Abstract
Cancer regression by gene-modified T cells bearing a chimeric antigen receptor (CAR) exodomain of mouse origin can be limited by the induction of transgene immunogenicity resulting in poor persistence and function in vivo. The development of functionally-active CAR of human origin can address this issue. Here, we constructed and evaluated fully human anti-mesothelin CARs comprised of a human mesothelin-specific single-chain antibody variable fragment (P4 scFv) coupled to T cell signaling domains. Primary human T cells expressing P4 CAR specifically produced proinflammatory cytokines, degranulated and exerted potent cytolytic functions when cultured with mesothelin-expressing tumors in vitro. P4 CAR T cells also mediated bystander killing of mesothelin-negative cancer cells during coculture. CAR reactivity was not abrogated by soluble tumor-secreted or recombinant mesothelin protein even at supraphysiological levels. Importantly, adoptive transfer of P4 CAR-expressing T cells mediated the regression of large, established tumor in the presence of soluble mesothelin in a xenogenic model of human ovarian cancer. Thus, primary human T cells expressing fully human anti-mesothelin CAR efficiently kill mesothelin-expressing tumors in vitro and in vivo and have the potential to overcome the issue of transgene immunogenicity that may limit CAR T cell trials that utilize scFvs of mouse origin.
Introduction
Successful T cell immunotherapeutic strategies are limited by the tolerance to self-antigens, rendering the identification and expansion of tumor-reactive T cells with high avidity for tumor-associated antigens difficult.1 Further, solid tumors often downregulate major histocompatibility complex (MHC) class I and/or other molecules related with the antigen processing machinery as a mechanism for evading immune response.2 To obviate these obstacles, tumor antigen-specific T cells have been engineered to express chimeric antigen receptors (CAR)—or “T bodies”— comprised of an antigen-specific single-chain antibody variable fragment (scFv) fused to intracellular signaling domains derived from receptors involved in lymphocyte activation.3 CARs can functionally redirect T cells with high specificity to various surface antigens on tumor cells independent of MHC restriction and antigen processing, and therefore bypass major mechanisms by which tumors escape immune recognition.
CARs targeting various tumor-associated antigens have been developed, characterized, and tested.4 Despite encouraging preclinical results, CAR therapy has had limited success in the clinic primarily due to poor long-term persistence of the engineered T cells following infusion to patients. This may be attributed in part to the frequent use of scFvs of mouse origin which renders these constructs susceptible to host immune recognition and responses against xenogeneic regions of the molecule. Xenogeneic responses have been observed in clinical trials of CAR therapy. For example, patients who received autologous T cells transduced to express a CAR of mouse origin mounted humoral immune responses against the transgene-bearing cells, which may have limited their persistence in vivo and their ability to respond against antigen-expressing tumor cells.5,6
Mesothelin is a glycosylphosphatidyl inositol-linked membrane glycoprotein overexpressed on the cell surface of mesothelioma, ovarian cancer as well as cancers of the pancreas, stomach, and lung.7,8,9 Mesothelin also exists as a soluble form and is a serum biomarker for lung, mesothelioma, and ovarian cancer.10,11,12 The biological function of mesothelin is still unclear; however mesothelin binds to CA125, a plasma glycoprotein on tumor cells, suggesting that mesothelin may contribute to peritoneal and pleural metastasis.13,14 Mesothelin expression is associated with chemoresistance, shorter disease-free survival, and worse overall survival of patients with epithelial ovarian cancer.15 Accordingly, mesothelin represents an attractive target for immune-based therapies. While vaccination with granulocyte macrophage-colony stimulating factor-transduced pancreatic cancer lines can induce in vivo mesothelin-specific CD8+ T cells with the capacity to kill mesothelin-expressing cancer cells in an MHC class I-restricted fashion,16 more recent work has shown that human T cells bearing an anti-human mesothelin CAR of mouse origin (referred to as SS1) exhibit MHC-independent effector functions in vitro and induce the regression of human mesothelioma xenografts in vivo in immunodeficient mice.17 Here, we address the potential issue of CAR transgene immunogenicity and report that primary human T cells can be efficiently transduced to express a fully human anti-mesothelin-specific CAR using lentiviral vectors, and that fully human CAR-transduced T cells demonstrate specific proinflammatory cytokine secretion and potent cytolytic activity in response to human cancer cells expressing mesothelin, resist soluble mesothelin inhibition, mediate bystander killing, and mediate regression of established human tumor in a xenogeneic mouse model of advanced ovarian cancer.
Results
CAR construction
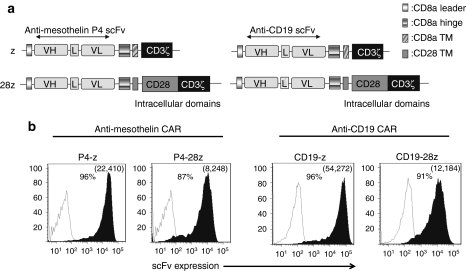
The human anti-human mesothelin-specific P4 scFv was selected for CAR construction based upon its high binding affinity and specificity for mesothelin (108–109/mol/l).18 P4 CAR constructs comprised the P4 scFv linked to a CD8α hinge and transmembrane region, followed by a CD3ζ signaling moiety alone (P4-z) or in tandem with the CD28 intracellular signaling motif were generated (P4-28z; Figure 1a). An anti-CD19 CAR containing CD3ζ alone or with CD28 signaling motifs in tandem (CD19-28z) was used as an antigen-specificity control.19 Primary human T cells were efficiently transduced with CAR lentiviral vectors with transduction efficiencies reproducibly above 90% (Figure 1b), and equilibrated to 80% by adding untransduced T cells for all functional assays.
Figure 1.
Generation and specific immune recognition by P4 anti-mesothelin CAR-transduced human T cells in vitro. (a) Schematic representation of P4 based chimeric antigen receptor (CAR) constructs containing the CD3ζ cytosolic domain alone (P4-z) or in combination with the CD28 costimulatory module (P4-28z). The anti-CD19-z and anti-CD19-28z CARs are shown. (b) P4 CAR expression (filled black histograms) was detected on human CD3-gated cells via recombinant mesothelin protein staining after transduction with lentivirus compared to untransduced T cells (open histograms). Anti-CD19 transduced T cells were detected using protein L followed by SA-PE. Transduction efficiencies are indicated with the percentage of CAR expression and the mean fluorescence intensity of the transduced populations in parentheses. L, linker; P4, antimesothelin scFv; VH, variable H chain; VL, variable L chain; SA-PE, streptavidin-phycoerythrin; scFv, single-chain antibody variable fragment; TM, transmembrane region.
