Abstract
Aging is a physiological process with a progressive decline of adaptation and functional capacity of the body. Bile acids (BAs) have been recognized as signaling molecules regulating the homeostasis of glucose, lipid, and energy. The current study characterizes the age-related changes of individual BA concentrations by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) in serum and liver of male and female C57BL/6 mice from 3 to 27 months of age. Total BA concentrations in serum increased 340% from 3 to 27 months in female mice, whereas they remained relatively constant with age in male mice. During aging, male and female mice shared the following changes: (1) BA concentrations in liver remained relatively constant; (2) the proportions of beta-muricholic acid (βMCA) increased and deoxycholic acid (DCA) decreased between 3 and 27 months in serum and liver; and (3) total BAs in serum and liver became more hydrophilic between 3 and 27 months. In female mice, (1) the mRNAs of hepatic BA uptake transporters, the Na+/taurocholate cotransporting polypeptide (Ntcp) and the organic anion transporting polypeptide 1b2 (Oatp1b2), decreased after 12 months, and similar trends were observed for their proteins; (2) the mRNA of the rate-limiting enzyme for BA synthesis, cholesterol 7α-hydroxylase (Cyp7a1), increased from 3 to 9 months and remained high thereafter. However, in male mice, Ntcp, Oatp1b2, and Cyp7a1 mRNAs remained relatively constant with age. In summary, the current study shows gender-divergent profiles of BA concentrations and composition in serum and liver of mice during aging, which is likely due to the gender-divergent expression of BA transporters Ntcp and Oatp1b2 as well as the synthetic enzyme Cyp7a1.
Introduction
Aging has become one of the most important global issues, because the elderly population (with chronological age of 65 years and older) are increasing, and it is estimated they will reach 22% of the population in 2050. Elderly people have an increased incidence of various age-related diseases, including liver and gastrointestinal (GI) diseases. The prevalence of chronic liver disease increases in the elderly, such as alcoholic liver disease, non-alcoholic fatty liver disease, viral hepatitis C, as well as hepatocellular carcinoma [1]. In addition, the risk of stomach cancer increases with age, and more than 90% of colon cancers were found in people over 50-years of age.
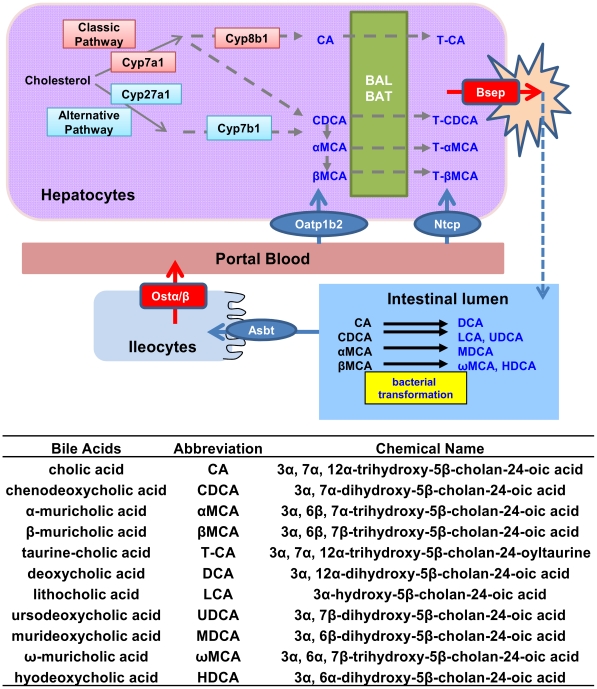
In the enterohepatic system, bile acids (BAs) play multifaceted physiological functions. Apart from their well-known roles for dietary lipid absorption and cholesterol homeostasis, BAs are increasingly appreciated as complex metabolic signaling molecules [2], regulating glucose, lipid, and energy metabolism. In humans, up to 95% of the BAs are efficiently recycled daily through the “enterohepatic circulation” (EHC) (Fig. 1), and only 5% are newly synthesized. Primary BAs are synthesized in the liver, namely cholic acid (CA) and chenodeoxycholic acid (CDCA) in humans. In rodents, CDCA can be hydroxylated into alpha-muricholic acid (αMCA), which is converted to beta-muricholic acid (βMCA) by 7-OH epimerization. In intestine, bacterial transformation of primary BAs occur to synthesize secondary BAs. CA is converted to its secondary BA deoxycholic acid (DCA), CDCA to lithocholic acid (LCA) and ursodeoxycholic acid (UDCA), αMCA to murideoxycholic acid (MDCA), and βMCA to ω-muricholic acid (ωMCA) and hyodeoxycholic acid (HDCA) [3], [4].
Figure 1. Scheme of the enterohepatic circulation (EHC) of bile acids (BAs) in mice.
Primary BAs are synthesized and conjugated in hepatocytes, and secreted into intestine to facilitate the fat digestion. Cyp7a1 is the rate-limiting enzyme for BA biosynthesis. Primary BAs are conjugated mainly with taurine in mice, by conjugating enzymes BAL and BAT. BAs are excreted into the canalicular bile by Bsep. In the intestinal lumen, BAs are transformed into secondary BAs by intestinal bacteria. BAs are efficiently reabsorbed by Asbt into ileocytes and effluxed into the portal blood by Ostα/β. Ntcp and Oatp1b2 take up BAs into hepatocytes. CA, CDCA, αMCA, and βMCA are the primary BAs in mice. DCA is the secondary BA of CA, LCA and UDCA are secondary BAs of CDCA, and ωMCA, MDCA, and HDCA are secondary BAs of α,βMCA.
Cholesterol 7α-hydroxylase (Cyp7a1) is the rate-limiting enzyme for BA biosynthesis in the liver [5]. Cyp8b1 catalyzes CA synthesis, and thus controls the ratio of CA to CDCA [6]. The alternative synthetic pathway of BA synthesis starts with side-chain oxidation by Cyp27a1 [7] and involves Cyp7b1 [8] to produce CDCA. BAs are conjugated with taurine or glycine by bile acid-CoA ligase (BAL) and bile acid-CoA∶amino acid N-acyltransferase (BAT) in liver. Mouse BAT is a taurine-specific conjugating enzyme [9]. BA transporters play important roles in promoting the EHC. In liver, conjugated BAs are taken up from the portal blood by the Na+/taurocholate cotransporting polypeptide (Ntcp) [10] and unconjugated BAs by basolateral transporters, such as the organic anion transporting polypeptide 1b2 (Oatp1b2) [11], and BAs and their conjugates are excreted into bile by the bile salt export pump (Bsep) [12]. In ileum, BAs are efficiently reabsorbed by the apical sodium-dependent bile acid transporter (Asbt) [13] and transported to the portal blood by the basolateral heterodimeric organic solute transporter alpha and beta (Ostα/β) [14].
