Abstract
Increased function of neuronal L-type voltage-sensitive Ca2+ channels (L-VSCCs) is strongly linked to impaired memory and altered hippocampal synaptic plasticity in aged rats. However, no studies have directly assessed L-VSCC function in any of the common mouse models of Alzheimer’s disease where neurologic deficits are typically more robust. Here, we used cell-attached patch-clamp recording techniques to measure L-VSCC activity in CA1 pyramidal neurons of partially dissociated hippocampal “zipper” slices prepared from 14-month-old wild-type mice and memory-impaired APP/PS1 double knock-in mice. Surprisingly, the functional channel density of L-VSCCs was significantly reduced in the APP/PS1 group. No differences in voltage dependency and unitary conductance of L-VSCCs were observed. The results suggest that mechanisms for Ca2+ dysregulation can differ substantially between animal models of normal aging and models of pathological aging.
Keywords: Calcium dyshomeostasis, Alzheimer’s disease, hippocampus, cognitive impairment
1. Introduction
Central to the Ca2+ hypothesis of brain aging is the concept of “Ca2+-as-mediator” of neurologic dysfunction associated with age and age-related diseases, such as Alzheimer’s disease (AD) [1–7]. Extensive support for the Ca2+ hypothesis comes from studies on both normal and pathologic aging using a variety of animal models and diverse experimental approaches. However, whether some Ca2+ signaling mechanisms contribute primarily to normal aging deficits or are more relevant for disease symptoms remains largely unknown. Elevated activity of L-type voltage sensitive Ca2+ channels (L-VSCCs) in hippocampal neurons [8] has provided one of the most robust links to altered membrane excitability, impaired synaptic function, and cognitive decline in animal models of normal aging (for review see [6]). In contrast, no studies have measured neuronal L-VSCC currents in any of the common mouse models of AD. Here, we used the partially-dissociated hippocampal “zipper” slice preparation and cell-attached patch-clamp to assess L-VSCC properties in CA1 pyramidal neurons of mid-age (14–15 mos) wild-type mice and memory-deficient AD (i.e. APP/PS1) mice. The results revealed a marked reduction in L-VSCC activity in the APP/PS1 group, suggesting that Ca2+ dysregulation may be independent of L-VSCC function in some transgenic mouse models of AD.
2. Materials and methods
2.1. Transgenic mice
Homozygous male APPNLh/NLh X PS-1P264L/P264L mice, maintained on a CD-1/129 background, were used at 14-months-of-age. These mice deposit humanized Aβ by 6 months, and exhibit extensive plaque pathology by 14 mos [9]. Wild-type mice were initially obtained from matings between APP/PS1 heterozygotes, and then maintained as a separate line. Mice were maintained on a 12h:12h light-dark cycle and had ad libitum access to rodent chow. All procedures were compliant with the guidelines of the University of Kentucky institutional animal use committee and the American Association for Accreditation of Laboratory Animal Care.
2.2 One-way active avoidance
On each trial, mice were placed in the dark compartment of a standard two compartment active avoidance chamber. After a seven second grace period, a 25 s 0.8 mA shock was delivered through the floor of the dark compartment and escape time to the light compartment was recorded. Mice were placed in a temporary holding cage for 60 s between trials. An escape time ≤ 7 s was considered to be an “avoidance”. The percent of successful avoidances for each mouse was averaged across four trials on each day (four days total), normalized to day 1 performance levels for each mouse, and compared across training days and genotype using repeated measures ANOVA (p < 0.05).
