Abstract
Objectives
To examine the potential utility of echocardiography and NT-proBNP for heart failure (HF) risk stratification in concert with a validated clinical HF risk score in older adults.
Background
Without clinical guidance, echocardiography and natriuretic peptides have suboptimal test characteristics for population-wide HF risk stratification. However, the value of these tests has not been examined in concert with a clinical HF risk score.
Methods
We evaluated the improvement in 5-year HF risk prediction offered by adding an echocardiographic score or/and NT-proBNP levels to the clinical Health ABC HF Risk Score (base model) in 3752 participants of the Cardiovascular Health Study (age, 72.6±5.4 years; 40.8% men; 86.5% white). The echocardiographic score was derived as the weighted sum of independent echocardiographic predictors of HF. We assessed changes in Bayesian information criterion (BIC), C index, integrated discrimination improvement (IDI), and net reclassification improvement (NRI). We examined also the weighted NRI across baseline HF risk categories under multiple scenarios of event versus nonevent weighting.
Results
Reduced left ventricular ejection fraction, abnormal E/A ratio, enlarged left atrium, and increased left ventricular mass, were independent echocardiographic predictors of HF. Adding the echocardiographic score and NT-proBNP levels to the clinical model improved BIC (echocardiography: −43, NT-proBNP: −64.1, combined: −68.9; all p<0.001) and C index (baseline 0.746; echocardiography: +0.031, NT-proBNP: +0.027, combined: +0.043; all p<0.01) and yielded robust IDI (echocardiography: 43.3%, NT-proBNP: 42.2%, combined: 61.7%; all p<0.001), and NRI (based on Health ABC HF risk groups; echocardiography: 11.3%; NT-proBNP: 10.6%, combined: 16.3%; all p<0.01). Participants at intermediate risk by the clinical model (5% to 20% 5-yr HF risk; 35.7% of the cohort) derived the most reclassification benefit. Echocardiography yielded modest reclassification when used sequentially after NT-proBNP.
Conclusions
In older adults, echocardiography and NT-proBNP offer significant HF risk reclassification over a clinical prediction model, especially for intermediate risk individuals.
Keywords: epidemiology, heart failure, risk score, risk prediction, risk stratification
Subclinical changes in cardiac structure and function, including left ventricular (LV) hypertrophy, enlargement, wall motion abnormalities, diastolic dysfunction, or reduced ejection fraction (EF), and left atrial enlargement, often precede development of manifest (Stage C) heart failure (HF) (1). These changes, detected mostly through echocardiography, have been strongly associated with HF risk in large cohort studies (2–7) Similarly, elevated natriuretic peptide levels are associated with structural and functional cardiac abnormalities (8), and in turn with HF risk (8–11). However, the value of echocardiography and natriuretic peptides for HF risk stratification has not been assessed in concert with a validated clinical risk score. Moreover, due to suboptimal test characteristics from a screening perspective and concerns regarding costs and consequences of unnecessary testing (4,12–16), these tests are not recommended currently as standalone tools for identification of individuals at high risk for HF. However, guidance by a clinical risk score, e.g. the Health ABC HF Risk Score (17,18), may render these tests more appealing as risk stratification tools for targeted population groups.
We hypothesized that echocardiography and N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels, used individually or in combination (simultaneously or sequentially), will have incremental predictive value over the clinical Health ABC HF Risk Score for 5-year HF risk assessment in older adults and yield clinically relevant risk reclassification. To evaluate this hypothesis, we analyzed data from 3752 participants of the Cardiovascular Health Study (CHS) with available echocardiography and NT-proBNP data.
METHODS
Study Population
The design of CHS has been previously published (19). Participants were non-institutionalized persons 65 to 100 years old recruited from Medicare eligibility lists. An original cohort of 5201 persons was recruited in 1989-90, and a second cohort of 687 African-Americans was recruited in 1992-93. The present study included participants with available (1) baseline NT-proBNP levels and (2) echocardiographic data, obtained at baseline for the original and at year 2 for the second cohort. From the 4522 participants with available baseline NT-proBNP levels we excluded (1) participants with prevalent HF at baseline (n=212) and those from the second cohort with prevalent HF at year 2 (n=35); (2) those with missing data on Health ABC HF Risk Score variables (n=162); and (3) those with missing data on echocardiography (n=361). In all, 3752 participants were included in the primary analysis. In a secondary analysis, we examined 2538 participants with available quantitative M-mode data on LV dimensions and mass.
Definition of Risk Factors and Health ABC HF Score
We calculated the Health ABC HF Risk Score and categorized participants into <5%, 5–10%, 10–20%, >20% 5-yr HF risk, as previously described (17,18). To incorporate the second CHS cohort, we calculated the score at baseline for these participants; however, echocardiography for these participants was available at year 2. For calculation of Health ABC HF Risk Score, classification of prevalent CHD was based on self-report of coronary revascularization, myocardial infarction, or angina (20). Smoking was classified as current, past, or never. The Minnesota 3.1 code was used to classify electrocardiographic LV hypertrophy (21). The core laboratory at University of Vermont, Colchester, VT, analyzed fasting serum chemistry.