BAs can regulate their own homeostasis [15], [16]. BAs activate their nuclear receptor farnesoid X receptor (FXR) in liver, which transactivates small heterodimer partner (SHP). SHP subsequently forms inactive heterodimer with liver related homologue-1 (LRH-1) [17], resulting in decreased transcription of Cyp7a1. In addition, BAs also activate FXR in the intestine, which induces fibroblast growth factor 15 (Fgf15), an intestinal hormone that travels through the circulation to the liver and down-regulates Cyp7a1 transcription [18], [19].
There is little known about BA homeostasis during aging. Limited reports about BA metabolism in aged humans or rats show inconsistent results. BAs are important signaling molecules, whose homeostasis if disrupted can lead to various age-related diseases, such as metabolic syndrome, cholestatic liver diseases, intestinal bacterial overgrowth and infection, and colorectal cancer. However, BAs are cytotoxic when present in abnormally high concentrations [15]. Therefore, altering BA concentration and composition might be a potential anti-aging intervention.
The current study aims to describe the effect of aging on BA composition and concentration, and determine the molecular mechanism with respect to the expression of genes involved in BA homeostasis in mice. To address these questions, both male and female mice at nine ages, from 3- to 27-months old, were used for serum and tissue collection for BA, mRNA, and protein quantification. Substantial information on the changes of BA concentrations and composition in male and female mice during aging was obtained, as well as possible regulatory mechanisms for these changes.
Materials and Methods
Ethics statement
Mice were housed according to guidelines of the Institutional Animal Care and Use Committee at the University of Kansas Medical Center, and procedures were carried out in compliance with standards for use of laboratory animals. Animal experiments performed in this study have been approved by the Institutional Animal Care and Use Committee at the University of Kansas Medical Center (protocol 2011-1969).
Chemicals and reagents
The sources of individual BA standards and internal standards are described previously by Zhang and Klaassen [16]. Rabbit anti-rat Ntcp antibody (K4), which has cross-reactivity with mouse Ntcp, was a generous gift from Bruno Steiger (University Hospital, Zurich, Switzerland). A polyclonal antibody to mouse Oatp1b2 was developed in our laboratory. β-Actin antibody (ab8227) was purchased from Abcam, Inc. (Cambridge, MA). Goat anti-rabbit IgG horseradish peroxidase-linked secondary antibody was purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals and reagents, unless indicated, were purchased from Sigma-Aldrich (St. Louis, MO).
Animals
Male and female C57BL/6 mice of various ages were purchased from the National Institute of Aging (Bethesda, MD) and acclimated for at least one month before tissue collections. Mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC)-accredited facility with a 14-h light/10-h dark-cycle, temperature-, and humidity-controlled environment and given ad libitum access to water and standard rodent chow (Harlan Teklad 8604; Harlan Teklad, Madison, WI). At 3, 6, 9, 12, 15, 18, 21, 24, and 27 months of age, mice (n = 5–7) were anesthetized with pentobarbital (50 mg/kg), and blood was collected from the suborbital vein. After cervical dislocation, liver and ileum (posterior one third of small intestine) were removed, snap-frozen in liquid nitrogen, and stored at −80°C. Tissue collections were between 9:00 and 12:00 in the morning, to decrease the variations due to circadian rhythm of BAs [20]. These studies were approved by the Institutional Animal Care and Use Committee at the University of Kansas Medical Center.
BA extraction from serum and liver
Internal standards (40 µg/ml d4-G-CDCA and 20 µg/ml d4-CDCA in MeOH) were added to the samples, and BAs were extracted from liver tissue using methods reported by Zhang and Klaassen [16]. For serum samples, methanol (MeOH) was added for protein precipitation. One ml of MeOH was added to 50 µl of serum spiked with 5 µl IS, vortexed, and centrifuged at 12,000 g for 10 min. The supernatant was aspirated, evaporated under vacuum, and reconstituted in 50 µl of 50% MeOH.
Quantification of BAs by ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS)
The conditions of liquid chromatography and mass spectrometry analysis were previously reported by Zhang and Klaassen [16]. Major individual BAs quantified were T-CA, T-CDCA, T-αMCA, T-βMCA, T-DCA, T-LCA, T-UDCA, T-MDCA, T-ωMCA, T-HDCA, CA, CDCA, αMCA, βMCA, DCA, LCA, UDCA, MDCA, ωMCA, and HDCA. The concentrations of individual BAs were summed to derive the concentrations of conjugated, unconjugated, and total BAs.
Total RNA Isolation
Total RNA was isolated from liver and ileum tissues using RNA Bee reagent (Tel-Test Inc., Friendswood, TX) following the manufacturer's protocol. The concentration of total RNA in each sample was quantified spectrophotometrically at 260 nm.
Multiplex Suspension Assay
The mRNA expression of genes of interest in liver was determined by Panomics 2.0 QuantiGene Plex technology (Panomics/Affymetrix Inc., Fremont, CA). Individual gene information can be found on Panomics Web site (http://www.panomics.com) with Panel numbers 21095, 21197, and 21151. Fluorescence was analyzed using a Bio-Plex 200 system array reader with Luminex 100 X-MAP technology, and data were acquired using Bio-Plex data manager software 5.0 (Bio-Rad, Hercules, CA). The mRNA of target genes were normalized to Gapdh.
Preparation of Crude Membrane Fractions
Livers were homogenized in ST buffer (0.25 M sucrose, 10 mM Tris•HCl, pH 7.4) containing protease inhibitors and centrifuged at 100,000 g for 60 min at 4°C. The membrane pellet was rinsed and resuspended with ST buffer with protease inhibitor. Protein concentrations were determined using Pierce protein assay reagents accordingly to the manufacturer's instructions (Pierce Biotechnology, Rockford, IL).
Western Blot Analysis
Western blots of Ntcp and Oatp1b2 were performed as previously described with minor modifications [21]. Primary antibodies were diluted in blocking buffer as follows: Ntcp (K4, 1∶2000) and Oatp1b2 (1∶1000). Membranes were stripped and reprobed with β-actin antibody (ab8227, 1∶10000) as the loading control. Intensities of protein bands were determined using the Image J software (National Institute of Health).
Statistical Analysis
Data are presented as mean ± SEM. Data were analyzed by one-way ANOVA, followed by Duncan's post-hoc test, differences being considered significant at p<0.05 (daggers † represent differences from 3 months of age in male mice, double daggers ‡ represent differences from 3 months of age in female mice). Asterisks (*) represent gender differences between male and female mice, determined by student t-test (p<0.05). Pound signs (#) indicate differences of BA proportions between 3 and 27 months.
