Abstract
Offspring from families with multiple cases of alcohol dependence have a greater likelihood of developing alcohol dependence (AD) and related substance use disorders. Greater susceptibility for developing these disorders may be related to structural differences in brain circuits that influence the salience of rewards or modify the efficiency of information processing and AD susceptibility. We examined the cerebellum of 71 adolescent/young adult high-risk (HR) offspring from families with multiple cases of alcohol dependence (multiplex families), and 60 low-risk (LR) controls with no family history of alcohol or drug dependence who were matched for age, gender, socioeconomic status and IQ, with attention given to possible effects of personal use of substances and maternal use during pregnancy. Magnetic resonance images were acquired on a General Electric 1.5-Tesla scanner and manually traced (BRAINS2) blind to clinical information. GABRA2 and BDNF variation were tested for their association with cerebellar volumes. High-risk offspring from multiplex AD families showed greater total volume of the cerebellum and total gray matter (GM), in comparison with LR controls. An interaction between allelic variation in GABRA2 and BDNF genes was associated with GM volumes, suggesting that inherited variation in these genes may promote early developmental differences in neuronal proliferation of the cerebellum.
Keywords: Cerebellum, High-risk, BDNF, GABRA2, Development, MRI
1. Introduction
The cerebellum has long been considered an important region for motor control, coordination, and motor learning (Thach, 1996; Bastian et al., 1998). Recently, the cerebellum has been viewed as an integral part of a cortico-cerebellar-thalamic-cortical circuit that is involved in coordination and modulation of cortical activity (Desmond and Fiez, 1998; Andreasen and Pierson, 2008). Neuroim-aging studies show that the cerebellum has a critical role in directed attention, many types of memory, facial recognition, theory of mind attribution, and emotional monitoring (Kim et al., 1994; Andreasen et al., 1995a, b; Fiez et al., 1995; Andreasen et al., 1996; Allen et al., 1997; Andreasen et al., 1999; Turner et al., 2007). Also, the cerebellum may facilitate cognitive processing through its role in monitoring of time perception (Keele and Ivry, 1990). In addition, there is now a large body of evidence suggesting that the cerebellum contributes to other higher order functions (Parkins, 1997; Schmahmann and Sherman, 1998; Desmond, 2001; Castellanos et al., 2002; Heyder et al., 2004) including personality characteristics (Schmahmann and Sherman, 1998) and emotional control (Turner et al., 2007).
A number of studies now suggest that brain morphology is associated with familial genetic risk for alcohol dependence even in individuals without substantial exposure to alcohol or drugs (Hill et al., 2001; Benegal et al., 2007; Hill et al., 2007; Venkatasubramanian et al., 2007; Hill et al., 2009). Among the brain regions that have been implicated in alcohol use disorders is the cerebellum. An association between cerebellar volume and alcohol use disorders has been established based both on clinical and neuropathological findings in alcohol dependent individuals (Zahr et al., 2010) and in their high risk relatives (Hill et al., 2007).
Previously, we reported that familial risk for alcohol dependence is associated with greater total cerebellar volume in comparison to low risk controls (Hill et al., 2007). Age-related changes in cerebellar gray matter appeared to differ by risk group as well, with low-risk controls showing a steeper slope for reduced gray matter with age. These findings appeared to suggest developmental delay in achieving age-appropriate cerebellar gray matter volume possibly due to developmental change in gray matter that represents normal pruning with age that occurs during adolescence and young adulthood. In contrast to the greater cerebellar volume that is seen in association with familial risk, reduced volume of the cerebellar vermis has been reported for adolescent marihuana users with greater alcohol use (N=16) in comparison to 16 controls (Medina et al., 2010). Although reduction in cerebellar volume was not seen in a sample of adolescent and young adults with alcohol use disorder (AUD) studied by DeBellis et al. (2005), this negative finding may have been the result of differing methodology for measurement of the cerebellar vermis. At any rate, the findings by Medina et al. (2010) show that the reduction of cerebellar volume that is often seen in older alcohol dependent subjects (Sullivan et al., 2003; Chanraud et al., 2011) can be observed even in youth.
Because we had previously observed risk group differences in a much smaller sample of 34 individuals (Hill et al., 2007), our first goal was to determine if risk group differences in cerebellar volume (total and segmented volumes) would be seen in an expanded sample. A large sample of high- and low-risk offspring was available because of an ongoing longitudinal study of offspring from multiplex alcohol dependence families and control families.
Our second goal was to identify genetic variation associated with cerebellar volume. Candidate genes were chosen based on their implicated role in the etiology of alcohol dependence and CNS growth. Cerebellum volumes (total, white matter and gray matter) were tested for association with genotypic variation in brain derived neurotrophic factor (BDNF) and GABRA2 the gene that encodes the gamma aminobutyric acid subunit 2. Consistent with its role as a nerve growth factor, variation in the VAL/MET alleles of BDNF is associated with smaller volume of the hippocampus in healthy controls (Bueller et al., 2006), and in first episode schizophrenia patients and controls (Szeszko et al., 2005). Additionally, VAL/MET variation in the BDNF gene has been reported to be associated with cerebellar gray matter volume (Agartz et al., 2006), one of the regions in which BDNF expression has been well documented (Levi-Montalcini., 1987). GABRA2 also appears to act as a nerve growth factor and has been associated with cerebellar development (Takayama and Inoue, 2004; Fiszman, 2005; Jelitai and Madarasz, 2005). Additionally, because both BDNF and GABRA2 have been implicated in the etiology of alcohol dependence (Edenberg et al., 2004; Janak et al., 2006; Matthews et al., 2007), these genes appeared to be good candidates for uncovering a possible relationship between alcohol dependence risk and cerebellar volume.
Although developmental changes in cortical organization and functioning have been studied extensively, cerebellar morphology and functioning have received less attention from a developmental perspective. Therefore, a third goal was to provide a description of cerebellar age-related changes by gender and by familial/genetic risk group.
2. Methods
2.1. Participants
The sample included 71 high-risk subjects from multiplex for alcohol dependence families and 60 low-risk control subjects (Table 1). The groups did not differ in mean age, IQ, education, gender, body mass index (BMI) or hand preference, though high risk offspring had larger intracranial volumes (ICV).
Table 1.
Demographic characteristics of high and low-risk adolescents and young adults.
| High risk (N=71)
|
Low risk (N=60)
|
F | d.f. | p | |||
|---|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | ||||
| Age | 18.31 | 4.18 | 17.75 | 5.80 | 0.41 | 1, 130 | NS |
| Intracranial volume (cc1,2,3) | 1480.94 | 115.43 | 1453.46 | 108.78 | 0.75 | 1, 36.2 | NS |
| Males | |||||||
| Intracranial volume (cc1,2,4) | 1342.31 | 104.66 | 1278.44 | 101.21 | 6.16 | 1, 45.2 | 0.02 |
| Females | |||||||
| SES5 | 42.36 | 11.42 | 45.41 | 10.56 | 2.46 | 1, 128 | NS |
| IQ6 | 109.24 | 13.71 | 112.90 | 17.20 | 1.82 | 1, 129 | NS |
| BMI Male1,2,3 | 24.67 | 5.16 | 23.01 | 3.77 | 2.07 | 1, 54 | NS |
| BMI Female1,2,4 | 23.18 | 4.97 | 25.76 | 6.33 | 3.40 | 1, 65 | NS |
| Number right-handed, (%) right-handed | 68 (95.8) | 56 (93.3) | 0.707 | 1 | NS | ||
| Alcohol or drug abuse/dependence8 | 19 | 5 | |||||
Age range for high-risk individuals was 9–28 years and 8–29 years for low-risk controls.
Analysis of risk, gender and risk by gender showed a significant difference by gender and risk. Accordingly, results are presented by gender.
There was a total of 65 males (37 high risk and 28 low risk).
There was a total of 66 females (34 high risk and 32 low risk).
Hollingshead Four-Factor Index Index (61).
Subjects were administered the Peabody Picture Vocabulary Test to determine IQ.
Chi square value.
