Abstract
Integrin-mediated cell adhesion stimulates a cascade of signaling pathways that control cell proliferation, migration, and survival, mostly through tyrosine phosphorylation of signaling molecules. p130Cas, originally identified as a major substrate of v-Src, is a scaffold molecule that interacts with several proteins and mediates multiple cellular events after cell adhesion and mitogen treatment. Here, we describe a novel p130Cas-associated protein named p140Cap (Cas-associated protein) as a new tyrosine phosphorylated molecule involved in integrin- and epidermal growth factor (EGF)-dependent signaling. By affinity chromatography of human ECV304 cell extracts on a MBP-p130Cas column followed by mass spectrometry matrix-assisted laser desorption ionization/time of flight analysis, we identified p140Cap as a protein migrating at 140 kDa. We detected its expression in human, mouse, and rat cells and in different mouse tissues. Endogenous and transfected p140Cap proteins coimmunoprecipitate with p130Cas in ECV304 and in human embryonic kidney 293 cells and associate with p130Cas through their carboxy-terminal region. By immunofluorescence analysis, we demonstrated that in ECV304 cells plated on fibronectin, the endogenous p140Cap colocalizes with p130Cas in the perinuclear region as well as in lamellipodia. In addition p140Cap codistributes with cortical actin and actin stress fibers but not with focal adhesions. We also show that p140Cap is tyrosine phosphorylated within 15 min of cell adhesion to integrin ligands. p140Cap tyrosine phosphorylation is also induced in response to EGF through an EGF receptor dependent-mechanism. Interestingly expression of p140Cap in NIH3T3 and in ECV304 cells delays the onset of cell spreading in the early phases of cell adhesion to fibronectin. Therefore, p140Cap is a novel protein associated with p130Cas and actin cytoskeletal structures. Its tyrosine phosphorylation by integrin-mediated adhesion and EGF stimulation and its involvement in cell spreading on matrix proteins suggest that p140Cap plays a role in controlling actin cytoskeleton organization in response to adhesive and growth factor signaling.
INTRODUCTION
Integrins are extracellular matrix (ECM) receptors formed by alpha and beta subunits, which mediate cell-matrix adhesion. Integrin-dependent adhesion stimulates multiple signaling pathways that modulate actin cytoskeleton organization, cell motility, cell growth, and the ability of cells to escape from apoptosis (Miranti and Brugge, 2002). Integrin-dependent signaling requires tyrosine phosphorylation of cytoplasmic proteins such as p125 focal adhesion kinase (Fak) and Src family kinases, resulting in the activation of downstream signaling pathways such as the extracellular signal-regulated kinases 1 and 2 (Defilippi et al., 1997a; Giancotti and Ruoslahti, 1999; Damsky and Ilic, 2002; Giancotti and Tarone, 2003). On adhesion to ECM p125Fak is phosphorylated on tyrosine 397, exposing high-affinity binding sites for the SH2 domain of c-Src (Schaller et al., 1994; Schlaepfer et al., 1997). Src activation is required for the phosphorylation of the adaptor protein p130Cas (Tachibana et al., 1997) and of focal adhesion components such as paxillin, and p125Fak itself, thus contributing to integrin-mediated signaling propagation (Ruest et al., 2001; Hanks et al., 2003; Hsia et al., 2003).
Integrins are also known to cooperate with growth factor receptors either by enhancing signaling pathways in response to insulin, platelet-derived growth factor, epidermal growth factor (EGF), fibroblast growth factor, and vascular endothelial growth factor (Vuori and Ruoslahti, 1994; Miyamoto et al., 1996; Schwartz and Baron, 1999; Assoian and Schwartz, 2001) or by directly phosphorylating growth factor receptors in a ligand-independent manner (Rusciano et al., 1996; Sundberg and Rubin, 1996; Wang et al., 1996; Moro et al., 1998, 2002).
p130Cas is an ubiquitous protein originally identified as a phosphorylated protein associated with v-Crk (Matsuda et al., 1990; Sakai et al., 1994) and v-Src (Reynolds et al., 1989). p130Cas is extensively tyrosine phosphorylated in nontransformed cells in response to adhesion to ECM (Vuori and Ruoslahti, 1995; Harte et al., 1996; Nakamoto et al., 1996) and to growth factor stimuli (Casamassima and Rozengurt, 1997; Ojaniemi and Vuori, 1997). As a consequence of its phosphorylation, it is involved in the linkage of actin cytoskeleton to the extracellular matrix during cell migration, cell invasion, and cell transformation (for reviews, see Defilippi et al., 1997a, 1997b; Giancotti and Ruoslahti, 1999; O'Neill et al., 2000; Bouton et al., 2001). We have recently shown that upon integrin engagement, p130Cas is tyrosine phosphorylated (Moro et al., 1998), and its presence is required for integrin-dependent activation of EGFR. In the early phases of cell adhesion, p130Cas is also recruited on the cell membrane in a macromolecular complex with integrins, EGFR, and the c-Src kinase (Moro et al., 2002).
p130Cas modular structure is characterized by an amino-terminal Src homology 3 domain (SH3), a proline-rich region, a large substrate binding domain with 15 repeats of the YXXP sequence, a serine-rich region, and a carboxy-terminal domain with an additional proline-rich sequence. On the p130Cas molecule, phosphorylated tyrosines in the YXXP sequence, the proline-rich region and the SH3 domain are docking sites for multiple interacting effector proteins. p125Fak, the Fak related protein Pyk2, Src family kinases, the adaptor Crk, and the phosphatases PTP1B and PTP-PEST have been demonstrated to interact with distinct domains of p130Cas both in vivo and in vitro (O'Neill et al., 2000; Bouton et al., 2001). How these complexes contribute to the role of p130Cas in cell proliferation, migration, and survival is still poorly understood.
In the present study, we isolated p140Cap as a novel p130Cas-associated protein. p140Cap colocalizes with actin stress fibers and with p130Cas in membrane ruffling. Its tyrosine phosphorylation upon integrin-mediated adhesion and EGF treatment and its ability to modulate cell spreading on fibronectin suggest a role for this molecule in regulating the interplay between integrins and growth factor receptor signaling.
MATERIALS AND METHODS
Cell Lines
Human ECV304, HeLa, and HEK293 epithelial cells and murine neuroblastoma NIE115 were obtained from American Type Culture Collection (Manassas, VA) and cultured in DMEM medium supplemented with 10% heat-inactivated fetal calf serum (FCS) and penicillin/streptomycin (100 U/ml and 100 μg/ml, respectively) at 37°C in a 5% CO2 atmosphere. Human breast carcinoma T47D and neuroblastoma SH-SY5Y cells, obtained from American Type Culture Collection, were cultured in RPMI 1640 medium supplemented with 10% FCS and antibiotics. Fisher rat thyroid (FRT) cells (kind gift of Dr. Lucio Nitsch, Napoli, Italy) were cultured in Coon's F12 medium supplemented with 10% FCS and antibiotics. NIH3T3 cells (kind gift of Dr. A. Eva, Genova, Italy) were cultured in DMEM medium supplemented with 10% heat-inactivated CS (Colorado) and penicillin/streptomycin (100 U/ml and 100 μg/ml, respectively) at 37°C in a 5% CO2 atmosphere. Tissue culture media, fetal calf serum, and antibiotics were obtained from Invitrogen (Carlsbad, CA).
Reagents and Antibodies
Polyclonal and monoclonal antibodies were produced against a MBP-p140Cap recombinant protein obtained in Escherichia coli by fusing the sequence corresponding to amino acids 808-1026 of human KIAA1684 to the maltose binding protein (MBP). This sequence was cloned by reverse transcription-polymerase chain reaction (RT-PCR) from ECV304 RNA as a 654-bp fragment and inserted into the EcoRI site of pMAL-C2 vector. The MBP-p140Cap protein was injected into rabbits and mice, and the resulting antibodies were characterized by Western blotting and immunoprecipitation on ECV304 cell extracts. To test antibody specificity, preadsorption with MBP-p140Cap fusion protein was performed.
Rabbit polyclonal serum, previously adsorbed on an MBP-CNBr-Sepharose column, was purified by affinity chromatography on a MBP-p140Cap CNBr-Sepharose column by 12-h incubation at 4°C. After extensive washing with phosphate-buffered saline (PBS), bound antibodies were eluted with 0.1 M glycine pH 3 and subjected to dialysis against PBS overnight. Monoclonal antibodies (mAbs) 2A8 and 1A11 were affinity purified on protein A-Sepharose as described previously (Ey et al., 1978) and the purity of the antibodies was determined to be >95%.
Antibodies to Crk and p130Cas were purchased from BD Transduction Laboratories (Lexington, KY), and antibody PY99 to phosphotyrosine was from Santa Cruz Biotechnology (Santa Cruz, CA). mAb L230 to the αv integrin subunit was described previously (Moro et al., 2002). mAb 9E10 to the Myc epitope was purchased from American Type Culture Collection. Fluorescein-labeled phalloidin and rhodamine-conjugated anti-rabbit and antimouse antibodies were from Sigma-Aldrich (St. Louis, MO). Fluorescein-conjugated anti-rabbit antibodies were from Jackson ImmunoResearch Laboratories (West Grove, PA).
Fibronectin was purified from human plasma as described previously (Defilippi et al., 1994). Human recombinant EGF and phorbol-12-myristate-13-acetate (PMA) were from Sigma-Aldrich. Coomassie Brilliant Blue, CNBr-activated Sepharose, protein A-Sepharose, protein G-Sepharose, nitrocellulose, and films were from Amersham Biosciences (Piscataway, NJ). Tyrphostin AG1478 was from Calbiochem (San Diego, CA).
