Abstract
Manipulation of hematopoietic stem/progenitor cells (HSPCs) ex vivo is of clinical importance for stem cell expansion and gene therapy applications. However, most cultured HSPCs are actively cycling, and show a homing and engraftment defect compared with the predominantly quiescent noncultured HSPCs. We previously showed that HSPCs make contact with osteoblasts in vitro via a polarized membrane domain enriched in adhesion molecules such as tetraspanins. Here we show that increased cell cycling during ex vivo culture of HSPCs resulted in disruption of this membrane domain, as evidenced by disruption of polarity of the tetraspanin CD82. Chemical disruption or antibody-mediated blocking of CD82 on noncultured HSPCs resulted in decreased stromal cell adhesion, homing, and engraftment in nonobese diabetic/severe combined immunodeficiency IL-2γnull (NSG) mice compared with HSPCs with an intact domain. Most leukemic blasts were actively cycling and correspondingly displayed a loss of domain polarity and decreased homing in NSG mice compared with normal HSPCs. We conclude that quiescent cells, unlike actively cycling cells, display a polarized membrane domain enriched in tetraspanins that mediates homing and engraftment, providing a mechanistic explanation for the homing/engraftment defect of cycling cells and a potential new therapeutic target to enhance engraftment.
Introduction
Since the discovery and purification of the first hematopoietic cytokines more than 2 decades ago, ex vivo culture techniques have been developed for stem cell expansion and gene therapy applications. The ability to expand hematopoietic stem/progenitor cells (HSPCs) could widen the availability and improve the efficacy of cord blood (CB) transplantation, speed recovery from cytopenias after transplantation, and ensure engraftment in mismatched or nonmyeloablative allogeneic transplantation. Genetic manipulation of HSPCs with retroviral vectors requires ex vivo stimulation with cytokines to maintain viability and to stimulate progression through the cell cycle, allowing nuclear membrane dissolution and vector access to chromosomal DNA. Genetic modification of HSPCs with lentiviral vectors, despite less reliance on cell cycle progression for vector access to chromatin, also requires cytokine stimulation for efficient transduction.1,2 However, a loss of in vivo repopulating stem cell function after a relatively brief culture in stimulatory cytokines has been demonstrated in murine studies,3,4 the rhesus autologous transplantation model,5 and nonobese diabetic/severe combined immunodeficiency (NOD/SCID) mouse studies using human CB cells or mobilized peripheral blood (MPB) stem cells.6,7
The mechanism for this phenomenon remains incompletely understood, but it has been proposed to result from changes in the expression or function of cell-surface molecules including chemokine receptors such as CXCR4, integrins such as very late antigen-4 (VLA-4), tetraspanins such as CD82, selectins, or leukosialins.8 These molecules function in the complex processes of homing and engraftment to the bone marrow (BM) by participating in the intimate physical contact of the HSPCs with the osteoblastic and endothelial components of the microenvironment.9–11 Using live cell imaging approaches, we previously showed that HSPCs make prolonged contact with the osteoblastic surface via a polarized membrane domain enriched in prominin 1, VLA-4, and tetraspanin proteins.12
Tetraspanins are part of a large family of evolutionarily conserved 4-transmembrane domain proteins. They facilitate the assembly of specialized molecular aggregates on plasma and intracellular membranes known as tetraspanin-enriched microdomains (TEM) that consist of tetraspanins as well as other membrane and cytosolic proteins, such as receptor tyrosine kinases, integrins, and adaptor proteins that are integral to signaling cascades. Depending on the nature of the interacting proteins, tetraspanins have been implicated in the control of cellular migration, adhesion, and signaling.13–16 CD82 has been shown to be an important member of the tetraspanin superfamily of glycoproteins and appears to function by modulating the levels, trafficking, or activity of its interacting partners in the TEM. In the context of cancer, CD82, also known as Kai1, associates with integrins on the surfaces of various tumor cells, and its expression is linked to metastasis suppression.17 CD82 can also be found in cells of the immune system,18 and has been shown to be highly expressed on the majority (up to 95%) of CD34+ cells isolated from healthy BM, CB, and MPB samples whereas only moderate expression was detected on normal mature peripheral blood cells.19 Similarly, CD82 was overexpressed in CD34+ blasts isolated from patients with acute myeloid leukemia (AML), and in leukemic cells from patients with chronic myeloid leukemia (CML) in accelerated or blastic phase, and chronic lymphocytic leukemia (CLL).19 These observations suggested a role of CD82 in normal and malignant hematopoiesis. Analogous to normal HSPCs, it has been proposed that leukemic cells are hierarchically organized populations of cells with only a small fraction of these cells capable of initiating leukemia.20–23 These rare cells are enriched in the CD34+ cell population and rely on interactions with the BM microenvironment to control their self-renewal and differentiation.24–28
In this study, we analyzed the distribution of CD82 as an indicator of domain polarity in normal and malignant CD34+ cells, and investigated the functional significance of this organized membrane domain for homing and engraftment of HSPCs to the BM microenvironment.
Methods
Patients and healthy donors
Cells from patients with AML (n = 5), CML (n = 1) and ALL (n = 1) were obtained from peripheral blood, BM, or leukapheresis products before chemotherapy treatment or hematopoietic stem cell transplant. Human CD34+ cells were also collected from healthy donors who received G-CSF (10 μg/Kg, Filgrastim) for 5 days, given as a single daily subcutaneous injection with leukapheresis initiated on the morning of day 5. Large volume (15 L) leukapheresis procedures were performed with a model CD-3000 Plus continuous-flow apheresis device (Fenwal Division).
