Cardiovascular diseases remain the major cause of death in the western world.1 Stem and progenitor cell (SPC)-based therapies in animal models and human trials in recent years have suggested promising therapeutic potential and drawn intense public interest. Possible beneficial mechanisms of cell-based therapies include generation of new and mature ventricular cardiomyocytes (cardiomyogenesis), recruitment of endogenous SPCs for cardiomyogenesis, and salvage of native myocardium through paracrine, angiogenic, and anti-apoptotic effects. 2, 3 We refer readers to several excellent reviews for an overview of cell-based therapies for cardiac diseases.2–5 Here we discuss potential electrophysiological challenges and arrhythmic risks posed by cell therapies designed to replace damaged myocardium. Although adverse outcomes after cell therapies, such as lethal arrhythmias, occurred in early clinical trials of skeletal myoblast (SkM) transplantation, 5–8 recent clinical trials using various sources of SPCs, novel modes of cell delivery, better cell selection, and different timing of cell transplantation did not show any significant increase in arrhythmic incidence. It is tempting to conclude on this basis that cell-based therapies are “safe” and non-arrhythmogenic (reviewed in 2, 4); however, the negligible production of new cardiomyocytes (CMs) documented for many SPCs employed make it difficult to extrapolate their safety records towards future trials in which substantial numbers of new CMs might be generated or introduced. Clinical trials to test cardiomyocyte replacement (cardiomyogenesis), long a goal of SPC-based therapy, are bound to take place in the near future, thanks to many investigators who are developing technologies for efficient generation of CMs from SPCs and for increasing their retention after transplantation. In weighing the benefits of replacement therapies, we should not overlook the fact that SPC-derived CMs (SPC-CMs) exhibit variable electrophysiological (EP) properties and are typically immature as compared to adult CMs (see below). This immaturity of primitive SPC-CMs remains a major hurdle towards developing safe cell therapies. In anticipation of improved cardiomyogenesis in future cell therapies, we will review here developmental and electrophysiological challenges, common to all SPC-CMs, that need to be resolved in order to improve the maturation of primitive SPC-CMs and to avoid unwanted risks of increasing arrhythmic incidence (pro-arrhythmia) after transplantation.
Concerns about Maturation Status of Transplanted Cells
Lack of Regional and Timely Developmental Cues for Subtype Specification and Maturation
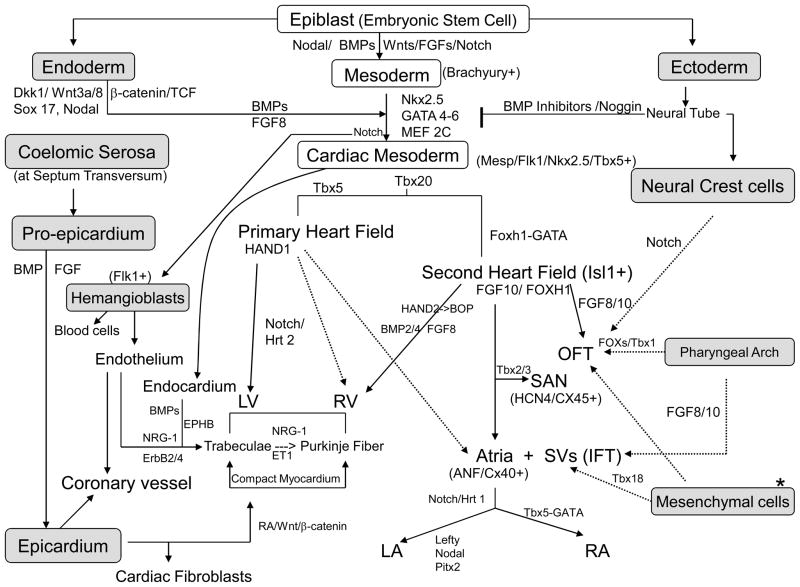
Research in cardiac morphogenesis has provided valuable insights into basic mechanisms controlling cardiomyogenesis, and these are beginning to be applied to direct differentiation of cardiomyocytes from SPCs (reviewed in 9–13). Cardiomyocytes develop from cardiogenic mesoderm (Nkx2.5+ and Mesp1/2+) during embryonic development. In addition to the primary cardiac mesoderm, there are other sources of cells that are recruited to join the mature myocardium during cardiac development, including a second wave of cardiogenic mesoderm, known as the secondary heart field, that gives rise to the outflow tract, right ventricle, and portions of the atria.11, 14 Cardiomyocytes in the developing fetal heart continue to proliferate, despite functional contraction, but mitotic activity declines quickly after birth (few weeks in rat) 15 and continued growth of the heart is largely due to an increase in myocyte size.9 In human, cardiomyocytes reach adult size at ages between 10 to 20 years.16 The prevailing view that the heart does not regenerate has slowly given way to the idea that some degree of endogenous regeneration occurs through replacement of dead and damaged cells, in particular after injury.9, 17 Three aspects of cardiac development are relevant to cell replacement therapy: differentiation, subtype-specification, and maturation of cardiomyocytes. In a simple schematic, Figure 1 illustrates some of many factors and genes that work in a coordinated, region-specific and temporally defined fashion to determine the fate of various subtypes of cardiomyocytes during cardiogenesis. Not detailed are various extra-cardiac cells, such as pharyngeal mesodermal cells, mesenchymal cells at arterial and venous poles, and possibly circulating hematopoietic and/or endothelial progenitor cells (HPCs and/or EPCs), all contributing to or influencing the formation of a functional myocardium. During early development when many of the cell lineage decisions are being made, a number of regulatory factors are diffusible proteins derived from other tissues, such as pharyngeal endoderm, neural crest and pro-epicardium. A number of key factors that dictate chamber identity are diagrammed in Figure 2, illustrating that many subtle temporal and regional cues determine the subtype specification of cardiomyocytes, such as atrial, ventricular and pacemaking CMs, and direct their maturation during normal cardiac development.18–19 How these regional and temporal cues influence the expression of various types of ion channels remains to be explored.
Figure 1.
A simple schematic of molecular pathways of cardiogenesis. See reviews in 9–13 for details. Shaded areas indicate the extra-cardiac cell sources. Dkk1 indicates Dickkopf1; Sox17, an endodermal transcription factor; TCF, T cell factor; BMP, Bone morphogenetic protein; Wnts, Wingless related factors; Nkx, NK family of Homeobox genes; MEF2C, myocyte-specific enhancer-binding factor 2C; Tbx, T-box transcription factors; Flk1, kinase insert domain protein receptor; Isl1: LIM homeodomain transcription factor islet1; FGF, Fibroblast growth factor; EphB, Ephrin-B; PitX, Pituitary homeobox family; NRG-1, neuregulin-1; RA, retinoid acid; ET1, endothelin-1; Hrt, hairy-related transcription factor; BOP, CD8b-opposite transcription factor; Foxh1, forkhead DNA binding transcription factor; Fox, forkhead transcription factor; and * denotes mesenchymal cells from multiple sources.
Figure 2.
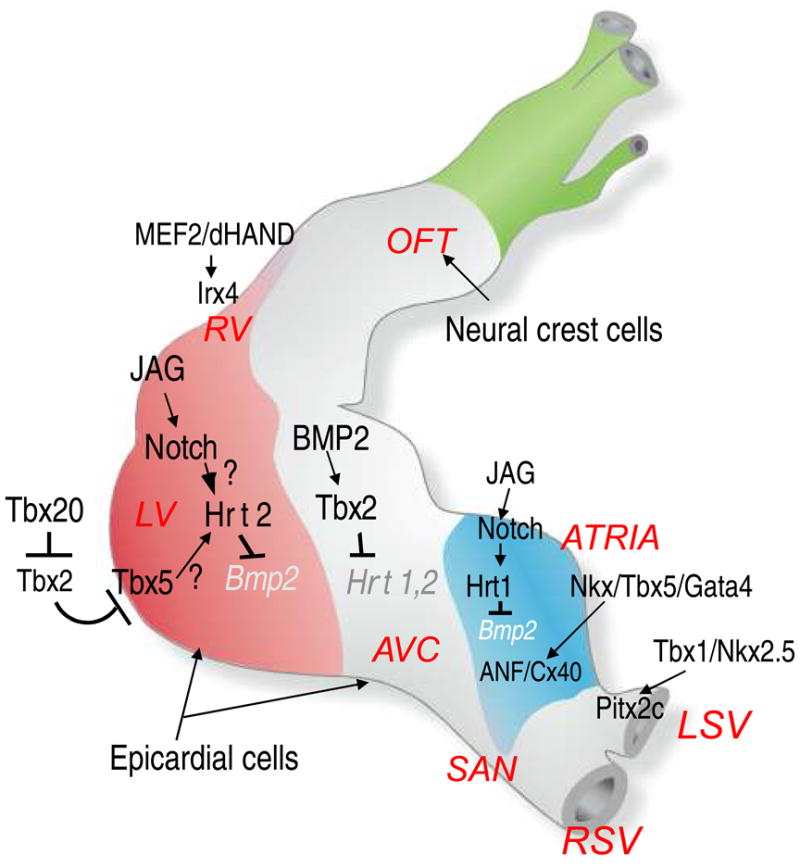
A diagram of genetic and signaling pathways involved in chamber specification of myocardial development. JAG indicates a notch ligand, Jagged; Irx, Iroquois-related homeobox protein; see Figure 1 legend for the definition of other abbreviations. Adapted from reference 18 with permission.