2.3. Cell-attached patch clamp recording in hippocampal zipper slices
Preparation of partially dissociated “zipper” slices from mice was carried out as described by earlier studies on adult guinea pigs [10] and aged rats [8, 11, 12]. Mice were decapitated after CO2 asphyxiation, and brains placed briefly in ice-cold oxygenated (95%O2/5%CO2) artificial cerebral spinal fluid (ACSF) containing (in mM): 114 NaCl, 2.5 KCl, 2 MgCl2, 30 NaHCO3, 10 Glucose, and 0.1 CaCl2 (pH 7.4). Coronal slices (300 μm) were made in ice-cold ACSF using a Vibratome® as described [13] and transferred to prewarmed (32° C), oxygenated ACSF containing 2 mM CaCl2 and 0.7 mg/ml pronase for 30 minutes. Slices were washed twice in ACSF followed by an additional 15 min in ACSF containing 0.5 mg/ml thermolysine. After two more washes in ACSF, slices were bathed for 1–4 h in enzyme-free, ACSF with 2mM CaCl2. To expose CA1 pyramidal neurons for patch clamp recordings, slices were nicked at CA1 stratum pyramidale near the border of the subiculum using a scalpel blade, then transferred to a 2 mL analyzing cup containing ~1 mL of Ca2+-free ACSF and gently shaken by hand until clear dissociation was observed along CA1 stratum pyramidale.
Glass recording pipettes (5.6 ± 0.14 MΩ) were coated with Sylgard® and fire-polished immediately before use. External recording solution contained (in mM): 140 K+-gluconate, 3 MgCl2, 10 glucose, 10 EGTA, and 10 HEPES, pH = 7.35, osmolarity = 300 mOsm. Pipette solution contained (in mM): 20 BaCl2, 90 choline Cl, 10 TEA Cl, and 10 HEPES, 0.0005 Bay K 8644 (L-VSCC activator), pH = 7.35, osmolarity = 290 mOsm. Junction potentials were nulled in the bath and tip resistances determined prior to obtaining a giga-ohm seal from cleanly exposed CA1 neurons. I/V curves were constructed by successively stepping (150 ms) the membrane voltage (Vm) in 10 mV increments from holding (−70 mV) to +40 mV. Current amplitudes (i) of clearly resolvable L-VSCC openings were also measured at different Vm levels (i.e. −70 to + 10 mV; one sec duration steps) to assess slope conductance (in pS) of L-VSCCs. The Vm was then stepped from −70 to the maximal activating voltage (0 or +10mV) a total of 90 times (10 s interstep interval) to find the maximal instantaneous current (Imax, see below) and to generate an average ensemble current for each patch. Currents were leak-subtracted off-line using hyperpolarizing pulses, identical in duration and opposite in polarity. Current density (pA/μm2) was derived by dividing the average ensemble current by the patch area (a=12.6(1/R+0.018), where ‘a’ is the patch area and ‘R’ is the pipette resistance [14]. The method of maximum instantaneous openings was used to estimate the total number of channels in a patch (N) by dividing Imax by the single channel average amplitude (i.e. N = Imax/i) as described [8, 15]. This value was then divided by patch area to estimate channel density (N/μm2). All recordings were obtained at room temperature, filtered at 2kHz, and digitized at 5 kHz using an Axoclamp 1D patch-clamp amplifier and Clampex software. All VSCC properties were measured offline using Clampfit software and compared between genotypes using unpaired, two-tailed Student’s T tests (p < 0.05).
3. Results
3.1 2xTg mice show impairments on a one-way active avoidance task
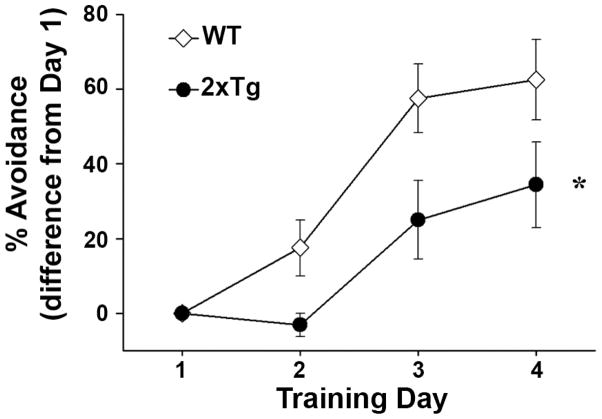
At 14 mos, 10 male WT and 7 male 2xTg mice were trained across four days on a standard one-way active avoidance task (4 trials/day, see Methods). Optimal performance on this task requires a functionally intact hippocampus [16]. As shown in Figure 1, the percent of trials in which mice exhibited a successful avoidance was calculated on each day and normalized to day 1 performance. WT and 2xTg animals showed nearly identical performance levels on day 1. While both groups exhibited a higher percentage of avoidances with each successive training day (p < 0.001), 2xTg mice were generally outperformed by their WT counterparts (p < 0.05). Thus, like many other AD transgenic models, the APP/PS1 double knock-in mice used here exhibit a measurable cognitive deficit.