Mononuclear cells were separated using Ficoll-Hypaque density gradient centrifugation (MP Biomedicals). The apheresis products were enriched for CD34+ cells by immunomagnetic bead affinity elution with a magnetic cell selection (Isolex 300I) and cryopreserved in PlasmaLyte (Baxter) supplemented with 7% human serum albumin (Baxter), 1.5% hetastarch (B. Braun), 20 mcg/mL DNAse (Genentech), 30 U/mL preservative free heparin (APP Pharmaceuticals), and 5% dimethyl-sulfoxide (Sigma-Aldrich) according to standard protocols. Samples from patients with AML and CML were sorted for CD34+ cells by fluorescence-activated cell sorter (FACS) using an Aria II instrument (Becton Dickinson). All patients and healthy donors gave written informed consent on treatment protocols approved by the National Institutes of Health (NIH) Institutional Review Board in accordance with the Declaration of Helsinki.
Fixation, immunostaining, and cell imaging
Normal and leukemic human hematopoietic cells were fixed with 4% paraformaldehyde for 15 minutes at room temperature followed by a wash with 1× PBS. Cells were permeabilized and blocked at room temperature with a tween-20/BSA/PBS solution and stained at 4°C for approximately 15 hours with anti-CD45 (Calbiochem), anti-CD34 (Becton Dickinson), anti-CXCR4 (Calbiochem), anti-CD49d (Calbiochem), and anti-CD82 (Addgene) antibodies. Cells were washed gently with 1xPBS followed by incubation with Alexa-488 goat anti–mouse antibody (Invitrogen) or Cy3-goat anti–rabbit antibody (Jackson ImmunoResearch) for 1 hour at room temperature. Cells were washed after secondary antibody with 1×PBS and mounted in Fluoromount-G (Southern Biotech) for imaging using a Zeiss LSM 510 confocal microscope (Carl Zeiss) with excitation wavelengths of 488 or 543 nm and a 63× oil immersion objective (numerical aperture = 1.2). Image analysis was performed manually using the Zeiss LSM 510 Version 4.2 software (Carl Zeiss Inc) by an investigator who was blind to the source samples.
Disruption of the membrane domain with methyl-β-cyclodextrin
Human CD34+ cells (1 × 106) were incubated for 30 minutes at 37°C with 10mM methyl-β-cyclodextrin (MβCD) in X-vivo media (Lonza) supplemented with SCF 100 ng/mL, Flt3L 100 ng/mL, and TPO 100 ng/mL. For the control group, cells were incubated in X-vivo media with cytokines in the presence of MβCD vehicle alone. Cell counts and viability were determined by trypan blue exclusion staining using a Vi-Cell XR cell viability analyzer (Beckman Coulter).
CFC assay
Human CD34+ cells were plated in duplicate in methylcellulose (MethoCult GF+H4435; StemCell Technologies) at a concentration of 500 cells/mL at 37°C in 5% CO2. Between days 10 and 14, colonies of more than 50 cells were counted and identified morphologically.
Transplantation of normal and leukemic human hematopoietic cells into immune-deficient mice
Nonobese diabetic/severe combined immunodeficiency IL-2γnull (NSG) mice were purchased from The Jackson Laboratory and maintained at the NIH under specific pathogen-free conditions. For homing studies, 8- to 10-week-old mice (n = 3-5 mice/group) were sublethally irradiated (300 cGy), transplanted intravenously with 1 × 106 G-CSF mobilized CD34+ cells from healthy donors, or 1 × 106 CD34+ cells from the peripheral blood or BM of patients with myeloid leukemias (AML/MDS, CML), and killed 16 hours after transplantation. For long-term engraftment studies, sublethally irradiated mice (n = 3-12 mice/group) were transplanted with 1 × 104 CD34+ cells from healthy donors, and killed 8 weeks after transplantation. The animal experiments were approved by the Animal Care and Use Committee of the National Institutes of Health.
Analysis of human cell homing and engraftment
Six bones were collected from each mouse for analysis of human cell homing and engraftment in the murine BM. In select mice, homing was also examined in the peripheral blood, spleen, liver, and lungs. The BM and various organs of engrafted mice were analyzed by flow cytometric analysis with anti–human CD34 and CD45 antibodies conjugated to phycoerythrin, using an LSRII instrument (BD Biosciences). In each experiment, cells from a nontransplanted mouse were stained with the same antibodies as a negative control.
Cell cycle analysis
Cells (0.5-1 × 106 CD34+) from healthy donors and patients with myeloid leukemias (AML/MDS, CML) or unfractionated peripheral blood from 1 patient with lymphoid leukemia (ALL) were stained with Hoechst 6.5 μg/mL (Invitrogen) at 37°C for 45 minutes in Hanks balanced salt solution (HBSS; Mediatech) supplemented with FCS 10% (Sigma-Aldrich), HEPES 20mM (Mediatech), and glucose 1 g/L (Mediatech). Pyronin Y 1 μg/mL (Sigma-Aldrich) was added and the incubation continued for an additional 45 minutes at 37°C. Cell luminescence was measured using flow cytometry (LSRII instrument; BD Biosciences). The fraction of cells in the G0, G1, and S/G2/M phases of the cell cycle was determined by gating on cell populations defined by their DNA and RNA contents.
