Abstract
Mycobacterium tuberculosis is a facultative intracellular pathogen that parasitizes macrophages by modulating properties of the Mycobacterium-containing phagosome. Mycobacterial phagosomes do not fuse with late endosomal/lysosomal organelles but retain access to early endosomal contents by an unknown mechanism. We have previously reported that mycobacterial phosphatidylinositol analog lipoarabinomannan (LAM) blocks a trans-Golgi network-to-phagosome phosphatidylinositol 3-kinase-dependent pathway. In this work, we extend our investigations of the effects of mycobacterial phosphoinositides on host membrane trafficking. We present data demonstrating that phosphatidylinositol mannoside (PIM) specifically stimulated homotypic fusion of early endosomes in an ATP-, cytosol-, and N-ethylmaleimide sensitive factor-dependent manner. The fusion showed absolute requirement for small Rab GTPases, and the stimulatory effect of PIM increased upon partial depletion of membrane Rabs with RabGDI. We found that stimulation of early endosomal fusion by PIM was higher when phosphatidylinositol 3-kinase was inhibited by wortmannin. PIM also stimulated in vitro fusion between model phagosomes and early endosomes. Finally, PIM displayed in vivo effects in macrophages by increasing accumulation of plasma membrane-endosomal syntaxin 4 and transferrin receptor on PIM-coated latex bead phagosomes. In addition, inhibition of phagosomal acidification was detected with PIM-coated beads. The effects of PIM, along with the previously reported action of LAM, suggest that M. tuberculosis has evolved a two-prong strategy to modify its intracellular niche: its products block acquisition of late endosomal/lysosomal constituents, while facilitating fusion with early endosomal compartments.
INTRODUCTION
Mycobacterium tuberculosis is an intracellular parasite that survives in infected macrophages by modulating phagosomal biogenesis and maturation. After phagocytosis, mycobacteria reside in phagosomes that do not acquire late endosomal and lysosomal characteristics (Armstrong and Hart, 1971; Russell et al., 2002). Another important aspect of the mycobacterial phagosome is that the vacuole seems to retain access to markers derived from plasma membrane (Clemens and Horwitz, 1996; Russell et al., 1996; Sturgill-Koszycki et al., 1996), allowing acquisition of nutrients, such as iron (Schaible et al., 2002), by M. tuberculosis.
The mycobacterial factors affecting phagosome maturation are only now beginning to be identified (Vergne et al., 2003a). In recent studies (Fratti et al., 2001, 2003b), lipoarabinomannan (LAM), a heavily glycosylated phosphatidylinositol exclusively produced by mycobacteria, has been shown to interfere with phagosomal acquisition of late endosomal constituents. LAM, and a number of other mycobacterial lipids, have been reported to traffic within the infected macrophages and intercalate into the host cell endomembranes (Beatty et al., 2000). One of the M. tuberculosis glycolipids shown to impregnate host cell endomembranes is phosphatidylinositol mannoside (PIM) (Figure 1A), a biosynthetic precursor of LAM. PIM is abundantly produced by mycobacteria and represents 56% of all phospholipids in the mycobacterial cell wall (Goren, 1984).
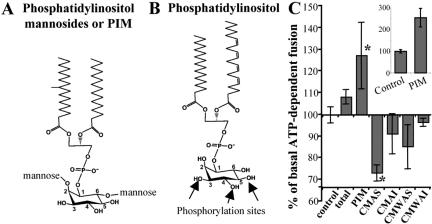
Figure 1.
Effects of mycobacterial lipids on early endosomal fusion. Structures of phosphatidylinositol mannosides PIM (A) and a typical mammalian phosphatidylinositol (B). PIM is glycosylated with single mannose residues at positions 2 and 6 of the inositol ring, whereas positions 3, 4, and 5 are unsubstituted. In eukaryotic cells, phosphatidylinositol can be phosphorylated at the positions 3, 4, or 5 on the inositol headgroup as indicated by arrows. (C) Early endosomal fusion was carried out in the presence of 40 μg/ml mycobacterial lipids, and 2 mg/ml cytosol, for 1 h at 37°C. CMAS and CMAI: lipids soluble in chloroform/methanol (2/1) (CM) and soluble (AS) or insoluble (AI) in acetone. CMWAS and CMWAI: lipids soluble in chloroform/methanol/water (10/10/3) (CMW) and soluble (AS) or insoluble (AI) in acetone. Total, unfractionated lipids extracted from M. tuberculosis. Inset, stimulatory effect of PIM upon preincubation of PIM with cytosol and ATP, before the initiation of endosomal fusion. Shown is the percentage of ATP-dependent fusion (1 mg/ml cytosol) in the presence or absence of PIM preincubated with cytosol and ATP-regenerating system for 20 min. Bars, SEM asterisk, p < 0.05 (ANOVA).
Historically, inositol phospholipids were first recognized as general components of biological membranes by studies carried out in mycobacteria (Anderson and Roberts, 1930; Ballou and Lee, 1964). Over many years of research, it has become evident that phosphatidylinositols and their different phosphorylated forms are important regulatory molecules in eukaryotic cells. Numerous studies have shown that phosphoinositides play important roles in signal transduction, cytoskeletal organization, and, more recently, in regulating discrete membrane trafficking events in organellar biogenesis (for reviews, see De Camilli et al., 1996; Corvera et al., 1999; Gillooly et al., 2001; Simonsen et al., 2001). Phosphoinositides are substrates for modifying enzymes, such as phosphatidylinositol kinases, phosphatases, and phospholipases. Phosphatidylinositol (PI), when phosphorylated in various combinations at the 3, 4, or 5 positions of the inositol ring (Figure 1B), earmarks the organelles or their membrane domains for subsequent trafficking events. One manifestation of these phenomena is the recruitment of membrane fusion effector proteins containing specific phosphoinositide binding domains (Simonsen et al., 2001).
Phosphatidylinositol 3-phosphate (PI3P) is an example of phosphoinositides relevant for phagosomal maturation in the context of M. tuberculosis interference with the intracellular trafficking in infected macrophages (Fratti et al., 2001). PI3P recruits the endosomal tethering molecule EEA1 to the endocytic organelles (Simonsen et al., 1998). EEA1 is essential for phagosomal maturation into phagolysosomes (Fratti et al., 2001; Vieira et al., 2002), and its recruitment to M. tuberculosis phagosomes is altered as a part of the mycobacterial phagosome maturation block (Fratti et al., 2001). The mycobacterial heavily glycosylated phosphatidylinositol analog LAM inhibits EEA1 recruitment to phagosomal membranes (Fratti et al., 2001), which leads to a disruption of delivery of lysosomal hydrolases and Vo H+ATPase from the trans-Golgi network (TGN) to the phagosome (Fratti et al., 2003b). These effect of LAM, exerted via a block in [Ca2+]c rise (Vergne et al., 2003b), while explaining at least in part the mycobacterial phagosome maturation block, also open the question of how mycobacteria maintain the interactions with early endosomes. The latter phenomenon has been postulated to exist based on the observed delivery of transferrin receptor and iron to mycobacterial phagosomes (Clemens and Horwitz, 1996; Sturgill-Koszycki et al., 1996; Schaible et al., 2002)
Based on the pivotal role that phosphatidylinositols play in intracellular signaling and trafficking in mammalian cells (De Camilli et al., 1996; Corvera et al., 1999; Gillooly et al., 2001; Simonsen et al., 2001), combined with the observations that M. tuberculosis produces PI analogs, such as LAM and PIM, which intercalate into the endomembranes of infected macrophages (Beatty et al., 2000), we have continued our investigations (Fratti et al., 2001, 2003b; Vergne et al., 2003b) of whether and how mycobacterial phosphoinositides modulate interactions of M. tuberculosis phagosome with endocytic and biosynthetic organelles of the host cell. In this work, we report a surprising and previously unappreciated activity of PIM. We show that this mycobacterial phospholipid stimulates homotypic fusion of early endosomes. Moreover, PIM enhances fusion between phagosomes and early endosomes. We also report that PIM affects phagosomal properties in vivo.
