Abstract
Although large cholangiocytes exert their functions by activation of cyclic adenosine 3′,5′-monophosphate (cAMP), Ca2+-dependent signaling regulates the function of small cholangiocytes. Histamine interacts with four receptors, H1–H4HRs. H1HR acts by Gαq activating IP3/Ca2+, whereas H2HR activates Gαs stimulating cAMP. We hypothesize that histamine increases biliary growth by activating H1HR on small and H2HR on large cholangiocytes. The expression of H1–H4HRs was evaluated in liver sections, isolated and cultured (normal rat intrahepatic cholangiocyte culture (NRIC)) cholangiocytes. In vivo, normal rats were treated with histamine or H1–H4HR agonists for 1 week. We evaluated: (1) intrahepatic bile duct mass (IBDM); (2) the effects of histamine, H1HR or H2HR agonists on NRIC proliferation, IP3 and cAMP levels and PKCα and protein kinase A (PKA) phosphorylation; and (3) PKCα silencing on H1HR-stimulated NRIC proliferation. Small and large cholangiocytes express H1–H4HRs. Histamine and the H1HR agonist increased small IBDM, whereas histamine and the H2HR agonist increased large IBDM. H1HR agonists stimulated IP3 levels, as well as PKCα phosphorylation and NRIC proliferation, whereas H2HR agonists increased cAMP levels, as well as PKA phosphorylation and NRIC proliferation. The H1HR agonist did not increase proliferation in PKCα siRNA-transfected NRICs. The activation of differential signaling mechanisms targeting small and large cholangiocytes is important for repopulation of the biliary epithelium during pathologies affecting different-sized bile ducts.
Keywords: biliary epithelium, biogenic amines, cAMP, heterogeneity, IP3, secretin receptor
Cholangiocytes, which line intrahepatic and extrahepatic bile ducts,1,2 modify canalicular bile while it is being delivered from the bile canaliculus to the small intestine.3,4 Secretin stimulates secretion of ductal bile by interaction with secretin receptors (SRs)1,3 that increases the synthesis of cyclic adenosine 3′,5′-monophosphate (cAMP), phosphorylation of protein kinase A (PKA), opening of cystic fibrosis transmembrane conductance regulator with subsequent activation of the Cl−/HCO3− anion exchanger 2 (AE2), leading to enhanced bicarbonate secretion into bile.1,3,5–7
Cholangiocytes, which are constitutively mitotically dormant, 8,9 markedly proliferate or are damaged in human cholangiopathies10 and in animal models of cholestasis, including bile duct ligation (BDL) and acute carbon tetrachloride (CCl4) administration.11 As cholangiocytes are the only cells expressing SR in the rodent liver,12 changes in the expression of this receptor are key for functionally evaluating the degree of biliary growth/loss.3,8,9,11,13,14 For example, treatment of normal rats with forskolin induces cholangiocyte hyperplasia and increases secretin-stimulated choleresis13 that is similar to that seen in the BDL model.3 In models of biliary damage (eg, after acute CCl4 administration), there is loss of secretin-stimulated ductal secretion.11 Models of biliary proliferation/loss are critically important to better understand the development of cholangiopathies, including primary biliary cirrhosis and primary sclerosing cholangitis. Two main signaling pathways are important for regulating cholangiocyte proliferation: the IP3/Ca2+- and the cAMP-dependent signaling pathways.8,9,11,14–16 These two signaling pathways regulate both small (IP3/Ca2+) and large (cAMP) cholangiocyte growth/loss in rodent models of cholestasis.8,9,11,14–16
Histamine is derived from the conversion of histidine to histamine through the enzyme L-histidine decarboxylase,17 and interacts with four G protein-coupled receptors: H1HR, H2HR, H3HR and H4HR.18,19 Although endogenous histamine elicits a proliferative effect in different cell types,20,21 the four histamine receptors (HRs) interact with independent G proteins to stimulate varying signaling pathways producing varying effects on cells.22 The H1HR couples to Gαq mobilizing Ca2+-dependent signaling in a number of cells including cholangiocytes,15,22 whereas the H2HR mainly interacts with Gαs stimulating a cAMP-dependent pathway.23 These two receptors stimulate the growth of numerous cell types, including cholangiocytes.15,21 This is in contrast to H3 and H4HRs that have inhibitory effects on cell growth (including cholangiocytes)16 by Gαi coupling that inhibits cAMP synthesis.16 In our study, we tested the hypotheses that (1) histamine stimulates cholangiocyte proliferation of normal rats and (2) an H1HR agonist stimulates the proliferation of small cholangiocytes by activation of IP3/Ca2+ signaling, whereas H2HR agonists increase the proliferation of large cholangiocytes by an intracellular mechanism requiring the activation of cAMP-dependent signaling.
MATERIALS AND METHODS
Materials
Reagents were purchased from Sigma Chemical (St Louis, MO), unless otherwise indicated. The HR agonists, histamine trifluoromethyl toluidide (HTMT dimaleate, H1HR agonist), 15 amthamine dihydrobromide (H2HR agonist)24 and (R)-(α)-(−)-methylhistamine dihydrobromide (RAMH, a H3HR agonist)16 were obtained from Tocris Bioscience (Ellisville, MO). The monoclonal mouse antibody against proliferating cellular nuclear antigen (PCNA, clone PC10, cat. no. M0879) was purchased from Dako (Kyoto, Japan). The substrate for γ-glutamyl transpeptidase (γ-GT), N-(γ-L-glutamyl)-4-methoxy-2-naphthylamide was purchased from Polysciences (Warrington, PA). Antibodies for HR subtypes were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), and previously used in rat liver sections and in rat and mouse cholangiocytes.15,16 In particular, H1HR (clone A-20, cat. no. sc-33970) is an affinity-purified goat polyclonal antibody raised against a peptide mapping within a cytoplasmic domain of H1HR of human origin. H2HR (clone H-70, cat. no. sc-50314) is a rabbit polyclonal antibody raised against amino acids 290–359 mapping within a C-terminal cytoplasmic domain of H2HR of human origin. H3HR (clone C-20, cat. no. sc-17921) is an affinity-purified goat polyclonal antibody raised against a peptide mapping near the C terminus of H3HR of human origin. H4HR (clone Q-20, cat. no. sc-33965) is an affinity-purified goat polyclonal antibody raised against a peptide mapping within a cytoplasmic domain of H4HR of human origin. The goat polyclonal affinity-purified phosphorylated-PKCα antibody (Ser 657, cat. no. sc-12356) was raised against a short aminoacid sequence containing phosphorylated Ser 657 of PKCα of human origin (Santa Cruz Biotechnology). PKCα (H-7, sc-8393) is a mouse monoclonal antibody specific for an epitope mapping between amino acids 645 and 672 at the C terminus of PKCα of human origin (Santa Cruz Biotechnology). The goat polyclonal affinity-purified pPKA IIα reg antibody (Ser 96, sc-12905-R) was raised against a short amino-acid sequence containing phosphorylated Ser 96 of p-PKA IIα reg of human origin (Santa Cruz Biotechnology). The PKAα cat affinity-purified rabbit polyclonal antibody (C-20, sc-903, Santa Cruz Biotechnology) against total PKA was raised against a peptide mapping at the C terminus of PKAα cat of human origin. The RNeasy mini kit to purify total RNA from cholangiocytes was purchased from Qiagen (Valencia, CA). The RIA kits for measurement of intracellular cAMP ([125I] Biotrak Assay System, RPA509) and IP3 (D-myo-inositol 1,4,5-trisphosphate (IP3) [3H] Biotrak Assay System, TRK1000) levels were purchased from GE Healthcare (Piscataway, NJ).