Adhesion assay
The osteoblastic cell line SaOS-2 (American Type Culture Collection) was prepared for adhesion assays according to the manufacturer's recommendations by washing twice with PBS. CD34+ cells were introduced to monolayers at a final concentration of 10 000 cells/100uL/well and allowed to incubate for 4 hours at 37°C in X-Vivo media. The nonadherent cells were removed in 3 subsequent washes and counted using a hemocytometer to determine the number of adherent cells remaining in each well. As a control, osteoblast monolayers alone were washed and released cells were counted to identify any background contamination.
Function blocking CD82 antibody assay
Human CD34+ cells (1 × 106) were resuspended in 1 mL PBS and incubated on ice for 45 minutes in the presence of 1 ug/mL CD82 (Abcam) or control IgG antibodies (Santa Cruz Biotechnology). Cells were washed twice with 1× PBS, and secondary antibodies (Invitrogen) were added at a concentration of 1 ug/mL. After an incubation of 30 minutes on ice, cells were washed and incubated in X-vivo with cytokines for 2 hours at 37°C before using in an adhesion assay, cell cycle analysis, immunofluorescence imaging, or transplanting into NSG mice.
Statistics
Data were analyzed using the unpaired Student t test using Sigma Plot or KaleidaGraph Version 4.03 software. All of the statistical tests were 2 sided. A P value of .05 was considered to be statistically significant.
Results
A polarized membrane domain found on normal HSPCs
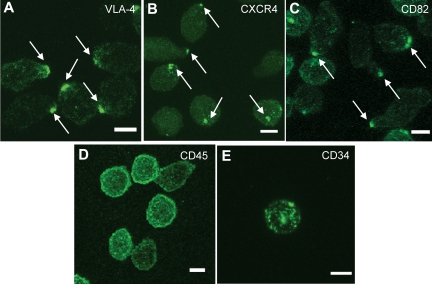
To explore membrane domain organization and its regulation in actively cycling cultured normal HSPCs, we first examined the baseline surface distribution of various adhesion molecules on noncultured human MPB CD34+ cells from healthy donors using confocal microscopy. Scoring at least 100 cells, we found that VLA-4 (Figure 1A), CXCR4 (Figure 1B), and CD82 (Figure 1C), displayed a polarized distribution on the plasma membrane. The polarized localization of these markers was specific because other surface markers, CD45 (Figure 1D) and CD34 (Figure 1E), were distributed throughout the plasma membrane. On average, 47 ± 12% (range 30%-63%) of human MPB CD34+ cells from 7 independent healthy donors (age 19-48, n = 4 males, n = 3 females) showed domain polarity, as evidenced by a polarized distribution of CD82.
Figure 1.
A polarized membrane domain is found on normal human HSPCs. (A-E) Immunofluorescence labeling of human MPB CD34+ cells from a normal volunteer with antibodies for VLA-4 (A), CXCR4 (B), and CD82 (C) shows a polarized distribution of the membrane proteins on the majority of the cells, whereas CD45 (D) and CD34 (E) are distributed uniformly on the plasma membrane. These images are representative of the polarized molecule phenotypes observed in more than 100 CD34+ cells. Scale bars in panels A through E equal 5 μm.
Ex vivo culture of normal HSPCs in stimulatory cytokines results in increased cell cycling and disruption of domain polarity
To test the impact of ex vivo culture on domain polarity and homing in NSG mice, CD34+ cells from healthy donors were cultured for 4 days in the presence of stimulatory cytokines (stem cell factor [SCF], fms-like tyrosine kinase-3 [FLT3], and thrombopoietin [TPO]). For comparison, CD34+ cells were also maintained in nonstimulatory culture conditions for 4 days with SCF alone, a cytokine that promotes survival without inducing proliferation or differentiation. The presence of polarized domains, as evidenced by CD82 polarity, was scored at days 0 and 4 on at least 100 cells by confocal microscopy, and the cycling status of the cells was determined by double staining with Hoechst and Pyronin Y. In one experiment, 1 × 106 CD34+ cells from each group were also injected intravenously into NSG mice and the ability of the cells to home to the BM microenvironment was measured by flow cytometric detection of human cells using anti-CD45 staining 16 hours after injection.