Echocardiographic Assessment in CHS
Two-dimensional echocardiography was performed at baseline for the original cohort and at year 2 for the second cohort. Studies were centrally interpreted at the University of California, Irvine (22). We classified LV systolic function as normal, borderline, or abnormal, corresponding to EF ≥0.55, 0.45 to 0.54, and <0.45, respectively (23). Based on previous findings from CHS showing values <0.7 and >1.5 to be associated with increased HF risk (4), the mitral inflow E to A peak velocity ratio (E/A ratio) was classified into <0.7, 0.7 to 1.5, and >1.5. Left atrial enlargement was based on American Society of Echocardiography (ASE) recommended M-mode antero--posterior diameter of >23 mm/m2 (24). Quantitative LV M-mode measurements were available in 3410 participants of the original cohort, with age being the strongest correlate of missing data (23), and 405 participants of the second cohort. LV mass was calculated with the Devereux formula (25). To define LV hypertrophy and enlargement we used gender-specific cut-offs proposed by ASE; 95 g/m2 for women and 115 g/m2 for men for LV mass; and >32 mm/m2 for women and >31 mm/m2 for men for LV diameter at diastole (24).
Natriuretic Peptide Assay
NT-proBNP was measured with the Elecsys 2010 system (Roche Diagnostics, Indianapolis, IN) in serum collected at baseline. Coefficient of variation was 2% to 5% and range was 5 to 35,000 pg/ml. In a previous analysis from CHS (9), NT-proBNP >190pg/ml optimally predicted HF risk and cardiovascular mortality; we therefore used this cut-off for additional analyses.
Definition of Incident Heart Failure
Methods used to assess cardiovascular events in CHS, including HF, have been reported previously (26,27). An HF event was confirmed if, in addition to a physician diagnosis, there were (1) documented symptoms (e.g. shortness of breath, fatigue, orthopnea, paroxysmal nocturnal dyspnea) and physical signs (e.g. edema, pulmonary rales, gallop rhythm) consistent with HF; (2) supporting clinical findings like pulmonary edema on chest X-ray; or (3) therapy for HF, including diuretics, digitalis, angiotensin-converting enzyme inhibitors, or beta-blockers. In the current analysis, incident HF was defined as adjudicated first hospitalization for HF.
Statistical Analysis
NT-proBNP values were log-transformed prior to analyses because of lognormal distribution. To identify echocardiographic variables that predict HF independently of clinical risk factors, we used the Health ABC HF Risk Score as a covariate in multivariable Cox proportional hazards models and followed an iterative, clinically guided approach to reach the final echocardiography-added model. The final set of echocardiographic predictors was treated subsequently as a single score (weighted sum of covariates) to assess model improvement.
To assess the incremental value of NT-proBNP and echocardiographic score for 5-year HF risk prediction, we used Health ABC HF Risk Score as the base clinical model and calculated: (1) changes in the Bayesian information criterion (BIC), a likelihood-based measure of model fit that penalizes for complexity (28); (2) changes in the C index; (3) the integrated discrimination improvement (IDI), which quantifies improvement in prognostic separation (29,30); and relative IDI (rIDI), which quantifies the relative contribution of an added variable to separation (30); (4) the net reclassification improvement (NRI), a clinically-oriented measure of reclassification that considers any correct upwards movement of events (in the new vs. the old model) and any downwards movement of non-events as model improvement and vice versa (29). To facilitate prospective interpretation, we used the censored data versions of IDI (31) and NRI (32).The Kaplan-Meier estimates for 5-year HF incidence served as observed event rates. We calculated the continuous NRI, which counts all movements of probabilities and does not rely on categories (NRI0), and the NRI based on 5-year risk groups (i.e., <5%, 5–10%, 10–20%, and >20%) (17), which counts only movements of predicted probabilities that result in a change in risk category (NRIcat). Because event and non-event NRI components are weighted by event incidence (32), and thus NRI is not comparable across risk categories, we calculated the weighted NRI for events and non-events (wNRI) (32) to compare reclassification across baseline risk categories. Currently, no established thresholds for action regarding HF risk exist, and thus, the relative merit of correctly classifying an event vs. a non-event is unknown. We calculated thus wNRI assigning weights from 1:1 (equal importance) to 16:1 (events 16× more important). The NRI formulas (NRI0, NRIcat, and wNRI) are provided in the Online Data Supplement and Supplemental Table 1.
To examine the incremental value of NT-proBNP and echocardiographic score for risk prediction among participants with normal LV ejection fraction at baseline, we replicated the analyses in the subcohort of participants with LVEF ≥0.55. Analyses were performed with STATA 11 (StataCorp LP, TX).
RESULTS
Clinical Characteristics and HF Incidence
Average age was 72.6±5.4 years; 40.8% were men; 86.5% were white. Table 1 summarizes the clinical characteristics of participants by Health ABC HF risk group. There were 286 incident HF events during the first 5 years of follow-up, corresponding to 8.0% 5-year HF incidence.
Table 1.