Most cellular cardiomyocyte replacement strategies have focused on using immature cardiomyocytes or committed progenitors since the adult heart seems unlikely to produce the complex array of signaling molecules needed to direct cardiogenic differentiation of uncommitted SPCs. Embryonic stem cells are the extreme case in that they are derived from and resemble cells of pre-implantation embryos, form teratomas upon transplantation, and appear to require a recapitulation of the cardiogenic differentiation program that occurs in the developing embryo to yield CMs. 3, 20 It is unrealistic to expect the adult heart to provide such a vast array of developmental cues, and even less so in the correct sequence. Whether the damaged adult heart produces factors 21 capable of stimulating clinically meaningful differentiation of extra-cardiac SPCs, such as HPCs, also remains controversial. 3 Studies reporting induced differentiation of extra-cardiac SPCs by juxtaposition with adult myocardium in vitro or after implantation in vivo have largely relied on co-localizing cardiomyocyte immunostaining with lineage labels, yet discrepancies in experimental protocols and estimates of differentiation efficiency vary to the extent that the premise of transdifferentiation of extra-cardiac SPCs into CMs is a matter of considerable dispute.3 Therefore, considering the broader picture, it is clear that the question of the optimal developmental stage to implant cells remains unresolved for most SPCs and that the answer is likely to vary depending on the particular SPC. Transplanting uncommitted cells could result in poor differentiation, or even form a tumor, as in the case of highly proliferative cells such as embryonic stem cells (ESCs). Since late stage differentiation to a fully matured VM population has not been achieved (see below), and these cells are probably too fragile to survive implantation, it would seem, therefore, that immature cardiomyocytes or committed progenitors offer the most promise. However, the ability of adult myocardium to support further development, maturation and EP subtype specification of such cells remains poorly probed and in all likelihood will need to be studied further to elucidate potential means of post-transplant augmentation in order to avoid unwanted risks.2–5
Future Electrophysiological Challenges in Cardiomyogenesis
Gap Junction Distribution Mismatch
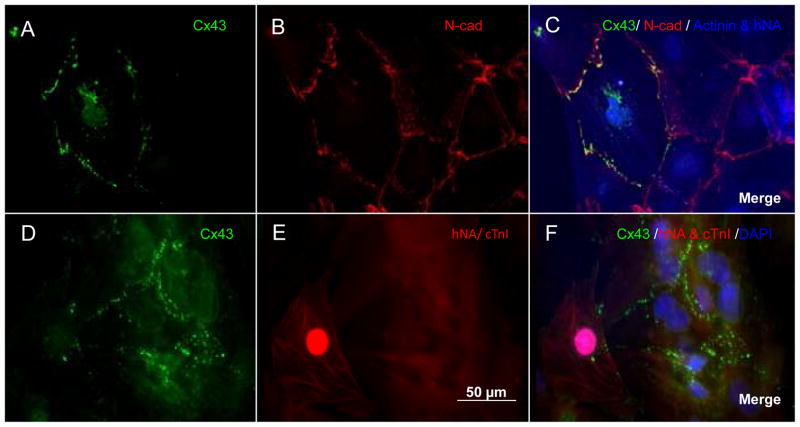
Gap junctions, formed by various types of connexins (Cxs) are important for cardiomyocyte coupling and electrical conduction. In adult ventricular cardiomyocytes (VMs), Cxs are mostly confined to intercalated discs for longitudinal conduction.22 In contrast, Cxs in normal neonatal VMs and, pathologically, at border zones of myocardial infarction (MI) distribute over the entire perimeter of myocytes in a punctate pattern.23,24 It takes 6 years for normal human neonatal myocytes to completely acquire the adult pattern of gap junction distribution.23 Additionally, pathologically disturbed distribution of gap junctions has been linked to arrhythmogenesis in MI, ventricular hypertrophy, atrial fibrillation and aging hearts.25 Not surprisingly, gap junction distribution of SPC-CMs resembles that of neonatal cardiomyocytes (Figure 3), which have slower conduction velocities.26 Moreover, cadherin and gap junction connections between SPC-CMs and host myocytes in most co-cultures and animal models are randomly distributed at the contact interfaces without proper alignment 27–29 (Figure 3). Action potential (AP) propagation across these interfaces is unpredictable, inhomogeneous, and, consequently, might result in increased anisotropy and reentrant arrhythmias. 27 Little is known about the developmental and transcriptional control of myocardial Cx expression, 30 particularly the major Cx of VMs, Cx43. Furthermore, SkM-derived myotubes do not express cadherin or Cx43,31 and do not electromechanically couple to host myocytes.32 As a result, conduction slowing and induction of arrhythmia after SkM transplantation have been demonstrated (see below).
Figure 3.
Expression patterns of connexin 43 (Cx43) in human embryonic stem cell-derived cardiomyocytes (hESC-CMs) and neonatal rat ventricular myocytes (NRVMs). Images are of hESC-CMs in cardiospheres (A–C) and in co-cultures with NRVMs (D–F). hESC-CMs were stained with anti-Cx43 (green) (A), anti-N-cadherin (red) (B), anti-α-actinin (blue), and anti-human nuclear antigen (hNA, blue) (merged in C). In the co-culture of hESC-CMs and NRVMs, myocytes were immunostained with anti-Cx43 (green) (D), anti-hNA and cardiac troponin I (cTnI) (both in red, E), as well as DAPI for nuclear staining (blue) (merged in F). In E, F, only human cells are positive for anti-hNA staining (red). hESC-CMs are variable in size. Cxs 43 of both hESC-CMs and NRVMs distribute in a punctate and neonatal-like pattern at the cell contact surfaces.
More importantly, ion channels, electrical dispersion, and tissue architecture including fibroblasts all contribute to normal and abnormal cardiac conduction.33 Therefore, it is necessary to examine many other cellular and tissue attributes in order to avoid an over-simplified view of cardiac conduction and to provide a true evaluation of functional integration of SPC-CMs after cell transplantation.
Cell Size and Shape Mismatch
Other than gap junctions, cell size has been shown to be a major determinant of impulse propagation and maximal rate of AP depolarization (Vmax = dV/dtmax). 34 Adult VMs have a cylindrical and elongated shape, contracting along a single axis with a positive force-frequency relationship.35 Neonatal cardiomyocytes and pacemaker cells, however, have a fusiform or spindle-like appearance.34 In comparison, most SPCs and SPC-CMs used for clinical trials or animal models display irregular cell shapes (neonatal-like, Figure 3) with their mean size (< 50–60 μM) 26,36–37 smaller than adult VMs. 15,34 In contrast, SkM-derived myotubes are larger than VMs in size 38,39 and display fusiform shapes with characteristics of both skeletal and cardiac cells. 40 Also, some SPCs, e.g. hematopoietic stem cells, may not have significant potential for regenerating myocytes but survive in the damaged heart and contribute to other cell types.3 Therefore, in addition to immature Cx connections between donor and host myocardial cells, cell size mismatch per se and subsequent changes in interstitial space and/or resistance could cause slower conduction and depolarization source-sink mismatch, both of which facilitate reentry.34 Size match and cellular alignment of engrafted cells is rarely achieved in most reports, 29,41–42 therefore, translational research into methods for stimulating maturation, alignment and electrical coupling of regenerated cardiomyocytes will be required.42
Macroscopic myocardial fiber alignment mismatch
Macroscopically, the left ventricular muscle fibers can be separated into three layers. The superficial layer runs a slightly slanting vertical course, the midlayer fibers form a circular pattern, and the endocardial (deep) layer lies mainly vertically. These three fiber layers work in a coordinate fashion with the supporting fibrous matrix to create a twisting motion for generating efficient cardiac ejection. 43 The alignment of the injected SPC-CMs with the host muscle fibers has been shown to be discordant or, in rare occasions, concordant. 20 Currently, there is no established method to align the injected cells into a single muscle layer. Misaligned muscle fibers could create dyssynchronous contractions, leading to inefficient pumping function, inappropriate stretch and local electrical dispersion. 44
Acton potential mismatch
Ion channels are important for excitation-contraction (EC) coupling, pulse generation, signal transduction, and cell differentiation/proliferation/maturation.45 Altered properties of sodium (Na+), potassium (K+) and calcium (Ca2+) channels are found in failing hearts and at the epicardial border zone of infarcted myocardium, which has been linked to the stabilization of ventricular tachycardia (VT) circuits. 46,47 The AP duration (APD) in human hearts during the first few years of life increases by about 20% despite a 16-fold increase in heart weight and a 2.4-fold increase in myocardial cell size.15,48 Adult VMs are usually quiescent (although excitable) and characterized by hyperpolarized maximal diastolic potential (MDP), relatively long APD and the absence of spontaneous phase 4 depolarization. 49
Table 1 is a literature search summary of data from electrophysiological characterizations of SPC-CMs. Compared to EP properties of adult cardiomyocytes (CMs), Table 1 depicts that most SPC-CMs, at best, exhibit APs similar to fetal or neonatal CMs. 37,50 Regardless of cell sources, these heterogeneous APs are commonly categorized as sinus node- (or pacemaker-), atrial-, and ventricular-like CMs based on overall EP morphologies. 53 Implanting such immature SPC-CMs with variable APDs relative to aging adult CMs may not be beneficial. 71 Furthermore, the ability of HPC or EPCs to form electrophysiologically functional CMs by transdifferentiation remains controversial (discussed in 3). Other types of extra-cardiac SPCs, such as mesenchymal stem cells (MSCs) from bone marrow (BM), 58 and adipose tissue derived stem cells, 63 have been reported to yield heterogeneous populations of CMs with various AP shapes (see Table 1), albeit at very low incidence. Also, SkM-derived myotubes obtained from cultures or after engraftment into myocardium displayed very short APD, fast afterdepolarization, and bursting neuronal-like activities (similar to trains of early-afterdepolarization in Torsades de Pointes).32 Thus far, no studies have demonstrated that a uniform and mature EP phenotype could be derived from any SPC-CM in vitro or in vivo (Table 1). Thus, we expect that the EP heterogeneity of SPC-CMs will likely pose substantial challenges for developing a safe cell replacement therapy. A multidisciplinary evaluation of functional properties of SPC-CMs after cell transplantation, including ion channel distributions and their development patterns, would help identify the proper EP phenotype of SPC-CMs for future cell therapies.
TABLE 1.