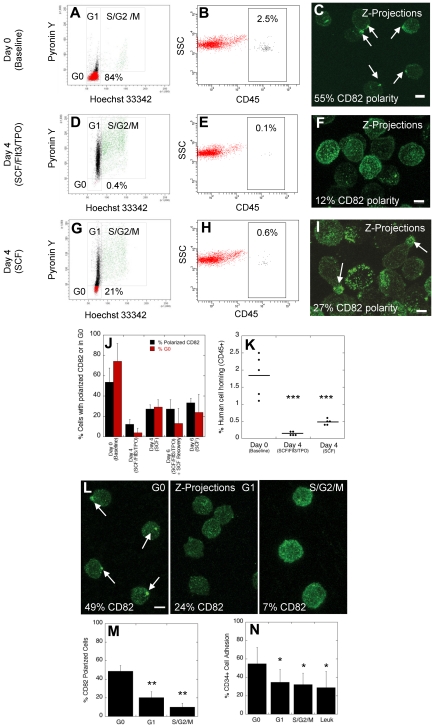
At baseline (day 0), the majority of CD34+ cells (84%) were in the G0 phase of the cell cycle (Figure 2A), up to 2.5% of human cells were detected in the mouse BM 16 hours after transplantation (Figure 2B), and 55% of the cells had polarized domains (Figure 2C). After 4 days in stimulatory cytokines, few cells (0.4%) remained quiescent in G0 (Figure 2D), only rare cells (0.1%) homed to the mouse BM (Figure 2E), and although all cells continued to express CD82 as assessed by flow cytometry (supplemental Figure 1; available on the Blood Web site; see the Supplemental Materials link at the top of the online article), only 12% of the cells maintained their domain polarity (Figure 2F). VLA-4 distribution was similar to that of CD82 with a polarized enrichment of membrane VLA-4 at day 0 that was mostly redistributed throughout the plasma membrane at day 4 (supplemental Figure 2). Cells cultured in SCF alone for 4 days were more frequently (21%) in G0 (Figure 2G), more cells (0.6%) homed to the mouse BM (Figure 2H), and correspondingly more cells (27%) were detected with polarized domains (Figure 2I) compared with cells cultured in stimulatory cytokines. Overall, when data from 4 independent CD34+ cell donors were analyzed, the percentage of cells with polarized domains correlated closely with the percentage of cells in G0 (Figure 2J, day 0 and 4). Furthermore, domain polarity correlated with the homing ability of CD34+ cells (Figure 2K). Therefore, ex vivo culture of HSPCs in stimulatory cytokines results in increased cell cycling, decreased homing, and disruption of membrane domain polarity.
Figure 2.
Disruption of polarized membrane domains in cycling normal human HSPCs. (A-C) Analysis of the cell cycling status (A), homing in NSG mice (B), and membrane domain polarity (C) of normal human MPB CD34+ cells before ex vivo culture (day 0). (D-F) Analysis of the cell cycling status (D), homing in NSG mice (E), and membrane domain polarity (F) of normal human MPB CD34+ cells after ex vivo culture for 4 days in stimulatory conditions in the presence of SCF, FLT3, and TPO. (G-I) Analysis of the cell cycling status (G), homing in NSG mice (H), and membrane domain polarity (I) of normal human MPB CD34+ cells after ex vivo culture for 4 days in nonstimulatory conditions in the presence of SCF alone. (J) Percentage of human CD34+ cells with polarized domain (± SEM) and percentage of cells in the G0 phase of the cell cycle (± SEM) for cells at baseline, and cells cultured for 4 to 6 days in stimulatory and/or nonstimulatory conditions (n = 4 donors). (K) Percentage of human cell homing in NSG mice 16 hours after IV injection of uncultured cells or cells cultured for 4 days in stimulatory or nonstimulatory conditions (t test; horizontal bars represent average human cell homing). (L) Immunofluorescence labeling of human CD34+ cells with CD82 antibodies after sorting cells in the G0, G1, and S/G2/M phases of the cell cycle. (M) Percentage of cells in G0, G1, and S/G2/M phases of the cell cycle with polarized CD82 (± SEM, n = 3 donors, t test). (N) Percentage of normal CD34+ cells in G0, G1, and S/G2/M phases of the cell cycle and percentage of leukemic blast cells from patients with AML, CML, and ALL (Leuk) adhering to osteoblasts in vitro (± SEM, n = 3 donors, t test). Horizontal bars in K represent means. Scale bars in panels C, F, I, and L equal 5 μm (*P ≤ .05; **P < .01; ***P < .001).
Studies in mice and in rhesus macaques have shown that cells cultured for 6 days, with exposure to stimulatory cytokines (SCF, FLT3, and TPO) for the first 4 days, followed by an additional 2 day-culture in nonstimulatory conditions (SCF alone) show a partial rescue in engraftment defect.5,29 We hypothesized that reorganization of the membrane proteins into a polarized domain after culture in SCF alone may explain the partial rescue in engraftment defect. When normal CD34+ cells were cultured for 2 days in SCF alone after the initial 4 day culture in stimulatory cytokines, we observed a 2.2-fold increase in the percentage of cells with polarized membrane domains and a 3.3-fold increase in the percentage of cells in G0 compared with CD34+ cells cultured for 4 days under stimulatory conditions without an additional 2 day culture in SCF alone (Figure 2J, day 6, SCF/Flt3/TPO + SCF recovery). These percentages approached those obtained when cells were cultured for 6 days under nonstimulatory conditions (Figure 2J, day 6, SCF). CD34+ cells cultured for an additional 2 days in SCF alone were injected intravenously in NSG mice and homing of these cells was compared with cells cultured for 4 days in SCF, Flt3, and TPO. A partial rescue (1.2-fold) in homing was observed with cells cultured for an additional 2 days in SCF alone, but the difference between both groups did not reach statistical significance (data not shown, P = .2).
We next confirmed the data obtained after ex vivo culture of human CD34+ cells by sorting cells in each phase of the cell cycle. As shown in a representative experiment (Figure 2L), the majority of cells in G0 showed domain polarity. In contrast, polarity was not detected in the majority of cycling cells in either the G1 or S/G2/M phases of the cell cycle. Figure 2M summarizes data from 3 independent donors, illustrating a disruption of domain polarity in actively cycling cells compared with quiescent cells. Consistent with this observation and the known cell adhesion properties of CD82,15 an important component of the domain, we detected decreased osteoblastic adhesion in vitro comparing cycling HSPCs to cells in G0 (Figure 2N). In combination, these data illustrate a strong correlation between quiescence, increased membrane domain polarity and HSPC adhesion.