Affinity Chromatography and Mass Spectrometry Analysis
A cDNA fragment of p130Cas cDNA (kind gift of S. Hanks, Nashville, VA) was cloned in the EcoRI site of pMAL-C2 vector, and the resulting MBP-p130CasΔN recombinant protein, encompassing aa 361-895, was immobilized on CNBr-activated Sepharose 4B. As control, the MBP protein alone was bound to CNBr-activated Sepharose 4B.
ECV304 cells were grown to confluence and extracted with lysis buffer (1% NP-40, 150 mM NaCl, 20 mM Tris-HCl pH 7.5, 1 mM sodium orthovanadate, 10 μg/ml each of leupeptin, pepstatin, and aprotinin). MBP-p130CasΔN or MBP columns were incubated with 60 mg of cell extract for 12 h at 4°C. Columns were washed with PBS, and eight fractions were eluted with 0.1 M glycine pH 3. One tenth of each fraction was analyzed by SDS-PAGE and fractions 4-6 were combined and loaded onto 5-10% SDS-PAGE gradient. Gels were stained with Coomassie Blue, and the identified bands were excised and analyzed by matrix-assisted laser desorption ionization/time of flight (MALDI-TOF) mass spectroscopy. Bands were destained overnight with a solution of 50 mM ammonium bicarbonate, 40% ethanol. The protein was digested in gel with trypsin (Promega, Madison, WI) according to Hellman et al. (1995), except that the bands had been washed three times with acetonitrile before drying them in a speed vacuum concentrator. For MALDITOF mass spectrometry, aliquots of 0.5 μl of the peptide mixtures were applied to a target disk and allowed to air dry. Subsequently, 0.5 μl of matrix solution [1% (wt:vol) α-cyano-4-hydroxycinnamic acid in 50% acetonitrile, 0.1% trifluoroacetic acid] was applied to the dried sample and again allowed to dry. Spectra were obtained using a Biflex III MALDI-TOF spectrometer (Bruker, Bremen, Germany). For interpretation of the protein fragments, PeptIdent (http://www.expasy.ch/tools/peptident.html), MS-Fit (http://falcon.ludwig.ucl.ac.uk/MS-Fit.html), and Profound (http://prowl.rockefeller.edu/cgibin/ProFound) software was used.
Total RNA and RT-PCR Analysis
Total RNA was extracted from frozen brain tissue by using the RNAeasy total RNA kit (QIAGEN, Valencia, CA) according to manufacturer's instructions. Quantification and purity of the RNA was assessed by A260/A280 absorption, and RNA quality was assessed by agarose gel electrophoresis. Five micrograms of RNA template was reverse-transcribed into cDNA (MV-MLV reverse transcriptase enzyme; Invitrogen) in a final volume of 100 μl. The cDNA templates were amplified by PCR with specific primers.
Amplification of exon 1a and 1b with exon 2 were performed with the following primers: A1HF (5′-GGGCCGAGCGAGGAGC-3′) for exon 1a, A2F (5′-CATGCAGCCCTGGCAGTG-3′) for exon 1b, and B1R2 (5′-CGCTTGCGGTCAGCATCC-3′) for exon 2. Amplicons were analyzed by 2% agarose gel electrophoresis with a 1-kb DNA ladder (Invitrogen).
Analysis of p140Cap Expression in Cells and Tissues
For Western blot analysis, ECV304, T47D, FRT, N1E115, and HeLa cells were extracted by sonication and boiling in a solution containing 60 mM Tris-Cl pH 6.8, 2% SDS, 1 mM Na3VO4, 10 mM NaF, 10 μg/ml leupeptin, 0.4 μg/ml pepstatin, 0.1 U/ml aprotinin.
For tissue analysis organs from adult mice were frozen and triturated in liquid nitrogen and extracted in the same buffer as described above. Proteins were analyzed by 6% SDS-PAGE and Western blotting by using standard procedures, by detection with peroxidase-conjugated secondary antibodies and chemoluminescent reagent.
DNA Constructs and Transfection
For expression in mammalian cells, full-length mouse p140Cap cDNA (kind gift of Dr. Consalez, DIBIT, Milan, Italy) was cloned into the BamHI site of pCDNA3.1/Myc-Hys expression vector (Invitrogen) and sequenced. Full-length mouse p140Cap cDNA was cloned into the EcoRI-KpnI sites of pEGFP-N2 vector (BD Biosciences Clontech, Palo Alto, CA) and sequenced. p140-ΔCT expression vector carrying a deletion of the carboxy-terminal region was obtained by digestion with Xho restriction endonucleases of the full-length p140Cap cDNA. The resulting Xho-fragment was then cloned into the Xho site of pCDNA3.1/Myc-Hys and in pEGFP-N2 (BD Biosciences Clontech) expression vector and sequenced.
HEK293 cells were transfected by calcium phosphate precipitation by conventional procedures. Twenty-four hours after transfection, the medium was changed to DMEM containing 10% fetal bovine serum, and cells were incubated for 24 h before processing. When indicated, cells were serum deprived for the last 15 h.
Pull-Down Experiments
Five cDNA fragments coding for distinct p140Cap regions were amplified by PCR from full-length mouse p140Cap cDNA and cloned into EcoRI restriction site of pGEX-5 × 1 vector. Starting from the ATG codon, we generated the following p140Cap fragments: 1-305 aa (Cap1), 305-568 aa (Cap2), 568-788 aa (Cap3), 788-1000 aa (Cap4), and 1000-1217 aa (Cap5). As positive control, a GST-Crk-SH2 protein was produced from the pGex-Crk-SH2 cDNA (kind gift of Dr. K. Vuori, La Jolla, Ca). To produce YXXP (aa117-418), Ab1-Ab2 (aa361-378) and CT (aa544-874) recombinant p130Cas proteins, YXXP, and CT fragments were obtained by digestion by using BamHI and EcoRI sites of pRK5-YXXP and pRK5-CT (kind gift of A.H. Bouton, Charlottesville, VA) and cloned into the same sites of pGEX-4T3 (Amersham Biosciences). The Ab1-Ab2 fragment was amplified by PCR from full-length mouse p130Cas cDNA and cloned into EcoRI restriction site of pGEX-5 × 1 vector. Recombinant GST-fusion proteins were produced in E. coli. Approximately 0.4 mg of each protein in bacterial lysis buffer (1 mM EDTA, 100 mM NaCl, 50 mM Tris-HCl pH 7.5, 5% glycerol, 0.1% Triton X-100, 1 mM EDTA, 10 μg/ml each of leupeptin, pepstatin, and aprotinin) was bound to 100 μl of 50% glutathione-Sepharose beads for 30 min to 4°C, washed three times with glutathione S-transferase (GST)-fish buffer (10% glycerol, 50 mM Tris pH 7.4, 100 mM NaCl, 1% NP-40, 2 mM MgCl2, 10 μg/ml each of leupeptin, pepstatin, and aprotinin). For pull-down experiments untransfected or p140Cap transfected HEK293 cells were washed in ice-cold PBS and extracted in 750 μl of GST-fish buffer. Then, 0.5 mg of HEK293 cell extracts was incubated with 10 μl of GST-fusion protein beads at 4°C for 1 h. After three washes in GST-fish buffer, beads were eluted in 20 μl of Laemmli sample buffer, and the eluted complexes run on 6% SDS-PAGE and immunoblotted with anti p130Cas mAb or with anti p140Cap polyclonal antibodies.
Immunoprecipitation and Immunoblotting
ECV304 and transfected HEK293 cells were extracted with 1% Nonidet NP-40 lysis buffer as indicated above. Cell lysates were centrifuged at 13,000 × g for 10 min, and the supernatants were collected and assayed for protein concentration by using the Bio-Rad protein assay method (Bio-Rad, Hercules, CA). For immunoprecipitation experiments, precleared lysates were incubated for 2 h at 4 C with 5 μg/ml purified antibodies or 5 μl of preimmune sera in the presence of 20 μl of protein A or protein G-Sepharose beads. The beads were washed three times with 1 ml of Tris-buffered saline 0.5% Triton X-100 and once with 1 ml of Tris-buffered saline 0.5% Triton X-100-0.1% SDS, and the immunoprecipitates were eluted by boiling the beads in 2× Laemmli sample buffer for 5 min. The immunoprecipitates were resolved on 6% SDS-polyacrylamide gel and transferred onto nitrocellulose for immunoblotting, reacted with specific antibodies, and then detected with peroxidase-conjugated secondary antibodies and chemiluminescent enhanced chemiluminescence reagent. When appropriate, the nitrocellulose membranes were stripped according to manufacturer's recommendations and reprobed.
Cell Adhesion Assay and Growth Factor Treatment
HEK293 cells were transfected with pcDNA3.1-p140Cap cDNA as described above for 48 h and serum deprived for the last 24 h. Cells were than incubated either with 20% FCS or 50 ng/ml recombinant EGF in serum-free medium for the indicated times. Inhibition of EGF receptor kinase activity was performed by adding 250 nM tyrphostin AG1478 to cells. For cell adhesion assay, cells were detached by gentle pipetting, washed twice in serum-free DMEM, and kept in suspension or plated for different times on 10 μg/ml αv integrin antibody-coated dishes or 10 μg/ml fibronectin-coated dishes as described previously (Moro et al., 2002). Cells were then washed with PBS and extracted in radioimmunoprecipitation buffer (0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate 1 mM Na3VO4, 10 μg/ml leupeptin, 4 μg/ml pepstatin, and 0.1 U/ml aprotinin). One milligram of protein extract was immunoprecipitated and immunoblotted as described above.
