Abstract
Platelets are essential for normal hemostasis, but close regulation is required to avoid the destructive effects of either inappropriate platelet activation or excessive responses to injury. Here, we describe a novel complex comprising the scaffold protein, spinophilin (SPL), and the tyrosine phosphatase, SHP-1, and show that it can modulate platelet activation by sequestering RGS10 and RGS18, 2 members of the regulator of G protein signaling family. We also show that SPL/RGS/SHP1 complexes are present in resting platelets where constitutive phosphorylation of SPL(Y398) creates an atypical binding site for SHP-1. Activation of the SHP-1 occurs on agonist-induced phosphorylation of SHP-1(Y536), triggering dephosphorylation and decay of the SPL/RGS/SHP1 complex. Preventing SHP-1 activation blocks decay of the complex and produces a gain of function. Conversely, deleting spinophilin in mice inhibits platelet activation. It also attenuates the rise in platelet cAMP normally caused by endothelial prostacyclin (PGI2). Thus, we propose that the role of the SPL/RGS/SHP1 complex in platelets is time and context dependent. Before injury, the complex helps maintain the quiescence of circulating platelets by maximizing the impact of PGI2. After injury, the complex gradually releases RGS proteins, limiting platelet activation and providing a mechanism for temporal coordination of pro thrombotic and antithrombotic inputs.
Introduction
Platelet responses to most agonists are mediated by G protein–coupled receptors, giving rise to the intracellular events that trigger platelet aggregation and granule exocytosis.1 It has been known for some time that signaling by G proteins in platelets is subject to regulation by extrinsic factors arising from endothelial cells, especially nitric oxide and prostacyclin (PGI2).2 However, intrinsic modulators of platelet activation also exist, including members of the RGS (regulator of G protein signaling) family,3 proteins that suppress G protein signaling by accelerating the hydrolysis of GTP bound to active Gα.4,5 In contrast to nitric oxide and PGI2, RGS proteins are thought to have their effect once activation has begun; hence, the gain of function that we observed when an RGS-insensitive variant of Gi2α was introduced into platelets.3
This inhibitory role for RGS proteins produces a potential conundrum: although preventing unwarranted platelet activation is desirable, preventing the rapid onset of the hemostatic response to injury is not. We have, therefore, sought the means by which the onset of signal suppression by RGS proteins can be delayed, allowing signaling to begin. That search brought us to spinophilin (SPL or neurabin-II), a 130-kDa scaffold protein originally identified in screens for brain proteins that can bind to the serine/threonine phosphatase, PP1,6 and F-actin,7 and subsequently found to associate with other proteins as well,8 including a limited set of G protein–coupled receptors and RGS proteins.9–12 Prior evidence suggests that one region of interaction with SPL lies in the third cytoplasmic loop of susceptible G protein–coupled receptors, allowing SPL to compete with β-arrestin for receptor binding. However, the relation of SPL to G protein–dependent signaling is not fully understood, leaving unanswered critical questions such as the mechanism by which the formation and dissolution of a putative SPL/RGS complex might be regulated and what effect this might have on cellular events.
Here, we show for the first time that SPL is expressed in quiescent human and mouse platelets where it is associated with the protein tyrosine phosphatase, SHP-1, and the RGS proteins, RGS10 and RGS18. Together they form a previously unrecognized SPL/RGS/SHP1 complex in which an unpaired, constitutively phosphorylated ITIM centered on SPL Y398 performs an atypical role: supporting the binding, but not the activation, of SHP-1. We also show that activation of Src family tyrosine kinases by thrombin leads to phosphorylation of a regulatory tyrosine residue (Y536) in SHP-1, activating SHP-1 bound to SPL and triggering the subsequent dephosphorylation and dissociation of the pSPL/RGS/SHP1 complex. We propose that the activation of SHP-1 previously bound to tyrosine-phosphorylated SPL provides a link between receptor activation, decay of the SPL/RGS complex, and release of RGS proteins. The effect of these events is suggested by the consequences of manipulating them: preventing dissociation of the complex produces a gain of function, whereas preventing formation of the complex by knocking out SPL in mice produces a net loss of function by impairing responses to PGI2 in resting platelets and reducing Gq-dependent signaling in activated platelets. The data suggest that the pSPL/RGS/SHP1 complex serves as a context-dependent modulator of platelet function, permitting the initial response to injury in quiescent platelets by sequestering RGS proteins and then releasing them after platelet activation begins.
Methods
Materials
PGI2, apyrase, and ADP were from Sigma-Aldrich. Thrombin was from Hematologic Technologies Inc. Convulxin (CVX) was from Alexis Biochemicals. U46619 was from Calbiochem. Collagen was from Chrono-log. pEx39Not+ containing the cDNA encoding rat SPL was a gift from Dr Patrick Allen (Yale University). Human SHP-1 in pCDNA3.1 was constructed from a pGEX-2T plasmid encoding a GST-SHP-1 fusion protein,13 which was a gift from Dr Benjamin Neel (Ontario Cancer Institute). Human RGS10 and RGS18 in pCDNA3.1+ were purchased from the Missouri University of Science and Technology. Goat anti-SPL (A-20), mouse anti-RGS10 (A-8), anti-pSHP-1 (Ser591), and anti–pSHP-1 (Y536) were from Santa Cruz Biotechnology. Monoclonal anti-SPL (612166) was from Becton Dickinson. Rabbit anti-RGS18 (LS-C785) was from LifeSpan Biosciences. Anti-pTyr Abs 4G10 and PY20 were from Upstate. Mouse (9B11) and rabbit (71D10) anti-Myc were from Cell Signaling Technology. Rabbit polyclonal Gqα Ab no. 0945 was provided by Dr David Manning (University of Pennsylvania).14 NSC-87877 was from Calbiochem. SPL−/− mice were generously provided by Dr Paul Greengard (Rockefeller University).15 The mice had been backcrossed into C57 Bl/6 ≥ 7 times. Comparisons were made between SPL+/+ and SPL−/− mice produced by breeding heterozygotes and performed with protocols approved by the Institutional Animal Care and Use Committee.
Platelet aggregation
Blood was taken from the inferior vena cava of anesthetized mice (100 mg/kg Nembutal) with the use of a heparinized syringe (150 U/mL; 1:9 dilution with blood). Blood was diluted 1:1 with HEPES-Tyrode buffer, and spun at 129g for 7 minutes to prepare platelet-rich plasma. Platelet counts (Beckman-Coulter Z1) were adjusted to 2.5 × 108/mL with autologous platelet-poor plasma. Aggregation was observed in a dual-channel Chrono-log lumi-aggregometer.
Isolation of human platelets
Blood obtained from healthy donors with the use of protocols approved by the University of Pennsylvania Institutional Review Board was anticoagulated 1:5 with ACD (65mM Na3 citrate/70mM citric acid/100mM dextrose, pH 4.4) and centrifuged at 129g for 20 minutes to obtain platelet-rich plasma. Gel-filtered platelets were isolated with Sepharose 2B (Amersham Pharmacia Biotech). Washed platelets were prepared by sedimentation at 341g for 10 minutes. Platelets were washed with HEN (150mM NaCl/1mM Na2EDTA/10mM HEPES, pH 6.5) containing 1μM PGI2 and 1 U/mL apyrase and resuspended in modified Tyrode buffer (137mM NaCl/20mM HEPES/5.6mM glucose/1 g/L BSA/1mM MgCl2/2.7mM KCl/3.3mM/NaH2PO4, pH 7.4).