Results
Total BA Concentrations in Serum during Aging
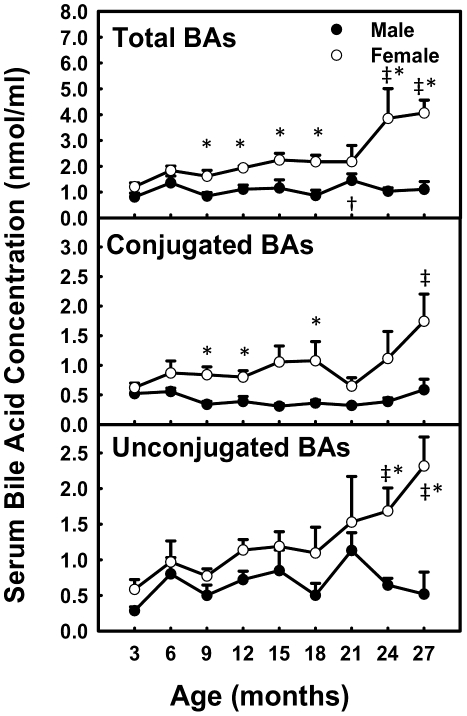
Total BA concentrations in serum remained relatively constant in male mice, whereas in female mice, they increased 340% from 3 to 27 months of age, due to an increase in both conjugated (280%) and unconjugated (400%) BAs (Fig. 2). Female mice had higher total BA concentrations than male mice from 9 (190%) to 27 (370%) months.
Figure 2. Total BA concentrations in serum during aging of male and female mice.
Data are presented as means ± SEM of 5–7 mice. Daggers (†) represent statistically significant difference from the value at 3 months of age during aging of male mice. Double daggers (‡) represent statistically significant difference from the value at 3 months of age during aging of female mice. Age-dependent differences were considered at p<0.05 by one-way ANOVA, followed by Duncan's post-hoc test. Asterisks (*) represent statistically significant difference between male and female mice at respective ages during aging (p<0.05), by student t-test.
Conjugated BAs in Serum during Aging
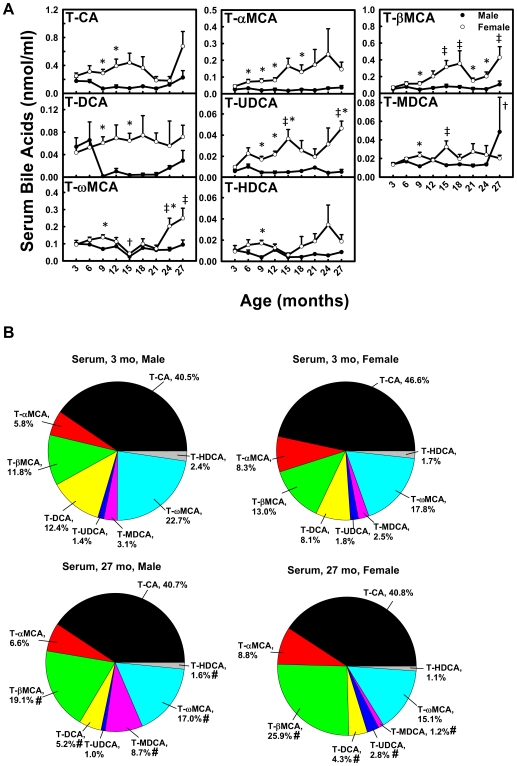
In male mice, the concentrations of most conjugated BAs (T-CA, T-αMCA, T-βMCA, T-UDCA, and T-HDCA) remained relatively constant during aging (Fig. 3A). In female mice, the concentrations of three BAs increased markedly between 3 and 27 months, namely T-βMCA (610%), T-UDCA (470%), and T-ωMCA (260%). However, the concentrations of T-CA, T-αMCA, T-DCA, T-MDCA, and T-HDCA remained relatively constant with age.
Figure 3. Concentrations of conjugated BAs in serum during aging of male and female mice.
(A) Concentrations of individual conjugated BAs in serum during aging and (B) proportions of them in all conjugated BAs in serum at 3 and 27 months of age in male and female mice. Data are presented as means ± SEM of 5–7 mice. Daggers (†) represent statistically significant difference from the value at 3 months of age during aging of male mice. Double daggers (‡) represent significant difference from the value at 3 months of age during aging of female mice. Age-dependent differences were considered at p<0.05 by one-way ANOVA, followed by Duncan's post-hoc test. Asterisks (*) represent statistically significant difference between male and female mice at respective ages during aging (p<0.05), by student t-test. In panel B, pound signs (#) represent differences of BA proportions between 3 and 27 months.
The composition of conjugated BAs in serum changed during aging (Fig. 3B). In male mice, the proportions of T-βMCA (11.8%→19.1%) and T-MDCA (3.1%→8.7%) increased, whereas T-DCA (12.4%→5.2%) and T-HDCA (2.4%→1.6%) decreased between 3 and 27 months of age. In female mice, the proportions of T-βMCA (13.0%→25.9%) and T-UDCA (1.8%→2.8%) increased, whereas T-MDCA (2.5%→1.2%) and T-DCA (8.1%→4.3%) decreased between 3 and 27 months.
Unconjugated BAs in Serum during Aging
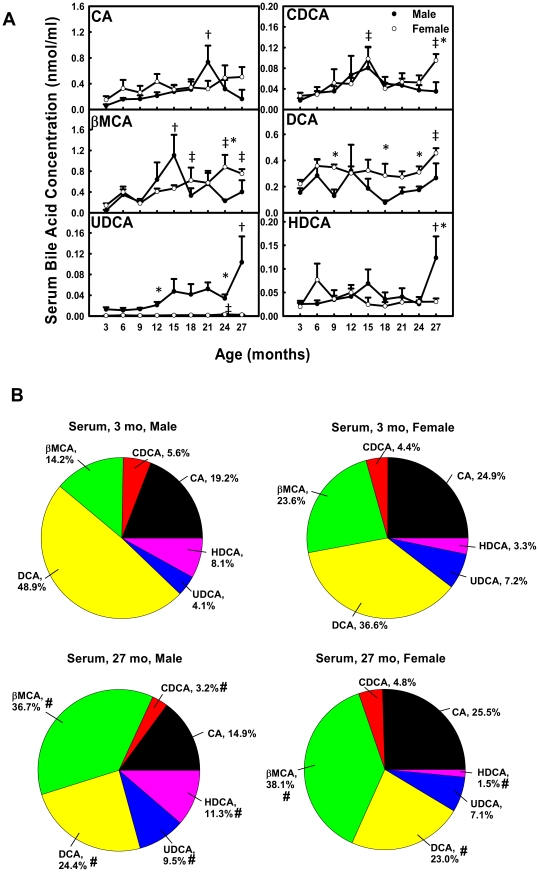
In male mice, the concentration of UDCA increased 800% from 3 to 27 months of age (Fig. 4A). CA increased (12.1-fold) from 3 to 21 months and decreased thereafter. Similarly, βMCA increased 24.6-fold from 3 to 15 months and decreased thereafter, and HDCA increased 480% from 3 to 27 months and decreased thereafter. CDCA and DCA remained relatively constant with age. In female mice, gradual age-dependent increase in concentrations of βMCA (530%) and DCA (210%) was observed between 3 and 27 months. CDCA was higher at 15 (370%) and 27 (360%) months than at 3 months of age. UDCA was 430% higher at 24 than 3 months. CA and HDCA remained relatively constant with age.
Figure 4. Concentrations of unconjugated BAs in serum during aging of male and female mice.