Colocalization Experiments and Cell Spreading Analysis
Glass coverslips were coated with 10 μg/ml fibronectin overnight at 4°C. ECV304 cells were detached from the plates, replated on the coverslips in the presence of DMEM supplemented with 10% fetal bovine serum, and left to adhere for 12 h. When indicated, 100 nM PMA was added to the medium for 1 h. Coverslips were washed in PBS, fixed for 15 min with 4% p-formaldehyde, and permeabilized for 1 min with PBS-0.1% Triton X-100. Coverslips were saturated for 30 min with 5% goat serum in PBS-0.1% Triton X-100 and incubated with primary antibodies (10 μg/ml) at room temperature for 60 min. After three washes in PBS-0.1% Triton X-100, coverslips were incubated with fluorescein- or rhodamine-labeled secondary antibodies (3 μg/ml) or phalloidin (2 μg/ml) for 1 h. The coverslips were washed five times in PBS-0.1% Triton X-100 for 5 min and mounted on glass slides with Mowiol.
For cell spreading experiments, NIH3T3 and ECV304 cells were transfected with the indicated expression vectors (see “DNA Constructs and Transfection”) carrying either the full-length p140Cap or the p140Cap-5′ with LipofectAMINE (Invitrogen) according to manufacturer's instructions. Forty-eight hours after transfection, cells were detached by 5 mM EDTA treatment, washed twice in serum-free DMEM, and plated for different times on 10 μg/ml fibronectin-coated glass coverslips. Cells were then washed in PBS, fixed for 15 min with 4% p-formaldehyde, and subjected to immunofluorescence analysis. Cell images were visualized on an IX70 Olympus microscope with an Uplan FI 100× oil objective and analyzed with IAS 2000 software (Delta Sistema, Rome, Italy). In the p130Cas colocalization experiments, cells were analyzed under a confocal microscope TCS SPII scanner (Leika, Heerbrugg, Switzerland) connected to an inverted microscope (Leika). The specificity of each staining was verified by setting two distinct not overlapping emission wavelengths. Images were processed using LCS software package (Leika).
RESULTS
Identification of a New p130Cas-associated Protein
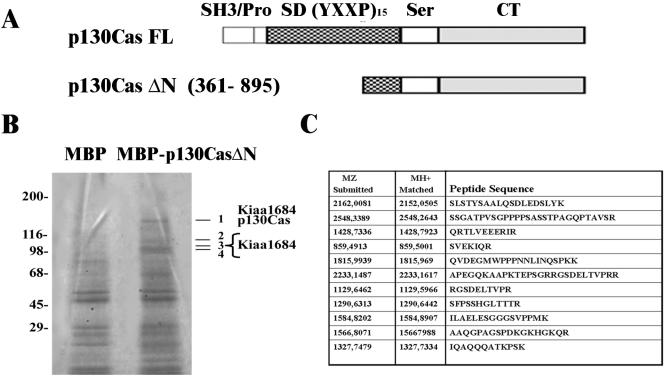
p130Cas has been demonstrated to associate with several signaling molecules, thus participating in different cellular processes such as cell migration, cell transformation, and cell adhesion (O'Neill et al., 2000; Bouton et al., 2001). Affinity chromatography experiments were performed to identify new interactors of p130Cas, whose schematic structure, with distinct domains depicted in it, is represented in the diagram in Figure 1A. The region of p130Cas from amino acids 361-895, containing part of the substrate domain, the serinerich region, and the carboxy-terminal domain, was fused to MBP and the resulting fusion protein (p130CasΔN) was immobilized on CnBr-activated Sepharose 4B (Figure 1A). Affinity chromatography was performed on ECV304 cell extracts, and the eluted fractions were run on 5-15% SDS-PAGE gradient and stained with Coomassie Colloidal Blue. As shown in Figure 1B, distinct bands migrating in a range between 140 and 96 kDa were selectively recovered from MBP-p130CasΔN column. Four of them (Figure 1B, 1-4) were excised from the gel and subjected to MALDI-TOF mass spectrometry analysis. From 18 tryptic peptides derived from band 1, seven were identified as p130Cas itself, which has been previously shown to homodimerize (Law et al., 1996). In addition, 11 peptides present in the same band, listed in Figure 1C, corresponded to a partial protein sequence (KIAA1684) derived from an unknown human gene (GenBank accession no. BAB21775). Tryptic digestion of bands 2-4 led to the identification of the same protein (our unpublished data), suggesting that these bands might either represent proteolytic fragments or distinct isoforms of the protein detected in band 1. The corresponding cDNA of human KIAA1684 was uncompleted; however blasting the partial sequence in GenBank revealed that this cDNA is highly homologous to those encoding for an unpublished mouse protein p140 (GenBank accession no. NP_061361) and for the rat SNIP protein (GenBank accession no. NP_062251) (Chin et al., 2000). The molecular mass of 140 kDa of band 1 was compatible with a complete KIAA1684 protein, suggesting that this protein might be the human ortholog of mouse p140 and rat SNIP proteins. Therefore, these data show that the human unknown KIAA1684 protein corresponds to the p140 band selectively eluted from MBP-p130Cas column, suggesting that the two proteins might associate into the cells.
Figure 1.
Identification of KIAA1684 protein as a p130Cas-associated protein by affinity chromatography and mass spectrometry MALDITOF analysis. (A) Schematic diagram of the structural domains of full-length (FL) or truncated (ΔN) p130Cas molecules. (B) ECV304 cell lysates were loaded on MBP or MBP-p130CasΔN-Sepharose columns, and eluted fractions 4-6 were collected and run on a 5-10% SDS-PAGE gradient. Proteins migrating with molecular masses around 98-130 kDa were specifically detected by Coomassie Blue staining in the MBP-p130CasΔN eluted fractions (right line) but not in the MBP-eluted ones (left line). Bands 1-4 were excised and analyzed by mass spectrometry MALDI-TOF. Molecular mass markers are shown on the left. (C) List of peptide sequences derived from MALDI-TOF spectra of band 1 that led to identification of the KIAA1684 protein. The results are representative of three independent experiments.
Analysis of the Expression of KIAA1684 and p140 Proteins
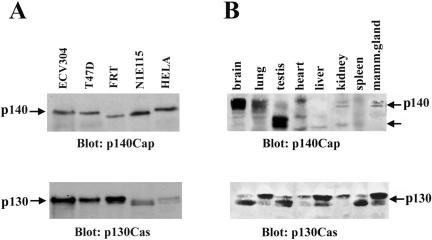
To analyze the pattern of expression of the KIAA1684 protein, a fragment containing the region from amino acids 808-1026 was cloned by RT-PCR from ECV304 mRNA and the obtained fusion protein used to generate polyclonal and monoclonal antibodies (see details in MATERIALS AND METHODS). Purified antibodies were tested on extracts of human ECV304, mammary breast carcinoma T47D, and cervix carcinoma HeLa epithelial cells and found to detect a major band migrating at 140 kDa (Figure 2A). A similar band was also observed in FRT epithelial cells and in a murine neuroblastoma N1E115 cells (Figure 2A), showing that the antibodies against the human protein recognized also the rat and mouse endogenous p140 orthologs. The antibodies specificity was determined by competing with the immunogenic fusion protein (our unpublished data). Antibodies also recognized a p140 protein in HEK293 cells transfected with the full-length mouse p140 cDNA (kind gift of Dr. Consalez, Milan, Italy) (Figure 4B).
Figure 2.
Expression of p140Cap protein in cells and tissues. (A) ECV304, T47D, FRT, HeLa epithelial cells, and N1E115 neuroblastoma cells were detergent extracted and analyzed by immunoblotting with anti-p140Cap-purified polyclonal antibodies. The blot was reprobed with p130Cas mAb (bottom). (B) Equal amounts of homogenates (100 μg of protein for lane) from the indicated mouse tissues were analyzed by immunoblotting by using the anti-p140Cap antibodies (top). Proteins migrating as a doublet at 140 and 116 kDa, indicated by the arrows, were specifically detected in brain, lung, kidney, mammary gland, and testis. The blot was reprobed with p130Cas mAb (bottom). p140Cap migration as a single band or a doublet was shown to be dependent on the length of the gels. The results are representative of five independent experiments.
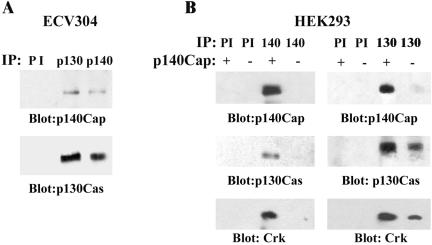
Figure 4.
In vivo interaction between p130Cas and p140Cap. (A) ECV304 cell lysates were immunoprecipitated both with preimmune serum (PI), p130Cas mAb, and p140Cap polyclonal antibodies. Western blotting analysis was performed using anti-p140Cap (top) or p130Cas antibodies (bottom). (B) HEK293 cells either untransfected (-) or transfected with p140Cap cDNA (+) were immunoprecipitated with PI, p130Cas, or p140Cap antibodies. Immunoprecipitates were run on a 6% SDS-PAGE and Western blotted with anti-p140Cap (top) and anti-p130Cas antibodies (middle). The blots were reprobed with anti-Crk antibody (bottom). The results are representative of seven independent experiments.
The term p140Cap (p130Cas-associated protein) will be used in all the following experiments to identify both the human and the mouse p140 proteins.
To test the in vivo expression of p140Cap, different adult mouse tissues were frozen in liquid nitrogen, smashed, and lysed as indicated in Figure 2B. Western blotting analysis by using polyclonal antibodies on tissue lysates showed that p140Cap is mainly expressed in brain, testis, and epithelial-rich tissues such as mammary gland, lung, and kidney. Interestingly, the antibodies recognized a double band at 140 kDa in brain and at 116 kDa in testis, suggesting the existence of different tissue-specific isoforms. Northern blot analysis on adult tissues confirmed the presence of two transcripts of different lengths in brain and testis (our unpublished data). p140Cap migrated as a single band or a doublet (panel A and B) and this mobility was shown to be dependent on the length of the gels. All the cells and tissues positive for p140Cap expression also expressed a high amount of p130Cas (Figure 2, A and B). Therefore, we identify p140Cap as a novel human and mouse protein that is mainly present in brain, testis, and in epithelial-rich tissues as different tissue-specific isoforms.
