Abstract
SIRT1 is an NAD+-dependent histone deacetylase implicated in the establishment of the primitive hematopoietic system during mouse embryonic development. However, investigation of the role of SIRT1 in adult hematopoiesis has been complicated by the high perinatal mortality of SIRT1-deficient mice (SIRT1−/−). We performed a comprehensive in vivo study of the hematopoietic stem cell (HSC) compartment in adult SIRT1−/− mice and show that, apart from anemia and leukocytosis in older mice, the production of mature blood cells, lineage distribution within hematopoietic organs, and frequencies of the most primitive HSC populations are comparable to those of wild-type littermate controls. Furthermore, we show that SIRT1-deficient BM cells confer stable long-term reconstitution in competitive repopulation and serial transplantation experiments. The results of the present study rule out an essential physiologic role for cell-autonomous SIRT1 signaling in the maintenance of the adult HSC compartment in mice.
Introduction
SIRT1 is a mammalian homolog of the yeast NAD+-dependent protein deacetylase Sir2, and both genes have been implicated in gene silencing, heterochromatin formation, cell survival, metabolism, and development.1–3 Even though SIRT1 and its orthologs have been proposed to serve as key mediators of health benefits and lifespan extension conferred by calorie restriction,4–10 direct evaluation of the role of SIRT1 in aging of mammals has been complicated by high perinatal mortality of SIRT1 knockout mice. In all strains, SIRT1 deficiency led to several developmental defects and severely reduced body size, with only a fraction of mice surviving to weaning.11–14
We hypothesized that the increased mortality of SIRT1−/− mice could be at least partially attributed to failure of hematopoiesis, and suspected that SIRT1 could be involved in controlling hematopoietic stem cell (HSC) self-renewal properties, stress responses, and aging. Furthermore, our group is developing antilymphoma agents based on SIRT1 inhibition,15 and we wondered whether SIRT1 down-regulation might adversely affect the HSC pool. Consistent with these concerns, a recent study showed that SIRT1 deficiency leads to impaired mouse embryonic stem cell (mESC) differentiation into hematopoietic progeny in vitro, indicating a possible role for SIRT1 in the development of primitive hematopoiesis in mouse embryos.16
Several SIRT1 knockout models have been established using different targeting strategies in various genetic backgrounds, with the common feature of almost universal embryonic or perinatal mortality in the inbred 129SV background but a significant fraction of mice surviving to adulthood in other genetic backgrounds.11–14 Specifically, the deletion of SIRT1 exon 4 in the C57BL/6 background allowed some of the mice to survive to weaning while conferring the same gross phenotype of SIRT1 deletion.14 In the present study, we used the latter model to generate a large cohort of adult mice to study hematopoietic functions of SIRT1 in vivo, and show that frequencies and functional properties of SIRT1−/− HSCs are comparable to those of their wild-type littermate controls.
Methods
SIRT1−/− mice
Mice carrying a deletion of exon 4 have been described previously.14 Briefly, a targeting construct for generating the conditional SIRT1 allele was electroporated into (129 × 1/SvJ × 129S1/Sv) F1-derived R1 mESCs, which were injected into C57BL/6 blastocysts. The chimeras were bred with C57BL/6 mice and the resulting heterozygotes crossed with CMV-Cre+ C57BL/6 mice to generate mice with constitutive deletion of SIRT1, which were crossed again with C57BL/6 and subsequently interbred to generate homozygous SIRT1−/− and littermate control mice. All mouse experiments were conducted according to Fred Hutchinson Cancer Research Center Institutional Animal Care and Use Committee–approved protocols.
Immunophenotyping
BM, thymus, and spleen cells were obtained as single-cell suspensions and stained with the appropriate Abs in 1:100 dilutions. A full list of Abs used (BD Biosciences) is available on request. Immunophenotypes were analyzed using an LSR flow cytometer (BD Biosciences) and a Vantage Cell Sorter (BD Biosciences), as described previously.17–19
Transplantation experiments
BM cells were collected from CD45.2+ SIRT1−/− mice and their littermate controls and injected into lethally irradiated (900-1000 cGy) CD45.1+ recipients via the tail vein. FACS analysis of the peripheral blood was performed at 4-week intervals after BM transplantation.