Polarized membrane domains are functionally important for homing and engraftment
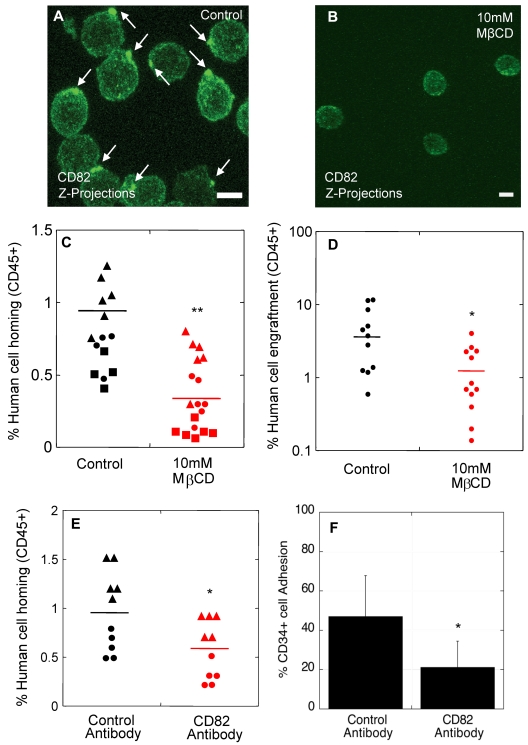
We previously showed that cholesterol and cytoskeletal-based processes are important for the polarized distribution of HSPC surface components.12 When HSPCs were exposed to the cholesterol-sequestering agent, methyl-β-cyclodextrin (MβCD), polarity of CD82 (Figure 3A-B) and other surface molecules normally enriched in the polarized domain12 was disrupted, and the components of the domain were redistributed into punctuate clusters across the membrane. To better understand the significance of polarity of these surface markers for homing and engraftment, we disrupted the polarized membrane domains on normal noncultured human CD34+ cells by exposure to 10mM MβCD for 30 minutes before transplantation into NSG mice. Human CD34+ cells in the control group were exposed to vehicle alone for 30 minutes. To rule out an impact of MβCD on CD34+ cell viability and on the functional ability of these cells to differentiate, a portion of the MβCD-treated and control CD34+ cells were analyzed in standard viability and colony forming cell (CFC) assays. In all experiments (n = 3 homing studies, n = 2 engraftment studies), there was no impact of MβCD exposure on CD34+ cell viability (96.7% vs 97.8%; P = .52). Similarly, the colony forming ability of MβCD-treated CD34+ cells (average 410 CFC/1000 CD34+ cells) was unchanged compared with the vehicle control (average 434 CFC/1000 CD34+ cells; P = .89).
Figure 3.
Polarized membrane domains are functionally important for homing and engraftment. (A-B) CD82 immunofluorescence labeling of human MPB CD34+ cells exposed to MβCD vehicle alone (A) or 10mM MβCD (B). (C) Percentage of human cell homing in NSG mice 16 hours after transplantation of control or MβCD-treated CD34+ cells (Donor 1 ▴, Donor 2 ●, Donor 3 ■, t test). (D) Percentage of human cell engraftment in NSG mice 2 months after transplantation of control or MβCD-treated CD34+ cells (t test). (E) Percentage of human cell homing in NSG mice 16 hours after transplantation of human CD34+ cells treated with isotype control or CD82 antibodies (Donor 1 ▴, Donor 2 ●, t test). (F) Percentage of human cells incubated with isotype control or CD82 antibodies adhering to osteoblasts in vitro (n = 3 donors, t test). Horizontal bars in panels C, D, and E represent means. Scale bars in panels A and B equal 5 μm (*P < .05; **P < .00001).
For homing studies, 1 × 106 human CD34+ cells from 3 independent healthy donors were transplanted per mouse, and homing of the cells was analyzed in the BM, peripheral blood, spleen, lungs, and liver of the animals 16 hours after transplantation. In the vehicle control group (Figure 3C), an average of 0.78% human CD34+ cells were detected in the mouse BM compared with 0.32% in the MβCD-treated group (Figure 3C). No transplanted cells were detected in the peripheral blood or other tissues analyzed in both control and MβCD groups with a detection limit of 0.1% (data not shown).
For engraftment studies, 1 × 104 normal human CD34+ cells incubated for 30 minutes in the presence or absence of MβCD were transplanted per NSG mouse, and human cell engraftment in the BM was analyzed by flow cytometry 8 weeks after transplantation. Exposure to MβCD before transplantation resulted in significantly lower human cell engraftment (1.4%) compared with untreated cells (4.8%; Figure 3D), suggesting that the polarized membrane domain is important for engraftment of long-term repopulating cells.
