Abstract
The biomechanical characteristics of stiff knee gait following neurological injury include decreased knee flexion velocity at toe-off, which may be due to exaggerated quadriceps activity. The neuromuscular mechanism underlying this abnormal activity is unclear, although hyperexcitable heteronymous reflexes may be a source of impaired coordination. The present study examines the contribution of reflex activity from hip flexors on knee extensors following stroke and its association with reduced swing-phase knee flexion during walking. Twelve individuals poststroke and six control subjects were positioned in supine on a Biodex dynamometer with the ankle and knee held in a static position. Isolated hip extension movements were imposed at 60, 90, and 120°/s through a 50° excursion to end-range hip extension. Reflexive responses of the rectus femoris (RF), vastus lateralis (VL), and vastus medialis (VM) were quantified during and after the imposed hip rotation. Gait analysis was also performed for all subjects in the stroke group. In subjects with stroke, imposed hip extension evoked a brief reflexive response in the quadriceps, followed by a heightened level of sustained activity. The initial response was velocity dependent and was larger in the stroke group than in the control group. In contrast, the prolonged response was not velocity dependent, was significantly greater in the VL and RF in subjects with stroke, and, importantly, was correlated to decreased swing-phase knee flexion. Hyperexcitable heteronymous connections from hip flexors to knee extensors appear to elicit prolonged quadriceps activity and may contribute to altered swing-phase knee kinematics following stroke.
INTRODUCTION
Following neurological injury, such as stroke, muscle activity patterns during walking are often compromised, resulting in decreased gait speed, postural instability, reduced energy efficiency, and kinematic abnormalities. Substantial efforts have thus been made to discern the biomechanical and neuromuscular characteristics of abnormal walking patterns after neurological injury. Biomechanically, “spastic paretic stiff-legged gait” has been attributed to decreased knee flexion velocity at toe-off (Anderson et al. 2004; Goldberg et al. 2003). Knee flexion velocity typically increases rapidly during the period of double support, suggesting that events occurring during this period may be particularly critical to achieving sufficient swing-phase knee flexion. Conversely, inappropriate vasti activity during the double support period is purported to be the most powerful antagonist to knee flexion velocity in people with neurologic injury (Goldberg et al. 2004); yet, despite the evidence linking inappropriate knee extensor activity with swing-phase knee kinematics (Kerrigan et al. 1991), the neuromuscular mechanism responsible for generating abnormal quadriceps activity is not well understood.
The double support period of the leg initially in stance includes peak hip extension, while the knee begins flexing in preparation for toe-off. Therefore one explanation for the presence of elevated inappropriate quadriceps activity after stroke is the misinterpretation at the spinal level of sensory (e.g., group I and group II afferents) information originating from the hip proprioceptors, resulting in the production of spastic knee extensor activity. Sensory information from hip proprioceptors is known to be important in modulating stepping patterns after neurological insult (Grillner and Rossignol 1978; Hiebert et al. 1996). The fact that peak hip extension occurs during double support suggests that the stretch of the hip flexors may contribute to the presence of inappropriate uniarticular vasti activity. Multijoint reflex responses involving excitation of knee extensors have been reported previously in people with stroke. For example, common peroneal stimulation at group I and group II strength elicits elevated early and late facilitation of quadriceps H-reflexes in patients with poststroke hemiplegia as compared with unimpaired subjects (Marque et al. 2001; Maupas et al. 2004). This divergent excitation to muscles crossing different joints may result from increased excitability of propriospinal neurons following stroke (Mazevet et al. 2003). Despite these findings, the functional consequence of exaggerated multijoint reflexes in the lower extremity following stroke is not well described.
In individuals with spinal cord injury (SCI), exaggerated multijoint, heteronymous reflex responses are well known to contribute to abnormal muscle activation patterns. Specifically, lengthening the hip flexor muscles by passive hip extension movements elicits long-lasting hip flexor activity, with concomitant knee and ankle extensor activity (Schmit and Benz 2002; Steldt and Schmit 2004), which is consistent with the clinical description of an extensor spasm (Benz et al. 2005; Kuhn 1950; Little et al. 1989). Such data are in direct contrast to the well-established role of hip position afferents for facilitating limb flexion during terminal stance phase of locomotion in spinal or decerebrate cats (Grillner and Rossignol 1978; Hiebert et al. 1996), or infants during treadmill stepping (Pang and Yang 2000). The combined findings suggest a reorganization of heteronymous reflex function after neurological injury, which may increase knee extensor activity in response to hip extension. During gait, this reorganization of multijoint reflex coupling between hip extension (as observed during terminal stance) and enhanced knee extensor activity may contribute to the commonly observed deficits in “stiff-legged gait.”
The presence of heteronymous stretch reflex activity from the hip flexors to knee extensors in subjects after stroke has not been demonstrated previously. Further, the contribution of such heteronymous stretch reflex activity to abnormal gait kinematics in subjects with neurological injury has not been established. The purpose of this study was to examine the effect of an isolated imposed hip extension movement on knee extensor muscle activity in individuals with stroke and the potential relation of these heteronymous reflexes to kinematic abnormalities during gait. We hypothesized that the presence of increased quadriceps activity during and/or after an imposed hip extension movement is correlated to the presence of stiff knee gait (i.e., reduced knee flexion in swing).
METHODS
Subjects
Twelve subjects (eight females, four males between 28 and 75 yr old, mean = 52, SD = 11 yr old) with chronic poststroke hemiparesis (>6 mo) were recruited for testing (Stroke group; see Table 1). Six of these subjects exhibited right-side hemiparesis. All stroke subjects demonstrated clinical symptoms consistent with an ischemic or hemorrhagic unilateral brain lesion resulting in sensory motor dysfunction ≥6 mo before testing. Subjects were excluded if they had a history of severe osteoporosis, cardiorespiratory diseases (e.g., cardiac arrhythmia, uncontrolled hypertension, chronic emphysema), unhealed decubiti, or significant residual cognitive or communication deficits that could impede the understanding of the purpose and procedures of the study. The individuals with stroke exhibited a variety of walking patterns yielding swing-phase knee flexion values that ranged from nearly “normal” to clinical stiff-knee gait. A group of six unimpaired control subjects (two females, four males; age = 39 ± 10 yr old) underwent identical reflex testing (Control group). All subjects were informed of the purpose and procedures of the study and signed informed consent forms approved by the Institutional Review Boards of Northwestern University before testing.
TABLE 1.