Figure 1.
2xTg mice show deficits on a standard one-way active avoidance task. Line graph shows mean ± SEM percent avoidances on each training day (normalized to Day 1 performance) for WT and 2xTg mice. *indicates significantly poorer performance in 2xTg mice across all training days (p < 0.05, repeated measures ANOVA).
3.2 Neuronal L-VSCC currents are reduced in 2xTg mice
Hippocampal zipper slices were prepared from 13 of the behaviorally characterized mice shown in Figure 1 (WT, n =7; Tg, n =6). On-cell patch clamp analysis of L-VSCC currents from CA1 pyramidal neurons were made as described previously by our labs [8, 11, 12]. Thirty-five patches were recorded across both groups (WT, n =19; 2xTg, n =16). No differences in pipette tip resistance, seal resistance, or the maximal activating voltage (i.e. the membrane potential associated with maximal L-VSCC activity) were observed (Table 1). For all L-VSCC properties measured, values were averaged across patches within each mouse and used for statistical comparisons (i.e. n = # of mice/group). Representative L-VSCC ensemble currents during step depolarizations from −70 to +10 mV in patches taken from a WT and 2xTg mouse are shown in Figure 2A, and the average ± SEM ensemble peak current density (pA/μm2) is shown in Figure 2B. The results revealed a significant reduction in maximal L-VSCC current density in the 2xTg group (p < 0.05). No change in L-VSCC voltage dependency was observed. Analysis of single channel properties indicated similar unitary L-VSCC current (i) amplitudes across Vm levels for both groups (Figure 2C) and no differences in slope conductance were found. Using the method of maximal instantaneous openings to estimate the total number of channels (N) per patch [8, 15], we observed a greater than two-fold reduction in L-VSCC channel density (N/μm2) for the 2xTg group. These results suggest that lower L-VSCC activity in 2xTg mice is largely due to a reduction in the number of functional channels in the plasma membrane.
Table 1.
| Number of patches | Number of mice | Tip Resistance (MΩ) | Seal Resistance (MΩ) | Maximal Activation Voltage (mV) | |
|---|---|---|---|---|---|
| WT | 19 | 7 | 5.71 ± 0.23 | 22 ± 2.57 | 6.67 ± 1.98 |
| 2×Tg | 16 | 6 | 5.58 ± 0.16 | 28.67 ± 4.84 | 6.88 ± 1.5 |
Tip and cell parameter values expressed as mean ± SEM. No group differences were observed.
Figure 2.
CA1 neuronal L-VSCC activity is reduced in 2xTg mice. A, Representative L-VSCC current traces (top 3 waveforms), and the average ensemble current (lowest trace) generated from 90-step WWwas elicited by stepping the patch membrane from −70 to +10 mV for 150 ms. Scale bars: 20pA/50ms. B, Mean ± SEM peak ensemble current density (pA/μm2) in each genotype group. C, Mean ± SEM unitary L-VSCC current amplitudes (i in pA) measured at different membrane potentials (Vm in mV). No genotype differences in slope conductance were observed. D, Mean ± SEM functional channel density in cell-attached patches from each genotype. *significant reduction relative to WT mice (p < 0.05, two-tailed unpaired T tests).