Number of cases meeting criteria for alcohol or drug abuse or dependence before their scan. Diagnoses were made using the age appropriate diagnostic instrument, KSADS for those under age 19 and CIDI for those 19 or greater.
2.2. Risk group status
Multiplex alcohol dependence (AD) families were identified through the presence of two adult brothers with AD. Extensive in person and family history information was available for these targeted families (Table 2). The multiplex sampling strategy used in this study resulted in a high density of AD in the targeted pedigrees as previously described (Hill et al., 2008). Offspring from the brother pairs and their siblings in the multiplex families provided the high-risk offspring for the present report. Psychiatric status of the “marrying in” side of the offspring’s family was also obtained. Although not all offspring had a father or mother with a lifetime diagnosis of AD, the sampling design insured that each high-risk offspring had an average of four first and second degree relatives who were alcohol dependent. Among the high-risk offspring, 66% of the fathers and 19.7% of the mothers had AD (see Table 2). In addition, low-risk control families were identified through two adult brothers without alcohol or drug dependence. Parental AD among low-risk offspring was infrequent (<5%) but did occur where “marrying in” spouses had AD.
Table 2.
Alcohol and drug dependence among fathers and mothers of high- and low-risk offspring.
| High risk (N=71)
|
Low risk (N=60)
|
|||||
|---|---|---|---|---|---|---|
| Yes | No | Unknown | Yes | No | Unknown | |
| Father alcohol dependent | 47 (66.2) | 16 (22.5) | 8 (11.3) | 3 (5) | 49 (81.7) | 8 (13.3) |
| Father drug dependent | 18 (25.4) | 34 (47.9) | 19 (26.8) | 1 (1.7) | 48 (80.0) | 11 (18.3) |
| Mother alcohol dependent | 14 (19.7) | 54 (76.1) | 3 (4.2) | 0 (0) | 54 (90) | 6 (10) |
| Mother drug dependent | 3 (4.2) | 62 (87.3) | 6 (8.5) | 0 (0) | 53 (88.3) | 7 (11.7) |
High-risk target families identified through an affected male proband pair. The affected and unaffected siblings of the pair were also eligible for inclusion. Offspring of the proband pairs and their siblings form the basis of this report.
Although some high-risk offspring did not have an alcohol-dependent father, the familial density of alcohol dependence for these offspring was greater than that typically seen in unselected families of alcohol-dependent individuals because their uncles and grandparents were frequently alcohol dependent.
Low-risk target families were selected for absence of alcohol dependence in two generations. The alcohol- and drug-dependent fathers seen among the control families resulted from these individuals marrying into the target families.
2.3. Contaminating variables
In order to control for variables other than familial/genetic risk that might influence brain morphology, careful attention was paid to personal characteristics of the subjects including their personal history of substance use and other psychiatric disorders (Tables 3 and 4). Similarly, prenatal history of use of substances by mothers was obtained.
Table 3.
Psychiatric diagnosis for participants scanned in childhood (ages 8–18 years old) by familial risk group status. All diagnoses were based on outcome of K-SADS a interviews with parent and child.
| High risk (N=33) | Low risk (N=34) | |
|---|---|---|
| Alcohol or drug abuse | 2 (6.1) | 1 (2.9) |
| Alcohol or drug dependence | 2 (5.7) | 1 (2.9) |
| Either abuse or dependence | 3 (9.1) | 1 (2.9) |
| Anxiety disorders | 5 (15.2) | 4 (11.8) |
| Depressionb | 6 (18.2) | 1 (2.9) |
| Attention deficit hyperactivity disorder (ADHD)c | 8 (24.2) | 1 (2.9) |
| Oppositional/conduct disorder | 5 (15.2) | 2 (5.9) |
Kiddie version Schedule for Affective Disorders and Schizophrenia.
A significantly greater proportion of high- than low-risk offspring met criteria for depression in childhood (χ2=4.16, d.f.=1, p=0.041).
A significantly greater proportion of high- than low-risk offspring met criteria for ADHD in childhood (χ2=6.53, d.f.=1, p=0.011).
Table 4.
Cumulative psychiatric diagnoses for participants scanned as young adults (19 years or older). (All diagnoses were based on outcome of a CIDIa interview if performed after age 19 or K-SADS interview performed between the ages of 8–18).
| High risk (N=38) | Low risk (N=26) | |
|---|---|---|
| Alcohol or drug abuse | 13 (34.2) | 3 (11.5) |
| Alcohol or drug dependence | 12 (31.6) | 7 (26.9) |
| Either abuse or dependenceb | 13 (34.2) | 3 (11.5) |
| Anxiety disorders | 13 (34.2) | 8 (30.8) |
| Depression | 9 (23.7) | 3 (11.5) |
| Attention deficit hyperactivity disorder (ADHD)c | 4 (11.8) | 0 (0) |
| Oppositional/conduct disorderc | 9 (26.5) | 0 (0) |
Composite International Diagnostic Interview.
A significantly greater proportion of high than low risk offspring met criteria for substance use disorder defined as either abuse or dependence on alcohol or drugs (χ2=4.23, d.f.=1, p=0.04).
This diagnosis was available only from the K-SADS.
2.3.1. Prenatal use of substances
Mothers of both high- and low-risk offspring were administered a structured interview designed to assess quantity and frequency of use of alcohol, drugs and cigarettes during pregnancy. Although the assessment was retrospective, being obtained at the child’s first longitudinal assessment and usually before the age of 12 years, the information obtained would appear to be valid. Comparison of prospective and retrospective data for drinking during pregnancy has shown retrospective data to be valid (Griesler and Kandel, 1998). Moreover, follow-up of women for 4 and 5 years following their pregnancies has shown substantial reliability (r=0.53 and 0.67, respectively) between reports obtained during pregnancy and those obtained following the pregnancy (Ernhart et al., 1988; Jacobson et al., 1991).
2.3.2. Personal history of psychiatric disorders
All participants are enrolled in an ongoing longitudinal study that has followed youngsters from childhood through young adulthood. Extensive clinical information was available for determining if any psychiatric disorder including substance use disorder was present by the time the magnetic resonance imaging (MRI) assessment was performed. Children/adolescents were assessed yearly with the Kiddie version Schedule for Affective Disorders and Schizophrenia (K-SADS) (Chambers et al., 1985) using separate interviews of parent and offspring to determine the presence or absence of Axis I DSM-III diagnoses. (DSM-III was the current methodology at the initiation of the study in 1989.) Young adults were assessed using the Composite International Diagnostic Interview (CIDI) diagnostic instrument (Janca et al., 1992) to obtain DSM-IV Axis I diagnoses. There were no exclusion criteria applied based on presence of psychiatric disorder.
2.4. Informed consent and safety monitoring
All participants signed informed consent documents after having the study explained to them. All were screened to insure absence of ferromagnetic metal in or on their body. All female subjects were screened for absence of pregnancy using Icon® 25 hCG pregnancy kits. A neuroradiologist reviewed any scan considered suspicious for abnormality.
2.5. Image acquisition
All subjects were scanned on a GE 1.5 T GE scanner located in the Department of Radiology MR Research Center. T1 weighted axial images with slice thickness of 1.5 mm were obtained using a three-dimensional spoiled gradient recalled echo in the steady state sequence (3D SPGR) (TE=5, TR=24, fiip angle=45°, acquisition matrix=192×256, NEX=1, FOV=24 cm). Slices were resliced in the coronal plane through the anterior commissures to provide a more reproducible guide for image orientation. Additionally, axial proton density and T2 weighted images were obtained covering the whole brain at a slice thickness of 5 mm, slice gap=0 mm ([double echo spin echo, TE=17 ms and 102 ms; TR=3000 ms], acquisition matrix=256×192, NEX=1, FOV=24 cm). Obtaining the dual echo study enabled us to adequately address segmentation.