Demographics of subjects in stroke group
| Subject | Age | Lesion Location | Time Since Stroke, mo | Berg Balance Score | Comfortable Gait Speed, m/s |
|---|---|---|---|---|---|
| S1 | 52 | Right hemisphere | 228 | 53 | 0.55 |
| S2 | 75 | Left hemisphere | 54 | 45 | 0.59 |
| S3 | 40 | Right hemisphere | 89 | 53 | 0.70 |
| S4 | 57 | Left hemisphere | 27 | 45 | 0.64 |
| S5 | 56 | Right hemisphere, hemorrhagic | 119 | 55 | 1.18 |
| S6 | 57 | Left basal ganglia | 46 | 52 | 0.30 |
| S7 | 53 | Left internal carotid, ischemic | 235 | 53 | 0.75 |
| S8 | 48 | Right MCA, ischemic | 47 | 52 | 0.49 |
| S9 | 50 | Left posterior insula and putamon, ischemic | 22 | 47 | 0.27 |
| S10 | 51 | Left corona radiate and posterior limb of the internal capsule | 28 | 54 | 0.88 |
| S11 | 59 | Right hemisphere | 51 | 52 | 0.52 |
| S12 | 28 | Right hemisphere, hemorrhagic | 10 | 55 | 0.57 |
Reflex testing and analysis
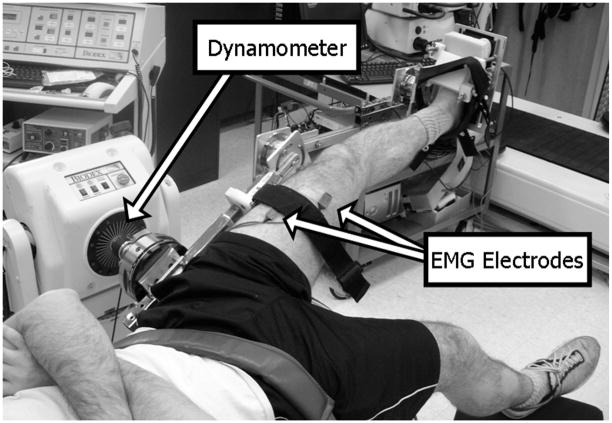
Detection of the presence of hyperexcitable heteronymous stretch reflexes from the hip flexors to the uniarticular knee extensors was accomplished by imposing isolated hip extension movements using a Biodex System 3 (Biodex Medical Equipment, Shirley, NY). Evaluation of the role of passive hip movement on uniarticular knee extensor excitability began with the subject positioned in supine and the pelvis secured firmly to the table with a strap placed across the iliac crests to prevent pelvic rotation. The knee and ankle were fixed in a static posture by strapping the hemiparetic limb securely within a custom-designed, modifiable full-leg brace (Fig. 1). The leg brace was fixed to the Biodex so that the motor’s axis of rotation was aligned with the flexion/extension axis of rotation of the hip. Proper alignment was verified visually by an absence of leg translation during manual movement of the leg brace, ensuring that observations of a muscle response about the knee were due solely to rotation at the hip.
FIG. 1.
Subject setup in supine. Hip is rotated into extension while the knee and ankle are held static.
The hip was rotated through a 50° excursion from flexion to extension, ending 1–2° from maximum hip extension. The end range of motion was determined by the point at which the subject reported a muscle stretch in the upper thigh or by the tester noting a firm/muscular end-feel. All subjects denied the presence of pain or discomfort with the hip extension movement. The average start position for the Stroke group was 36 ± 6° of hip flexion, whereas the Control group began at an average of 30 ± 6° of hip flexion. The tested knee of both groups was fixed in about 10° of flexion and the ankle was about 15° of plantarflexion. The knee was flexed to about 10° to approximately match the paretic knee’s position during the second double support period (before toe-off). Increasing knee flexion would also increase the relative tension on the RF, and perhaps not elicit a sufficient stretch of the uniarticular hip flexors (Van Dillen et al. 2000). The Biodex motor rotated the hip into extension at 60, 90, and 120°/s to simulate a range of peak angular velocities observed during normal gait (Granata et al. 2000). At the end of the imposed hip extension rotation, the limb was held in the extended position for 5 s before rotating back to the start (flexed) position. Electromyographic (EMG) measurement of rectus femoris (RF), vastus lateralis (VL), and vastus medialis (VM) muscle responses was recorded using active surface electrodes (DE 2.1, Delsys, Boston, MA).
Fifteen trials were repeated at each tested velocity (i.e., 60, 90, and 120°/s) with about 1-min rest between trials. During the first 5 trials of each tested velocity, subjects were asked to relax as much as possible during the imposed movement. The final 10 trials at each velocity were performed with the subjects preactivating the hip flexors (including the RF) at about 5–10% of their maximum voluntary isometric contraction (MVIC) (Crago et al. 1976). This preactivation level was maintained during the imposed hip extension movement to ensure an adequate stretch of the hip flexors, and subjects were instructed to relax several seconds after the end of the movement.
During all trials, the hip joint position, torque, and velocity were recorded from the Biodex. Simultaneous collection of EMG data occurred at 1,000 Hz, following amplification (×10K) and band-pass filtering between 20 and 450 Hz. Analysis of muscle activity was performed using custom-designed software (LabVIEW 7, National Instruments, Austin, TX). A linear envelope was created from the EMG data by full-wave rectifying the signals, and then filtering with a phase-corrected, eighth-order, low-pass Butterworth filter with a cutoff frequency of 10 Hz. The linear envelope was normalized to the peak recorded during the MVIC collected before performing the reflex testing. The MVIC was performed while the subjects were seated (i.e., hip at ~70 – 80° and the knee flexed to ~10°), to allow for comparison of RF, VL, and VM muscle activity between groups. The normalized EMG for each muscle was combined into an ensemble average for the 5 relaxed trials, as well as for the 10 preactivated trials, at each velocity. The individual muscle responses were compared between groups for each condition (i.e., relaxed and preactivated).
Gait testing and analysis
All subjects in the Stroke group returned on a different day to undergo gait analysis to determine lower extremity kinematics during overground walking. Subjects did not use an ankle foot orthosis (AFO) during the gait testing session. As subjects walked across a 10-m walkway, the motions of the lower extremity segments were tracked with a passive eight-camera motion capture system (Motion Analysis, Santa Rosa, CA) collecting at 120 Hz. Joint centers were located by 1 inch (25.4 mm), retro reflective markers affixed to the posterior sacrum and bilaterally over the anterior superior iliac spines (ASIS), medial and lateral femoral condyles, and medial and lateral malleoli. Rigid thermoplastic shells, each with three markers firmly affixed, were attached to the lateral aspect of the thigh and shank and covered with an elastic wrap to minimize movement between the shell and the bone to track the limb segments during walking. The foot was tracked by markers placed on the posterior heel counter of the shoe and over the second metatarsal head. Subjects walked at their self-selected velocity, but were asked to maintain a consistent gait velocity across trials. The marker trajectories were low-pass filtered at 6 Hz (EvaRT, Motion Analysis) to calculate the relative three-dimensional positions and intersegmental joint angles using a rigid body analysis and normalized to a stride cycle (OrthoTrak 6.2.4, Motion Analysis) (Grood and Suntay 1983).
