Abstract
Background
Because of the anatomic complexity of the pelvis, there is no standard surgical treatment for giant cell tumors (GCTs) of the pelvic bones, especially in the periacetabular region. Treatment options include intralesional curettage with or without adjunctive techniques and wide resection. The best surgical treatment of a pelvic GCT remains controversial.
Questions/purposes
We compared wide resection and intralesional excision in terms of (1) local control, (2) function, and (3) complications.
Methods
We retrospectively identified 27 patients with periacetabular benign GCTs who underwent surgery from July 1999 to July 2009. Intralesional surgery was performed in 13 patients and wide resection in 14 patients. We determined surgical complications, local disease control, and Musculoskeletal Tumor Society (MSTS) 93 functional score. The minimum followup was 18 months (mean, 50 months; range, 18–121 months).
Results
Four of 13 patients who had intralesional surgery and none of 14 who had wide resection had local recurrence. The mean functional score was 24 for the 13 patients who underwent intralesional surgery and 22 for the 14 patients who had wide resection. One minor and one major complication occurred among patients who underwent intralesional surgery and one minor and six major complications occurred among patients who underwent wide resection.
Conclusions
Even with a higher complication rate with wide resection and prosthetic reconstruction, we believe the lower local recurrence rate makes wide resection a reasonable option for patients with extensive and/or aggressive GCTs involving the acetabulum.
Level of Evidence
Level IV, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
Pelvic giant cell tumors (GCTs) are rare, accounting for only 1.5% to 6.1% of bone GCTs [31, 35, 44]. The relatively few articles on GCTs of the pelvis report only five to 19 cases [5, 14, 23, 25, 30, 34, 37]. Owing to their infrequency, the best treatment of pelvic GCTs is controversial. Although a GCT is benign, the aggressiveness of the tumor leads to local recurrence in 7% to 75% of patients [8, 26, 27, 29]. If the tumor is located in a complex region, such as the periacetabular region and spinal column, recurrence of the tumor often makes it unresectable. The risk of malignant transformation ranges from 7% to 25% [13, 22, 33], and 2% to 9.1% metastasize either after radiation therapy [8, 32] or after several local recurrences [15, 24, 32].
Because of the complex anatomy of the pelvic region and the variable aggressiveness of GCTs, there is no standard treatment procedure for pelvic GCTs, especially in the periacetabular region. Treatment options include radiation therapy [11, 12], intralesional curettage with or without adjunctive techniques [5, 14, 23, 25, 30, 34], and wide resection [5, 14, 25, 30, 34]. Radiation eliminates surgical complications but may cause local injuries such as early and late skin changes, late pathologic fractures, and neuritis [11, 12, 25, 36]. Although curettage preserves the integrity of the pelvis, the local recurrence rate in this region ranges from 6.3% to 43% [5, 14, 25, 30, 34], especially in the acetabulum. Wide resection is intended to prevent local recurrence [5, 14, 25, 30, 34], but it increases surgical morbidity with complications such as superficial infections (skin sloughs and fistulas), deep infection, hematoma, functional deficits, and problems resulting from reconstruction for iliofemoral stability [16, 18, 21, 28]. The scarce literature on this topic provides limited guidance for surgeons to achieve maximum local control and low surgical morbidity in patients with pelvic GCTs.
We compared intralesional excision and wide resection in terms of local control, function, and complications.