Baseline Clinical Characteristics According to Health ABC HF Risk Group
| Characteristic | Health ABC 5-yr HF Risk | |||
|---|---|---|---|---|
| <5% | 5–10% | 10–20% | >20% | |
| N=2228 | N=907 | N=433 | N=184 | |
| Age, years | 70.6 (3.9) | 74.4 (5.4) | 76.5 (6.0) | 79.3 (6.9) |
| Men, % | 34.0 | 48.2 | 56.1 | 51.6 |
| White, % | 88.5 | 83.7 | 83.8 | 82.6 |
| Smoking | ||||
| Current, % | 6.3 | 15.3 | 16.4 | 22.8 |
| Former, % | 42.3 | 42.1 | 41.8 | 33.2 |
| Coronary heart disease | 3.4 | 25.3 | 50.8 | 66.9 |
| Left ventricular hypertrophy*, % | 1.2 | 3.9 | 11.5 | 24.5 |
| Systolic blood pressure, mmHg | 129 (17) | 143 (21) | 149 (23) | 158 (25) |
| Heart rate, beats/min | 63 (10) | 66 (12) | 68 (13) | 74 (18) |
| Creatinine, mg/dl | 0.97 (0.23) | 1.09 (0.28) | 1.21 (0.39) | 1.38 (0.64) |
| Fasting glucose, mg/dl | 105 (22) | 117 (40) | 125 (46) | 139 (85) |
| Albumin, g/dl | 4.04 (0.28) | 3.97 (0.28) | 3.96 (0.30) | 3.86 (0.28) |
| Total cholesterol, mg/dl | 215 (38) | 211 (40) | 206 (39) | 206 (43) |
by ECG
Echocardiographic Findings and NT-proBNP Levels
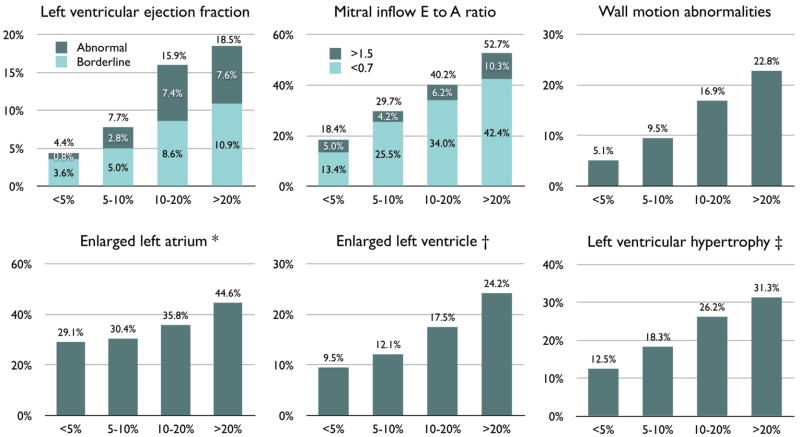
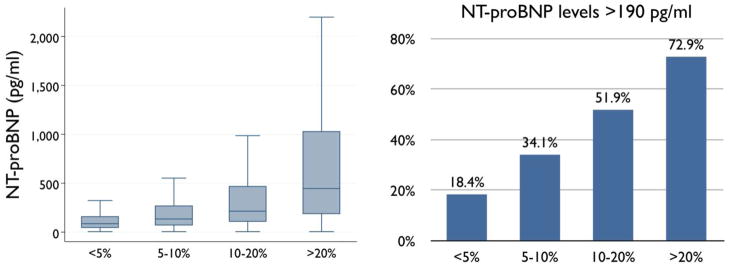
Figure 1 presents the prevalence of echocardiographic abnormalities according to clinically projected HF risk by the Health ABC HF Risk Score. Left atrial diameter, LV diameters, and LV mass, all had strong increasing trends across Health ABC HF risk groups (Table 2). Abnormal EF (<0.45%) or restrictive-type diastolic (E/A >1.5) dysfunction was present in 5.8%, 6.5%, 11.5%, and 16.8% of participants in the escalating HF risk groups. Absolute NT-proBNP levels and prevalence of increased (>190 pg/ml) NT-proBNP levels both had strong increasing trends across clinical HF risk groups (Figure 2).
Figure 1. Abnormal echocardiographic findings according to projected heart failure risk.
Prevalence of abnormal echocardiographic findings according to projected Health ABC HF Risk in 3752 participants without heart failure at baseline. M-mode left ventricular diameter and mass were available in 2538 participants. *Indexed left atrial diameter >23 mm/m2 (24). †Indexed internal diameter at diastole >32 mm/m2 for women, >31 mm/m2 for men (24). ‡Indexed left ventricular mass >95 g/m2 for women, >115 g/m2 for men (24). Parameters indexed for body surface area. P<0.001 for linear trend across categories for all parameters.
Table 2.
Quantitative Echocardiographic Characteristics According to Health ABC HF Risk
| Characteristic | Health ABC 5-yr HF Risk Group | |||||
|---|---|---|---|---|---|---|
| N available | <5% | 5–10% | 10–20% | >20% | P for trend | |
| Left atrial diameter index, mm/m2 | 3752 | 21.2 (3.5) | 21.5 (3.8) | 22.2 (4.3) | 23.0 (4.3) | <0.001 |
| LV diastolic diameter index, mm/m2 | 2538 | 27.3 (3.4) | 27.4 (3.8) | 27.8 (4.0) | 28.2 (4.6) | 0.024 |
| LV systolic diameter index, mm/m2 | 2538 | 15.6 (3.1) | 16.2 (3.6) | 16.6 (4.3) | 17.2 (4.5) | <0.001 |
| LV mass index, g/m2 | 2538 | 79 (20) | 85 (23) | 93 (30) | 94 (33) | <0.001 |
LV=left ventricular
Figure 2. NT-proBNP levelsaccording to projected heart failure risk.
Distribution of NT-proBNP levels (left) and prevalence of increased (>190pg/ml) (9) NT-proBNP levels (right) according to projected Health ABC HF Risk. P<0.001 for linear trend across categories in both analyses.