Primary human P4 CAR T cells exert antigen-specific function in vitro
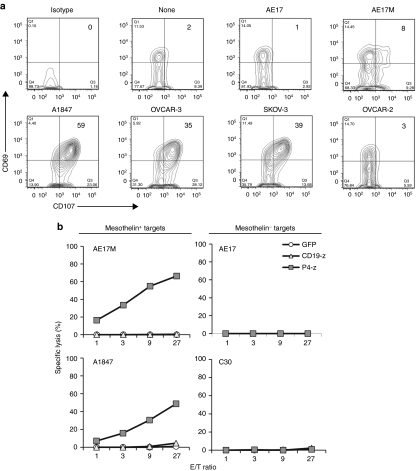
We first tested whether soluble or immobilized mesothelin protein stimulation induces P4-z CAR-transduced T cell activation in vitro. Soluble mesothelin protein did not induce activation of P4 CAR T cells, even at high protein concentrations (5 µg/ml). However, cross-linking of the CAR by immobilized mesothelin protein resulted in robust T cell activation and secretion of high levels of interferon-γ (IFN-γ) (Figure 2a). To evaluate the ability of P4-z CAR T cells to respond to mesothelin expressed on the cell surface, AE17, a mouse malignant mesothelioma cell line, was modified to express human mesothelin (AE17M; Figure 2b).20 P4-z CAR T cells secreted IFN-γ in response to AE17M cells, but not to parental AE17 cells (Figure 2c). Since ovarian cancers frequently express mesothelin,21 a panel of established human ovarian cancer cell lines of disparate human leukocyte antigen -haplotype that expressed surface mesothelin at varying levels (A1847, OVCAR-3, SKOV-3, OVCAR-5) or not at all (C30 and OVCAR-2) was assembled for functional assays (Figure 2b). P4-z CAR-transduced T cells secreted IFN-γ, macrophage inflammatory protein-1α (MIP-1α), tumor necrosis factor-α and interleukin-2 (IL-2) in response to mesothelin+ tumor lines stimulation, but not when stimulated with mesothelinneg lines (Figure 2c). Low levels of IL-4 (<25 pg/ml) and higher levels of IL-10 (<700 pg/ml) were secreted by P4 CAR T cells in response to mesothelin-expressing cancer cells (data not shown). P4-z CAR T cells recognized and responded to stimulation by all human ovarian cancer cells expressing detectable mesothelin on their surface (Figure 2d). The amount of IFN-γ secreted correlated with the level of surface protein expressed by tumor cells (Figure 2e). Control T cells transduced to express green fluorescent protein (GFP) or the CD19-z CAR did not produce cytokines after mesothelin+ tumor stimulation, demonstrating the need for antigen-specificity.
Figure 2.
Mesothelin redirected T cells secrete Th1 proinflammatory cytokines in response to plate-bound mesothelin or tumor cell surface-associated mesothelin. (a) Transduced T cells respond against immobilized but not soluble mesothelin. P4-z T cells or control CD19-z and GFP T cells (105 cells/well) were incubated with either 5 µg/ml of soluble or plate-immobilized mesothelin. After overnight incubation, culture supernatants were analyzed for human Th1/Th2 cytokines using cytometric bead array technology. Concentration of IFN-γ was expressed in pg/ml. (b) Surface mesothelin expression (solid black histograms) by various human ovarian cancer cell lines was evaluated by flow cytometry. The native mouse malignant mesothelioma cell line AE17 which does not express human mesothelin was engineered to express high surface levels of human mesothelin (AE17M) as shown by flow cytometry; isotype antibody control (open gray histograms). (c) Primary human T cells transduced with P4-z preferentially produce Th1 cytokines after stimulation with mesothelin+ cancer cell lines. Transduced T cells (105 CAR+ T cells) were cultured alone (none) or stimulated overnight with an equal number of human mesothelin+ AE17M and A1847 or antigen-negative AE17 and C30 cancer cell lines. Cell-free supernatant from three independent cultures was harvested and pooled after ~20 hours of incubation and the indicated human Th1/Th2 cytokines were quantified using cytometric bead array technology. Values represent cytokine concentration (pg/ml). (d) Antigen-stimulated IFN-γ secretion by P4-z but not CD19-z or GFP T cells following overnight incubation with ovarian cancer cell lines expressing different levels of surface mesothelin. Mean IFN-γ concentration ± SEM (pg/ml) from triplicate cultures is shown. (e) Correlation of mesothelin expression (mean fluorescence intensity; MFI) on mesothelin-expressing tumor cells was plotted versus the production of IFN-γ by P4-z CAR-transduced T cells cocultured with these tumor cells. CAR, chimeric antigen receptor; GFP, green fluorescent protein; IFN, interferon; IL, interleukin; MIP, macrophage inflammatory protein; Th1, T helper 1; Th2, T helper 2; TNF, tumor necrosis factor.
Primary human P4 CAR T cells exhibit potent cytolytic function
Degranulation is a quantitative indicator of lytic function by T cells.22 P4 CAR CD8+ T cells degranulated in response to ovarian cancer cell lines expressing mesothelin (A1847, OVCAR-3, SKOV-3) or AE17M, with upregulated surface coexpression of mobilized CD107 (lysosomal-associated membrane protein 1) and the activation-associated marker CD69, but not when stimulated with mesothelinneg lines (AE17 and OVCAR-2; Figure 3a). AE17M stimulation reproducibly resulted in moderate CD107 upregulation and substantial apoptosis of CAR T cells (data not shown). CD107 expression was restricted to T cells expressing the P4 chimeric receptors (data not shown). Mock-T cells and anti-CD19 CAR T cells did not degranulate in response to mesothelin+ cells (data not shown). In 4 hours chromium release assays, P4-z CAR T cells specifically lysed AE17M cells but not the parental AE17 line (Figure 3b). P4-z CAR T cells also directly and efficiently lysed the mesothelin+ human ovarian cancer cell line A1847, but not mesothelinneg C30 cells (Figure 3b). CD19-z CAR or GFP transduced T cells showed no cytotoxic activity against the same target cells, excluding alloreactivity or nonspecific lysis. These results were reproducible in extended 18-hour assays, with increased specific lysis from P4 CAR T cells (Supplementary Figure S1). Anti-CD19 CAR T cells did however lyse CD19+K562 cells, demonstrating their capacity to respond (Supplementary Figure S2).
Figure 3.
Cytolytic activity of anti-mesothelin lentiviral vector-engineered T cells. (a) P4 CAR T cells degranulate and express T cell activation markers in response to mesothelin-specific stimulation. P4 CAR T cells were cultured without target cells (none) or with the indicated mesothelin- negative or positive tumor cell targets for 5 hours, while being stained by an anti-CD107a, b antibody conjugated with FITC. After the incubation period, T cells were stained for CD8 and CD69 and analyzed by flow cytometry. (b) Antigen-specific killing of mesothelin+ tumor cells by P4 CAR T cells. Primary human T cells transduced to express either P4-z or CD19-z CARs or GFP were cocultured with Cr51-labeled native AE17, AE17M, A1847, and C30 cell lines for 4 hours at the indicated effector to target ratio. Percent specific target cell lysis was calculated as (experimental– spontaneous release) ÷ (maximal – spontaneous release) × 100. Error bars indicate standard deviation. CAR, chimeric antigen receptor; E/T ratio, effector cell to target cell ratio; GFP, green fluorescent protein; FITC, fluorescein isothiocyanate.