Results and Discussion
We reported previously that mice carrying a homozygous deletion of SIRT1 exon 4 were small at birth and exhibited the hallmarks of SIRT1 deficiency, with only a third of them surviving to weaning.14 We observed that the increased mortality, which we were not able to attribute to a single gross defect, continued until the age of 7 months, after which time survival became similar to their wild-type littermate controls (Figure 1A). This observation suggests that SIRT1 may play a more important role during early development and growth than it does later in life.
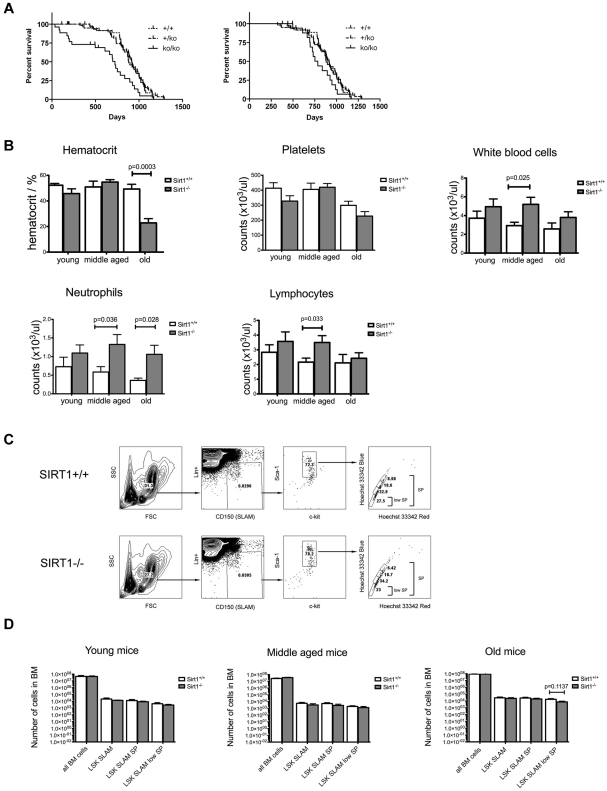
Figure 1.
Long-term follow-up of SIRT1−/− mice reveals adequate mature blood cell production with normal frequencies of primitive HSCs in the BM. (A) Survival curves for the SIRT1−/− (full line), SIRT1+/− (dashed line), and SIRT1+/+ (dotted line) mice that survived to weaning (left) and up to 7 months of age (right). (B) Quantitative analysis of hematocrit, WBCs, and platelets in the peripheral blood of SIRT1−/− (gray) and SIRT1+/+ (white) mice. Three age groups were analyzed: young (1-3 months) middle-aged (3-10 months) and old (10-20 months); n = 10-12 per group. Bars represent means ± SEMs. (C) FACS analysis of the primitive HSC compartment, identified by low expression of lineage markers (Lin), high expression of Sca-1, c-kit, and CD150 surface molecules, and specific Hoechst 33342 staining pattern. One representative sample for SIRT1−/− and SIRT1+/+ group is shown. (D) Absolute numbers (as per 4 limbs per mouse) of Lin-Sca-1+Kit+CD150+ (LSK SLAM), LSK SLAM SP, and LSK SLAM SPlow populations within the bone marrow of SIRT1−/− (gray) and SIRT1+/+ (white) mice in three age groups: 1- to 3-month old (young), 4- to 6-month old (middle-aged) and older than 12 months (old mice). Bars represent means ± SEM, n = 5-10 per group. No statistically significant differences observed.