Experimental Models
Male Fisher rats (weighing 150–175 g) were purchased from Charles River (Wilmington, MA) and maintained in a temperature-controlled environment (20–22°C) with 12:12-h light–dark cycles. Animals were fed ad libitum standard chow and had free access to drinking water. Our studies were performed in normal rats treated by daily IP injections with vehicle (0.9% NaCl), histamine (0.5 mg/kg of BW),25 HTMT dimaleate (H1HR agonist, 0.5 mg/kg of BW),26 amthamine dihydrobromide (H2HR agonist, 0.5 mg/kg of BW),27 RAMH (H3HR agonist, 10 mg/kg of BW)16 or the H4HR agonist, clobenpropit (10 mg/kg of BW)28 for 1 week. Before each experimental procedure, animals were injected with sodium pentobarbital (50 mg/kg weight, IP). Study protocols were performed in compliance with the institutional guidelines set forth by the Institutional Animal Care and Use Committee (Scott and White Texas A&M Health Science Center).
Purified Cholangiocytes and Normal Rat Intrahepatic Cholangiocyte Cultures
Virtually pure cholangiocytes were isolated by immunoaffinity separation13,16,29 with a monoclonal antibody (a gift from Dr R Faris) against an unidentified antigen expressed by all intrahepatic rat cholangiocytes.29 Cell count and viability (~97%) were measured by trypan blue exclusion. Purity (~99%) was assessed by histochemistry for γ-GT.30 The in vitro experiments were performed in freshly isolated cholangiocytes and our polarized normal rat intrahepatic cholangiocyte culture (NRICs).31 Cell number and frequency distribution of NRIC were measured using an automated cell counter (Cellometer Auto T4, Nexcelom Bioscience).32
Assessment of Histamine Receptor Expression
We evaluated the expression of HR subtypes by (1) immunohistochemistry15,33 in normal liver sections; (2) immunofluorescence15 in NRIC smears; and (3) RT-PCR15 (1 μg total RNA) and immunoblots (10 μg protein) from freshly isolated normal cholangiocytes and NRICs. Immunohistochemistry in liver sections with the selected HR antibodies (1:100 dilution) was performed as described previously. 15,16 For all immunoreactions, negative controls (with pre-immune serum substituted for the primary antibody) were included. Light microscopy photographs of liver sections were taken by Leica Microsystems DM 4500 B Light Microscopy (Leica Microsystems, Weltzlar, Germany) using a Jenoptik Prog Res C10 Plus Videocam (Jena, Germany). Immunofluorescence for HR subtypes in NRICs was performed as described previously.15 After staining, images were visualized using a confocal microscope (Olympus IX-71; Olympus, Tokyo, Japan). For all immunoreactions, appropriate negative controls were used.
Standard RT-PCR conditions were used for measurement of the mRNA expression for H1–H4R in pooled cholangiocytes (formed by small and large cholangiocytes)31 and NRICs: 10 min at 95°C, 15 s at 95°C, 30 s at 55°C and 30 s at 72°C, 30 cycles at 4°C. Specific primers designed against the rat H1HR (NM_000861, expected fragment length 157 bp), H2HR (NM_022304, expected fragment length 181 bp), H3HR (NM_053506, expected fragment length 180 bp), H4HR (NM_131909, expected fragment length 181 bp) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH, NM_002046, the housekeeping gene,34 expected fragment length 175 bp) were purchased from (SABiosciences, Frederick, MD). We used RNA from the rat brain and water as positive and negative controls, respectively. The protein expression of H1–H4HR was measured by immunoblots in protein (10 μg) lysates from the brain (positive control) and isolated small and large normal cholangiocytes. Immunoblots were normalized by β-actin.15,16
In Vivo Studies Evaluation of the Intrahepatic Bile Duct Mass of Small, Large and Pooled Ducts
In the selected groups of animals, cholangiocyte growth was measured in liver sections (4 μm thick) by evaluation of the percentage of intrahepatic small, large and pooled bile ducts stained for cytokeratin-19 (CK-19, a specific marker of the biliary epithelium).9,34 In each liver section, IBDM was measured as area occupied by CK-19-positive bile duct/total area × 100.35 Sections were analyzed using a BX-51 light microscope (Olympus) with a video cam (Spot Insight; Diagnostic Instrument Inc., Sterling Heights, MI) and processed using an Image Analysis System (IAS: Delta Sistemi, Rome, Italy).
Cholangiocyte proliferation was also evaluated by immunoblots for PCNA14 in protein (10 μg) lysates from the spleen (positive control) and isolated cholangiocytes from the selected experimental groups. Immunoblots were normalized by β-actin.15,16 Band intensity was determined by scanning video densitometry using the phospho-imager, Storm 860 (GE Healthcare) and the ImageQuant TL software version 2003.02 (GE Healthcare, Little Chalfont, Buckinghamshire, England).
Effect of In Vivo Administration of Histamine on Basal-and Secretin-Stimulated Bile and Bicarbonate Secretion and cAMP levels
We measured the effect of chronic in vivo administration of histamine to normal rats on basal- and secretin-stimulated bile secretion (in bile fistula rats)3,9 and cAMP levels (in purified cholangiocytes),13,14,16 two functional parameters of cholangiocyte proliferation.3,9,13,14 After anesthesia, rats were surgically prepared for bile collection as described previously.3,9 When steady-state bile flow was reached (60–70 min from the intravenous infusion of Krebs–Ringer–Henseleit solution, KRH), rats were infused with secretin (100nM) for 30 min, followed by intravenous infusion of KRH for 30 min. Bicarbonate concentration in bile was determined using an ABL 520 Blood Gas System (Radiometer Medical A/S, Copenhagen, Denmark).