Genomic Organization of p140Cap
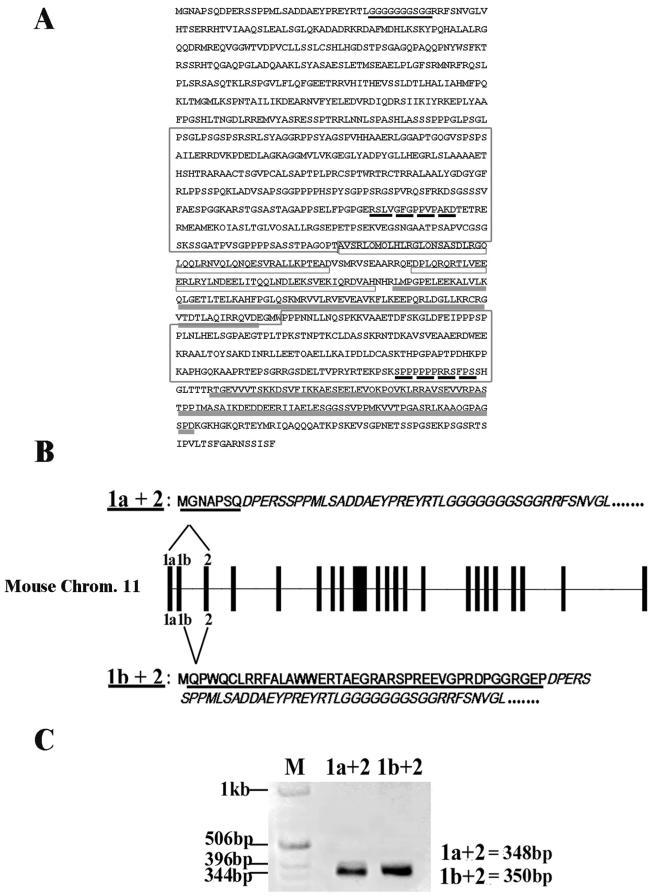
Analysis of the p140Cap structural motifs showed that the molecule did not contain a signal sequence nor a transmembrane domain and presented a glycine-rich region, two proline-rich motifs, two coil-coiled domains, and two C-terminal charged amino acid-rich regions (Figure 3A). However, mouse p140Cap cDNA encodes for an amino-terminal region that is distinct from that of the rat SNIP, suggesting the existence of two N-terminus alternative isoforms. Thus, different approaches were taken to test this hypothesis. Blasting mouse p140Cap cDNA on the mouse genome (www.ensembl.org) allowed the mapping of a single p140 gene on chromosome 11 with a coding sequence spanning on 22 exons (our unpublished data). However, blasting the first 163 bases of the mouse 5′-untranslated cDNA on the human genome revealed the presence of an upstream additional exon that we called 1a (Figure 3B). Similarly, blasting the rat cDNA on the human genome showed that the 5′-untranslated region matched to another upstream exon (1b) located between 1a and 2. To test whether the two putative 1a and 1b exons found on the human genome were also present in the mouse genome, PCR analysis was performed on mouse genomic DNA by using as primers oligonucleotides complementary to either the human 1a or 1b sequences. The products of the PCR amplification were sequenced and shown to be identical to the 1a and 1b human sequences. These results were further confirmed by RT-PCR analysis on mouse brain RNA. Amplification with the 1a and 1b primers and an internal primer for exon 2 and subsequent sequencing, demonstrated that the exon 1a and 1b are alternatively present on mouse cDNA. In fact as shown in Figure 3C, the product of the amplification of exon 1a with exon 2 never included the sequence of exon 1b, although the amplification of exon 1b with exon 2 was equally found. All these results together demonstrate that the p140 mouse gene encodes for at least two distinct isoforms with different amino-terminal domains based on alternative splicing of the exons 1a and 1b. In the following experiments, the 1a containing isoform of the p140Cap was used.
Figure 3.
Genomic organization of p140Cap mouse gene. (A) Amino acid sequence of p140Cap. Indicated are the predicted glycine-rich region (underlined in dark gray), the proline-rich domains (boxed), the putative SH3 binding regions (dashed black underlined), the coiled-coil domains (underlined in white), and the charged sequence (underlined in light gray). (B) p140Cap gene localized on chromosome 11 consists of 24 exons (top). The first two exons, 1a and 1b, are subjected to alternative splicing on exon 2 resulting in mRNA isoforms coding for two proteins that differ in their N-terminal portion (underlined). The sequence of exon 2 is only partially reproduced. (C) RT-PCR products of the two isoforms of the p140Cap gene was performed on total RNA extracted from mouse brain. cDNA was subjected to PCR by using specific oligonucleotides on 1a, 1b, and 2 exon sequences. The molecular weights of 1a + 2 (348-base pair) and 1b + 2 (350-base pair) amplicons are shown. Note the absence of an amplicon of 651 base pairs that would have been represented 1a + 1b + 2. DNA molecular weight ladder is shown on the left. The results are representative of two independent experiments.
In Vivo Association of p140Cap and p130Cas
To obtain evidences for the in vivo association between p140Cap and p130Cas, the endogenous proteins were coimmunoprecitated from ECV304 cell extracts by using either p130Cas or p140Cap antibodies. The immunoprecipitates were run on a 6% SDS-PAGE and blotted. As shown in Figure 4A, antibodies to p140Cap were able to immunoprecipitate p130Cas and vice versa, indicating that the endogenous proteins associate in an immunoprecipitable complex in ECV304 cells. To further assess the association of p140Cap with p130Cas, coimmunoprecipitation experiments were also performed in HEK293 cells transfected with p140Cap expression vector. As shown in Figure 4B, transfected p140Cap associated with endogenous p130Cas. In addition p140Cap and p130Cas immunocomplexes also contained the adaptor protein Crk, a known interactor of p130Cas, indicating that p140Cap and p130Cas take part in a macromolecular complex.
Together, these data clearly demonstrate that both endogenous and transfected p140Cap associate with p130Cas in a macromolecular complex in which the Crk protein is also present.
p140Cap Associates to p130Cas through Its Carboxy-terminal Domain
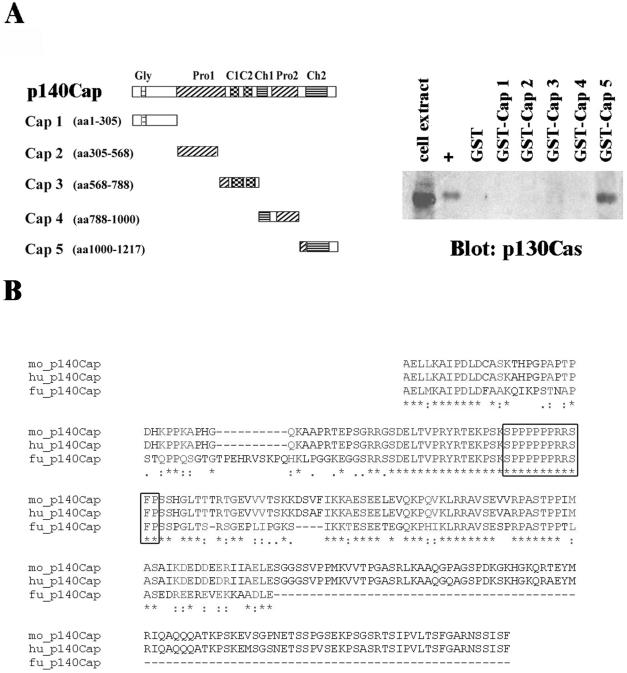
To define the protein regions required for the association between p140Cap and p130Cas, we generated five GST-fusion proteins bearing different portions of p140Cap (Figure 5A, left). The five fragments of p140Cap were generated by PCR from mouse p140 cDNA and cloned into GST-vector. The resulting GST-fusion proteins were immobilized on glutathione beads and used to affinity purify endogenous p130Cas from HEK293 cell extracts. Proteins eluted from the different columns were resolved on SDS-PAGE and subjected to Western blotting analysis by using antibodies against p130Cas. A recombinant protein expressing the Crk-SH2 domain, known to bind the substrate domain of p130Cas (Sakai et al., 1994), was used as positive control. As shown in Figure 5A, right, among all the GST-fusion proteins used, only the one reproducing the carboxy-terminal part of p140Cap (Cap 5) was able to pull down p130Cas from HEK293 cell lysates. The Cap 5 fragment contained a proline-rich region and a charged amino acid stretch, which by sequence comparison, was shown to be highly conserved among human, mouse, and fish species (Figure 5B). As an alternative approach to demonstrate the interaction between p130Cas and the p140Cap carboxy terminal region, HEK293 cells were transfected with Myc-p140Cap full-length cDNA or with a mutant Myc-p140Cap cDNA deleted of the Cap 5 region (indicated as 140-5′). Cell extracts were immunoprecipitated with antibodies to p130Cas, and the immunoprecipitates were blotted with anti-Myc antibodies. Figure 5C clearly demonstrated that only the full-length p140Cap was recovered in the p130Cas immunoprecipitates, whereas the mutant was not. Together, these data confirmed that the carboxy terminal region of p140Cap is required for the association between the two molecules.