MATERIALS AND METHODS
Materials
Purified Rab-GDP-dissociation inhibitor (GDI) was a gift from Drs. G.J. Quellhorst and M. Wessling-Resnick, Harvard University. 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid (BAPTA), anti-avidin antibody, wortmannin, phosphatidylinositol 3-phosphate, and phosphatidylinositol 4,5-bisphosphate were from Calbiochem (San Diego, CA). N-ethylmaleimide (NEM), guanosine 5′-O-(3-thio)triphosphate (GTPγS), phosphatidylinositol, and other products used for in vitro fusion were from Sigma.
Fractionation of Lipids, Purification of Phosphatidylinositol Mannosides, and Preparation of Lipid Suspensions and Lipid-coated Beads
Mycobacterium tuberculosis H37Rv (ATCC 27299) cells grown to late log phase in glycerol alanine salts medium (Takayama et al., 1975) were harvested, inactivated by γ-irradiation (Covert et al., 2001), and lyophilized. Dried Mycobacterium bovis BCG Pasteur (TMC 1011) was a kind gift from Dr. Patrick J. Brennan (Colorado State University). The dried cells were extracted at room temperature for 24 h with CHCl3/CH3OH (2:1) at 30 ml/g of cells. The cells and organic extract were separated by filtration through Whatman 17 CHR chromatography paper. The filtrate was dried suspended in a biphasic mixture of CHCl3/CH3OH/H2O (4:2:1) (Folch et al., 1957), and the lower organic phase was recovered. This material was termed the CM total lipid extract. The extracted cells were recovered from the filter paper and extracted a second time for 24 h at room temperature with CHCl3/CH3OH/H20 (10:10:3) to recover polar glycolipids and lipoglycans (Besra et al., 1997). This extract was termed the total polar lipid extract (CMW).
A portion of the dried CM total lipid and the total polar lipid extracts (CMW) were placed on ice, cold (-20°C) acetone was added to each at 30 ml/g of lipid, and the material was thoroughly suspended. Each acetone trituration was centrifuged at 10,000 × g, 4°C for 20 min. The acetone supernatants were collected and the insoluble pellet washed twice more with cold acetone. The supernatants of each lipid sample were pooled and dried along with the acetone-insoluble pellets. This resulted in acetone soluble (AS) and acetone insoluble fractions (AI) of the CM total lipid and CMW extracts.
To isolate PIM, the acetone-insoluble fraction of the CM total lipid extract from the M. bovis BCG Pasteur was applied to a column (3.5 × 35 cm) of silica (Merck AG, Darmstadt, Germany) equilibrated with CHCl3/CH3OH (9:1). Lipids were eluted with 500-ml step gradients of increasing CH3OH concentrations (10, 20, 30, 40, and 50%) in CHCl3. The resulting fractions were examined by silica gel thin-layer chromatography (TLC) in CHCl3/CH3OH/H20 (60:30:6) and resolved lipids visualized by spraying with α-napthol and 10% CuSO4 in 8% H2SO4 (Kates, 1986). The 40 and 50% CH3OH fractions were enriched for PIM. These fractions were pooled and separated by preparative TLC by using a 20 × 20-cm glass-backed silica gel TLC plate (Merck AG) developed in CHCl3/CH3OH/H20 (60:30:6). Individual lipid bands were visualized using iodine vapors (Dawson et al., 1993) and scraped from the TLC plates. The purified lipids (PIM) were extracted from the silica gel in CHCl3/CH3OH (2:1). The purified PIM was compared with standards by one-dimensional TLC, two-dimensional TLC, and gas chromatography (Brennan and Ballou, 1968; Khoo et al., 1995).
Lipid suspension were prepared by solubilization of lipids in dimethyl sulfoxide (DMSO), injection of this solution into phosphate-buffered saline (PBS) while vortexing, followed by sonication (final lipid concentration 0.8 mg/ml in 8% DMSO in PBS). Standard PIM concentration used was 40 μg/ml, as described previously with phosphoinositides (Mayer et al., 2000; Defacque et al., 2002) with the exception of experiments with PIM preincubation with the cytosol, when 80 μg/ml PIM was used, and when stated otherwise.
Lipid-coated beads were prepared as described previously (Kang and Schlesinger, 1998). Latex beads were washed in NaHCO3, pH 9.6, and incubated for 2 h at 37°C in the presence or absence of PIM in NaHCO3.
Preparation of Endosomes and Cytosol for In Vitro Fusion
The murine macrophage-like cell line J774 was maintained in DMEM supplemented with 4 mM l-glutamine and 5% fetal bovine serum. Suspended J774 cells were incubated with either 1.7 mg/ml biotinylated horseradish peroxidase (b-HRP) or 3.3 mg/ml avidin at 37°C in 150 mM NaCl, 20 mM HEPES/KOH, pH 7.4, 5.5 mM glucose, 1 mg/ml bovine serum albumin (BSA) for 5 min (early endosomes loading) or for 20-min pulse and 40-min chase (late endosomes loading). Cells were washed several times with PBS, PBS with 5 mg/ml BSA, and then homogenized in 250 mM sucrose, 3 mM imidazole, pH 7.2, 0.5 mM EGTA, cocktail of protease inhibitors (10 μg/ml N-tosyl-l-lysine chloromethyl ketone, 1.79 μg/ml E64, 1 nM pepstatin, 10 μg/ml leupeptin), and a postnuclear supernatant (PNS) was prepared as described previously (Via et al., 1997). PNS was quickly frozen in liquid nitrogen and stored at -80°C. Endosome enriched fractions were prepared by diluting 1 volume of rapidly thawed PNS with 4 volumes of 250 mM sucrose, 20 mM HEPES/KOH, pH 7.2, 0.5 mM EGTA, and protease inhibitors. The diluted PNS was centrifuged 1min at 37,000 × g for early endosomes or at 25,000 × g for late endosomes. The supernatant was recovered and was centrifuged 5 min at 50,000 × g. The pellet of the second centrifugation was used as a fraction enriched for early endosomes (Mayorga et al., 1991). For cytosol preparation, PNS from J774 was centrifuged 1 h at 150,000 × g at 4°C and the supernatant was aliquoted, quickly frozen in ethanol/dry ice, and stored at -80°C.
Homotypic Early Endosome Fusion Assay
Avidin- and b-HRP-loaded early endosomes (∼0.5 mg/ml) were combined at 4°C in the presence of cytosol (1-3 mg/ml) and 60 μg/ml biotinylated-insulin (b-insulin), as a scavenger for external avidin leaked from endosomes, and incubated at room temperature, for 20 min with PIM, other lipids, or carrier (0.8% DMSO). Fusion reactions were performed in 250 mM sucrose, 1 mM dithiothreitol, 0.5 mM EGTA, 20 mM HEPES/KOH, pH 7.2, with an ATP-regenerating system (1 mM ATP, 8 mM creatine phosphate, 28 U/ml creatine phosphokinase) or ATP-depleting system (10 mM glucose, 10 U/ml apyrase). Reactions were initiated by rising the temperature to 37°C and stopped by placing the tubes on ice. After 5 min on ice, endosomes were solubilized in 1% (vol/vol) Triton X-100, 0.1% (wt/vol) SDS, 50 μg/ml b-insulin for 30 min at 0°C and diluted in 0.05% (vol/vol) Triton X-100, 50 mM NaCl, 10 mM Tris-HCl, pH 7.5, 1 mg/ml heparin. Samples were centrifuged at 12,000 × g, for 5 min and the supernatants assayed for avidin-b-HRP complexes by using precoated anti-avidin enzyme-linked immunosorbent assay plates (Braell, 1992). HRP activity was measured by fluorescence in a Gemini fluorescent plate reader by using QuantaBlu substrate from Pierce Chemical (Rockford, IL), and excitation and emission wavelengths of 320 and 405 nm, respectively. To determine the level of extravesicular probes, early endosome fractions containing b-HRP or avidin were mixed, incubated for 1 h at 37°C, in the absence of ATP and b-inusulin, and then processed as described above for fusion assay.