After purification, cholangiocytes (1×105 cells) were incubated at 37°C for 1 h (to regenerate membrane proteins damaged by proteolytic enzymes during cell isolation)5 and subsequently stimulated at room temperature for 5 min with 0.2% bovine serum albumin (BSA) or secretin (100nM solution containing 0.2% BSA) before evaluation of cAMP levels by RIA.13,14,16
In Vitro Studies Evaluation of Cholangiocyte Proliferation and Signaling Mechanisms
Effects of histamine, H1HR or H2HR agonists (in the absence or presence of specific HR antagonists or inhibitors of IP3/Ca2+ or cAMP signaling) on cholangiocyte proliferation and signaling mechanisms were evaluated in NRICs.31 To evaluate dose and time dependency, NRICs were treated with 0.2% BSA (basal) or histamine (10, 50 and 100 μM) for 24 and 48 h before evaluating cell proliferation using the CellTiter 96 Aqueous One Solution Cell Proliferation Assay (MTS)15 (see above). In separate sets of experiments, NRICs were treated at 37°C for 24 h with: (1) 0.2% BSA; (2) histamine (10 μM) in the absence or presence of pre-incubation with terfenadine (H1HR antagonist, 10 μM),15 or cimetidine (H2HR antagonist, 10 μM)36 or terfenadine+cimetidine (both at 10 μM+histamine, 10 μM); or (3) HTMT dimaleate (H1HR agonist, 10 μM),15 amthamine dihydrobromide (H2HR agonist, 10 μM),37 RAMH (H3HR agonist, 10 μM)16 or clobenpropit (H4HR agonist, 10 μM)37 before evaluation of proliferation by MTS assays.15 To determine whether the stimulatory effects of histamine (the endogenous neurotransmitter) on NRIC proliferation are mediated by activation of H1 (mediated by Ca2+ signaling) and/or H2 (regulated by cAMP signaling) HRs, we stimulated NRICs at 37°C for 24 h with (1) 0.2% BSA; (2) histamine (10 μM); or (3) HTMT dimaleate (10 μM),15 or amthamine dihydrobromide (10 μM)37 in the absence or presence of pre-incubation with BAPTA/AM (5 μM),15 Gö6976 (1 μM, Ca2+-dependent PKCα inhibitor)38 or Rp-cAMPs (100 μM, PKA inhibitor).13
Measurement of IP3 and cAMP Levels and Phosphorylation of PKC and PKA
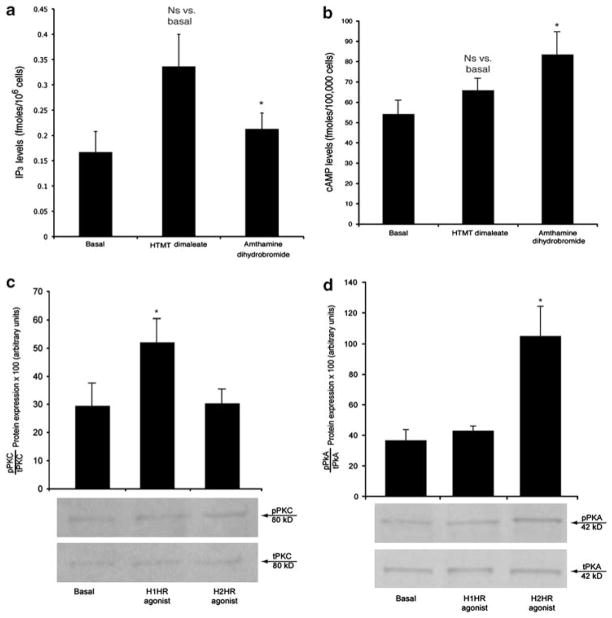
We performed experiments in NRICs aimed to demonstrate that HTMT dimaleate increases IP3 levels and PKC phosphorylation, whereas amthamine dihydrobromide enhances cAMP levels and PKA phosphorylation. For evaluation of cAMP levels, NRICs were treated at room temperature for 5 min5,13 with (1) 0.2% BSA or (2) HTMT dimaleate (10 μM)15 or amthamine dihydrobromide (10 μM) before evaluation of cAMP levels by RIA.5,13 For measurements of IP3 levels, NRICs were treated at room temperature for 10 min with (1) 0.2% BSA or (2) HTMT dimaleate (10 μM)15 or amthamine dihydrobromide (10 μM) before evaluation of IP3 levels by RIA.15
Before evaluation of phosphorylation of PKC or PKA,39 NRICs were treated at room temperature for 90 min with (1) 0.2% BSA or (2) HTMT dimaleate (10 μM)15 or amthamine dihydrobromide (10 μM).37
Effect of PKCα Silencing on NRIC Proliferation
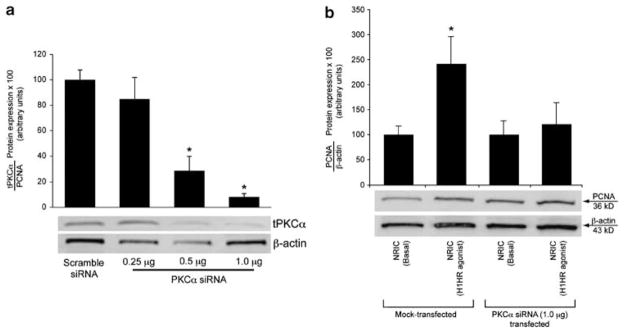
As (1) IP3/Ca2+-dependent PKCα has a key role in the regulation of cholangiocyte functions14,39,40 and (2) HTMT dimaleate increases IP3 levels and PKCα phosphorylation (see the ‘Results’ section), we evaluated the effect of PKCα silencing on NRIC proliferation after treatment of these cells with HTMT dimaleate. NRICs were plated into 6-well plates and allowed to adhere overnight. siRNA transfection (0.25–1 μg of PKCα siRNA was used) was performed according to the instructions provided by Santa Cruz Biotechnology. Diluted siRNA duplexes were added to the cells and allowed to incubate for 5 h at 37°C in a CO2 incubator. For controls, a scrambled siRNA duplex (Santa Cruz Biotechnology) was added to corresponding wells. The extent of PKCα silencing was evaluated by measuring the protein expression of total PKCα in transfected vs control NRICs by immunoblots (see above).15 PCNA protein expression was evaluated in NRIC cells treated with 0.2% BSA or HTMT dimaleate (10 μM) for 24 h in the absence or presence of PKCα siRNA (1.0 μg).
Statistical Analysis
All data are expressed as mean±s.e.m. Differences between groups were analyzed by Student’s unpaired t-test when two groups were analyzed and ANOVA when more than two groups were analyzed, followed by an appropriate post hoc test.
RESULTS
Histamine Receptor Expression
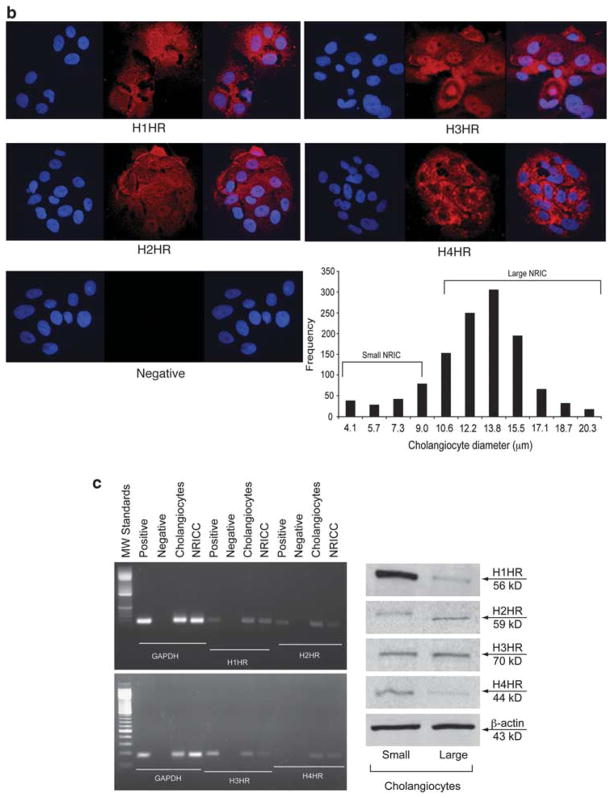
By immunohistochemistry in liver sections, both small (yellow arrow) and large (red arrow) bile ducts express the four HR subtypes (H1–H4HRs) (Figure 1a). By immunofluorescence, NRICs (which are formed by small and large cholangiocytes as evaluated by frequency size distribution)31 (Figure 1b) expressed the four HRs (Figure 1b). DAPI staining is seen in blue, whereas the red staining represents the specific HR. A merged image is also supplied along with negative staining (Figure 1b). By RT-PCR, purified pooled (containing small and large cells) cholangiocytes from normal rats and NRICs expressed H1–H4HRs (Figure 1c). In addition, by immunoblots, purified small and large normal cholangiocytes expressed H1–H4HRs (Figure 1c).
Figure 1.