Electrophysiological properties of stem and progenitor cell-derived cardiomyocytes.
| Cell Source | HR | AP type | MDP | Vmax | Auto. | APD90 | APA | INa | If | ICaL | IK1 | IK-DR | IKto | B-R | Ach-R | Arrhyth. | Ca Oscil. | CX43 | Connect. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (bpm) | (mV) | (V/s) | (mV) | (HCN) | ||||||||||||||||
| Large mammals Adult CM | ||||||||||||||||||||
| Schram49 | SAN | 70 | Nodal/P | −50 | <2–15 | high | 200 | 70 | No | large | strong | min. | IKr,IKs | min. | Y | Y | Physiol. | Y | N | Y |
| Atria | min. | A | (−70~−80) | 150–300 | min. | 150 | 100–110 | large | small | mod. | mod. | IKr,IKs | Y | Y | Y | N | N | Y | Y | |
| V | N | V | −80 | 200–300 | no | 200–300 | 140 | strong | none | large | large | IKr,IKs | Y | Y | Y | N | N | Y | Y | |
| AVN | 40–50 | AVN | −64 | <20 | mod. | <120 | 90 | small | large | large | min. | IKr,IKs | Y | Y | Y | Y | Y | N | Y | |
| Purkinje | <30 | Pur | −90 | 400–800 | low | 290 | 130 | strong | large | small | large | IKr | Y | Y | ? | Y | Y | ? | Y | |
| Human fetal CM | ||||||||||||||||||||
| Mummery37 | hFetal V | 0.8 HZ | fetal V | −38.5 | 8.9 | low | 370 | 69 | ND | ND | Y | ND | ND | ND | Y | Y | ND | Y | ND | ND |
| hFetal A | 1Hz | fetal A | −35 | 1.2 | mod. | 164.9 | 57.2 | ND | ND | Y | ND | ND | ND | Y | Y | ND | Y | ND | ND | |
| Murine Fetal CM | ||||||||||||||||||||
| Halbach50 | mFetal V | min. | fetel V | −55 | <15 | min. | 125 | ~60 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Y | Y |
| Murine ESC-CM: see reviews51–52 | ||||||||||||||||||||
| Human Embryonic SC-CM | ||||||||||||||||||||
| He53 | hESC | 69 | A | −52.6 | 11.5 | Y | 131 | 78.5 | ND | ND | ND | ND | IKr | ND | Y | ND | EAD,DAD | ND | ND | ND |
| 47 | V | −53.9 | 13.2 | Y | 247 | 85.3 | Y(Irreg.) | |||||||||||||
| 70 | Nodal | −49.2 | 6.9 | Y | 168 | 68.5 | ||||||||||||||
| Mummery37 | hESC | 90 | A | −38.7 | 8.5 | Y | 121.8 | 60.8 | ND | ND | Y | ND | ND | ND | Y | Y | ND | Y | ND | ND |
| 36 | V | −48 | 7 | Y | 436.4 | 80 | ||||||||||||||
| 72 | P | −20.8 | 2.6 | Y | 134 | 32 | ||||||||||||||
| Xu54 | hESC | 40–50 | ND | ND | ND | Y | ND | ND | ND | ND | Y | ND | ND | ND | Y | ND | ND | Y | ND | ND |
| Satin55 | hESC | 54–94 | P, V | ~−60 | 12 | Y | ~450 | 100 | Y | Y | Y | N | ND | ND | Y | Y | ND | Y | Y | Y |
| Santiani56 | hESC | 26–36 | A & V | ~−70 | 4.2–6.0 | Y | 200–341 | 80–100 | ND | Y | Y | Y | IKr | Y | Y | ND | ND | ND | ND | ND |
| Mesenchymal SC-CM | ||||||||||||||||||||
| Takeda57 | hBM-MSC | 66–70 | V, Nodal | −50 | ND | Y | 165–346 | 47–64 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Y | N |
| Makino58 | mBMSC | 108–950 | V | −59.5 | ND | Y | 52 | 71 | ND | ND | Y | ND | ND | ND | Y | ND | ND | ND | Y | ND |
| 143–788 | Nodal | −55 | ND | Y | 42 | 58.5 | ND | ND | ND | ND | ND | ND | Y | ND | ND | ND | Y | ND | ||
| Nishiyama28 | hUCB-MSC | 36 | A or V | −54 | ND | Y | 186 | 57 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Y | N |
| BMPC (non-Mesenchym.) CM | ||||||||||||||||||||
| Badorff59 | hEPC CD34+ | ? | ND | ND | ND | ? | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Y | Y | ND | Y |
| Lagostena 60 | mBM c-kit+ | NA | NA | (−55~−65) | NA | N | NA | NA | N | ND | N | Subset | Subset | Subset | ND | ND | NA | N | N | N |
| Rota61 | mBM c-kit+ | ND | ? | ND | ND | Y | ND | ND | Y | ND | ND | Y | Y | ND | ND | ND | ? | Y | Y | Y |
| Adipose Derived SC-CM | ||||||||||||||||||||
| Bai62 | hASC | 28–47 | P | ND | ND | Y | ND | ND | ND | ND | <1% | ND | ND | Y | ND | ND | ND | Y | ND | ND |
| Planat-Bérard63 | mASC | 7Hz | A, V, P | (−40~−60) | 1.8 | Y | 60–300 | 68 | ND | ND | ND | ND | ND | ND | Y | Y | ND | ND | Y | ND |
| Yamada64 | mCD29+ | 167–490 | P | −56.5 | ND | Y | 50 | 57.5 | ND | ND | Y | ND | ND | ND | Y | ND | ND | ND | ND | ND |
| Adult Spermatogonial SC-CM | ||||||||||||||||||||
| Guan65 | mASpSC | 48 | A, Purkinje | −57 | 44, 49 | Y | 162, 287 | 88, 96 | Y | ND | Y | ND | ND | ND | Y | ND | Y(Irreg.) | Y | Y | Y |
| V | −54 | 31.6 | Y | 314 | 93 | |||||||||||||||
| P | −38 | 2.5 | Y | 227 | 63 | |||||||||||||||
| Intermed. | −43 | 2.9 | Y | 340 | 74 | |||||||||||||||
| Resident CPCs | ||||||||||||||||||||
| Laugwitz14 | mIsl1+ | 1Hz | A, N | ~−60 | ~60 | Y | ~220 | 80 | ND | ND | ND | ND | Y | ND | Y | ND | ND | Y | ND | Y |
| Matsuura 66 | mSca-1+ | 132 | ND | ND | ND | ~1% | ND | ND | ND | ND | ND | ND | ND | ND | Y | ND | Y(Irreg.) | Y | Y | ND |
| Smith67 | h&p c-kit+ | 0 | P, V | −50, −80 | ND | N | ~400 | 75, 110 | Y | ND | Y | Y | ND | ND | ND | ND | Y(Irreg.) | Y | Y | Y |
| Goumans68 | hSca-1+ | 40 | V | −67 | 39 | Y | 483 | ~90 | ND | ND | Y | ND | ND | ND | Y | ND | ND | Y | Y | Y |
| Skeletal Myoblast | ||||||||||||||||||||
| Leobon32 | rSkM | ND | Skeletal | ND | ND | rare | 1–5.6 | ND | ND | ND | ND | ND | ND | ND | ND | ND | EAD | ND | ND | N |
| Winitsky69 | mSpoc cell | 1–8 Hz | Skeletal | −60 | fast | High | ~5 | 110–150 | Y | ND | Y | ND | ND | ND | Y | ND | Y(Irreg.) | Y | ND | ND |
| Itabashi39 | rSkM | 2–5Hz | Skeletal | ND | fast | 0.3 | short | ~100 | ND | ND | N | ND | ND | ND | ND | Y | Y | Y(Fib.) | N | N |
| Placental SC-CM | ||||||||||||||||||||
| Okamoto70 | hPDEMS | 63 | P, V | −52 | 4.5 | Y | 209 | 64 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | Y | N |
CM indicates cardiomyocyte; h, human; m, murine; r, rat; p, pig; HR, heart rate; AP, action potential; MDP, maximal diastolic potential; Auto, automaticity; APD90, AP duration at 90% of repolarization; APA, AP amplitude; INa, NA+ current; If (HCN), pacemaker current mediated by hyperpolarization-activated cyclic nucleotide-gated channel; ICaL, L-type Ca2+ current; IK1, inward-rectifier K+ current; IK-DR, delay-rectifier K+ current; IKr/IKs, rapid and sustained component of IK-DR; IKto, transient outward K+ current; B-R, beta-adrenergic response; Ach-R, cholinergic response; Arrhyth., arrhythmic activities; EAD, early after-depolarization; DAD, delayed after-depolarization; CA Oscil., intracellular Ca2+ oscillation shown by Ca2+ imaging; Cx43, Cx43 by staining; Connect., intercellular connections with host cells shown by dye transfer; SAN, sinoatrial node; A, atria; V, ventricle; AVN, atrioventricular node; Pur., Purkinje cell; P, pacemaker cell; Intermed., intermediate type; min., minimal; mod., moderate; Y, positive result; N, negative result; ND, not determined; NA, not applicable; Irreg., irregular rhythm; ESC, embryonic stem cells; MSC, mesenchymal stem cell; BM, bone-marrow; UCB, umbilical cord blood-derived; BMPC, Bone marrow derived progenitor cell; EPC, Endothelial progenitor cell: ASC, adipose derived stem cell; ASpSC, adult spermatogonial stem cell; CPC, cardiac progenitor cell; Isl1, islet-1; Sca-1, stem cell antigen-1; SkM, Skeletal myoblast; PDEMC: placenta-derived extraembryonic mesodermal cell.
Transmural and Regional Dispersion of Ventricular Repolarization
Intrinsic transmural and regional electrical heterogeneity in VMs has been demonstrated in human hearts. Based on transmural locations, VMs in large animals display different APDs and compositions of ion channels.72 For example, compared to VMs located within the endo-myocardial layer, epi-myocardial VMs depict larger IKto (transient outward K+ current) with shorter APDs. Differences in EP properties of these myocardial cells create transmural dispersion of repolarization (TDR) and their differential responses to pharmacologic agents, heart rate changes, neural inputs, and myocardial injury could increase TDR, which has been linked to arrhythmogenesis in genetic disorders, e.g. long QT and Brugada syndromes. 72 In failing hearts, decreased coupling between subepi- and mid-myocardium also increases APD dispersion and may facilitate arrhythmia.73
Furthermore, levels and activities of K+ channel subtypes responsible for various phases of repolarization, such as IKto, ultra-rapid (IKUR), delayed-rectifier (IKS and IKr) and inward-rectifier (IK1) currents, undergo significant changes during embryonic 74 and postnatal 75 development, yet regulation of their developmental changes is not well elucidated. Given that alterations in these K+ currents are responsible for regional and transmural heterogeneity of ventricular repolarization, unselective transplantation of immature SPC-CMs into undefined layers or regions of myocardium would be expected to increase local or regional TDR and might promote arrhythmia. 71 Thus, the intrinsic transmural and regional electrical heterogeneity of human hearts poses particular problems for cell-based therapies if myocardial repair becomes a reality and a substantial number of transplanted SPC-CMs survive. Furthermore, a greater understanding of the developmental changes of K+ channels in implanted cells will help in developing electrophysiologically compatible SPC-CMs for cell therapies.