Phagosome-Early Endosomes Fusion Assay
Macrophages were incubated for 30 min at 37°C, 5% CO2 with streptavidin magnetic particles (Polysciences, Warrington PA) (1/45) in 150 mM NaCl, 20 mM HEPES pH 7.4, 6.5 mM glucose, 1 mg/ml BSA. After three washes with cold PBS, cells were lysed by passing through 22-gauge needles connected to a two-syringe apparatus. PNSs were generated and phagosomes were isolated using a magnetic separator (Polysciences) and resuspend in 250 mM sucrose, 3 mM imidazole, pH 7.2, 0.5 mM EGTA, protease inhibitors. Early endosomes loaded with b-HRP were prepared as described above. Early endosome fraction (0.6 mg/ml) and phagosomes (0.8 mg/ml) were combined at 4°C in the presence of cytosol (2 mg/ml) and 140 μg/ml biotinylated-insulin (b-insulin), as a scavenger. Fusion reactions were performed in 250 mM sucrose, 1 mM dithiothreitol, 0.5 mM EGTA, 20 mM HEPES/KOH, pH 7.2, with an ATP-regenerating system or ATP-depleting system. Reactions were initiated by rising the temperature to 37°C, incubating for 1 h and stopped by placing the tubes on ice. Phagosomes were isolated with the magnetic separator, resuspended in 1% (vol/vol) Triton X-100, 0.1% (wt/vol) SDS, 100 μg/ml b-insulin, diluted in 0.05% (vol/vol) Triton X-100, 50 mM NaCl, 10 mM Tris-HCl, pH 7.5, 1 mg/ml heparin and incubated at 4°C for 30 min to lyse the membranes. Particles were washed two times with 1% (vol/vol) Triton X-100, 0.1% (wt/vol) SDS, 50 mM NaCl, 10 mM Tris-HCl, pH 7.5, 1 mM EDTA, 0.5% fish skin gelatin, and then two times with PBS. HRP activity bound to streptavidin particles was measured as described above.
Fluorescence Microscopy
J774 cells grown on glass coverslips and allowed to phagocytize latex beads. Synchronization of infection was achieved by centrifugation of particles onto macrophages. Macrophages were fixed with 3.7% paraformaldehyde, followed by membrane permeabilization by using 0.2% saponin. Permeabilized cells were incubated with primary antibody, syntaxin 4 (from A. Hubbard and P. Tuma, Johns Hopkins University, Baltimore, MD) or transferrin receptor (Santa Cruz Biotechnology, Santa Cruz, CA), followed by secondary Alexa 568-conjugated antibody. LysoTracker Red DN-99 (Molecular Probes, Eugene, OR) staining was carried out as described previously (Via et al., 1998). The presence of syntaxin 4 on phagosomes was scored by morphological criteria, as described previously (Fratti et al., 2001, 2003b) and the formation of a rim-like structure (red channel) around latex bead phagosomes (green channel) was scored as a positive event. Images were collected and processed using LSR Esprit or Ultraview for acquisition of confocal images and Adobe PhotoShop software. The effect of mycobacterial lipids on the accumulation of syntaxin 4 was verified by extracting lipid coated latex beads with Triton X-100. Relative to control beads, Syntaxin 4 colocalization with Triton X-100-extracted beads was unaffected.
Statistical Analysis
All statistical analyses were calculated using Fisher's protected least significant difference post hoc test (analysis of variance, ANOVA) (SuperANOVA 1.11; Abacus Concepts, Berkeley, CA). P values of ≤0.05 were considered significant.
RESULTS
Mycobacterial Phosphatidylinositol Mannoside PIM Stimulates Early Endosomal Fusion
An in vitro system to monitor early endosomal fusion by using purified organelles has been established previously (Mayorga et al., 1991; Braell, 1992). In this assay, two populations of purified endosomes, separately loaded with either avidin or b-HRP, are mixed in the presence of cytosol and ATP, to allow tethering, docking, and fusion. Organellar fusion is then quantitated using an HRP fluorescent assay after capture of avidin-b-HRP complexes that form only upon the mixing of the lumenal contents of the fusing endosomes. Purified endosomal fractions were preincubated for 20 min with a panel of M. tuberculosis products, including PIM, before initiating fusion reactions by adding salt and ATP-regenerating system. Different lipid fractions from M. tuberculosis showed for the most part inhibitory or minor stimulatory effects on endosomal fusion (Figure 1C), whereas PIM increased early endosomal fusion (Figure 1C). PIM-dependent stimulation was 25% when PIM was added to the mixture at the outset of the fusion PIM reaction (Figure 1C, main panel). When the cytosol and PIM were preincubated in the presence of ATP for 20 min before the initiation of the fusion reaction, the stimulatory effect activity of PIM was further augmented to a 2.5-fold increase in fusion (Figure 1C, inset). Figure 2A shows that the observed stimulatory effect of PIM on endosomal fusion was ATP and cytosol dependent. Because, the fusion induced by PIM required ATP and cytosolic factors, PIM did not act simply as a fusogenic lipid bypassing regulated vesicle fusion (Hinkovska-Galcheva et al., 1998). We also tested whether PIM potentially acted as a detergent causing nonspecific content leak from vesicles. When the amount of probe outside the vesicles was measured, extraorganellar HRP levels were the same in the presence or absence of PIM (Figure 2A, inset).
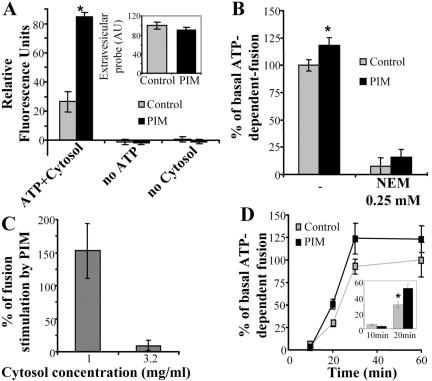
Figure 2.
PIM stimulates early endosomal fusion in ATP-, NSF-, and cytosol-dependent manner. (A) In vitro fusion was carried out, in the presence or absence of 80 μg/ml PIM for 30 min. Reactions contained: 1 mg/ml cytosol or no cytosol (control); ATP regenerating system or an ATP-depleting system (no ATP). Inset, quantitation of extravesicular probes (avidin and biotinylated HRP), in the presence or absence of 80 μg/ml PIM. (B) NSF dependency of PIM-stimulated fusion. Endosomes and cytosol were preincubated for 20 min with 0.25 mM NEM followed by initiating fusion reaction with 1 mg/ml cytosol and incubation for 30 min. (C) Cytosol concentration effects on PIM-mediated stimulation of endosomal fusion. Fusion reaction was allowed to occur for 30 min in the presence of different concentrations of cytosol in the presence or absence of 80 μg/ml PIM. (D) PIM effects on rate and extent of endosomal fusion. Fusion was conducted in the presence of 2 mg/ml cytosol. Inset, percentage of ATP-dependent fusion in the presence or absence of PIM at 10 and 20 min. All percent values are given relative to the control fusion reaction without PIM. Bars, SEM asterisk, p < 0.05 (ANOVA).