Statistical analysis
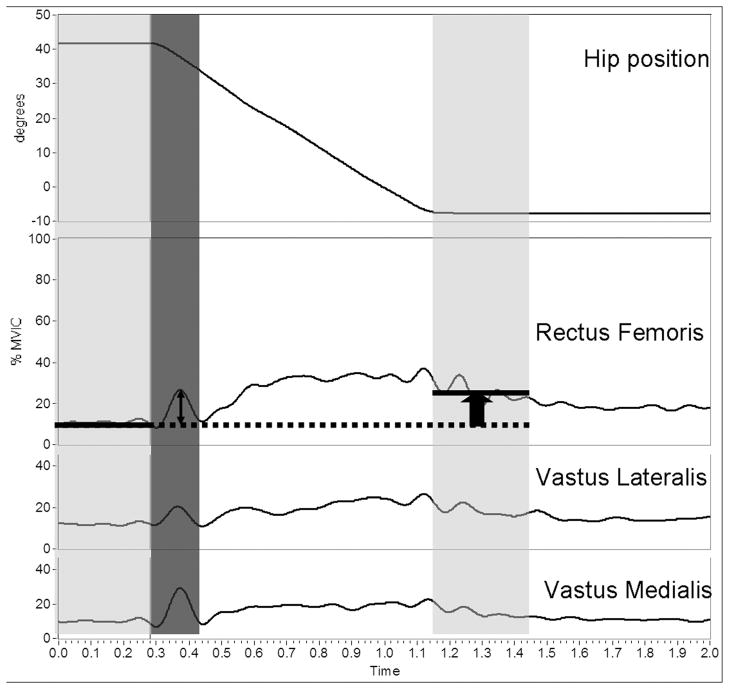
Statistical analyses were performed with SPSS (SPSS 10.0, Chicago, IL). Outcome variables from the imposed hip extension movement (Fig. 2) were calculated from the ensemble average of normalized EMG linear envelopes (Fig. 3) and expressed relative to a baseline, identified as the average muscle activity during the 250-ms period immediately before hip movement initiation. We were particularly interested in 1) the phasic reflex response, quantified as the initial peak reflexive response of the RF, VL, and VM muscles measured during the 150-ms period immediately after the initiation of limb movement; and 2) the tonic or prolonged response, calculated as the difference in EMG magnitude between the baseline average (250 ms before hip movement) and the final position average in muscle activity (identified as the 250 ms immediately after termination of hip extension). Analyses were performed during both relaxed and preactivated conditions. Group means and SDs of muscle activity were compared using a two-way (group by imposed hip velocity) repeated-measures ANOVA (repeated for imposed hip velocity). If differences were found to be significant, Bonferroni-corrected post hoc testing was performed. Gait kinematic variables included midstance peak hip extension and swing-phase peak knee flexion angles, as well as the peak hip extension velocity and the knee flexion velocity at toe-off. Paired t-tests were used to compare kinematic variables between limbs. Finally, Pearson product moment correlations were used to relate peak initial and prolonged reflex responses to gait kinematics (i.e., peak knee flexion during swing and knee flexion velocity at toe-off). All statistical tests were two sided and a significance level of P < 0.05 was used.
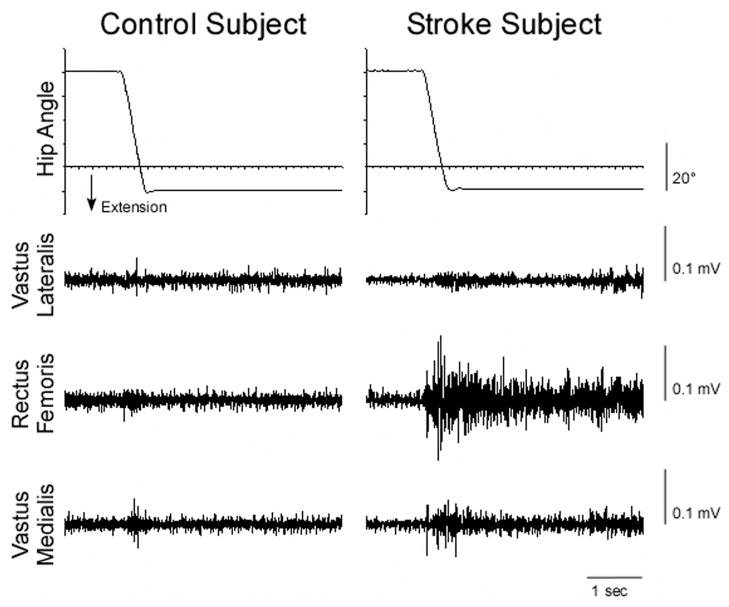
FIG. 2.
Example of muscle responses for a Control (left) and Stroke subject (right) during a “preactivated” trial.
FIG. 3.
Variables obtained during reflex trials. Top trace: hip extension movement. Bottom 3 traces: ensemble average of the linear envelope of rectus femoris (RF), vastus lateralis (VL), and vastus medialis (VM). Peak response is chosen within 150 ms of the initiation of movement (dark gray). Prolonged response is the difference in averaged activity from pre- to postmovement (light gray).
RESULTS
Reflex responses
Following the initiation of the imposed hip movement, the stretched RF muscle as well as the unstretched VL and VM exhibited a brief reflexive response followed by a sustained EMG signal that was particularly evident in the Stroke group, as demonstrated in Fig. 3. The quadriceps response to the hip extension movement was generally larger in the Stroke group than in the Control group during both the relaxed and preactivated conditions. In addition, the quadriceps reflex responses were generally larger during the preactivated trials compared with the relaxed trials.
Relaxed trials
During the relaxed trials, small-amplitude reflex responses were observed in both Stroke and Control groups. An analysis of the initial peak reflexive response revealed a significant main effect for imposed velocity for the VL (ANOVA: P < 0.005); however, a significant velocity × group interaction (ANOVA: P < 0.05) suggested that the groups did not respond similarly. Post hoc testing related to VL muscle activity demonstrated that the Stroke group exhibited a significantly greater peak than the Control group at 90°/s (P < 0.05) and 120°/s (P < 0.01), although no difference was observed between the groups at 60°/s (P = 0.56). Despite nonsignificant main effects for group for the peak responses of the RF (ANOVA: P = 0.06) and VM (ANOVA: P = 0.30) (Fig. 4A), the Stroke group exhibited larger responses on average with group differences ranging from 0.9 to 5.1%MVIC for the RF, and 0.1 to 4.0%MVIC for the VM, depending on the test velocity.
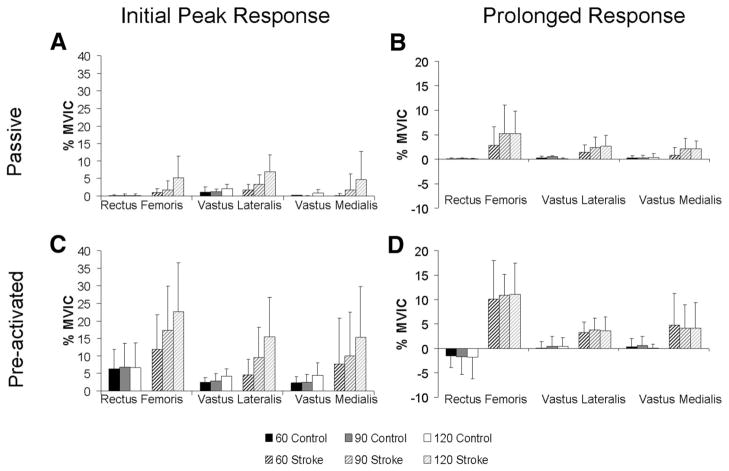
FIG. 4.
Group means and SDs for the initial peak reflexive response for (A) relaxed and (C) preactivated trials for the Control and Stroke groups at 60, 90, and 120°/s. Prolonged response followed the initial peak response. Group means and SDs for the prolonged response for the (B) relaxed and (D) preactivated trials for the Control and Stroke groups at 60, 90, and 120°/s.