Cytosolic calcium
Mouse platelets were isolated and washed as described under “Platelet aggression,” incubated with 10 μg/mL Fura-2 AM in Tyrode buffer for 45 minutes at room temperature, washed once with HEN buffer, and resuspended in Tyrode buffer at 5 × 107 platelets/mL. Chinese hamster ovary (CHO) cells were transfected with Myc-SPL, RGS18, and either wild-type (WT) SHP-1 or SHP-1 Y536F; resuspended in PBS containing 1mM CaCl2 and 0.1% BSA; and incubated with 10 μg/mL Fura-2 AM for 30 minutes at 37° in the dark. Cells were then washed twice with PBS/CaCl2 buffer and diluted to 106cells/mL in PBS/CaCl2 buffer. Changes in cytosolic Ca2+ were detected with an SLM/Aminco model AB2 fluorescence spectrophotometer with excitation at 340 and 380 nm and emission at 510 nm.
Rap1 activation
Platelets were washed with 10mM HEPES pH 6.5, 1mM EDTA, 150mM NaCl, 1μM PGE1 and then resuspended in HEPES-Tyrode buffer before use. Each experiment used blood from 2 mice of each genotype adjusted to ≥ 3.0 × 108 platelets/mL and a total volume of 0.25 mL. After incubation with indicated amounts of PAR4 agonist peptide (AYPGKF) for 5 minutes at room temperature, platelets were lysed with Rap1 activation lysis buffer containing protease inhibitors and spun in a microcentrifuge at high speed for 10 minutes at 4°C to remove cell debris. Lysates were removed and processed with the Rap1 Activation Assay kit from Millipore. Quantitation was performed with ImageJ Version 1.42q software (National Institutes of Health).
cAMP formation
Except for the basal cAMP determinations, washed platelets (2.5 × 108/mL) were incubated for 30 minutes at 37°C with 500μM 3-isobutyl-1-methylxanthine to inhibit cAMP phosphodiesterase activity. Platelets were stimulated with 15μM PGI2 and ADP for 10 minutes as indicated. The reaction was stopped with the addition of 1 volume of 10% ice-cold TCA after which the samples were mixed by vortexing, lysed by rapid freezing and thawing, and then spun to remove precipitates. cAMP was measured with the Biotrak EIA system from GE Healthcare. Samples used to measure basal cAMP levels were not incubated with 3-isobutyl-1-methylxanthine, PGI2, or ADP.
Vascular injury
In the carotid artery injury model, the right common carotid artery of 8- to 12-week-old mice was exposed by blunt dissection and placed in contact for 2 minutes with a strip of no. 1 Whatman filter paper soaked with 10% FeCl3. After rinsing with saline, blood flow was recorded with a Doppler flow probe for 30 minutes and the time to first occlusion was determined.16
For additional materials and methods, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
Spinophilin associates with RGS18 and RGS10 in resting platelets and CHO cells
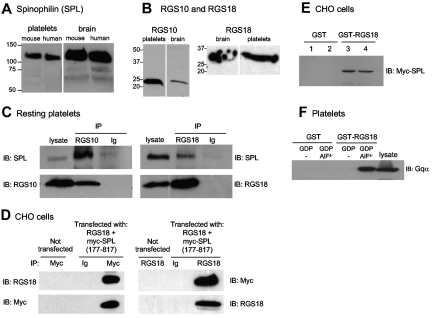
Western blot analyses show that SPL is expressed in human and mouse platelets, comigrating with brain SPL (Figure 1A). RNA transcripts encoding as many as 10 RGS proteins have been reported in platelets.17–22 However, we were able to detect only 2 (RGS10 and RGS18; Figure 1B), which is consistent with transcriptome data.23 Although not reported previously, the following observations indicate that SPL can bind to both of these RGS proteins: in resting platelets and serum-starved CHO cells, immunoprecipitating RGS10 or RGS18 coprecipitated SPL (Figure 1C-D). Conversely, precipitating epitope-tagged SPL from CHO cells coprecipitated RGS18 (Figure 1D). Finally, GST-RGS18 (but not GST), retrieved Myc-tagged SPL from CHO cell lysates (Figure 1E). CHO cells were also used to map the site of interaction of RGS18 with SPL (supplemental Figure 1). Binding localized to SPL (586-664), which is distinct from the previously reported RGS2 binding site.9 In terms of specificity, RGS10 and RGS18 have been shown to interact with Giα family members; RGS18 can bind Gqα as well (Figure 1F).20,24 In the studies that follow, we have chosen to focus primarily on RGS18, but we present evidence that RGS10 behaves in a similar manner.
Figure 1.
Formation of an SPL/RGS complex in resting platelets and CHO cells. (A) Western blot analysis of SPL in platelets and brain. Note that all 4 lysates were run on a single gel, and, indicated by the white line, a marker lane and an empty lane were excised. (B) Western blot analyses of RGS10 and RGS18 in lysates prepared from human platelets and human brain. The vertical white line indicates where an intervening lane was excised. (C) Platelet lysates were precipitated with anti-RGS10, anti-RGS18, or rabbit nonimmune immunoglobulin (Ig) and then probed as indicated. (D) Lysates were prepared from CHO cells cotransfected with RGS18 and Myc-tagged SPL (residues 177-817, Myc-SPL). Proteins were precipitated with anti-Myc, anti-RGS18, or nonimmune globulin (Ig), and then probed as indicated. Each example is representative of ≥ 2 experiments. (E) Lysates prepared from duplicate sets of CHO cells transfected with full-length Myc-SPL were incubated with GST (lanes 1 and 2) or GST-RGS18 (lanes 3 and 4). Proteins that became bound to glutathione beads were probed with anti-Myc. (F) Platelet lysates supplemented with GDP ± AlF4− as indicated were incubated with GST or GST-RGS18. Proteins that became bound to glutathione beads were probed with anti-Gqα. Experimental details are in supplemental Methods.
Agonist-selective dissociation of the SPL/RGS complex during platelet activation
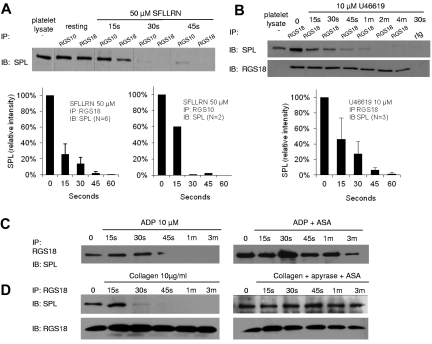
Platelets were incubated with either the agonist peptide, SFLLRN (to activate PAR-1 thrombin receptors), U46619 (TxA2 [thromboxane A2] receptors), ADP (P2Y1 and P2Y12), or collagen (GPVI/FcRγ-chain). SFLLRN and U46619 are potent activators of Gq-mediated events in platelets, ADP is less so, and collagen is not at all except indirectly by causing the release of TxA2 and ADP.1 SFLLRN and U46619 triggered dissociation of the SPL/RGS complex, reaching completion 30-45 seconds after agonist addition (Figure 2A-B). ADP also caused dissociation of the complex, but this effect was reduced by pretreating the platelets with aspirin (ASA), suggesting that it is mediated by released TxA2 (Figure 2C). Similarly, collagen also caused dissociation of the complex, but ASA and apyrase (the latter added to hydrolyze released ADP) blocked this effect (Figure 2D).
