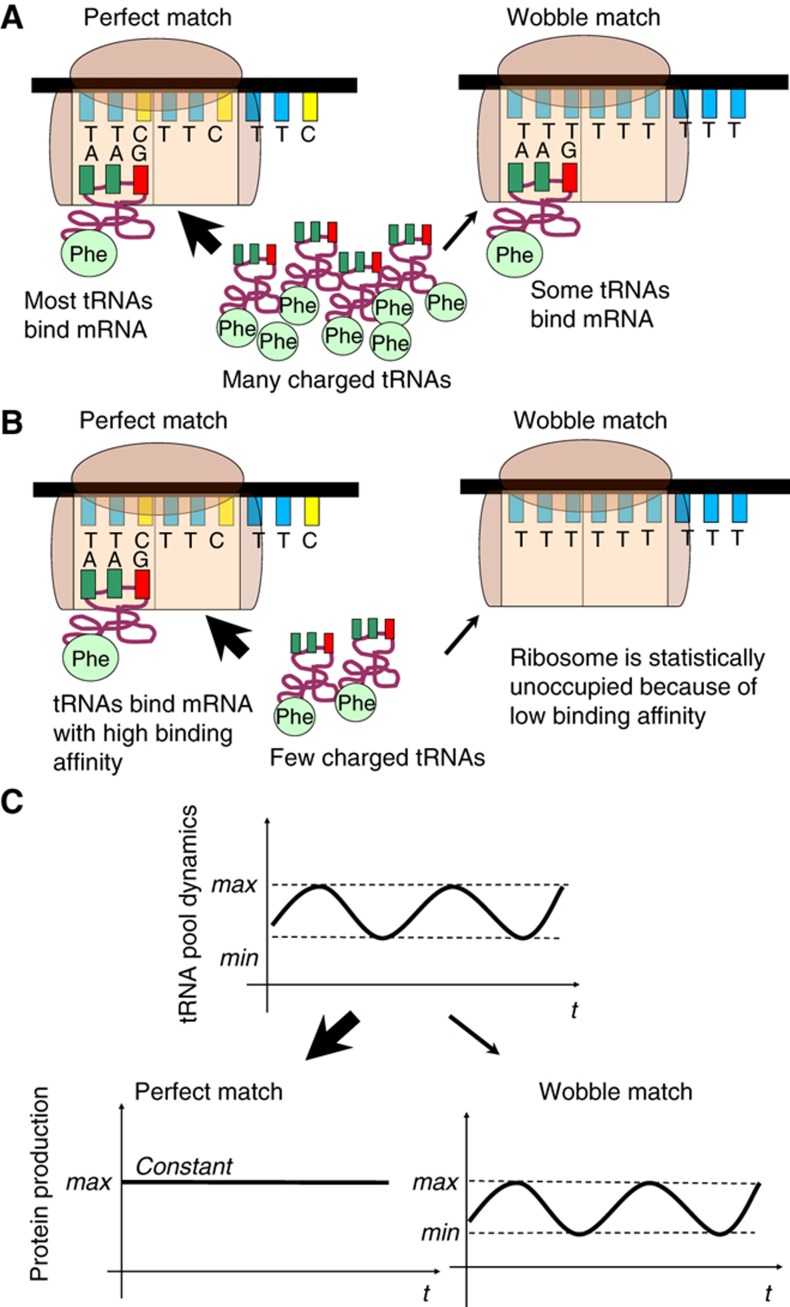
To describe how temporal changes in the tRNA pool can lead to the translational regulation mathematically (Figure 4), we concentrated only on two processes: amino-acid charging of tRNAs by aaRSs (producing aminoacyl-tRNAs or ‘aa-tRNAs’); and cognate or ‘wobble’ aa-tRNA binding to mRNAs. The rate of transport of aa-tRNAs species to a ribosomal A site, the intrinsic kinetics of peptidyl transfer, ribosome concentration and their translocation were not considered in this model.
Figure 4.
A schematic presentation of the additional level of protein translation regulation via the tRNA pool. (A) The translation of poly-TTC and poly-TTT chains (used as an example) when the pool of charged tRNAs includes many TTC-tRNAPhe. (B) Changes in the translation rate of poly-TTC and poly-TTT chains if few TTC-tRNAPhe are available. (C) The oscillating tRNA pool may produce cell cycle-dependent translation of genes, which use wobble codon–anticodon base pairing. The translation rate of proteins using optimal codons stays constant.