We next examined whether PIM-mediated stimulation of fusion acts via the involvement of known membrane fusion factors, such as N-ethylmaleimide sensitive factor (NSF). NSF is critical for membrane fusion as it primes the soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs) for subsequent formation of trans-SNARE complexes leading to organellar fusion (Haas, 1998). Figure 2B shows that PIM did not promote fusion in the presence of the NSF inhibitor N-ethylmaleimide. This, along with the ATP and cytosol dependence of PIM effects, demonstrates that PIM acts by enhancing regulated early endosomal fusion. The magnitude of the stimulatory effect by PIM was cytosol concentration dependent, because at higher concentrations of cytosol, PIM did not increase endosomal fusion (Figure 2C). A kinetic analysis using intermediate concentrations of cytosol (2 mg/ml) showed that PIM acted by both increasing the rate of fusion (Figure 2D and inset) and enhancing the extent of fusion (Figure 2D).
Specific Stimulation of Homotypic Early Endosomal Fusion by PIM
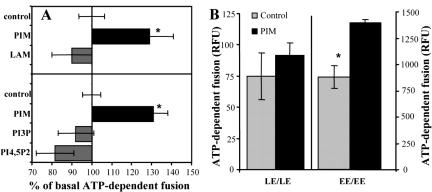
The stimulation of fusion was specific for PIM, as ManLAM, a polyglycosylated form of PIM, did not enhance fusion (Figure 3A). We also tested two eukaryotic phosphatidylinositols, PI3P and phosphatidylinositol 4,5-bisphosphate (PIP2), known to participate in early endosomal (Simonsen et al., 1998) or vacuolar (Mayer et al., 2000) fusion. However, in contrast to PIM, PI3P and PIP2 did not enhance early endosomal fusion (Figure 3A).
Figure 3.
Specificity of PIM-stimulated endosomal fusion. (A) Fusion was conducted in the presence of 40 μg/ml mycobacterial lipids (top) or 80 μg/ml lipids (bottom). (B) Fusion was conducted for 30 min, in the presence of 80 μg/ml PIM and 2 mg/ml cytosol, by using early endosomes (EE) or late endosomes (LE) loaded with biotinylated-HRP or avidin. Bars, SEM asterisk, p < 0.05 (ANOVA).
Most of the regulated fusion events in the cell are NSF dependent. Within the endocytic pathway, these include homotypic early endosomal (Diaz et al., 1988; Holroyd et al., 1999) or late endosomal fusion events (Haas, 1998; Mullock et al., 1998). To examine whether PIM acts specifically at a certain stage, or, alternatively, indiscriminately activates any regulated endosomal fusion, we compared its effects on early versus late homotypic endosomal fusion. In contrast to its stimulatory effect on early endosomes, PIM did not enhance fusion between late endosomes (Figure 3B), indicating that the effects of PIM are specific for early endosomal fusion.
Fusion Stimulation by PIM Requires Rab GTPases
The endosomal GTPase Rab5 is essential for early endosomal fusion. Rab5 recruits to endosomes several Rab5-interacting components and downstream effectors important for membrane tethering and fusion (Gorvel et al., 1991). In the absence of Rab5, endosomal fusion is inhibited (Barbieri et al., 1998). Rabs can be removed from membranes by adding the protein known as GDI, which plucks Rabs from organellar membranes (Soldati et al., 1993; Barbieri et al., 1998). Figure 4A shows that the stimulatory effect of PIM was preserved at intermediate concentrations of GDI, reducing Rab levels but not completely eliminating Rabs from membranes. Thus, PIM stimulates fusion when either membrane components (Rabs) or cytosolic factors controlling this process become limiting. The enhanced stimulation of membrane fusion by PIM when cytosolic components necessary for fusion are in short supply is evident from experiments with different amounts of cytosol in the fusion reaction (Figure 2C). When endosomes were preincubated with higher concentrations of GDI expected to remove most of the Rabs from membranes, this not only further inhibited early endosomal fusion but also it fully abrogated the stimulatory effect of PIM. Thus, PIM stimulates only a Rab-dependent reaction and cannot promote Rab-less membrane fusion.
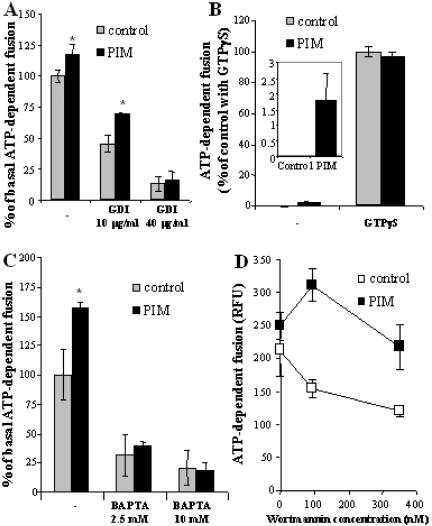
Figure 4.
Analysis of the step at which PIM stimulates early endosomal fusion. Endosome preparations and cytosol, were preincubated for 20 min with indicated concentrations of GDI (A), GTPγS (25 μM) (B), BAPTA (C), or wortmannin (D). Fusion was carried out for 30 min, in the presence of 1 mg/ml cytosol (0.5 mg/ml cytosol was used for GTPγS experiment). Inset, percentage of ATP-dependent fusion (0.5 mg/ml cytosol) in the presence or absence of PIM. Bars, SEM asterisk, p < 0.05 (ANOVA).
Next, we tested the effect of GTPγS on PIM-mediated stimulation of fusion. It has been reported that, at low cytosol concentration (0.5 mg/ml), GTPγS stimulates fusion of early endosomes by acting most likely on Rab GTPases (Mayorga et al., 1989). For example, GTPγS locks Rab5 in its active, GTP-bound form, thus facilitating the recruitment from the cytosol of Rab5 effectors, such as EEA1, which facilitate fusion (Simonsen et al., 1998). It is also important to note that GTP hydrolysis by Rabs is not necessary for endosomal fusion but its conversion to GDP is rather a means to terminate the activation cycle (Rybin et al., 1996). As expected, at low concentrations of cytosol, no detectable fusion occurred in the control (Figure 4B). However, in the presence of PIM, endosomal fusion was evident (Figure 4B, inset). On addition of GTPγS, which in its own right strongly stimulated fusion, PIM lost its enhancing effect. These results indicate that PIM can stimulate fusion if Rab GTPases are not constitutively activated by other means.
PIM-stimulated Fusion Requires Release of Intravesicular Ca2+
Several reports have indicated that Ca2+ release from vesicles plays a role in fusion events (Peters and Mayer, 1998; Holroyd et al., 1999; Pryor et al., 2000). Fusion subsequent to Ca2+ release from vesicular lumen into the cytosol can be countered by adding the fast-acting Ca2+ chelator BAPTA at 10 mM, but not with a slower Ca2+ chelator such as EGTA (Peters and Mayer, 1998; Pryor et al., 2000). When tested, in the presence of BAPTA, PIM could not stimulate fusion (Figure 4C). These results indicate that PIM-mediated stimulation of early endosomal fusion requires Ca2+. Because the fusion reaction contained 0.5 mM EGTA, a slow-acting Ca2+ chelator, the Ca2+ required for the fusion must have originated from the endosomes, as reported previously (Peters and Mayer, 1998; Holroyd et al., 1999; Pryor et al., 2000). The absence of any stimulatory effects of PIM in the presence of lower concentration of BAPTA (2.5 mM), i.e., under conditions that permitted 30% of the reaction to occur, suggests that PIM acts in fusion upstream of the release of intravesicular Ca2+.