Figure 2.
Dissociation of the SPL/RGS complex during platelet activation by thrombin and TxA2 mimetics but not by ADP or collagen. (A-B) Human platelets were incubated with the PAR1 agonist, SFLLRN, or the TxA2 mimetic, U46619, after which lysates were precipitated with anti-RGS10 or anti-RGS18 before being probed for SPL. Error bars represent SEM for the number of replicates indicated if N > 2. The vertical lines in panel A indicate grouping of the results at each time point, not rearrangement of the lanes. (C) Platelets were incubated with ADP ± ASA. (D) Platelets were incubated with collagen ± apyrase and ASA. (C-D) Results are representative of 2 studies each performed with separate donors.
Spinophilin is tyrosine phosphorylated in resting platelets and undergoes dephosphorylation during platelet activation
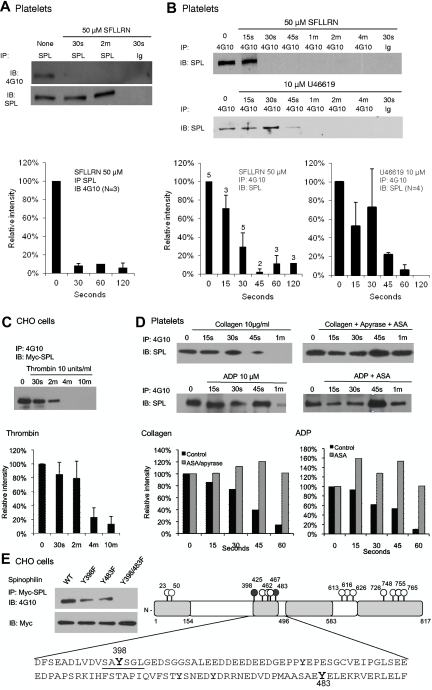
Although tyrosine phosphorylation of SPL has not previously been described, mammalian SPL contains 14 conserved tyrosine residues. Western blot analysis shows that SPL is tyrosine phosphorylated in resting platelets and becomes dephosphorylated when platelets are activated, dephosphorylation occurring in the same time frame as dissociation of the SPL/RGS complex (Figure 3A-B). Dephosphorylation was also observed in CHO cells expressing Myc-tagged SPL (Figure 3C), but as will be shown in supplemental Figure 3A, only when the CHO cells were cotransfected with SHP-1, which they do not normally express. In contrast, ADP and collagen had little, if any, effect on SPL tyrosine phosphorylation in platelets when secondary formation of TxA2 was prevented (Figure 3D). Thus, the agonists that cause dissociation of the pSPL/RGS complex are those that also cause dephosphorylation of SPL.
Figure 3.
Phosphorylation and selective agonist-induced dephosphorylation of SPL Y398 and Y483. (A) Lysates from human platelets incubated with SFLLRN were precipitated with anti-SPL, then probed with a phosphotyrosine-specific Ab (4G10). The graph summarizes 3 studies (mean ± SEM). (B) Platelets were incubated with SFLLRN (N = 2-5) or U46619 (N = 4). Lysates were precipitated with 4G10 or a matched monoclonal control (Ig) and probed for SPL. (C) CHO cells were transfected with Myc-SPL (177-817) and SHP-1. Thrombin was added after an overnight incubation in serum-free medium beginning ∼ 30 hours after transfection (N = 4). (D) Platelets were incubated with collagen or ADP in the presence of ASA and/or apyrase as indicated (N = 2). (E) Phosphorylation sites. (Left) CHO cells were transfected with Myc-tagged, full-length WT SPL, or with Y398F, Y483F, and Y398/483F SPL variants. Lysates were precipitated with anti-Myc and probed for pTyr with 4G10 (N = 2). (Right) A representation of full-length SPL with all 14 tyrosine residues indicated. The sequence that includes Y398 and Y483 is shown. See supplemental Figure 1 for a map of protein binding domains that have been identified in SPL.
Spinophilin forms a complex with SHP-1
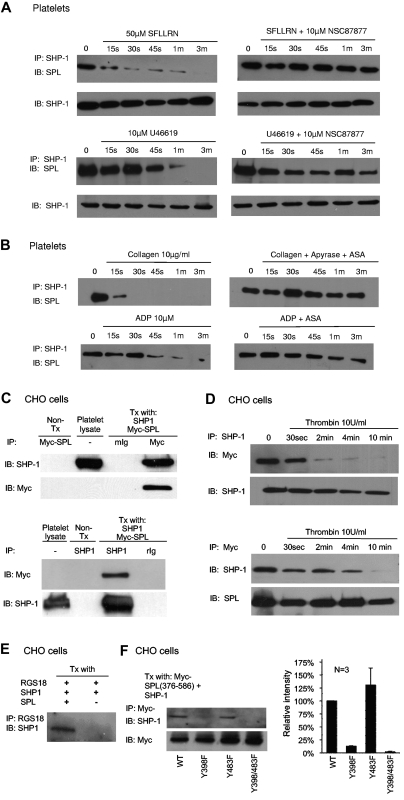
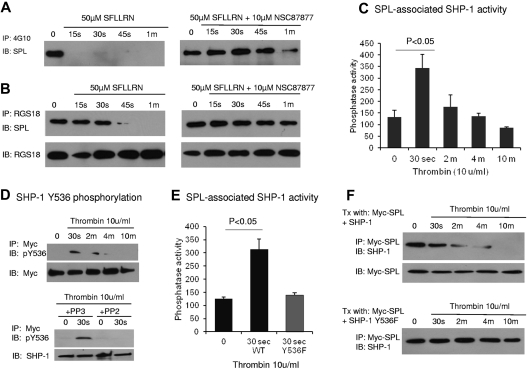
With the use of site-directed mutagenesis, we determined that the sites of phosphorylation in SPL are Y398 and Y483 (Figure 3E). Inspection of the sequence surrounding Y398 suggested that it could serve as an ITIM (I/V/L/SxY(p)xx(I/V/L) and potentially form a binding site for the nonreceptor protein tyrosine phosphatases, SHP-1 and SHP-2, by their tandem SH2 domains.25–27 ITIM domains typically come in pairs, allowing concurrent binding to tandem SH2 domains. However, Y483, the only other phosphorylated tyrosine residue in SPL, is located farther from Y398 than is usually the case for paired ITIM domains, and the sequence surrounding Y483 does not fit an obvious consensus sequence. In terms of potential ITIM partners for SPL, platelets express both SHP-1 and SHP-2, the latter being ubiquitously expressed, whereas SHP-1 is primarily expressed in hematopoietic cells.28,29 We began with SHP-1, probing for an association with SPL in lysates from resting human platelets (Figure 4A-B left side of the figure looking at the time 0 or unstimulated samples) and serum-starved CHO cells cotransfected with SHP-1 and Myc-tagged SPL (Figure 4C-D). As will be discussed below, addition of SFLLRN or U46619 caused dissociation of the SPL/SHP-1 complex, and an SHP-1 inhibitor prevented dissociation. In both platelets and CHO cells, SPL coprecipitated with SHP-1 under conditions in which SPL also coprecipitates with RGS18. To determine whether SPL, RGS proteins, and SHP-1 form a ternary complex, CHO cells were transfected with RGS18 and SHP-1 with and without SPL. The results show that SHP-1 coprecipitates with RGS18 only when SPL is present (Figure 4E). Thus, these molecules form at least a ternary complex.