Echocardiographic Parameters, NT-proBNP, and HF Risk Prediction
Among the echocardiographic parameters examined, EF, enlarged left atrium, and E/A ratio were independent predictors of HF risk when added to Health ABC HF Risk Score (Table 3). In the subset with M-mode data on LV dimensions and mass available (N=2538), LV fractional shortening was a stronger predictor than EF, and LV mass index was an additional independent predictor of HF (Table 3). The weighted sum of echocardiographic parameters constituted the echocardiographic score used subsequently to determine risk stratification improvement. For completeness, we have created separate scores for the main cohort (EF, enlarged left atrium, and E/A ratio) and for the quantitative LV subset (fractional shortening, enlarged left atrium, and E/A ratio, LV mass) to test for incremental value, although only the main cohort parameters were converted to a point system for practical use.
Table 3.
Echocardiographic Predictors of Incident Heart Failure
| Univariate* | Multivariate* | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Main cohort (N=3752) | ||||
|
| ||||
| LV ejection fraction | ||||
| Borderline (0.45 to 0.54) | 1.20 (0.76–1.89) | 0.43 | 1.17 (0.74–1.84) | 0.50 |
| Abnormal (<0.45) | 3.48 (2.35–5.15) | <0.001 | 2.91 (1.96–4.33) | <0.001 |
| E/A ratio | ||||
| <0.7 | 1.61 (1.24–2.10) | <0.001 | 1.56 (1.19–2.03) | 0.001 |
| >1.5 | 3.23 (2.25–4.63) | <0.001 | 2.70 (1.87–3.09) | <0.001 |
| Enlarged LA (>23 mm/m2) | 1.60 (1.27–2.03) | <0.001 | 1.50 (1.18–1.91) | 0.001 |
| Wall motion abnormalities (yes, no) | 1.54 (1.13–2.11) | 0.007 | - | - |
|
| ||||
| Quantitative LV subset (N=2538) | ||||
|
| ||||
| LV ejection fraction | ||||
| Borderline (0.45 to 0.54) | 1.47 (0.84–2.57) | 0.17 | - | - |
| Abnormal (<0.45) | 3.78 (2.22–6.46) | <0.001 | - | - |
| LV fractional shortening, per 5% decrease | 1.23 (1.13–1.34) | <0.001 | 1.16 (1.07–1.27) | 0.001 |
| E/A ratio | ||||
| <0.7 | 1.98 (1.42–2.77) | <0.001 | 1.72 (1.22–2.42) | 0.002 |
| >1.5 | 3.27 (2.08–5.14) | <0.001 | 2.92 (1.84–4.63) | <0.001 |
| Enlarged LA (>23 mm/m2) | 1.55 (1.15–2.08) | 0.004 | 1.42 (1.04–1.93) | 0.028 |
| LV end-diastolic diameter, per cm/m2 | 1.69 (1.18–2.43) | 0.005 | - | - |
| LV end-systolic diameter, per cm/m2 | 2.40 (1.71–3.36) | <0.001 | - | - |
| LV mass index, per 10 g/m2 | 1.16 (1.10–1.21) | <0.001 | 1.11 (1.06–1.17) | <0.001 |
Adjusted for Health ABC HF Risk Score; LV=left ventricular; LA=left atrium
NT-proBNP levels were independently associated with 5-year HF risk when added to Health ABC HF Risk Score; hazard ratio (HR) per natural log-increase of NT-proBNP was 1.61 (95% confidence interval [CI]: 1.44, 1.81; p<0.001). Increased NT-proBNP as a binary variable (>190pg/ml) was associated with 2.7-fold increased risk (95% CI: 2.1, 3.6; p<0.001).
Both NT-proBNP and echocardiographic score significantly improved fit, discrimination, and risk classification when added to Health ABC HF Risk Score (Table 4). Combined NT-proBNP and echocardiography had further incremental value over each modality alone, achieving further improvements in integrated discrimination (0.064 or 61.7% relative increase) and net reclassification (35.9% for continuous NRI and 16.3% for category-based NRI). The impact of echocardiography was stronger when quantitative LV parameters were considered (Table 4).
Table 4.
Added Value of Echocardiographic Score and NT-proBNP for Heart Failure Risk Prediction
| Statistic | BIC* | P (ΔBIC) | C | P (ΔC) | IDI (rIDI)† | ‡ NRI0 | P (NRI0) | ‡ NRIcat | P (NRIcat) |
|---|---|---|---|---|---|---|---|---|---|
| Main cohort (N=3752) | |||||||||
|
| |||||||||
| Health ABC HF Score | 4424.8 | Reference | 0.746 | Reference | Reference | Reference | Reference | Reference | Reference |
| Echocardiography added | 4381.8 | <0.001 | 0.777 | <0.001 | 0.044 (42.2%) | 31.8% | <0.001 | 10.6% | 0.007 |
| NT-proBNP added | 4360.7 | <0.001 | 0.773 | 0.004 | 0.045 (43.3%) | 31.7% | <0.001 | 11.3% | 0.007 |
| Echocardiography and NT-proBNP added | 4355.9 | <0.001 | 0.789 | <0.001 | 0.064 (61.7%) | 35.9% | <0.001 | 16.3% | <0.001 |
|
| |||||||||
| Quantitative LV subset (N=2538) | |||||||||
|
| |||||||||
| Health ABC HF Score | 2607.5 | Reference | 0.744 | Reference | Reference | Reference | Reference | Reference | Reference |
| Echocardiography added | 2532.2 | <0.001 | 0.798 | <0.001 | 0.056 (53.7%) | 39.7% | <0.001 | 18.8% | 0.001 |
| NT-proBNP added | 2570.1 | <0.001 | 0.774 | 0.014 | 0.033 (32.5%) | 26.6% | 0.001 | 12.1% | 0.024 |
| Echocardiography and NT-proBNP added | 2520.1 | <0.001 | 0.805 | <0.001 | 0.075 (72.8%) | 50.2% | <0.001 | 24.5% | <0.001 |
BIC: Bayesian information criterion.