Persistent Automaticity of SPC-CMs
SPC-CMs are a heterogeneous population of cells with variable APDs. Most SPC-CMs display significant phase-4 depolarization with spontaneous automaticity (Table 1). In fact, human ESC-CMs after implantation can form a pulse-generating focus with functional connections to host myocytes and have been used to develop so-called biological pacemakers. 29 These studies demonstrated that these implanted SPC-CMs could easily become automatic foci for frequent premature ventricular complexes (PVCs) observed in most clinical trials of cell therapies. Furthermore, Purkinje-like cells with automaticity have been derived from SPCs in culture (Table 1). Purkinje cells could be the prevalent trigger foci for ventricular arrhythmias or contribute to reentrant circuits in post-infarct VTs. 76 These reports raise the concern that persistent automaticity of SPC-CMs could be an unwanted result of cell therapies.
Immature Intracellular Calcium Handling Capability
Intracellular calcium homeostasis is important for optimal cell function and EC coupling. Imbalance of the intracellular Ca2+ handling ability through disease processes (heart failure and ischemia/reperfusion) or drugs can lead to electrical alternans 77 and delayed after-depolarization (DAD), which might induce triggered activity. DAD is the main mechanism for right ventricular outflow tract tachycardia and triggering foci located in Purkinje fibers. During mouse embryonic and postnatal development, expression of ryanodine receptor (RyR2), sarcoplasmic reticulum (SR) Ca2+ pump (SERCA2), phospholamban (PLB) and L-type Ca2+ channel currents (ICaL) increases with cardiac maturation yet Na+/Ca2+ exchanger levels remain constant or decrease slightly. 78 In embryonic CMs, Ca2+ influx through Ca2+ channels is the main source for EC coupling. The SR matures postnatally and plays a major role in EC coupling through the Ca2+–induced Ca2+ release apparatus. 79 In human ESC-CMs, these Ca2+ handling receptors have been shown to be functionally or developmentally immature. 35, 80 Dysfunctional Ca2+ handling by defective RyR2 and calsequestrin has been linked to exercise-induced sudden death in catecholaminergic polymorphic VT and triggered arrhythmia in patients with heart failure. 81 Reports in recent literature are just beginning to study Ca2+ handling capabilities of various SPC-CMs and might offer insights towards ways to enhance the maturation of intracellular Ca2+ handling apparatus of SPC-CMs.
Uncontrolled Sympathetic Hyperinnervation Induced by Cell Transplantation
Sympathetic nerve sprouting has been suggested to be one of the mechanisms of arrhythmogenesis (VF and AF). 82 Direct injection of mesenchymal stem cells into the infarct zone increases sympathetic nerve density throughout the ventricle. 83 Taken together, these results suggest the possibility that cell therapies may induce heterogeneous and unpredictable sympathetic hyper-innervation, which could facilitate cardiac arrhythmia. In addition, the magnitude of SPC-CM responses to adrenergic and cholinergic stimuli has not yet been compared to host myocytes. For instance, L-type Ca2+ channels undergo developmental changes in their degree of responses to beta-adrenergic modulation. 84 If these SPC-CMs respond to neurohormonal stimuli differently from host myocardium, implanted cells might become arrhythmogenic during emotional stress or exercise.
Arrhythmogenic Potential Related to the Injection or Transplant Sites
Injected SPCs usually form islands or clusters of cells with questionable or imperfect coupling to host myocardium. 32, 50 Such islands of implanted cells may generate abnormal automaticity and increase dispersion of conduction and refractoriness. In a dog MI model, SkM transplantation caused slow impulse propagation at sites of injection. 85 In a rabbit MI model, injection of SkM in the infarct border zone led to erratic ventricular ectopy, 86 and injection of SkMs in the scar center elicited negative LV remodeling and late ventricular ectopies. 87 Intramyocardial injection of bone marrow mononuclear cells (BMMNCs) in a rat chronic heart failure model 88 also generated cell clusters in the infarct border zone, which most likely were responsible for the increased ventricular arrhythmias. Myocardial injection per se may also lead to scarring. Compared to myocardial injection, intravenous or intracoronary delivery of SPCs seems to be relatively safe in this regard. However, in a swine acute MI model, intravenous delivery of MSCs resulted in shortened effective refractory periods (ERPs) and increased slope of ERP restitution, which would facilitate ventricular arrhythmias. 89 Moreover, implanted SPCs (especially MSCs) could also generate fibroblasts with unknown effects on arrhythmogenesis. Therefore, although implanted SPCs might provide a range of beneficial effects, we must remain cognizant that they could induce electrical heterogeneity at the implant site and lead to arrhythmias.
Arrhythmic Potentials of immature SPC-CMs in Animal Models and Cultures
In animal models and cultured cells, a number of studies have suggested the pro-arrhythmic potential of cell-based therapy. In cell culture models, cultures of human and murine ESC-CMs exhibited heterogeneous EP phenotypes, immature APDs, immature gap junction distributions and spontaneous automaticity, which predispose these ESC-CMs towards propagation failure and triggered arrhythmias.26, 37, 53, 90–91 Immature intracellular Ca2+ handling machinery of these ESC-CMs further increases the pro-arrhythmic potential of these ESC-CMs. 35, 81 Moreover, co-cultures of ≥ 10% MSCs 27 or human SkMs 38 with neonatal rat VMs (NRVMs) resulted in an arrhythmogenic substrate that was characterized by decreased conduction velocity and easily inducible reentrant arrhythmias.27 These arrhythmias could be decreased by the genetically induced expression of Cx43. 38 Sheets of SkM-derived myotubes also displayed automaticity and caused fibrillation-like cardiomyocyte contractions through stretch-activated channels in a co-culture model. 39
In animal models, intravenous MSC therapy after acute MI in a swine model improved EF, shortened ventricular ERP, induced uncontrolled sympathetic nerve sprouting, and increased the slope of ERP restitution curves, which may increase susceptibility to ventricular arrhythmias. 83,89 In a rat MI model, transplantation of myoblasts, but not BMMNCs, resulted in increased susceptibility of inducible VTs by PES. 92 Injection of SkMs in dog and infusion of SkMs and BM-MSC in rabbit models slowed local ventricular conduction and increased propagation heterogeneity. 85, 93 The relationship of cell retention sites relative to the scars after SkM transplantation further complicates the issue of arrhythmogenic potential of cell therapies (see above).
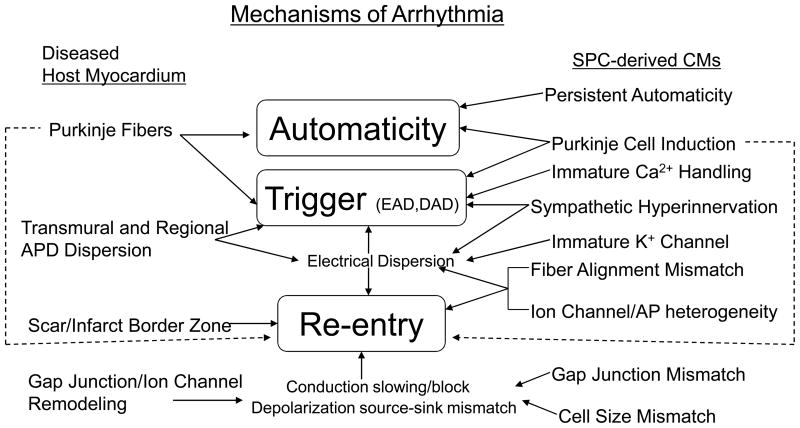
An important but rarely performed investigation after cell transplantation in animal models involves isolating SPC-CMs from host hearts for maturation studies. Halbach and colleagues recently used a mouse heart slice preparation to assess the properties and functional integration of implanted mouse fetal cardiomyocytes (FCMs) after cryoinjury. 50 Implanted FCMs in the center of the injured region showed no electrical integration and retained fetal AP phenotypes. Some so-called integrated FCMs formed gap junction connections with surrounding viable host myocardium, but these connections displayed a punctate, neonatal pattern of Cx43 distribution. With the immature pattern of integration, conduction blocks occurred at FCM-host myocardium junctions due to failed impulse propagation. These integrated FCMs also displayed immature EP properties. Because this pro-arrhythmic potential and immature development occurs for FCMs, similar results would be expected to occur for immature SPC-CMs. Of note, another study using the same murine model with FCM transplants demonstrated improved short-term cardiac function. 94 Therefore, arrhythmogenesis might be independent from cardiac contractile improvement conferred by cell therapy. Despite benign safety profiles of recent clinical trials, results from these animal models and cultures provide support that adverse EP outcomes could be present if substantial numbers of SPC-CMs survive after transplantation (Figure 4).
Figure 4.
Summary of potential arrhythmogenic mechanisms of cell-based therapies.
A Brief Review of Arrhythmic Risks in Clinical Trials
Clinical trials of cell therapy demonstrated mixed results regarding safety and efficacy, which have been reviewed elsewhere. 2–5 Early clinical trials of SkM transplantation demonstrated significant ventricular arrhythmias. 6–8 Recent trials with SkM transplants, however, reported lower incidence of ventricular arrhythmias 95–97 (but also see 98). Mechanisms of cell therapies are complex and the reasons for a decreased incidence of ventricular arrhythmia in recent trials remains to be determined. For instance, prophylactic use of amiodarone may have reduced the incidence of arrhythmias.95 Among various types of SPC sources, infusion of selective types of BMMNCs seems to have provided modest improvement of cardiac function without serious pro-arrhythmic effects.99–101 However, the ability of such stem cells to generate cardiomyocytes is limited and thus the ameliorating effect is likely due to other beneficial mechanisms.2–3 Although other side effects of cell therapy, such as coronary restenosis and calcifications, occurred in a few studies, we will focus only on the discussion of arrhythmogenic potentials.