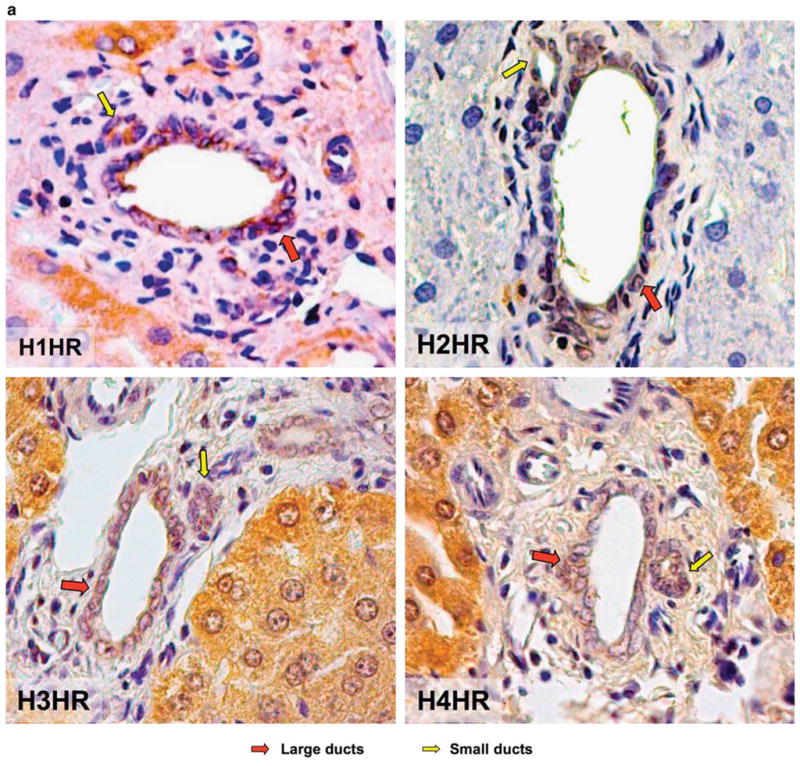
(a) By immunohistochemistry in liver sections, both small (yellow arrow) and large (red arrow) bile ducts express H1–H4HRs. Original magnification ×40. (b) Frequency size distribution shows that NRIC are formed by both small and large NRICs. NRIC by immunofluorescence (bar=20 μm); DAPI staining is seen in blue, whereas the red staining represents the specific histamine receptor. A merged image is shown along with negative staining. (c) By RT-PCR, purified pooled cholangiocytes from normal rats and NRICs expressed H1–H4HRs. By immunoblots, purified small and large normal cholangiocytes expressed H1–H4HRs.
Evaluation of Intrahepatic Bile Duct Mass
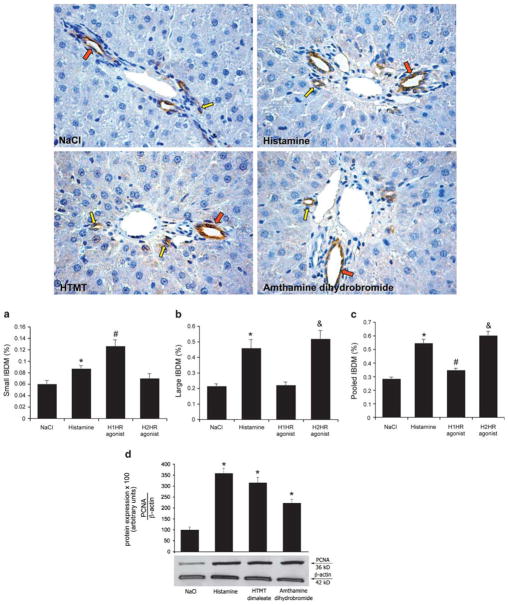
Chronic administration of histamine and the H1HR agonist, HTMT dimaleate, increased small IBDM of normal rats compared with saline-treated rats (Figure 2a); the H2HR agonist, amthamine dihydrobromide, did not alter small IBDM (Figure 2a). Histamine and amthamine dihydrobromide (but not HTMT dimaleate) increased large IBDM of normal rats compared with control rats (Figure 2b). Histamine, the H1 and the H2HRs agonists increased overall IBDM (Figure 2a–c). No changes were observed when normal rats were treated with RAMH or clobenpropit (not shown). There was a significant increase in PCNA protein expression in freshly isolated pooled cholangiocytes from normal rats treated with histamine, H1HR or H2HR agonists compared with vehicle-treated rats (Figure 2d). These results rule out the potential role of the H3HR and H4HR agonists in activating cholangiocyte proliferation.
Figure 2.
(a–c) Effect of chronic administration of saline, histamine, H1HR or H2HR agonists on small, large and pooled IBDM of normal rats. (a) Administration of histamine and the H1HR agonist, HTMT dimaleate increased small IBDM of normal rats compared with saline-treated rats; the H2HR agonist, amthamine dihydrobromide, did not alter small IBDM. (b) Histamine and amthamine dihydrobromide (but not HTMT dimaleate) increased large IBDM of normal rats compared with control rats. (c) The IBDM of pooled bile ducts was increased by histamine and both H1 and H2HR agonists. *P<0.05 vs small, large and pooled IBDM of normal rats. #P<0.05 vs small and pooled IBDM of normal rats. 5P<0.05 vs large and pooled IBDM of normal rats. Data are mean±s.e.m. Ten randomly selected portal tracts were evaluated in three different slides. *P<0.05 vs IBDM of normal rats treated with saline. (d) There was a significant increase in PCNA protein expression in freshly isolated pooled cholangiocytes from normal rats treated with histamine, H1HR or H2HR compared with vehicle-treated rats. *P<0.05 vs PCNA of cholangiocytes from normal rats treated with saline. Data are mean±s.e.m. of four blots from several different and cumulative preparations of cholangiocytes.
Effect of In Vivo Administration of Histamine on Basal-and Secretin-Stimulated Bile and Bicarbonate Secretion and cAMP Levels
Secretin increased intracellular cAMP levels in cholangiocytes from normal rats treated with saline (Figure 3). Chronic in vivo administration of histamine to normal rats increased both basal- and secretin-stimulated cAMP levels of cholangiocytes (a functional index of biliary growth)3,11,14 compared with their corresponding values of purified cholangiocytes from saline-treated normal rats (Figure 3). As previously shown,3 intravenous infusion of secretin did not increase bile and bicarbonate secretion in normal rats (Table 1). Secretin increased bile and bicarbonate secretion in normal rats treated with histamine compared with normal rats treated with saline (Table 1), demonstrating that histamine stimulates biliary hyperplasia by activation of cAMP-dependent signaling.
Figure 3.
Evaluation of basal- and secretin-stimulated cAMP levels in purified cholangiocytes from normal rats treated with saline or histamine for 1 week. Histamine increased both basal- and secretin-stimulated cAMP of normal cholangiocytes compared with cholangiocytes from normal rats treated with saline. &P<0.05 vs basal cAMP levels of cholangiocytes from normal rats treated with saline. *P<0.05 vs basal cAMP levels of cholangiocytes from saline-treated normal rats. #P<0.05 vs secretin-stimulated cAMP levels of cholangiocytes from saline-treated normal rats. Data are mean±s.e.m. of six experiments.
Table 1.
Measurement of basal- and secretin-stimulated bile flow, bicarbonate concentration and secretion in normal rats treated with saline or histamine for 1 week
| Treatment | Bile flow
|
Bicarbonate secretion
|
||
|---|---|---|---|---|
| Basal (ml/min per kg BW) | Secretin (ml/min per kg BW) | Basal (mEquiv./min per kg BW) | Secretin (mEquiv./min per kg BW) | |
| Normal rats+NaCl (n = 7) | 68.3±6.2 | 73.6±7.0 | 2.3±0.2 | 2.5±0.2 |
| Normal rats+histamine (n = 7) | 74.5±4.8 | 90.5±5.2* | 2.4±0.2 | 3.1±0.15* |
BW, body weight.
When steady spontaneous bile flow was reached (60–70min from the infusion of Krebs–Ringer–Henseleit (KRH), rats were infused for 30 min with secretin, followed by a final infusion of KRH for 30min. Data are mean±s.e.m.
P<0.05 (by Student’s unpaired t-test) vs the corresponding basal value of bile flow or bicarbonate secretion of normal rats treated with histamine for 1 week.