2.6. Region of interest analysis
Images were transferred from the MR Research Center to a computer workstation in our neuroimaging laboratory and regions of interest drawn using BRAINS2 (Magnotta et al., 2002), a software that provides valid and reliable volume measurements of specific structures by using a semiautomated segmentation approach. Two raters (SW and HC) blind to subject’s identity and risk group membership traced the volumes of the cerebellum and intracranial volume (ICV) for each subject. The Neural Net program (BRAINS2 [Magnotta et al., 2002]) provided landmarks for outlining each slice. Region of interest (ROI) manual tracing (approximately 70–75 slices) was performed in the coronal plane. BRAINS2 provides separate windows for coronal, axial, sagittal and three-dimensional views and provides automated segmentation of gray, white, and CSF volumes. Inter-rater reliability for total volume was 97.7%, 99.3% for gray matter and 95.8% for white matter.
BRAINS2 uses successive maximization of tissue into gray and white matter to optimize the kappa values obtained. Separation of tissue classes is performed using discriminant function analysis with the function then applied to the entire image. A kappa of 1.00 indicates perfect classification of gray matter, white matter, and CSF. All scans had Kappa values in the range of 0.92–0.98.
2.7. Morphometry of the cerebellum
Using guidelines established for the cerebellum using the Neural Net algorithm (Pierson et al., 2002), the midline was determined by using the vertex of the fourth ventricle at its most posterior point (coronal plane) and the midline of the corpus medullare. The cerebellar peduncles were excluded from the corpus medullare at the point where it emerged from beyond the gray matter of the cerebellar cortex (axial plane). The vermis was included in the cerebellum measurements.
The cerebral hemispheres, brainstem, and the CSF surrounding these structures were included in the ICV measure (Prasad et al., 2005). Intracranial volumes were calculated by summing areas of successive coronal slices, including gray and white matter and CSF volumes, and multiplying by slice thickness. Statistical analyses of cerebellar volumes (total, gray and white) were adjusted using total intracranial volume (ICV) minus the total volume of the cerebellum.
2.8. Genotyping
DNA was available for 103 Caucasian individuals (62 high-risk and 41 low-risk offspring) for whom structural MRI scans were assessed. Genetic variation in two genes was of interest: BDNF and GABRA2. These genes were chosen based on their potential to alter brain growth and development (Fiszman, 2005; Agartz et al., 2006) and known interaction in this process (Berninger et al., 1995).
2.8.1. BDNF
The BDNF genotyping was completed using the SNP rs6265 analyzed on the Biotage PSQ 96MA Pyrosequencer (Biotage AB, Uppsala, Sweden). An amplimer containing the polymorphism was generated by PCR in 96 well plates in a 50-μl total reaction volume, containing 10 ng of human genomic DNA; 1× GeneAmp® PCR Gold Buffer; 2.5 mM magnesium chloride; 200-μM dNTPs; 1 unit of AmpliTaq Gold™ taq polymerase; and 1 pmol of each of the unmodified forward primer 5′-GGACTCTGGAGAGCGTGAAT-3′ and the biotinylated reverse primer 5′-CCTCATCCAACAGCTCTTCTATC-3′. Thermal cycling included 45 cycles at an annealing temperature of 60 °C. The Biotage workstation was used to isolate the biotinylated single strand from the double strand PCR products. The isolated product was then sequenced using the complimentary sequencing primer 5′-GGCTGACACTTTCGAAC-3′. At the polymorphic site, the minor allele was detected by the presence of an A nucleotide whereas the major allele was detected by the presence of a G nucleotide.
2.8.2. GABRA2 — rs279871
This polymorphism was analyzed by PCR amplification using the unmodified forward primer 5′-AATCCAAACCCTGAAACACTTCT-3′ and the biotinylated reverse primer 5′-AGAAGGGATCAGAGGTAGAA-CAAA-3′. The Biotage PSQ 96MA Pyrosequencer and accompanying workstation were then utilized in order to isolate the biotinylated single-stranded from double-stranded PCR products. Subsequently, the isolated product was sequenced using the complimentary sequencing primer 5′-TGACATGTATGTGATATATT-3′. The wild type allele was detected by an A nucleotide at the polymorphic site, while the variant allele was detected by the presence of a G nucleotide.
2.9. Statistical analysis
Mixed model analyses were performed with fixed effects that included familial risk, gender, a gender by risk interaction term, with age and ICV (total ICV volume minus total cerebellum) as covariates. Each family was assigned a unique family ID that was used as a random effect in the model to control for multiple siblings within families (57.3% had 2 or more offspring).
3. Results
3.1. Personal psychiatric history and prenatal maternal use of substance
3.1.1. Psychiatric disorders — subjects scanned in childhood or in young adulthood
A greater incidence of psychopathology (depression, attention deficit hyperactivity disorder (ADHD), abuse and dependence) was seen in high-risk (HR) than low-risk (LR) offspring (Tables 3 and 4).
3.1.2. Prenatal use of substances
Mothers of both high- and low-risk offspring were interviewed concerning their use of alcohol or drugs during pregnancy and found to be free of heavy use during pregnancy. Drinking among mothers in both groups was quite low. A total of 76.3% reported no drinking with an additional 21.9% drinking less than 1 drink per day. A total of 2.7% reported using any drugs during pregnancy. Absence of cigarette use was reported by 77.8% of mothers. However, of those who smoked, a greater percentage were from high-risk families (35%) versus low-risk families (6.3%).
3.2. Group effects for total cerebellum volume
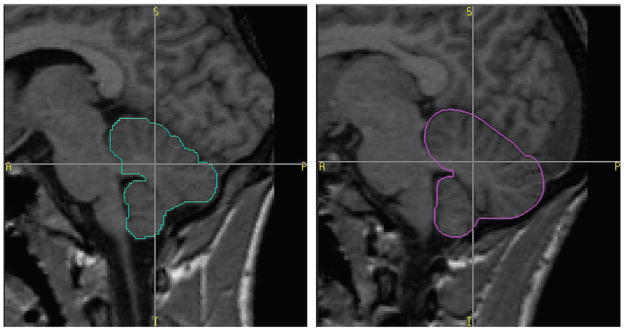
The effect of familial risk membership on total cerebellar volume was analyzed in a 2X2 model that assessed risk and gender and their interaction (Table 5A). Data were then removed for 24 cases who met criteria for alcohol or drug abuse or dependence (Table 5B), resulting in a significant risk group difference for total cerebellum volume with high-risk individuals having larger volumes of both. It was expected that removal of the 24 cases would increase risk group differences because alcohol dependence is associated with reduction of cerebellar volume (Zahr et al., 2010). To insure that prenatal exposure to alcohol did not influence our results, an analysis was performed for total cerebellar volume for the 107 cases in which familial risk was evaluated with gender, age, ICV, and prenatal alcohol use as covariates. This analysis continued to reveal risk differences (F =5.30, d.f. 1, 39.11, p =0.027). Typical high- and low-risk cerebellar tracings are shown in Fig. 1.
Table 5.
Effect of familial risk, gender and their interaction on total cerebellar volume, gray matter volume and white matter volume adjusting for ICV and age.
| Table 5A–Analysis for full sample (N=131)
|
Table 5B–Analysis for sample with no SUD prior to MRI scan (N=107)
|
|||||
|---|---|---|---|---|---|---|
| Full sample N=131
|
Sample with no SUD N=107a |
|||||
| F value | d.f. | p value | F value | d.f. | p value | |
| Total cerebellum | ||||||
| Risk | 3.65 | 1, 57.33 | 0.061 | 6.03 | 1, 41.39 | 0.018 |
| Gender | 4.64 | 1, 124.96 | 0.033 | 4.43 | 1, 101.94 | 0.038 |
| RiskXGender | <1 | 1, 123.75 | NS | <1 | 1, 101.28 | NS |
| ICV | 23.33 | 1, 118.70 | <0.001 | 25.17 | 1, 92.49 | <0.001 |
| Age | 2.21 | 1, 99.91 | 0.14 | 1.59 | 1, 73.08 | NS |
| Gray matter | ||||||
| Risk | 3.81 | 1, 62.05 | 0.056 | 4.88 | 1, 53.34 | 0.031 |
| Gender | 11.02 | 1, 124.65 | 0.001 | 10.32 | 1, 101.98 | 0.002 |
| RiskXGender | 1.12 | 1, 124.65 | NS | <1 | 1, 100.73 | NS |
| ICV | 12.49 | 1, 124.74 | 0.001 | 12.32 | 1, 97.07 | 0.001 |
| Age | <1 | 1, 100.97 | NS | <1 | 1, 82.90 | NS |
| White matter | ||||||
| Risk | <1 | 1, 125.0 | NS | <1 | 1, 102 | NS |
| Gender | 1.89 | 1, 125.0 | NS | 1.83 | 1, 102 | NS |
| RiskXGender | 1.63 | 1, 125.0 | NS | 1.43 | 1, 102 | NS |
| ICV | 13.19 | 1, 125.0 | <0.001 | 12.49 | 1, 102 | 0.001 |
| Age | 18.18 | 1, 125.0 | <0.001 | 12.88 | 1, 102 | 0.001 |
A total of 131 cases had MRI scans. A total of 24 cases meeting criteria for alcohol or drug abuse or dependence prior to the MRI scan were removed from the analyses resulting in 107 cases with no known dependence. Analyses were completed for this sample and are displayed in Table 5B.