4. Discussion
L-VSCC activity is potentiated during aging[8, 11] and plays a significant role in age-related alterations in synaptic function [17, 18], membrane excitability[19], and cognition [20, 21]. There are several lines of evidence to suggest that L-VSCCs may also contribute to the pathophysiology of AD. Postmortem AD brain is associated with increased radiolabel binding to L-VSCCs [22], and L-VSCC activity in cell cultures is elevated following delivery of pathogenic Aβ peptides [23–30]. However, the present study is the first to directly assess neuronal L-VSCC activity in a mouse model of AD. The mid-aged double knock-in Tg mice used here exhibit high brain tissue levels of both soluble and insoluble Aβ [9], as well as significant cognitive impairment (Figure 1). It was therefore somewhat surprising to observe a reduction, rather than an increase, in neuronal L-VSCC currents in Tg mice (Figure 2).
The mechanistic basis for reduced L-VSCC activity is not presently known, but it seems unlikely that 2xTg mice simply lack sensitivity to age-dependent mechanisms implicated in gain-of-function. The cyclic AMP dependent protein kinase (PKA) and the protein phosphatase calcineurin have each been proposed to augment L-VSCC activity during aging [11, 31, 32]. While evidence for impaired activation of PKA and/or PKA dependent processes has been reported in AD mice and/or cell cultures treated with Aβ [33, 34], calcineurin activity and signaling are upregulated in the same or similar models [35–38]. Moreover, previous work by Stutzmann et al on single and triple Tg models of AD revealed an age-dependent increase in the post-burst afterhyperpolarization, a Ca2+ dependent event requiring L-VSCC activation [39].
The findings of the present study may be more closely associated with functional changes associated with the PS1 mutation. PS1 is a resident protein of the endoplasmic reticulum (ER) and is functionally coupled to key ER Ca2+ release channels (e.g. IP3 receptors and ryanodine receptors) that are also juxtaposed to L-VSCCs. Indeed, it is well established that L-VSCCs operate in series with ER channels to mediate Ca2+-induced Ca2+ release (CICR) in neurons [40]. During aging, in which Ca2+ dysregulation is relatively mild, elevations in L-VSCC activity are clearly able to co-exist with elevations in CICR [41–43]. However, abnormal gating properties and expression levels of ER Ca2+ channels associated with PS1 mutations [44] may drive Ca2+ levels high enough to ultimately deliver negative feedback onto L-VSCCs, as shown previously for NMDA receptors [45]. Clearly, it will be important to conduct extensive investigations on singly transgenic mice (mice that express the human APP mutation, but lack mutant PS1, and vice versa) at multiple age points to determine the relative contributions of PS1 and aging to L-VSCC regulation. Further work on single knock-in mice may also offer increased relevancy to AD, since humans rarely if ever co-express APP and PS1 mutations.
In summary, the present study revealed a significant reduction in neuronal L-VSCC activity in a double knock-in mouse model of AD. These results were unexpected based on extensive evidence of enhanced L-VSCC activity in animal models of aging and cell culture models of amyloid toxicity. Our findings, together with previous work, suggest that the directional regulation of L-VSCC activity during AD depends on a complex interaction between aging, circulating Aβ levels, the presence of PS1 variants, and the degree of existing Ca2+ dysregulation, among other factors. Clearly, additional work on other Tg mouse models as well as human AD brain tissue will be necessary to fully determine the role of L-VSCCs in the pathophysiology of AD.
Highlights.
Increased activity of L-type Ca2+ channels is linked to cognitive decline with aging.
L channel activity has not been assessed in mouse models of Alzheimer’s disease (AD)
L channel activity was investigated in hippocampal “zipper” slices from APP/PS1 mice
Surprisingly, APP/PS1 mice showed reduced L channel activity compared to wild types
Ca2+ dysregulation may be independent of L channels in some mouse models of AD
Acknowledgments
Work supported by NIH grants AG033649 to O.T.; AG004542 and AG010836 to P.W.L.; and AG027297 to C.M.N. The authors wish to thank Dr. Eric Blalock for important technical assistance.