IDI: Integrated discrimination improvement; rIDI expresses the relative improvement.
NRI: Net reclassification improvement; NRI0 represents continuous NRI, which counts any movement of predicted probabilities; NRIcat is based on <5%, 5–10%, 10–20%, >20% categories and counts only movements that result in change in risk category.
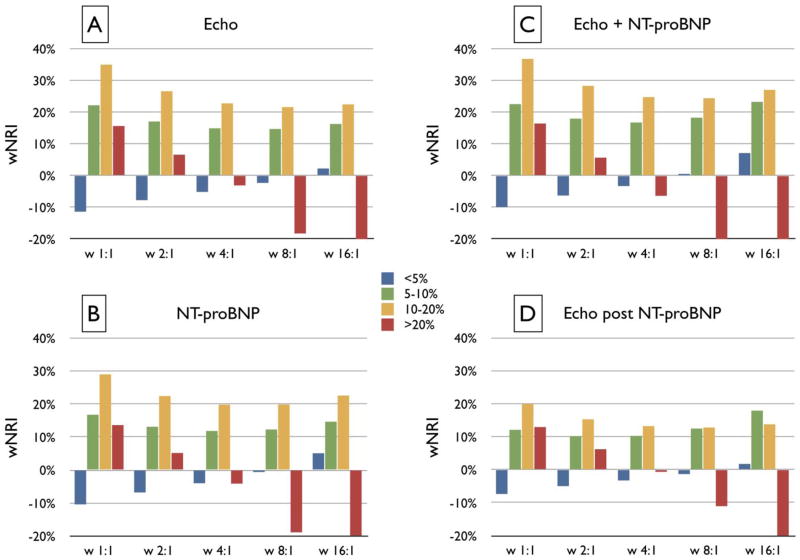
When the echocardiographic score was used sequentially after adding NT-proBNP to the base model, it still provided significant but less robust improvements in integrated discrimination (0.019 or 12.8% relative increase) and net reclassification (20.0% for continuous NRI and 7.9% for category-based NRI; both p<0.01). In the subset with quantitative LV data, echocardiography further improved classification compared to the NT-proBNP-added model (0.041 or 30.1% for IDI; 31.0% for continuous NRI [p<0.001]; and 13.2% for category-based NRI [p<0.01]).
Reclassification of Heart Failure Risk According to Baseline HF Risk Group
In participants with <5% projected risk, neither echocardiographic score nor NT-proBNP provided clinically relevant reclassification under a wide range of scenarios (Figure 3). In the 5%–10% category, clinically relevant reclassification was observed both with echocardiography (wNRI ≥15%) and NT-proBNP (wNRI >10%) across assigned weights. The 10%–20% HF risk category derived the most reclassification benefit both from echocardiography and NT-proBNP; wNRI was ≥20% for both modalities regardless of weights assigned to events vs nonevents. In the >20% risk category, reclassification gain was evident only when 1:1 weights were assigned. Modest synergistic gains were observed when echocardiography and NT-proBNP were used simultaneously. When risk was first refined by NT-proBNP, echocardiography provided gains over the NT-proBNP-added model (≥10% wNRI) in the 5%–10% and 10%–20% categories.
Figure 3. Net reclassification improvement by echocardiography and NT-proBNP according to baseline heart failure risk.
Net reclassification improvement (NRI) across the Health ABC HF Risk categories with addition of the echocardiographic score (A), NT-proBNP (B), or both (C) to the Health ABC HF model; in (D) echocardiographic score is added after refinement of risk with NT-proBNP. Five different scenarios are presented with varying weights (from 1:1 to 16:1) for correct reclassification of events vs. nonevents, representing increasing importance of events; 1:1 denotes that correct reclassification is equally important for events and nonevents whereas 16:1 denotes that correct reclassification of an event is 16 times more important than correct reclassification of a nonevent. Clinically relevant correct reclassification (indicated by >10% positive bars) was observed with echocardiography (A) and NT-proBNP (B) across assigned weights for participants with baseline 5%–10% (green bars) or 10%–20% (yellow bars) heart failure risk as clinically determined by the Health ABC HF Risk Score. Modest synergistic gains were observed when NT-proBNP and echocardiography were used simultaneously (C). When risk was first refined by NT-proBNP (D), echocardiography provided modest incremental benefit. wNRI: average weighted NRI, the weighted percentage of participants correctly reclassified.
Echocardiography and NT-proBNP in Participants with Normal Left Ventricular Ejection Fraction
Among the 3480 participants with LVEF ≥0.55, there were 236 incident HF events during the first 5 years of follow-up, corresponding to 7.1% 5-year HF incidence. Both echocardiography and NT-proBNP conferred significant reclassification of heart failure risk in these participants (Supplemental Table 2). When measured by the IDI, the magnitude of reclassification benefit by echocardiography or NT-proBNP in this subcohort was diminished relative to that observed in the entire study cohort, because risk variance was lower in this relatively healthier subcohort. However, both the continuous and the categorical NRI for echocardiography were practically unaffected. NT-proBNP exhibited slightly smaller NRI values relative to the entire cohort. In the subset with quantitative LV measurements and LVEF ≥0.55 (N=2384), the reclassification properties of echocardiography were enhanced.