First, the prevalence of arrhythmic events increases with the severity of heart failure.102 Trials with SkM transplantations were mostly conducted in patients with left ventricular ejection fraction (LVEF) <36% and ventricular arrhythmias occurred more frequently post procedures. Whether these increased arrhythmic events were a reflection of the severity of underlying diseases or the result of intervention remains debatable. 103 Many recent trials with BMMNC, such as ASTAMI, 104 REPAIR-AMI, 105 were conducted on patients with MI and a LVEF more than 40–45% (reviewed in 99–101). This latter group of patients displays low incidence of arrhythmia-related events or death, 102 which may not allow a firm conclusion about arrhythmogenic risks due to lack of statistical power of these studies. Concomitant coronary revascularization, bypass-surgery and medications with anti-arrhythmic effects (such as beta blockers, statins, and ACE inhibitors) would further reduce the incidence of arrhythmias. 99 Consequently, a much larger number of patients with low arrhythmia-related events might be needed in future trials in order to obtain a firm conclusion that the procedure is safe.
Second, most trials applied electrocardiograms, or 24-hour Holter monitoring to determine arrhythmic events at follow-ups. These simple techniques may not be sufficient to detect arrhythmic events based on recent experience in defining the success of atrial fibrillation ablations. 106 Other monitoring techniques such as event monitors, insertable long-term recorders, microvolt T-wave alternans and invasive EP studies with programmed electrical stimulation (PES) protocols 107 should be included, especially for trials with SkM or ESC transplantations. If defibrillators (ICD) or pacemakers were implanted, the specifics of arrhythmia detection algorithms and types of detected arrhythmias should be reported. Inadequate device programming will underestimate arrhythmic events especially with concomitant use of amiodarone. In addition, lack of electrical therapy delivered from an ICD is not the same as lack of increased arrhythmic events. More detailed evaluation of the electrophysiological and arrhythmic consequences of cell-based therapies will improve future trial designs.
Third, human neonatal CMs take 6–10 years to reach adult form in terms of size, shape and gap junction distributions (see above). The developmental status of myocardial protein expression in most SPCs after transplantation in clinical trials is incompletely characterized. In one rare clinical instance, a postmortem study of a patient who received the BMMNC transplant revealed that pericytes just started expressing myocardial proteins at 11 months after cell therapy. 108 While the significance of this result remains to be established, it may suggest that longer follow-up times after cell transplantation are needed because of the extended time required for SPC-CMs to mature completely.
Fourth, most trials with positive outcomes did not provide evidence of true cardiomyogenesis from surviving SPCs. Even if cardiomyocyte differentiation from SPCs did occur, the frequency would expectedly be very low (discussed in 3) with unclear EP maturation of these SPC-CMs. Moreover, positive outcomes reported from cell transplantation trials showing modest improvement in EF without increasing arrhythmic events might simply indicate that the beneficial effects of cell therapies are from mechanisms other than cardiomyogenesis. In fact, small numbers of surviving, immature SPC-CMs might explain the safety records of recent trials. If a sufficient number of injected cells were to survive and differentiate into immature cardiomyocytes (>10%, see above), they might be pro-arrhythmic. Therefore, the arrhythmogenic potential of cell therapies may depend on the balance between cell retention and cardiomyogenesis on one hand versus other beneficial mechanisms of donor cells on the other.
Concluding Remarks and Future Perspective
This article is intended to raise awareness of possible pro-arrhythmic consequences, stimulate further mechanistic research, help in designing safer clinical trials, enhance better clinical monitoring, and improve the efficacy of cell-based therapy with the ultimate goal of avoiding arrhythmogenic side effects. Current concerns reside in a lack of complete understanding of cardiac differentiation, specification, and maturation, especially regarding EP properties of SPC-CMs. Most SPC-CMs possess immature and diverse EP phenotypes with undefined ion channel compositions. Molecular and genetic factors that regulate ion channel development are mostly unknown. Also, it is unlikely that ischemic or failing hearts would provide a full cardiomyogenic environment for maturation of injected SPCs, and this is aggravated by the variability of diseased myocardium across patients after myocardial damage. In this regard, it is of utmost importance to develop an efficient means of ensuring that a physiologically relevant population of VMs will mature normally and integrate properly in patients’ hearts. Since normal maturation takes years to complete, it would also be important to develop methods to accelerate maturation in order to reduce unwanted risks during the period of electrophysiological and structural incompatibility. Furthermore, since paracrine effects are the potential beneficial mechanisms of cell therapy, research to develop therapies with the responsible factors 41, 109 might offer a near-term opportunity to improve cell survival and angiogenesis. Thus, much more carefully designed basic research and clinical trials are needed to provide mechanistic insights into various types of cell therapies before they can be considered safe for widespread application. 110
To reach the aforementioned goals, we recommend a multi-disciplinary approach for future clinical and animal studies, including a cardiac electrophysiologist with expertise in ventricular arrhythmogenesis and a cardiac developmental biologist, to ensure proper trial design, arrhythmia monitoring and interpretation, as well as proper understanding of myocardial cell differentiation, maturation and integration. Additionally, injected SPCs in any trial should be isolated or evaluated for EP characterization several months to years after cell transplantation. For clinical trials, an EP study (EPS) with PES protocols in ventricles, especially for patients with LVEF <40%, 111 should be performed during transplantation and at follow-up to provide more information regarding EP consequences of these experimental cell therapies. Dobutamine or isoproterenol infusion during follow-up stress tests or EPS should also be considered to monitor triggered activities and exercise-induced arrhythmias from newly formed CMs. An event monitor or an ICD/pacemaker with proper arrhythmia detection settings will be very informative about the frequency and occurrence of ventricular arrhythmias. Microvolt T wave alternans test 112 may provide a further assessment of sudden death risk.
Lastly, it is important to highlight several recent scientific breakthroughs for the readers because they have made cell-based myocardial repair close to reality: Combining genetic/prosurvival factors with cell-based therapy 41 may improve the efficacy of cell replacement and avoid arrhythmogenic potentials. Induced pluripotent stem cell technology reported recently 113 opens the door for future patient-specific, cell-based therapies. 114 Cardiac tissue engineering also demonstrates promises for developing myocardium and bioartificial hearts ex vivo for cell replacement therapies. 115
Acknowledgments
Due to quickly expanding literature in the field of progenitor/stem cell-based therapies, we could not cover every paper published and we express our sincere regret if we missed any important paper or research work.
Funding Sources: This work was supported by an Early Career Development Award from American College of Cardiology Foundation, Bechtel Trusts & Foundation Grant, and California Institute of Regenerative Medicine (CIRM) Grants (RS1-00171-1 and RT1-01143) to H-S.V.C., and by NIH grants (R37 HL059502 and R01 HL083463), CIRM (RC1-00132-1), Exelixis, Inc., and the Mathers Charitable Foundation to M. M.
Footnotes
Disclosures: Dr. Chen and Dr. Mercola received research grants from agencies listed in funding sources. Dr. Kim has nothing to disclose.
References
- 1.National Institutes of Health, National Heart, Lung, and Blood Institute. Fact Book Fiscal Year 2005. Bethesda, Md: National Institutes of Health; 2006. [Google Scholar]
- 2.Dimmeler S, Burchfield J, Zeiher AM. Cell-based therapy of myocardial infarction. Arterioscler Thromb Vasc Biol. 2008;28:208–16. doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 3.Murry CE, Reinecke H, Pabon LM. Regeneration gaps: observations on stem cells and cardiac repair. J Am Coll Cardiol. 2006;47:1777–85. doi: 10.1016/j.jacc.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Boyle AJ, Schulman SP, Hare JM, Oettgen P. Is stem cell therapy ready for patients? Stem Cell Therapy for Cardiac Repair. Ready for the Next Step. Circulation. 2006;114:339–52. doi: 10.1161/CIRCULATIONAHA.105.590653. [DOI] [PubMed] [Google Scholar]
- 5.Oettgen P, Boyle AJ, Schulman SP, Hare JM. Cardiac Stem Cell Therapy. Need for Optimization of Efficacy and Safety Monitoring. Circulation. 2006;114:353–8. doi: 10.1161/CIRCULATIONAHA.106.639385. [DOI] [PubMed] [Google Scholar]