(A) Concentrations of individual unconjugated BAs in serum during aging and (B) proportions of them in all unconjugated BAs in serum at 3 and 27 months of age in male and female mice. Data are presented as means ± SEM of 5–7 mice. Daggers (†) represent statistically significant difference from the value at 3 months of age during aging of male mice. Double daggers (‡) represent significant difference from the value at 3 months of age during aging of female mice. Age-dependent differences were considered at p<0.05 by one-way ANOVA, followed by Duncan's post-hoc test. Asterisks (*) represent statistically significant difference between male and female mice at respective ages during aging (p<0.05), by student t-test. In panel B, pound signs (#) represent differences of BA proportions between 3 and 27 months.
The composition of unconjugated BAs in serum also changed during aging (Fig. 4B). In male mice, the proportions of βMCA (14.2%→36.7%), UDCA (4.1%→9.5%), and HDCA (8.1%→?>11.3%) increased, whereas CDCA (5.6%→3.2%) and DCA (48.9%→24.4%) decreased between 3 and 27 months of age. In female mice, the proportion of βMCA (23.6%→38.1%) increased, whereas DCA (36.6%→23.0%) and HDCA (3.3%→1.5%) decreased between 3 and 27 months.
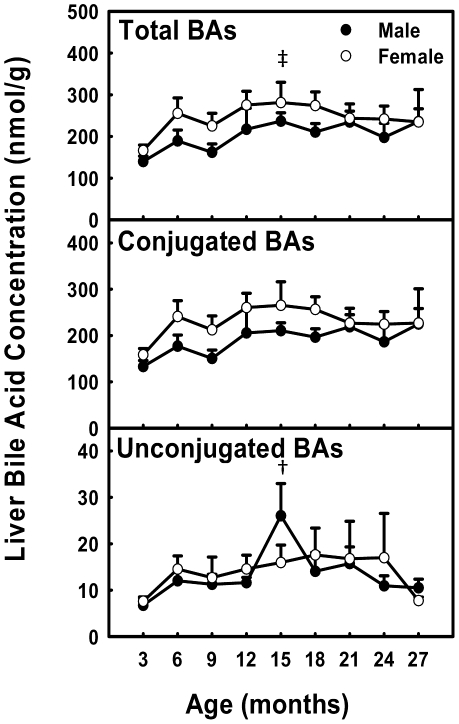
Total BA Concentrations in Liver during Aging
The concentrations of total, conjugated, and unconjugated BAs in liver remained relatively constant with age, except a small increase (170%) of total BAs at 15 months of age in female mice, and a small increase (390%) of unconjugated BAs at 15 months in male mice (Fig. 5).
Figure 5. Total BA concentrations in liver during aging of male and female mice.
Data are presented as means ± SEM of 5–7 mice. Daggers (†) represent statistically significant difference from the value at 3 months of age during aging of male mice. Double daggers (‡) represent significant difference from the value at 3 months of age during aging of female mice. Age-dependent differences were considered at p<0.05 by one-way ANOVA, followed by Duncan's post-hoc test. Asterisks (*) represent statistically significant difference between male and female mice at respective ages during aging (p<0.05), by student t-test.
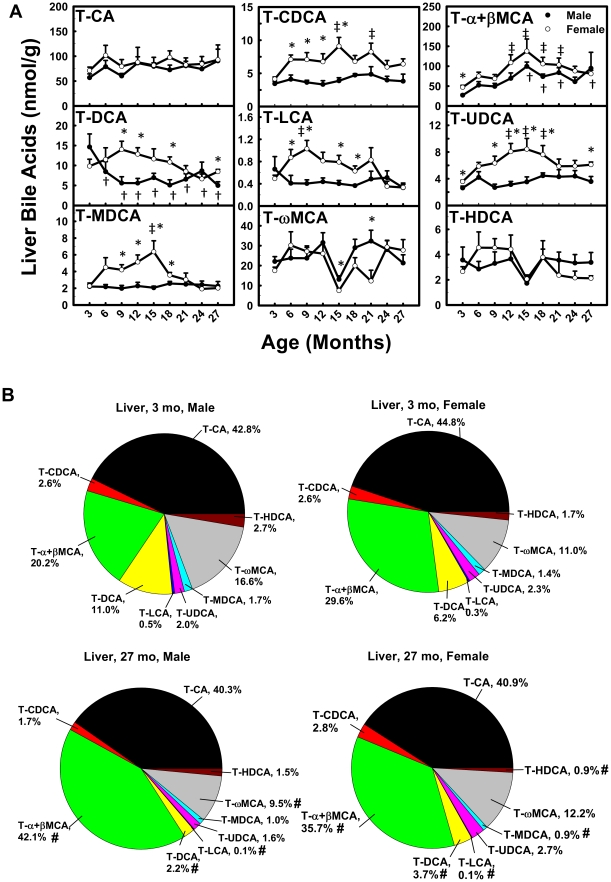
Conjugated BAs in Liver during Aging
In male mice, the concentration of T-α+βMCA gradually increased (350%), whereas T-DCA gradually decreased (65.8%) between 3 and 27 months of age (Fig. 6A). T-CA, T-CDCA, T-LCA, T-UDCA, T-MDCA, T-ωMCA, and T-HDCA remained relatively constant with age. In female mice, the concentrations of T-CDCA, T-α+βMCA, T-UDCA, and T-MDCA doubled between 3 and 15 months, and decreased thereafter. Similarly, T-LCA doubled between 3 and 9 months and decreased thereafter. T-CA, T-DCA, T-ωMCA, and T-HDCA remained relatively constant with age.
Figure 6. Concentrations of conjugated BAs in liver during aging of male and female mice.
(A) Concentrations of individual conjugated BAs in liver during aging and (B) proportions of them in all conjugated BAs in liver at 3 and 27 months of age in male and female mice. Data are presented as means ± SEM of 5–7 mice. Daggers (†) represent statistically significant difference from the value at 3 months of age during aging of male mice. Double daggers (‡) represent significant difference from the value at 3 months of age during aging of female mice. Age-dependent differences were considered at p<0.05 by one-way ANOVA, followed by Duncan's post-hoc test. Asterisks (*) represent statistically significant difference between male and female mice at respective ages during aging (p<0.05), by student t-test. In panel B, pound signs (#) represent differences of BA proportions between 3 and 27 months.
The composition of conjugated BAs in liver changed during aging (Fig. 6B). In male mice, the proportion of T-α+βMCA (20.2%→42.1%) increased, whereas T-DCA (11.0%→2.2%), T-LCA (0.5%→0.1%), and T-ωMCA (16.6%→9.5%) decreased between 3 and 27 months of age. In female mice, the proportion of T-α+βMCA (29.6%→35.7%) increased, whereas T-DCA (6.2%→3.7%), T-LCA (0.3%→0.1%), T-MDCA (1.4%→0.9%), and T-HDCA (1.7%→0.9%) decreased between 3 and 27 months.