We next evaluated whether disruption of CD82 specifically on CD34+ cells using function-blocking antibodies would impact the homing process in NSG mice. CD34+ cells incubated with CD82 antibodies showed a ∼ 2-fold decrease in homing (Figure 3E) compared with control cells incubated with isotype antibodies. Similarly, we detected a 2.5-fold decrease in osteoblastic adhesion when cells were incubated with CD82 antibodies compared with the control group (Figure 3F). This observed decrease in homing and adhesion was not because of CD82 antibody effects on membrane domain organization and polarity (supplemental Figure 3A-B), or to changes in cell cycle status (supplemental Figure 3C), or in expression of other surface markers known to be important for HSPC homing, including CXCR4, VLA-4, and CD44 (supplemental Figure 3D). Similarly, CD82 antibodies had no impact on cell viability and did not result in an increase in CD82 internalization (data not shown). We speculate that CD82 antibody treatment disrupts homing and adhesion by blocking CD82 interacting proteins critical to these processes. Taken together, these data suggest that an intact CD82-enriched polarized membrane domain is necessary for optimal homing of normal human CD34+ cells to, and adhesion within, the BM microenvironment.
Loss of membrane domain polarity in blast cells from leukemic patients
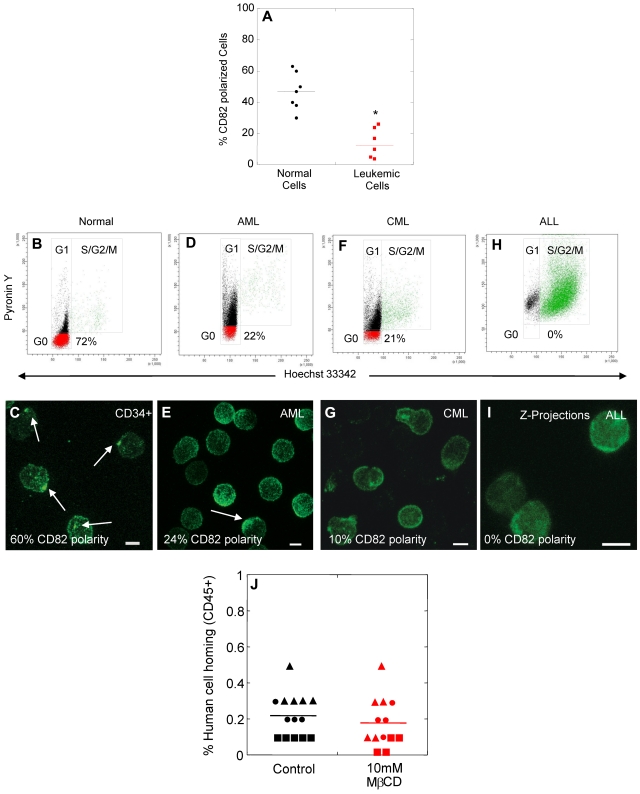
We next investigated whether polarized membrane domains could be found on blast cells from patients with AML (n = 5), CML (n = 1), and ALL (n = 1). Although approximately half of the HSPCs from healthy donors showed domain polarity (average 47%, range 30%-63%), the majority of blast cells from leukemic patients (> 95% CD34+ cells in myeloid leukemias) lacked domain polarity (average 12%, range 0%-26%; Figure 4A). We hypothesized that the decreased percentage of leukemic blasts with polarized domains may be related to increased cell cycling. Compared with a healthy donor with 72% of CD34+ cells in the G0 phase of the cell cycle (Figure 4B) and 60% of cells with polarized domains (Figure 4C), only 22% of cells were quiescent in a patient with AML (Figure 4D), and correspondingly the percentage of leukemic blasts with polarized domains was lower (24%, Figure 4E). Similar results were obtained with CD34+ cells derived from a patient diagnosed with CML blast crisis (Figure 4F-G). Interestingly, in a patient with ALL, no leukemic blasts were detected in the G0 phase of the cell cycle (Figure 4H) and likewise no cells with a polarized domain were identified (Figure 2I). These data indicate a loss of membrane domain polarity in actively cycling blast cells from patients with AML, CML, and ALL, similar to actively cycling normal HSPCs after ex vivo culture. Consistent with these data, leukemic blasts showed decreased abilities to adhere to osteoblasts in vitro compared with quiescent HSPCs (Figure 2N). Leukemic blasts (Figure 4J control) also exhibited decreased homing in NSG mice compared with untreated, normal CD34+ cells (Figure 3C, control, P = .0000002), but showed similar homing capacity to normal CD34+ cells treated with MβCD (Figures 3C, MβCD; P = .12). Exposure of leukemic blasts to MβCD had no impact on their homing ability, consistent with the absence of a polarized membrane domain on the majority of these cells before MβCD treatment (Figures 4J, MβCD).
Figure 4.
Loss of membrane domain polarity in blast cells from leukemic patients. (A) Percentage of normal human CD34+ cells and leukemic blasts with membrane domain polarity. (B-I) Analysis of the cell cycling status (B,D,F,H), and membrane domain polarity (C,E,G,I) of normal human MPB CD34+ cells (B-C), and blast cells from patients with AML (D-E), CML (F-G), or ALL (H-I). (J) Percentage of human cell homing in NSG mice 16 hours after transplantation of control or MβCD-treated leukemic blasts (AML ▴, CML ●, AML/MDS ■, t test, P > .1). Scale bars in panels C, E, G, and I equal 5 μm. Horizontal bars in panels A and J represent means (*P < .0001).