- 6.Menasché P, Hagège AA, Vilquin JT, Desnos M, Abergel E, Pouzet B, Bel A, Sarateanu S, Scorsin M, Schwartz K, Bruneval P, Benbunan M, Marolleau JP, Duboc D. Autologous skeletal myoblast transplantation for severe postinfarction left ventricular dysfunction. J Am Coll Cardiol. 2003;41:1078–83. doi: 10.1016/s0735-1097(03)00092-5. [DOI] [PubMed] [Google Scholar]
- 7.Siminiak T, Kalawski R, Fiszer D, Jerzykowska O, RzeŸniczak J, Rozwadowska N, Kurpisz M. Autologous skeletal myoblast transplantation for the treatment of postinfarction myocardial injury: phase I clinical study with 12 months of follow-up. Am Heart J. 2004;148:531–7. doi: 10.1016/j.ahj.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 8.Smits PC, van Geuns RJ, Poldermans D, Bountioukos M, Onderwater EE, Lee CH, Maat AP, Serruys PW. Catheter-based intramyocardial injection of autologous skeletal myoblasts as a primary treatment of ischemic heart failure: clinical experience with six-month follow-up. J Am Coll Cardiol. 2003;42:2063–9. doi: 10.1016/j.jacc.2003.06.017. [DOI] [PubMed] [Google Scholar]
- 9.Olson EN, Schneider MD. Sizing up the heart: development redux in disease. Genes Dev. 2003;17:1937–56. doi: 10.1101/gad.1110103. [DOI] [PubMed] [Google Scholar]
- 10.Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258:1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 11.Eisenberg LM, Markwald RR. Cellular recruitment and the development of the myocardium. Dev Biol. 2004;274:225–32. doi: 10.1016/j.ydbio.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 12.Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–35. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- 13.Foley AC, Gupta RW, Guzzo RM, Korol O, Mercola M. Embryonic heart induction. Ann N Y Acad Sci. 2006;1080:85–96. doi: 10.1196/annals.1380.008. [DOI] [PubMed] [Google Scholar]
- 14.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–53. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zak R. Development and proliferative capacity of cardiac muscle cells. Circ Res. 1974;35:17–26. [PubMed] [Google Scholar]
- 16.Roberts JT, Wearn JT. Quantitative changes in the capillary-muscle relationship in human hearts during normal growth and hypertrophy. AM. Heart J. Cell proliferation during cardiac growth. Am J Cardiol. 1941;21:617–633. [Google Scholar]
- 17.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–70. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 18.Rutenberg JB, Fischer A, Jia H, Gessler M, Zhong TP, Mercola M. Developmental patterning of the cardiac atrioventricular canal by Notch and Hairy-related transcription factors. Development. 2006;133:4381–90. doi: 10.1242/dev.02607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet. 2008;9:49–61. doi: 10.1038/nrg2279. [DOI] [PubMed] [Google Scholar]
- 20.Caspi O, Huber I, Kehat I, Habib M, Arbel G, Gepstein A, Yankelson L, Aronson D, Beyar R, Gepstein L. Transplantation of human embryonic stem cell-derived cardiomyocytes improves myocardial performance in infarcted rat hearts. J Am Coll Cardiol. 2007;50:1884–93. doi: 10.1016/j.jacc.2007.07.054. [DOI] [PubMed] [Google Scholar]
- 21.Vandervelde S, van Luyn MJ, Tio RA, Harmsen MC. Signaling factors in stem cell-mediated repair of infarcted myocardium. J Mol Cell Cardiol. 2005;39:363–76. doi: 10.1016/j.yjmcc.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 22.Saffitz JE, Kanter HL, Green KG, Tolley TK, Beyer EC. Tissue-specific determinants of anisotropic conduction velocity in canine atrial and ventricular myocardium. Circ Res. 1994;74:1065–1070. doi: 10.1161/01.res.74.6.1065. [DOI] [PubMed] [Google Scholar]
- 23.Peters NS, Severs NJ, Rothery SM, Lincoln C, Yacoub MH, Green CR. Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium. Circulation. 1994;90:713–25. doi: 10.1161/01.cir.90.2.713. [DOI] [PubMed] [Google Scholar]
- 24.Peters NS. New insights into myocardial arrhythmogenesis: Distribution of gap junctional coupling in normal, ischemic and hypertrophied human hearts. Clin Sci (Lond) 1996;90:447–52. doi: 10.1042/cs0900447. [DOI] [PubMed] [Google Scholar]
- 25.Peters NS, Wit AL. Gap junction remodeling in infarction: does it play a role in arrhythmogenesis? J Cardiovasc Electrophysiol. 2000;11:488–90. doi: 10.1111/j.1540-8167.2000.tb00348.x. [DOI] [PubMed] [Google Scholar]
- 26.Kehat I, Gepstein A, Spira A, Itskovitz-Eldor J, Gepstein L. High-resolution electrophysiological assessment of human embryonic stem cell-derived cardiomyocytes: a novel in vitro model for the study of conduction. Circ Res. 2002;91:659–61. doi: 10.1161/01.res.0000039084.30342.9b. [DOI] [PubMed] [Google Scholar]
- 27.Chang MG, Tung L, Sekar RB, Chang CY, Cysyk J, Dong P, Marbán E, Abraham MR. Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation. 2006;113:1832–41. doi: 10.1161/CIRCULATIONAHA.105.593038. [DOI] [PubMed] [Google Scholar]
- 28.Nishiyama N, Miyoshi S, Hida N, Uyama T, Okamoto K, Ikegami Y, Miyado K, Segawa K, Terai M, Sakamoto M, Ogawa S, Umezawa A. The significant cardiomyogenic potential of human umbilical cord blood-derived mesenchymal stem cells in vitro. Stem Cells. 2007;25:2017–24. doi: 10.1634/stemcells.2006-0662. [DOI] [PubMed] [Google Scholar]
- 29.Kehat I, Khimovich L, Caspi O, Gepstein A, Shofti R, Arbel G, Huber I, Satin J, Itskovitz-Eldor J, Gepstein L. Electromechanical integration of cardiomyocytes derived from human embryonic stem cells. Nat Biotechnol. 2004;22:1282–9. doi: 10.1038/nbt1014. [DOI] [PubMed] [Google Scholar]
- 30.Teunissen BE, Bierhuizen MF. Transcriptional control of myocardial connexins. Cardiovasc Res. 2004;62:246–55. doi: 10.1016/j.cardiores.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Reinecke H, Poppa V, Murry CE. Skeletal muscle stem cells do not transdifferentiate into cardiomyocytes after cardiac grafting. J Mol Cell Cardiol. 2002;34:241–9. doi: 10.1006/jmcc.2001.1507. [DOI] [PubMed] [Google Scholar]
- 32.Leobon B, Garcin I, Menasche P, Vilquin JT, Audinat E, Charpak S. Myoblasts transplanted into rat infarcted myocardium are functionally isolated from their host. Proc Natl Acad Sci USA. 2003;100:7808–7811. doi: 10.1073/pnas.1232447100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kléber AG, Rudy Y. Basic mechanisms of cardiac impulse propagation and associated arrhythmias. Physiol Rev. 2004;84:431–88. doi: 10.1152/physrev.00025.2003. [DOI] [PubMed] [Google Scholar]
- 34.Spach MS, Heidlage JF, Barr RC, Dolber PC. Cell size and communication: role in structural and electrical development and remodeling of the heart. Heart Rhythm. 2004;1:500–15. doi: 10.1016/j.hrthm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Dolnikov K, Shilkrut M, Zeevi-Levin N, Gerecht-Nir S, Amit M, Danon A, Itskovitz-Eldor J, Binah O. Functional properties of human embryonic stem cell-derived cardiomyocytes: intracellular Ca2+ handling and the role of sarcoplasmic reticulum in the contraction. Stem Cells. 2006;24:236–45. doi: 10.1634/stemcells.2005-0036. [DOI] [PubMed] [Google Scholar]
- 36.Valina C, Pinkernell K, Song YH, Bai X, Sadat S, Campeau RJ, Le Jemtel TH, Alt E. Intracoronary administration of autologous adipose tissue-derived stem cells improves left ventricular function, perfusion, and remodelling after acute myocardial infarction. Eur Heart J. 2007;28:2667–2677. doi: 10.1093/eurheartj/ehm426. [DOI] [PubMed] [Google Scholar]
- 37.Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R, Tertoolen L. Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells. Circulation. 2003;107:2733–40. doi: 10.1161/01.CIR.0000068356.38592.68. [DOI] [PubMed] [Google Scholar]
- 38.Abraham MR, Henrikson CA, Tung L, Chang MG, Aon M, Xue T, Li RA, O’ Rourke B, Marbán E. Antiarrhythmic engineering of skeletal myoblasts for cardiac transplantation. Circ Res. 2005;97:159–67. doi: 10.1161/01.RES.0000174794.22491.a0. [DOI] [PubMed] [Google Scholar]
- 39.Itabashi Y, Miyoshi S, Yuasa S, Fujita J, Shimizu T, Okano T, Fukuda K, Ogawa S. Analysis of the electrophysiological properties and arrhythmias in directly contacted skeletal and cardiac muscle cell sheets. Cardiovasc Res. 2005;67:561–70. doi: 10.1016/j.cardiores.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 40.Taylor DA, Atkins BZ, Hungspreugs P, Jones TR, Reedy MC, Hutcheson KA, Glower DD, Kraus WE. Regenerating functional myocardium: improved performance after skeletal myoblast transplantation. Nat Med. 1998;4:929–33. doi: 10.1038/nm0898-929. [DOI] [PubMed] [Google Scholar]
- 41.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 42.Miyahara Y, Nagaya N, Kataoka M, Yanagawa B, Tanaka K, Hao H, Ishino K, Ishida H, Shimizu T, Kangawa K, Sano S, Okano T, Kitamura S, Mori H. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–65. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 43.Torrent-Guasp F, Kocica MJ, Corno AF, Komeda M, Carreras-Costa F, Flotats A, Cosin-Aguillar J, Wen H. Towards new understanding of the heart structure and function. Eur J Cardiothorac Surg. 2005;27:191–201. doi: 10.1016/j.ejcts.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 44.Hansen DE, Craig CS, Hondeghem LM. Stretch-induced arrhythmias in the isolated canine ventricle. Evidence for the importance of mechanoelectrical feedback. Circulation. 1990;81:1094–105. doi: 10.1161/01.cir.81.3.1094. [DOI] [PubMed] [Google Scholar]