The aminoacylation reaction is achieved in two steps (Ibba and Söll, 2004). First, the amino acid is activated by the attack of a molecule of ATP at the [alpha]-phosphate, giving rise to an aminoacyl-adenylate intermediate and an inorganic pyrophosphate. Second, the amino acid is transferred to the 3′-terminal ribose of the cognate tRNA, yielding an aa-tRNA and AMP:
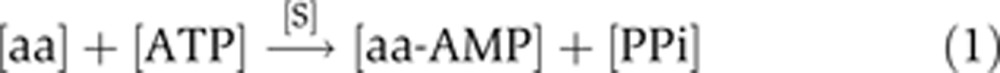 |
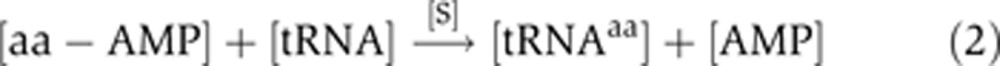 |
Assuming that the cells are not under amino-acid starvation, the production rate of the charged tRNA (tRNAaa) is proportional to a concentration of the corresponding aaRS (S), the amino acid (aa), and ATP:
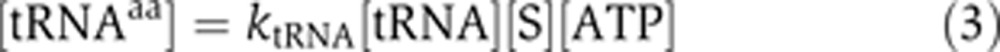 |
where ktRNA is a charging rate of tRNA per synthetase and ATP molecule (Ibba and Söll, 2004).
Assuming the termination rate as a constant, VT, for simplicity and using the Michaelis–Menten kinetics, the observed rate of mRNA translation is
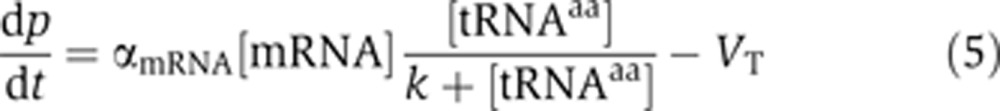 |
where αmRNA is an mRNA-specific translation constant, and k is the codon–anticodon affinity of a tRNA.
For simplicity, assume that we have two mRNAs in equal concentration: mRNATTC is a poly-TTC chain and mRNATTT is a poly-TTT (Figure 4). The TTC codon binds the cognate tRNAPhe ‘strongly’ to the corresponding anticodon GAA (GAA-tRNAPhe), while the TTT codon does not have a cognate tRNA and binds to the same GAA-tRNAPhe ‘weakly’ (Figure 4A and B). ([mRNATTC]= [mRNATTT]=[mRNA]). (It is routine that we write the anticodon sequence from 5′ to 3′.) The energetic difference between ‘strong’ and ‘weak’ binding was evaluated using the HyTher program (Watkins and SantaLucia, 2005). Since the translation rate of a protein is proportional to the production rate of the complex mRNA∷tRNAaa, the production rates of the proteins are
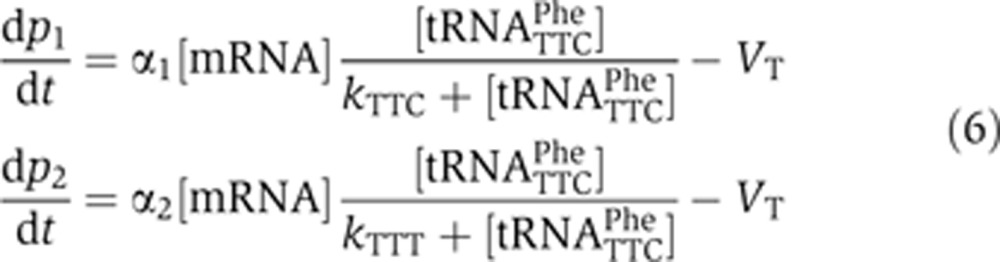 |
For the wobble and perfect matches, at steady state let us assume that codon–anticodon affinities fulfill: kTTC≪[tRNATTC]≪kTTT:
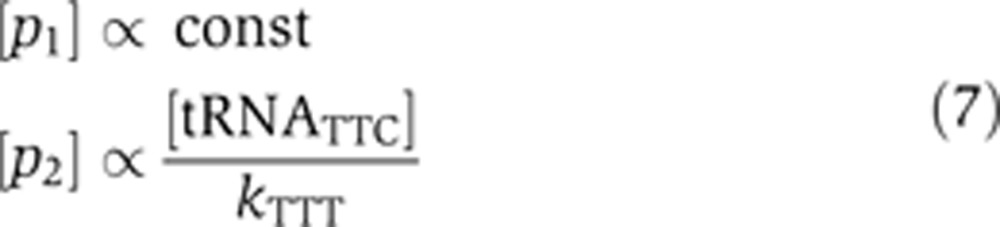 |
This implies that the production rate of p1, which has the mRNATTC as a precursor, does not depend on the concentration of charged tRNA, whereas that of p2, with mRNATTT, is directly proportional to the concentration of GAA-tRNAPhe (Figure 4C). In other words, the mathematical model shows that the pool of the charged tRNAs can specifically affect the translation rate of proteins encoded by non-optimal codons rather than by optimal codons during a cell cycle. This is also supported by the observation that we found a much higher proportion of non-optimal codons in cell cycle-regulated genes (57%) compared with their non-cell cycle-regulated paralogs (37%) that encode protein products with similar sequences (Gauthier et al, 2008, 2010; Supplementary Table 10).