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Disterhoft JF, Moyer JR, Jr, Thompson LT. The calcium rationale in aging and Alzheimer's disease. Evidence from an animal model of normal aging. Ann N Y Acad Sci. 1994;747:382–406. doi: 10.1111/j.1749-6632.1994.tb44424.x. [DOI] [PubMed] [Google Scholar]
- 2.Foster TC, Norris CM. Age-associated changes in Ca(2+)-dependent processes: relation to hippocampal synaptic plasticity. Hippocampus. 1997;7:602–612. doi: 10.1002/(SICI)1098-1063(1997)7:6<602::AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 3.Khachaturian ZS. The role of calcium regulation in brain aging: reexamination of a hypothesis [published erratum appears in Aging (Milano) 1989;1(2):II] Aging (Milano) 1989;1:17–34. doi: 10.1007/BF03323872. [DOI] [PubMed] [Google Scholar]
- 4.Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226:1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- 5.Stutzmann GE. The pathogenesis of Alzheimers disease is it a lifelong "calciumopathy"? Neuroscientist. 2007;13:546–559. doi: 10.1177/1073858407299730. [DOI] [PubMed] [Google Scholar]
- 6.Thibault O, Gant JC, Landfield PW. Expansion of the calcium hypothesis of brain aging and Alzheimer's disease: minding the store. Aging Cell. 2007;6:307–317. doi: 10.1111/j.1474-9726.2007.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bezprozvanny I, Mattson MP. Neuronal calcium mishandling and the pathogenesis of Alzheimer's disease. Trends Neurosci. 2008;31:454–463. doi: 10.1016/j.tins.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thibault O, Landfield PW. Increase in single L-type calcium channels in hippocampal neurons during aging. Science. 1996;272:1017–1020. doi: 10.1126/science.272.5264.1017. [DOI] [PubMed] [Google Scholar]
- 9.Anantharaman M, Tangpong J, Keller JN, Murphy MP, Markesbery WR, Kiningham KK, St Clair DK. Beta-amyloid mediated nitration of manganese superoxide dismutase: implication for oxidative stress in a APPNLH/NLH X PS-1P264L/P264L double knock-in mouse model of Alzheimer's disease. Am J Pathol. 2006;168:1608–1618. doi: 10.2353/ajpath.2006.051223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray R, Fisher R, Spruston N, Johnston D. In Vitro. Wiley; New York: 1990. Preparations of the vertebrate central nervous system. [Google Scholar]
- 11.Norris CM, Blalock EM, Chen KC, Porter NM, Thibault O, Kraner SD, Landfield PW. Hippocampal 'zipper' slice studies reveal a necessary role for calcineurin in the increased activity of L-type Ca(2+) channels with aging. Neurobiol Aging. 2010;31:328–338. doi: 10.1016/j.neurobiolaging.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brewer LD, Dowling AL, Curran-Rauhut MA, Landfield PW, Porter NM, Blalock EM. Estradiol reverses a calcium-related biomarker of brain aging in female rats. J Neurosci. 2009;29:6058–6067. doi: 10.1523/JNEUROSCI.5253-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mathis DM, Furman JL, Norris CM. Preparation of Acute Hippocampal Slices from Rats and Transgenic Mice for the Study of Synaptic Alterations during Aging and Amyloid Pathology. J Vis Exp. 2011 doi: 10.3791/2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakmann B, Neher E. Patch clamp techniques for studying ionic channels in excitable membranes. Annu Rev Physiol. 1984;46:455–472. doi: 10.1146/annurev.ph.46.030184.002323. [DOI] [PubMed] [Google Scholar]
- 15.Porter NM, Thibault O, Thibault V, Chen KC, Landfield PW. Calcium channel density and hippocampal cell death with age in long- term culture. J Neurosci. 1997;17:5629–5639. doi: 10.1523/JNEUROSCI.17-14-05629.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olton DS, Isaacson RL. Hippocampal lesions and active avoidance. Physiol Behav. 1968;3:719–724. [Google Scholar]