DISCUSSION
In this study, we demonstrated that echocardiography and NT-proBNP could have incremental value over a validated clinical model for HF risk prediction in older adults. Improvements in HF risk prediction were not limited to formal improvements in model fit and discrimination, but also resulted in clinically relevant risk reclassification among the 35.7% of participants deemed to be at intermediated risk (5%–10% and 10%–20% predicted 5-year HF risk) by the clinical score. Of note, LV diastolic function proxy parameters (E/A ratio and left atrial size) were independent determinants of HF risk in this cohort. The risk reclassification potential of echocardiographic parameters and NT-proBNP was also evident among participants with normal (≥0.55) LVEF at baseline. Quantitative assessment of LV mass further strengthened the value of echocardio-graphy for risk reclassification; this was more pronounced in participants with normal LVEF, potentially because LV mass plays a central role in HF risk determination in these individuals.
There is a fundamental difference between screening for subclinical disease and risk stratification for future disease. The detection of the former is by definition the ‘end-product’, e.g. breast cancer, which necessitates treatment. The latter needs further evidence for effective prevention strategies and action thresholds to be clinically relevant. Currently, besides treatment of asymptomatic LV systolic dysfunction, there are no specific HF prevention strategies beyond usual risk factor management, e.g. hypertension. However, most participants who developed HF in CHS did not have reduced EF at baseline (4), and the prevalence of asymptomatic LV systolic dysfunction in the community is too low to justify population-wide screening (16). In fact, even combining abnormal systolic (EF <0.45) or restrictive-type diastolic (E/A >1.5) dysfunction would miss 80% of CHS participants who developed HF within 5 years. On the other hand, if all echocardiographic predictors in our study (any degree of LV systolic or diastolic dysfunction or left atrial enlargement) were considered as subclinical disease, the yield would be too high (43%, 53%, 65%, and 80% for the ordered Health ABC HF Risk Score groups) to be clinically meaningful. Thus, screening for asymptomatic abnormalities is unlikely to be very effective by itself for HF prevention in the general population of older adults.
In line with previous studies (4,6,38), the echocardiographic markers associated with HF risk in our study were related to both systolic and diastolic LV function. Despite adjustment for the Health ABC HF Risk Score, which incorporates clinical risk factors associated with diastolic dysfunction (17), echocardiographic surrogates of diastolic dysfunction were strongly associated with HF risk in our study, highlighting the importance of diastolic dysfunction when considering incorporation of echocardiography in HF prevention efforts (39). Although we cannot suggest that echocardiography be used for HF risk stratification at this point, considering that a number of patients undergo echocardiography for other indications, we provide a modification table for the Health ABC HF Risk Score based on echocardiographic findings (Supplemental Table 3).
Similar to echocardiography, most studies have concluded that natriuretic peptides are not cost-effective for detection of asymptomatic structural heart disease (33,34). This is rooted in the fact that natriuretic peptides are conceptually used to select individuals for echocardiographic screening (16), bearing all the limitations noted above. Based on our results, the effect of echocardiographic screening on HF prevention would still be modest at best, even after NT-proBNP-based selection (16,35). On the other hand, NT-proBNP refined risk assessment in our study, in line with observations from younger cohorts (36,37). Currently, no data support use of NT-proBNP as a HF risk tool in unselected older adults. However, our findings underscore the potential of a strategy incorporating natriuretic peptides for intermediate-risk individuals when considering HF prevention efforts. In this respect, we provide a practical approach to refine HF risk based on NT-proBNP levels if these are available (Supplemental Table 4).
Abnormal systolic or restrictive-type diastolic LV dysfunction often has causes amenable to treatment (ischemia, cardiomyopathy, LV hypertrophy, valvular disease etc). Therefore, it might be worth screening with echocardiography older adults with projected 10%–20% or >20% 5-year HF risk. In these groups, which combined constituted 16.5% of our cohort; abnormal systolic or restrictive-type diastolic dysfunction was present in 11.5% and 16.8%, respectively. Based on analyses showing that prevalence rates of >5% in the target population are likely to result in cost-effective echocardiographic screening (35), the Health ABC HF Risk Score might serve as a useful selection tool. Similarly, incorporation of NT-proBNP in the Health ABC HF Risk Score for screening selection appears to be an attractive target for future investigations.
What are the practical implications of our findings? First, there are currently no prevention strategies for individuals at increased HF risk, barring treatment of asymptomatic LV dysfunction or individual risk factors. Thus, it is difficult to translate improvement in HF risk classification into actionable items or cost-effectiveness gains at his point. This gap highlights the need for prospective data on HF prevention strategies. Second, we demonstrated that echocardiographic screening with a “subclinical disease” approach alone would fail to detect most patients at risk for HF, even for a 5-year horizon. A strategy providing HF risk assessment and risk factor modification based on projected HF risk, and potentially novel preventive interventions, would likely be more effective from a population perspective. Echocardiography and natriuretic peptide testing might be reserved for selected individuals. Such a strategy, however, would need prospective validation. Third, extrapolating from the CHD prevention paradigm, it may be reasonable at this point to follow-up more closely individuals at >10% 5-year HF risk, with the aim to intensify risk factor control, monitor for symptoms, and potentially screen with echocardiography for LV dysfunction based on the rationale described above.