Anti-mesothelin CAR T cells mediate bystander killing of mesothelin-negative tumor cells
Since established cell lines may not accurately represent the heterogeneity of complex solid tumor samples, mesothelin expression was evaluated using immunohistochemical analysis in 18 cases of high-grade ovarian serous carcinomas in a tissue microarray. All cases contained at least one primary lesion and one metastatic site. Mesothelin was expressed at various levels among the tumor sites, ranging from undetectable (score 0) to strong staining (score 3+ Figure 4a). Mesothelin was expressed in at least one primary site in all (18/18) cases (Figure 4b), and 94% (17/18) of cases showed mesothelin expression at all primary and metastatic sites. Of all sites, 93% (63/68) expressed mesothelin at some level, and mesothelin scores were generally similar across metastatic and primary sites (Supplementary Figure S3). Heterogeneity in the expression levels of mesothelin protein among the different sites was present in 56% (10/18) of cases. Consistent with existing data,23 regional diversity in mesothelin expression level was observed, with some areas of tumor being devoid of detectable mesothelin expression, suggesting that cancer cells in these regions may be resistant to the direct cytotoxic effects of P4 CAR T cells. We therefore labeled antigen-negative tumor cells (C30 and AE17) with chromium and cocultured them with P4 CAR T cells in the presence or absence of mesothelin+ tumor cells (A1847 and AE17M) to evaluate whether P4 CAR T cells can elicit bystander killing of mesothelinneg tumor cells. No killing of mesothelin-negative cells was detected after 4 hours in either the presence or absence of mesothelin+ tumor cells (Figure 4c). However, after 18 hours of coculture, bystander killing of the mesothelinneg tumor cells by P4 CAR T cells was detected but only in the presence of mesothelin+ tumor targets (Figure 4c). In A1847 human tumor cell cultures, bystander killing was T cell dose-dependent. Importantly, the level of chromium released by bystander killing substantially exceeded the level of spontaneous release in control cultures of tumor alone, excluding the likelihood of chromium reuptake by mesothelin+ cancer cells. Direct killing of mesothelin+ targets by P4 CAR T cells was also observed and was not inhibited by the presence of mesothelinneg targets. Lastly, the bystander effects observed were not due to tumor-secreted mesothelin transfer from antigen-positive to antigen- negative cells (Supplementary Figure S4).
Figure 4.
Bystander killing induced by P4-redirected T cells. (a) Mesothelin immunostaining showing regional diversity of mesothelin expression in high-grade papillary serous ovarian adenocarcinoma. Original magnification was ×200. (b) Heatmap illustration of mesothelin expression level in primary and metastatic ovarian carcinoma cases. (c) P4-z CAR T cells were cocultured with antigen-negative Cr51-labeled AE17 (upper) or C30 tumor cells (lower) in the presence or absence of unlabelled antigen-positive AE17M (upper) or A1847 cells (lower), respectively, for 4 hours (left panels) and 18 hours (right panels) at the indicated effector to target ratio. As positive controls, P4-z CAR T cells were cocultured with antigen-positive Cr51-labeled AE17M (upper) or A1847 tumor cells (lower) in the presence or absence of unlabelled antigen-negative AE17 (upper) or C30 cancer cells (lower), respectively. Percent specific target cell lysis was calculated as (experimental − spontaneous release) ÷ (maximal − spontaneous release) × 100. Error bars indicate standard deviation. CAR, chimeric antigen receptor; E/T ratio, effector cell to target cell ratio.
Tumor-stimulated response by P4 CAR T cells is not inhibited by tumor-secreted mesothelin
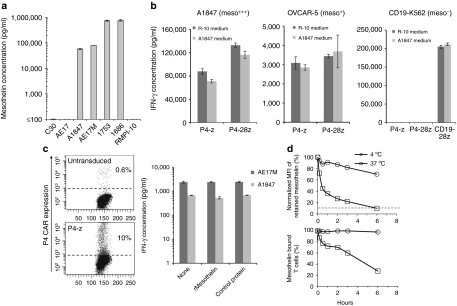
Ovarian cancers secrete high levels of soluble mesothelin protein which is frequently found in the serum and ascites fluid of patients, and represents a biomarker of disease.10,12,24 Consistent with these results, we found high levels of soluble mesothelin (~1 µg/ml) in ascites fluid collected from patients with epithelial ovarian cancer (Figure 5a). High levels of soluble mesothelin were also secreted by cancer cell lines expressing surface mesothelin (A1847 and AE17M), but not mesothelinneg cells (C30 and AE17), in overnight culture (Figure 5a). To determine whether soluble mesothelin blocks P4 CAR T cell activity, cocultures were established in the presence or absence of tumor-secreted mesothelin from cell-free A1847 tumor supernatants (~25 ng/ml final concentration). First (P4-z) or second generation (P4-28z) CAR T cells cocultured with mesothelin+ A1847 cells retained their IFN-γ response in the presence of tumor-derived soluble mesothelin (Figure 5b). Similar results were observed from cocultures of P4 CAR T cells and OVCAR-5 as target cells, which are mesothelinlow (Figure 2b). CD19-redirected T cells also retained their activity against CD19+K562 target cells in the presence of soluble mesothelin protein. Additional coculture assays of P4-z CAR T cells with mesothelin+ cancer cells (A1847 and AE17M) were performed in the presence of recombinant mesothelin protein at supraphysiological levels (5 µg/ml); fivefold higher concentrations than that measured in patient-derived ascites fluid (Figure 5a). To more rigorously assess blocking capacity, P4 CAR T cells were also diluted tenfold with untransduced T cells before coculture to reduce their frequency to 10% of total T cells (Figure 5c). Again, no blocking of P4 CAR T cell tumor recognition and IFN-γ secretion by recombinant mesothelin protein was detected (Figure 5c). Control protein at identical concentration also did not block T cell responses. We next longitudinally measured by flow cytometry the dissociation of biotinylated-mesothelin protein that was prebound to P4-z CAR T cells in the presence of tenfold higher concentration of unbiotinylated protein and incubated at either 37 °C or 4 °C, as a surrogate indicator of CAR affinity. A modest dissociation of biotinylated mesothelin (30%) was detected over 6 hours at 4 °C based upon mean fluorescence intensity, although all CAR T cells remained bound at some level (Figure 5d). At 37 °C progressive, substantial loss of detectable surface protein was evident within 10 minutes and fully displaced to background levels (90%) after 6 hours, showing the transient nature of P4 CAR binding to soluble mesothelin protein under standard culture conditions that may provide the opportunity for CAR re-engagement with surface bound antigen.
Figure 5.
P4 CAR T cells exhibit effector functions against tumor cells in the presence of recombinant or tumor-derived mesothelin. (a) Soluble mesothelin is present in the supernatant of mesothelin expressing tumor cells and in the ovarian cancer-derived ascites fluid. Tumor-free cell supernatants or ascites fluid were analyzed for the presence of soluble mesothelin using ELISA assay as described in Materials and Methods. (b) Anti-mesothelin T cells were incubated with mesothelin+ tumor cells in the presence of A1847 mesothelin rich medium or RPMI 10% FBS medium. After overnight incubation, supernatants were assayed for IFN-γ by ELISA (results depict the mean ± SEM of triplicate wells). (c) T cells were incubated overnight with AE17M and A1847 tumor cells in the presence or absence of soluble mesothelin or control. The amount of IFN-γ in culture supernatants was determined using ELISA (results depict the mean ± SEM of triplicate wells). (d) Mesothelin dissociation assay. P4-z CAR T cells were labeled with recombinant biotinylated mesothelin and then incubated at 37 °C or 4 °C in a time course in the presence of a tenfold excess of nonbiotinylated mesothelin. Antigen retention on the cell surface was assessed by flow cytometry by adding SA-PE after the end of each culture period. (Upper) Percent retained mesothelin (y-axis) was normalized and scored as mean fluorescence intensity (MFI) postincubation ÷ preincubation MFI × 100. (Lower) The percentage of prebound CD3+ T cells with positive mesothelin binding compared to similarly treated untransduced T cells from the same donor as control was plotted on the y-axis. ELISA, enzyme-linked immunosorbent assay; FBS, fetal bovine serum; IFN, interferon; SA-PE, streptavidin-phycoerythrin.