Discussion
We show that polarity of membrane domains enriched in molecules known to be important for adhesion to the microenvironment, namely the tetraspanin CD82, is required for efficient homing and engraftment of normal HSPCs to the BM. Increased cell cycling during ex vivo culture of HSPCs resulted in disruption of the organization of this membrane domain, as evidenced by loss of CD82 polarity, providing, to our knowledge, the first mechanistic explanation for the homing and engraftment defect of cells cultured ex vivo compared with noncultured cells. Polarized membrane domains may enhance the process of anchorage of HSPC to the BM microenvironment by clustering functionally related molecules and/or tightly packing a critical number of specific molecules at the plasma membrane. Thus, these polarized domains may allow a more specific and robust detection of cell adhesion receptors, establishing strong multivalent ligand-receptor interactions between HSPC and niche components, and resulting in improved homing/engraftment. Therapeutic strategies to enhance membrane domain polarity may improve homing and engraftment in clinical applications such as nonmyeloablative allogeneic stem cell transplantation, or CB transplant where the number of repopulating stem cells is limiting. Various approaches to enhance membrane domain polarity are under investigation, including use of ingenol 3,20-dibenzoate (IDB), known to up-regulate expression of tetraspanins on human CD34+ cells,30,31 and cell culture under hypoxic conditions, which is thought to favor maintenance of HSPCs in the more quiescent G0/G1 phases of the cell cycle.32
In contrast to normal noncultured HSPCs, the majority of blast cells from patients diagnosed with AML, CML, and ALL were actively cycling and correspondingly displayed minimal domain polarity. Although the functional significance of this finding remains to be fully elucidated, we hypothesize that the small population of leukemic blasts that maintains a polarized membrane domain may represent a more quiescent population, with an increased homing ability and a leukemia-initiating capacity. Demonstration of this hypothesis would require sorting of the leukemic cells with a polarized domain followed by transplantation in immune-deficient animals (eg, NSG mice) to assess the long-term repopulating ability of these cells. This could be done using technologies that combine high-resolution microscopy with the fluorescence sensitivity of flow cytometry, to allow cell sorting based on morphologic characteristics, such as membrane domain polarity. Various systems combine microscopy and flow cytometry but the currently available technology does not have cell-sorting capability. With the development of new sorting technologies based on morphologic features for cells in suspension culture, further characterization of membrane domain polarity on leukemic populations, and its impact on homing and engraftment will offer new insight into tumor-host interactions and suggest novel therapeutic options.
Supplementary Material
Acknowledgments
The authors thank Leigh Samsel and Dr Phil McCoy from the National Heart, Lung, Blood Institute (NHLBI) flow core facility, and Keyvan Keyvanfar from the Hematology Branch flow facility for their assistance with flow sorting and analysis. They are also grateful to Dr David Stroncek and the department of transfusion medicine staff for the apheresis and CD34+ cell selection from healthy donors. They also thank Dr Minoo Battiwalla for providing samples from patients with leukemia.
This work was supported by the intramural research programs of the NHLBI (C.E.D. and A.J.B.), the National Institute of Child Health and Human Development (J.L.S.), and the Center for Cancer Research, National Cancer Institute of the NIH (A.S.W.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the paper.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.L., J.M.G., and R.D. designed and performed the experimental procedures, and analyzed the data; A.L. and J.M.G. wrote the paper and prepared the figures; B.I. and A. Cantilena helped with the NSG transplantation studies; A. Cerf provided help with confocal microscopy; A.J.B. and A.S.W. provided leukemic samples; and J.L.-S. and C.E.D. designed the experiments and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.M.G. is Department of Pathology, University of New Mexico Health Sciences Center, Albuquerque, NM.
Correspondence: Cynthia E. Dunbar, NHLBI, NIH, 9000 Rockville Pike, Bldg 10CRC, Rm 4E-5132, Bethesda, MD 20892; e-mail: dunbarc@nhlbi.nih.gov.