PI3-Kinase Inhibition with Wortmannin Enhances Stimulatory Effect of PIM on Endosomal Fusion
One of the characterized Rab5 effectors is hVPS34, a type III PI3-kinase (Christoforidis et al., 1999b). Early endosomal fusion has been shown to depend on this PI3-kinase, which produces phosphatidylinositol 3-phosphate on early endosomal membranes essential for the recruitment of the organelle-tethering and SNARE-organizing protein EEA1 (Simonsen et al., 1998; Christoforidis et al., 1999a). Wortmannin is a specific inhibitor of mammalian PI3-kinases when used at 10-100 nM concentrations. As expected, wortmannin at all concentrations tested (95-350 nM) inhibited fusion (Figure 4D). In the presence of 100 nM wortmannin, PIM showed a dramatic increase in fusion relative to the untreated, control level reaction (Figure 4D). PIM also showed stimulatory effects at higher concentrations of wortmannin, but the magnitude of its effects was the highest at concentration of the inhibitor specifically affecting the PI3-kinases. These results show that PIM can stimulate early endosomal fusion even when PI3-kinase activity is inhibited by pharmacological means, and thus, PI3P production is limited.
PIM Stimulates Phagosome-Early Endosome Fusion In Vitro
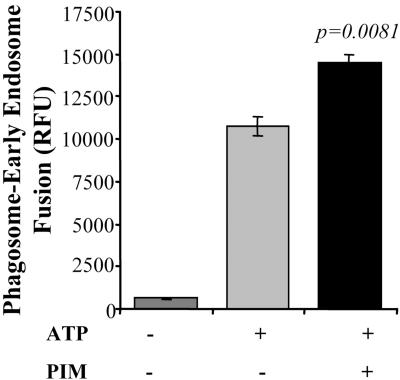
We next investigated the effect of PIM on fusion between phagosomes and early endosomes, in vitro, using a sensitive assay described in MATERIALS AND METHODS. PIM was added exogenenously to the system and preincubated for 20 min with cytosol, phagosomes and early endosomes before initiating fusion. Figure 5 shows that PIM stimulates the ATP-dependent fusion between phagosomes and early endosomes.
Figure 5.
PIM stimulates phagosome-early endosomes fusion in vitro. Phagosome, cytosol and endosome preparations were preincubated for 20 min at room temperature in absence or presence of 80 μg/ml PIM, and then fusion was carried out at 37C for 1 h in presence or absence of ATP. Bars, SEM.
In Vivo Effects of PIM
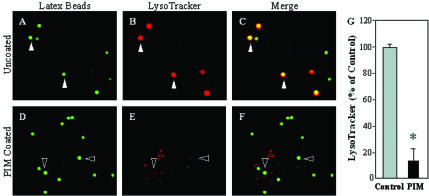
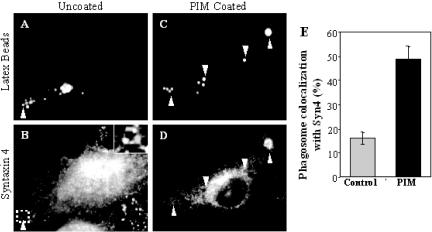
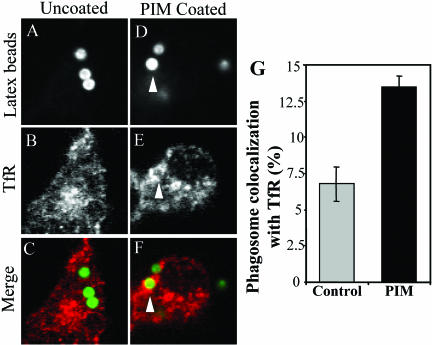
To test whether the observed PIM effects on in vitro endosomal and phagosomal fusion have consequences on phagosomes in vivo, we applied the previously published approach of coating 1-μm latex beads with mycobacterial glycolipids (Kang and Schlesinger, 1998; Fratti et al., 2001, 2003b). PIM-coated beads were incubated with macrophages and markers of phagosomal maturation were examined. We observed two effects with PIM-coated latex beads relative to the uncoated control: 1) PIM coating prevented acidification of phagosomes, determined by reduced staining with the acidotropic fluorescent dye LysoTracker DN-99 (Figure 6); and 2) recycling endosomal and plasma membrane SNARE syntaxin 4 (Bennett et al., 1993; Band et al., 2002) (Figure 7) as well as transferrin receptor (Figure 8) were found to accumulate to higher extent on phagosomes containing PIM-coated latex beads relative to the uncoated bead control. PIM-coated bead phagosomes showed the same level of syntaxin 6 (Fratti et al., 2003b) and lysobisphosphatidic acid (our unpublished data) as uncoated bead phagosomes, indicating that PIM does not directly affect acquisition of late endosomal markers via the previously shown TGN-to-phagosome pathway (Fratti et al., 2003b). These findings are consistent with PIM having a highly specialized biological activity in vivo, limited to promoting interactions with early endosomes that may deliver nutrients in mycobacterial phagosomes and maintain a relatively nonacidic pH.
Figure 6.
PIM reduces acidification of latex bead phagosomes. Shown is epifluorescence microscopy of latex bead phagosomes in J774 cells. (A and D) Green fluorescence of latex beads. (B and E) LysoTracker staining carried out by incubating J774 cells with the dye 2 h before infection and throughout the experiment. (C and F) Merged images of green and red fluorescence. Yellow color illustrates the acidification of phagosomes. Cells contained either mock-treated control latex beads (A-C), or PIM-coated latex beads (D-F) as described in MATERIALS AND METHODS. Solid arrowheads exemplify colocalization of latex beads and LysoTracker staining. Hollow arrowheads exemplify phagosomes that are not acidified. (G) Quantitation of LysoTracker colocalization with phagosomes containing uncoated control beads or PIM-coated beads (30 min postinfection). The data are means ± SE of three separate experiments. *p < 0.001.
Figure 7.
M. tuberculosis PIM causes syntaxin 4 accumulation on latex bead phagosomes. Shown is epifluorescence microscopy of latex bead phagosomes in J774 cells. (A and C) Green fluorescence of latex beads. (B and D) Red immunofluorescence of syntaxin 4. Latex beads were coated with purified M. tuberculosis H37Rv PIM and phagocytosed by J774 cells for 30 min. On phagocytosis, cells were fixed, permeabilized, and labeled with anti-syntaxin 4 antibody followed by Alexa 568-conjugated secondary antibody. Arrowheads indicated colocalization of latex beads with syntaxin 4. Quantitation of syntaxin 4 colocalization is shown numerically in (E). The data are means ± SE of three separate experiments (n = 826 phagosomes for control and n = 208 phagosomes for PIM).
Figure 8.
M. tuberculosis PIM causes transferrin receptor accumulation on latex bead phagosomes. Shown is confocal microscopy of latex bead phagosomes in J774 cells. (A and D) Green fluorescence of latex beads. (B and E) Red immunofluorescence of transferrin receptor. (C and F) Merged images of green and red fluorescence. Latex beads were coated with purified M. tuberculosis H37Rv PIM and phagocytosed by J774 cells for 30 min. On phagocytosis, cells were fixed, permeabilized, and labeled with anti-transferrin receptor antibody followed by Alexa-568-conjugated secondary antibody. Arrowheads indicate colocalization of latex beads with transferrin receptor. Quantitation of transferrin receptor colocalization is shown numerically in G. The data are means ± SE of three separate experiments (n = 400 for control and n = 400 for PIM).
DISCUSSION
M. tuberculosis produces a panoply of lipids, including several glycosylated phosphatidylinositol species (Lee et al., 1996), some of which have been shown to traffic within the endomembranes of infected macrophages (Beatty et al., 2000). One of them, LAM, a heavily glycosylated phosphatidylinositol, has been shown to participate in the arrest of phagolysosome biogenesis (Fratti et al., 2001, 2003b). The mechanism of LAM action, which involves inhibition of [Ca2+]c rise, has recently been reported (Vergne et al., 2003b). In the present study, we have presented evidence that the much less glycosylated LAM precursor PIM, stimulates homotypic early endosomal fusion and phagosomeearly endosome fusion. This stimulatory effect is specific for early endosomes and has all the characteristics of regulated fusion events because it is dependent on cytosol, ATP, NSF, Rabs, and Ca2+.