Antitumor activity of primary human T cells expressing P4 anti-mesothelin CAR in vivo
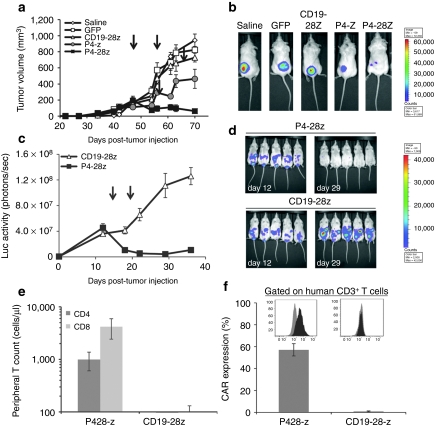
To evaluate the capacity of P4 CAR T cells to induce regression of large, established human tumors in vivo, second generation P4-28z CAR T cells were used, since it has been previously shown that costimulated CAR T cells exhibit enhanced effector functions in vivo.25,26,27 P4-28z CAR T cells used for infusion showed specific and enhanced IFN-γ secretion as well as IL-2 and tumor necrosis factor-α in response to mesothelin+ tumor stimulation in vitro, compared with P4-z CAR T cells, confirming their functional reactivity (Supplementary Figure S5a,b). NOD/SCID/γ-chain−/− (NSG) mice with established subcutaneous A1847 tumors (≥150 mm3) received intratumoral injections of CAR T cells on days 47 and 57 post-tumor inoculation. Tumor growth was modestly inhibited in mice receiving P4-z CAR T cells (P = 0.07), compared to saline, CD19-28z CAR T cells or GFP T cell control groups 3 weeks after first T cell dose (Figure 6a). In contrast, mice receiving P4-28z T cells experienced rapid tumor regression which was significantly better than P4-z T cells (P < 0.05), indicating that incorporation of CD28 signals enhances net antitumor activity in vivo (Figure 6a,b). Furthermore, a xenogeneic model of advanced intraperitoneal metastatic cancer was established to evaluate the functional activity of P4 CAR T cells against tumor in a more physiologically relevant compartment. NSG mice were inoculated intraperitoneally with A1847 fLuc+ cells. Two weeks postinoculation, two intravenous injections of P4-28z T cells resulted in rapid tumor regression in all mice (Figure 6c,d). Disease progression occurred in all mice receiving CD19-28z T cells. Mice treated with P4-28z CAR did not develop distended abdomens or ascites, and exhibited a profound enhancement in tumor-related survival (P < 0.05) with no cases of tumor-related mortality. In the CD19-28z control group, all mice had to be euthanized due to disease progression 32–45 days post first T cell infusion (data not shown). Three weeks after the first T cell dose, peripheral blood CD8+ T and CD4+ T cell counts from mice injected with P4-28z T cells were significantly higher than in the CD19-28z group (P < 0.05; Figure 6e). Mice that received P4-28z CAR T cell transfer also had increased persistence of human T cells bearing surface scFv (57%), compared to CD19-28z CAR T cell-treated mice (0.82%; Figure 6f). Similar differences in blood counts and CAR expression were observed in mice with subcutaneous tumors (Supplementary Figure S5c,d).
Figure 6.
Mesothelin re-directed T cells exert potent effector functions in vivo. (a) Rapid regression of large pre-established tumors in vivo by P4-28z CAR T cells: effect of the CD28 costimulatory signaling domain. NSG mice bearing established subcutaneous tumor were treated with intratumoral injections of 5 × 106 P4-z and P4-28z CAR+ T cells or control CD19-28z and GFP T cells or saline on day 45 and 55. Tumor growth was assessed by caliper measurement. Tumors treated with P4-28z CAR-transduced T cells (~75% CAR expression) rapidly regressed (arrows indicate days of T cell infusion); tumors treated with saline, GFP or CD19-28z CAR-transduced T cells did not regress 3 weeks post-first T cell dose (P < 0.05). Equal doses of P4-z CAR-transduced T cells (~75% CAR expression) only slowed the tumor growth (P = 0.05). Results are expressed as a mean tumor volume (mm3 ± SEM) with n = 5 for all groups. (b) A1847 fLuc+ bioluminescence signal was decreased in P4-z and P4-28z CAR-treated mice compared with the CD19-z and the control treatment groups 3 weeks after the first T cell dose. (c) P4-28z T cells inhibit tumor outgrowth and ascites formation in A1847 murine model of peritoneal carcinomatosis. NSG mice received intraperitoneal injection of 10 × 106 A1847 fLuc+ tumor cells and were randomized into two groups before beginning therapy with 10 × 106 T cells expressing P4-28z or CD19-28z via intravenous infusion on day 14 and 19 after tumor inoculation. Tumor growth was monitored by bioluminescence imaging (days 12 and 29 shown). Photon emission from fLuc+ tumor cells was quantified and the mean ± SEM bioluminescence signal determined with n = 5 for both groups. (d) A1847 fLuc+ bioluminescence signal was rapidly decreased and reached the background luminescence level in P4-28z CAR-treated mice compared with the CD19-28z 2 weeks after the first T cell dose. (e) Stable persistence of P4 CAR-engineered human T cells in vivo. Peripheral blood was collected 3 weeks after the first T cell infusion and quantified for the absolute number of human CD4+ and CD8+ T cells/µl of blood. Mean cell count ± SEM is shown with n = 5 for all groups. (f) Surface CAR expression on persisting P4+ and CD19-specific human CD3+ T cells derived from the blood of treated mice measured by flow cytometry. Mean CAR+ expression frequency per CD3+ T cell ± SEM per group is shown with n = 5 for all groups. CAR, chimeric antigen receptor; GFP, green fluorescent protein; NSG, NOD/SCID/γ-chain−/−.
Discussion
To date, only one other mesothelin-specific CAR has been reported,17 the SS1 CAR, whose specificity is derived from the mouse anti- human mesothelin scFv SS1.28 SS1 CAR T cells exert potent effector functions against cancer cell lines expressing mesothelin in vitro and eradicate established mesotheliomas in preclinical studies.17 Two phase I studies29,30 of SS1 coupled to Pseudomonas exotoxin A (PE38) have shown antitumor activity in subjects with mesothelin+ tumors and a chimeric antibody-based on SS1 scFv (MORAb-009) remains under investigation in a phase II study for mesothelioma and pancreatic cancer ("http://www.morphotek.com/page2597.aspx). Still, human anti-mouse antibody responses have not been assessed in these SS1-treated subjects. However, the mouse origin of the SS1 scFv predicts its propensity for inducing xenogeneic responses upon transfer to human subjects.