- 17.Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J Neurosci. 1998;18:3171–3179. doi: 10.1523/JNEUROSCI.18-09-03171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thibault O, Hadley R, Landfield PW. Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: relationship to impaired synaptic plasticity. J Neurosci. 2001;21:9744–9756. doi: 10.1523/JNEUROSCI.21-24-09744.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moyer JR, Jr, Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. J Neurophysiol. 1992;68:2100–2109. doi: 10.1152/jn.1992.68.6.2100. [DOI] [PubMed] [Google Scholar]
- 20.Deyo RA, Straube KT, Disterhoft JF. Nimodipine facilitates associative learning in aging rabbits. Science. 1989;243:809–811. doi: 10.1126/science.2916127. [DOI] [PubMed] [Google Scholar]
- 21.Veng LM, Mesches MH, Browning MD. Age-related working memory impairment is correlated with increases in the L-type calcium channel protein alpha1D (Cav1.3) in area CA1 of the hippocampus and both are ameliorated by chronic nimodipine treatment. Brain Res Mol Brain Res. 2003;110:193–202. doi: 10.1016/s0169-328x(02)00643-5. [DOI] [PubMed] [Google Scholar]
- 22.Coon AL, Wallace DR, Mactutus CF, Booze RM. L-type calcium channels in the hippocampus and cerebellum of Alzheimer's disease brain tissue [In Process Citation] Neurobiol Aging. 1999;20:597–603. doi: 10.1016/s0197-4580(99)00068-8. [DOI] [PubMed] [Google Scholar]
- 23.Davidson RM, Shajenko L, Donta TS. Amyloid beta-peptide (A beta P) potentiates a nimodipine-sensitive L-type barium conductance in N1E-115 neuroblastoma cells. Brain Res. 1994;643:324–327. doi: 10.1016/0006-8993(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 24.Fu H, Li W, Lao Y, Luo J, Lee NT, Kan KK, Tsang HW, Tsim KW, Pang Y, Li Z, Chang DC, Li M, Han Y. Bis(7)-tacrine attenuates beta amyloid-induced neuronal apoptosis by regulating L-type calcium channels. J Neurochem. 2006;98:1400–1410. doi: 10.1111/j.1471-4159.2006.03960.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim S, Rhim H. Effects of amyloid-beta peptides on voltage-gated L-type Ca(V)1.2 and Ca (V)1.3 Ca (2+) channels. Mol Cells. 2011 doi: 10.1007/s10059-011-0075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramsden M, Henderson Z, Pearson HA. Modulation of Ca2+ channel currents in primary cultures of rat cortical neurones by amyloid beta protein (1–40) is dependent on solubility status. Brain Res. 2002;956:254–261. doi: 10.1016/s0006-8993(02)03547-3. [DOI] [PubMed] [Google Scholar]
- 27.Scragg JL, Fearon IM, Boyle JP, Ball SG, Varadi G, Peers C. Alzheimer's amyloid peptides mediate hypoxic up-regulation of L-type Ca2+ channels. FASEB J. 2005;19:150–152. doi: 10.1096/fj.04-2659fje. [DOI] [PubMed] [Google Scholar]
- 28.Ueda K, Shinohara S, Yagami T, Asakura K, Kawasaki K. Amyloid beta protein potentiates Ca2+ influx through L-type voltage-sensitive Ca2+ channels: a possible involvement of free radicals. J Neurochem. 1997;68:265–271. doi: 10.1046/j.1471-4159.1997.68010265.x. [DOI] [PubMed] [Google Scholar]
- 29.Webster NJ, Ramsden M, Boyle JP, Pearson HA, Peers C. Amyloid peptides mediate hypoxic increase of L-type Ca2+ channels in central neurones. Neurobiol Aging. 2006;27:439–445. doi: 10.1016/j.neurobiolaging.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Weiss JH, Pike CJ, Cotman CW. Ca2+ channel blockers attenuate beta-amyloid peptide toxicity to corical neurons in culture. J Neurochem. 1994;62:372–375. doi: 10.1046/j.1471-4159.1994.62010372.x. [DOI] [PubMed] [Google Scholar]