In Vitro Studies Evaluation of Cholangiocyte Proliferation and Signaling Mechanisms
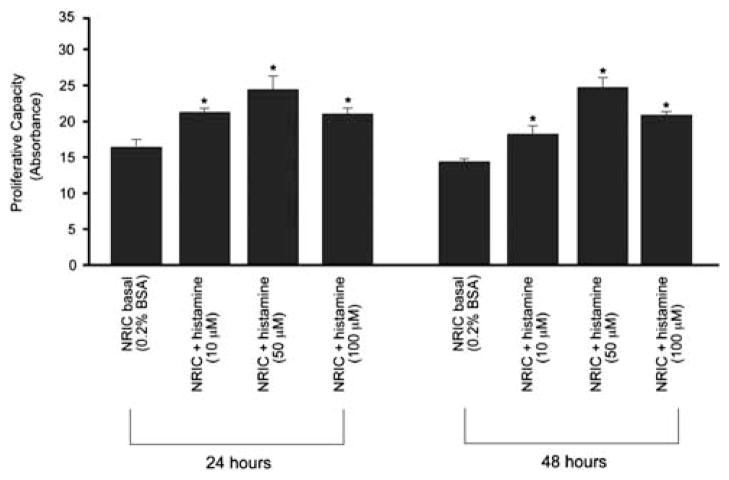
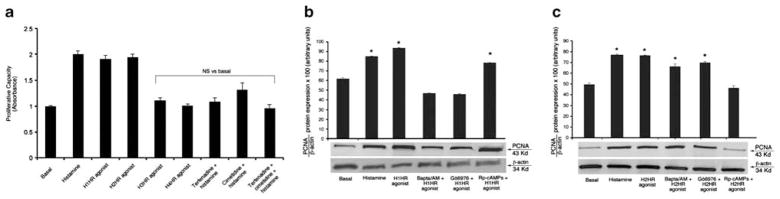
Dose- and time-response experiments demonstrated that histamine increases NRIC proliferation (by MTS assays) compared with BSA-treated NRICs (Figure 4). As the stimulatory effects of histamine on cholangiocyte proliferation were seen as early as after 24 h of incubation, all other stimulations were performed at 24 h at the histamine concentration of 10 μM, the smallest dose at which we observed a stimulatory effect on NRIC proliferation. Furthermore, we performed experiments aimed to demonstrate which HR subtypes regulate histamine-stimulated NRIC proliferation. Histamine, H1HR and H2HR (but not H3HR or H4HR) agonists increased NRIC proliferation (Figure 5a). Histamine stimulation of NRIC proliferation was blocked by terfenadine (H1HR antagonist), cimetidine (H2HR antagonist) and at the same extent by terfenadine+cimetidine (Figure 5a), demonstrating that both H1HR (by activation of Ca2+ signaling; see Figure 6) and H2HR (by activation of cAMP signaling; see Figure 6) mediate histamine-induced increase in NRIC proliferation (Figure 5a). Furthermore, we demonstrated that (1) H1HR and H2HR (in addition to histamine) agonists stimulate NRIC proliferation (Figure 5b and c); (2) H1HR-induced NRIC proliferation was blocked by BAPTA/AM and Gö6976 but not by Rp-cAMPs (Figure 5b); and (3) H2HR-induced NRIC proliferation was blocked by Rp-cAMPs but not BAPTA/AM and Gö6976 (Figure 5c).
Figure 4.
Dose- and time-response experiments demonstrated that histamine (at 10, 50 and 100 μM) increase NRIC proliferation (by MTS) compared with NRIC treated with BSA. *P<0.05 vs proliferation of NRIC treated in vitro with 0.2% BSA (basal). Data are mean±s.e.m. of seven experiments.
Figure 5.
(a) Histamine, H1HR and H2HR (but not H3HR or H4HR) agonists increased NRIC proliferation. Histamine stimulation of NRIC proliferation was partly blocked by terfenadine and cimetidine. *P<0.05 vs proliferation of NRIC treated in vitro with 0.2% BSA (basal). Data are mean±s.e.m. of seven experiments. H1HR agonist=HTMT dimaleate; H2HR agonist=amthamine dihydrobromide; H3HR agonist=RAMH; H4HR=clobenpropit. (b and c) By PCNA immunoblots, we demonstrated that: (b and c) H1HR and H2HR agonists (in addition to histamine) stimulate NRIC proliferation; (b) H1HR-induced NRIC proliferation is blocked by BAPTA/AM and Gö6976 but not Rp-cAMPs; and (c) H2HR-induced NRIC proliferation is blocked by Rp-cAMPs but not BAPTA/AM and Gö6976. *P<0.05 vs proliferation of NRIC treated in vitro with 0.2% BSA (basal). Data are mean±s.e.m. of six experiments. H1HR agonist=HTMT dimaleate; H2HR agonist=amthamine dihydrobromide.
Figure 6.
Effect of H1HR and H2HR agonists on (a) IP3 levels and (b) cAMP levels, (c) PKC phosphorylation and (d) PKA phosphorylation in NRIC. We demonstrated that HTMT dimaleate (a H1HR agonist) increases IP3 levels (a) and PKC (panel c) phosphorylation, whereas amthamine dihydrobromide (a H2HR agonist) enhances cAMP levels (b) and PKA phosphorylation (d). *P<0.05 vs proliferation of NRIC treated in vitro with 0.2% BSA (basal). Data are mean±s.e.m. of six experiments.
Measurement of IP3 and cAMP Levels and PKC and PKA
We demonstrated that HTMT dimaleate (H1HR agonist) increases IP3 levels (Figure 6a) and PKC phosphorylation (Figure 6c), whereas amthamine dihydrobromide (H2HR agonist) enhances cAMP levels (Figure 6b) and PKA phosphorylation (Figure 6d). HTMT dimaleate did not alter cAMP levels of NRIC, whereas amthamine dihydrobromide did not change IP3 levels of NRIC (Figure 6a and b).
Effect of PKCα Silencing on NRIC Proliferation
With the finding that the H1HR agonist increased phosphorylation of PKCα, we sought to pinpoint the role of Ca2+-dependent PKCα (that has a key role in biliary growth)14,39–44 on NRICs (after stimulation with HTMT dimaleate) using siRNA transfection. In PKCα-siRNA (1.0 μg)-transfected cells (90% knockdown efficiency, Figure 7a), HTMT dimaleate failed to increase NRIC proliferation (Figure 7b). As expected, in scrambled-transfected NRICs, HTMT dimaleate increased NRIC proliferation (Figure 7b).
Figure 7.
(a, b) Effect of (a) PKCα silencing on the (b) proliferation (by PCNA immunoblots) of NRIC after treatment with 0.2% BSA (basal) or HTMT dimaleate. In scrambled-transfected NRIC, HTMT dimaleate increased NRIC proliferation. In PKCα-siRNA (1.0 μg) transfected cells (90% silenced efficiency), HTMT dimaleate failed to increase NRIC proliferation. *P<0.05 vs proliferation of NRIC treated in vitro with 0.2% BSA (basal). Data are mean±s.e.m. of four experiments. H1HR agonist=HTMT dimaleate.
DISCUSSION
We have demonstrated that intrahepatic bile ducts, freshly isolated cholangiocytes and NRICs express H1–H4HRs. We have shown that histamine increases normal cholangiocyte growth by interaction with H1HR and H2HR by activation of both IP3/Ca2+- and cAMP-dependent mechanisms, respectively. Furthermore, the H1HR agonist increases NRIC proliferation by activation of IP3/Ca2+/PKCα-dependent signaling likely targeting small Ca2+-dependent NRIC. When PKCα was silenced, the H1HR agonist did not increase NRIC proliferation. In contrast, the H2HR (that signals through activation of Gαs)23 induces normal cholangiocyte growth by activation of a cAMP/PKA-dependent mechanism (independent of PKCα) by likely targeting large cAMP-dependent NRICs.