Figure 4.
Dissociation of the SPL/SHP1 complex in response to thrombin and TxA2 but not collagen or ADP. (A) Human platelets were incubated with SFLLRN or U46619 ± the SHP-1/SHP-2 inhibitor, NSC87877, and then precipitated with anti–SHP-1 before being probed for SPL (N = 4 for SFLLRN; 2 for U46619). (B) Human platelets were incubated with collagen or ADP ± ASA and apyrase as indicated in the figure, and then precipitated with anti–SHP-1 before being probed for SPL (N = 3 for collagen; 2 for ADP). (C) Lysates were prepared from CHO cells cotransfected with SHP-1 and Myc-SPL (N = 2). Proteins were precipitated with (top) anti-Myc or nonimmune globulin (Ig), and then probed with SHP-1 and Myc-SPL, or (bottom) precipitated with anti–SHP-1 and probed for Myc-SPL and SHP-1. (D) CHO cells were transfected with Myc-SPL and SHP-1. Thrombin was added after an overnight incubation in serum-free medium beginning ∼ 30 hours after transfection. Proteins were precipitated with (top) SHP-1, and then probed with Myc-SPL and SHP-1, or (bottom) were precipitated with anti-Myc and probed for SHP-1 and Myc-SPL (N = 3). (E) CHO cells were transfected with RGS18 and SHP-1 ± full-length SPL and then serum-starved. Proteins were precipitated with anti-RGS18 and probed for SHP-1 (N = 3). (F) CHO cells were transfected with SHP-1 and a Myc-tagged SPL fragment encompassing 376-586 in which Y398 and/or Y483 were replaced with Phe as indicated. Proteins were precipitated with anti-Myc and probed for SHP-1, after which time they were reprobed for Myc as indicated. (Left) Representative experiment. (Right) Summary of 3 experiments (mean ± SEM).
SHP-1 binding site on SPL is an unpaired ITIM
The di-Y398/483F substitution that abolished the tyrosine phosphorylation of full-length SPL (shown in Figure 3E) also reduces the association of SHP-1 with SPL (supplemental Figure 2A), suggesting that phosphorylation of one or both of these tyrosine residues is required for SHP-1 binding. However, note that the reduction was incomplete, suggesting that there is an additional interaction site. This additional site maps to the C-terminus of SPL in a fragment that encompasses residues 586-817 (supplemental Figure 2B). Although this region includes 7 tyrosine residues, none resemble an ITIM and none appear to be phosphorylated.
The relative contributions of pY398 and pY483 were assessed with a SPL fragment (residues 376-586) that encompasses both tyrosines, but not the secondary SHP-1 binding site located nearer to the SPL C-terminus. When transfected into CHO cells, SPL (376-586) associated with SHP-1, as did the same fragment with Y483 replaced with Phe. Replacing both tyrosine residues abolished SHP-1 binding, as did replacing Y398 alone (Figure 4F). Thus, it appears that the single phosphorylated ITIM in SPL is both necessary and sufficient for the binding of SHP-1 to SPL. However, as will be shown below, it is not sufficient to activate the phosphatase, which is the normal outcome when SHP-1 and SHP-2 bind to paired ITIMs.29 Thus, the interaction of SHP-1 with SPL is atypical in this regard.
pSPL/SHP1 complex dissociates when platelets are activated
As already noted, SHP-1 is bound to SPL in resting platelets (Figure 4A-B) and serum-starved CHO cells (Figure 4C). Adding SFLLRN or U46619 caused dissociation of the complex in platelets (Figure 4A), as did incubating CHO cells with thrombin (Figure 4D). In contrast, when secondary TxA2 formation was prevented, neither collagen nor ADP induced complex dissociation (Figure 4B). Thus, the agonists that cause dissociation of SHP-1 from SPL in platelets are the same ones that trigger the dephosphorylation of SPL and the release of RGS proteins from SPL.
SHP-1 activation is required for dephosphorylation and dissociation of the pSPL/RGS/SHP1 complex
To investigate the role of SPL-bound SHP-1, we preincubated platelets with SHP-1/SHP-2 inhibitor, NSC-8787730 and then stimulated with either SFLLRN or U46619. The inhibitor prevented the dephosphorylation of SPL (Figure 5A). It also prevented release of SHP-1 and RGS18 from SPL (Figures 4A and 5B). This suggests that activation of SHP-1 is required for all of these events. As a further test, we transfected CHO cells with SPL in the presence and absence of SHP-1 and found that thrombin caused dephosphorylation of SPL only when SHP-1 was present (supplemental Figure 3A). Because CHO cells express SHP-2 endogenously (supplemental Figure 3B),31 these observations indicate that (1) activation of SHP-1 is needed for dephosphorylation and dissociation of the pSPL/RGS/SHP1 complex, and (2) SHP-2 is unable to substitute for SHP-1.
Figure 5.
SHP-1 activation links thrombin to dephosphorylation and dissociation of the pSPL/RGS/SHP1 complex. (A-B) Human platelets were incubated with SFLLRN ± NSC87877 to inhibit SHP-1 and then precipitated with the phosphotyrosine-specific Ab, 4G10 (A), or anti-RGS18 (B) before being probed for SPL. (C) CHO cells were transfected with Myc-SPL and SHP-1 (not tagged). Thrombin was added after an overnight incubation in serum-free medium beginning ∼ 30 hours after transfection. Phosphatase activity was measured in anti-Myc precipitates (mean ± SEM; N = 3). (D top) Similar to panel C except that the anti-Myc precipitate was probed with a pY536-specific SHP-1 Ab. (D bottom) Platelets were preincubated for 10 minutes with either the inhibitor of Src family kinases, PP2, or the inactive congener, PP3, and then stimulated with thrombin as indicated (N = 2). (E) Similar to panel C except that the cells were cotransfected with Myc-SPL and either SHP-1 or SHP-1 Y536F. SPL-associated phosphatase activity was measured (mean ± SEM; N = 3). (F) CHO cells were cotransfected with Myc-SPL and either WT SHP-1 or SHP-1 Y536F. The anti-Myc precipitate was probed for SHP-1 (representative of 2 experiments).
Thrombin activates SHP-1 bound to SPL by causing phosphorylation of SHP-1 Y536
To determine whether thrombin activates the SHP-1 bound to pSPL, we cotransfected CHO cells with Myc-tagged SPL and SHP-1, immunoprecipitated SPL, and assayed phosphatase activity in the precipitate. There was a 3-fold increase in phosphatase activity 30 seconds after the addition of thrombin followed by a gradual decrease (Figure 5C). Because this increase was only observed when SHP-1 was present, unanticipated coprecipitation of a different tyrosine phosphatase is unlikely (supplemental Figure 3C).