Fig. 1.
Region of interest outlines of a typical low-risk male participant (blue outline) and of a typical high-risk male participant (red). Both male participants were 16 years old at the time the scans were obtained.
3.3. Group effects for cerebellar gray matter volumes
In the initial 2X2 statistical model performed, gender was found to be statistically significant but familial risk was marginally significant (Table 5A). Because exposure to alcohol and other drugs can be expected to reduce cerebellar volume, it was important to remove this confounder effect, which might mask the familial risk group differences that were the focus of interest. Removal of data for the 24 alcohol and drug exposed cases resulted in significant risk group differences for cerebellar gray matter (GM) with high-risk offspring having larger cerebellar GM volume than low-risk offspring (Table 5B). Gender was also significant with GM greater for males than females.
3.4. Group effects for white matter volumes
The mixed model analysis did not find an association between risk status, gender or their interaction for cerebellar white matter for the full sample (Table 5A), or for the sample with exposed cases removed (Table 5B).
3.5. Effects of BDNF and GABRA2 variation on cerebellar gray matter volume
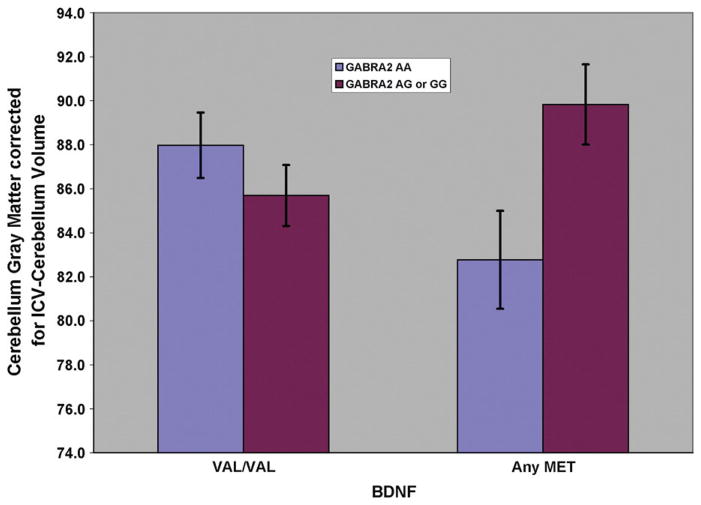
Analyses were performed to determine if variation in the BDNF or GABRA2 genes, alone or in combination, influenced gray or white matter volume. Using gray matter volume as the dependent variable, a mixed model analysis was performed in which GABRA2 variation (those with 1 or 2 copies of the minor allele versus those with none) and genetic variation in the BDNF gene that similarly contrasted individuals with 1 or 2 copies of the minor allele versus those with none. The model included control for ICV using a modified ICV index (ICV minus cerebellum volume). A significant effect for GABRA2 or BDNF alone was not seen. However, the interaction between the two genes showed a significant association with gray matter volume (F=7.15, d.f.=1, 64.2, p=0.009) (Fig. 2). Because differences in genotype by familial risk group might have explained these results, a statistical model was run in which risk group was entered as a dummy variable to covary the effects of risk status. Results continued to be significant (F=6.90, d.f.=1, 60.3, p=0.011). Unlike the results for cerebellar gray, significant effects for white matter were not seen.
Fig. 2.
Cerebellar gray matter volume for male and female subjects genotyped for GABRA2 and BDNF (N=103). Note that the effect of having at least one copy of the minor G allele is influenced by the presence of having any Met allele of the BDNF gene.
3.6. Cerebellum and age related regression analyses
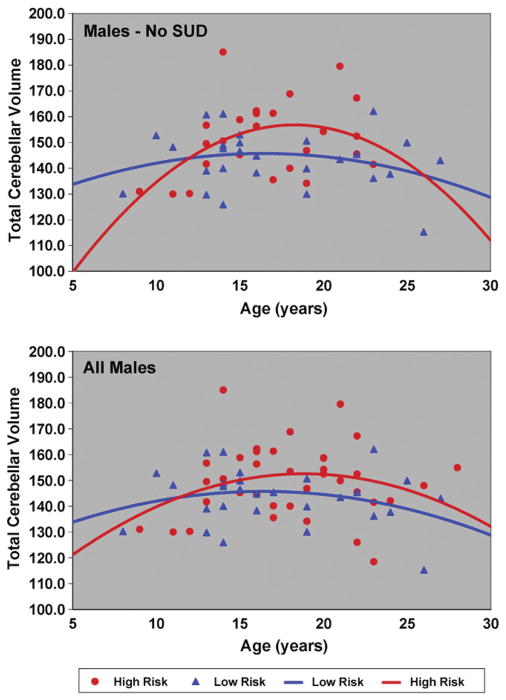
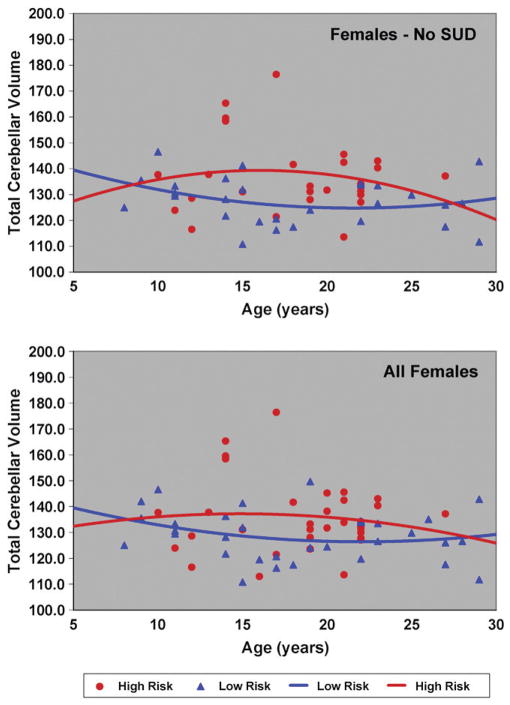
Because familial risk group differences in total and GM cerebellar volumes were found, it was of interest to determine if differing developmental trajectories might be seen by risk group. First, curve fitting models were used to determine the best fit solution for all 131 male and female high- and low-risk cases. Data for the 24 offspring with substance use disorder were then removed and curve fitting models applied to the remaining 107 cases (Table 6). For total cerebellar volume, a quadratic model provided the best fit for the total sample, and for the male only sample (Fig. 3), though neither linear nor quadratic models fit the data for females (Fig. 4). For males, removal of the SUD cases improved the significance of the fit (Table 6). For gray matter volumes, the quadratic models provided a statistically significant fit for the combined sample of males and females. Separate analyses performed for the male and female samples showed that the quadratic model provided a good fit to the data for the males but not the females (Table 6). White matter age related changes for the 131 subjects were significant for both the linear and quadratic models. Analysis within each gender showed a significant fit for white matter for females that was not seen for males.
Table 6.