Figure 5.
p140Cap binds to p130Cas through the carboxy-terminal domain. A, left, a schematic representation of full-length p140Cap and of recombinant GST-p140Cap fragments. Right, pull down of p130Cas from HEK293 cell lysates on immobilized GST, GST-CrkSH2 (+), and GST-P140Cap fusion proteins. Bound proteins were eluted and analyzed by 8% SDS-PAGE and immunoblotted by using p130Cas mAb. The results are representative of two independent experiments. (B) Multiple alignment of human (Hu), mouse (Mo), and fugu (Fu) amino acids of the Cap5 region. *, single, fully conserved residues,:, conservation of strong groups;., conservation of weak groups. Box: highly conserved proline-rich region. (C) HEK293 cells were transfected either with full-length p140Cap or with p140-ΔCT mutant deleted of the region associating with p130Cas. Immunoprecipitation was performed using anti-p130Cas antibodies and immunoprecipitates were run on a 6% SDS-PAGE and blotted with anti-myc antibodies (D) Left, schematic diagram of different domains of full-length p130Cas and of GST-p130CasΔ, GST-YXXP, GST-Ab1-Ab2, and GST-CT (from top to bottom) used for pull-down assays. Left, pull down of p140Cap from p140Cap-transfected HEK293 cell lysates bound to GST, GST-YXXP, GST-Ab1-Ab2 and GST-CT fusion proteins immobilized on glutathione-Sepharose beads. Bound proteins were eluted and analyzed by 8% SDS-PAGE and immunoblotted using p140Cap polyclonal antibodies.
To identify the region of p130Cas involved in the association between the two molecules, three different GST-fusion proteins bearing different parts of p130Cas (Figure 5D, left) were used to purify p140Cap from transfected HEK293 cell extracts. As shown in Figure 5D, right panel, both GST-Ab1-Ab2 and GST-CT were able to pull down endogenous p140Cap, whereas GST-YXXP protein was not. As indicated in the schematic diagram in Figure 5D (left), the two p130Cas fragments that associate to p140Cap presented an overlapping region encompassing amino acids 544-678. Interestingly, this shared region contains consensus sequences for the interaction with SH3 and SH2 domains and several stretches of charged amino acids that might participate to the association of p130Cas and p140Cap.
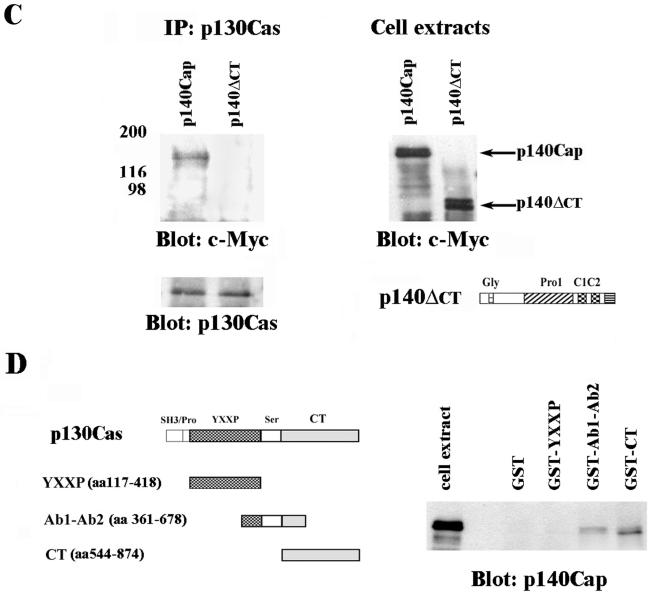
p140Cap Localization in ECV304 Cells
To assess p140Cap localization, immunofluorescence experiments were performed. ECV304 cells were detached from the dishes and replated on fibronectin for 12 h in the presence of serum. As shown in Figure 6, b and e, p140Cap, in addition to a cytoplasmic punctuate staining enriched in the perinuclear region, is specifically localized on tiny cables that extends toward the periphery of the cells and at the cellular edges. Similar staining patterns were obtained using either purified polyclonal or monoclonal antibodies to p140Cap. As shown by phalloidin staining (a), p140Cap-positive fibers correspond to actin filaments and cortical actin. The specificity of each staining was verified by using confocal analysis with two distinct nonoverlapping emission wavelengths. Interestingly, costaining with antibodies specific to paxillin, which is predominantly localized in focal adhesions, showed that these adhesive structures were negative for the p140Cap antibodies (our unpublished data). Therefore, these data indicate that p140Cap associates with actin cytoskeleton but it is excluded from focal adhesions. In addition the p140Cap cytoplasmic punctuate positive structures were found to significantly overlap with p130Cas staining (d-f). To further analyze the colocalization of p140Cap and p130Cas, cells were treated with 100 nM PMA, a well recognized strategy for inducing the formation of cellular extensions after growth factors deprivation. The addition of PMA induces lamellipodial formation and actin assembly (Downey et al., 1992; Chew et al., 2002). In our experimental conditions, this treatment induced a massive reorganization of actin in lamellipodia-like structures. Confocal analysis showed that membrane ruffles were intensively stained by antibodies to p140Cap as well as to p130Cas (g and h), indicating that p140Cap and p130Cas colocalize in specific membrane microdomains as indicated by the merging in i.
Figure 6.
Subcellular localization of p140Cap and colocalization with p130Cas. (A) ECV304 cells were detached from culture dishes and plated for 12 h on fibronectin-coated coverslips in presence of serum. Some coverslips were treated with 100 nM PMA for 1 h (g-i). Cells were then fixed, permeabilized, and double labeled using anti-p140Cap purified polyclonal antibody (b, e, and h), phalloidin (Phd) (a), or anti-p130Cas mAb (d and g). p140Cap colocalization with stress fibers is indicated in the inset of the overlay (c), with a higher magnification. d, e, g, and h show confocal analysis of cells double stained with p130Cas and p140Cap in growing conditions (d and e) or in presence of PMA (g and h). The overlay staining is shown in c, f, and i. In the overlay (i), the inset represents an enlargement of the area indicated by the arrow. The figures are representative of four independent experiments.
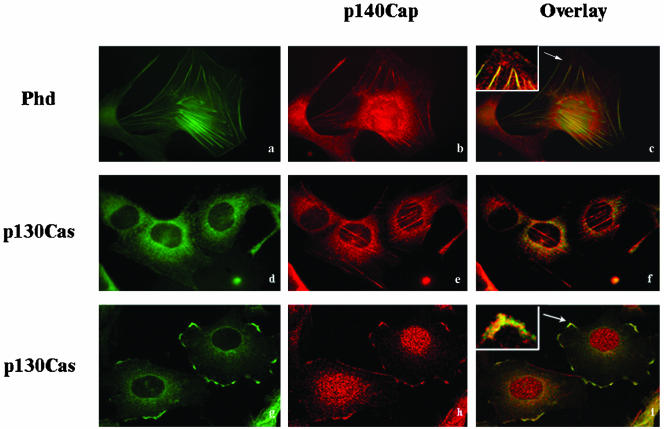
p140Cap Is Tyrosine Phosphorylated after Integrin-mediated Adhesion and EGF Treatment
Tyrosine phosphorylation is required for activation of downstream signaling events upon integrin-dependent adhesion and growth factors stimulation. p130Cas is strongly tyrosine phosphorylated in response to adhesive stimuli, neuropeptides, and growth factors (Casamassima and Rozengurt, 1997; Defilippi et al., 1997a; O'Neill et al., 2000; Bouton et al., 2001).
Because analyses of the amino acids composition of the p140Cap protein showed that among the tyrosine residues present in the molecule, two are likely target of tyrosine kinases, we tested whether p140Cap might be tyrosine phosphorylated in response to adhesion. As shown in Figure 7A, HEK293 cells transfected with p140Cap were detached from the plates and replated on integrin ligand for different times or kept in suspension, as described previously (Moro et al., 2002). Although cells in suspension did not show phosphorylation of p140Cap, plating of cells triggered the rapid tyrosine phosphorylation of p140Cap within 15 min of adhesion with a maximal peak at 60 min. Similarly, cell plating on fibronectin and immunoprecipitation with anti-phosphotyrosine antibodies confirmed that the tyrosine phosphorylation of p140Cap was rapid, peaking at 60 min and decreasing at basal levels after 240 min (Figure 7B). In the same extracts, tyrosine phosphorylation of p130Cas followed a similar rapid time course upon cell adhesion on fibronectin (Figure 7B), indicating that the two events might be concomitant. These data identify p140Cap as a novel target of integrin-dependent activation of tyrosine kinases. In addition in HEK293 transfected cells, p140Cap was tyrosine phosphorylated in basal culture condition in presence of 10% serum. Serum deprivation completely abolished the level of tyrosine phosphorylation of transfected p140Cap (Figure 7C), suggesting that phosphorylation is dependent on mitogens and growth factors present in the serum. In fact, cell treatment with 50 ng/ml EGF induced a rapid tyrosine phosphorylation of p140Cap within 15 min of stimulation (Figure 7C). The tyrosine phosphorylation of p140Cap was strongly reduced when EGF stimulus was given in combination with 250 nM AG1478 tyrfostin, a specific inhibitor of EGFR kinase (Figure 7D), indicating that EGF-dependent p140Cap tyrosine phosphorylation relies on EGFR kinase activity. Together, these results show that p140Cap is a substrate of integrin and growth factor signaling.