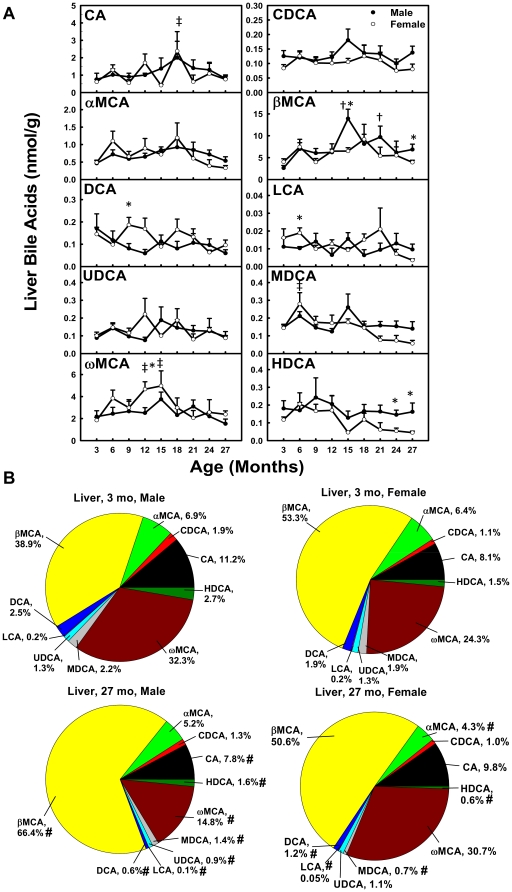
Unconjugated BAs in Liver during Aging
In male mice, The concentration of βMCA increased (530%) from 3 to 15 months and decreased 50.8% thereafter (Fig. 7A). In female mice, CA was 390% higher at 18 than 3 months, and ωMCA increased (170%) between 3 and 15 months and decreased 52.1% thereafter. CDCA, αMCA, DCA, LCA, UDCA, and HDCA remained relatively constant with age in both genders.
Figure 7. Concentrations of unconjugated BAs in liver during aging of male and female mice.
(A) Concentrations of individual unconjugated BAs in liver during aging and (B) proportions of them in all unconjugated BAs in liver at 3 and 27 months of age in male and female mice. Data are presented as means ± SEM of 5–7 mice. Daggers (†) represent statistically significant difference from the value at 3 months of age during aging of male mice. Double daggers (‡) represent significant difference from the value at 3 months of age during aging of female mice. Age-dependent differences were considered at p<0.05 by one-way ANOVA, followed by Duncan's post-hoc test. Asterisks (*) represent statistically significant difference between male and female mice at respective ages during aging (p<0.05), by student t-test. In panel B, pound signs (#) represent differences of BA proportions between 3 and 27 months.
The composition of unconjugated BAs in liver changed during aging (Fig. 7B). In male mice, the proportion of βMCA (38.9%→66.4%) increased, whereas CA (11.2%→7.8%), DCA (2.5%→0.6%), LCA (0.2%→0.1%), UDCA (1.3%→0.9%), MDCA (2.2%→1.4%), ωMCA (32.3%→14.8%), and HDCA (2.7%→1.6%) decreased between 3 and 27 months. In female mice, αMCA (6.4%→4.3%), DCA (1.9%→1.2%), LCA (0.2%→0.05%), MDCA (1.9%→0.7%), and HDCA (1.5%→0.6%) decreased between 3 and 27 months.
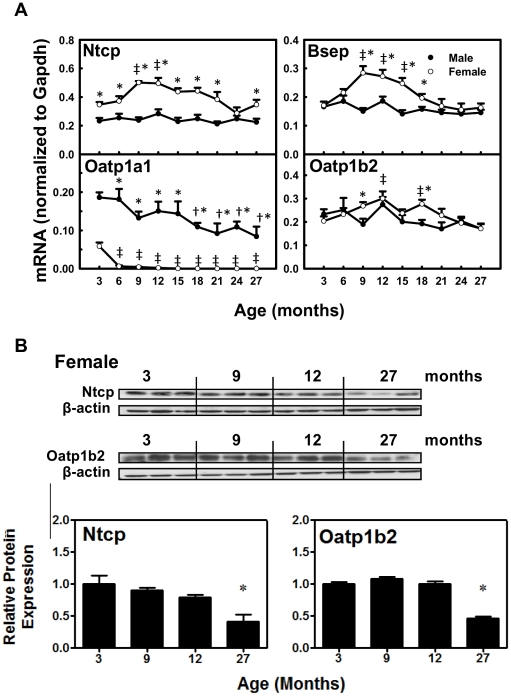
The mRNAs and Proteins of BA Hepatic Transporters during Aging
The mRNAs of BA uptake transporter Ntcp and efflux transporter Bsep remained relatively constant with age (Fig. 8A). Whereas they shared an age-dependent expression pattern in female mice, which was first an increase from 3 to 9 months (Ntcp: 140%; Bsep: 170%) and then a decrease from 9 to 27 months (Ntcp: 30.8%; Bsep: 42.8%). The mRNA of uptake transporter Oatp1b2 for unconjugated BAs remained constant with age in male mice, whereas in female mice, it increased 150% from 3 to 12 months and decreased 43% thereafter. The mRNA of male-predominantly expressed uptake transporter Oatp1a1 in male mice decreased gradually (54.9%) between 3 and 27 months. Interestingly, Oatp1a1 mRNA in female mice decreased markedly (89.4%) from 3 to 6 months and remained extremely low thereafter.
Figure 8. The mRNAs and proteins of BA transporters in livers during aging of male and female mice.
A. The mRNAs of Ntcp, Bsep, Otap1a1, and Oatp1b2 during aging in male and female livers. Data are presented as means ± SEM of 5–7 mice, by normalization to Gapdh mRNA. B. Protein abundance of Ntcp and Oatp1b2 in several representative ages in female livers. Western blots for Ntcp (∼50 kDa) and Oatp1b2 (∼75 kDa) were performed using liver membrane protein fractions (40 µg protein/lane) from livers of female mice at 3, 9, 12, and 27 months of age. β-Actin (∼45 kDa) was used as loading control for each transporter. Bars represent the relative protein expression ± SEM of 3 mice. Daggers (†) represent statistically significant difference from the value at 3 months of age during aging of male mice. Double daggers (‡) represent significant difference from the value at 3 months of age during aging of female mice. Age-dependent differences were considered at p<0.05 by one-way ANOVA, followed by Duncan's post-hoc test. Asterisks (*) represent statistically significant difference between male and female mice at respective ages during aging (p<0.05), by student t-test.
As shown in Fig. 8B, decreased proteins in female livers were observed by western blots at 27 months for Ntcp (59%) and Oatp1b2 (53.4%), confirming the decreased expression of these two uptake transporters at a later stage of life in female mice.