Best fit model for total, gray and white matter volumes for full sample (N=131) and sample with no SUD prior to MRI scan (N=107).
| Full sample (N=131)
|
Sample with no SUD (N=107)
|
|||||
|---|---|---|---|---|---|---|
| F value | d.f. | p value | F value | d.f. | p value | |
| Total cerebellum | ||||||
| Linear model | ||||||
| Total sample | 1.05 | 1, 129 | NS | 1.42 | 1, 106 | NS |
| Females | <1.00 | 1, 64 | NS | 1.24 | 1, 54 | NS |
| Males | <1.00 | 1, 63 | NS | <1.00 | 1, 50 | NS |
| Quadratic | ||||||
| Total sample | 2.78 | 2, 128 | 0.07 | 3.86 | 2, 105 | 0.024 |
| Females | <1.00 | 2, 63 | NS | <1.00 | 2, 53 | NS |
| Males | 2.37 | 2, 62 | 0.10 | 3.51 | 2, 49 | 0.038 |
| Gray matter | ||||||
| Linear model | ||||||
| Total sample | <1.00 | 1, 129 | NS | <1.00 | 1, 106 | NS |
| Females | 1.02 | 1, 64 | NS | <1.00 | 1, 54 | NS |
| Males | <1.00 | 1, 63 | NS | <1.00 | 1, 50 | NS |
| Quadratic | ||||||
| Total sample | 4.31 | 2, 128 | 0.015 | 5.77 | 2, 105 | 0.004 |
| Females | 1.41 | 2, 63 | NS | 1.11 | 2, 53 | NS |
| Males | 3.21 | 2, 62 | 0.047 | 6.33 | 2, 49 | 0.004 |
| White matter | ||||||
| Linear model | ||||||
| Total sample | 13.42 | 1, 129 | <0.001 | 10.47 | 1, 106 | 0.002 |
| Females | 10.79 | 1, 64 | 0.002 | 9.62 | 1, 54 | 0.003 |
| Males | 2.38 | 1, 63 | NS | <1.00 | 1, 50 | NS |
| Quadratic | ||||||
| Total sample | 6.85 | 2, 128 | 0.001 | 5.36 | 2, 105 | 0.006 |
| Females | 6.13 | 2, 63 | 0.004 | 5.45 | 2, 53 | 0.007 |
| Males | 1.23 | 2, 62 | NS | <1.00 | 2, 49 | NS |
Fig. 3.
Total cerebellar volume by age is shown for high and low risk male subjects. The upper panel shows the trajectories obtained for the 52 males without SUD prior to the MRI scan. The lower panel shows all 65 male subjects and includes those with SUD (N=13).
Fig. 4.
Cerebellar gray volume by age is shown for female high- and low-risk subjects. The upper panel shows the trajectories obtained for the 55 females without substance use disorder (SUD) prior to the MRI scan. The lower panel shows all 66 female subjects and includes those with SUD prior to the scan (N=11).
In summary, we find that total cerebellar volume for males appears to peak later than it does for females as may be seen by comparing Figs. 3 and 4. Also, low-risk males appear to reach peak volumes earlier than high-risk males (Fig. 3). A similar trend can be observed for the high- and low-risk females (Fig. 4).
4. Discussion
It is now well-known that offspring of parents with alcohol dependence (AD) have an increased risk for developing alcohol and drug dependence by young adulthood (Hill et al., 2008; Kendler et al., 2008). With a number of morphological differences now being identified in high-risk offspring (see Tessner and Hill, 2010, for review), it is clear that some of these variations may provide clues to the neurobiological underpinnings for addiction. It is also clear that alcohol has neuropathological effects on neuronal integrity in adults (Sullivan and Pfefferbaum, 2005) and adolescents (DeBellis et al., 2005). Reduced hippocampal volume (DeBellis et al., 2000) and smaller prefrontal cortex volume (DeBellis et al., 2005) along with neurocognitive changes (Brown et al., 2000) have been reported in adolescent-onset alcohol use disorders. What is not always clear is whether the differences observed are a consequence of exposure or, through the effects of familial risk factors, are antecedent to the development of alcohol dependence. Alternatively, the effects of risk and exposure may be in opposite directions so that removal of cases with substance use disorders may be necessary to observe familial risk group differences as was the case in the present study. To illustrate, adolescents with significant alcohol exposure show reduced hippocampal volume (DeBellis et al., 2000) that is not seen in high-risk male offspring with minimal alcohol exposure (Hill et al., 2001). However, other regional alterations in morphology have been observed in high-risk offspring with only minimal alcohol or drug exposure including reduced volume of the right amygdala (Hill et al., 2001), and the right orbitofrontal cortex (OFC) (Hill et al., 2009).
Both total and gray matter volumes of the cerebellum are greater in high-risk offspring from multiplex for alcohol dependence families in comparison to controls. The results are unlikely to be due to the personal diagnosis of offspring as no single diagnosis contributed more than 25% of cases with the exception of substance use disorders. Analyses controlling for the effects of exposure to alcohol and drugs in these adolescent and young adult participants suggest that the greater volume seen in individuals with high familial loading for alcohol dependence is present before drug and alcohol use are initiated. How these morphological changes may be related to greater susceptibility to alcohol dependence and other substance use disorders is currently unknown. However, it is clear that adults with long histories of alcohol dependence display disruption of frontocerebellar circuitry (Sullivan et al., 2003). Other regions such as the orbitofrontal cortex have been studied far more extensively as candidate regions for addiction susceptibility because of their involvement in disinhibitory processes (Dom et al., 2005). The present results suggest that the cerebellum may play an important role in addiction susceptibility as well.
In addition to structural alterations, functional changes in the cerebellum have also been observed in association with addiction. In a PET study using a visual–spatial recognition task with delayed response and monetary reward, Martin-Soelch et al. (2001) found greater activation of the left cerebellum in an opiate addicted group in comparison to controls. Also, greater activation in the right superior cerebellum and left frontal cortex has been reported for alcohol dependent subjects in comparison to controls when performing a Sternberg working memory task (Desmond et al., 2003). Additionally, cocaine users in comparison to controls have more difficulty inhibiting their responses in a GO/NO GO inhibition task with varying working memory load which is accompanied by greater activation in the left cerebellum (Hester and Garavan, 2004). Taken together, these studies suggest that individuals susceptible to substance use disorders may have altered cognitive functioning involving the cerebellum.
In addition to the structural and functional cerebellar alterations seen across different addictions, there is reason to believe that cerebellar development may be altered in those with highest risk for developing an addiction through familial/genetic loading. Cortical development has been studied extensively allowing for description of the overall process. Briefly, during adolescence and young adulthood changes in brain structure and refinement of brain organization lead to significant changes in cognitive, social, and emotional behavior (Casey et al., 2005; Yurgelun-Todd, 2007). White matter volume appears to increase well into adulthood while gray matter volume tends to increase in childhood and adolescence followed by a decrease (Jernigan et al., 1991; Pfefferbaum et al., 1994; Giedd et al., 1996a,b; Sowell et al., 2004), with females reaching their peak 1–2 years earlier than males (Lenroot and Giedd, 2006). Because cortical development appears to follow a pattern that subserves the needs of the organism, primary motor, sensory and visual areas mature earlier than those supporting more complex cognitive functions such as the association areas (Gogtay et al., 2004). Although these developmental changes in cortical organization and functioning have been studied extensively, developmental changes in cerebellar morphology and functioning have received less attention.
Previous reports indicate that the cerebellum undergoes substantial development during childhood and adolescence, first increasing to a peak volume and then subsequently declining (Castellanos et al., 2002; Keller et al., 2003; Raz et al., 2003; Mackie et al., 2007; Tiemeier et al., 2010), peaking approximately 2 years later than cerebral volume (Giedd et al., 2009). In addition to an overall larger volume seen in males than females, peak volumes differ by gender with girls peaking at approximately 12 years and boys at about 15 years of age (Tiemeier et al., 2010). White matter volume of the cerebellum increases through adolescence with a peak of approximately 17 years in females and 22 years in males. In the present study, we find that total cerebellar volume for males appears to peak later than it does for females in agreement with the Tiemeier et al. findings. Moreover, we find that when the contaminating influence of alcohol and drug exposure that is brought about by a substance use disorder diagnosis is removed, the developmental curves for high- and low-risk offspring more clearly show a difference in developmental trajectories by risk group. This difference appears to show that low-risk males reach peak volumes earlier than high-risk males. A similar trend can be observed for the females, with high-risk females showing a somewhat different developmental trajectory than low-risk females.