Discussion
We have presented a comprehensive analysis of cell cycle-regulated codon usage and a mathematical model describing a mechanism of translational regulation through changes in the charged tRNA pool during the cell cycle in four eukaryotes. The model was illustrated for the very simple situation, in which only two mRNAs encoding poly-phenylalanyl exist, one using the perfect-matching codon for a tRNA and the other using the wobble match. If many charged Phe-tRNAPhe molecules are present in the cell, both mRNAs will be efficiently translated. However, if only a few charged Phe-tRNAPhe molecules are available, the mRNA with optimal (perfectly matched) codons will be translated, while the mRNA with non-optimal (wobbly matched) ones will be unable to compete for charged tRNAs and will thus only be translated very slowly. The independence of translation rate on codon usage if many charged tRNAs are present may explain the lack of correlation between codon bias and a certain protein expression level published by Kudla et al (2009).
Cell cycle-dependent changes in the pool of charged tRNAs, due to oscillations in aaRS protein levels, tRNAs, and the cellular ATP concentration, are likely to explain why non-optimal codon usage is only associated with efficient translation during the cell-cycle phases when many charged tRNAs are available. The idea that codon usage may provoke cell cycle-regulated translation is supported by the observation that three sets of cell cycle-regulated genes in humans, and in other eukaryotes, together with another set of cell cycle-regulated human proteins, all have an overrepresentation of non-optimal codons.
Our findings are consistent with the ‘recycling’ principle of tRNAs presented recently (Cannarozzi et al, 2010): once non-optimal codons are used for amino acids encoding cell cycle-regulated genes, the subsequent codons for these amino acids are likely be the same. Notably, the novelty of our findings is that genes may gain the functional advantage of preferably using wobbling and non-optimal codons to create oscillations during the cell cycle. In addition, we found that genes cycling in the G1 phase of the cell cycle prefer optimal codons even at the beginning of their coding sequences (Supplementary Table 8). Therefore, the early elongation ‘ramp’ and primary slow translation proposed recently (Tuller et al, 2010) does not have advantages for the G1 phase genes, when we have shown that the tRNA level decreases to a minimum. Finally, Tsutsumi et al (2007) showed that the modified base inosine in tRNA used for wobble base pairing is vital for the G1/S and G2/M cell-cycle transitions in Schizosaccharomyces pombe. These findings explain the significant preferences for non-optimal codons that adapt the wobble codon–anticodon base pairing found in cell cycle-regulated genes in yeast.
The aaRSs are remarkable examples of proteins that are dynamic during the cell cycle, although their mRNA transcripts do not cycle. It is tempting to speculate that perhaps most aaRSs have cycling protein dynamics, considering that the preference for non-optimal codons is observed for all members of the aaRS family except for methionine and tryptophan. However, this does not have to be the case, since the ATP concentration varies during the cell cycle (Orfanoudakis et al, 1987), which in itself will affect the concentration of charged tRNAs in a cell cycle-dependent manner. Indeed, in the steady state, the cycling of ATP and tRNA levels together provides a mechanism to generate oscillating levels of the charged aa-tRNA.
The codon preference will lead to cycling production rates for a protein in the same way as cycling mRNA levels affect cycling protein synthesis rate. But since the half-life of a protein is usually far longer than the cell cycle, this is not sufficient to cause large-amplitude change of the level of cycling proteins. For that to happen, the protein must be actively degraded at some point of the cell cycle. Thus, a protein with no degradation signals is unlikely to cycle significantly, even if it has strong codon preferences to non-optimal codons.