The mRNAs of BA Synthetic Enzymes in Liver during Aging
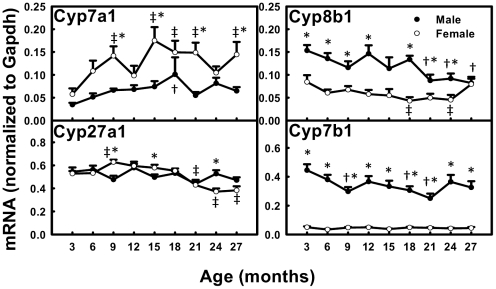
The mRNA of the rate-limiting enzyme Cyp7a1 for BA synthesis increased 290% from 3 to 18 months in male mice and tended to decrease thereafter, whereas in female mice, it increased 300% from 3 to 15 months and remained high thereafter (Fig. 9). Cyp8b1 decreased 46.5% between 3 and 27 months in male mice, whereas in female mice, it decreased 46.6% between 3 and 24 months, and then increased thereafter. Cyp27a1 remained relatively constant with age in male mice, whereas in female mice, it increased 120% from 3 to 9 months and then decreased 39.0% thereafter. Cyp7b1 decreased 43.8% between 3 and 21 months and tended to increase thereafter in male mice, whereas it was relatively constant with age in female mice.
Figure 9. The mRNAs of BA synthetic enzymes in livers during aging of male and female mice.
Data are presented as means ± SEM of 5–7 mice, by normalization to Gapdh mRNA. Cyp, cytochrome P450. Daggers (†) represent statistically significant difference from the value at 3 months of age during aging of male mice. Double daggers (‡) represent significant difference from the value at 3 months of age during aging of female mice. Age-dependent differences were considered at p<0.05 by one-way ANOVA, followed by Duncan's post-hoc test. Asterisks (*) represent statistically significant difference between male and female mice at respective ages during aging (p<0.05), by student t-test.
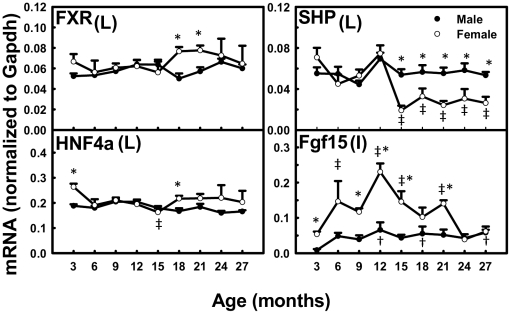
The mRNAs of Regulators of Cyp7a1 Transcription during Aging
In liver, the mRNA of FXR and HNF4α remained relatively constant with age in both genders (Fig. 10). SHP was relatively constant with age in male mice, whereas in female mice, it markedly decreased (74.1%) from 12 to 15 months and remained low thereafter. In ileum, the mRNA of Fgf15 gradually increased 670% between 3 and 27 months in male mice, whereas in female mice, it increased 430% between 3 and 12 months and decreased 73.3% thereafter.
Figure 10. The mRNAs of regulators of Cyp7a1 transcription during aging of male and female mice.
Figure shows mRNA data for FXR, SHP, and HNF4α in liver (L) and Fgf15 in ileum (I). Data are presented as means ± SEM of 5–7 mice, by normalization to Gapdh mRNA. Daggers (†) represent statistically significant difference from the value at 3 months of age during aging of male mice. Double daggers (‡) represent significant difference from the value at 3 months of age during aging of female mice. Age-dependent differences were considered at p<0.05 by one-way ANOVA, followed by Duncan's post-hoc test. Asterisks (*) represent statistically significant difference between male and female mice at respective ages during aging (p<0.05), by student t-test.
Discussion
The present study demonstrates a female-specific increase of total BA concentrations in serum during aging of mice. Both conjugated and unconjugated BAs in serum increase with age in female mice, whereas they remain relatively constant with age in male mice (Fig. 2). It has been reported that there were no age-related changes between young (8 weeks) and middle-aged (12 months) male rats in biliary BA secretion, distribution of BAs in the bile and intestine, nor the turnover frequency of BAs [22], and in addition, the biliary BA secretion in aged (106 weeks) male rats was similar to younger rats [23]. The finding of the present study that concentrations of total BAs in both serum and liver are similar in young and aged male mice is consistent with the previous reports in aged male rats. In humans, fasting concentrations of conjugated and unconjugated serum BAs were similar between 12 younger (mean age 37 years, range 22–59 years; seven female, five male) and 12 elder (mean age 67 years, range 60–82 years; seven female, five male) subjects [24]. This finding in humans is different from the present results in mice, possibly because 1) there are species differences in BA composition and BA conjugation between humans and mice, 2) the small number of subjects compromises the conclusion from the human study, and 3) possible changes of BAs in female subjects may be missed because male and female subjects were not analyzed separately in the human study.
Total BAs in serum and liver become more hydrophilic during aging in both male and female mice. Heuman [25] and Wang et al. [26] have reported the order of hydrophobicity indices of individual BAs as follows: T-ωMCA (∼−0.9)<T-αMCA (−0.84)<T-βMCA (−0.78)<T-MDCA (∼−0.6)<T-UDCA (−0.47)<T-HDCA (−0.35)<T-CA (0.00)<T-CDCA (+0.46)<T-DCA (+0.59)<T-LCA (+1.00), ωMCA (∼−0.77)<αMCA (∼−0.7)<βMCA (∼−0.65)<MDCA (∼−0.47)<UDCA (−0.31)<HDCA (∼−0.22)<CA (+0.13)<CDCA (+0.59)<DCA (+0.72)<LCA (+1.13). The hydrophobicity index (HI) of individual BAs and their proportions in biological samples are used to predict the HI of total BAs at physiological pH. Total BAs become more hydrophilic during aging, because the HI of total BAs decreases between 3 and 27 months in serum (M: −0.096→−0.24; F: −0.095→−0.20) and liver (M: −0.27→−0.42; F: −0.32→−0.38). The decrease is largely due to the increased proportion of hydrophilic βMCA and decreased proportion of hydrophobic DCA (Figs. 3B, 4B, 6B, and 7B).
The changes of individual BAs during aging in the present study provide important evidence that BAs may function as markers for longevity. A very intriguing finding in the long-lived lit/lit mice is that they had increased concentrations of several BAs in the serum, and feeding CA to wild-type mice reproduced the expression profiles of xenobiotic metabolism genes observed in the long-lived mice, which might increase resistance to stress and alleviate age-related tissue damage [27]. Females had a longer life expectancy than males in many species, including humans [28]. As to laboratory animals, female Wistar rats lived on average 14% longer than males, and female BALB/cJ mice lived much longer than males. The median life span of female mice in a heterogeneous background was shown to be longer than males at three research sites [29]. C57BL/6J mice are long-lived mice compared to other inbred strains, and females tended to live longer than males, but this was not statistically significant, possibly due to the small number of animals [30]. In the present study, serum concentrations of T-CA, T-αMCA, T-βMCA, T-DCA, and T-UDCA are higher in female C57BL/6 mice, and increase during aging in female mice (Fig. 3A). The long-lived lit/lit mice were shown to have increased βMCA, CDCA, LCA, DCA, CA, and UDCA in serum [27]. Therefore, increased serum concentrations of some individual BAs, such as CA, βMCA, DCA, and UDCA might correlate with the tendency of increased longevity in female C57BL/6 mice. UDCA is used clinically to treat gallstones. In cancer cells, UDCA induced senescence through increased histone hypoacetylation and inhibiting telomerase activity [31]. In the present study, T-UDCA in serum increases 470% between 3 and 27 months of age in female mice (Fig. 3A). UDCA in serum increases 800% between 3 and 27 months in male mice, and increases 430% between 3 and 24 months in female mice (Fig. 4A). The increased serum concentrations of UDCA during aging indicate that UDCA in serum may be an important marker of longevity.