The present results support our previous work with a sample of 34 male participants in finding larger cerebellar volume (total and gray matter) in high-risk offspring from multiplex for alcohol dependence families (Hill et al., 2007). Importantly, variation in SNPs within the BDNF and GABRA2 genes show evidence for gene/gene interaction in altering cerebellar volume. In order to better understand the relationship between cerebellar volume, genotypic variance and familial risk, preliminary analyses were performed relating BDNF and GABRA2 genotypes and familial risk. An association for BDNF was not found. For GABRA2, the rs279871 SNP showed a significant association between risk status and presence of the “2” allele (χ2= 7.34, d.f.=1, p=0.007). Because of modest sample size, we view this result as preliminary. However, it does suggest that risk group differences in GABRA2 in association with BDNF variation may promote cerebellar growth resulting in larger cerebellar volume in high-risk offspring.
It is possible that risk group differences at the time these participants were scanned might represent developmental differences in the high- and low-risk groups. Age-regression analyses showed significant age-related changes over the age range studied with total cerebellar volume and gray matter volume following a quadratic pattern and white matter a more linear trend. As noted in previous reports (Giedd et al., 2009), cerebellar volumes of males and females differed significantly. Comparison of high- and low-risk subjects without substance use disorder shows that high-risk subjects, especially males, may be delayed in reaching full cerebellar volume before a subsequent reduction in volume with age is seen. These results support our previous results in showing what appeared to be a delay in gray matter reduction with age in the high-risk male sample studied.
Of particular interest were our findings concerning the association between variation in GABRA2 and BDNF genes and cerebellar volume. These data provide for the first time an indication that specific genes that have been associated with alcohol dependence risk (Edenberg et al., 2004; Janak et al., 2006) may be influential in determining cerebellar growth and differentiation. In addition, there is abundant evidence that these two genes may be operating in early development and beyond to influence cerebellar development. Although GABA is the principal inhibitory neurotransmitter in the nervous system, GABA appears to have excitatory effects, inducing immature cerebellar granule cells to proliferate. This is accomplished when GABA depolarizes these cells allowing intracellular calcium to be increased and phosphorylation of MAPK and CAMKII to occur which then leads to trophic effects in these neurons (Fiszman, 2005). BDNF mRNA expression increases by approximately one-third from infancy to adulthood, rising during adolescence and peaking during young adulthood (Webster et al., 2002). Accordingly, it appeared plausible that BDNF might influence growth and development of cells within the cerebellum, a region that does not reach peak volume until adolescence or young adulthood. BDNF has also been reported to influence the volume of the hippocampus (Szeszko et al., 2005; Bueller et al., 2006) and posterior superior cerebellar vermis (Agartz et al., 2006) through its trophic effects on neurons.
In addition to the specific effects that each of these genes have on neuronal development, a number of studies point to the interaction of these two genes in neuronal development of the cerebellum. Evidence for these epistatic effects includes in vitro studies showing that BDNF accelerates cerebellar granule cell gene expression and modulates cell differentiation (Lin et al., 1998; Bulleit and Hsieh, 2000). Also, there is evidence that BDNF may be involved in the maturation of the GABAergic system, particularly in the hippocampus, by promoting differentiation and formation of synapses and cell survival (Berninger et al., 1995). A direct effect of BDNF on GABAA receptors has now been demonstrated. In a novel approach, GABAA receptors were microtransplanted from human epileptic brain tissue to Xenopus oocytes (Palma et al., 2005). With this technique, the oocytes acquire human GABAA receptors which can then be tested for response to BDNF. In this experiment, exposure to BDNF increased the currents generated by the “epileptic” GABAA receptors. With demonstrated effects for each of these genes alone and together in cerebellar development, we view the present results showing an interaction of these genes on cerebellar gray matter volume as quite plausible. What is unclear is how the presence of the GABRA2 minor allele (1 or 2 copies) protects cerebellar cells from tissue reduction effects often associated with the BDNF Met allele.
4.1. Limitations of the study
One limitation of the present report is that age-related cerebellar relationships were determined using cross-sectional data. However, data were collected for 131 high- and low-risk individuals across a broad age range (8–28) allowing for description of developmental patterns for total, gray and white matter in childhood, adolescence and young adulthood. Another limitation of the study was its focus on total cerebellar volumes for the statistical analyses presented. Functional variation among the cerebellar lobes is well documented (Andreasen and Pierson, 2008). Use of a single SNP to characterize genetic variation of the BDNF and GABRA2 genes might also be viewed as a limitation. However, among several GABRA2 SNPs studied, the rs279871 SNP was highly related to alcohol dependence (Edenberg et al., 2004). Similarly, allelic variation in the BDNF SNP rs6265 and regional variation in brain volume is well documented (Szeszko et al., 2005; Agartz et al., 2006; Bueller et al., 2006).
Another possible limitation of our analysis was that some offspring were exposed to alcohol, cigarettes or other drugs used by the mothers while they were in utero. Although the ideal situation would be to have offspring without such exposure when evaluating the effects of familial risk, this may not be possible due to the base rate of such exposures in the general population. However, the rates of alcohol use for mothers during their pregnancies observed in this sample are similar to those seen in the general population. Using data from a survey of eight obstetric clinics in Southeastern Michigan, Flynn et al. (2003) found that 81.9% of the mothers reported drinking less than 1 drink per week. In our sample, 76.3% reported no drinking during pregnancy. Smoking during pregnancy was reported by 22.2% of our sample in comparison to national survey data for the US and England in which 12–20% of mothers continue to smoke during pregnancy (ONS, 2006; Gilman et al., 2008). These considerations suggest that the risk group differences observed for cerebellar volume are minimally influenced by the effects of prenatal use of substances by the mothers of these offspring.
4.2. Conclusion
In summary, these results suggest that familial risk group differences are associated with differences in cerebellar morphology that may be due to altered developmental trajectories that appear to be influenced by genetic variation in BDNF and its influence on GABRA2 signaling in early development and/or altered gene expression through adolescence and young adulthood.
Acknowledgments
This research was supported by NIAAA grants AA018289, AA05909, AA 08082, and AA015168 to SYH.