The finding that normal rat cholangiocytes and NRICs express H1–H4HRs are supported by previous studies in normal mouse15 and BDL rat cholangiocytes.16 Moreover, we have previously demonstrated the expression of H3HR in hyperplastic cholangiocytes and cholangiocarcinoma cells, and defined the inhibitory role of H3HR in the growth of hyperplastic and neoplastic cholangiocytes.16,45 HRs are also expressed in the liver by hepatocytes that are activated by endogenous histamine to release a wide array of effects.46,47 For example, histamine activates IP3 synthesis in guinea pig hepatocytes by activation of H1HR47 and stimulates glycogenolysis in hepatocytes by interaction with H1HR by activating the Ca2+ messenger system.46
In animal models of cholangiocyte hyperplasia/loss, both IP3/Ca2+- and cAMP-dependent signaling pathways regulate the proliferative activities of small and large cholangiocytes, respectively.3,8,9,16 For example, in the cholestatic BDL model, there is marked proliferation of large cholangiocytes by activation of cAMP signaling.3,8,16 With regard to IP3/Ca2+-dependent signaling, stimulation of small cholangiocyte growth (eg, after feeding of the bile salt, taurocholate) depends on the activation of the Ca2+-dependent PKCα.41 Moreover, activation of α1-adrenergic receptors stimulate the proliferation of small mouse cholangiocytes through Ca2+-dependent activation of NFAT2 and Sp1.48 In this study, we demonstrate that histamine stimulates small and large biliary growth by the differential activation of both Ca2+ and cAMP signaling. One possible explanation for the differential effects induced by histamine is likely due to the interaction with specific HRs expressed by different-sized cholangiocytes, ie, small and large. Indeed, we have shown that activation of H1HR increases the proliferation of small cholangiocytes by activation of IP3/Ca2+-CaMK I signaling.15 In addition, activation of H3HR causes decreased large cholangiocyte hyperplasia by downregulation of cAMP-dependent signaling.16 Based on this, we propose that histamine-induced increases in the proliferation of both small and large cholangiocytes are mediated by activation of IP3/Ca2+ signaling in small cholangiocytes and NRICs (by likely interaction with H1HR) and cAMP transduction pathways in large cholangiocytes and NRICs (presumably mediated by H2HR). On the basis of this rationale and these previous findings, we also propose that the increase in secretin-stimulated choleresis (observed after in vivo administration of histamine to normal rats) likely depends on activation of H2HR in large cholangiocytes, the only biliary cell type in the liver that functions by a cAMP-dependent mechanism.1,6,8,11
We next performed in vitro studies in polarized NRICs (which contain both small and large cholangiocytes, Figure 1b) and demonstrated that histamine activation of NRIC proliferation is blocked by terfenadine (H1HR antagonist), cimetidine (H2HR antagonist) and at the same extent by simultaneous treatment with terfenadine+cimetidine. A shortcoming of this in vitro experiment is represented by the fact that both H1HR and H2HR antagonists block completely the stimulatory effects of histamine on NRIC proliferation, although one of the two receptors (H1HR or H2HH) are still active during treatment with these two inhibitors. We provide this explanation supported by our previous findings.14,39,42,44,49,50 As we have previously demonstrated that cross-talk between Ca2+- and cAMP-dependent signaling coordinately mediates biliary secretion and growth,14,39,42,44,49,50 we propose that blockage of Ca2+ (by the H1HR antagonist) and cAMP (by the H2HR antagonist) signaling may reciprocally inhibit cAMP and Ca2+ signaling, respectively. This explanation is also supported by the fact that the inhibitory effects of terfenadine and cimetidine (when administered together) on NRIC proliferation were not additive.
We have also shown that (1) histamine stimulation of NRIC proliferation is dependent on the activation of both the IP3/Ca2+ and the cAMP signaling pathways (blockage by both Ca2+ chelators and specific PKC and PKA inhibitors); (2) H1HR stimulation of small cholangiocyte growth depends on the activation of IP3/Ca2+/PKCα signaling (proliferation blocked by Ca2+ chelators and PKC but not by PKA inhibitors); and (3) H2HR stimulation of large NRIC proliferation is mediated by cAMP-dependent PKA signaling (blockage by only PKA inhibitors). Further confirmation of differential signaling was found with evaluation of intracellular cAMP and IP3 levels, as well as PKCα and PKA phosphorylation. H1HR stimulation activates IP3 levels and PKCα phosphorylation, whereas H2HR increases cAMP levels and PKA phosphorylation. The importance of signaling through differential pathways is important because in cholangiopathies, there is proliferation/damage of only specific bile ducts of different sizes.8,10,11,13,15,34 Cholangiopathies target both small and large cholangiocytes,8,10,11,34 and, thus, it may be important for histamine to have the ability to modulate cholangiocyte proliferation and/or loss through the activation of HRs that will induce stimulatory (H1 and H2H) and inhibitory (H3 and H4HR) effects on biliary growth.
After finding that H1HR activates Ca2+-dependent PKCα and knowing that PKCα has a critical role in cholangiocyte function,14,39,41,42,50 we knocked down PKCα expression and evaluated the effect of HTMT dimaleate on NRIC proliferation. As no commercially kits are available for silencing PKA, we could not evaluate the role of PKA silencing on H2HR-induced large NRIC proliferation. Similar to what we have previously shown45 in cholangiocarcinoma cells using the H3 agonist, RAMH, we found here that the reduction of PKCα in NRICs ablates the stimulatory effects of HTMT dimaleate. PKCα has a critical role in the function of numerous cells and the loss of PKCα signaling can have a negative or positive impact on the proliferation of a number of epithelial including cholangiocytes.14,39–41,45,50 For example, PKCα has a stimulatory role in wound healing of human corneal epithelial cells after hepatocyte growth factor stimulation.51 In cancer, PKCα has a stimulatory role enhancing the growth of certain cancers,52 whereas the activation of PKCα modulates the inhibition of cholangiocarcinoma growth.42,45 Further studies are necessary to understand why activation of PKCα in biliary cells induces either inhibition or activation of hyperplastic and neoplastic biliary growth and secretin-stimulated ductal secretion (a functional index of biliary hyperplasia). 14,39,41–43,50 One potential explanation for the differential effects of PKCα on biliary growth may depend on the type of receptor (eg, M3 acetylcholine, insulin, gastrin or α1-adrenergic) or transporter (eg, Na+-dependent apical bile acid transporter) that are upregulated or downregulated after vagotomy, the treatment with the selected gastrointestinal hormone or bile salt feeding.14,39,41–43,50 A possible shortcoming of our study is that PKCα may be not the only factor as other Ca2+-dependent (βI, βII and γ) and Ca2+-independent (δ, ε, θ, η and ζ) PKC isoforms may be involved in H1HR stimulation of biliary growth.
Taken together, these findings implicate histamine and HRs as key factors in the regulation of normal cholangiocyte proliferation and function. Although endogenous histamine may activate numerous signaling mediators and pathways, it may be more important that stimulation of specific HRs is able to induce specific effects in target cells (possibly in small and large subpopulations of cholangiocytes) using different signaling pathways. In clinical situations in which certain subpopulations of cells are affected, the usage of specific agonists may help in the development of therapeutic treatments. For example, in a cholestatic state in which inflamed cholangiocytes are overproliferating, the usage of the H3HR agonist RAMH might aid in patient therapy to help decrease cholangiocyte proliferation. On the other hand, in a case of damaged or cholangiocyte loss, treatment with either an H1HR or H2HR agonist may increase proliferation when warranted and necessary for recovery.
As histamine is released by mast cells,53 further studies are warranted to evaluate how mast cells may be contributing to cholangiocyte proliferation, a topic that is a topic of future work in our laboratory. In support of this novel topic, it has been demonstrated that during cholestasis, mast cell quantities increase.53 Increased mast cell quantity would likely lead to increased histamine release into the microenvironment, thus modulating biliary functions. Furthermore, ongoing studies from our laboratory aim to demonstrate that bile acids (that have a key role in the regulation of biliary functions and stimulate histamine secretion from mast cells)40,41,44,50,54 affect cholangiocyte growth by releasing histamine from proliferating mast cells.