Interesting findings from the present study show gender-divergent changes of expression of BA hepatic uptake and efflux transporters during aging of mice. In female mice, BA uptake transporters Ntcp and Oatp1b2 have decreased expression from about 12 to 27 months (Fig. 8A and 8B), and the BA efflux transporter Bsep has decreased expression from 9 to 27 months of age (Fig. 8A). In contrast, in male mice, the expression of Ntcp, Oatp1b2, and Bsep remains constant during aging. Female-predominant Ntcp expression in young adult mice is due to the inhibitory effect of male-pattern growth hormone secretion [32]. The lower sodium-dependent uptake of taurocholate in hepatocytes from female rats was partially due to female-specific lower expression of Ntcp [33]. The gender-divergent expression changes of Ntcp and Oatp1b2 during aging in the present study might be regulated by gender differences in growth hormone secretion patterns, or changes of female sex hormones with the cease of rodent estrous cycles. In contrast with the changes of BA uptake transporters, the mRNAs of BA-conjugating enzymes (BAL and BAT) and ileal BA transporters (Asbt and Ostα/β) remain relatively constant with age in both genders (data not shown). Decreased expression of uptake transporters Ntcp and Oatp1b2 likely contributes to the accumulation of BAs in serum with aging in female mice.
In addition to the decreased expression of BA uptake transporters, increased Cyp7a1 expression probably contributes to the increased concentrations of total BAs in serum during aging in female mice. The present study shows Cyp7a1 mRNA increases from 3 to 9 months of age, and remains at a high level thereafter in female mice, whereas in male mice, it remains constant during aging (Fig. 9). However, the present data are opposite to previous reports in human livers showing decreased cholesterol 7α-hydroxylation in the elderly [34], [35] and an inverse correlation between age and CYP7A1 mRNA levels [36] in both male and female subjects. There are probably species differences in BA changes during aging between human and mice, and the reliability of the findings in the human study is compromised by the low correlation coefficient (about −0.6) and the small number of subjects.
The present study characterizes the mRNA changes of several regulators for Cyp7a1 transcription during aging. SHP mRNA remains at low levels from 15 to 27 months compared to that at 3 months in female mice, and is inversely correlated with Cyp7a1 mRNA during aging in female mice (Fig. 10). This can be explained by the down-regulation of Cyp7a1 transcription by the FXR-SHP pathway. The Fgf15 signaling is also important for down-regulating Cyp7a1 transcription. Mice with liver-conditional knockout of HNF4α have reduced mRNA of Cyp7a1 [37], indicating that HNF4α is probably a positive regulator for Cyp7a1 transcription in mice. However, the mRNAs of neither Fgf15 nor HNF4α correlate with the elevated Cyp7a1 mRNA (Fig. 10). Therefore, in the present models, we conclude that Cyp7a1 transcription appears to be regulated by SHP inhibition during aging.
The current study provides a comprehensive description of the age-related changes of BA composition and concentration in serum and liver of male and female C57BL/6 mice from 3 to 27 months of age. The major findings are that (1) total BA concentrations in serum increase during aging in female mice, whereas they remain relatively constant in male mice; (2) livers maintain constant concentrations of BAs during aging in both genders; (3) total BAs in serum and liver become more hydrophilic during aging in both genders, largely due to the increased proportion of βMCA and decreased proportion of DCA between 3 and 27 months; (4) mRNAs of Ntcp and Oatp1b2 decrease from 9 to 27 months in female mice, whereas they remain relatively constant in male mice; (5) Cyp7a1 mRNA increases from 3 to 9 months and remains at high levels thereafter in female mice, which inversely correlates with SHP mRNA during aging. In male mice, however, Cyp7a1 mRNA remains constant with age. Therefore, the female-specific increased total BAs in serum during aging appear to result from a female-specific BA-related gene expression pattern, which is the decreased BA uptake transporters, Ntcp and Oatp1b2, and the increased rate-limiting enzyme for BA synthesis Cyp7a1.
Acknowledgments
The authors acknowledge Dr. Nancy Berman for help in obtaining mice for the study, Drs Youcai Zhang and Rachel Chennault for technical assistance, and all graduate students and fellows in Dr. Klaassen's lab for help in tissue collection and critical review of the manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by National Institutes of Health grants ES-009649, ES-019487, and DK-081461. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Frith J, Jones D, Newton JL. Chronic liver disease in an ageing population. Age Ageing. 2009;38:11–18. doi: 10.1093/ageing/afn242. [DOI] [PubMed] [Google Scholar]
- 2.Li T, Chiang JY. Bile Acid signaling in liver metabolism and diseases. J Lipids. 2012;2012:754067. doi: 10.1155/2012/754067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hofmann AF. The enterohepatic circulation of bile acids in mammals: form and functions. Front Biosci. 2009;14:2584–2598. doi: 10.2741/3399. [DOI] [PubMed] [Google Scholar]
- 4.Martin FP, Dumas ME, Wang Y, Legido-Quigley C, Yap IK, et al. A top-down systems biology view of microbiome-mammalian metabolic interactions in a mouse model. Mol Syst Biol. 2007;3:112. doi: 10.1038/msb4100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiang JY. Regulation of bile acid synthesis. Front Biosci. 1998;3:176. doi: 10.2741/a273. [DOI] [PubMed] [Google Scholar]
- 6.Chiang JY. Bile acid regulation of hepatic physiology: III. Bile acids and nuclear receptors. Am J Physiol Gastrointest Liver Physiol. 2003;284:G349–356. doi: 10.1152/ajpgi.00417.2002. [DOI] [PubMed] [Google Scholar]
- 7.Cali JJ, Russell DW. Characterization of human sterol 27-hydroxylase. A mitochondrial cytochrome P-450 that catalyzes multiple oxidation reaction in bile acid biosynthesis. J Biol Chem. 1991;266:7774–7778. [PubMed] [Google Scholar]
- 8.Li-Hawkins J, Lund EG, Turley SD, Russell DW. Disruption of the oxysterol 7alpha-hydroxylase gene in mice. J Biol Chem. 2000;275:16536–16542. doi: 10.1074/jbc.M001811200. [DOI] [PubMed] [Google Scholar]