Figure 7.
p140Cap is tyrosine phosphorylated in response to adhesion, serum, or EGF treatment. (A) p140Cap-transfected HEK293 cells, serum-deprived for 24 h, were detached and plated for different times on dishes coated with mAb L230 against the αv integrin subunit or kept in suspension for 30 min. Cells were detergent extracted at the indicated times, and extracts were immunoprecipitated using antibodies to p140Cap. The immunoprecipitates were blotted with anti-phosphotyrosine antibody (top) and reblotted with antibodies to p140Cap (bottom). (B) Cells treated as described in A, kept in suspension or plated on FN for the indicated times, were extracted and immunoprecipitated using antibodies to phosphotyrosine (p-Tyr), and the immunoprecipitates were blotted with anti-p140Cap antibodies (top) and reblotted with antibodies to p130Cas (bottom). (C) Cells were treated with 20% FCS or with 50 ng/ml human recombinant EGF for 30 min. (D) Cells were treated with 50 ng/ml human recombinant EGF for 30 min in the presence of 250 nM AG1478. The p140Cap immunoprecipitates were blotted with anti-phosphotyrosine antibody (top) and reblotted with antibodies to p140Cap (bottom). The results are representative of three independent experiments.
p140Cap Affects NIH3T3 and ECV304 Cell Spreading
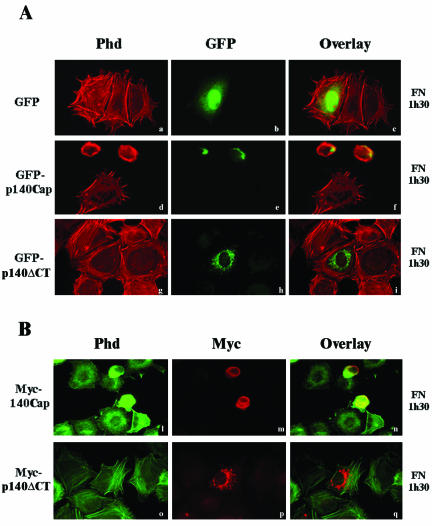
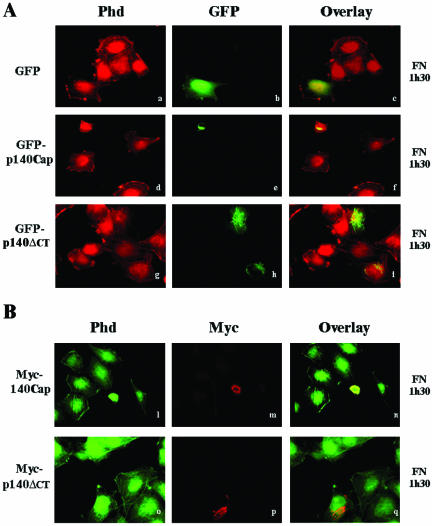
Integrin binding to the extracellular matrix induces cell flattening and the organization of the cytoskeleton leading to cell spreading. We analyzed the effects of the expression of p140Cap in the ability of NIH3T3 fibroblasts to spread on fibronectin. NIH3T3 cells that do not express endogenous p140Cap were transfected with empty pEGFP, the pEGFP-p140Cap vector, or the pEGFP-140-5′, lacking the Cap 5 region (see also Figure 5, A and C). Forty-eight hours after transfection, cells were detached and allowed to adhere to fibronectin-coated glass coverslips for 1.5 h in the presence of serum and stained with phalloidin for actin stress fibers organization. As shown in Figure 8A (d-f) cells positive for GFP-p140Cap adhered to the fibronectin matrix but did not spread after 1.5 h of adhesion, remaining completely rounded. On the contrary, the untransfected cells in the same field or the GFP-expressing cells (a-c) showed a flatten morphology and a defined actin staining. The absence of actin organization in p140Cap-transfected cells clearly indicated that expression of p140Cap in NIH3T3 cells severely impaired the early phases of cell flattening on extracellular matrix. In contrast, NIH3T3 cells expressing the mutant GFP-p140-ΔCT protein, which is unable to interact with p130Cas (Figure 5C), did not affect the ability to spread on fibronectin and to organize actin cytoskeleton after 1.5 h of plating (g-i). These data indicate that overexpression of p140Cap in the early phases of cell adhesion prevented cells from spreading on extracellular matrix. The fact that the mutant lacking the domain of association with p130Cas did not alter cell spreading suggests that the association of p140Cap and p130Cas might be relevant for the inhibition of cell spreading. The same effect on cell spreading and actin organization was also observed in NIH3T3 cells transfected with wild-type or mutant p140Cap Myc-tagged expression vectors (Figure 8B, l-q), indicating that different tags do not modify the ability of p140Cap protein to affect cell spreading. The impaired cell spreading observed in Figure 8, A and B, was not due to a toxic effect of GFP-p140Cap or of 140Cap-Myc expression as cell spreading was progressively restored after 12 h of plating on fibronectin (our unpublished data). Similar results were obtained by transfecting ECV304 cells with the same GFP-p140Cap and Mycp140Cap constructs used in the experiments described above. Although ECV304 cells do express endogenous p140Cap, the overexpression of p140Cap was able to affect cell spreading and actin cytoskeleton organization. As shown in Figure 9, A and B (d-l), GFP-p140Cap-transfected ECV304 cells remained rounded compared with the untransfected control. As already seen in NIH3T3 cells, deletion of the carboxy-terminal domain (Figure 9A, g, h, and l) was sufficient to rescue the effect on cell spreading. Therefore, these data show that p140Cap overexpression prevents NIH3T3 and ECV304 cell spreading on the extracellular matrix during the early phases of cell adhesion and that the binding domain that mediate the association between p130Cas and p140Cap is required for this effect.
Figure 8.
Overexpression of p140Cap affects cell spreading in NIH3T3 cells. (A) NIH3T3 cells were transfected with pEGFP-N2 (a-c), pEGFP-p140Cap (d-f), or pEGFP-p140-ΔCT cDNAs (g-i). Forty-eight hours after transfection, cells were detached from culture dishes and plated for 1.5 h on fibronectin-coated glass coverslips. Cells were than fixed, permeabilized, and stained using rhodamine-labeled phalloidin (Phd). The overlay staining is shown in c, f, and i. The images are representative of three independent experiments. (B) NIH3T3 cells were transfected with pCDNA3.1/Myc-Hys-p140Cap (l-n) or with mutant pCDNA3.1/Myc-Hys-p140-ΔCT expression vectors (o-q). Forty-eight hours after transfection, cells were detached from culture dishes and plated for 1.5 h on fibronectin-coated glass coverslips and then double stained using fluorescein-labeled phalloidin (l and o) or and rhodamine-labeled Myc antibodies (m and p). The overlay staining is shown in n and q.
Figure 9.
Overexpression of p140Cap affects cell spreading in ECV304 cells. (A and B) ECV 304 cells were treated, transfected, and stained as described in Figure 8, A and B.
DISCUSSION
In this work, we describe the identification and characterization of p140Cap, a novel protein associating with the adaptor molecule p130Cas. p140Cap is highly expressed in brain, testis, and epithelial-rich tissues such as lung and mammary gland. Its immunolocalization into ECV304 cells plated on fibronectin showed that p140Cap codistributes with actin filaments, is absent from focal adhesion structures and colocalizes with p130Cas in membrane ruffles. We also show that p140Cap is tyrosine phosphorylated after integrin-dependent adhesion or EGF treatment and that its expression in NIH3T3 and ECV304 cells inhibits early phases of cell spreading on fibronectin. Therefore, p140Cap is a new molecule involved in integrinand growth factor-mediated signaling that modulate the ability of cells to spread on matrix proteins likely by its association with p130Cas and actin stress fibers.
p140Cap is expressed both in normal epithelial cells, such as bladder ECV304 and FRT, in transformed breast carcinoma T47D, and in cervix carcinoma HeLa cells. It is also expressed in cells of neuroectodermal origin such as human SH-SY5Y (our unpublished data) and mouse NIE115 neuroblastoma cells, indicating that the molecule is widely distributed. In vivo, the p140Cap protein is detectable in mouse brain, testis, kidney, lung, and mammary gland. Interestingly, the mouse protein found in the brain and in the epithelial tissues migrates at 140 kDa, whereas that from testis at 116 kDa. The brain, but not the testis protein, is selectively recognized by a mAb raised against the carboxyterminal region of the protein, suggesting the existence of tissue-specific isoforms probably derived from an alternative splicing in this region. Therefore, the p140Cap protein is expressed in both human and mouse cells and tissues. Indeed, sequence comparison between human, mouse, rat, fish, and Drosophila genomes revealed the existence of ortholog genes, coding for highly homologous proteins in all these species (www.ensembl.org). The comparison of the recently published mouse and human genomic sequences allowed us to identify two alternative exons located in the 5′ region of the gene. These exons named 1a and 1b, once spliced on exon 2, give rise to two distinct amino-terminal domains. The 1a-2 spliced isoform corresponds to the unpublished mouse sequence p140, whereas the 1b-2 version is the mouse homolog of the rat SNIP protein (Chin et al., 2000). Thus, it is possible to conclude that the p140Cap proteins are evolutionary conserved and exist in at least two different N-terminal alternative spliced forms. The recently identified rat SNIP protein, selectively expressed in brain and testis, was shown to be resistant to extraction with nonionic detergents such as NP-40, Triton X-100, or caotropic agents such as urea (Chin et al., 2000). In contrast, in our experimental conditions, we were able to immunoprecipitate endogenous p140Cap from distinct tissues and cell types. Even if the different properties of the antibodies used to immunoprecipitate SNIP and p140Cap are taken into account, the solubility observed for p140Cap might also be related to different localization of specific isoforms.