- 45.Lory P, Bidaud I, Chemin J. T-type calcium channels in differentiation and proliferation. Cell Calcium. 2006;40:135–46. doi: 10.1016/j.ceca.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 46.Baba S, Dun W, Cabo C, Boyden PA. Remodeling in cells from different regions of the reentrant circuit during ventricular tachycardia. Circulation. 2005;112:2386–96. doi: 10.1161/CIRCULATIONAHA.105.534784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nattel S, Maguy A, Le Bouter S, Yeh YH. Arrhythmogenic Ion-Channel Remodeling in the Heart: Heart Failure, Myocardial Infarction, and Atrial Fibrillation. Physiol Rev. 2007;87:425–456. doi: 10.1152/physrev.00014.2006. [DOI] [PubMed] [Google Scholar]
- 48.Lakatta EG, Sollott SJ. Perspectives on mammalian cardiovascular aging: humans to molecules. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:699–721. doi: 10.1016/s1095-6433(02)00124-1. [DOI] [PubMed] [Google Scholar]
- 49.Schram G, Pourrier M, Melnyk P, Nattel S. Differential distribution of cardiac ion channel expression as a basis for regional specialization in electrical function. Circ Res. 2002;90:939–5. doi: 10.1161/01.res.0000018627.89528.6f. [DOI] [PubMed] [Google Scholar]
- 50.Halbach M, Pfannkuche K, Pillekamp F, Ziomka A, Hannes T, Reppel M, Hescheler J, Müller-Ehmsen J. Electrophysiological maturation and integration of murine fetal cardiomyocytes after transplantation. Circ Res. 2007;101:484–92. doi: 10.1161/CIRCRESAHA.107.153643. [DOI] [PubMed] [Google Scholar]
- 51.Boheler KR, Czyz J, Tweedie D, Yang HT, Anisimov SV, Wobus AM. Differentiation of pluripotent embryonic stem cells into cardiomyocytes. Circ Res. 2002;91:189–201. doi: 10.1161/01.res.0000027865.61704.32. [DOI] [PubMed] [Google Scholar]
- 52.Sachinidis A, Fleischmann BK, Kolossov E, Wartenberg M, Sauer H, Hescheler J. Cardiac specific differentiation of mouse embryonic stem cells. Cardiovasc Res. 2003;58:278–91. doi: 10.1016/s0008-6363(03)00248-7. [DOI] [PubMed] [Google Scholar]
- 53.He JQ, Ma Y, Lee Y, Thomson JA, Kamp TJ. Human embryonic stem cells develop into multiple types of cardiac myocytes: action potential characterization. Circ Res. 2003;93:32–9. doi: 10.1161/01.RES.0000080317.92718.99. [DOI] [PubMed] [Google Scholar]
- 54.Xu C, Police S, Rao N, Carpenter MK. Characterization and enrichment of cardiomyocytes derived from human embryonic stem cells. Circ Res. 2002;91:501–8. doi: 10.1161/01.res.0000035254.80718.91. [DOI] [PubMed] [Google Scholar]
- 55.Satin J, Kehat I, Caspi O, Huber I, Arbel G, Itzhaki I, Magyar J, Schroder EA, Perlman I, Gepstein L. Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes. J Physiol. 2004;559:479–96. doi: 10.1113/jphysiol.2004.068213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sartiani L, Bettiol E, Stillitano F, Mugelli A, Cerbai E, Jaconi ME. Developmental changes in cardiomyocytes differentiated from human embryonic stem cells: a molecular and electrophysiological approach. Stem Cells. 2007;25:1136–44. doi: 10.1634/stemcells.2006-0466. [DOI] [PubMed] [Google Scholar]
- 57.Takeda Y, Mori T, Imabayashi H, Kiyono T, Gojo S, Miyoshi S, Hida N, Ita M, Segawa K, Ogawa S, Sakamoto M, Nakamura S, Umezawa A. Can the life span of human marrow stromal cells be prolonged by bmi-1, E6, E7, and/or telomerase without affecting cardiomyogenic differentiation? J Gene Med. 2004;6:833–45. doi: 10.1002/jgm.583. [DOI] [PubMed] [Google Scholar]
- 58.Makino S, Fukuda K, Miyoshi S, Konishi F, Kodama H, Pan J, Sano M, Takahashi T, Hori S, Abe H, Hata J, Umezawa A, Ogawa S. Cardiomyocytes can be generated from marrow stromal cells in vitro. J Clin Investig. 1999;103:697–70. doi: 10.1172/JCI5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Badorff C, Brandes RP, Popp R, Rupp S, Urbich C, Aicher A, Fleming I, Busse R, Zeiher AM, Dimmeler S. Transdifferentiation of blood-derived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–32. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 60.Lagostena L, Avitabile D, De Falco E, Orlandi A, Grassi F, Iachininoto MG, Ragone G, Fucile S, Pompilio G, Eusebi F, Pesce M, Capogrossi MC. Electrophysiological properties of mouse bone marrow c-kit+ cells co-cultured onto neonatal cardiac myocytes. Cardiovasc Res. 2005;66:482–92. doi: 10.1016/j.cardiores.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 61.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A. 2007;104:17783–8. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bai X, Ma J, Pan Z, Song YH, Freyberg S, Yan Y, Vykoukal D, Alt E. Electrophysiological properties of human adipose tissue-derived stem cells. Am J Physiol Cell Physiol. 2007;293:C1539–50. doi: 10.1152/ajpcell.00089.2007. [DOI] [PubMed] [Google Scholar]
- 63.Planat-Bénard V, Menard C, André M, Puceat M, Perez A, Garcia-Verdugo JM, Pénicaud L, Casteilla L. Spontaneous cardiomyocyte differentiation from adipose tissue stroma cells. Circ Res. 2004;94:223–9. doi: 10.1161/01.RES.0000109792.43271.47. [DOI] [PubMed] [Google Scholar]
- 64.Yamada Y, Yokoyama S, Wang XD, Fukuda N, Takakura N. Cardiac stem cells in brown adipose tissue express CD133 and induce bone marrow nonhematopoietic cells to differentiate into cardiomyocytes. Stem Cells. 2007;25:1326–1333. doi: 10.1634/stemcells.2006-0588. [DOI] [PubMed] [Google Scholar]
- 65.Guan K, Wagner S, Unsöld B, Maier LS, Kaiser D, Hemmerlein B, Nayernia K, Engel W, Hasenfuss G. Generation of functional cardiomyocytes from adult mouse spermatogonial stem cells. Circ Res. 2007;100:1615–25. doi: 10.1161/01.RES.0000269182.22798.d9. [DOI] [PubMed] [Google Scholar]
- 66.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, Komuro I. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem. 2004;279:11384–91. doi: 10.1074/jbc.M310822200. [DOI] [PubMed] [Google Scholar]
- 67.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marbán E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 68.Goumans MJ, de Boer TP, Smits AM, van Laake LW, van Vliet P, Metz CH, Korfage TH, Kats KP, Hochstenbach R, Pasterkamp G, Verhaar MC, van der Heyden MAG, de Kleijn D, Mummery CL, van Veen TAB, Sluijter JPG, Doevendans PA. TGF-β1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res. 2008;1:138–49. doi: 10.1016/j.scr.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 69.Winitsky SO, Gopal TV, Hassanzadeh S, Takahashi H, Gryder D, Rogawski MA, Takeda K, Yu ZX, Xu YH, Epstein ND. Adult murine skeletal muscle contains cells that can differentiate into beating cardiomyocytes in vitro. PLoS Biol. 2005;3:e87. doi: 10.1371/journal.pbio.0030087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okamoto K, Miyoshi S, Toyoda M, Hida N, Ikegami Y, Makino H, Nishiyama N, Tsuji H, Cui CH, Segawa K, Uyama T, Kami D, Miyado K, Asada H, Matsumoto K, Saito H, Yoshimura Y, Ogawa S, Aeba R, Yozu R, Umezawa A. ‘Working’ cardiomyocytes exhibiting plateau action potentials from human placenta-derived extraembryonic mesodermal cells. Exp Cell Res. 2007;313:2550–62. doi: 10.1016/j.yexcr.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 71.Bode F, Karasik P, Katus HA, Franz MR. Upstream stimulation versus downstream stimulation: arrhythmogenesis based on repolarization dispersion in the human heart. J Am Coll Cardiol. 2002;40:731–6. doi: 10.1016/s0735-1097(02)02008-9. [DOI] [PubMed] [Google Scholar]
- 72.Antzelevitch C. Modulation of transmural repolarization. Ann NY Acad Sci. 2005;1047:314–323. doi: 10.1196/annals.1341.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Poelzing S, Rosenbaum DS. Altered connexin43 expression produces arrhythmia substrate in heart failure. Am J Physiol Heart Circ Physiol. 2004;287:H1762–70. doi: 10.1152/ajpheart.00346.2004. [DOI] [PubMed] [Google Scholar]
- 74.Davies MP, An RH, Doevendans P, Kubalak S, Chien KR, Kass RS. Developmental changes in ionic channel activity in the embryonic murine heart. Circ Res. 1996;78:15–25. doi: 10.1161/01.res.78.1.15. [DOI] [PubMed] [Google Scholar]
- 75.Grandy SA, Trépanier-Boulay V, Fiset C. Postnatal development has a marked effect on ventricular repolarization in mice. Am J Physiol Heart Circ Physiol. 2007;293:H2168–77. doi: 10.1152/ajpheart.00521.2007. [DOI] [PubMed] [Google Scholar]
- 76.Bogun F, Good E, Reich S, Elmouchi D, Igic P, Tschopp D, Dey S, Wimmer A, Jongnarangsin K, Oral H, Chugh A, Pelosi F, Morady F. Role of Purkinje fibers in post-infarction ventricular tachycardia. J Am Coll Cardiol. 2006;48:2500–7. doi: 10.1016/j.jacc.2006.07.062. [DOI] [PubMed] [Google Scholar]
- 77.Weiss JN, Karma A, Shiferaw Y, Chen PS, Garfinkel A, Qu Z. From pulsus to pulseless: the saga of cardiac alternans. Circ Res. 2006;98:1244–53. doi: 10.1161/01.RES.0000224540.97431.f0. [DOI] [PubMed] [Google Scholar]
- 78.Liu W, Yasui K, Opthof T, Ishiki R, Lee JK, Kamiya K, Yokota M, Kodama I. Developmental changes of Ca(2+) handling in mouse ventricular cells from early embryo to adulthood. Life Sci. 2002;71:1279–92. doi: 10.1016/s0024-3205(02)01826-x. [DOI] [PubMed] [Google Scholar]
- 79.Tohse N, Seki S, Kobayashi T, Tsutsuura M, Nagashima M, Yamada Y. Development of excitation-contraction coupling in cardiomyocytes. Jpn J Physiol. 2004;54:1–6. doi: 10.2170/jjphysiol.54.1. [DOI] [PubMed] [Google Scholar]
- 80.Liu J, Fu JD, Siu CW, Li RA. Functional Sarcoplasmic Reticulum for Calcium Handling of Human Embryonic Stem Cell-Derived Cardiomyocytes: Insights for Driven Maturation. Stem Cells. 2007;25:3038–3044. doi: 10.1634/stemcells.2007-0549. [DOI] [PubMed] [Google Scholar]
- 81.Priori SG, Napolitano C. Intracellular calcium handling dysfunction and arrhythmogenesis: a new challenge for the electrophysiologist. Circ Res. 2005;97:1077–9. doi: 10.1161/01.RES.0000194556.41865.e2. [DOI] [PubMed] [Google Scholar]
- 82.Chen LS, Zhou S, Fishbein MC, Chen PS. New Perspectives on the Role of Autonomic Nervous System in the Genesis of Arrhythmias. J Cardiovasc Electrophysiol. 2007;18:123–7. doi: 10.1111/j.1540-8167.2006.00590.x. [DOI] [PubMed] [Google Scholar]