- 9.Falany CN, Fortinberry H, Leiter EH, Barnes S. Cloning, expression, and chromosomal localization of mouse liver bile acid CoA:amino acid N-acyltransferase. J Lipid Res. 1997;38:1139–1148. [PubMed] [Google Scholar]
- 10.Anwer MS. Cellular regulation of hepatic bile acid transport in health and cholestasis. Hepatology. 2004;39:581–590. doi: 10.1002/hep.20090. [DOI] [PubMed] [Google Scholar]
- 11.Csanaky IL, Lu H, Zhang Y, Ogura K, Choudhuri S, et al. Organic anion-transporting polypeptide 1b2 (Oatp1b2) is important for the hepatic uptake of unconjugated bile acids: Studies in Oatp1b2-null mice. Hepatology. 2011;53:272–281. doi: 10.1002/hep.23984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerloff T, Stieger B, Hagenbuch B, Madon J, Landmann L, et al. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem. 1998;273:10046–10050. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- 13.Shneider BL, Dawson PA, Christie DM, Hardikar W, Wong MH, et al. Cloning and molecular characterization of the ontogeny of a rat ileal sodium-dependent bile acid transporter. J Clin Invest. 1995;95:745–754. doi: 10.1172/JCI117722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballatori N, Fang F, Christian WV, Li N, Hammond CL. Ostalpha-Ostbeta is required for bile acid and conjugated steroid disposition in the intestine, kidney, and liver. Am J Physiol Gastrointest Liver Physiol. 2008;295:G179–G186. doi: 10.1152/ajpgi.90319.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song PZ, Zhang Y, Klaassen CD. Dose-response of five bile acids on serum and liver bile Acid concentrations and hepatotoxicty in mice. Toxicol Sci. 2011;123:359–367. doi: 10.1093/toxsci/kfr177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Klaassen CD. Effects of feeding bile acids and a bile acid sequestrant on hepatic bile acid composition in mice. J Lipid Res. 2010;51:3230–3242. doi: 10.1194/jlr.M007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell. 2000;6:517–526. doi: 10.1016/s1097-2765(00)00051-4. [DOI] [PubMed] [Google Scholar]
- 18.Kim I, Ahn SH, Inagaki T, Choi M, Ito S, et al. Differential regulation of bile acid homeostasis by the farnesoid X receptor in liver and intestine. J Lipid Res. 2007;48:2664–2672. doi: 10.1194/jlr.M700330-JLR200. [DOI] [PubMed] [Google Scholar]
- 19.Inagaki T, Choi M, Moschetta A, Peng L, Cummins CL, et al. Fibroblast growth factor 15 functions as an enterohepatic signal to regulate bile acid homeostasis. Cell Metab. 2005;2:217–225. doi: 10.1016/j.cmet.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Y-KJ, Guo GL, Klaassen CD. Diurnal Variations of Mouse Plasma and Hepatic Bile Acid Concentrations as well as Expression of Biosynthetic Enzymes and Transporters. PLoS ONE. 2011;6:e16683. doi: 10.1371/journal.pone.0016683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Csanaky IL, Aleksunes LM, Tanaka Y, Klaassen CD. Role of hepatic transporters in prevention of bile-acid toxicity after partial hepatectomy in mice. Am J Physiol Gastrointest Liver Physiol. 2009;297:G419–433. doi: 10.1152/ajpgi.90728.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchida K, Chikai T, Takase H, Nomura Y, Seo S, et al. Age-related changes of bile acid metabolism in rats. Arch Gerontol Geriatr. 1990;10:37–48. doi: 10.1016/0167-4943(90)90042-5. [DOI] [PubMed] [Google Scholar]
- 23.Uchida K, Nomura Y, Kadowaki M, Takase H, Takano K, et al. Age-related changes in cholesterol and bile acid metabolism in rats. J Lipid Res. 1978;19:544–552. [PubMed] [Google Scholar]
- 24.Salemans JM, Nagengast FM, Tangerman A, van Schaik A, Hopman WP, et al. Effect of ageing on postprandial conjugated and unconjugated serum bile acid levels in healthy subjects. Eur J Clin Invest. 1993;23:192–198. doi: 10.1111/j.1365-2362.1993.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 25.Heuman DM. Quantitative estimation of the hydrophilic-hydrophobic balance of mixed bile salt solutions. J Lipid Res. 1989;30:719–730. [PubMed] [Google Scholar]
- 26.Wang DQ, Tazuma S, Cohen DE, Carey MC. Feeding natural hydrophilic bile acids inhibits intestinal cholesterol absorption: studies in the gallstone-susceptible mouse. Am J Physiol Gastrointest Liver Physiol. 2003;285:G494–502. doi: 10.1152/ajpgi.00156.2003. [DOI] [PubMed] [Google Scholar]
- 27.Amador-Noguez D, Dean A, Huang W, Setchell K, Moore D, et al. Alterations in xenobiotic metabolism in the long-lived Little mice. Aging Cell. 2007;6:453–470. doi: 10.1111/j.1474-9726.2007.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vina J, Borras C, Gambini J, Sastre J, Pallardo FV. Why females live longer than males: control of longevity by sex hormones. Sci Aging Knowledge Environ. 2005;2005:pe17. doi: 10.1126/sageke.2005.23.pe17. [DOI] [PubMed] [Google Scholar]
- 29.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodrick CL. Life-span and the inheritance of longevity of inbred mice. J Gerontol. 1975;30:257–263. doi: 10.1093/geronj/30.3.257. [DOI] [PubMed] [Google Scholar]
- 31.Akare S, Jean-Louis S, Chen W, Wood DJ, Powell AA, et al. Ursodeoxycholic acid modulates histone acetylation and induces differentiation and senescence. Int J Cancer. 2006;119:2958–2969. doi: 10.1002/ijc.22231. [DOI] [PubMed] [Google Scholar]
- 32.Cheng X, Buckley D, Klaassen CD. Regulation of hepatic bile acid transporters Ntcp and Bsep expression. Biochem Pharmacol. 2007;74:1665–1676. doi: 10.1016/j.bcp.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simon FR, Fortune J, Iwahashi M, Bowman S, Wolkoff A, et al. Characterization of the mechanisms involved in the gender differences in hepatic taurocholate uptake. Am J Physiol. 1999;276:G556–565. doi: 10.1152/ajpgi.1999.276.2.G556. [DOI] [PubMed] [Google Scholar]
- 34.Bertolotti M, Abate N, Bertolotti S, Loria P, Concari M, et al. Effect of aging on cholesterol 7 alpha-hydroxylation in humans. J Lipid Res. 1993;34:1001–1007. [PubMed] [Google Scholar]
- 35.Einarsson K, Nilsell K, Leijd B, Angelin B. Influence of age on secretion of cholesterol and synthesis of bile acids by the liver. N Engl J Med. 1985;313:277–282. doi: 10.1056/NEJM198508013130501. [DOI] [PubMed] [Google Scholar]
- 36.Bertolotti M, Gabbi C, Anzivino C, Crestani M, Mitro N, et al. Age-related changes in bile acid synthesis and hepatic nuclear receptor expression. Eur J Clin Invest. 2007;37:501–508. doi: 10.1111/j.1365-2362.2007.01808.x. [DOI] [PubMed] [Google Scholar]
- 37.Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol. 2001;21:1393–1403. doi: 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]