In addition to its in vitro activity, we have observed PIM effects in vivo, by using latex beads coated with this glycolipid. The observation that syntaxin 4 and transferrin receptor are accumulating on phagosomes containing PIM is in keeping with the biochemical detection of increased fusion with early endosomal compartments. Although initial over-expression experiments with syntaxin 4 have localized this SNARE exclusively to the plasma membrane (Bennett et al., 1993), recent studies with endogenous syntaxin 4 have shown that a major fraction of this SNARE localizes to endosomal membranes in normal rat kidney cells, where it colocalizes partially with recycling endosomal markers (Band et al., 2002). The distribution of syntaxin 4 in macrophages detected in our studies matches well the observations in normal rat kidney cells reported by Band et al. (2002. The in vivo effects of PIM on preventing acidification of phagosomes, as detected by inhibition of staining with the dye LysoTracker DN-99, which is normally trapped in acidified phagosomes (Via et al., 1998), can be related to fusion with early endosomal compartments that are not acidic. Interestingly, the pH of mycobacterial phagosome (pH 6.4) has been reported to match that of the recycling endosome (Sturgill-Koszycki et al., 1994). This block of acidification does not seem to be related to the prevention of phagosomes to interact with late endosomal marker as lysobisphosphatidic acid and syntaxin 6 colocalize to the same extent with uncoated and PIM-coated bead phagosomes (our unpublished data) (Fratti et al., 2003b).
The survival of M. tuberculosis in phagocytic cells rests on the ability of the tubercle bacillus to balance the block of phagosomal maturation with its need to access nutrients while residing in macrophages (Clemens and Horwitz, 1996; Russell et al., 1996; Vergne et al., 2003a). Whether both of these phenomena are achieved by a single effector molecule produced by M. tuberculosis or is a manifestation of separate processes, is not known. The observations with PIM, presented in this work, and the previously reported findings with LAM (Fratti et al., 2001), suggest a model in which distinct M. tuberculosis products manipulate separate intracellular membrane trafficking systems in the host cell. In the case of LAM and PIM, they exert two opposing but compatible and complementary actions: 1) LAM inhibits recruitment of effectors that normally promote delivery of lysosomal hydrolases and acidification systems (Simonsen et al., 1999; Fratti et al., 2001, 2003b; Gillooly et al., 2001; Vergne et al., 2003b); 2) PIM stimulates early endosomal fusion, most likely allowing the mycobacterial vacuole to gain access to endocytosed nutrients, including iron bound to transferrin. The opposing effects of LAM and PIM are also in keeping with the LAM effects on late endosomal, lysosomal, and TGN markers, in contrast to the absence of similar effects of PIM on delivery of markers from the TGN, as reported previously (Fratti et al., 2001, 2003b). This model could also explain an apparent paradox that the inhibitory action of LAM, although advantageous in terms of blocking maturation into the phagolysosome, by the same token diminishes the recruitment of early endosomal tethering molecules, i.e., EEA1. The inhibition of EEA1 recruitment would be expected to potentially interfere with acquisition by the pathogen of fresh nutrients from the endocytic pathway. However, the stimulatory action of PIM on early endosomal fusion reported here, most likely bypasses the negative effect of LAM, and compensates for the diminution of EEA1 on mycobacterial phagosomes (Fratti et al., 2001), thus generating a shunt for fusion with early endosomes.
Based on kinetic studies and the results obtained under conditions when various components necessary for endosomal fusion (cytosol, Rabs), were in a limited supply, it seems that PIM acts at a rate limiting step(s) in the fusion process. The experiments with GDI suggest that this rate-limiting step involves a Rab GTPase, most likely Rab5, because Rab5 controls early endosomal homotypic fusion and is a rate-limiting factor (Rybin et al., 1996). PIM effects on Rab GTPases could be either direct or indirect through stimulation of a GEF (e.g., Rin-1 or Rabex-5) (Horiuchi et al., 1997; Tall et al., 2001) or inhibition of a GAP (e.g., RN-Tre) (Lanzetti et al., 2000). This model is in keeping with the finding that PIM cannot further stimulate fusion under conditions when Rab GTPases are fully activated by GTPγS.
Our results indicate that PIM can stimulate fusion between phagosomes and early endosomes when it presented from the lumenal side (in vivo) or the cytosolic side (in vitro). During M. tuberculosis infection, PIM has been found intercalated into endomembranes (Beatty et al., 2000). However, its location within the lipid bilayer is still unknown. One study has shown that PIM binds to galectin-3, a galactoside-binding protein enriched on the cytosolic side of mycobacterial phagosomes (Beatty et al., 2002). We propose two models for how PIM could exert its activity 1) either directly on its target after translocation to the cytosolic side of the membrane, or 2) indirectly by modifying the biophysical properties of the membrane bilayer. For example, lipid-altered membrane fluidity has been shown to play a role in membrane association of Rab proteins, affecting the capacity of GDI to extract membrane-bound Rabs (Lebrand et al., 2002). Regarding the latter model, not only does the M. tuberculosis var. bovis BCG phagosome display abnormal Rab patterns (Via et al., 1997) but also it has unusually high levels of membrane-associated GDI (Fratti et al., 2003a), suggestive of potential alterations of the biophysical properties of the mycobacterial phagosomal membrane affecting Rab extractability.
We propose the following model in which mycobacterial lipids act to balance inhibitory and stimulatory activities controlling organellar biogenesis in macrophages: 1) PIM stimulates Rab5 to enhance early endosomal fusion. This enhancement is independent of PI3K activity, because PIM stimulates fusion even in the presence of wortmannin. This is in keeping with involvement of an active, GTP-bound Rab5, because constitutively ON Rab5 can bypass pharmacological inhibition of its downstream effector PI3K hVPS34 and promote early endosomal fusion (Li et al., 1995; Simonsen et al., 1998). It is also important to underscore the observed wortmannin insensitivity of PIM-stimulated fusion, because Mycobacterium phagosome shows stage-dependent, reduced levels of PI3P compared with model, latex bead phagosomes (Chua, Vergne, and Deretic, unpublished data). 2) LAM inhibits wortmannin-sensitive steps and effectors downstream of Rab5 activation and thus selectively diminishes delivery of lysosomal constituents from the TGN to the phagosome, a process that normally depends on EEA1-syntaxin 6 interactions (Fratti et al., 2003b). 3) The wortmannin-insensitive bypass caused by PIM, which activates early endosomal fusion even when PI3K activity is inhibited, reduces the need for high levels of EEA1 on mycobacterial phagosomes otherwise required to maintain interactions with the early endosomal compartments (Russell et al., 2002). As a net result, LAM and PIM act in coordination to prevent the delivery of lysosomal constituents without hurting the ability of the mycobacterial phagosome to gain access to essential nutrients such as iron (Clemens and Horwitz, 1996; Sturgill-Koszycki et al., 1996), which seems critical for the intracellular survival of M. tuberculosis (Schaible et al., 2002; Kelley and Schorey, 2003). Although different aspects of this model remain to be further examined, the biochemical definition of PIM activities presented here is another affirmation of the concept that lipids produced by M. tuberculosis have discrete and clearly significant effects on intracellular trafficking in mammalian cells.
Acknowledgments
We thank M. Wessling-Resnick for GDI. ManLAM, PIM1,2 and lipid fractions were prepared at Colorado State University with the support of National Institutes of Health, National Institute of Allergy and Infectious Diseases Contract NO1 AI-75320 entitled “Tuberculosis Research Materials and Vaccine Testing”. This work was supported by grant AI45148 from the National Institutes of Health.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-05-0307. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-05-0307.