Acknowledgments
This work was supported by the Dr Nicholas C Hightower Centennial Chair of Gastroenterology from Scott and White Hospital, a VA Research Career Scientist Award, a VA Merit award and NIH grants DK58411 and DK76898 to Dr Alpini, by a MIUR grant 2003060137_004 to the Department of Gastroenterology, a grant award from Health and Labour Sciences Research Grants for the Research on Measures for Intractable Diseases (from the Ministry of Health, Labour and Welfare of Japan) and from Grant-in-Aid for Scientific Research C (21590822) from JSPS to Dr Ueno, by University funds to Dr Onori and University Funds from University ‘Sapienza’ of Rome, MIUR grant 2009X84L84 and FIB grant #RAP10Z7FS to Professor Gaudio and an NIH K01 grant award (DK078532) to Dr DeMorrow.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Alpini G, Roberts S, Kuntz SM, et al. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–1643. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- 2.Glaser S, Gaudio E, Rao A, et al. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest. 2009;89:456–469. doi: 10.1038/labinvest.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alpini G, Lenzi R, Sarkozi L, et al. Biliary physiology in rats with bile ductular cell hyperplasia. Evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology. 1991;14:551–566. [PubMed] [Google Scholar]
- 5.Kato A, Gores GJ, LaRusso NF. Secretin stimulates exocytosis in isolated bile duct epithelial cells by a cyclic AMP-mediated mechanism. J Biol Chem. 1992;267:15523–15529. [PubMed] [Google Scholar]
- 6.Alpini G, Ulrich C, Roberts S, et al. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1997;272:G289–G297. doi: 10.1152/ajpgi.1997.272.2.G289. [DOI] [PubMed] [Google Scholar]
- 7.Banales JM, Arenas F, Rodriguez-Ortigosa CM, et al. Bicarbonate-rich choleresis induced by secretin in normal rat is taurocholate-dependent and involves AE2 anion exchanger. Hepatology. 2006;43:266–275. doi: 10.1002/hep.21042. [DOI] [PubMed] [Google Scholar]
- 8.Alpini G, Glaser S, Ueno Y, et al. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1998;274:G767–G775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- 9.LeSage G, Glaser S, Gubba S, et al. Regrowth of the rat biliary tree after 70% partial hepatectomy is coupled to increased secretin-induced ductal secretion. Gastroenterology. 1996;111:1633–1644. doi: 10.1016/s0016-5085(96)70027-6. [DOI] [PubMed] [Google Scholar]
- 10.Alpini G, Prall RT, LaRusso NF. The pathobiology of biliary epithelia. In: Arias IM, Boyer JL, Chisari FV, et al., editors. The Liver; Biology & Pathobiology. 4. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. pp. 421–435. [Google Scholar]
- 11.LeSage G, Glaser S, Marucci L, et al. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1289– G1301. doi: 10.1152/ajpgi.1999.276.5.G1289. [DOI] [PubMed] [Google Scholar]
- 12.Alpini G, Ulrich CD, Phillips JO, et al. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1994;266:G922–G928. doi: 10.1152/ajpgi.1994.266.5.G922. [DOI] [PubMed] [Google Scholar]
- 13.Francis H, Glaser S, Ueno Y, et al. cAMP stimulates the secretory and proliferative capacity of the rat intrahepatic biliary epithelium through changes in the PKA/Src/MEK/ERK1/2 pathway. J Hepatol. 2004;41:528–537. doi: 10.1016/j.jhep.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Glaser S, Benedetti A, Marucci L, et al. Gastrin inhibits cholangiocyte growth in bile duct-ligated rats by interaction with cholecystokinin-B/Gastrin receptors via D-myo-inositol 1,4,5-triphosphate-, Ca(2+)-, and protein kinase C alpha-dependent mechanisms. Hepatology. 2000;32:17–25. doi: 10.1053/jhep.2000.8265. [DOI] [PubMed] [Google Scholar]
- 15.Francis H, Glaser S, DeMorrow S, et al. Small mouse cholangiocytes proliferate in response to H1 histamine receptor stimulation by activation of the IP3/CaMK I/CREB pathway. Am J Physiol Cell Physiol. 2008;295:C499–C513. doi: 10.1152/ajpcell.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Francis H, Franchitto A, Ueno Y, et al. H3 histamine receptor agonist inhibits biliary growth of BDL rats by downregulation of the cAMP-dependent PKA/ERK1/2/ELK-1 pathway. Lab Invest. 2007;87:473–487. doi: 10.1038/labinvest.3700533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenthaler J, Guirard BM, Chang GW, et al. Purification and properties of histidine decarboxylase from Lactobacillus 30a. Proc Natl Acad Sci USA. 1965;54:152–158. doi: 10.1073/pnas.54.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Repka-Ramirez MS. New concepts of histamine receptors and actions. Curr Allergy Asthma Rep. 2003;3:227–231. doi: 10.1007/s11882-003-0044-3. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen T, Shapiro DA, George SR, et al. Discovery of a novel member of the histamine receptor family. Mol Pharmacol. 2001;59:427–433. doi: 10.1124/mol.59.3.427. [DOI] [PubMed] [Google Scholar]
- 20.Molina-Hernandez A, Velasco I. Histamine induces neural stem cell proliferation and neuronal differentiation by activation of distinct histamine receptors. J Neurochem. 2008;106:706–717. doi: 10.1111/j.1471-4159.2008.05424.x. [DOI] [PubMed] [Google Scholar]
- 21.Medina VA, Rivera ES. Histamine receptors and cancer pharmacology. Br J Pharmacol. 2010;161:755–767. doi: 10.1111/j.1476-5381.2010.00961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dickenson JM. Stimulation of protein kinase B and p70 S6 kinase by the histamine H1 receptor in DDT1MF-2 smooth muscle cells. Br J Pharmacol. 2002;135:1967–1976. doi: 10.1038/sj.bjp.0704664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitsuhashi M, Mitsuhashi T, Payan D. Multiple signaling pathways of histamine H2 receptors (Identification of an H2 receptor-dependent Ca2+ mobilization pathway in human HL-60 promyelocytic leukemia cells) J Biol Chem. 1989;264:18356–18362. [PubMed] [Google Scholar]
- 24.Schultheiss G, Hennig B, Schunack W, et al. Histamine-induced ion secretion across rat distal colon: involvement of histamine H1 and H2 receptors. Eur J Pharmacol. 2006;546:161–170. doi: 10.1016/j.ejphar.2006.07.047. [DOI] [PubMed] [Google Scholar]
- 25.Karavodin L, Jensen R, Sarno M, et al. Toxicology and toxicokinetics of acute and subchronic administration of histamine dihydrochloride in rats. Drug Chem Toxicol. 2003;26:35–49. doi: 10.1081/dct-120017556. [DOI] [PubMed] [Google Scholar]
- 26.Farzin D, Asghari L, Nowrouzi M. Rodent antinociception following acute treatment with different histamine receptor agonists and antagonists. Pharmacol Biochem Behav. 2002;72:751–760. doi: 10.1016/s0091-3057(02)00748-7. [DOI] [PubMed] [Google Scholar]
- 27.Coruzzi G, Gambarelli E, Bertaccini G, et al. Cardiovascular effects of the novel histamine H2 receptor agonist amthamine: interaction with the adrenergic system. Naunyn Schmiedebergs Arch Pharmacol. 1996;353:417–422. doi: 10.1007/BF00261438. [DOI] [PubMed] [Google Scholar]