The immunogenicity of mouse transgenes is noted in numerous trials of adoptive immunotherapy using autologous T cells modified to express mouse-derived scFvs or tumor antigen-specific T cell receptors (TCRs).5,31,32,33 Transfer of T cells outfitted with xenogeneic CARs to immunocompetent subjects induces transgene-specific immune responses, which may limit the persistence and function of the gene-modified cells.5,31,32 Humoral responses are often noted. Induction of both humoral and cellular immune responses have been reported in patients treated with ex vivo-engineered anti-CAIX CAR T cells.33 Such responses were directed against the xenogeneic complementarity-determining and framework regions of the CAR variable domains. Although lymphodepleting chemotherapy can transiently disable endogenous T and B cell responses, transfer of autologous T cells bearing a murine anti-CEA TCR to patients with metastatic colorectal cancer after lymphodepleting preconditioning still induced anti-mouse TCR-specific IgG antibodies that was capable of impairing the functionality of carcinoembryonic antigen (CEA)-specific TCR T cells in vitro.34 Although a significant correlation between transgene immunogenicity and efficacy has not been established, the above mentioned clinical experiences rationalize the construction of receptors from human derivatives to minimize the immunogenic potential of CARs and optimize therapy. Our CAR construct utilizes the human anti-mesothelin P4 scFv, originally developed by selective enrichment of a yeast-display human scFv library,35 which demonstrates high specificity with binding of Meso-Ig protein detectable in the range of ng/ml.18
An important issue concerning antibody-based therapies directed against mesothelin is the stimulatory or possible inhibitory effect of soluble antigen on the ability to target membrane-bound mesothelin, particularly in ovarian cancer patients where high levels of soluble mesothelin is present in serum or ascites fluid.10,12,24 Immobilized mesothelin protein was able to activate P4 CAR T cells, a feature not detectable when soluble mesothelin was used. Until now, resistance of anti-mesothelin CAR T cells has not been tested. P4 CAR T cells challenged with ovarian cancer cells expressing high or low levels of mesothelin-resisted functional inhibition by soluble mesothelin protein, even at supraphysiological levels. Resistance was confirmed by their capacity to mediate the regression of established tumors in vivo in the presence of high levels of cancer-secreted mesothelin in the serum of treated mice (59 ng/ml ± 2.87 ng/ml, n = 3, data not shown). Our findings expand upon prior reports,36,37,38 and suggest a CAR's ability to resist soluble antigen blockade lies in its ability to disengage soluble antigen at physiological temperature, and the need for cross-linking of a critical mass of CAR receptors on the T cell surface which is provided by immobilized, but not soluble, mesothelin protein.
The P4 anti-mesothelin CAR allows for the direct cytolytic destruction of antigen-positive tumor cells, however, tumor antigens are often heterogeneously expressed among particular cancer histologies. Our immunohistochemical analysis of multiple serous adenocarcinoma tumor sections reveals disparate regional mesothelin expression with little to no detectable mesothelin expression in certain tumor areas. We find that antigen-activated P4 CAR T cells may exert effector functions capable of inducing bystander killing of cancer cells not expressing the target antigen. Soluble factors released by CAR-activated effector cells are indeed able to inhibit the growth of tumor cells lacking a cognate antigen.39 The mechanism accounting for CAR-mediated bystander cytotoxicity in our study is unknown, however, our results suggest that antigen-less tumor cells within a heterogeneous field of mesothelin-expressing tumor may be susceptible to indirect destruction by mesothelin-redirected T cells that has previously reacted against adjacent antigen-positive cancer cells. However, bystander activity may cause damage to the surrounding normal tissue. Thus, fine-tuning of CAR specificity and activity is critical.
P4 CAR T cells control large, well-established tumors in immunodeficient mice. Consistent with multiple reports,19,40,41 first generation CAR possessing only CD3z signaling had modest impact on tumor outgrowth in vivo, while second generation CAR comprising CD3z fused to a CD28 costimulatory domain mediated tumor regression in vivo.27 T cells modified to express an irrelevant anti-CD19 CAR or GFP were unable to alter tumor growth demonstrating the high specificity of the CAR system and ruling out the possibility of xenogeneity as the source of the tumor response. Persistence of human T cells was highest in mice treated with P4 CARs compared with control CD19-28z T cells demonstrating that antigen exposure is sufficient to support preferential CAR-transduced T cell engraftment and persistence in vivo. However, the persistence of P4 CAR T cells was independent of the addition of the CD28 costimulatory domain, as noted previously.40
Our results show that primary human T-cells expressing a fully human CAR-targeting mesothelin are highly effective in response controlling large, well-established tumors. Although the safety and efficacy of mesothelin-directed CAR therapy has yet to be tested in the clinic, the fully human P4 CAR described here is well positioned to resist soluble protein inhibition, elude transgene immunogenicity and maximize antitumor efficacy of adoptively transferred T cells in vivo.
Materials and Methods
Anti-mesothelin CAR construction. The pTOR002 plasmid containing the anti-mesothelin scFv P418,42 was used as a template for PCR amplification of a 795-bp P4 fragment using the following primers: 5′-GCGAGATCTCAGGTACAGCTGCAGCAGTC-3′ (BglII is underlined) and 5′-CGCGCTAGC GGAGAGGACGGTCAGTTGGG-3′ (NheI is underlined). The resulting PCR product containing a BglII site on the 5′- end and a NheI site on the 3′- end was digested with the relevant enzymes. Third generation self-inactivating lentiviral expression vectors pELNS previously described were digested with BamHI and NheI to create compatible cohesive ends and gel purified. The digested PCR products were then inserted into the pELNS vector containing CD3z or CD28-CD3z T cell signaling domains in which transgene expression is driven by the elongation factor-1α (EF-1α) promoter. The resulting construct was designated pELNS-P4-z/CD28-z.
Recombinant lentivirus production. High-titer replication-defective lentiviral vectors were produced and concentrated as previously described. 293T human embryonic kidney cells were seeded at 10 × 106 per T-150 tissue culture flask 24 hours before transfection. All plasmid DNA were purified using the QIAGEN Endo-free Maxi prep kit (Qiagen, Valencia, CA). Cells were transfected with 7 µg pVSV-G (VSV glycoprotein expression plasmid), 18 µg of µg pRSV.REV (Rev expression plasmid), 18 µg of pMDLg/p.RRE (Gag/Pol expression plasmid), and 15 µg of pELNS transfer plasmid using Express Inn (Open Biosytems, Huntsville, AL). The viral supernatant was harvested at 24 and 48 hours post-transfection. Viral particles were concentrated and resuspended in 0.4 ml by ultracentrifugation for 3 hours at 25,000 rpm with a Beckman SW28 rotor (Beckman Coulter, Fullerton, CA).
Human T cell transduction. Primary human T cells, which were purchased from the Human Immunology Core at University of Pennsylvania, were isolated from healthy volunteer donors following leukapheresis by negative selection. All specimens were collected under a University Institutional Review Board-approved protocol, and written informed consent was obtained from each donor. T cells were cultured in complete media (RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 µg/ml streptomycin sulfate, 10-mmol/l HEPES), and stimulated with anti-CD3 and anti-CD28 mAbs coated beads (Invitrogen, Carlsbad, CA) as described.43 Twelve to twenty-four hours after activation, T cells were transduced with lentiviral vectors at multiplicity of infection of ~5–10. CD4+ and CD8+ T cells used for in vivo experiments were mixed at 1:1 ratio, activated, and transduced. Human recombinant interleukin-2 (Novartis, St Louis, MO) was added every other day to a 50 IU/ml final concentration. Cell density of 0.5–1 × 106 cells/ml was maintained. Rested engineered T cells were adjusted for identical transgene expression before functional assays.
Cell lines. Lentivirus packaging was performed in the immortalized normal fetal renal 293T cell line purchased from American Type Culture Collection (Manassas, VA). Human cell lines used in immune-based assays include the established human ovarian cancer cell lines SKOV3, A1847, OVCAR3, OVCAR5, OVCAR-2, and C30. For bioluminescence assays, target cancer cell lines were transfected to express firefly luciferase (fLuc), enriched by antibiotic selection positive expression by bioluminescence imaging. For specificity controls, the mouse malignant mesothelioma cell line, AE17, was transduced with lentivirus to express human mesothelin (AE17-M). K562, a human erythroleukemic cell line, CD19-expressing K562 (CD19+K562) cells, and mesothelin/CD19-expressing K562 (Meso+CD19+K562) cells were kindly provided by Carl June (University of Pennsylvania). 293T cells and tumor cell lines were maintained in RPMI-1640 (Invitrogen) supplemented with 10% (vol/vol) heat-inactivated FBS, 2 mmol/l L-glutamine, and 100 µg/ml penicillin, and 100 U/ml streptomycin. All cell lines were routinely tested for mycoplasma contamination.