In summary, cell cycle-regulated genes contain a significant overrepresentation of non-optimal codons, adapting to wobble codon–anticodon base pairing. Protein translation rates are in part controlled by the availability of charged tRNAs, which in turn depends on the concentration of ATP, tRNAs, and aaRSs, which have been shown to oscillate during the cell cycle. We propose a competitive mechanism to explain how the presence of many non-optimal codons in some mRNAs can induce cell cycle-dependent protein expression. Thus, it is thus tempting to speculate that the absence of tRNA genes with certain anticodons in the human genome, and the preference for the resulting wobble codon–anticodon base pairing in cell cycle-regulated genes, may serve as a hitherto unknown regulatory control in the cell cycle.
Materials and methods
Data sets
Three sets of human cell cycle-regulated genes were studied here (Jensen et al, 2006): 63 cell cycle-regulated genes identified through single gene studies (the B1 set), 438 genes with E2F transcription factor binding sites in their promoter regions (the B2 set), and the 600 most significantly oscillating genes according to DNA microarray expression data (the top-600 set; Whitfield et al, 2002). Similar sets were considered for Schizosaccharomyces pombe, S. cerevisiae, and Arabidopsis thaliana (see Supplementary information and Jensen et al, 2006).
Codon preferences calculation
The codon usage table (CUT) was calculated using cDNA sequences of all annotated human genes. The codon preference of a specific codon, CP, was calculated with the following formula:
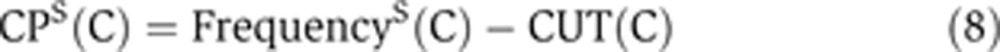 |
where FrequencyS(C) is a relative frequency of the codon, C, with respect to all codons in genes from a given data set S (namely the B1, B2, top-600, non-cycling genes with cell-cycle phenotype; Mukherji et al, 2006, or non-cycling genes with cycling orthologs; Jensen et al, 2006). Finally, CUT(C) is the global frequency of the codon C in human genes.
Bootstrapping and the P-value calculation
In all, 10 000 bootstrap samples were generated for each list of cell cycle-regulated genes from all the annotated genes in a given organism. The random sampling was first performed such that the CAI distribution of each bootstrap sample matched that of the cell cycle-regulated genes. All genes for a particular organism were binned based on their CAI. We then counted number of genes from each bin in the actual observed sample, and generate bootstrap samples by randomly sampling the same number of genes from each CAI bin, thereby ensuring that the overall CAI distribution is preserved in the bootstrapped samples.
The second bootstrap sampling ensured that the GC content distribution of the bootstrap samples matched that of the cell cycle-regulated genes. The P-values were calculated for each codon by comparing its usage in the set of cell cycle-regulated genes with the empirical distribution obtained from the bootstrap samples. The codon preference was considered as significant if the P-values were <0.01 for at least two sets of the cell cycle-regulated genes.
The CCCS
For each human gene, the cell-cycle codon score (CCCS) was calculated as a sum of the top-600 codon-preference values over all codons in the cDNA of the gene, normalized to the length of the cDNA:
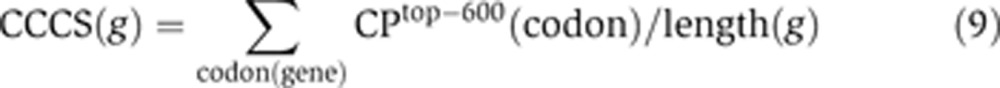 |
where for every codon of a gene, g, the codon preference in the top-600 set, CPtop-600(codon), is calculated by the formula 7 above. Thus, the CCCS of a specific gene evaluates how well the codon usage matches that of the top-600 cell cycle-regulated human genes.
The codon–anticodon affinity
The low and high codon–anticodon binding affinities were found using the accurate thermodynamics data of Watkins and SantaLucia (2005) for the synonymous codons of the same amino acid. For instance, for the G-T wobble anticodon:codon base-pairing cases, namely, XXG:XXC and XXG:XXT (or simply, G:C and G:T, G:C>G:T), have a ‘high’ and ‘low’ binding affinity, respectively (Table I). Moreover, according to the thermodynamics data the affinity trend for the I:X pairs for inosine wobble base pairing is I:C>I:A⩾I:T (Watkins and SantaLucia, 2005). Therefore, I:C has a high binding affinity and I:A, I:T low binding affinities among the I:X pairs of the same amino acid (Table I). Finally, for the codons AGA, AGG of arginine, TTA, TTG of leucine, CAA, CAG of glutamine, and GAA, GAG of glutamic acid, the affinity trend is C:G>U:A, and thus, C:G and U:A are the high and low binding affinities, correspondingly (Table I). The cell cycle-regulated genes have strong consistent preferences for codons adapting the wobble anticodon:codon base pairing, G:T, I:A and I:T.