In addition to the observed initial reflexive responses, prolonged muscle activity was also seen in the tested muscles. In general, the magnitude of reflex EMG activity in the Stroke group remained elevated after the imposed movement, whereas the Control group’s muscle activity did not. Specifically, the VL exhibited a significant main effect for group (ANOVA: P < 0.05), revealing greater prolonged VL activity in the Stroke group compared with the Control group (Fig. 4B). Likewise, the RF exhibited a significant main effect for group (ANOVA: P < 0.05) and imposed hip velocity (ANOVA: P < 0.05), as well as a significant group × velocity interaction (ANOVA: P < 0.05). Post hoc tests revealed that the RF of the Stroke group was significantly greater than the Control group at all tested velocities (60°/s: P < 0.05; 90°/s: P < 0.01; 120°/s: P < 0.005). No group differences were observed in the prolonged response of the VM muscle (ANOVA: P = 0.07), although the Stroke group exhibited a 0.6 –1.6%MVIC greater response than the Control group, depending on the imposed velocity.
Preactivated trials
Subjects preactivated the hip flexors before the imposed hip movement. No difference in preactivation level was observed between groups for the VL (P = 0.36), RF (P = 0.28), or VM muscles (P = 0.19). With the hip flexors preactivated to 5–10%MVIC, larger magnitude initial peak reflex responses were observed in the Stroke subject’s RF (P < 0.001), VL (P < 0.01), and VM (P < 0.01) muscles compared with the relaxed condition (Fig. 2). Additionally, the RF’s prolonged activity (P < 0.05) was significantly greater during the preactivated trials compared with the relaxed condition, although the VL (P = 0.07) and VM (P = 0.15) did not significantly increase with the hip flexors preactivated.
The magnitude of the Stroke group’s initial peak reflex response was generally observed to be larger than that of the Control group. Overall, there was a significant velocity × group interaction effect for the VL (ANOVA: P < 0.05) and RF (ANOVA: P < 0.05) muscles, suggesting that the Stroke and Control groups responded to the imposed hip perturbation at different velocities in a different manner (Fig. 4C). Specifically, the Stroke group’s initial reflex response was strongly velocity dependent for the VL (ANOVA: P = 0.001), RF (ANOVA: P < 0.001), and VM (ANOVA: P < 0.001) muscles, whereas the Control group exhibited a velocity-dependent response for the VL only (ANOVA: P < 0.005). As such, the difference in the response magnitude between groups was accentuated at greater imposed velocities. At the two slowest test velocities (60 and 90°/s), no significant differences were observed between the groups for any recorded muscles (group differences ranged from 2.1 to 10.7%MVIC). However, for the fastest velocity (120°/s), all tested muscles (VL: P < 0.01; RF: P < 0.05; VM: P < 0.05) exhibited a significantly greater initial response in the Stroke group compared with the Control group.
Similar to the relaxed condition, a prolonged facilitation in muscle activity was observed in the Stroke group following hip extension with the hip flexors preactivated. A significant main effect for group was observed for the VL (ANOVA: P = 0.005) and RF (ANOVA: P < 0.001) muscles, revealing that the long-lasting EMG activity observed in the Stroke group was significantly greater than that of the Control group for these muscles. Although no group difference was observed for the VM (ANOVA: P = 0.09) muscle, the trend was similar to the responses of the VL and RF (Fig. 4D). The imposed test velocity had no significant effect on the magnitude of the prolonged reflex response for any of the tested muscles (all P values > 0.50).
Gait kinematics
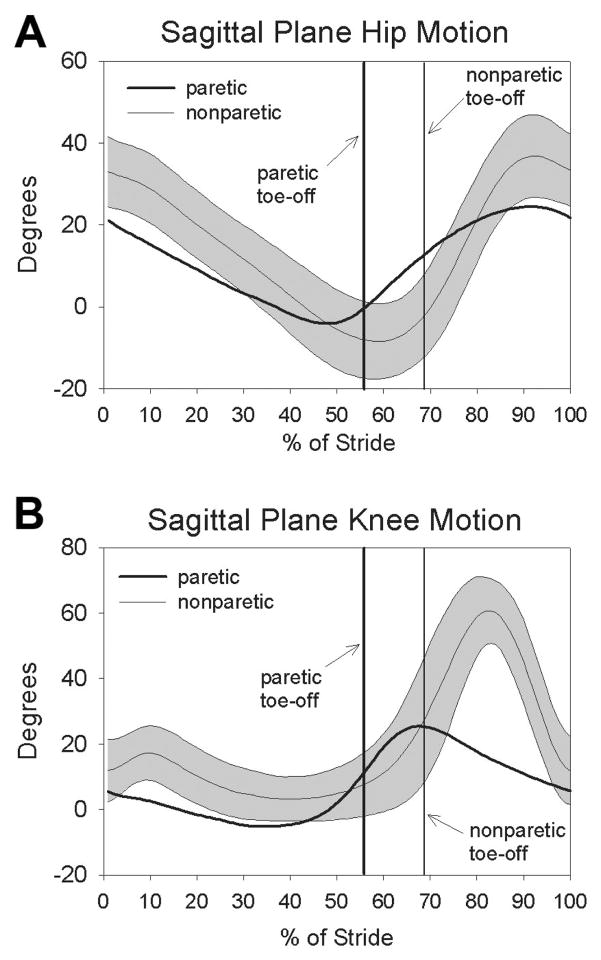
The subjects with stroke walked at an average of 0.6 ± 0.3m/s. During overground gait, differences in peak stance-phase hip extension between paretic (−8.4 ± 9.1°) and non-paretic limbs (−11.4 ± 8.2°) was not significant (P = 0.19) (Fig. 5A). In addition, no difference was observed between the paretic limb’s peak hip extension (−8.4 ± 9.1°) during walking and the hip extension imposed by the Biodex motor during reflex testing (−13.6 ± 6.1°; P = 0.18). Angular hip extension velocity, however, was slower on the paretic (49 ± 21°/s) versus nonparetic side (73 ± 29°/s; P < 0.05) during walking. During swing, the subjects flexed the paretic knee to 28 ± 15°, which was significantly less than the nonparetic peak knee flexion of 64 ± 8° (P < 0.001) (Fig. 5B). The individuals with stroke also flexed their hip significantly less during swing phase on the paretic side (23 ± 9°) compared with the nonparetic side (36 ± 10°; P < 0.001). No significant relationship was observed between the peak hip flexion angle and the peak knee flexion angle during swing (R = 0.44; P = 0.15). Knee flexion velocity at toe-off was also significantly reduced on the paretic side (163 ± 92°/s) compared with the nonparetic side (259 ± 93°/s; P < 0.001). The knee flexion velocity at toe-off correlated significantly with the peak knee flexion angle during swing (R = 0.87, P < 0.001).
FIG. 5.
Sagittal plane hip (A) and knee (B) kinematics for the Stroke group’s paretic (thick line) and nonparetic sides (thin line ±1 SD).