Others have shown that SHP-1 activity can be regulated by both tyrosine and serine phosphorylation.28,32,33 Phosphorylation of Y536 and Y564 stimulates phosphatase activity with Y536 phosphorylation having the greater effect.32 With the use of an Ab specific for SHP-1 pY536, we found that SPL-associated SHP-1 became phosphorylated within 30 seconds of thrombin addition, after which SHP-1 phosphorylation, like SHP-1 phosphatase activity, declined (Figure 5D top). Preincubating the cells with PP2 to inhibit Src family kinase activity34 prevented the increase in SHP-1(Y536) phosphorylation. The inactive congener, PP3, had no effect (Figure 5D bottom). In contrast, although we were able to detect serine-phosphorylated SHP-1 in total cell lysate with the use of an Ab specific for pSer591, we were not able to detect serine-phosphorylated SHP-1 in the fraction of SHP-1 that is bound to SPL (supplemental Figure 4). Therefore, SPL-bound SHP-1 appears to be regulated by tyrosine not by serine phosphorylation.
Finally, to determine whether SHP-1 (Y536) phosphorylation provides a necessary link between thrombin receptor activation and the increase in SPL-associated SHP-1 phosphatase activity, we cotransfected CHO cells with SPL and an SHP-1 variant in which Y536 was replaced with Phe. The variant showed no increase in phosphatase activity in response to thrombin (Figure 5E) and, like the WT protein in the presence of the SHP-1 inhibitor, failed to dissociate from SPL when thrombin was added (Figure 5F).
Knocking out SPL in mice inhibits signaling in platelets
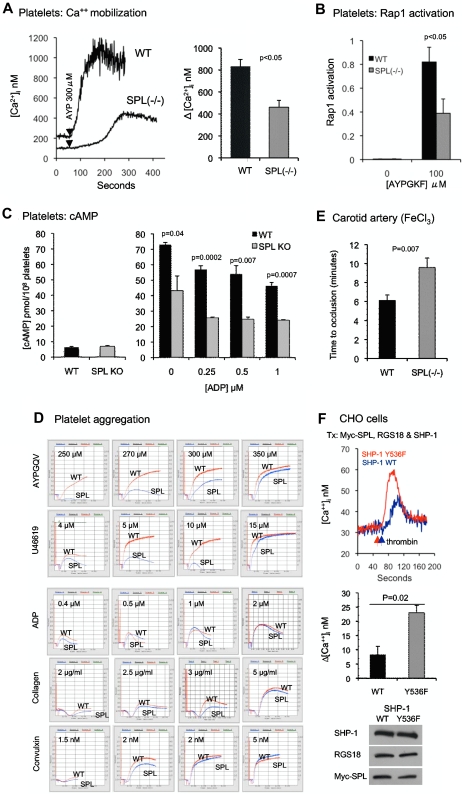
SPL−/− mice have a mild growth defect, diminished brain size, impaired memory, dysregulated dendritic spine formation, and defects in glutaminergic and dopaminergic transmissions.15,35,36 Their platelet counts are normal (mean ± SD, 1.35 ± 0.23 × 108/mL) compared with WT platelets (mean ± SD, 1.38 ± 0.34 × 108/mL), as is expression of RGS10 and RGS18 in SPL−/− platelets (supplemental Figure 5). If association with SPL promotes the interaction of RGS proteins with activated G proteins, then the SPL knockout would be expected to produce a gain of function. However, if association with SPL inhibits the interaction of RGS proteins with G proteins, then the SPL knockout would be predicted to cause a loss of function. To determine which is the case, we measured changes in the platelet cytosolic Ca2+ concentration in response to the PAR4 (thrombin receptor) agonist, AYPGKF. Figure 6A shows that in control platelets AYPGKF evokes a rapid increase in cytosolic Ca2+, peaking at > 1μM. By comparison, the Ca2+ increase in SPL−/− platelets was delayed, occurred more slowly, and rose only half as high. No effect of SPL deficiency was observed on the platelet response to collagen measured in the presence of ASA and apyrase (supplemental Figure 6).
Figure 6.
The SPL knockout produces a loss of function, whereas preventing SHP-1 activation produces a gain of function. (A) Ca2+ mobilization. (Left) Platelets from matched WT and SPL−/− mice stimulated with the PAR4 agonist peptide, AYPGKF. (Right) Summary of data from 3 studies (mean ± SEM). (B) Rap1 activation. Rap1-GTP levels were measured in unstimulated platelets and in platelets stimulated with AYPGKF for 5 minutes (mean ± SEM; N = 3). (C) cAMP formation. (Left) Basal cAMP concentration in SPL−/− platelets (N = 3) and matched controls (N = 8). (Right) cAMP levels in platelets preincubated with PGI2 and stimulated with ADP at the final concentrations indicated (mean ± SEM; N = 3). (D) Aggregation traces comparing platelets from SPL−/− and matched control mice (WT). These data are representative of 8-10 separate experiments. (E) Time to carotid artery occlusion after a 2-minute exposure to 10% FeCl3 in 6 SPL−/− mice and 9 matched WT controls. (F top) CHO cells transfected with Myc-SPL (177-817), RGS18, and either WT or Y536F SHP-1. After loading with Fura-2, the cells were stimulated with thrombin and changes in the cytosolic Ca2+ were recorded. (F middle) Summary of 3 experiments (mean ± SEM). (F bottom) Western blot analyses showing equal protein expression in cells receiving WT and Y536F SHP-1.
As a second index of Gq-dependent signaling, we measured Rap1 activation.37 The increase in Rap1-GTP caused by AYPGKF was only half as great in SPL−/− platelets as in controls (Figure 6B). Thus, the SPL knockout clearly reduces Gq-mediated signaling in platelets. These results indicate that a primary role for SPL in platelets is to inhibit, rather than promote, the interaction of RGS proteins with Gq.
Because RGS10 and RGS18 can also bind to Gi family members, we examined the effects of the SPL knockout on cAMP levels in resting platelets and in platelets stimulated with PGI2 in the presence or absence of ADP. Basal cAMP levels were normal in SPL−/− platelets, and there was no reduction in the ability of ADP to inhibit PGI2-stimulated cAMP formation (Figure 6C). However, the increment in cAMP levels observed on stimulation with PGI2 in the absence of ADP was only half as great in SPL−/− platelets as in controls (mean ± SEM, 36.3 ± 9.5 vs 65.4 ± 0.5 pmol/108 platelets after subtracting background; N = 3; P = .038). These results show that SPL is not linked to Gi-mediated ADP signaling in platelets, but they also suggest that the RGS proteins that associate with SPL in platelets may attenuate activation of adenylyl cyclase as RGS2 has been reported to do.38,39
Knocking out SPL impairs platelet function in vitro and in vivo
On the basis of the observations above, we predicted that SPL deficiency would have complex effects on global platelet function because the absence of SPL would leave RGS proteins free to affect events that are antithrombotic (cAMP formation) as well as those that are prothrombotic (Gq signaling). Stated differently, the defects we observed in Ca2+ regulation and Rap1 activation in SPL−/− platelets in vitro would be expected to translate into an activation defect in vivo, but a reduction in the ability of PGI2 to raise platelet cAMP levels would be expected to produce a gain of function.40
To determine the net effect of these 2 potentially opposing consequences of the SPL knockout, SPL−/− platelet function was tested in vitro and in vivo. In the aggregation assays, which are performed in the absence of a source of PGI2, platelets were stimulated with AYPGQV, U46619, ADP, and 2 collagen receptor agonists, collagen and CVX. A pronounced rightward shift in the dose/response curves for AYPGQV and U46619 was observed in the knockout (Figure 6D). In comparison, the aggregation responses to ADP, collagen, and CVX were essentially normal, especially when taking into account the secondary contribution of released TxA2 when platelets are stimulated with collagen and other CVX. Thus, the knockout impaired responses to agonists that can trigger dissociation of the pSPL/RGS/SHP1 complex but had no effect on the agonists that do not.