Copy number of tRNA genes
The gene copy number for different types of human tRNAs was obtained from the Genomic tRNA database (http://lowelab.ucsc.edu/GtRNAdb: Lowe and Eddy, 1997). The main assumption was that the number of tRNA genes is a true representation of the tRNA abundance within the cell. Many previous studies have shown that this assumption is not unfounded (Duret, 2000; Comeron, 2004; Lavner and Kotlar, 2005).
Isolation of tRNA during the yeast cell cycle
The CDC-15 yeast strain, which contains a temperature-sensitive cdc15 gene (Johnson and Blobel, 1999), was used to obtain cell cycle-synchronized cells. The cdc15 gene encodes the protein CDC-15, which controls the timing of cell division (Johnson and Blobel, 1999).
An overnight culture of CDC-15 grown at 21°C in YPD media was used to inoculate a 50-ml culture, which was grown to OD600 to ∼1.0. The 50-ml culture was diluted by YPD to 500 ml to an OD600 of 0.2, and then grown for ∼15 h at 21°C until an OD600 of 0.6 was reached. At this time, the culture displayed heterogeneous phenotypes when examined under a microscope and it was shifted to 37°C for 3 h to arrest cdc-15. The cell-cycle arrest was confirmed by a microscope analysis and the cells had a homogeneous phenotype. The culture was then shifted back to 25°C, which was termed T0. An aliquot of the cultured was removed at T0 and every 30 min after T0 to extract tRNA.
The extraction of tRNA
A total of 13 tRNA samples were prepared from the cell culture following a previously published procedure (Whipple et al, 2011). Yeast cells from each sample were spun down and resuspended on ice in 150 μl of the RNA elution solution (0.3 M sodium acetate (pH 4.5), 10 mM EDTA). An aliquot of glass beads (∼0.5 ml) was added to the cell suspension, and the cells were vortexed four times for 15 s each and extracted three times with an equal volume of phenol saturated in the RNA elution buffer for 15 s. After centrifugation at 5 K for 10 min at 4°C, the aqueous phase of the phenol extraction was recovered and after centrifugation at 13.2 K r.p.m. for 4 min at 4°C, the aqueous phase was again recovered and the tRNA in the aqueous phase was ethanol precipitated and collected by centrifugation. The cell suspension in the phenol extraction was back-extracted with 100 μl of the RNA elution buffer and the tRNA in the suspension was further precipitated by ethanol and collected by centrifugation. The tRNA pellets were resuspended in 20 μl of RNA elution buffer, combined and precipitated by ethanol one more time. The final tRNA pellet was resuspended in 20 μl of RNA elution buffer to determine the concentration by absorption at OD260 (1 OD=40 mg), before it was stored at −70°C.
Supplementary Material
Supplementary Tables S1-10, Supplementary Figures S1-2
Acknowledgments
We thank Mark G Safro for the useful discussions on the aminoacyl-tRNA synthetases, Avi Mayo for assistance with constructing the mathematical model, Ariel A Cohen for a help with the dynamic data analysis, and Alex Sigal, Daniel Koster and Uri Alon for many helpful remarks. We thank also the Uri Alon's laboratory for the hospitality and great support. The work carried out in this study was in part funded by the Kahn Family Foundation, the Novo Nordisk Foundation Center for Protein Research and Reinholdt W Jorck og Hustrus Fond. The work of MFM is supported by the Horowitz Center for Complexity Science.
Author contributions: MFM designed the study and experiments, analyzed and interpreted the data, wrote and revised the manuscript. TD and LC performed experiments involving the YFP-tagged aminoacyl-tRNA synthetases. LJJ analyzed and interpreted the data and helped write the manuscript. TC, TI and YMH performed experiments concerning total tRNA expression levels and also revised the manuscript.
Footnotes
The authors declare that they have no conflict of interest.