Correlation of reflex measures and gait kinematics
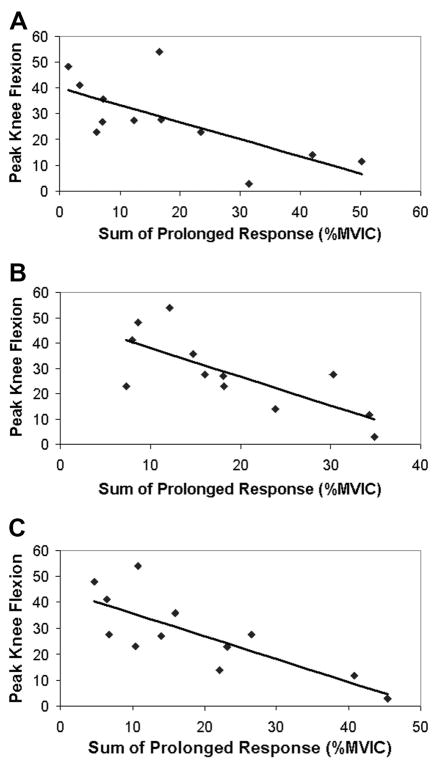
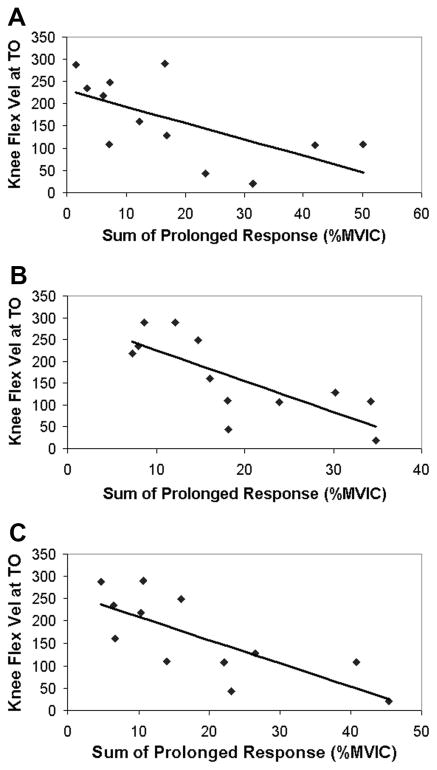
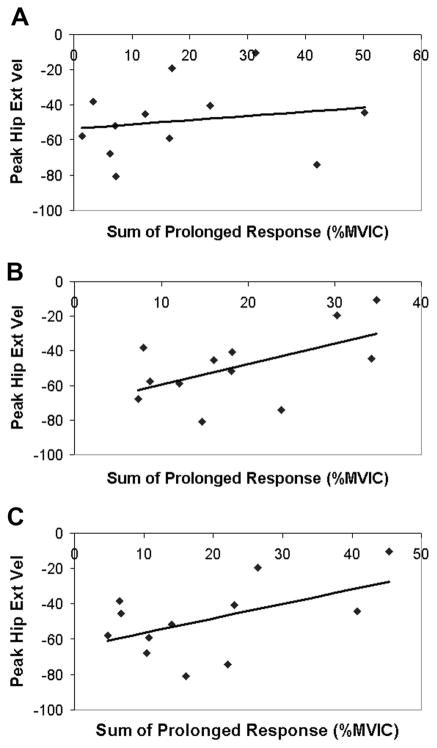
Paretic knee kinematics were correlated to the muscle responses observed after an isolated imposed hip extension movement. Because the individual muscle’s prolonged responses were correlated to each other (R values between 0.68 and 0.86), the sum of the prolonged responses of the quadriceps muscles (VL + RF + VM) was used as the independent variable for the correlation with kinematic measures. The sum of the prolonged responses of the preactivated VL, RF, and VM was significantly correlated to swing-phase peak knee flexion angle and the strength of the relationship improved with higher test speeds (60°/s: R = −0.70, P < 0.05; 90°/s: R = −0.75, P < 0.005; 120°/s: R = −0.79, P < 0.005) (Fig. 6). Likewise, the sum of the prolonged response of the preactivated VL, RF, and VM muscles was negatively related to knee flexion velocity at toe-off (60°/s: R = −0.63, P < 0.05; 90°/s: R = −0.76; P < 0.005; 120°/s: R = −0.75; P < 0.005) (Fig. 7). In addition, the sum of the prolonged response of the preactivated quadriceps muscles elicited at 90°/s was negatively related to the peak hip extension velocity (R = −0.56; P = 0.05) during walking, although the responses elicited by the 60°/s (R = −0.18; P = 0.57) and 120°/s (R = −0.52; P = 0.08) hip movements were not (Fig. 8). Although the prolonged response of the quadriceps during isolated testing was significantly related to the impaired limb’s movement pattern during walking, the initial peak reflexive response was not related to the peak knee flexion angle at any tested velocity (all P values >0.20). In addition, initial peak and prolonged reflex responses were not correlated to peak hip extension angle or gait speed.
FIG. 6.
Relationship between swing-phase peak knee flexion angle and prolonged response of the VL, RF, and VM muscles during “preactivated” trials at (A) 60°/s, (B) 90°/s, and (C) 120°/s.
FIG. 7.
Relationship between knee flexion velocity at toe-off and the prolonged response of the VL, RF, and VM muscles during the “preactivated” trials at (A) 60°/s, (B) 90°/s, and (C) 120°/s.
FIG. 8.
Relationship between peak hip extension velocity (deg/seg) and prolonged response of the VL, RF, and VM muscles during “preactivated” trials at (A) 60°/s, (B) 90°/s, and (C) 120°/s.
DISCUSSION
These data demonstrate that an imposed hip extension movement contributes to heightened, long-lasting quadriceps muscle activity in individuals with stroke that is correlated to knee kinematics during walking. These results provide insight into the potential mechanisms accounting for “spastic stiff-legged gait,” including the possible role of heteronymous reflexes from hip flexors onto knee extensors.
Role of heteronymous reflexes in spastic gait
The heightened response of the uniarticular knee extensor muscles (i.e., VL and VM) to an imposed hip movement suggests the presence of multijoint, heteronymous excitatory reflexes from the hip proprioceptors to the knee musculature. Given the RF’s anatomical position as a hip flexor and knee extensor, it is tempting to attribute the uniarticular vasti’s response to a stretch of the biarticular RF alone. In an analogous situation, however, group I afferents from the biarticular gastrocnemius muscle do not facilitate the uniarticular soleus muscle (Mao et al. 1984). Additionally, hip position afferents from numerous hip muscles are known to mediate lower extremity responses (Kriellaars et al. 1994), providing substantial redundancy in the sensorimotor system, suggesting that hip afferents from muscles other than the RF contribute to the initiation of the vasti’s response. Descriptions of multijoint reflexes in animal models are pervasive in the literature (Nichols 1999) in addition to a recent report of a group II–mediated, excitatory connection between the ankle and knee musculature in unimpaired humans (Marchand-Pauvert et al. 2005). In comparison, individuals with stroke are well known to exhibit elevated single-joint reflex responses (i.e., spasticity; Lance 1980) and, more recently, have revealed augmented heteronymous reflex responses between the ankle and knee (Marque et al. 2001; Maupas et al. 2004). Heteronymous reflex activity between the hip and knee joints, however, has not been described previously in unimpaired subjects or individuals with stroke, although individuals with SCI demonstrate analogous behaviors (Schmit and Benz 2002). The current results suggest similar, abnormal hip– knee reflex coupling between those with SCI and those with stroke, which may be related to spinal reflex reorganization after injury to the descending tracts.
Origin of enhanced multijoint reflexes in people with stroke
The multijoint reflexes observed in the current study were characterized by both an early velocity-mediated component and a prolonged component that was not velocity mediated and that persisted beyond the duration of the hip movement. The velocity dependence of the initial response is consistent with classically defined spasticity about a single joint (Lance 1980) and suggests a response to the hip flexor’s velocity-sensitive (group Ia) muscle spindle activity. However, both single-joint spasticity (Nadeau et al. 1999a) and the early velocity-mediated component of the heteronymous reflex responses described here have questionable roles in the performance of functional tasks (Patten et al. 2004), in particular, locomotion (however, see Damiano et al. 2006). In contrast to the early component, the prolonged reflex component was not velocity mediated and was highly correlated to knee kinematics in the Stroke group.