A loss of function was also seen when an oxidative injury was produced by applying FeCl3 to a surgically exposed carotid artery.16 This model is known to be sensitive to PGI2 formation as indicated by the substantial increase in thrombosis observed in mice that lack PGI2 receptors.40 Here, we found that the average time to occlusion was ∼ 6 minutes in control mice, increasing ∼ 57% to almost 10 minutes in SPL−/− mice (P = .007; Figure 6E). These results indicate that deleting SPL produces a net loss of platelet function despite attenuation of the inhibitory effect of PGI2.
Preventing RGS protein release from the SPL/RGS/SHP1 complex produces a gain of function
Because knocking out SPL produces a net loss of function, we next asked whether preventing dissociation of the complex would produce a gain of function. This was accomplished by replacing WT SHP-1 in CHO cells with the Y536F variant that can bind to SPL but is incapable of being activated. As already noted, this variant blocks dissociation of the pSPL/RGS/SHP-1 complex (Figure 5F). CHO cells were transfected with Myc-tagged SPL, RGS18, and either WT SHP-1 or the SHP-1 Y536F variant. Changes in cytosolic Ca2+ concentration were measured after the addition of thrombin. A gain of function was observed in cells expressing the SHP-1 Y536F variant (Figure 6F). Thus, in contrast to the knockout, which precludes formation of the pSPL/RGS/SHP1 complex, preventing SHP-1 activation results in an increase in Gq-dependent signaling.
Discussion
Achieving an optimal platelet response to vascular injury means finding a balance between an inadequate response that will not achieve hemostasis and an overly robust response that can lead to arterial occlusion. The present study is part of an ongoing effort to understand the intrinsic mechanisms that modulate signaling in platelets so that an optimal response can be achieved. Having recently established that RGS proteins play an inhibitory role in platelets,3 we asked whether the negative feedback provided by RGS proteins might itself be coordinated so that it limits, rather than prevents, platelet accumulation after injury.
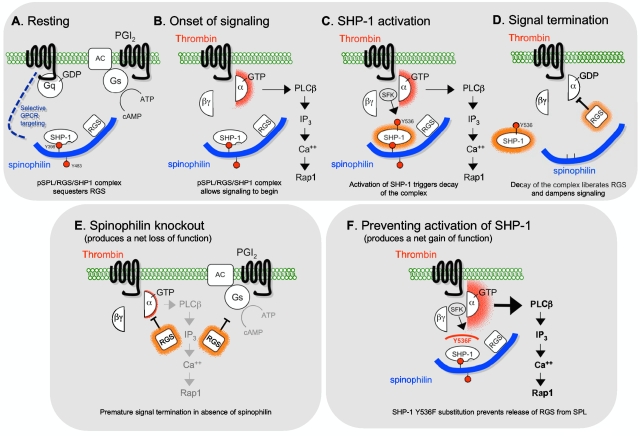
Figure 7A through D shows a working model that encompasses our observations. In resting platelets, RGS10 and RGS18 are bound to SPL that is phosphorylated on Y398 and Y483. On the basis of the sequence surrounding Y398 and our finding that replacing it with Phe blocks SHP-1 binding, we propose that phosphorylated Y398 is part of an unpaired ITIM that can support SHP-1 binding. SHP-1 typically binds to paired ITIMs, which engage both of its SH2 domains, activating the phosphatase catalytic domain by disengaging it from inhibitory intramolecular interactions with the N-terminal SH2 domain.27 However, this is clearly not the case for SPL whereby only one ITIM is present and the SHP-1 which is bound to SPL is inactive. Thus, we propose that SPL is an atypical binding partner for SHP-1, one in which the single SPL ITIM domain engages the C-terminal SH2 domain in SHP-1, allowing SHP-1 to bind without being activated (supplemental Figure 7). Instead, activation of SPL-bound SHP-1 requires phosphorylation of SHP-1 Y536, an event most probably mediated by a Src family member on the basis of both our observations and the literature.32,41,42 Although examined for different reasons, Pasquet et al suggested that the activation of Src family kinases in platelets by thrombin occurs downstream of a Gq-mediated increase in platelet Ca2+.33 If so, then this closes a loop in which the onset of signaling activates SHP-1, releasing RGS proteins from SPL and, therefore, dampening signaling.
Figure 7.
A model for the role of the pSPL/RGS/SHP1 complex in platelets. (A) The data indicate that in resting platelets SPL, SHP-1, and RGS proteins exist as a complex in which SPL is phosphorylated on Y398 and Y483. The specificity of subsequent events arises from a requirement for Gq-dependent signaling and/or selective interactions between SPL and the receptors for thrombin and TxA2. (B) Platelet activation by thrombin or TxA2 leads to platelet activation and (C) phosphorylation of SHP-1 Y536, activating the phosphatase. (D) SHP-1 activation triggers dephosphorylation of SPL and dissociation of the SPL/RGS/SHP1 complex, releasing RGS proteins that inhibit signaling by Gq. (E) The SPL knockout mimics aspects of the late activation state in platelets, making RGS proteins available prematurely and giving rise to the delayed signaling and loss of function phenotype that we observed. (F) Conversely, blocking SHP-1 activation by substituting Phe for Tyr 536 prevents dephosphorylation and dissociation of the pSPL/RGS/SHP1 complex, sequestering the RGS proteins and producing the observed gain of function.
As noted earlier, overexpression of RGS proteins inhibits signaling, whereas preventing RGS interactions with activated Gi2α in platelets produced a gain of function.3 The loss of function that we observed in SPL−/− platelets suggests that RGS10 and RGS18 bound to SPL are functionally sequestered in resting platelets and that their release on platelet activation allows them preferential access to activated Gqα, dampening signaling (Figure 7E). In the absence of SPL, Gq-mediated signaling is suppressed prematurely. Conversely, preventing SHP-1 activation by replacing SHP-1 Y536 with Phe produces a gain of function by preventing the release of RGS proteins (Figure 7F).
In addition to inhibiting Gq-mediated signaling, knocking out SPL also attenuates the ability of PGI2 to raise cAMP levels in platelets. Others have shown that RGS2, an RGS family member related to RGS18, can suppress cAMP formation by directly interacting with adenylyl cyclase type III,38 the isoform expressed in platelets.23,43 Given that report and the observations presented here, we propose that in circulating platelets the ability of SPL to sequester RGS proteins also facilitates a maximal response to PGI2, helping to maintain the platelets in a quiescent state. The attenuated response to PGI2 that we observed in the knockout may, as already noted, partially mitigate the effects of SPL deficiency on platelet activation in vivo.