References
- Akashi H, Eyre-Walker A (1998) Translational selection and molecular evolution. Curr Opin Genet Dev 8: 688–693 [DOI] [PubMed] [Google Scholar]
- Bernstein JA, Khodursky AB, Lin PH, Lin-Chao S, Cohen SN (2002) Global analysis of mRNA decay and abundance in Escherichia coli at single-gene resolution using two-color fluorescent DNA microarrays. Proc Natl Acad Sci USA 99: 9697–9702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brackley CA, Romano MC, Thiel M (2011) The dynamics of supply and demand in mRNA translation. PLoS Comput Biol 7: e1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannarozzi G, Schraudolph NN, Faty M, von Rohr P, Friberg MT, Roth AC, Gonnet P, Gonnet G, Barral Y (2010) A role for codon order in translation dynamics. Cell 141: 355–367 [DOI] [PubMed] [Google Scholar]
- Cho RJ, Campbell MJ, Winzeler EA, Steinmetz L, Conway A, Wodicka L, Wolfsberg TG, Gabrielian AE, Landsman D, Lockhart DJ, Davis RW (1998) A genome-wide transcriptional analysis of the mitotic cell cycle. Mol Cell 2: 65–73 [DOI] [PubMed] [Google Scholar]
- Coghlan A, Wolfe K (2000) Relationship of codon bias to mRNA concentration and protein length in Saccharomyces cerevisiae. Yeast 16: 1131–1145 [DOI] [PubMed] [Google Scholar]
- Cohen AA, Geva-Zatorsky N, Eden E, Frenkel-Morgenstern M, Issaeva I, Sigal A, Milo R, Cohen-Saidon C, Liron Y, Kam Z, Cohen L, Danon T, Perzov N, Alon U (2008) Dynamic proteomics of individual cancer cells in response to a drug. Science 322: 1511–1516 [DOI] [PubMed] [Google Scholar]
- Comeron J (2004) Selective and mutational patterns associated with gene expression in humans: influences on synonymous composition and intron presence. Genetics 167: 1293–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crick FH (1966) Codon—anticodon pairing: the wobble hypothesis. J Mol Biol 19: 548–555 [DOI] [PubMed] [Google Scholar]
- Csikász-Nagy A (2009) Computational systems biology of the cell cycle. Brief Bioinform 10: 424–434 [DOI] [PubMed] [Google Scholar]
- Dittmar K, Sørensen M, Elf J, Ehrenberg M, Pan T (2005) Selective charging of tRNA isoacceptors induced by amino-acid starvation. EMBO Rep 6: 151–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmar KA, Goodenbour JM, Pan T (2006) Tissue-specific differences in human transfer RNA expression. PLoS Genet 2: e221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DA, Raval A, Wilke CO (2006) A single determinant dominates the rate of yeast protein evolution. Mol Biol Evol 23: 327–337 [DOI] [PubMed] [Google Scholar]
- Drummond DA, Wilke CO (2008) Mistranslation-induced protein misfolding as a dominant constraint on coding-sequence evolution. Cell 134: 341–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duret L (2000) tRNA gene number and codon usage in the C. elegans genome are co-adapted for optimal translation of highly expressed genes. Trends Genet 16: 287–289 [DOI] [PubMed] [Google Scholar]
- Duret L, Mouchiroud D (1999) Expression pattern and, surprisingly, gene length shape codon usage in Caenorhabditis, Drosophila, and Arabidopsis. Proc Natl Acad Sci USA 96: 4482–4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francklyn C, Perona JJ, Puetz J, Hou YM (2002) Aminoacyl-tRNA synthetases: versatile players in the changing theater of translation. RNA 8: 1363–1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francklyn CS, First EA, Perona JJ, Hou YM (2008) Methods for kinetic and thermodynamic analysis of aminoacyl-tRNA synthetases. Methods 44: 100–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futcher B, Latter G, Monardo P, McLaughlin C, Garrels J (1999) A sampling of the yeast proteome. Mol Cell Biol 19: 7357–7368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier NP, Jensen LJ, Wernersson R, Brunak S, Jensen TS (2010) Cyclebase.org: version 2.0, an updated comprehensive, multi-species repository of cell cycle experiments and derived analysis results. Nucleic Acids Res 38: D699–D702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier NP, Larsen ME, Wernersson R, de Lichtenberg U, Jensen LJ, Brunak S, Jensen TS (2008) Cyclebase.org—a comprehensive multi-organism online database of cell-cycle experiments. Nucleic Acids Res 36: D854–D859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gingold H, Pilpel Y (2011) Determinants of translation efficiency and accuracy. Mol Syst Biol 7: 