References
- Agartz I, Sedvall GC, Terenius L, Kulle B, Frigessi A, Hall H, Jonsson EG. BDNF gene variants and brain morphology in schizophrenia. American Journal of Medical Genetics Part B Neuropsychiatric Genetics. 2006;141B:513–523. doi: 10.1002/ajmg.b.30338. [DOI] [PubMed] [Google Scholar]
- Allen G, Buxton R, Wong E, Courchesne E. Attentional activation of the cerebellum independent of motor involvement. Science. 1997;275:1940–1943. doi: 10.1126/science.275.5308.1940. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biological Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Arndt S, Cizadlo T, Hurtig R, Rezai K, Watkins GL, Ponto LL, Hichwa RD. Short-term and long-term verbal memory: a positron emission tomography study. Proceedings of the National Academy of Sciences of the United States of America. 1995a;92:5111–5115. doi: 10.1073/pnas.92.11.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Cizadlo T, Arndt S, Rezai K, Watkins GL, Boles-Ponto LL, Hichwa RD. PET studies of memory: novel versus practiced free recall of word lists. NeuroImage. 1995b;2:296–305. doi: 10.1006/nimg.1995.1037. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Arndt S, Cizadlo T, Hurtig R, Rezai K, Watkins GL, Ponto LL, Hichwa RD. Neural substrates of facial recognition. Journal of Neuropsychiatry and Clinical Neuroscience. 1996;8:139–146. doi: 10.1176/jnp.8.2.139. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, O’Leary DS, Paradiso S, Cizadlo T, Arndt S, Watkins GL, Boles-Ponto LL, Hichwa RD. The cerebellum plays a role in conscious episodic memory retrieval. Human Brain Mapping. 1999;8:226–234. doi: 10.1002/(SICI)1097-0193(1999)8:4<226::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastian AJ, Mink JW, Kaufman BA, Thach WT. Posterior vermal split syndrome. Annals of Neurology. 1998;44:601–610. doi: 10.1002/ana.410440405. [DOI] [PubMed] [Google Scholar]
- Benegal V, Antony G, Venkatasubramanian G, Jayakumar PN. Grey matter abnormalities and externalizing symptoms in subjects at high risk for alcohol dependence. Addiction Biology. 2007;12:122–132. doi: 10.1111/j.1369-1600.2006.00043.x. [DOI] [PubMed] [Google Scholar]
- Berninger B, Marty S, Zafra F, Berzaghi MDP, Thoenen H. GABAergic stimulation switches from enhancing to repressing BDNF expression in rat hippocampal neurons during maturation in vivo. Development. 1995;121:2327–2335. doi: 10.1242/dev.121.8.2327. [DOI] [PubMed] [Google Scholar]
- Brown SA, Tapert SF, Granholm E, Delis DC. Neurocognitive functioning of adolescents: effects of protracted alcohol use. Alcoholism: Clinical and Experimental Research. 2000;24:164–171. [PubMed] [Google Scholar]
- Bueller JA, Aftab M, Sen S, Gomez-Hassan D, Burmister M, Zubieta JK. BDNF Val66 allele is associated with reduced hippocampal volume in healthy subjects. Biological Psychiatry. 2006;59:812–815. doi: 10.1016/j.biopsych.2005.09.022. [DOI] [PubMed] [Google Scholar]
- Bulleit RF, Hsieh T. MEK inhibitors block BDNF-dependent and independent expression of GABAA receptor subunit mRNAs in cultured mouse cerebellar granule neurons. Developmental Brain Research. 2000;119:1–10. doi: 10.1016/s0165-3806(99)00119-4. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Tottenham N, Liston C, Durston S. Imaging the developing brain: what have we learned about cognitive development? Trends in Cognitive Science. 2005;9:104–110. doi: 10.1016/j.tics.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, Jeffries NO, Greenstein DK, Clasen LS, Blumenthal JD, James RS, Ebens CL, Walter JM, Zijdenbos A, Evans AC, Giedd JN, Rapoport JL. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. Journal of the American Medical Association. 2002;288:1740–1748. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini P, Tabrizi MA, Davies M. The assessment of affective disorders in children and adolescents by semi-structured interview. Archives of General Psychiatry. 1985;42:696–702. doi: 10.1001/archpsyc.1985.01790300064008. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Pfefferbaum A, Sullivan EV. Disruption of functional connectivity of the default-mode network in alcoholism. Cerebral Cortex. 2011;21 (10):2272–2281. doi: 10.1093/cercor/bhq297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157:733–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- DeBellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcoholism: Clinical and Experimental Research. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Desmond JE. Cerebellar involvement in cognitive function: evidence from neuroimaging. International Review of Psychiatry. 2001;13:283–294. [Google Scholar]
- Desmond JE, Fiez JA. Neuroimaging studies of the cerebellum: language, learning and memory. Trends in Cognitive Science. 1998;2:355–362. doi: 10.1016/s1364-6613(98)01211-x. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Chen SHA, DeRosa E, Pryor MR, Pfefferbaum A, Sullivan EV. Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. NeuroImage. 2003;19:1510–1520. doi: 10.1016/s1053-8119(03)00102-2. [DOI] [PubMed] [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, Van Den Brink W. Substance use disorders and the orbitofrontal cortex: systematic review of behavioural decision-making and neuroimaging studies. British Journal of Psychiatry. 2005;187:209–220. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- Edenberg HJ, Dick DM, Xuei X, Tian H, Almasy L, Bauer LO, Crowe RR, Goate A, Hesselbrock V, Jones K, Kwon J, Li TK, Nurnberger JI, Jr, O’Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. American Journal of Human Genetics. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernhart CB, Morrow-Tlucak M, Sokol RJ, Martier S. Under-reporting of alcohol use in pregnancy. Alcoholism: Clinical and Experimental Research. 1988;12:506–511. doi: 10.1111/j.1530-0277.1988.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raichle ME, Miezin FM, Petersen SE, Tallal P, Katz WF. PET studies of auditory and phonological processing: effects of stimulus characteristics and task demands. Trends in Cognitive Science. 1995;7:357–375. doi: 10.1162/jocn.1995.7.3.357. [DOI] [PubMed] [Google Scholar]
- Fiszman ML. Insights into GABA functions in the developing cerebellum. International Review of Neurobiology. 2005;71:95–112. doi: 10.1016/s0074-7742(05)71004-7. [DOI] [PubMed] [Google Scholar]
- Flynn HA, Marcus S, Barry KL, Blow FC. Rates and correlates of alcohol use among pregnant women in obstetrics clinics. Alcoholism: Clinical and Experimental Research. 2003;27:81–87. doi: 10.1097/01.ALC.0000046595.47491.37. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Vaituzis AC, Vauss YC, Hamburger SD, Kaysen D, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex. 1996a;6:551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Vaituzis AC, Hamburger SD, Lange N, Rajapakse JC, Kaysen D, Vauss YC, Rapoport JL. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18. The Journal of Comparative Neurology. 1996b;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Lalonde FM, Celano MJ, White SL, Wallace GL, Lee NR, Lenroot RK. Anatomical brain magnetic resonance imaging of typically developing children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:465–470. doi: 10.1097/CHI.0b013e31819f2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman SE, Breslau J, Subramanian SV, Hitsman B, Koenen KC. Social factors, psychopathology and maternal smoking during pregnancy. American Journal of Public Health. 2008;98:448–453. doi: 10.2105/AJPH.2006.102772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesler PC, Kandel DB. The impact of maternal drinking during and after pregnancy on the drinking of adolescent offspring. Journal of Studies on Alcohol. 1998;59:292–304. doi: 10.15288/jsa.1998.59.292. [DOI] [PubMed] [Google Scholar]
- Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. Journal of Neuroscience. 2004;24:11017–11022. doi: 10.1523/JNEUROSCI.3321-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyder K, Suchan B, Daum I. Cortico-subcortical contributions to executive control. Acta Psychologica. 2004;115:271–289. doi: 10.1016/j.actpsy.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Hill SY, DeBellis MD, Keshavan MS, Lowers L, Shen S, Hall J, Pitts T. Right amygdala volume in adolescent and young adult offspring from families at high risk for developing alcoholism. Biological Psychiatry. 2001;49:894–905. doi: 10.1016/s0006-3223(01)01088-5. [DOI] [PubMed] [Google Scholar]