Thus, it appears that activation of SPL-bound SHP-1 provides a link between receptor activation and dissociation of the SPL/RGS complex. The selectivity of the link is reflected by the precise correspondence between the agonists that cause dephosphorylation and dissociation of the pSPL/RGS/SHP1 complex and those whose effects are impaired in SPL−/− platelets. The lack of an effect on collagen signaling is perhaps to be expected because collagen receptors are not coupled to G proteins. ADP, however, activates 2 G protein–coupled receptors on platelets, one of which, P2Y1, interacts with Gq.1 However, P2Y1 is not expressed at high levels on platelets, and ADP is not as robust an activator of Gq as are thrombin and TxA2, which may keep it from activating the kinase that phosphorylates SHP-1.1 Alternatively, P2Y1 may not be one of the subset of G protein–coupled receptors to which SPL can bind. The lack of an effect on responses to some agonists appears to rule out a global defect in platelet function in the SPL−/− platelets, but a further possibility, which is that loss of SPL results in a selective decrease in PAR4 and TxA2 receptor expression, cannot be fully excluded at this time as a contributing factor to the results we obtained.
Although here we have focused on platelets, SPL is widely expressed, opening the possibility that the conclusions apply to other types of cells as well. SHP-1, however, is primarily expressed in hematopoietic cells.29,44 Previous investigators working on nonhematopoietic cells have proposed that SPL helps to promote the interaction of RGS proteins with G proteins.45 Consistent with this hypothesis, α-adrenergic receptor signaling is inhibited less well by SPL in cells lacking RGS2, and, conversely, RGS2 is less effective at inhibiting signaling in cells that lack SPL.9 This does not appear to be the case in platelets whereby the SPL knockout produces a net loss of function. Because SHP-2 does not appear to bind to SPL, the differences between cell types may reflect the presence or absence of SHP-1.
In conclusion, the present studies describe a novel mechanism by which a complex of SHP-1 and SPL can regulate responses to selected agonists and PGI2 in platelets by retaining and releasing RGS proteins. Putting our observations together with what is already known about extrinsic regulators of platelet function such as PGI2 leads us to suggest that the role of SPL occurs in 3 phases. In circulating platelets that have not been exposed to activators, the phosphorylated SPL/RGS/SHP-1 complex helps to maintain the quiescent state by maximizing the effects of endothelial PGI2. In acutely activated platelets, the complex helps to prevent premature termination of Gq-mediated signaling within platelets by restraining RGS proteins. Finally, in platelets that have been turned on long enough for signaling to initiate decay of the SPL/RGS/SHP-1 complex, SPL helps to place a limit on the extent of platelet accumulation by increasing the local concentration of available RGS18 and RGS10, targeting the RGS proteins to the vicinity of receptors able to interact directly with SPL. In other words, the pSPL/RGS/SHP1 complex provides not only a regulatory link but also a mechanism for conferring signaling selectivity and coordinating prothrombotic and antithrombotic inputs.
Supplementary Material
Acknowledgments
The authors thank Honghua Yang for her technical assistance and Dr Mark Kahn for his critical reading of the manuscript and helpful suggestions.
This work was supported by the National Institutes of Health (grants HL40387 and HL93123 to L.F.B.; grant GM39561 to R.R.N.; and T32 HL07439 to R.S.). T.J.S. was supported by an American Heart Association postdoctoral fellowship (0525630U).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: P.M., A.C., R.S., M.C., H.K., A.J.S., and T.J.S. performed experiments; P.M., A.C., R.S., and T.J.S. designed research, analyzed results, and made figures; R.R.N., D.K.N., and L.F.B. designed research and contributed new reagents or analytical tools; and P.M. and L.F.B. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lawrence F. Brass, University of Pennsylvania, 915 BRB II/III, 421 Curie Blvd, Philadelphia, PA 19104; e-mail: brass@mail.med.upenn.edu.
References
- 1.Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006;99(12):1293–1304. doi: 10.1161/01.RES.0000251742.71301.16. [DOI] [PubMed] [Google Scholar]
- 2.Mustard JF, Kinlough-Rathbone RL, Packham MA. Prostaglandins and platelets. Annu Rev Med. 1980;31:89–96. doi: 10.1146/annurev.me.31.020180.000513. [DOI] [PubMed] [Google Scholar]
- 3.Signarvic RS, Cierniewska A, Stalker TJ, et al. RGS/Gi2alpha interactions modulate platelet accumulation and thrombus formation at sites of vascular injury. Blood. 2010;116(26):6092–6100. doi: 10.1182/blood-2010-05-283846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xie GX, Palmer PP. How regulators of G protein signaling achieve selective regulation. J Mol Biol. 2007;366(2):349–365. doi: 10.1016/j.jmb.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bansal G, Druey KM, Xie Z. R4 RGS proteins: regulation of G-protein signaling and beyond. Pharmacol Ther. 2007;116(3):473–495. doi: 10.1016/j.pharmthera.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allen PB, Ouimet CC, Greengard P. Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci U S A. 1997;94(18):9956–9961. doi: 10.1073/pnas.94.18.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Satoh A, Nakanishi H, Obaishi H, et al. Neurabin-II/spinophilin. An actin filament-binding protein with one PDZ domain localized at cadherin-based cell-cell adhesion sites. J Biol Chem. 1998;273(6):3470–3475. doi: 10.1074/jbc.273.6.3470. [DOI] [PubMed] [Google Scholar]
- 8.Sarrouilhe D, di Tommaso A, Metaye T, Ladeveze V. Spinophilin: from partners to functions. Biochimie. 2006;88(9):1099–1113. doi: 10.1016/j.biochi.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Wang X, Zeng W, Soyombo AA, et al. Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nat Cell Biol. 2005;7(4):405–411. doi: 10.1038/ncb1237. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Zeng W, Kim MS, Allen PB, Greengard P, Muallem S. Spinophilin/neurabin reciprocally regulate signaling intensity by G protein-coupled receptors. EMBO J. 2007;26(11):2768–2776. doi: 10.1038/sj.emboj.7601701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith FD, Oxford GS, Milgram SL. Association of the D2 dopamine receptor third cytoplasmic loop with spinophilin, a protein phosphatase-1-interacting protein. J Biol Chem. 1999;274(28):19894–19900. doi: 10.1074/jbc.274.28.19894. [DOI] [PubMed] [Google Scholar]
- 12.Richman JG, Brady AE, Wang Q, Hensel JL, Colbran RJ, Limbird LE. Agonist-regulated interaction between alpha2-adrenergic receptors and spinophilin. J Biol Chem. 2001;276(18):15003–15008. doi: 10.1074/jbc.M011679200. [DOI] [PubMed] [Google Scholar]