Our study has several limitations. First, definition of incident HF was based on admissions and this may have underestimated HF incidence. However, because of central adjudication, underestimation is most likely distributed evenly across risk groups and thus our reclassification estimates should be relatively unbiased. On the other hand, we cannot exclude the possibility of ascertainment bias in determining the relative importance of the echocardiographic measures. Participants with possible clinical HF were perhaps more likely to be admitted if a clinically performed echocardiogram revealed LV systolic dysfunction (as opposed to isolated left atrial dilatation or increased LV mass). This could partly explain why LVEF was strongly associated with HF risk compared with other echocardiographic measures. Second, diastolic function by echocardiography was assessed by the E/A ratio and, indirectly, by the left atrial volume. These measures do not reflect contemporary assessment or classification of diastolic dysfunction. Third, our study population comprised of white and black participants, and blacks were under-represented. Thus our findings may not be applicable to other races. Finally, we studied participants aged 65 or older; thus our findings may not be applicable to younger populations.
In conclusion, echocardiography and NT-proBNP significantly reclassify 5-year HF risk in older adults when added to a clinical model, mainly among intermediate risk individuals. A strategy incorporating echocardiography or NT-proBNP for these individuals may be worth investigating prospectively. Echocardiography at this point might be reserved for those at >10% projected 5-year HF risk because of the prevalence of potentially treatable underlying abnormalities in this group. However, a strategy based solely on screening for asymptomatic abnormalities is unlikely to have dramatic impact on HF prevention in older adults. An alternative strategy, based on risk stratification and tiered preventive interventions, might be more effective and warrants further investigation.
Supplementary Material
Acknowledgments
Research Support: The research reported in this article was supported by contract numbers N01-HC-85239, N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, N01-HC-45133, grant number HL080295 from NHLBI and grant number AG-023269 from NIA, with additional contribution from NINDS. Additional support was provided through AG-15928, AG-20098, and AG-027058 from NIA, HL-075366 from NHLBI, and the University of Pittsburgh Claude D. Pepper Older Americans Independence Center P30-AG-024827. This project was also partially funded by an Emory University Heart and Vascular Board grant entitled ‘Novel Risk Markers and Prognosis Determination in Heart Failure’ and Public Health Service Grant UL1 RR025008 from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources.
ABRREVIATIONS
- ASE
American Society of Echocardiography
- CHD
coronary heart disease
- CHS
Cardiovascular Health Study
- EF
ejection fraction
- HF
heart failure
- LV
left ventricular
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
Footnotes
Relationships with Industry: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hunt S. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure) J Am Coll Cardiol. 2005;46:e1–82. doi: 10.1016/j.jacc.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 2.Vasan RS, Larson MG, Benjamin EJ, Evans JC, Levy D. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med. 1997;336:1350–5. doi: 10.1056/NEJM199705083361903. [DOI] [PubMed] [Google Scholar]
- 3.Gardin JM, McClelland R, Kitzman D, et al. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study) Am J Cardiol. 2001;87:1051–7. doi: 10.1016/s0002-9149(01)01460-6. [DOI] [PubMed] [Google Scholar]
- 4.Aurigemma G, Gottdiener J, Shemanski L, Gardin J, Kitzman D. Predictive value of systolic and diastolic function for incident congestive heart failure in the elderly: the cardiovascular health study. J Am Coll Cardiol. 2001;37:1042–8. doi: 10.1016/s0735-1097(01)01110-x. [DOI] [PubMed] [Google Scholar]
- 5.Gottdiener JS, Kitzman DW, Aurigemma GP, Arnold AM, Manolio TA. Left atrial volume, geometry, and function in systolic and diastolic heart failure of persons > or =65 years of age (the cardiovascular health study) Am J Cardiol. 2006;97:83–9. doi: 10.1016/j.amjcard.2005.07.126. [DOI] [PubMed] [Google Scholar]
- 6.de Simone G, Gottdiener J, Chinali M, Maurer M. Left ventricular mass predicts heart failure not related to previous myocardial infarction: the Cardiovascular Health Study. Eur Heart J. 2008;29:741–7. doi: 10.1093/eurheartj/ehm605. [DOI] [PubMed] [Google Scholar]
- 7.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–55. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–63. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 9.deFilippi C, Christenson R, Gottdiener J, Kop W, Seliger S. Dynamic cardiovascular risk assessment in elderly people. The role of repeated N-terminal pro-B-type natriuretic peptide testing. J Am Coll Cardiol. 2010;55:441–50. doi: 10.1016/j.jacc.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKie P, Cataliotti A, Lahr B, et al. The prognostic value of N-terminal pro-B-type natriuretic peptide for death and cardiovascular events in healthy normal and stage A/B heart failure subjects. J Am Coll Cardiol. 2010;55:2140–7. doi: 10.1016/j.jacc.2010.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emberson J, Ng L, Armitage J, Bowman L, Parish S, Collins R. N-terminal Pro-B-type natriuretic peptide, vascular disease risk, and cholesterol reduction among 20,536 patients in the MRC/BHF heart protection study. J Am Coll Cardiol. 2007;49:311–9. doi: 10.1016/j.jacc.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 12.Wang T, Levy D, Benjamin E, Vasan R. The epidemiology of “asymptomatic” left ventricular systolic dysfunction: implications for screening. Ann Intern Med. 2003;138:907–16. doi: 10.7326/0003-4819-138-11-200306030-00012. [DOI] [PubMed] [Google Scholar]