- 31.Davare MA, Hell JW. Increased phosphorylation of the neuronal L-type Ca(2+) channel Ca(v)1.2 during aging. Proc Natl Acad Sci U S A. 2003;100:16018–16023. doi: 10.1073/pnas.2236970100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Norris CM, Blalock EM, Chen KC, Porter NM, Landfield PW. Calcineurin enhances L-type Ca(2+) channel activity in hippocampal neurons: increased effect with age in culture. Neuroscience. 2002;110:213–225. doi: 10.1016/s0306-4522(01)00574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M. Reversal of long-term dendritic spine alterations in Alzheimer disease models. Proc Natl Acad Sci U S A. 2009;106:16877–16882. doi: 10.1073/pnas.0908706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O. Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell. 2006;126:775–788. doi: 10.1016/j.cell.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 35.Dineley KT, Hogan D, Zhang WR, Taglialatela G. Acute inhibition of calcineurin restores associative learning and memory in Tg2576 APP transgenic mice. Neurobiol Learn Mem. 2007;88:217–224. doi: 10.1016/j.nlm.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reese LC, Zhang W, Dineley KT, Kayed R, Taglialatela G. Selective induction of calcineurin activity and signaling by oligomeric amyloid beta. Aging Cell. 2008;7:824–835. doi: 10.1111/j.1474-9726.2008.00434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norris CM, Kadish I, Blalock EM, Chen KC, Thibault V, Porter NM, Landfield PW, Kraner SD. Calcineurin triggers reactive/inflammatory processes in astrocytes and is upregulated in aging and Alzheimer's models. J Neurosci. 2005;25:4649–4658. doi: 10.1523/JNEUROSCI.0365-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu HY, Hudry E, Hashimoto T, Kuchibhotla K, Rozkalne A, Fan Z, Spires-Jones T, Xie H, Arbel-Ornath M, Grosskreutz CL, Bacskai BJ, Hyman BT. Amyloid beta induces the morphological neurodegenerative triad of spine loss, dendritic simplification, and neuritic dystrophies through calcineurin activation. J Neurosci. 2010;30:2636–2649. doi: 10.1523/JNEUROSCI.4456-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer's disease mice. J Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chavis P, Fagni L, Lansman JB, Bockaert J. Functional coupling between ryanodine receptors and L-type calcium channels in neurons. Nature. 1996;382:719–722. doi: 10.1038/382719a0. [DOI] [PubMed] [Google Scholar]
- 41.Gant JC, Chen KC, Norris CM, Kadish I, Thibault O, Blalock EM, Porter NM, Landfield PW. Disrupting function of FK506-binding protein 1b/12.6 induces the Ca(2)+-dysregulation aging phenotype in hippocampal neurons. J Neurosci. 2011;31:1693–1703. doi: 10.1523/JNEUROSCI.4805-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gant JC, Sama MM, Landfield PW, Thibault O. Early and simultaneous emergence of multiple hippocampal biomarkers of aging is mediated by Ca2+-induced Ca2+ release. J Neurosci. 2006;26:3482–3490. doi: 10.1523/JNEUROSCI.4171-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar A, Foster TC. Enhanced long-term potentiation during aging is masked by processes involving intracellular calcium stores. J Neurophysiol. 2004;91:2437–2444. doi: 10.1152/jn.01148.2003. [DOI] [PubMed] [Google Scholar]
- 44.Stutzmann GE, Mattson MP. Endoplasmic reticulum Ca(2+) handling in excitable cells in health and disease. Pharmacol Rev. 2011;63:700–727. doi: 10.1124/pr.110.003814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y, Greig NH, Yu QS, Mattson MP. Presenilin-1 mutation impairs cholinergic modulation of synaptic plasticity and suppresses NMDA currents in hippocampus slices. Neurobiol Aging. 2009;30:1061–1068. doi: 10.1016/j.neurobiolaging.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]