Although group I and group II hip afferents likely contribute to initiation of the early component of the reflex response, the precise neurophysiological mechanisms underlying the prolonged reflex responses to an imposed stretch are less clear. An increased reflex excitability of the group II pathways could have accounted for the larger prolonged reflex response in the Stroke group. Both test groups were exposed to a similar hip flexor perturbation and thus similar peripheral input (e.g., group II) during the static hold, although the groups responded differently to the imposed stretch of the hip muscles. Because peripheral inputs were assumed to be similar, differences in modulation at spinal/supraspinal levels likely contributed to differences in motor output. Although group II afferents likely contributed to the prolonged response, the long-lasting knee extensor activity in the individuals with Stroke may not be accounted for solely by group II afferent input. Although the excitatory drive from the group II afferents remains elevated during the hold portion, the discharge rate of the secondary endings diminishes during the static portion of a ramp-hold perturbation (Fischer and Schafer 2000), suggesting that other mechanisms may contribute to the long-lasting knee extensor activity as well.
Another explanation for the prolonged muscle activity following stretch is the presence of nonlinear, ionic currents in spinal interneurons and/or motoneurons, which can both amplify and prolong motor output after brief afferent inputs (Kiehn and Eken 1998). The persistent inward currents that underlie the long-lasting depolarization and discharge (i.e., plateau potentials) of these spinal neurons are often facilitated by descending serotonergic and noradrenergic inputs from brain stem (reticular) nuclei. The presence of prolonged motor outputs after a brief synaptic input has previously been observed in humans without neurological injury (Kiehn and Eken 1997) as well as in individuals with SCI (Gorassini et al. 2004; Hornby et al. 2003, 2006). Similar responses have likewise been observed in decerebrate animal preparations (Crone et al. 1988; Hounsgaard et al. 1988), which, much like a cortical stroke, have the potential for disinhibition of the brain stem. Such a disinhibition may facilitate modulation of α-motoneurons or low-threshold spinal interneurons (Hammar et al. 2004) to generate long-lasting motor activity indicative of underlying persistent inward currents. Further neurophysiological testing is certainly required to investigate the potential contributions of these currents to abnormal motor strategies after stroke.
Role of reflexive knee extensor activity in walking
The presence of exaggerated heteronymous reflex activity between hip flexors and knee extensors in individuals with stroke may have important functional consequences. In particular, the heightened quadriceps muscle responses that persist following hip extension were correlated with reduced knee flexion velocity at toe-off as well as the peak knee flexion angle attained during the swing phase of walking. Although this correlation does not imply “cause and effect,” it provides evidence that the quadriceps response to isolated hip extension appears to be related to knee kinematics during walking. The time spent in the double support period at the end of the paretic limb’s stance phase for the subjects in this study was 223 ± 86 ms. This corresponds well to the 250-ms time chosen to evaluate the prolonged reflex response to imposed hip movements and indicates that the muscle activity we observed in an isolated setting could have a direct impact on the specific phase of the gait cycle purported to be crucial to attaining sufficient knee flexion. A potential limitation, however, is that if there is a critical amount of hip extension required to elicit the quadriceps response and that threshold was achieved in supine, but not in walking, then the reflex would not contribute to decreased swing-phase knee flexion. Amounts of hip extension during walking and reflex testing, however, were not significantly different (P = 0.18), but additional work will need to be performed to establish whether such a critical amount of hip extension exists to trigger the quadriceps activity. It was also observed that the hip extension velocity during walking was inversely related to the magnitude of the quadriceps responses triggered by faster imposed hip movements. Peak hip extension occurs in late stance, during the double support phase of the leg initially in stance. Although the knee joint normally flexes during this period in unimpaired individuals, even small increases in vasti activity during double support can produce substantial reductions in knee flexion velocity, the marker for subsequent swing-phase knee flexion (Goldberg et al. 2004). A potential limitation of our data is that we did not record quadriceps EMG from the Stroke group during the walking analysis. Although this may potentially limit our interpretation of the data, there is ample evidence to demonstrate that the rectus femoris and the vasti are overactive during terminal stance/early swing (Kerrigan et al. 1991; Remy-Neris et al. 2003; Sung and Bang 2000; Waters et al. 1979). The results presented here suggest a possible neurophysiologic mechanism to explain this overactivity, although future research is certainly warranted to confirm this hypothesis. Additionally, it is certainly possible that other impairments in muscle function may also contribute to the observed reduction in swing-phase knee flexion.
Although overactivity of the quadriceps muscle is often assumed to be the cause of stiff-knee gait, the mechanical coupling between the joints of the leg suggests that alterations in muscle function at other joints in the lower extremity also influence knee flexion during swing. For instance, there has been evidence that reductions in either hip or/and ankle moments at toe-off limit subsequent swing-phase knee flexion (Goldberg et al. 2004; Kerrigan et al. 1998; Olney and Richards 1996; Piazza and Delp 1996). At the ankle, both overactivity of the soleus muscle (Neptune et al. 2001) or inadequate ankle power generation at toe-off (Kerrigan et al. 2001) are thought to lead to reduced swing-phase knee flexion. The contribution of these variables to the decreased swing-phase knee flexion observed in this study is not known. It is possible that these variables, in addition to the heightened reflex response of the quadriceps, substantially contributed to decreased swing-phase knee flexion.
Others have noted that strong hip flexion can adequately compensate for the diminished push-off at the ankle (Nadeau et al. 1999b), although reduced hip flexor moments have been tied to diminished swing-phase knee flexion (Olney and Richards 1996; Piazza and Delp 1996). It is possible, then, that decreased hip flexion was a factor in the decreased swing-phase knee flexion observed in the present study. Despite this prior evidence, peak hip flexion and peak knee flexion angles during swing were not related in our Stroke group. Clearly, however, there are many potential mechanisms, in addition to reflex coupling of the hip and knee reported here, that may contribute to the stiff-knee gait pattern often seen after stroke. Future studies should address the importance of each of these potential mechanisms and attempt to understand how these mechanisms may differ in subgroups of the poststroke population.
The observation that preactivation of the hip flexor muscles (with RF) facilitate the RF reflex response to imposed hip extension may also have implications for gait. During swing initiation, flexion of the leg is driven, in part, by activation of the hip muscles, which occurs near peak hip extension. Despite the RF’s anatomical position as a hip flexor, emerging evidence suggests that activation of the RF muscle will actually accelerate the hip into extension by dynamic coupling with the knee (Hernandez et al. 2007; Neptune et al. 2004). With respect to the hip extensor, we speculate that the presence of increased RF activity may actually contribute to a reduction in hip flexion around toe-off (Kerrigan et al. 2001). Additionally, the presence of RF activity during both terminal stance of walking (Waters et al. 1979) and during the preactivated condition of our reflex testing suggests that we are producing comparable behaviors. The fact that hip flexion (with activation of the RF) facilitates the inappropriate prolonged RF activity in an isolated setting may prompt individuals with stroke to unconsciously reduce the net hip flexor moment during walking. Such a strategy may be ineffective, however, in increasing knee flexion because a larger hip flexor moment is thought to be important for facilitating knee flexion during swing (Kerrigan et al. 1998). In addition, some individuals with stroke need to increase hip flexion moments to compensate for plantarflexor weakness (Nadeau et al. 1999b). Such an increase in hip flexor activation, however, appears to augment the reflex excitability of the RF under isolated settings. Inappropriate knee extensor activity, initiated by hip extension and facilitated by hip flexor activation before swing, may therefore provide a neurophysiological basis for the commonly observed kinematic walking impairments in subjects with stroke.