- 13.Plutzky J, Neel BG, Rosenberg RD. Isolation of a src homology 2-containing tyrosine phosphatase. Proc Natl Acad Sci U S A. 1992;89(3):1123–1127. doi: 10.1073/pnas.89.3.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butkerait P, Zheng Y, Hallak H, et al. Expression of the human 5-hydroxytryptamine1A receptor in Sf9 cells. Reconstitution of a coupled phenotype by co-expression of mammalian G protein subunits. J Biol Chem. 1995;270(31):18691–18699. doi: 10.1074/jbc.270.31.18691. [DOI] [PubMed] [Google Scholar]
- 15.Feng J, Yan Z, Ferreira A, et al. Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci U S A. 2000;97(16):9287–9292. doi: 10.1073/pnas.97.16.9287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu L, Bergmeier W, Wu J, et al. Regulated surface expression and shedding support a dual role for semaphorin 4D in platelet responses to vascular injury. Proc Natl Acad Sci U S A. 2007;104(5):1621–1626. doi: 10.1073/pnas.0606344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yowe D, Weich N, Prabhudas M, et al. RGS18 is a myeloerythroid lineage-specific regulator of G-protein-signalling molecule highly expressed in megakaryocytes. Biochem J. 2001;359(pt 1):109–118. doi: 10.1042/0264-6021:3590109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagata Y, Oda M, Nakata H, Shozaki Y, Kozasa T, Todokoro K. A novel regulator of G-protein signaling bearing GAP activity for Galphai and Galphaq in megakaryocytes. Blood. 2001;97(10):3051–3060. doi: 10.1182/blood.v97.10.3051. [DOI] [PubMed] [Google Scholar]
- 19.Kim SD, Sung HJ, Park SK, et al. The expression patterns of RGS transcripts in platelets. Platelets. 2006;17(7):493–497. doi: 10.1080/09537100600758123. [DOI] [PubMed] [Google Scholar]
- 20.Gagnon AW, Murray DL, Leadley RJ. Cloning and characterization of a novel regulator of G protein signalling in human platelets. Cell Signal. 2002;14(7):595–606. doi: 10.1016/s0898-6568(02)00012-8. [DOI] [PubMed] [Google Scholar]
- 21.Garcia A, Prabhakar S, Hughan S, et al. Differential proteome analysis of TRAP-activated platelets: involvement of DOK-2 and phosphorylation of RGS proteins. Blood. 2004;103(6):2088–2095. doi: 10.1182/blood-2003-07-2392. [DOI] [PubMed] [Google Scholar]
- 22.Berthebaud M, Riviere C, Jarrier P, et al. RGS16 is a negative regulator of SDF-1-CXCR4 signaling in megakaryocytes. Blood. 2005;106(9):2962–2968. doi: 10.1182/blood-2005-02-0526. [DOI] [PubMed] [Google Scholar]
- 23.Rowley JW, Oler A, Tolley ND, et al. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood. 2011;118(14):e101–e111. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt TW, Fields TA, Casey PJ, Peralta EG. RGS10 is a selective activator of Galphai GTPase activity. Nature. 1996;383(6596):175–177. doi: 10.1038/383175a0. [DOI] [PubMed] [Google Scholar]
- 25.Barrow AD, Trowsdale J. You say ITAM and I say ITIM, let's call the whole thing off: the ambiguity of immunoreceptor signalling. Eur J Immunol. 2006;36(7):1646–1653. doi: 10.1002/eji.200636195. [DOI] [PubMed] [Google Scholar]
- 26.Ravetch JV, Lanier LL. Immune inhibitory receptors. Science. 2000;290(5489):84–89. doi: 10.1126/science.290.5489.84. [DOI] [PubMed] [Google Scholar]
- 27.Neel BG, Gu H, Pao L. The ‘Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28(6):284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 28.Jones ML, Craik JD, Gibbins JM, Poole AW. Regulation of SHP-1 tyrosine phosphatase in human platelets by serine phosphorylation at its C terminus. J Biol Chem. 2004;279(39):40475–40483. doi: 10.1074/jbc.M402970200. [DOI] [PubMed] [Google Scholar]
- 29.Lorenz U. SHP-1 and SHP-2 in T cells: two phosphatases functioning at many levels. Immunol Rev. 2009;228(1):342–359. doi: 10.1111/j.1600-065X.2008.00760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen L, Sung SS, Yip ML, et al. Discovery of a novel shp2 protein tyrosine phosphatase inhibitor. Mol Pharmacol. 2006;70(2):562–570. doi: 10.1124/mol.106.025536. [DOI] [PubMed] [Google Scholar]
- 31.Bjorbaek C, Buchholz RM, Davis SM, et al. Divergent roles of SHP-2 in ERK activation by leptin receptors. J Biol Chem. 2001;276(7):4747–4755. doi: 10.1074/jbc.M007439200. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, Shen K, Lu W, Cole PA. The role of C-terminal tyrosine phosphorylation in the regulation of SHP-1 explored via expressed protein ligation. J Biol Chem. 2003;278(7):4668–4674. doi: 10.1074/jbc.M210028200. [DOI] [PubMed] [Google Scholar]
- 33.Pasquet JM, Quek L, Pasquet S, et al. Evidence of a role for SHP-1 in platelet activation by the collagen receptor glycoprotein VI. J Biol Chem. 2000;275(37):28526–28531. doi: 10.1074/jbc.M001531200. [DOI] [PubMed] [Google Scholar]
- 34.Hanke JH, Gardner JP, Dow RL, et al. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271(2):695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- 35.Allen PB, Zachariou V, Svenningsson P, et al. Distinct roles for spinophilin and neurabin in dopamine-mediated plasticity. Neuroscience. 2006;140(3):897–911. doi: 10.1016/j.neuroscience.2006.02.067. [DOI] [PubMed] [Google Scholar]
- 36.Stafstrom-Davis CA, Ouimet CC, Feng J, Allen PB, Greengard P, Houpt TA. Impaired conditioned taste aversion learning in spinophilin knockout mice. Learn Mem. 2001;8(5):272–278. doi: 10.1101/lm.42101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crittenden JR, Bergmeier W, Zhang Y, et al. CalDAG-GEFI integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10(9):982–986. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- 38.Sinnarajah S, Dessauer CW, Srikumar D, et al. RGS2 regulates signal transduction in olfactory neurons by attenuating activation of adenylyl cyclase III. Nature. 2001;409(6823):1051–1055. doi: 10.1038/35059104. [DOI] [PubMed] [Google Scholar]
- 39.Salim S, Sinnarajah S, Kehrl JH, Dessauer CW. Identification of RGS2 and type V adenylyl cyclase interaction sites. J Biol Chem. 2003;278(18):15842–15849. doi: 10.1074/jbc.M210663200. [DOI] [PubMed] [Google Scholar]
- 40.Murata T, Ushikubi F, Matsuoka T, et al. Altered pain perception and inflammatory response in mice lacking prostacyclin receptor. Nature. 1997;388(6643):678–682. doi: 10.1038/41780. [DOI] [PubMed] [Google Scholar]
- 41.Li RY, Gaits F, Ragab-Thomas JM, et al. Protein tyrosine phosphatase SHP-1 fails to associate with cytoskeleton but is normally phosphorylated upon thrombin stimulation of thrombasthenic platelets. Thromb Haemost. 1997;77(1):150–154. [PubMed] [Google Scholar]
- 42.Hibbs ML, Harder KW, Armes J, et al. Sustained activation of Lyn tyrosine kinase in vivo leads to autoimmunity. J Exp Med. 2002;196(12):1593–1604. doi: 10.1084/jem.20020515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Katsel PL, Tagliente TM, Schwarz TE, Craddock-Royal BD, Patel ND, Maayani S. Molecular and biochemical evidence for the presence of type III adenylyl cyclase in human platelets. Platelets. 2003;14(1):21–33. doi: 10.1080/0953710021000062905. [DOI] [PubMed] [Google Scholar]
- 44.Jones ML, Poole AW. Protein tyrosine phosphatases. Methods Mol Biol. 2004;273:169–178. doi: 10.1385/1-59259-783-1:169. [DOI] [PubMed] [Google Scholar]
- 45.Abramow-Newerly M, Roy AA, Nunn C, Chidiac P. RGS proteins have a signalling complex: interactions between RGS proteins and GPCRs, effectors, and auxiliary proteins. Cell Signal. 2006;18(5):579–591. doi: 10.1016/j.cellsig.2005.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.