Cytokine release assays. Cytokine release assays were performed by coculture of 1 × 105 T cells with either soluble or immobilized yeast-derived recombinant mesothelin or 1 × 105 target cells per well in triplicate in 96-well round bottom plates in a final volume of 200 µl of T cell media. After 20–24 hours, coculture supernatants were assayed for presence of IFN-γ using an ELISA Kit, according to manufacturer's instructions (Biolegend, San diego, CA). Values represent the mean of triplicate wells. IL-2, IL-4, IL-10, tumor necrosis factor-α and MIP-1α cytokines were measured by flow cytometry using Cytokine Bead Array, according to manufacturer's instructions (BD Biosciences, San Jose, CA).
Cytotoxicity assays. 51Cr release assays were performed as described.44 Target cells were labeled with 100 µCi 51Cr at 37 µC for 1.5 hours. Target cells were washed three times in phosphate-buffered saline (PBS), resuspended in complete media at 105 viable cells/ml and 100 µl added per well of a 96-well V-bottom plate. Effector cells were washed twice in complete media and added to wells at the given ratios. Plates were quickly centrifuged to settle cells, and incubated at 37 °C in a 5% CO2 incubator for 4 or 18 hours after which time the supernatants were harvested, transferred to a LumaPlate and counted using a 1450 Microbeta Liquid Scintillation Counter (Perkin-Elmer, Waltham, MA). For the bystander cytotoxicity assays, 51Cr labeled mesothelin-negative target cells were mixed with unlabeled mesothelin-positive targets cells at a ratio 1:1 for a final concentration of 105 viable cells/ml before being incubated with the effector T cells at the given ratios. Spontaneous 51Cr release was evaluated in target cells incubated with medium alone. Maximal 51Cr release was measured in target cells incubated with sodium dodecyl sulfate at a final concentration of 2% (vol/vol). Percent specific lysis was calculated as (experimental − spontaneous lysis/maximal − spontaneous lysis) × 100.
Measurement of soluble secreted mesothelin. Cell culture supernatants, ascites fluids, and NSG mice-derived serum were analyzed for their mesothelin concentration using the Human Mesothelin DuoSet Kit (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions.
Blocking assays. Mesothelin-positive A1847 tumor cells were seeded at wells of a 96-well U-bottom plate at 2 × 105 cell/200 µl and cultured overnight. The next day the media was removed and cells were washed once with PBS or left untreated. P4 CAR T cell were then added to the tumor cells left untreated or to whom fresh medium was added and the percentage of inhibition of tumor recognition was calculated. To calculate the background inhibition possibly derived from immunoinhibitory cytokines anti-CD19BBZ T cells were resuspended in either A1847 conditioned media prior their addition to adhered and washed K562-CD19 cells. For the blocking experiment using recombinant mesothelin P4 CARs were incubated with the A1847 or AE17M cells in the presence of 5 µg/ml recombinant mesothelin and the IFN-γ secretion was determined after an overnight coculture.
Immunohistochemistry. Institutional review board approval was obtained. We retrieved records from 18 consecutive patients with metastatic papillary serous ovarian cancer (FIGO stage IIB and above) undergoing primary resection at our institution between 2005 and 2008. Slides were reviewed and annotated and paraffin-embedded tissue blocks were selected to construct a tissue microarray of primary and metastatic tumors. Including primary sites and metastases, a total of 72 tumor deposits were represented on the array. A mean of 3.7 sites were included per patient. The most common metastatic sites included omentum, peritoneum (e.g., cul-de-sac), uterine serosa, and bowel wall. For each block, triplicate 0.6 mm cores of tumor were placed on a tissue microarray. Paraffin sections of the array (5 µm) were stained with anti-mesothelin (1:10, cat #MS-1320; NeoMarkers, Fremont, CA) according to standard protocols in our laboratory. Mesothelin expression in each core was scored by light microscopy at ×200 magnification using a semiquantitative scale ranging from 0 to 3.
Antigen dissociation assay. P4 CAR or untransduced T cell were harvested, washed once with fluorescent-activated cell sorting buffer and stained with 0.5 µg/ml yeast-derived biotinylated-mesothelin protein18 for 30 minutes at 4 °C. Then the cells were washed two times before the addition of 5 µg/ml of nonbiotinylated mesothelin competitor and incubation at a 4 °C or 37 °C for different time points (0 < t > 6 hours). At indicated time points cells were removed from 4 °C or 37 °C, washed again, labeled with biotinylated streptavidin, washed and analyzed for percent mesothelin-positive and mean fluorescence intensity by flow cytometry.
Xenograft model of ovarian cancer. All animals were obtained from the Stem Cell and Xenograft Core of the Abramson Cancer Center, University of Pennsylvania. Six to 12-week-old NSG mice were bred, treated, and maintained under pathogen-free conditions in-house under University of Pennsylvania IACUC approved protocols. For an established ovarian cancer model, 6- to 12-week-old female NSG mice were inoculated subcutaneously with 1 × 106 A1847 fLuc+ cells on the flank on day 0. After tumors become palpable at about 6 weeks, human primary T cells were activated, and transduced as described above. After 2 weeks of T cell expansion, when the tumor burden was ~150–250 mm3, mice were injected intratumorally with T cells. Tumor dimensions were measured with calipers, and tumor volumes calculated using the formula V = 1/2(length × width2), where length is the greatest longitudinal diameter and width is the greatest transverse diameter. Animals were imaged before T cell transfer and about every week thereafter to evaluate tumor growth. Photon emission from fLuc+ cells was quantified using the “Living Image” software (Xenogen, Alameda, CA) for all in vivo experiments. For the intraperitoneal model of ovarian cancer, 8 to 12-week-old NSG mice were injected intraperitoneally with 10 × 106 A1847 fLuc+ cells. Two weeks after peritoneal inoculation, mice bearing established A1847 tumors received intravenously administered T cells. Mice were sacrificed when they became distressed and moribund. To monitor the extent of tumor progression, the mice were imaged weekly. In all models, five mice were randomized per group before treatment.
Bioluminescence imaging. Tumor growth was also monitored by Bioluminescent imaging. Bioluminescent imaging was performed using Xenogen IVIS imaging system and the photons emitted from fLuc-expressing cells within the animal body were quantified using Living Image software (Xenogen). Briefly, mice bearing A1847 fLuc+ tumor cells were injected intraperitoneally with D-luciferin (150 mg/kg stock, 100 µl of D-luciferin per 10 g of mouse body weight) suspended in PBS and imaged under isoflurane anesthesia after 5~10 minutes. A pseudocolor image representing light intensity (blue, least intense; red, most intense) was generated using Living Image. Bioluminescent imaging findings were confirmed at necropsy.