A potential limitation to the current results is that different head/trunk orientations were used for the acquisition of heteronymous reflexes (e.g., supine) and the gait analysis (e.g., upright). The vestibular system’s influence on spinal circuitry may therefore be altered, affecting both volitional (Lewek et al. 2006) and reflex activity (Rossi et al. 1988; Trimble 1998). Specifically, extensor circuits appear to be inhibited with the body in supine relative to an upright posture (Lewek et al. 2006; Rossi et al. 1988; Trimble 1998). During an upright task such as walking, the magnitude of the reflex response has the potential to be augmented beyond what was observed in supine. Other influences, such as a prolonged load through the limb, are also known to suppress extensor pathways in the lower leg (Trimble 1998), although load-release (e.g., toe-off) can further increase knee extensor activity (Wu and Schmit 2006). Thus any response observed in a supine, unloaded condition appears to convey an underestimation of extensor reflex activity compared with what might be observed during gait. Nevertheless, because all subjects in both groups experienced the same postures, the strength of the relationship between the observed reflex response and kinematics during gait should remain fundamentally unaffected.
The current data suggest an important reorganization of the reflex regulation that may influence gait after stroke. Although others have emphasized the importance of attaining hip extension during terminal stance to maximize subsequent hip flexion after neurological injury (Behrman et al. 2005) our data suggest that concomitant knee extensor activity may be triggered as a result of a rapid hip extension movement.
Acknowledgments
We thank T. Hayes, J. Moore, and H. Roth for valuable assistance with data collection and subject recruitment and V. Mercer for critical review of this manuscript.
GRANTS
This work was supported by the American Heart Association, National Institute of Child Health and Human Development Grant 5T32HD-007418, and National Institute on Disability and Rehabilitation Research Grant H133G-040065.
References
- Anderson FC, Goldberg SR, Pandy MG, Delp SL. Contributions of muscle forces and toe-off kinematics to peak knee flexion during the swing phase of normal gait: an induced position analysis. J Biomech. 2004;37:731–737. doi: 10.1016/j.jbiomech.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Behrman AL, Lawless-Dixon AR, Davis SB, Bowden MG, Nair P, Phadke C, Hannold EM, Plummer P, Harkema SJ. Locomotor training progression and outcomes after incomplete spinal cord injury. Phys Ther. 2005;85:1356–1371. [PubMed] [Google Scholar]
- Benz EN, Hornby TG, Bode RK, Scheidt RA, Schmit BD. A physiologically based clinical measure for spastic reflexes in spinal cord injury. Arch Phys Med Rehabil. 2005;86:52–59. doi: 10.1016/j.apmr.2004.01.033. [DOI] [PubMed] [Google Scholar]
- Crago PE, Houk JC, Hasan Z. Regulatory actions of human stretch reflex. J Neurophysiol. 1976;39:925–935. doi: 10.1152/jn.1976.39.5.925. [DOI] [PubMed] [Google Scholar]
- Crone C, Hultborn H, Kiehn O, Mazieres L, Wigstrom H. Maintained changes in motoneuronal excitability by short-lasting synaptic inputs in the decerebrate cat. J Physiol. 1988;405:321–343. doi: 10.1113/jphysiol.1988.sp017335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damiano DL, Laws E, Carmines DV, Abel MF. Relationship of spasticity to knee angular velocity and motion during gait in cerebral palsy. Gait Posture. 2006;23:1–8. doi: 10.1016/j.gaitpost.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Anderson FC, Pandy MG, Delp SL. Muscles that influence knee flexion velocity in double support: implications for stiff-knee gait. J Biomech. 2004;37:1189–1196. doi: 10.1016/j.jbiomech.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Ounpuu S, Delp SL. The importance of swing-phase initial conditions in stiff-knee gait. J Biomech. 2003;36:1111–1116. doi: 10.1016/s0021-9290(03)00106-4. [DOI] [PubMed] [Google Scholar]
- Gorassini MA, Knash ME, Harvey PJ, Bennett DJ, Yang JF. Role of motoneurons in the generation of muscle spasms after spinal cord injury. Brain. 2004;127:2247–2258. doi: 10.1093/brain/awh243. [DOI] [PubMed] [Google Scholar]
- Granata KP, Abel MF, Damiano DL. Joint angular velocity in spastic gait and the influence of muscle-tendon lengthening. J Bone Joint Surg Am. 2000;82:174–186. doi: 10.2106/00004623-200002000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner S, Rossignol S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Res. 1978;146:269–277. doi: 10.1016/0006-8993(78)90973-3. [DOI] [PubMed] [Google Scholar]
- Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105:136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- Hammar I, Bannatyne BA, Maxwell DJ, Edgley SA, Jankowska E. The actions of monoamines and distribution of noradrenergic and serotoninergic contacts on different subpopulations of commissural interneurons in the cat spinal cord. Eur J Neurosci. 2004;19:1305–1316. doi: 10.1111/j.l460-9568.2004.03239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez A, Dhaher YY, Thelen DG. In vivo measurement of dynamic rectus femoris function at postures representative of early swing phase. J Biomech. doi: 10.1016/j.jbiomech.2007.07.011. http://doi:10.1016/j.jbiomech.2007.07.011. [DOI] [PMC free article] [PubMed]
- Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol. 1996;75:1126–1137. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Hornby TG, Kahn JH, Wu M, Schmit BD. Temporal facilitation of spastic stretch reflexes following human spinal cord injury. J Physiol. 2006;571:593–604. doi: 10.1113/jphysiol.2005.102046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornby TG, Rymer WZ, Benz EN, Schmit BD. Windup of flexion reflexes in chronic human spinal cord injury: a marker for neuronal plateau potentials? J Neurophysiol. 2003;89:416–426. doi: 10.1152/jn.00979.2001. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrigan DC, Gronley J, Perry J. Stiff-legged gait in spastic paresis. A study of quadriceps and hamstrings muscle activity. Am J Phys Med Rehabil. 1991;70:294–300. [PubMed] [Google Scholar]
- Kerrigan DC, Karvosky ME, Riley PO. Spastic paretic stiff-legged gait: joint kinetics. Am J Phys Med Rehabil. 2001;80:244–249. doi: 10.1097/00002060-200104000-00002. [DOI] [PubMed] [Google Scholar]