481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenbour JM, Pan T (2006) Diversity of tRNA genes in eukaryotes. Nucleic Acids Res 34: 6137–6146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouy M, Gautier C (1982) Codon usage in bacteria: correlation with gene expressivity. Nucleic Acids Res 10: 7055–7074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham R, Gautier C, Gouy M, Jacobzone M, Mercier R (1981) Codon catalog usage is a genome strategy modulated for gene expressivity. Nucleic Acids Res 9: r43–r74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H, Fiers W (1982) Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene 18: 199–209 [DOI] [PubMed] [Google Scholar]
- Ibba M, Söll D (2004) Aminoacyl-tRNAs: setting the limits of the genetic code. Genes Dev 18: 731–738 [DOI] [PubMed] [Google Scholar]
- Ikemura T (1981) Correlation between the abundance of Escherichia coli transfer RNAs and the occurrence of the respective codons in its protein genes: a proposal for a synonymous codon choice that is optimal for the E. coli translational system. J Mol Biol 151: 389–409 [DOI] [PubMed] [Google Scholar]
- Ikemura T (1982) Correlation between the abundance of yeast transfer RNAs and the occurrence of the respective codons in protein genes. Differences in synonymous codon choice patterns of yeast and Escherichia coli with reference to the abundance of isoaccepting transfer RNAs. J Mol Biol 158: 573–597 [DOI] [PubMed] [Google Scholar]
- Ikemura T (1985) Codon usage and tRNA content in unicellular and multicellular organisms. Mol Biol Evol 2: 13–34 [DOI] [PubMed] [Google Scholar]
- Jensen L, Jensen T, de Lichtenberg U, Brunak S, Bork P (2006) Co-evolution of transcriptional and post-translational cell-cycle regulation. Nature 443: 594–597 [DOI] [PubMed] [Google Scholar]
- Johnson ES, Blobel G (1999) Cell cycle-regulated attachment of the ubiquitin-related protein SUMO to the yeast septins. J Cell Biol 147: 981–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaya S, Yamada Y, Kudo Y, Ikemura T (1999) Studies of codon usage and tRNA genes of 18 unicellular organisms and quantification of Bacillus subtilis tRNAs: gene expression level and species-specific diversity of codon usage based on multivariate analysis. Gene 238: 143–155 [DOI] [PubMed] [Google Scholar]
- Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, Calcagno AM, Ambudkar SV, Gottesman MM (2007) A ‘silent’ polymorphism in the MDR1 gene changes substrate specificity. Science 315: 525–528 [DOI] [PubMed] [Google Scholar]
- Kotlar D, Lavner Y (2006) The action of selection on codon bias in the human genome is related to frequency, complexity, and chronology of amino acids. BMC Genomics 7: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudla G, Murray A, Tollervey D, Plotkin J (2009) Coding-sequence determinants of gene expression in Escherichia coli. Science 324: 255–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner D, Bähler J (2008) Translational control of gene expression from transcripts to transcriptomes. Int Rev Cell Mol Biol 271: 199–251 [DOI] [PubMed] [Google Scholar]
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, Funke R, Gage D, Harris K, Heaford A, Howland J, Kann L, Lehoczky J, LeVine R, McEwan P, McKernan K et al. (2001) Initial sequencing and analysis of the human genome. Nature 409: 860–921 [DOI] [PubMed] [Google Scholar]
- Lavner Y, Kotlar D (2005) Codon bias as a factor in regulating expression via translation rate in the human genome. Gene 345: 127–138 [DOI] [PubMed] [Google Scholar]
- Liljenström H, von Heijne G (1987) Translation rate modification by preferential codon usage: intragenic position effects. J Theor Biol 124: 43–55 [DOI] [PubMed] [Google Scholar]
- Liljenström H, von Heijne G, Blomberg C, Johansson J (1985) The tRNA cycle and its relation to the rate of protein synthesis. Eur Biophys J 12: 115–119 [DOI] [PubMed] [Google Scholar]
- Lowe TM, Eddy SR (1997) tRNAscan-SE: a program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res 25: 955–964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makhoul C, Trifonov E (2002) Distribution of rare triplets along mRNA and their relation to protein folding. J Biomol Struct Dyn 20: 413–420 [DOI] [PubMed] [Google Scholar]