Flow cytometric analysis. The following monoclonal antibodies were used for phenotypic analysis: PE mouse anti-human CD3; FITC anti-human CD4; APC anti-human CD8; PE-anti-human CD45; APC-Cy7 anti-human CD69; FITC anti-human CD107a; and FITC anti-human CD107b. 7-Aminoactinomycin D (7-AAD) was used for viability staining. All monoclonal antibodies were purchased from BD Biosciences. In T cell transfer experiments, peripheral blood was obtained via retro-orbital bleeding and stained for the presence of human CD45, CD4, and CD8 T cells. After gating on the human CD45+ population, the CD4+ and CD8+ subsets were quantified using TruCount tubes (BD Biosciences) with known numbers of fluorescent beads as described in the manufacturer's instructions. Tumor cell surface expression of mesothelin was performed using soluble P4 anti-mesothelin scFv followed by PE-labeled streptavidin. T cell surface expression of the P4 or anti-CD19 CAR was evaluated using V5-tagged recombinant mesothelin followed by Alexa-647 conjugated anti-V5 monoclonal antibody (AbD Serotec, Raleigh, NC) or biotinylated protein L (GenScript, Piscataway, NJ) followed by PE-labeled streptavidin respectively. Acquisition and analysis was performed using a BD FACS CANTO II with DIVA software (BD Biosciences).
Degranulation assay. The degranulation assay was performed as earlier described22 with minor modifications. Target cells (1 × 105) were cocultured with an equal number of effector cells in 0.1 ml per well in a 96-well plate in triplicate. Control wells contained either T cells alone. Anti-CD107a and Ab Anti-CD107b (10 µl per well) or IgG1 conjugated to FITC (BD Biosciences) were added in addition to 1 µl/sample of monensin (BD Biosciences) and incubated for 4–5 hours at 37 °C. Cells were washed two times with PBS, stained for expression of the P4 CAR, CD8, and CD69 and analyzed on a FACS DIVA II.
Statistical analysis. Statistical analysis was performed using two-way repeated measures analysis of variance for the tumor burden (tumor volume, photon counts). Student's t-test was used to evaluate differences in absolute numbers of transferred T cells, cytokine secretion, and specific cytolysis. Kaplan–Meier survival curves were compared using the log-rank test. GraphPad Prism 4.0 (GraphPad Software, La Jolla, CA) was used for the statistical calculations. P < 0.05 was considered significant.
SUPPLEMENTARY MATERIAL Figure S1. Antigen-specific killing of mesothelin–positive tumor cells by P4 CAR T cells in 18-hour 51Cr-release assay. Figure S2. Antigen-specific killing of CD19+ targets by anti-CD19 CAR T cells 4 and 18 hours post co-culture. Figure S3. Expression levels of mesothelin protein among the different serous ovarian adenocarcinoma sites in individual cases. Figure S4. Mesothelin is not transferred from antigen-positive to antigen-negative cells. Figure S5. In vitro cytokine secretion and in vivo persistence of first and second generation P4 CAR T cells.
Acknowledgments
The authors gratefully acknowledge Drs Carl H. June, Michael Kalos, Gwenn Danet-Desnoyers, Steven M. Albelda, Edmund K. Moon, Michael C. Milone, and De-Gang Song, as well as Denarda Dangaj, for helpful suggestions, discussions and reagents. This work was supported by grants from the W.W. Smith Charitable Trust, the Sandy Rollman Ovarian Cancer Foundation and the Joint Fox Chase Cancer Center, and University of Pennsylvania Ovarian Cancer SPORE (P50 CA083638).
Supplementary Material
Antigen-specific killing of mesothelin–positive tumor cells by P4 CAR T cells in 18-hour 51Cr-release assay.
Antigen-specific killing of CD19+ targets by anti-CD19 CAR T cells 4 and 18 hours post co-culture.
Expression levels of mesothelin protein among the different serous ovarian adenocarcinoma sites in individual cases.
Mesothelin is not transferred from antigen-positive to antigen-negative cells.
In vitro cytokine secretion and in vivo persistence of first and second generation P4 CAR T cells.
REFERENCES
- Mansoor W, Gilham DE, Thistlethwaite FC., and, Hawkins RE. Engineering T cells for cancer therapy. Br J Cancer. 2005;93:1085–1091. doi: 10.1038/sj.bjc.6602839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han LY, Fletcher MS, Urbauer DL, Mueller P, Landen CN, Kamat AA.et al. (2008HLA class I antigen processing machinery component expression and intratumoral T-Cell infiltrate as independent prognostic markers in ovarian carcinoma Clin Cancer Res 143372–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross G, Waks T., and, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci USA. 1989;86:10024–10028. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jena B, Dotti G., and, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood. 2010;116:1035–1044. doi: 10.1182/blood-2010-01-043737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw MH, Westwood JA, Parker LL, Wang G, Eshhar Z, Mavroukakis SA.et al. (2006A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer Clin Cancer Res 1220 Pt 16106–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JR, Digiusto DL, Slovak M, Wright C, Naranjo A, Wagner J.et al. (2007Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma Mol Ther 15825–833. [DOI] [PubMed] [Google Scholar]
- Chang K., and, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci USA. 1996;93:136–140. doi: 10.1073/pnas.93.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE.et al. (2001Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE) Clin Cancer Res 73862–3868. [PubMed] [Google Scholar]
- Ho M, Bera TK, Willingham MC, Onda M, Hassan R, FitzGerald D.et al. (2007Mesothelin expression in human lung cancer Clin Cancer Res 131571–1575. [DOI] [PubMed] [Google Scholar]
- Scholler N, Fu N, Yang Y, Ye Z, Goodman GE, Hellström KE.et al. (1999Soluble member(s) of the mesothelin/megakaryocyte potentiating factor family are detectable in sera from patients with ovarian carcinoma Proc Natl Acad Sci USA 9611531–11536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson BW, Creaney J, Lake R, Nowak A, Musk AW, de Klerk N.et al. (2005Soluble mesothelin-related protein–a blood test for mesothelioma Lung Cancer 49 (suppl. 1S109–S111. [DOI] [PubMed] [Google Scholar]
- Hassan R, Remaley AT, Sampson ML, Zhang J, Cox DD, Pingpank J.et al. (2006Detection and quantitation of serum mesothelin, a tumor marker for patients with mesothelioma and ovarian cancer Clin Cancer Res 12447–453. [DOI] [PubMed] [Google Scholar]
- Rump A, Morikawa Y, Tanaka M, Minami S, Umesaki N, Takeuchi M.et al. (2004Binding of ovarian cancer antigen CA125/MUC16 to mesothelin mediates cell adhesion J Biol Chem 2799190–9198. [DOI] [PubMed] [Google Scholar]