- Hill SY, Muddasani S, Prasad K, Steinhauer S, Scanlon J, McDermott M, Keshavan M. Cerebellar volume in offspring from multiplex alcohol dependence families. Biological Psychiatry. 2007;61:41–47. doi: 10.1016/j.biopsych.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Locke-Wellman J, Lowers L. Psychopathology in childhood and adolescence: a prospective study of offspring from multiplex alcoholism families. Psychiatry Research. 2008;160:155–166. doi: 10.1016/j.psychres.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Wang S, Kostelnik B, Carter H, Holmes B, McDermott M, Zezza N, Stiffler S, Keshavan MS. Disruption of orbitofrontal cortex laterality in offspring from multiplex alcohol dependence families. Biological Psychiatry. 2009;65:129–136. doi: 10.1016/j.biopsych.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL, Sokol RJ, Martier SS, Ager JW, Kaplan MG. Maternal recall of alcohol, cocaine and marihuana use during pregnancy. Neurotoxicology and Teratology. 1991;13:535–540. doi: 10.1016/0892-0362(91)90062-2. [DOI] [PubMed] [Google Scholar]
- Janak PH, Wolf FW, Herberlein U, Pandey SC, Logrip ML, Ron D. BIG news in alcohol addiction: new findings on growth factor pathways BDNF, insulin, and GDNF. Alcoholism: Clinical and Experimental Research. 2006;30:214–221. doi: 10.1111/j.1530-0277.2006.00026.x. [DOI] [PubMed] [Google Scholar]
- Janca A, Robins LN, Cottler LB, Early TS. Clinical observation of assessment using the Composite International Diagnostic Interview (CIDI). An analysis of the CIDI field trials B wave II at the St. Louis site. British Journal of Psychiatry. 1992;160:815–818. doi: 10.1192/bjp.160.6.815. [DOI] [PubMed] [Google Scholar]
- Jelitai M, Madarasz E. The role of GABA in the early neuronal development. International Review of Neurobiology. 2005;71:27–62. doi: 10.1016/s0074-7742(05)71002-3. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Trauner DA, Hesselink JR, Tallal PA. Maturation of human cerebrum observed in vivo during adolescence. Brain. 1991;114:2037–2049. doi: 10.1093/brain/114.5.2037. [DOI] [PubMed] [Google Scholar]
- Keele SW, Ivry R. Does the cerebellum provide a common computation for diverse tasks? A timing hypothesis. Annals of the New York Academy of Sciences. 1990;608:179–207. doi: 10.1111/j.1749-6632.1990.tb48897.x. [DOI] [PubMed] [Google Scholar]
- Keller A, Castellanos FX, Vaituzis AC, Jeffries NO, Giedd JN, Rapoport JL. Progressive loss of cerebellar volume in childhood-onset schizophrenia. American Journal of Psychiatry. 2003;160:128–133. doi: 10.1176/appi.ajp.160.1.128. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of General Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SG, Ugurbil K, Strick PL. Activation of a cerebellar output nucleus during cognitive processing. Science. 1994;265:949–951. doi: 10.1126/science.8052851. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Brain development in children and adolescents: insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Levi-Montalcini R. The nerve growth factor 35 years later. Science. 1987;237:1154–1162. doi: 10.1126/science.3306916. [DOI] [PubMed] [Google Scholar]
- Lin X, Cui H, Bulleit RF. BDNF accelerates gene expression in cultured cerebellar granule neurons. Brain Research. Developmental Brain Research. 1998;105:277–286. doi: 10.1016/s0165-3806(97)00193-4. [DOI] [PubMed] [Google Scholar]
- Mackie S, Shaw P, Lenroot R, Pierson R, Greenstein DK, Nugent TF, III, Sharp WS, Giedd JN, Rapoport JL. Cerebellar development and clinical outcome in attention deficit hyperactivity disorder. American Journal of Psychiatry. 2007;164:647–655. doi: 10.1176/ajp.2007.164.4.647. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreason NC, O’Leary DS, Yuh WTC, Heckel D. Structural MR image processing using BRAINS2 toolbox. Computerized Medical Imaging and Graphics. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Martin-Soelch C, Chevalley AF, Kunig G, Missimer J, Magyar S, Mino A, Scultz W, Leenders KL. Changes in reward-induced brain activation in opiate addicts. European Journal of Neuroscience. 2001;14:1360–1368. doi: 10.1046/j.0953-816x.2001.01753.x. [DOI] [PubMed] [Google Scholar]
- Matthews AG, Hoffman EK, Zezza N, Stiffler S, Hill SY. The role of the GABRA2 polymorphism in multiplex alcohol dependence families with minimal comorbidity: Within-family association and linkage analyses. Journal of Studies on Alcohol and Drugs. 2007;68:625–633. doi: 10.15288/jsad.2007.68.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Nagel BJ, Tapert SF. Abnormal cerebellar morphometry in abstinent adolescent marihuana users. Psychiatry Research: Neuroimaging. 2010;182:152–159. doi: 10.1016/j.pscychresns.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Office for National Statistics. Statistics on Smoking: England 2006. ONS; 2006. [Google Scholar]
- Palma E, Torchia G, Limatola C, Trettel F, Arcella A, Cantore G, DiGennaro G, Manfredi M, Esposito V, Quarato PP, Miledi R, Eusebi F. BDNF modulates GABAA receptors microtransplanted from the human epileptic brain to Xenopus oocytes. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1667–1672. doi: 10.1073/pnas.0409442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkins EJ. Cerebellum and cerebrum in adaptive control and cognition: a review. Biological Cybernetics. 1997;77:79–87. doi: 10.1007/s004220050369. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky RB, Lim KO. A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Archives of Neurology. 1994;51:874–887. doi: 10.1001/archneur.1994.00540210046012. [DOI] [PubMed] [Google Scholar]
- Pierson R, Carlson PW, Sears LL, Alicata D, Magnotta V, O’Leary D, Andreasen NC. Semiautomated measurement of cerebellar subregions on MR images. NeuroImage. 2002;17:61–76. doi: 10.1006/nimg.2002.1207. [DOI] [PubMed] [Google Scholar]
- Prasad KMR, Sahni SD, Rohm BR, Keshavan MS. Dorsolateral prefrontal cortex morphology and short-term outcome in first episode schizophrenia. Psychiatry Research Neuroimaging. 2005;140:147–155. doi: 10.1016/j.pscychresns.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Dahle C, Head D, Acker JD. Differential age-related changes in the regional metencephalic volumes in humans: a 5-year follow-up. Neuroscience Letters. 2003;349:163–166. doi: 10.1016/s0304-3940(03)00820-6. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. Longitudinal mapping of cortical thickness and brain growth in normal children. Journal of Neuroscience. 2004;38:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology. 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin P, Parks MH, Desmond JE, Chen SHA, Pryor MR, DeRosa E, Pfefferbaum A. Disruption of frontocerebellar circuitry and function in alcoholism. Alcoholism: Clinical and Experimental Research. 2003;27:301–309. doi: 10.1097/01.ALC.0000052584.05305.98. [DOI] [PubMed] [Google Scholar]
- Szeszko PR, Lipsky R, Mentschel C, Robinson D, Gunduz-Bruce H, Sevy S, Ashtari M, Napolitano B, Bilder RM, Goldman D, Malhotra AK. Brain-derived neurotrophic factor val66met polymorphism and volume of the hippocampal formation. Molecular Psychiatry. 2005;10:631–636. doi: 10.1038/sj.mp.4001656. [DOI] [PubMed] [Google Scholar]
- Takayama C, Inoue Y. GABAeric signaling in the developing cerebellum. Anatomical Science International. 2004;79:124–136. doi: 10.1111/j.1447-073x.2004.00081.x. [DOI] [PubMed] [Google Scholar]
- Tessner K, Hill SY. Neural circuitry associated with risk for alcohol dependence. Neuropsychology Review. 2010;20:1–20. doi: 10.1007/s11065-009-9111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thach WT. On the specific role of the cerebellum in motor learning and cognition: clues from PET activation and lesion studies in humans. Behavioral and Brain Sciences. 1996;19:411–431. [Google Scholar]
- Tiemeier H, Lenroot RK, Greenstein DK, Tran L, Pierson R, Giedd JN. Cerebellum development during childhood and adolescence: a longitudinal morphometric MRI study. NeuroImage. 2010;49:63–70. doi: 10.1016/j.neuroimage.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BM, Paradiso S, Marvel C, Pierson R, Ponto LLB, Hichwa RD, Robinson RG. The cerebellum and emotional experience. Neuropsychologia. 2007;45:1331–1341. doi: 10.1016/j.neuropsychologia.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatasubramanian G, Antony G, Reddy U, Reddy V, Jayakumar PN, Benegal V. Corpus callosum abnormalities associated with greater externalizing behaviors in subjects at high risk for alcohol dependence. Psychiatry Research: Neuroimaging. 2007;156:209–215. doi: 10.1016/j.pscychresns.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Webster MJ, Weicker CS, Herman MM, Kleinman JE. BDNF mRNA expression during postnatal development, maturation and aging of the human prefrontal cortex. Developmental Brain Research. 2002;139:139–150. doi: 10.1016/s0165-3806(02)00540-0. [DOI] [PubMed] [Google Scholar]
- Yurgelun-Todd D. Emotional and cognitive changes during adolescence. Current Opinion in Neurobiology. 2007;17:251–257. doi: 10.1016/j.conb.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Zahr NM, Pitel AL, Chanraud S, Sullivan EV. Contributions of studies on alcohol use disorders to understanding cerebellar function. Neuropsychology Review. 2010;20:280–289. doi: 10.1007/s11065-010-9141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]