References
- 1.Case SS, Price MA, Jordan CT, et al. Stable transduction of quiescent CD34+CD38- human hematopoietic cells by HIV-1-based lentiviral vectors. Proc Natl Acad Sci U S A. 1999;96:2988–2993. doi: 10.1073/pnas.96.6.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutton RE, Reitsma MJ, Uchida N, Brown PO. Transduction of human progenitor hematopoietic stem cells by human immunodeficiency virus type 1-based vectors is cell cycle dependent. J Virol. 1999;73:3649–3660. doi: 10.1128/jvi.73.5.3649-3660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters SO, Kittler EL, Ramshaw HS, Quesenberry PJ. Ex vivo expansion of murine marrow cells with interleukin-3 (IL-3), IL-6, IL-11, and stem cell factor leads to impaired engraftment in irradiated hosts. Blood. 1996;87:30–37. [PubMed] [Google Scholar]
- 4.Peters SO, Kittler ELW, Ramshaw HS, Quesenberry PJ. Murine marrow cells expanded in culture with IL-3, IL-6, IL-11, and SCF acquire an engraftment defect in normal hosts. Exp Hematol. 1995;23:461–466. [PubMed] [Google Scholar]
- 5.Takatoku M, Sellers S, Agricola BA, et al. Avoidance of stimulation improves engraftment of cultured and retrovirally transduced hematopoietic cells in primates. J Clin Invest. 2001;108(3):447–455. doi: 10.1172/JCI12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gan OI, Murdoch B, Larochelle A, Dick JE. Differential maintenance of primitive human SCID-repopulating cells, clonogenic progenitors, and long-term culture-initiating cells after incubation on human bone marrow stromal cells. Blood. 1997;90(2):641–650. [PubMed] [Google Scholar]
- 7.Gothot A, van der Loo JCM, Clapp DW, Srour EF. Cell cycle-related changes in repopulating capacity of human mobilized peripheral blood CD34+ cells in non-obese diabetic/ severe combined immune-deficient mice. Blood. 1998;92:2641–2649. [PubMed] [Google Scholar]
- 8.Becker PS, Nilsson SK, Li Z, et al. Adhesion receptor expression by hematopoietic cell lines and murine progenitors: modulation by cytokines and cell cycle status. Exp Hematol. 1999;27(3):533–541. doi: 10.1016/s0301-472x(98)00037-x. [DOI] [PubMed] [Google Scholar]
- 9.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 10.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 11.Zhang J, Niu C, Ye L, et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425(6960):836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 12.Gillette JM, Larochelle A, Dunbar CE, Lippincott-Schwartz J. Intercellular transfer to signalling endosomes regulates an ex vivo bone marrow niche. Nat Cell Biol. 2009;11(3):303–311. doi: 10.1038/ncb1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charrin S, Le NF, Silvie O, Milhiet PE, Boucheix C, Rubinstein E. Lateral organization of membrane proteins: tetraspanins spin their web. Biochem J. 2009;420(2):133–154. doi: 10.1042/BJ20082422. [DOI] [PubMed] [Google Scholar]
- 14.Hemler ME. Targeting of tetraspanin proteins–potential benefits and strategies. Nat Rev Drug Discov. 2008;7(9):747–758. doi: 10.1038/nrd2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miranti CK. Controlling cell surface dynamics and signaling: how CD82/KAI1 suppresses metastasis. Cell Signal. 2009;21(2):196–211. doi: 10.1016/j.cellsig.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 16.Yáñez-Mó M, Barreiro O, Gordon-Alonso M, Sala-Valdes M, Sanchez-Madrid F. Tetraspanin-enriched microdomains: a functional unit in cell plasma membranes. Trends Cell Biol. 2009;19(9):434–446. doi: 10.1016/j.tcb.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Tsai YC, Weissman AM. Dissecting the diverse functions of the metastasis suppressor CD82/KAI1. FEBS Lett. 2011;585(20):3166–3173. doi: 10.1016/j.febslet.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarrant JM, Robb L, van Spriel AB, Wright MD. Tetraspanins: molecular organisers of the leukocyte surface. Trends Immunol. 2003;24(11):610–617. doi: 10.1016/j.it.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 19.Burchert A, Notter M, Dietrich MH, et al. CD82 (KAI1), a member of the tetraspan family, is expressed on early haemopoietic progenitor cells and up-regulated in distinct human leukaemias. Br J Haematol. 1999;107(3):494–504. doi: 10.1046/j.1365-2141.1999.01741.x. [DOI] [PubMed] [Google Scholar]
- 20.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 21.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112(13):4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 22.Greaves M. Cancer stem cells: back to Darwin? Semin Cancer Biol. 2010;20(2):65–70. doi: 10.1016/j.semcancer.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Wang JC, Dick JE. Cancer stem cells: lessons from leukemia. Trends Cell Biol. 2005;15(9):494–501. doi: 10.1016/j.tcb.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 24.Colmone A, Amorim M, Pontier AL, Wang S, Jablonski E, Sipkins DA. Leukemic cells create bone marrow niches that disrupt the behavior of normal hematopoietic progenitor cells. Science. 2008;322(5909):1861–1865. doi: 10.1126/science.1164390. [DOI] [PubMed] [Google Scholar]
- 25.Iwasaki H, Suda T. Cancer stem cells and their niche. Cancer Sci. 2009;100(7):1166–1172. doi: 10.1111/j.1349-7006.2009.01177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med. 2006;12(10):1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 27.Krause DS, Lazarides K, von Andrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12(10):1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 28.Li L, Neaves WB. Normal stem cells and cancer stem cells: the niche matters. Cancer Res. 2006;66(9):4553–4557. doi: 10.1158/0008-5472.CAN-05-3986. [DOI] [PubMed] [Google Scholar]
- 29.Li CL, Johnson GR. Stem cell factor enhances the survival but not the self-renewal of murine hematopoietic long-term repopulating cells. Blood. 1994;84(2):408–414. [PubMed] [Google Scholar]
- 30.Desmond R, Dunfee A, Racke F, Dunbar CE, Larochelle A. CD9 up-regulation on CD34+ cells with ingenol 3,20-dibenzoate does not improve homing in NSG mice. Blood. 2011;117(21):5774–5776. doi: 10.1182/blood-2011-01-332031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leung KT, Chan KY, Ng PC, Lau TK, Chiu WM, Tsang KS, et al. The tetraspanin CD9 regulates migration, adhesion, and homing of human cord blood CD34+ hematopoietic stem and progenitor cells. Blood. 2011;117(6):1840–1850. doi: 10.1182/blood-2010-04-281329. [DOI] [PubMed] [Google Scholar]
- 32.Danet GH, Pan Y, Luongo JL, Bonnet DA, Simon MC. Expansion of human SCID-repopulating cells under hypoxic conditions. J Clin Invest. 2003;112(1):126–135. doi: 10.1172/JCI17669. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.