- Mukherji M, Bell R, Supekova L, Wang Y, Orth A, Batalov S, Miraglia L, Huesken D, Lange J, Martin C, Sahasrabudhe S, Reinhardt M, Natt F, Hall J, Mickanin C, Labow M, Chanda S, Cho C, Schultz P (2006) Genome-wide functional analysis of human cell-cycle regulators. Proc Natl Acad Sci USA 103: 14819–14824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orfanoudakis G, Baltzinger M, Meyer D, Befort N, Ebel J, Befort J, Remy P (1987) Cell cycle variations of dinucleoside polyphosphates in synchronized cultures of mammalian cells. Mol Cell Biol 7: 2444–2450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmley JL, Huynen MA (2009) Clustering of codons with rare cognate tRNAs in human genes suggests an extra level of expression regulation. PLoS Genet 5: e1000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percudani R, Pavesi A, Ottonello S (1997) Transfer RNA gene redundancy and translational selection in Saccharomyces cerevisiae. J Mol Biol 268: 322–330 [DOI] [PubMed] [Google Scholar]
- Plotkin JB, Kudla G (2011) Synonymous but not the same: the causes and consequences of codon bias. Nat Rev Genet 12: 32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattner P, Brooks AN, Lowe TM (2005) The tRNAscan-SE, snoscan and snoGPS web servers for the detection of tRNAs and snoRNAs. Nucleic Acids Res 33: W686–W689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp PM, Tuohy TM, Mosurski KR (1986) Codon usage in yeast: cluster analysis clearly differentiates highly and lowly expressed genes. Nucleic Acids Res 14: 5125–5143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal A, Danon T, Cohen A, Milo R, Geva-Zatorsky N, Lustig G, Liron Y, Alon U, Perzov N (2007) Generation of a fluorescently labeled endogenous protein library in living human cells. Nat Protoc 2: 1515–1527 [DOI] [PubMed] [Google Scholar]
- Sigal A, Milo R, Cohen A, Geva-Zatorsky N, Klein Y, Alaluf I, Swerdlin N, Perzov N, Danon T, Liron Y, Raveh T, Carpenter AE, Lahav G, Alon U (2006a) Dynamic proteomics in individual human cells uncovers widespread cell-cycle dependence of nuclear proteins. Nat Methods 3: 525–531 [DOI] [PubMed] [Google Scholar]
- Sigal A, Milo R, Cohen A, Geva-Zatorsky N, Klein Y, Liron Y, Rosenfeld N, Danon T, Perzov N, Alon U (2006b) Variability and memory of protein levels in human cells. Nature 444: 643–646 [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG (2009) Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell 136: 731–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman PT, Sherlock G, Zhang MQ, Iyer VR, Anders K, Eisen MB, Brown PO, Botstein D, Futcher B (1998) Comprehensive identification of cell cycle-regulated genes of the yeast Saccharomyces cerevisiae by microarray hybridization. Mol Biol Cell 9: 3273–3297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi S, Sugiura R, Ma Y, Tokuoka H, Ohta K, Ohte R, Noma A, Suzuki T, Kuno T (2007) Wobble inosine tRNA modification is essential to cell cycle progression in G(1)/S and G(2)/M transitions in fission yeast. J Biol Chem 282: 33459–33465 [DOI] [PubMed] [Google Scholar]
- Tuller T, Carmi A, Vestsigian K, Navon S, Dorfan Y, Zaborske J, Pan T, Dahan O, Furman I, Pilpel Y (2010) An evolutionarily conserved mechanism for controlling the efficiency of protein translation. Cell 141: 344–354 [DOI] [PubMed] [Google Scholar]
- Watkins NJ, SantaLucia JJ (2005) Nearest-neighbor thermodynamics of deoxyinosine pairs in DNA duplexes. Nucleic Acids Res 33: 6258–6267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weygand-Durasevic I, Ibba M (2010) Cell biology. New roles for codon usage. Science 329: 1473–1474 [DOI] [PubMed] [Google Scholar]
- Whipple JM, Lane EA, Chernyakov I, D'Silva S, Phizicky EM (2011) The yeast rapid tRNA decay pathway primarily monitors the structural integrity of the acceptor and T-stems of mature tRNA. Genes Dev 25: 1173–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield M, Sherlock G, Saldanha A, Murray J, Ball C, Alexander K, Matese J, Perou C, Hurt M, Brown P, Botstein D (2002) Identification of genes periodically expressed in the human cell cycle and their expression in tumors. Mol Biol Cell 13: 1977–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright SI, Yau CB, Looseley M, Meyers BC (2004) Effects of gene expression on molecular evolution in Arabidopsis thaliana and Arabidopsis lyrata. Mol Biol Evol 21: 1719–1726 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables S1-10, Supplementary Figures S1-2