- 83.Pak HN, Qayyum M, Kim DT, Hamabe A, Miyauchi Y, Lill MC, Frantzen M, Takizawa K, Chen LS, Fishbein MC, Sharifi BG, Chen PS, Makkar R. Mesenchymal stem cell injection induces cardiac nerve sprouting and increased tenascin expression in a Swine model of myocardial infarction. J Cardiovasc Electrophysiol. 2003;14:841–8. doi: 10.1046/j.1540-8167.2003.03124.x. [DOI] [PubMed] [Google Scholar]
- 84.Liu W, Yasui K, Arai A, Kamiya K, Cheng J, Kodama I, Toyama J. beta-adrenergic modulation of L-type Ca2+-channel currents in early-stage embryonic mouse heart. Am J Physiol. 1999;276:H608–13. doi: 10.1152/ajpheart.1999.276.2.H608. [DOI] [PubMed] [Google Scholar]
- 85.Fouts K, Fernandes B, Mal N, Liu J, Laurita KR. Electrophysiological consequence of skeletal myoblast transplantation in normal and infarcted canine myocardium. Heart Rhythm. 2006;3:452–61. doi: 10.1016/j.hrthm.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 86.Soliman A, Krucoff M, Crater S, Morimoto Y, Taylor D. Cell location may be a primary determinant of safety after myoblast transplantation into the infarcted heart (abstr) J Am Coll Cardiol. 2004;43:15A. [Google Scholar]
- 87.McCue JD, Swingen C, Feldberg T, Caron G, Kolb A, Denucci C, Prabhu S, Motilall R, Breviu B, Taylor DA. The real estate of myoblast cardiac transplantation: negative remodeling is associated with location. J Heart Lung Transplant. 2008;27:116–23. doi: 10.1016/j.healun.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fukushima S, Varela-Carver A, Coppen SR, Yamahara K, Felkin LE, Lee J, Barton PJ, Terracciano CM, Yacoub MH, Suzuki K. Direct intramyocardial but not intracoronary injection of bone marrow cells induces ventricular arrhythmias in a rat chronic ischemic heart failure model. Circulation. 2007;115:2254–61. doi: 10.1161/CIRCULATIONAHA.106.662577. [DOI] [PubMed] [Google Scholar]
- 89.Price MJ, Chou CC, Frantzen M, Miyamoto T, Kar S, Lee S, Shah PK, Martin BJ, Lill M, Forrester JS, Chen PS, Makkar RR. Intravenous mesenchymal stem cell therapy early after reperfused acute myocardial infarction improves left ventricular function and alters electrophysiologic properties. Int J Cardiol. 2006;111:231–9. doi: 10.1016/j.ijcard.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 90.Zhang YM, Hartzell C, Narlow M, Dudley SC., Jr Stem cell-derived cardiomyocytes demonstrate arrhythmic potential. Circulation. 2002;106:1294–9. doi: 10.1161/01.cir.0000027585.05868.67. [DOI] [PubMed] [Google Scholar]
- 91.Igelmund P, Fleischmann BK, Fischer IR, Soest J, Gryshchenko O, Böhm-Pinger MM, Sauer H, Liu Q, Hescheler J. Action potential propagation failures in long-term recordings from embryonic stem cell-derived cardiomyocytes in tissue culture. Pflugers Arch. 1999;437:669–79. doi: 10.1007/s004240050831. [DOI] [PubMed] [Google Scholar]
- 92.Fernandes S, Amirault JC, Lande G, Nguyen JM, Forest V, Bignolais O, Lamirault G, Heudes D, Orsonneau JL, Heymann MF, Charpentier F, Lemarchand P. Autologous myoblast transplantation after myocardial infarction increases the inducibility of ventricular arrhythmias. Cardiovasc Res. 2006;69:348–58. doi: 10.1016/j.cardiores.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 93.Chen M, Fan ZC, Liu XJ, Deng JL, Zhang L, Rao L, Yang Q, Huang DJ. Effects of autologous stem cell transplantation on ventricular electrophysiology in doxorubicin-induced heart failure. Cell Biol Int. 2006;30:576–82. doi: 10.1016/j.cellbi.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 94.Roell W, Lu ZJ, Bloch W, Siedner S, Tiemann K, Xia Y, Stoecker E, Fleischmann M, Bohlen H, Stehle R, Kolossov E, Brem G, Addicks K, Pfitzer G, Welz A, Hescheler J, Fleischmann BK. Cellular cardiomyoplasty improves survival after myocardial injury. Circulation. 2002;105:2435–2441. doi: 10.1161/01.cir.0000016063.66513.bb. [DOI] [PubMed] [Google Scholar]
- 95.Siminiak T, Fiszer D, Jerzykowska O, Grygielska B, Rozwadowska N, Kałmucki P, Kurpisz M. Percutaneous trans-coronary-venous transplantation of autologous skeletal myoblasts in the treatment of post-infarction myocardial contractility impairment: the POZNAN trial. Eur Heart J. 2005;26:1188–95. doi: 10.1093/eurheartj/ehi159. [DOI] [PubMed] [Google Scholar]
- 96.Dib N, Michler RE, Pagani FD, Wright S, Kereiakes DJ, Lengerich R, Binkley P, Buchele D, Anand I, Swingen C, Di Carli MF, Thomas JD, Jaber WA, Opie SR, Campbell A, McCarthy P, Yeager M, Dilsizian V, Griffith BP, Korn R, Kreuger SK, Ghazoul M, MacLellan WR, Fonarow G, Eisen HJ, Dinsmore J, Diethrich E. Safety and feasibility of autologous myoblast transplantation in patients with ischemic cardiomyopathy: four-year follow-up. Circulation. 2005;112:1748–55. doi: 10.1161/CIRCULATIONAHA.105.547810. [DOI] [PubMed] [Google Scholar]
- 97.Cleland JG, Coletta AP, Abdellah AT, Cullington D, Clark AL, Rigby AS. Clinical trials update from the American Heart Association 2007: CORONA, RethinQ, MASCOT, AF-CHF, HART, MASTER, POISE and stem cell therapy. Eur J Heart Fail. 2008;10:102–8. doi: 10.1016/j.ejheart.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 98.Menasché P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, Vilquin JT, Marolleau JP, Seymour B, Larghero J, Lake S, Chatellier G, Solomon S, Desnos M, Hagège AA. The Myoblast Autologous Grafting in Ischemic Cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- 99.Reffelmann T, Könemann S, Kloner RA. Promise of blood- and bone marrow-derived stem cell transplantation for functional cardiac repair: putting it in perspective with existing therapy. J Am Coll Cardiol. 2009;53:305–8. doi: 10.1016/j.jacc.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 100.Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA, Zuba-Surma EK, Al-Mallah M, Dawn B. Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis. Arch Intern Med. 2007;167:989–97. doi: 10.1001/archinte.167.10.989. [DOI] [PubMed] [Google Scholar]
- 101.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: a collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–7. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 102.Cleland JG, Chattopadhyay S, Khand A, Houghton T, Kaye GC. Prevalence and incidence of arrhythmias and sudden death in heart failure. Heart Fail Rev. 2002;7:229–42. doi: 10.1023/a:1020024122726. [DOI] [PubMed] [Google Scholar]
- 103.Peters NS. Arrhythmias after cell transplantation for myocardial regeneration: natural history or result of the intervention? J Cardiovasc Electrophysiol. 2005;16:1255–7. doi: 10.1111/j.1540-8167.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- 104.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grøgaard HK, Bjørnerheim R, Brekke M, Müller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 105.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Süselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM REPAIR-AMI Investigators. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–21. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 106.Piorkowski C, Kottkamp H, Tanner H, Kobza R, Nielsen JC, Arya A, Hindricks G. Value of different follow-up strategies to assess the efficacy of circumferential pulmonary vein ablation for the curative treatment of atrial fibrillation. J Cardiovasc Electrophysiol. 2005;16:1286–92. doi: 10.1111/j.1540-8167.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 107.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–8. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 108.Dohmann HF, Perin EC, Takiya CM, Silva GV, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Pascarelli BM, Assis IM, Dutra HS, Assad JA, Castello-Branco RV, Drummond C, Dohmann HJ, Willerson JT, Borojevic R. Transendocardial autologous bone marrow mononuclear cell injection in ischemic heart failure: postmortem anatomicopathologic and immunohistochemical findings. Circulation. 2005;112:521–6. doi: 10.1161/CIRCULATIONAHA.104.499178. [DOI] [PubMed] [Google Scholar]
- 109.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–19. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kovacic JC, Harvey RP, Dimmeler S. Cardiovascular Regenerative Medicine: Digging in for the Long Haul. Cell stem cell. 2007;1:628–633. doi: 10.1016/j.stem.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 111.Prystowsky EN, Nisam S. Prophylactic implantable cardioverter defibrillator trials: MUSTT, MADIT, and beyond. Multicenter Unsustained Tachycardia Trial. Multicenter Automatic Defibrillator Implantation Trial. Am J Cardiol. 2000;86:1214–5. doi: 10.1016/s0002-9149(00)01205-4. [DOI] [PubMed] [Google Scholar]
- 112.Chow T, Kereiakes DJ, Bartone C, Booth T, Schloss EJ, Waller T, Chung E, Menon S, Nallamothu BK, Chan PS. Microvolt T-wave alternans identifies patients with ischemic cardiomyopathy who benefit from implantable cardioverter-defibrillator therapy. J Am Coll Cardiol. 2007;49:50–8. doi: 10.1016/j.jacc.2006.06.079. [DOI] [PubMed] [Google Scholar]
- 113.Okita K, Ichisaka T, Yamanaka S. Generation of germline-competent induced pluripotent stem cells. Nature. 2007;448:313–7. doi: 10.1038/nature05934. [DOI] [PubMed] [Google Scholar]
- 114.Tulloch NL, Pabon L, Murry CE. Get with the (re)program: cardiovascular potential of skin-derived induced pluripotent stem cells. Circulation. 2008;118:472–5. doi: 10.1161/CIRCULATIONAHA.108.791442. [DOI] [PubMed] [Google Scholar]
- 115.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, Taylor DA. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]