- 28.Gantner F, Sakai K, Tusche MW, et al. Histamine h(4) and h(2) receptors control histamine-induced interleukin-16 release from human CD8(+) T cells. J Pharmacol Exp Ther. 2002;303:300–307. doi: 10.1124/jpet.102.036939. [DOI] [PubMed] [Google Scholar]
- 29.Ishii M, Vroman B, LaRusso NF. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989;97:1236–1247. doi: 10.1016/0016-5085(89)91695-8. [DOI] [PubMed] [Google Scholar]
- 30.Rutenburg AM, Kim H, Fischbein JW, et al. Histochemical and ultrastructural demonstration of γ-glutamyl transpeptidase activity. J Histochem Cytochem. 1969;17:517–526. doi: 10.1177/17.8.517. [DOI] [PubMed] [Google Scholar]
- 31.Alpini G, Phinizy JL, Glaser S, et al. Development and characterization of secretin-stimulated secretion of cultured rat cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1066–G1073. doi: 10.1152/ajpgi.00260.2002. [DOI] [PubMed] [Google Scholar]
- 32.Wadugu BA, Ng C, Bartley BL, et al. DNA interstrand cross-linking activity of (1-Chloroethenyl)oxirane, a metabolite of beta-chloroprene. Chem Res Toxicol. 2010;23:235–239. doi: 10.1021/tx9003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mancinelli R, Onori P, Gaudio E, et al. Follicle-stimulating hormone increases cholangiocyte proliferation by an autocrine mechanism via cAMP-dependent phosphorylation of ERK1/2 and Elk-1. Am J Physiol Gastrointest Liver Physiol. 2009;297:G11–G26. doi: 10.1152/ajpgi.00025.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mancinelli R, Franchitto A, Gaudio E, et al. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol. 2010;176:1790–1800. doi: 10.2353/ajpath.2010.090677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mancinelli R, Onori P, Gaudio E, et al. Taurocholate feeding to bile duct ligated rats prevents caffeic acid-induced bile duct damage by changes in cholangiocyte VEGF expression. Exp Biol Med (Maywood) 2009;234:462–474. doi: 10.3181/0808-RM-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghosh AK, Hirasawa N, Ohuchi K. Enhancement by histamine of vascular endothelial growth factor production in granulation tissue via H(2) receptors. Br J Pharmacol. 2001;134:1419–1428. doi: 10.1038/sj.bjp.0704372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Medina V, Croci M, Crescenti E, et al. The role of histamine in human mammary carcinogenesis: H3 and H4 receptors as potential therapeutic targets for breast cancer treatment. Cancer Biol Ther. 2008;7:28–35. doi: 10.4161/cbt.7.1.5123. [DOI] [PubMed] [Google Scholar]
- 38.Gaudio E, Barbaro B, Alvaro D, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology. 2006;130:1270–1282. doi: 10.1053/j.gastro.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 39.LeSage G, Marucci L, Alvaro D, et al. Insulin inhibits secretin-induced ductal secretion by activation of PKC alpha and inhibition of PKA activity. Hepatology. 2002;36:641–651. doi: 10.1053/jhep.2002.35537. [DOI] [PubMed] [Google Scholar]
- 40.Alpini G, Kanno N, Phinizy JL, et al. Tauroursodeoxycholate inhibits human cholangiocarcinoma growth via Ca2+-, PKC-, and MAPK-dependent pathways. Am J Physiol Gastrointest Liver Physiol. 2004;286:G973–G982. doi: 10.1152/ajpgi.00270.2003. [DOI] [PubMed] [Google Scholar]
- 41.Alpini G, Ueno Y, Glaser S, et al. Bile acid feeding increased proliferative activity and apical bile acid transporter expression in both small and large rat cholangiocytes. Hepatology. 2001;34:868–876. doi: 10.1053/jhep.2001.28884. [DOI] [PubMed] [Google Scholar]
- 42.Kanno N, Glaser S, Chowdhury U, et al. Gastrin inhibits cholangiocarcinoma growth through increased apoptosis by activation of Ca2+-dependent protein kinase C-alpha. J Hepatol. 2001;34:284–291. doi: 10.1016/s0168-8278(00)00025-8. [DOI] [PubMed] [Google Scholar]
- 43.LeSage G, Alvaro D, Glaser S, et al. Alpha-1 adrenergic receptor agonists modulate ductal secretion of BDL rats via Ca(2+)- and PKC-dependent stimulation of cAMP. Hepatology. 2004;40:1116–1127. doi: 10.1002/hep.20424. [DOI] [PubMed] [Google Scholar]
- 44.Marzioni M, Francis H, Benedetti A, et al. Ca2+-dependent cytoprotective effects of ursodeoxycholic and tauroursodeoxycholic acid on the biliary epithelium in a rat model of cholestasis and loss of bile ducts. Am J Pathol. 2006;168:398–409. doi: 10.2353/ajpath.2006.050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Francis H, Onori P, Gaudio E, et al. H3 histamine receptor-mediated activation of protein kinase Calpha inhibits the growth of cholangiocarcinoma in vitro and in vivo. Mol Cancer Res. 2009;7:1704–1713. doi: 10.1158/1541-7786.MCR-09-0261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mine T, Kojima I, Ogata E. Mechanism of glycogenolytic action of histamine in rat hepatocytes. Am J Physiol Gastrointest Liver Physiol. 1991;261:G1000–G1004. doi: 10.1152/ajpgi.1991.261.6.G1000. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Sainz JA, Macias-Silva M, Olivares-Reyes A, et al. Histamine activates phosphorylase and inositol phosphate production in guinea pig hepatocytes. Eur J Pharmacol. 1992;227:325–331. doi: 10.1016/0922-4106(92)90011-j. [DOI] [PubMed] [Google Scholar]
- 48.Alpini G, Franchitto A, DeMorrow S, et al. Activation of alpha(1) - adrenergic receptors stimulate the growth of small mouse cholangiocytes via calcium-dependent activation of nuclear factor of activated T cells 2 and specificity protein 1. Hepatology. 2011;53:628–639. doi: 10.1002/hep.24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Strazzabosco M, Fiorotto R, Melero S, et al. Differentially expressed adenylyl cyclase isoforms mediate secretory functions in cholangiocyte subpopulation. Hepatology. 2009;50:244–252. doi: 10.1002/hep.22926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alpini G, Baiocchi L, Glaser S, et al. Ursodeoxycholate and tauroursodeoxycholate inhibit cholangiocyte growth and secretion of BDL rats through activation of PKC alpha. Hepatology. 2002;35:1041–1052. doi: 10.1053/jhep.2002.32712. [DOI] [PubMed] [Google Scholar]
- 51.Sharma GD, Ottino P, Bazan NG, et al. Epidermal and hepatocyte growth factors, but not keratinocyte growth factor, modulate protein kinase Calpha translocation to the plasma membrane through 15(S)-hydroxyeicosatetraenoic acid synthesis. J Biol Chem. 2005;280:7917–7924. doi: 10.1074/jbc.M408852200. [DOI] [PubMed] [Google Scholar]
- 52.Aziz MH, Manoharan HT, Sand JM, et al. Protein kinase Cepsilon interacts with Stat3 and regulates its activation that is essential for the development of skin cancer. Mol Carcinog. 2007;46:646–653. doi: 10.1002/mc.20356. [DOI] [PubMed] [Google Scholar]
- 53.Francis H, Meininger CJ. A review of mast cells and liver disease: what have we learned? Dig Liver Dis. 2010;42:529–536. doi: 10.1016/j.dld.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 54.Quist RG, Ton-Nu HT, Lillienau J, et al. Activation of mast cells by bile acids. Gastroenterology. 1991;101:446–456. doi: 10.1016/0016-5085(91)90024-f. [DOI] [PubMed] [Google Scholar]