To determine whether lack of SIRT1 causes overt hematologic alterations, complete blood counts were analyzed for young, middle-aged, and old SIRT1−/− mice and their controls. In addition to markedly decreased hematocrit in the old group, which is consistent with a study showing SIRT1 knockouts to be anemic due to decreased erythropoietin production in the liver and kidneys,20 middle-aged and old SIRT1−/− mice exhibited significant leukocytosis (Figure 1B). Lymphocytosis and neutrophilia in SIRT1-deficient mice might reflect the perturbation of physiologic diurnal fluctuations in leukocyte counts,21,22 which is consistent with the proposed role of SIRT1 in the regulation of circadian rhythms,23,24 as well as possible dysregulation of cellular NAD+ supplies, which have been implicated directly in myeloid differentiation.25 The young group, which exhibited markedly increased mortality, did not demonstrate any deficiencies in mature cell production, making hematopoietic failure an unlikely cause of premature death in this age group. We also examined RBC and WBC precursors in the BM, spleen, and thymus and, apart from increased myeloid precursor counts in the BM corresponding to neutrophilia in the peripheral blood, found no significant differences between SIRT1−/− mice and their controls (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
We also examined the numbers of most primitive HSCs within the BM of SIRT1−/− mice. These cells can be immunophenotypically profiled by low expression of lineage markers, high expression of Sca-1 and Kit surface molecules (LSK cells),17 presence of the SLAM molecule CD150,18 and a specific Hoechst 33342 staining pattern (side population, SP)19 as LSK SLAM, LSK SLAM SP, and LSK SLAM SPlow populations (Figure 1C). As indicated in Figure 1D, we found no significant differences in sizes of these populations nor in overall BM cellularities between SIRT1−/− mice and their littermate controls regardless of the age group analyzed.
To determine how SIRT1−/− HSCs function in a stressful environment, we conducted competitive repopulation assays using 2-, 6-, and 16-month-old mice as donors. We transplanted 2 × 105 CD45.2+ SIRT1−/− or wild-type control BM cells together with 1 × 105 CD45.1+ rescue BM cells into lethally irradiated CD45.1+ mice and determined the fraction of CD45.2+ WBCs in the recipients' blood at 4, 8, and 16 weeks after transplantation. SIRT1−/− HSCs showed no disadvantage in the competitive transplantation experiment, with frequencies of donor cells comparable to wild-type controls after 4 and 8 weeks, showing normal short-term reconstitution, and 16 weeks, which is the time required for transplanted pluripotent HSCs to start participating in mature blood cell production (Figure 2A-B).
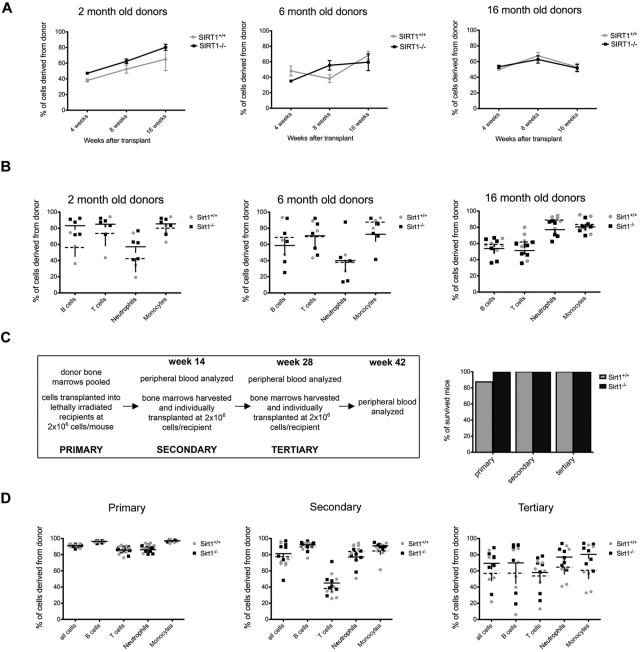
Figure 2.