- 13.Redfield M, Rodeheffer R, Jacobsen S, Mahoney D, Bailey K, Burnett JJ. Plasma brain natriuretic peptide to detect preclinical ventricular systolic or diastolic dysfunction: a community-based study. Circulation. 2004;109:3176–81. doi: 10.1161/01.CIR.0000130845.38133.8F. [DOI] [PubMed] [Google Scholar]
- 14.Bibbins-Domingo K, Ansari M, Schiller NB, Massie B, Whooley MA. Is B-type natriuretic peptide a useful screening test for systolic or diastolic dysfunction in patients with coronary disease? Data from the Heart and Soul Study. Am J Med. 2004;116:509–16. doi: 10.1016/j.amjmed.2003.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Lemos J, McGuire D, Khera A, et al. Screening the population for left ventricular hypertrophy and left ventricular systolic dysfunction using natriuretic peptides: results from the Dallas Heart Study. Am Heart J. 2009;157:746–53.e2. doi: 10.1016/j.ahj.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 16.Atherton J. Screening for left ventricular systolic dysfunction: is imaging a solution? JACC Cardiovasc Imaging. 2010;3:421–8. doi: 10.1016/j.jcmg.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 17.Butler J, Kalogeropoulos A, Georgiopoulou V, et al. Incident heart failure prediction in the elderly: the health ABC heart failure score. Circ Heart Fail. 2008;1:125–33. doi: 10.1161/CIRCHEARTFAILURE.108.768457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalogeropoulos A, Psaty BM, Vasan RS, et al. Validation of the health ABC heart failure model for incident heart failure risk prediction: the Cardiovascular Health Study. Circulation Heart failure. 2010;3:495–502. doi: 10.1161/CIRCHEARTFAILURE.109.904300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fried LP, Borhani NO, Enright P, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–76. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 20.Psaty BM, Kuller LH, Bild D, et al. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–7. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 21.Blackburn H. Classification of the electrocardiogram for population studies: Minnesota Code. J Electrocardiol. 1969;2:305–10. doi: 10.1016/s0022-0736(69)80120-2. [DOI] [PubMed] [Google Scholar]
- 22.Gardin JM, Wong ND, Bommer W, et al. Echocardiographic design of a multicenter investigation of free-living elderly subjects: the Cardiovascular Health Study. J Am Soc Echocardiogr. 1992;5:63–72. doi: 10.1016/s0894-7317(14)80105-3. [DOI] [PubMed] [Google Scholar]
- 23.Gardin JM, Siscovick D, Anton-Culver H, et al. Sex, age, and disease affect echocardiographic left ventricular mass and systolic function in the free-living elderly. The Cardiovascular Health Study. Circulation. 1995;91:1739–48. doi: 10.1161/01.cir.91.6.1739. [DOI] [PubMed] [Google Scholar]
- 24.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–8. doi: 10.1016/0002-9149(86)90771-x. [DOI] [PubMed] [Google Scholar]
- 26.Ives DG, Fitzpatrick AL, Bild DE, et al. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–85. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 27.Schellenbaum GD, Heckbert SR, Smith NL, et al. Congestive heart failure incidence and prognosis: case identification using central adjudication versus hospital discharge diagnoses. Ann Epidemiol. 2006;16:115–22. doi: 10.1016/j.annepidem.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Volinsky CT, Raftery AE. Bayesian information criterion for censored survival models. Biometrics. 2000;56:256–62. doi: 10.1111/j.0006-341x.2000.00256.x. [DOI] [PubMed] [Google Scholar]
- 29.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 30.Pencina MJ, D’Agostino RB, Vasan RS. Statistical methods for assessment of added usefulness of new biomarkers. Clin Chem Lab Med. 2010;48:1703–11. doi: 10.1515/CCLM.2010.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chambless L, Cummiskey C, Cui G. Several methods to assess improvement in risk prediction models: Extension to survival analysis. Stat Med. 2010 doi: 10.1002/sim.4026. [DOI] [PubMed] [Google Scholar]
- 32.Pencina M, D’Agostino RS, Steyerberg E. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2010 doi: 10.1002/sim.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nielsen O, McDonagh T, Robb S, Dargie H. Retrospective analysis of the cost-effectiveness of using plasma brain natriuretic peptide in screening for left ventricular systolic dysfunction in the general population. J Am Coll Cardiol. 2003;41:113–20. doi: 10.1016/s0735-1097(02)02625-6. [DOI] [PubMed] [Google Scholar]
- 34.Redfield M, Jacobsen S, Burnett JJ, Mahoney D, Bailey K, Rodeheffer R. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 35.Heidenreich P, Gubens M, Fonarow G, Konstam M, Stevenson L, Shekelle P. Cost-effectiveness of screening with B-type natriuretic peptide to identify patients with reduced left ventricular ejection fraction. J Am Coll Cardiol. 2004;43:1019–26. doi: 10.1016/j.jacc.2003.10.043. [DOI] [PubMed] [Google Scholar]
- 36.Smith J, Newton-Cheh C, Almgren P, et al. Assessment of conventional cardiovascular risk factors and multiple biomarkers for the prediction of incident heart failure and atrial fibrillation. J Am Coll Cardiol. 2010;56:1712–9. doi: 10.1016/j.jacc.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Velagaleti R, Gona P, Larson M, et al. Multimarker approach for the prediction of heart failure incidence in the community. Circulation. 2010;122:1700–6. doi: 10.1161/CIRCULATIONAHA.109.929661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takemoto Y, Barnes M, Seward J, et al. Usefulness of left atrial volume in predicting first congestive heart failure in patients > or = 65 years of age with well-preserved left ventricular systolic function. Am J Cardiol. 2005;96:832–6. doi: 10.1016/j.amjcard.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 39.From AM, Scott CG, Chen HH. The development of heart failure in patients with diabetes mellitus and pre-clinical diastolic dysfunction a population-based study. J Am Coll Cardiol. 2010;55:300–5. doi: 10.1016/j.jacc.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.