The association between p140Cap and p130Cas detected by affinity chromatography was confirmed in ECV304 cells by coimmunoprecipitation and pull-down experiments. By using recombinant p140Cap and p130Cas fragments, we demonstrated that the interaction between p140Cap and p130Cas is mediated by the last 217 amino acids of the p140Cap carboxy-terminal region. Conversely, p130Cas associates with p140Cap in the region encompassing amino acids 544-678. By ProScan domain prediction program, we identified in the p140Cap carboxy-terminal region a charged amino acid-rich domain and a proline-rich region, putative binding sites for the SH3 domains of Abl, Crk, and Itk proteins. Our analysis showed that both the proline and the charged amino acid-rich regions are highly conserved among vertebrates. The fact that during evolution these sequences have been specifically selected suggests that they could be relevant for the function of the p140Cap protein, possibly by mediating interaction with other molecules such as p130Cas. Similarly, the region of p130Cas that associates with the carboxy-terminal fragment of p140Cap in the pull-down experiments is characterized by a proline-rich region that can bind to the SH3 domains of several transducing molecules (Bouton et al., 2001) and by the presence of a tyrosine in position 668 that mediates binding to the Src SH2 domain (Nakamoto et al., 1997). The domains that lie on the associating portions of the p140Cap and p130Cas molecules do not provide evidence of a direct binding between the two proteins. Preliminary data of in vitro binding studies indicate that the Cap 5 fragment of p140Cap and the interacting regions on p130Cas do not bind to each other, suggesting that other interacting molecules might mediate the association between p140Cap and p130Cas. However, the presence of Crk, a known interactor of p130Cas, in the p140Cap immunoprecipitates indicates that additional molecules are also recruited in the complex together with p130Cas and p140Cap. In addition to Crk, several proteins directly interacting with p130Cas have been already described, among which the most extensively studied are p125Fak, PTP-PEST, PTP-1B (Polte and Hanks, 1995; Harte et al., 1996; Garton et al., 1997; Manie et al., 1997), and the Src family kinases (Astier et al., 1997; Manie et al., 1997; Tachibana et al., 1997). Their relevance, if any, to the organization of the p130Cas-p140Cap complex has yet to be defined.
Interestingly, in ECV304 cells plated on fibronectin in the presence of serum, p140Cap clearly localizes on stress fibers that extend throughout the cell body and on cortical actin (see examples in Figure 6, b and e), indicating an association of p140Cap with actin-enriched structures. In ECV304 cells spread on fibronectin, actin stress fibers end into focal adhesion structures, which by definition are the connecting point between the extracellular matrix and actin cytoskeleton (Burridge and Chrzanowska-Wodnicka, 1996; Sastry and Burridge, 2000). Although p140Cap strongly stains actin cables, it is completely excluded from focal adhesions (our unpublished data). The SNIP protein, the rat homolog of p140Cap, was also described to be tightly associated with actin cytoskeleton in differentiated PC12 pheochromocytoma cells (Chin et al., 2000). Thus, both in epithelial and neuroectodermal cells, the p140Cap is strictly connected to the actin cytoskeleton, suggesting a role in actin cytoskeleton remodeling. In addition to the colocalization with actin stress fibers, p140Cap seems to colocalize with p130Cas in a cytoplasmic punctuate staining as well as in specific membrane ruffle structures induced by PMA treatment (Downey et al., 1992; Chew et al., 2002). The association of p140Cap and p130Cas in the cytoplasm and in ruffles and the codistribution of p140Cap with actin cables strongly suggest that p140Cap plays an important role either in the structural organization of the actin cytoskeleton, which affects cell spreading on extracellular matrix, or in functional regulation of cell motility.
Finally, our results show that p140Cap is tyrosine phosphorylated in response to adhesion or soluble mitogens such as serum and EGF. In HEK293, EGF treatment induces a rapid tyrosine phosphorylation of transfected p140Cap, which is dependent on the kinase activity of the EGFR itself, as demonstrated by using the specific EGFR inhibitor AG1478. These results demonstrate that p140Cap is a novel downstream effector of the EGFR in response to its ligand, opening a new perspective for analysis of possible functional roles of p140Cap. Integrin-mediated adhesion induces strong phosphorylation of cellular proteins that have been shown to play multiple functions affecting cell polarity, migration, growth, and survival (Defilippi et al., 1997a; Giancotti and Ruoslahti, 1999; Damsky and Ilic, 2002; Giancotti and Tarone, 2003). Plating HEK293 cells on integrin ligands stimulates p140Cap phosphorylation within 15 min of cell adhesion, demonstrating that p140Cap tyrosine phosphorylation is also modulated by integrin-dependent adhesion. We and others have previously shown that adhesion to matrix protein activates c-Src kinase, p125Fak, and EGFR and induces Src-dependent tyrosine phosphorylation of p130Cas (Moro et al., 1998, 2002; for reviews, see Giancotti and Ruoslahti, 1999; Damsky and Ilic, 2002; Giancotti and Tarone, 2003). Our data also indicate that c-Src kinase activity and p130Cas adaptor are required for integrin-dependent EGFR activation (Moro et al., 2002). Both the c-Src kinase and the EGFR kinase could be responsible for integrin-dependent p140Cap tyrosine phosphorylation. Together, the phosphorylation of p140Cap by integrin-dependent adhesion or EGF and its association with p130Cas might represent a new example of cooperative signaling induced by growth factors and integrins in the organization of multiple cell responses. Intriguingly in different experimental conditions, p140Cap is associated to actin stress fibers or to ruffles. Therefore, the onset of p140Cap phosphorylation occurring upon cell-matrix adhesion or EGF stimulus and its association with p130Cas might be crucial in regulating distinct cell processes such as cell adhesion or membrane ruffling organization.
On integrin-mediated adhesion, cells attachment to extracellular matrix triggers a complex process of cytoskeleton remodeling that ultimately leads to cell spreading. Tyrosine phosphorylation has been demonstrated to be crucial in these adhesive events (Burridge and Chrzanowska-Wodnicka, 1996; Panetti, 2002). The functional role of p140Cap in regulating cell adhesion was studied by expressing the wild-type protein or the mutant lacking the carboxy-terminal domain that mediates the association with p130Cas. Our data showed that p140Cap plays a relevant role in the early phases of cell adhesion inhibiting NIH3T3 and ECV304 cell spreading on fibronectin. In fact, when cells were plated on fibronectin, p140Cap expression dramatically affects actin cytoskeleton organization, maintaining the cells in a round shape. This effect was abolished when the mutant p140-ΔCT was expressed, indicating that the region involved in p130Cas association is required for this effect. Therefore, these data led to the hypothesis that p140Cap might regulate cell spreading through sequestering p130Cas and/or outcompeting its interaction with other proteins involved in modulating spreading in the early phases of cell adhesion. Accordingly, it is well known that GTP loading of Rac is involved in cell spreading and membrane ruffles, through the formation of a CAS/Crk complex that activates DOCK180, an upstream effector of Rac (Klemke et al., 1998; Cheresh et al., 1999). Because both p130Cas and Crk participate in complex with p140Cap, a possible mechanism through which p140Cap affects cell spreading might account on inhibition of Rac activity during the early phases of cell adhesion. Alternatively, tyrosine phosphorylation of p140Cap and p130Cas that occur concomitantly with inhibition of cell spreading can represent an additional regulatory step in the ability of cells to spread on extracellular matrix. Together, these data indicate p140Cap as a mediator of integrin- and growth factor-dependent adhesion, involved in modulating cell shape through the association with p130Cas and the actin filaments.
Acknowledgments
We thank Dr. G. Consalez for the gift of the murine p140 cDNA, K. Vuori for the SH2-Crk domain p-GEX vector, A.H Bouton and S. Hanks for p130Cas constructs, and Dr. L. Nitsch for the FRT cells. We thank Tiziana Cravero and Federica Logrand for the technical assistance in the preparation of monoclonal and polyclonal antibodies. We thank Paola Bernabei and Simona Degani for help with the confocal microscope and Ferdinando Di Cunto for helping with the protein databank analysis. This work was supported by grants of the Italian Association for Cancer Research, Ministero deIl'Università e Ricerca Scientifica, cofinanziamento MURST, fondi ex-60%, and Fondi FIRB, Special project “Oncology”, Compagnia San Paolo/FIRMS, Torino, Italy, and Consiglio Nazionale delle Ricerche. S.C. is supported by a fellowship of the Fondazione Italiana Ricerca sul Cancro.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-09-0689. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-09-0689.
References
- Assoian, R.K., and Schwartz, M.A. (2001). Coordinate signaling by integrins and receptor tyrosine kinases in the regulation of G1 phase cell-cycle progression. Curr. Opin. Genet. Dev. 11, 48-53. [DOI] [PubMed] [Google Scholar]
- Astier, A., Avraham, H., Manie, S.N., Groopman, J., Canty, T., Avraham, S., and Freedman, A.S. (1997). The related adhesion focal tyrosine kinase is tyrosine-phosphorylated after beta1-integrin stimulation in B cells and binds to p130cas. J. Biol. Chem. 272, 228-232. [DOI] [PubMed] [Google Scholar]
- Bouton, A.H., Riggins, R.B., and Bruce-Staskal, P.J. (2001). Functions of the adapter protein Cas: signal convergence and the determination of cellular responses. Oncogene 20, 6448-6458. [DOI] [PubMed] [Google Scholar]
- Burridge, K., and Chrzanowska-Wodnicka, M. (1996). Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 12, 463-518. [DOI] [PubMed] [Google Scholar]
- Casamassima, A., and Rozengurt, E. (1997). Tyrosine phosphorylation of p130(cas) by bombesin, lysophosphatidic acid, phorbol esters, and platelet-derived growth factor. Signaling pathways and formation of a p130(cas)-Crk complex. J. Biol. Chem. 272, 9363-9370. [DOI] [PubMed] [Google Scholar]
- Cheresh, D.A., Leng, J., and Klemke, R.L. (1999). Regulation of cell contraction and membrane ruffling by distinct signals in migratory cells. J. Cell Biol. 146, 1107-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chew, C.S., Chen, X., Parente, J.A., Jr., Tarrer, S., Okamoto, C., and Qin, H.Y. (2002). Lasp-1 binds to non-muscle F-actin in vitro and is localized within multiple sites of dynamic actin assembly in vivo. J. Cell Sci. 115, 4787-4799. [DOI] [PubMed] [Google Scholar]
- Chin, L.S., Nugent, R.D., Raynor, M.C., Vavalle, J.P., and Li, L. (2000). SNIP, a novel SNAP-25-interacting protein implicated in regulated exocytosis. J. Biol. Chem. 275, 1191-1200. [DOI] [PubMed] [Google Scholar]
- Damsky, C.H., and Ilic, D. (2002). Integrin signaling: it's where the action is. Curr. Opin. Cell Biol. 14, 594-602. [DOI] [PubMed] [Google Scholar]
- Defilippi, P., Bozzo, C., Volpe, G., Romano, G., Venturino, M., Silengo, L., and Tarone, G. (1994). Integrin-mediated signal transduction in human endothelial cells: analysis of tyrosine phosphorylation events. Cell Adhes. Commun. 2, 75-86. [DOI] [PubMed] [Google Scholar]
- Defilippi, P., Gismondi, A., Santoni, A., and Tarone, G. (1997a). Integrins and Signal Transduction. Berlin: Springer.