SIRT1−/− BM cells confer stable reconstitution in competitive and serial transplantation experiments. (A) Percentages of CD45.2+ WBCs derived from 2- (left), 6- (middle), and 16 (right)–month-old SIRT1−/− (black) and SIRT1+/+ (gray) donors in the blood of lethally irradiated CD45.1+ recipients, as measured 4, 8, and 16 weeks after transplantation. Dots represent means ± SEM (n = 8-11 per age group). Recipients were transplanted with 2 × 105 whole BM donor cells together with 1 × 105 competitor recipient cells. (B) Percentages of donor-derived cells within B-cell (CD19+), T-cell (CD3+), neutrophil (Gr1+), and monocyte (F4/80+) populations in recipient bloods 16 weeks after transplantation. Each data point represents an individual animal; lines represent means ± SEM. The groups are as described in panel A. (C) Design of the serial transplantation experiment (left). BMs from 3 mice older than 18 months were pooled and transplanted into lethally irradiated CD45.1+ recipients (n = 7) at a dose of 2 × 106 cells without BM rescue cells. Four months later, recipient peripheral blood was analyzed by FACS, followed by harvesting whole BM cells and transplanting them individually into a new set of lethally irradiated hosts (n = 7) at the same cell dose (secondary transplantations). The same procedure was repeated after another 16-week period (tertiary transplantations). Also shown (C right) are survival rates for recipient mice transplanted with SIRT1−/− (black) and SIRT1+/+ (gray) BM cells. Bars represent the percentage of mice surviving at 16 weeks after transplantation. (D) Percentages of CD45.2+ donor cells in each WBC compartment of lethally irradiated CD45.1+ recipients 4 months after primary (left), secondary (middle), and tertiary (right) transplantation; each data point represents an individual animal; lines represent means ± SEM (n = 7 per group). The fraction of circulating donor cells gradually decreases during subsequent transplantations, indicating that donor cells are competing with the recipient cells that have survived irradiation.
We next performed a serial transplantation assay using donors older than 18 months to examine the effects of SIRT1 deletion on aging of HSCs and their ability to confer stable hematopoiesis over 3 subsequent transplantations. Four months after we transplanted 2 × 106 CD45.2+ whole BM cells without any competitors into CD45.1+ lethally irradiated recipients, the recipients' BMs were harvested and repetitively transplanted into new CD45.1+ lethally irradiated recipients (secondary and tertiary transplantations, Figure 2C). Recipients transplanted with BM from SIRT1−/− and SIRT1+/+ mice showed equal survival and equal degrees of donor-derived WBC reconstitution 4 months after each transplantation (Figure 2C-D), indicating that SIRT1-deficient HSCs do have a functional potential comparable to those of the wild-type controls. We also examined the frequencies of donor HSCs in recipient mice after harvesting their BM for subsequent transplantation, and saw no differences between the 2 groups (supplemental Figure 2).
In summary, our findings demonstrate that SIRT1 does not have an essential role in the maintenance of the HSC compartment in the adult mice. Evidence from a different mouse model indicated previously that, based on inadequate differentiation of SIRT1−/− mESCs into erythroid progenitors in vitro and the decreased numbers of progenitors under hypoxia ex vivo, SIRT1 plays a role in the establishment of primitive hematopoiesis during embryonic development, raising concern that hematopoiesis could also be affected in the adult, probably to lesser extent, by SIRT1 deletion.16 In the present study, we used a SIRT1 knockout model in a more survival-permissive genetic background and showed that, whereas these mice exhibited all of the stigmata of SIRT1 deletion, they did not exhibit any phenotypical or functional abnormalities in their HSC compartment. These results contradict the hypothesis that SIRT1 deletion confers an adverse effect on the adult HSC pool and, of particular interest to us, possible mechanism-based HSC toxicity associated with future pharmacologic inhibition of SIRT1 in adult humans.
Acknowledgments
The authors thank Drs Julian Simon, Eric Foss, and Elizabeth Kwan for carefully reading the manuscript and providing helpful suggestions.
This work was supported by the National Institutes of Health (grants U19 AI 067770 and CA 129132 to A.B. and P01HL084205 to I.B.) and by the American Cancer Society (grant RSG-04019 CNE to Y.G.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: V.L. designed and performed the experiments, analyzed the data, and wrote the manuscript; B.V.-F. designed the study and analyzed the data; H.L. and Y.G. performed and analyzed the survival curves; D.F. and C.N. provided vital analytical tools; and I.D.B. and A.B. conceived and designed the study, analyzed the data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonio Bedalov, MD, PhD, Clinical Research Division, Fred Hutchinson Cancer Research Center, 1100 Fairview Ave North, Seattle, WA 98109; e-mail: abedalov@fhcrc.org.