References
- Anderson, R.J., and Roberts, E.G. (1930). The chemistry of lipoids of tubercule bacilli. J. Biol. Chem. 89, 611-611. [Google Scholar]
- Armstrong, J.A., and Hart, P.D.A. (1971). Response of cultured macrophages to M. tuberculosis with observations of fusion of lysosomes with phagosomes. J. Exp. Med. 134, 713-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou, C.E., and Lee, Y.C. (1964). The structure of myoinositol mannoside from Mycobacterium tuberculosis. Biochemistry 72, 682-685. [DOI] [PubMed] [Google Scholar]
- Band, A.M., Ali, H., Vartiainen, M.K., Welti, S., Lappalainen, P., Olkkonen, V.M., and Kuismanen, E. (2002). Endogenous plasma membrane t-SNARE syntaxin 4 is present in rab11 positive endosomal membranes and associates with cortical actin cytoskeleton. FEBS Lett. 531, 513-519. [DOI] [PubMed] [Google Scholar]
- Barbieri, M.A., Hoffenberg, S., Roberts, R., Mukhopadhyay, A., Pomrehn, A., Dickey, B.F., and Stahl, P.D. (1998). Evidence for a symmetrical requirement for Rab5-GTP in in vitro endosome-endosome fusion. J. Biol. Chem. 273, 25850-25855. [DOI] [PubMed] [Google Scholar]
- Beatty, W.L., Rhoades, E.R., Hsu, D.K., Liu, F.T., and Russell, D.G. (2002). Association of a macrophage galactoside-binding protein with Mycobacterium-containing phagosomes. Cell Microbiol. 4, 167-176. [DOI] [PubMed] [Google Scholar]
- Beatty, W.L., Rhoades, E.R., Ullrich, H.J., Chatterjee, D., Heuser, J.E., and Russell, D.G. (2000). Trafficking and release of mycobacterial lipids from infected macrophages. Traffic 1, 235-247. [DOI] [PubMed] [Google Scholar]
- Bennett, M.K., Garcia-Arrara's, J.E., Elferink, L.A., Peterson, K., Fleming, A.M., Hazuka, C.D., and Scheller, R.H. (1993). The syntaxin family of vesicular transport receptors. Cell 74, 863-873. [DOI] [PubMed] [Google Scholar]
- Besra, B.S., Morehouse, C.B., Rittner, C.M., Waechter, C.J., and Brennan, P.J. (1997). Biosynthesis of mycobacterial lipoarabinomannan. J. Biol. Chem. 272, 18460-18466. [DOI] [PubMed] [Google Scholar]
- Braell, W.A. (1992). Detection of endocytic vesicle fusion in vitro, using assay based on avidin-biotin association reaction. Methods Enzymol. 219, 12-21. [DOI] [PubMed] [Google Scholar]
- Brennan, P., and Ballou, C.E. (1968). Biosynthesis of mannophosphoinositides by Mycobacterium phlei-enzymatic acylation of dimannophosphoinositides. J. Biol. Chem. 243, 2975-2984. [PubMed] [Google Scholar]
- Christoforidis, S., McBride, H.M., Burgoyne, R.D., and Zerial, M. (1999a). The Rab5 effector EEA1 is a core component of endosome docking. Nature 397, 621-625. [DOI] [PubMed] [Google Scholar]
- Christoforidis, S., Miaczynska, M., Ashman, K., Wilm, M., Zhao, L., Yip, S.C., Waterfield, M.D., Backer, J.M., and Zerial, M. (1999b). Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nat. Cell Biol. 1, 249-252. [DOI] [PubMed] [Google Scholar]
- Clemens, D.L., and Horwitz, M.A. (1996). The Mycobacterium tuberculosis phagosome interacts with early endosomes and is accessible to exogenously administered transferrin. J. Exp. Med. 184, 1349-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corvera, S., D'Arrigo, A., and Stenmark, H. (1999). Phosphoinositides in membrane traffic. Curr. Opin. Cell Biol. 11, 460-465. [DOI] [PubMed] [Google Scholar]
- Covert, B.A., Spencer, J.S., Orme, I.M., and Belisle, J.T. (2001). The application of proteomics in defining the T cell antigens of Mycobacterium tuberculosis. Proteomics 1, 574-586. [DOI] [PubMed] [Google Scholar]
- Dawson, R.M.C., Elliott, D.C., Elliott, W.H., and Jones, K.M. (1993). Data for Biochemical Research, 3rd ed., Clarendon Press, Oxford.
- De Camilli, P., Emr, S.D., McPherson, P.S., and Novick, P. (1996). Phosphoinositides as regulators in membrane traffic. Science 271, 1533-1539. [DOI] [PubMed] [Google Scholar]
- Defacque, H., Bos, E., Garvalov, B., Barret, C., Roy, C., Mangeat, P., Shin, H.W., Rybin, V., and Griffiths, G. (2002). Phosphoinositides regulate membrane-dependent actin assembly by latex bead phagosomes. Mol. Biol. Cell 13, 1190-1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz, R., Mayorga, L., and Stahl, P. (1988). In vitro fusion of endosomes following receptor-mediated endocytosis. J. Biol. Chem. 263, 6093-6100. [PubMed] [Google Scholar]
- Folch, J., Lees, M., and Stanley, G.H.S. (1957). A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 226, 497-509. [PubMed] [Google Scholar]
- Fratti, R.A., Backer, J.M., Gruenberg, J., Corvera, S., and Deretic, V. (2001). Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 154, 631-644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratti, R.A., Chua, J., and Deretic, V. (2003a). Induction of p38 mitogenactivated protein kinase reduces EEA1 recruitment to phagosomal membranes. J. Biol. Chem. 278, 46961-46967. [DOI] [PubMed] [Google Scholar]
- Fratti, R.A., Chua, J., Vergne, I., and Deretic, V. (2003b). Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc. Natl. Acad. Sci. USA 100, 5437-5442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillooly, D.J., Simonsen, A., and Stenmark, H. (2001). Phosphoinositides and phagocytosis. J. Cell Biol. 155, 15-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goren, M. (1984). Biosynthesis and structure of phospholipids and sulfatides. In: The Mycobacteria, Part A; Microbiology Series, vol. 15, ed. P. Kubica and L.G. Wayne, New York: Marcel Dekker, 370-415. [Google Scholar]
- Gorvel, J.P., Chavrier, P., Zerial, M., and Gruenberg, J. (1991). rab5 controls early endosome fusion in vitro. Cell 64, 915-925. [DOI] [PubMed] [Google Scholar]
- Haas, A. (1998). NSF-fusion and beyond. Trends Cell Biol. 8, 471-473. [DOI] [PubMed] [Google Scholar]
- Hinkovska-Galcheva, V.T., Boxer, L.A., Mansfield, P.J., Harsh, D., Blackwood, A., and Shayman, J.A. (1998). The formation of ceramide-1-phosphate during neutrophil phagocytosis and its role in liposome fusion. J. Biol. Chem. 273, 33203-33209. [DOI] [PubMed] [Google Scholar]
- Holroyd, C., Kistner, U., Annaert, W., and Jahn, R. (1999). Fusion of endosomes involved in synaptic vesicle recycling. Mol. Biol. Cell 10, 3035-3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi, H., et al. (1997). A novel Rab5 GDP/GTP exchange factor complexed to Rabaptin-5 links nucleotide exchange to effector recruitment and function. Cell 90, 1149-1159. [DOI] [PubMed] [Google Scholar]
- Kang, B.K., and Schlesinger, L.S. (1998). Characterization of mannose receptor-dependent phagocytosis mediated by Mycobacterium tuberculosis lipoarabinomannan. Infect. Immun. 66, 2769-2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates, M. (1986). Techniques of Lipidology: Isolation, Analysis and Identification of Lipids, 2nd ed., Elsevier, Amsterdam.