- Kerrigan DC, Roth SR, Riley PO. The modelling of adult spastic paretic stiff-legged gait swing period based on actual kinematic data. Gait Posture. 1998;7:117–124. doi: 10.1016/s0966-6362(97)00040-4. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Prolonged firing in motor units: evidence of plateau potentials in human motoneurons? J Neurophysiol. 1997;78:3061–3068. doi: 10.1152/jn.1997.78.6.3061. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Eken T. Functional role of plateau potentials in vertebrate motor neurons. Curr Opin Neurobiol. 1998;8:746–752. doi: 10.1016/s0959-4388(98)80117-7. [DOI] [PubMed] [Google Scholar]
- Kriellaars DJ, Brownstone RM, Noga BR, Jordan LM. Mechanical entrainment of fictive locomotion in the decerebrate cat. J Neurophysiol. 1994;71:2074–2086. doi: 10.1152/jn.1994.71.6.2074. [DOI] [PubMed] [Google Scholar]
- Kuhn RA. Functional capacity of the isolated human spinal cord. Brain. 1950;73:1–51. doi: 10.1093/brain/73.1.1. [DOI] [PubMed] [Google Scholar]
- Lance JW. Symposium synopsis. In: Feldman RG, Young RR, Koella WP, editors. Spasticity: Disordered Motor Control. Chicago, IL: Year Book Publishers; 1980. pp. 485–494. [Google Scholar]
- Lewek MD, Schmit BD, Hornby TG, Dhaher YY. Hip joint position modulates volitional knee extensor muscle activity after stroke. Muscle Nerve. 2006;34:767–774. doi: 10.1002/mus.20663. [DOI] [PubMed] [Google Scholar]
- Little JW, Micklesen P, Umlauf R, Britell C. Lower extremity manifestations of spasticity in chronic spinal cord injury. Am J Phys Med Rehabil. 1989;68:32–36. doi: 10.1097/00002060-198902000-00009. [DOI] [PubMed] [Google Scholar]
- Mao CC, Ashby P, Wang M, McCrea D. Synaptic connections from large muscle afferents to the motoneurons of various leg muscles in man. Exp Brain Res. 1984;56:341–350. doi: 10.1007/BF00236290. [DOI] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Nicolas G, Marque P, Iglesias C, Pierrot-Deseilligny E. Increase in group II excitation from ankle muscles to thigh motoneurones during human standing. J Physiol. 2005;566:257–271. doi: 10.1113/jphysiol.2005.087817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marque P, Simonetta-Moreau M, Maupas E, Roques CF. Facilitation of transmission in heteronymous group II pathways in spastic hemiplegic patients. J Neurol Neurosurg Psychiatry. 2001;70:36–42. doi: 10.1136/jnnp.70.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maupas E, Marque P, Roques CF, Simonetta-Moreau M. Modulation of the transmission in group II heteronymous pathways by tizanidine in spastic hemiplegic patients. J Neurol Neurosurg Psychiatry. 2004;75:130–135. [PMC free article] [PubMed] [Google Scholar]
- Mazevet D, Meunier S, Pradat-Diehl P, Marchand-Pauvert V, Pierrot-Deseilligny E. Changes in propriospinally mediated excitation of upper limb motoneurons in stroke patients. Brain. 2003;126:988–1000. doi: 10.1093/brain/awg088. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Arsenault AB, Gravel D, Bourbonnais D. Analysis of the clinical factors determining natural and maximal gait speeds in adults with a stroke. Am J Phys Med Rehabil. 1999a;78:123–130. doi: 10.1097/00002060-199903000-00007. [DOI] [PubMed] [Google Scholar]
- Nadeau S, Gravel D, Arsenault AB, Bourbonnais D. Plantarflexor weakness as a limiting factor of gait speed in stroke subjects and the compensating role of hip flexors. Clin Biomech (Bristol, Avon) 1999b;14:125–135. doi: 10.1016/s0268-0033(98)00062-x. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Kautz SA, Zajac FE. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J Biomech. 2001;34:1387–1398. doi: 10.1016/s0021-9290(01)00105-1. [DOI] [PubMed] [Google Scholar]
- Neptune RR, Zajac FE, Kautz SA. Muscle force redistributes segmental power for body progression during walking. Gait Posture. 2004;19:194–205. doi: 10.1016/S0966-6362(03)00062-6. [DOI] [PubMed] [Google Scholar]
- Nichols TR. Receptor mechanisms underlying heterogenic reflexes among the triceps surae muscles of the cat. J Neurophysiol. 1999;81:467–478. doi: 10.1152/jn.1999.81.2.467. [DOI] [PubMed] [Google Scholar]
- Olney SJ, Richards CL. Hemiparetic gait following stroke. Part I. Characteristics. Gait Posture. 1996;4:136–148. [Google Scholar]
- Pang MY, Yang JF. The initiation of the swing phase in human infant stepping: importance of hip position and leg loading. J Physiol. 2000;528:389–404. doi: 10.1111/j.1469-7793.2000.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten C, Lexell J, Brown HE. Weakness and strength training in persons with poststroke hemiplegia: rationale, method, and efficacy. J Rehabil Res Dev. 2004;41:293–312. doi: 10.1682/jrrd.2004.03.0293. [DOI] [PubMed] [Google Scholar]
- Piazza SJ, Delp SL. The influence of muscles on knee flexion during the swing phase of gait. J Biomech. 1996;29:723–733. doi: 10.1016/0021-9290(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Remy-Neris O, Tiffreau V, Bouilland S, Bussel B. Intrathecal baclofen in subjects with spastic hemiplegia: assessment of the antispastic effect during gait. Arch Phys Med Rehabil. 2003;84:643–650. doi: 10.1016/s0003-9993(02)04906-7. [DOI] [PubMed] [Google Scholar]
- Rossi A, Mazzocchio R, Scarpini C. Changes in Ia reciprocal inhibition from the peroneal nerve to the soleus alpha-motoneurons with different static body positions in man. Neurosci Lett. 1988;84:283–286. doi: 10.1016/0304-3940(88)90521-6. [DOI] [PubMed] [Google Scholar]
- Schmit BD, Benz EN. Extensor reflexes in human spinal cord injury: activation by hip proprioceptors. Exp Brain Res. 2002;145:520–527. doi: 10.1007/s00221-002-1134-5. [DOI] [PubMed] [Google Scholar]
- Steldt RE, Schmit BD. Modulation of coordinated muscle activity during imposed sinusoidal hip movements in human spinal cord injury. J Neurophysiol. 2004;92:673–685. doi: 10.1152/jn.00677.2003. [DOI] [PubMed] [Google Scholar]
- Sung DH, Bang HJ. Motor branch block of the rectus femoris: its effectiveness in stiff-legged gait in spastic paresis. Arch Phys Med Rehabil. 2000;81:910–915. doi: 10.1053/apmr.2000.5615. [DOI] [PubMed] [Google Scholar]
- Trimble MH. Postural modulation of the segmental reflex: effect of body tilt and postural sway. Int J Neurosci. 1998;95:85–100. doi: 10.3109/00207459809000652. [DOI] [PubMed] [Google Scholar]
- Van Dillen LR, McDonnell MK, Fleming DA, Sahrmann SA. Effect of knee and hip position on hip extension range of motion in individuals with and without low back pain. J Orthop Sports Phys Ther. 2000;30:307–316. doi: 10.2519/jospt.2000.30.6.307. [DOI] [PubMed] [Google Scholar]
- Waters RL, Garland DE, Perry J, Habig T, Slabaugh P. Stiff-legged gait in hemiplegia: surgical correction. J Bone Joint Surg Am. 1979;61:927–933. [PubMed] [Google Scholar]
- Wu M, Schmit BD. Spastic reflexes triggered by ankle load release in human spinal cord injury. J Neurophysiol. 2006;96:2941–2950. doi: 10.1152/jn.00186.2006. [DOI] [PubMed] [Google Scholar]