- Defilippi, P., Venturino, M., Gulino, D., Duperray, A., Boquet, P., Fiorentini, C., Volpe, G., Palmieri, M., Silengo, L., and Tarone, G. (1997b). Dissection of pathways implicated in integrin-mediated actin cytoskeleton assembly. Involvement of protein kinase C, Rho GTPase, and tyrosine phosphorylation. J. Biol. Chem. 272, 21726-21734. [DOI] [PubMed] [Google Scholar]
- Downey, G.P., Chan, C.K., Lea, P., Takai, A., and Grinstein, S. (1992). Phorbol ester-induced actin assembly in neutrophils: role of protein kinase C. J. Cell Biol. 116, 695-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ey, P.L., Prowse, S.J., and Jenkin, C.R. (1978). Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-Sepharose. Immunochemistry 15, 429-436. [DOI] [PubMed] [Google Scholar]
- Garton, A.J., Burnham, M.R., Bouton, A.H., and Tonks, N.K. (1997). Association of PTP-PEST with the SH3 domain of p130cas; a novel mechanism of protein tyrosine phosphatase substrate recognition. Oncogene 15, 877-885. [DOI] [PubMed] [Google Scholar]
- Giancotti, F.G., and Ruoslahti, E. (1999). Integrin signaling. Science 285, 1028-1032. [DOI] [PubMed] [Google Scholar]
- Giancotti, F.G., and Tarone, G. (2003). Positional control of cell fate through joint integrin/receptor protein kinase signaling. Annu. Rev. Cell Dev. Biol. 19, 173-206. [DOI] [PubMed] [Google Scholar]
- Hanks, S.K., Ryzhova, L., Shin, N.Y., and Brabek, J. (2003). Focal adhesion kinase signaling activities and their implications in the control of cell survival and motility. Front. Biosci. 8, D982-D996. [DOI] [PubMed] [Google Scholar]
- Harte, M.T., Hildebrand, J.D., Burnham, M.R., Bouton, A.H., and Parsons, J.T. (1996). p130Cas, a substrate associated with v-Src and v-Crk, localizes to focal adhesions and binds to focal adhesion kinase. J. Biol. Chem. 271, 13649-13655. [DOI] [PubMed] [Google Scholar]
- Hellman, U., Wernstedt, C., Gonez, J., and Heldin, C.H. (1995). Improvement of an “In-Gel” digestion procedure for the micropreparation of internal protein fragments for amino acid sequencing. Anal. Biochem. 224, 451-455. [DOI] [PubMed] [Google Scholar]
- Hsia, D.A., et al. (2003). Differential regulation of cell motility and invasion by FAK. J. Cell Biol. 160, 753-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke, R.L., Leng, J., Molander, R., Brooks, P.C., Vuori, K., and Cheresh, D.A. (1998). CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J. Cell Biol. 140, 961-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law, S.F., Estojak, J., Wang, B., Mysliwiec, T., Kruh, G., and Golemis, E.A. (1996). Human enhancer of filamentation 1, a novel p130cas-like docking protein, associates with focal adhesion kinase and induces pseudohyphal growth in Saccharomyces cerevisiae. Mol. Cell. Biol. 16, 3327-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manie, S.N., et al. (1997). Involvement of p130(Cas) and p105(HEF1), a novel Cas-like docking protein, in a cytoskeleton-dependent signaling pathway initiated by ligation of integrin or antigen receptor on human B cells. J. Biol. Chem. 272, 4230-4236. [DOI] [PubMed] [Google Scholar]
- Matsuda, M., Mayer, B.J., Fukui, Y., and Hanafusa, H. (1990). Binding of transforming protein, P47gag-crk, to a broad range of phosphotyrosine-containing proteins. Science 248, 1537-1539. [DOI] [PubMed] [Google Scholar]
- Miranti, C.K., and Brugge, J.S. (2002). Sensing the environment: a historical perspective on integrin signal transduction. Nat. Cell Biol. 4, E83-E90. [DOI] [PubMed] [Google Scholar]
- Miyamoto, S., Teramoto, H., Gutkind, J.S., and Yamada, K.M. (1996). Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 135, 1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro, L., et al. (2002). Integrin-induced epidermal growth factor (EGF) receptor activation requires c-Src and p130Cas and leads to phosphorylation of specific EGF receptor tyrosines. J. Biol. Chem. 277, 9405-9414. [DOI] [PubMed] [Google Scholar]
- Moro, L., Venturino, M., Bozzo, C., Silengo, L., Altruda, F., Beguinot, L., Tarone, G., and Defilippi, P. (1998). Integrins induce activation of EGF receptor: role in MAP kinase induction and adhesion-dependent cell survival. EMBO J. 17, 6622-6632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto, T., Sakai, R., Honda, H., Ogawa, S., Ueno, H., Suzuki, T., Aizawa, S., Yazaki, Y., and Hirai, H. (1997). Requirements for localization of p130cas to focal adhesions. Mol. Cell. Biol. 17, 3884-3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamoto, T., Sakai, R., Ozawa, K., Yazaki, Y., and Hirai, H. (1996). Direct binding of C-terminal region of p130Cas to SH2 and SH3 domains of Src kinase. J. Biol. Chem. 271, 8959-8965. [DOI] [PubMed] [Google Scholar]
- Ojaniemi, M., and Vuori, K. (1997). Epidermal growth factor modulates tyrosine phosphorylation of p130Cas. Involvement of phosphatidylinositol 3′-kinase and actin cytoskeleton. J. Biol. Chem. 272, 25993-25998. [DOI] [PubMed] [Google Scholar]
- O'Neill, G.M., Fashena, S.J., and Golemis, E.A. (2000). Integrin signalling: a new Cas(t) of characters enters the stage. Trends Cell Biol. 10, 111-119. [DOI] [PubMed] [Google Scholar]
- Panetti, T.S. (2002). Tyrosine phosphorylation of paxillin, FAK, and p130CAS: effects on cell spreading and migration. Front. Biosci. 7, d143-d150. [DOI] [PubMed] [Google Scholar]
- Polte, T.R., and Hanks, S.K. (1995). Interaction between focal adhesion kinase and Crk-associated tyrosine kinase substrate p130Cas. Proc. Natl. Acad. Sci. USA 92, 10678-10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, A.B., Kanner, S.B., Wang, H.C., and Parsons, J.T. (1989). Stable association of activated pp60src with two tyrosine-phosphorylated cellular proteins. Mol. Cell. Biol. 9, 3951-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruest, P.J., Shin, N.Y., Polte, T.R., Zhang, X., and Hanks, S.K. (2001). Mechanisms of CAS substrate domain tyrosine phosphorylation by FAK and Src. Mol. Cell. Biol. 21, 7641-7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusciano, D., Lorenzoni, P., and Burger, M.M. (1996). Constitutive activation of c-Met in liver metastatic B16 melanoma cells depends on both substrate adhesion and cell density and is regulated by a cytosolic tyrosine phosphatase activity. J. Biol. Chem. 271, 20763-20769. [DOI] [PubMed] [Google Scholar]
- Sakai, R., Iwamatsu, A., Hirano, N., Ogawa, S., Tanaka, T., Mano, H., Yazaki, Y., and Hirai, H. (1994). A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 13, 3748-3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry, S.K., and Burridge, K. (2000). Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp. Cell Res. 261, 25-36. [DOI] [PubMed] [Google Scholar]
- Schaller, M.D., Hildebrand, J.D., Shannon, J.D., Fox, J.W., Vines, R.R., and Parsons, J.T. (1994). Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol. Cell. Biol. 14, 1680-1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer, D.D., Broome, M.A., and Hunter, T. (1997). Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol. Cell. Biol. 17, 1702-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, M.A., and Baron, V. (1999). Interactions between mitogenic stimuli, or, a thousand and one connections. Curr. Opin. Cell Biol. 11, 197-202. [DOI] [PubMed] [Google Scholar]
- Sundberg, C., and Rubin, K. (1996). Stimulation of beta1 integrins on fibroblasts induces PDGF independent tyrosine phosphorylation of PDGF beta-receptors. J. Cell Biol. 132, 741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana, K., Urano, T., Fujita, H., Ohashi, Y., Kamiguchi, K., Iwata, S., Hirai, H., and Morimoto, C. (1997). Tyrosine phosphorylation of Crk-associated substrates by focal adhesion kinase. A putative mechanism for the integrin-mediated tyrosine phosphorylation of Crk-associated substrates. J. Biol. Chem. 272, 29083-29090. [DOI] [PubMed] [Google Scholar]
- Vuori, K., and Ruoslahti, E. (1994). Association of insulin receptor substrate-1 with integrins. Science 266, 1576-1578. [DOI] [PubMed] [Google Scholar]
- Vuori, K., and Ruoslahti, E. (1995). Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J. Biol. Chem. 270, 22259-22262. [DOI] [PubMed] [Google Scholar]
- Wang, R., Kobayashi, R., and Bishop, J.M. (1996). Cellular adherence elicits ligand-independent activation of the Met cell-surface receptor. Proc. Natl. Acad. Sci. USA 93, 8425-8430. [DOI] [PMC free article] [PubMed] [Google Scholar]