- Kelley, V.A., and Schorey, J.S. (2003). Mycobacterium's arrest of phagosome maturation in macrophages requires Rab5 activity and accessibility to iron. Mol. Biol. Cell 14, 3366-3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoo, K.H., Dell, A., Morris, H.R., Brennan, P.J., and Chatterjee, D. (1995). Structural definition of acylated phosphatidylinositol mannosides from Mycobacterium-tuberculosis-definition of a common anchor for lipomannan and lipoarabinomannan. Glycobiology 5, 117-127. [DOI] [PubMed] [Google Scholar]
- Lanzetti, L., Rybin, V., Malabarba, M.G., Christoforidis, S., Scita, G., Zerial, M., and Di Fiore, P.P. (2000). The Eps8 protein coordinates epidermal growth factor receptor signalling through Rac and trafficking through Rab5. Nature 408, 374-377. [DOI] [PubMed] [Google Scholar]
- Lebrand, C., Corti, M., Goodson, H., Cosson, P., Cavalli, V., Mayran, N., Faure, J., and Gruenberg, J. (2002). Late endosome motility depends on lipids via the small GTPase Rab7. EMBO J. 21, 1289-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, R.E., Brennan, P.J., and Besra, G.S. (1996). Mycobacterium tuberculosis cell envelope. Curr. Top. Microbiol. Immunol. 215, 1-27. [DOI] [PubMed] [Google Scholar]
- Li, G., D'Souza-Schorey, C., Barbieri, M.A., Roberts, R.L., Klippel, A., Williams, L.T., and Stahl, P.D. (1995). Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of Rab5. Proc. Natl. Acad. Sci. USA 92, 10207-10211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer, A., Scheglmann, D., Dove, S., Glatz, A., Wickner, W., and Haas, A. (2000). Phosphatidylinositol 4, 5-bisphosphate regulates two steps of homotypic vacuole fusion. Mol. Biol. Cell 11, 807-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayorga, L.S., Bertini, F., and Stahl, P.D. (1991). Fusion of newly formed phagosomes with endosomes in intact cells and in a cell-free system. J. Biol. Chem. 266, 6511-6517. [PubMed] [Google Scholar]
- Mayorga, L.S., Diaz, R., Colombo, M.I., and Stahl, P.D. (1989). GTP gamma S stimulation of endosome fusion suggests a role for a GTP-binding protein in the priming of vesicles before fusion. Cell Regul. 1, 113-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullock, B.M., Bright, N.A., Fearon, C.W., Gray, S.R., and Luzio, J.P. (1998). Fusion of lysosomes with late endosomes produces a hybrid organelle of intermediate density and is NSF dependent. J. Cell Biol. 140, 591-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, C., and Mayer, A. (1998). Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature 396, 575-580. [DOI] [PubMed] [Google Scholar]
- Pryor, P.R., Mullock, B.M., Bright, N.A., Gray, S.R., and Luzio, J.P. (2000). The role of intraorganellar Ca(2+) in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J. Cell Biol. 149, 1053-1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, D.G., Dant, J., and Sturgill-Koszycki, S. (1996). Mycobacterium avium-and Mycobacterium tuberculosis-containing vacuoles are dynamic, fusion-competent vesicles that are accessible to glycosphingolipids from the host cell plasmalemma. J. Immunol. 156, 4764-4773. [PubMed] [Google Scholar]
- Russell, D.G., Mwandumba, H.C., and Rhoades, E.E. (2002). Mycobacterium and the coat of many lipids. J. Cell Biol. 158, 421-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybin, V., Ullrich, O., Rubino, M., Alexandrov, K., Simon, I., Seabra, M.C., Goody, R., and Zerial, M. (1996). GTPase activity of Rab5 acts as a timer for endocytic membrane fusion. Nature 383, 266-269. [DOI] [PubMed] [Google Scholar]
- Schaible, U.E., Collins, H.L., Priem, F., and Kaufmann, S.H. (2002). Correction of the iron overload defect in beta-2-microglobulin knockout mice by lactoferrin abolishes their increased susceptibility to tuberculosis. J. Exp. Med. 196, 1507-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonsen, A., Gaullier, J.M., D'Arrigo, A., and Stenmark, H. (1999). The Rab5 effector EEA1 interacts directly with syntaxin-6. J. Biol. Chem. 274, 28857-28860. [DOI] [PubMed] [Google Scholar]
- Simonsen, A., Lippe, R., Christoforidis, S., Gaullier, J.M., Brech, A., Callaghan, J., Toh, B.H., Murphy, C., Zerial, M., and Stenmark, H. (1998). EEA1 links PI(3)K function to Rab5 regulation of endosome fusion. Nature 394, 494-498. [DOI] [PubMed] [Google Scholar]
- Simonsen, A., Wurmser, A.E., Emr, S.D., and Stenmark, H. (2001). The role of phosphoinositides in membrane transport. Curr. Opin. Cell Biol. 13, 485-492. [DOI] [PubMed] [Google Scholar]
- Soldati, T., Riederer, M.A., and Pfeffer, S.R. (1993). Rab GDI: a solubilizing and recycling factor for rab9 protein. Mol. Biol. Cell 4, 425-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgill-Koszycki, S., Schaible, U.E., and Russell, D.G. (1996). Mycobacterium-containing phagosomes are accessible to early endosomes and reflect a transitional state in normal phagosome biogenesis. EMBO J. 15, 6960-6968. [PMC free article] [PubMed] [Google Scholar]
- Sturgill-Koszycki, S., Schlesinger, P.H., Chakraborty, P., Haddix, P.L., Collins, H.L., Fok, A.K., Allen, R.D., Gluck, S.L., Heuser, J., and Russell, D.G. (1994). Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263, 678-681. [DOI] [PubMed] [Google Scholar]
- Takayama, K., Schnoes, H.K., Armstrong, E.L., and Boyle, R.W. (1975). Site of inhibitory action of isoniazid in the synthesis of mycolic acids in Mycobacterium tuberculosis. J. Lipid Res. 16, 308-317. [PubMed] [Google Scholar]
- Tall, G.G., Barbieri, M.A., Stahl, P.D., and Horazdovsky, B.F. (2001). Ras-activated endocytosis is mediated by the Rab5 guanine nucleotide exchange activity of RIN1. Dev. Cell 1, 73-82. [DOI] [PubMed] [Google Scholar]
- Vergne, I., Chua, J., and Deretic, V. (2003a). Mycobacterium tuberculosis Phagosome maturation arrest: selective targeting of PI3P-dependent membrane trafficking. Traffic 4, 600-606. [DOI] [PubMed] [Google Scholar]
- Vergne, I., Chua, J., and Deretic, V. (2003b). Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J. Exp. Med. 198, 653-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Via, L.E., Deretic, D., Ulmer, R.J., Hibler, N.S., Huber, L.A., and Deretic, V. (1997). Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by rab5 and rab7. J. Biol. Chem. 272, 13326-13331. [DOI] [PubMed] [Google Scholar]
- Via, L.E., Fratti, R.A., McFalone, M., Pagan-Ramos, E., Deretic, D., and Deretic, V. (1998). Effects of cytokines on mycobacterial phagosome maturation. J. Cell Sci. 111, 897-905. [DOI] [PubMed] [Google Scholar]
- Vieira, O.V., Botelho, R.J., and Grinstein, S. (2002). Phagosome maturation: aging gracefully. Biochem. J. 366, 689-704. [DOI] [PMC free article] [PubMed] [Google Scholar]