References
- 1.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature. 2000;403(6771):795–800. doi: 10.1038/35001622. [DOI] [PubMed] [Google Scholar]
- 2.Sinclair DA, Guarente L. Extrachromosomal rDNA circles–a cause of aging in yeast. Cell. 1997;91(7):1033–1042. doi: 10.1016/s0092-8674(00)80493-6. [DOI] [PubMed] [Google Scholar]
- 3.Vaquero A, Scher M, Lee D, Erdjument-Bromage H, Tempst P, Reinberg D. Human SirT1 interacts with histone H1 and promotes formation of facultative heterochromatin. Mol Cell. 2004;16(1):93–105. doi: 10.1016/j.molcel.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 4.Tissenbaum HA, Guarente L. Increased dosage of a sir-2 gene extends lifespan in Caenorhabditis elegans. Nature. 2001;410(6825):227–230. doi: 10.1038/35065638. [DOI] [PubMed] [Google Scholar]
- 5.Rogina B, Helfand SL. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101(45):15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guarente L. Sir2 links chromatin silencing, metabolism, and aging. Genes Dev. 2000;14(9):1021–1026. [PubMed] [Google Scholar]
- 8.Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18(1):12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434(7029):113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 10.Wood JG, Rogina B, Lavu S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430(7000):686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 11.McBurney MW, Yang X, Jardine K, et al. The mammalian SIR2alpha protein has a role in embryogenesis and gametogenesis. Mol Cell Biol. 2003;23(1):38–54. doi: 10.1128/MCB.23.1.38-54.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang RH, Sengupta K, Li C, et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell. 2008;14(4):312–323. doi: 10.1016/j.ccr.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng HL, Mostoslavsky R, Saito S, et al. Developmental defects and p53 hyperacetylation in Sir2 homolog (SIRT1)-deficient mice. Proc Natl Acad Sci U S A. 2003;100(19):10794–10799. doi: 10.1073/pnas.1934713100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Rajendran GK, Liu N, Ware C, Rubin BP, Gu Y. SirT1 modulates the estrogen-insulin-like growth factor-1 signaling for postnatal development of mammary gland in mice. Breast Cancer Res. 2007;9(1):R1. doi: 10.1186/bcr1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heltweg B, Gatbonton T, Schuler AD, et al. Antitumor activity of a small molecule inhibitor of human Sir2 enzymes. Cancer Res. 2006;66(8):4368–4377. doi: 10.1158/0008-5472.CAN-05-3617. [DOI] [PubMed] [Google Scholar]
- 16.Ou X, Chae HD, Wang RH, et al. SIRT1 deficiency compromises mouse embryonic stem cell hematopoietic differentiation, and embryonic and adult hematopoiesis in the mouse. Blood. 2011;117(2):440–450. doi: 10.1182/blood-2010-03-273011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kondo M, Wagers AJ, Manz MG, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 18.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 19.Goodell MA, Brose K, Paradis G, Conner AS, Mulligan RC. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J Exp Med. 1996;183(4):1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dioum EM, Chen R, Alexander MS, et al. Regulation of hypoxia-inducible factor 2alpha signaling by the stress-responsive deacetylase sirtuin 1. Science. 2009;324(5932):1289–1293. doi: 10.1126/science.1169956. [DOI] [PubMed] [Google Scholar]
- 21.Brown HE, Dougherty TF. The diurnal variation of blood leucocytes in normal and adrenalectomized mice. Endocrinology. 1956;58(3):365–375. doi: 10.1210/endo-58-3-365. [DOI] [PubMed] [Google Scholar]
- 22.Ohkura N, Oishi K, Sekine Y, et al. Comparative study of circadian variation in numbers of peripheral blood cells among mouse strains: unique feature of C3H/HeN mice. Biol Pharm Bull. 2007;30(6):1177–1180. doi: 10.1248/bpb.30.1177. [DOI] [PubMed] [Google Scholar]
- 23.Ramsey KM, Yoshino J, Brace CS, et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science. 2009;324(5927):651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science. 2009;324(5927):654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skokowa J, Lan D, Thakur BK, et al. NAMPT is essential for the G-CSF-induced myeloid differentiation via a NAD(+)-sirtuin-1-dependent pathway. Nat Med. 2009;15(2):151–158. doi: 10.1038/nm.1913. [DOI] [PubMed] [Google Scholar]