- Kaneko O, Gong L, Zhang J, Hansen JK, Hassan R, Lee B.et al. (2009A binding domain on mesothelin for CA125/MUC16 J Biol Chem 2843739–3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WF, Huang CY, Chang MC, Hu YH, Chiang YC, Chen YL.et al. (2009High mesothelin correlates with chemoresistance and poor survival in epithelial ovarian carcinoma Br J Cancer 1001144–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas AM, Santarsiero LM, Lutz ER, Armstrong TD, Chen YC, Huang LQ.et al. (2004Mesothelin-specific CD8(+) T cell responses provide evidence of in vivo cross-priming by antigen-presenting cells in vaccinated pancreatic cancer patients J Exp Med 200297–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM.et al. (2009Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains Proc Natl Acad Sci USA 1063360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan L, Gross JA, Nevin B, Urban N., and, Scholler N. Development and in vitro validation of anti-mesothelin biobodies that prevent CA125/Mesothelin-dependent cell attachment. Cancer Lett. 2007;255:263–274. doi: 10.1016/j.canlet.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Milone MC, Fish JD, Carpenito C, Carroll RG, Binder GK, Teachey D.et al. (2009Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo Mol Ther 171453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackaman C, Bundell CS, Kinnear BF, Smith AM, Filion P, van Hagen D.et al. (2003IL-2 intratumoral immunotherapy enhances CD8+ T cells that mediate destruction of tumor cells and tumor-associated vasculature: a novel mechanism for IL-2 J Immunol 1715051–5063. [DOI] [PubMed] [Google Scholar]
- Hassan R, Kreitman RJ, Pastan I., and, Willingham MC. Localization of mesothelin in epithelial ovarian cancer. Appl Immunohistochem Mol Morphol. 2005;13:243–247. doi: 10.1097/01.pai.00000141545.36485.d6. [DOI] [PubMed] [Google Scholar]
- Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M.et al. (2003Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation J Immunol Methods 28165–78. [DOI] [PubMed] [Google Scholar]
- Yen MJ, Hsu CY, Mao TL, Wu TC, Roden R, Wang TL.et al. (2006Diffuse mesothelin expression correlates with prolonged patient survival in ovarian serous carcinoma Clin Cancer Res 123 Pt 1827–831. [DOI] [PubMed] [Google Scholar]
- Hellstrom I. Mesothelin in serum. Biochem Biophys Res Commun. 2008;376:629; author reply 630. doi: 10.1016/j.bbrc.2008.08.080. [DOI] [PubMed] [Google Scholar]
- Alvarez-Vallina L., and, Hawkins RE. Antigen-specific targeting of CD28-mediated T cell co-stimulation using chimeric single-chain antibody variable fragment-CD28 receptors. Eur J Immunol. 1996;26:2304–2309. doi: 10.1002/eji.1830261006. [DOI] [PubMed] [Google Scholar]
- Beecham EJ, Ma Q, Ripley R., and, Junghans RP. Coupling CD28 co-stimulation to immunoglobulin T-cell receptor molecules: the dynamics of T-cell proliferation and death. J Immunother. 2000;23:631–642. doi: 10.1097/00002371-200011000-00004. [DOI] [PubMed] [Google Scholar]
- Kowolik CM, Topp MS, Gonzalez S, Pfeiffer T, Olivares S, Gonzalez N.et al. (2006CD28 costimulation provided through a CD19-specific chimeric antigen receptor enhances in vivo persistence and antitumor efficacy of adoptively transferred T cells Cancer Res 6610995–11004. [DOI] [PubMed] [Google Scholar]
- Chowdhury PS, Viner JL, Beers R., and, Pastan I. Isolation of a high-affinity stable single-chain Fv specific for mesothelin from DNA-immunized mice by phage display and construction of a recombinant immunotoxin with anti-tumor activity. Proc Natl Acad Sci USA. 1998;95:669–674. doi: 10.1073/pnas.95.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan R, Bullock S, Premkumar A, Kreitman RJ, Kindler H, Willingham MC.et al. (2007Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers Clin Cancer Res 135144–5149. [DOI] [PubMed] [Google Scholar]
- Kreitman RJ, Hassan R, Fitzgerald DJ., and, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15:5274–5279. doi: 10.1158/1078-0432.CCR-09-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers CH, Sleijfer S, Vulto AG, Kruit WH, Kliffen M, Debets R.et al. (2006Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience J Clin Oncol 24e20–e22. [DOI] [PubMed] [Google Scholar]
- Lamers CH, Langeveld SC, Groot-van Ruijven CM, Debets R, Sleijfer S., and, Gratama JW. Gene-modified T cells for adoptive immunotherapy of renal cell cancer maintain transgene-specific immune functions in vivo. Cancer Immunol Immunother. 2007;56:1875–1883. doi: 10.1007/s00262-007-0330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers CH, Willemsen R, van Elzakker P, van Steenbergen-Langeveld S, Broertjes M, Oosterwijk-Wakka J.et al. (2011Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells Blood 11772–82. [DOI] [PubMed] [Google Scholar]
- Parkhurst MR, Yang JC, Langan RC, Dudley ME, Nathan DA, Feldman SA.et al. (2011T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis Mol Ther 19620–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhaus MJ, Siegel RW, Opresko LK, Coleman JR, Feldhaus JM, Yeung YA.et al. (2003Flow-cytometric isolation of human antibodies from a nonimmune Saccharomyces cerevisiae surface display library Nat Biotechnol 21163–170. [DOI] [PubMed] [Google Scholar]
- Nolan KF, Yun CO, Akamatsu Y, Murphy JC, Leung SO, Beecham EJ.et al. (1999Bypassing immunization: optimized design of “designer T cells” against carcinoembryonic antigen (CEA)-expressing tumors, and lack of suppression by soluble CEA Clin Cancer Res 53928–3941. [PubMed] [Google Scholar]
- Hombach A, Heuser C, Sircar R, Tillmann T, Diehl V, Pohl C.et al. (1998An anti-CD30 chimeric receptor that mediates CD3-zeta-independent T-cell activation against Hodgkin's lymphoma cells in the presence of soluble CD30 Cancer Res 581116–1119. [PubMed] [Google Scholar]
- Westwood JA, Murray WK, Trivett M, Haynes NM, Solomon B, Mileshkin L.et al. (2009The Lewis-Y carbohydrate antigen is expressed by many human tumors and can serve as a target for genetically redirected T cells despite the presence of soluble antigen in serum J Immunother 32292–301. [DOI] [PubMed] [Google Scholar]
- Turatti F, Figini M, Alberti P, Willemsen RA, Canevari S., and, Mezzanzanica D. Highly efficient redirected anti-tumor activity of human lymphocytes transduced with a completely human chimeric immune receptor. J Gene Med. 2005;7:158–170. doi: 10.1002/jgm.647. [DOI] [PubMed] [Google Scholar]
- Carpenito C, Milone MC, Hassan R, Simonet JC, Lakhal M, Suhoski MM.et al. (2009Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains Proc Natl Acad Sci U S A 1063360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song D, Ye Q, Carpenito C, Poussin M, Wang LP, Ji C.et al. (2011Improved T cell survival provided by CD137 costimulatory signaling to folate receptor-redirected T cells results in tumor localization and eradication Cancer Res 714617–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholler N, Garvik B, Quarles T, Jiang S., and, Urban N. Method for generation of in vivo biotinylated recombinant antibodies by yeast mating. J Immunol Methods. 2006;317:132–143. doi: 10.1016/j.jim.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine BL, Bernstein WB, Connors M, Craighead N, Lindsten T, Thompson CB.et al. (1997Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells J Immunol 1595921–5930. [PubMed] [Google Scholar]
- Johnson LA, Heemskerk B, Powell DJ, Jr, Cohen CJ, Morgan RA, Dudley ME.et al. (2006Gene transfer of tumor-reactive TCR confers both high avidity and tumor reactivity to nonreactive peripheral blood mononuclear cells and tumor-infiltrating lymphocytes J Immunol 1776548–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antigen-specific killing of mesothelin–positive tumor cells by P4 CAR T cells in 18-hour 51Cr-release assay.
Antigen-specific killing of CD19+ targets by anti-CD19 CAR T cells 4 and 18 hours post co-culture.
Expression levels of mesothelin protein among the different serous ovarian adenocarcinoma sites in individual cases.
Mesothelin is not transferred from antigen-positive to antigen-negative cells.
In vitro cytokine secretion and in vivo persistence of first and second generation P4 CAR T cells.