Patients and Methods
We retrospectively reviewed the records of all 47 patients who had a primary benign GCT involving the acetabulum and who were treated with surgery from July 1999 to July 2009. The indications for surgery were: (1) no pelvic neurovascular involvement; (2) no visceral organ involvement; and (3) the ability to tolerate surgery. The relative contraindications for surgery were: (1) pelvic neurovascular involvement; (2) visceral organ involvement; and (3) inability to tolerate surgery. For this study we included patients with the following criteria: (1) those with lesions greater than 5 cm in maximum dimension measured by CT or MRI; (2) no prior treatments of the tumor; (3) complete clinical, radiographic, and pathologic records; and (4) minimum followup of 12 months after surgery. We excluded 20 of the 47 patients for the following reasons: five because their tumor sizes were smaller than 5 cm; 10 who had previous surgery in other hospitals and transferred to our hospital for treatment of local recurrence; four owing to insufficient clinical information; and one who was lost to followup. This left 27 patients for study. There were nine males and 18 females with a mean age of 29.2 years (range, 15–47 years) (Table 1). Thirteen patients were treated with intralesional surgery and 14 underwent wide resection. The minimum followup was 18 months (mean, 50 months; range, 18–121 months) for all 27 patients. Followup was 18 months (mean, 55 months; range, 18–121 months) for patients treated with intralesional surgery and 18 months (mean, 45 months; range, 18–120 months) for patients undergoing wide resection. We had prior approval of our Institutional Review Board.
Table 1.
Clinical data and surgical results for 27 patients with CGTs involving the acetabulum
| Patient number | Gender/age (years) | Location* | Grade† | Treatment | Margin | Reconstruction | Complication | Followup (months) | Function‡ | Recurrence or metastasis |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M/25 | II + III | III | Resection | Wide | Recycled + THA | 120 | 21 | ||
| 2 | F/25 | II + III | III | Resection | Wide | Hemipelvic | 61 | 16 | Multiple lung metastases | |
| 3 | F/20 | II + III | III | Resection | Wide | Hemipelvic | Delayed infection | 52 | 16 | |
| 4 | F/29 | I + II | II | Curettage | Intra-lesional | THA | 48 | 24 | ||
| 5 | F/36 | II + III | III | Resection + curettage | Intra-lesional | Hip fusion | 121 | 20 | ||
| 6 | M/40 | I + II | III | Resection | Wide | Recycled + THA | Wound healing disturbance | 47 | 22 | |
| 7 | F/27 | I + II | III | Resection | Wide | Hemipelvic | 64 | 23 | ||
| 8 | F/28 | II + III | III | Resection | Wide | Recycled + THA | Bone nonunion | 75 | 23 | |
| 9 | F/42 | I + II | III | Resection | Wide | Hemipelvic | 40 | 25 | ||
| 10 | M/27 | I + II | II | Curettage | Intra-lesional | THA | 80 | 25 | ||
| 11 | F/21 | II | II | Curettage | Intra-lesional | THA | Thrombosis | 119 | 24 | Recurrence |
| 12 | F/31 | II + III | II | Curettage | Intra-lesional | THA | 45 | 29 | Recurrence | |
| 13 | M/32 | II + III | III | Resection | Wide | Hemipelvic | Dislocation | 44 | 24 | |
| 14 | F/15 | II + III | III | Resection + curettage | Intra-lesional | Hip fusion | 113 | 18 | Recurrence | |
| 15 | M/35 | I +II + III | III | Resection + curettage | Intra-lesional | Hemipelvic | Wound healing disturbance | 41 | 24 | |
| 16 | F/47 | I + II | II | Curettage | Intra-lesional | THA | 30 | 24 | ||
| 17 | M/26 | II + III | III | Resection + curettage | Intra-lesional | Hemipelvic | 31 | 24 | ||
| 18 | F/15 | II + III | III | Resection | Wide | Hemipelvic | Would healing disturbance | 24 | 25 | |
| 19 | F/16 | II + III | III | Resection | Wide | Hemipelvic | 24 | 23 | ||
| 20 | F/47 | II + III | III | Resection | Wide | Hemipelvic | 23 | 22 | ||
| 21 | M/32 | I + II | II | Curettage | Intra-lesional | THA | 22 | 23 | Recurrence | |
| 22 | F/28 | I + II | III | Resection | Wide | Hemipelvic | 19 | 22 | ||
| 23 | M/26 | II + III | II | Curettage | Intra-lesional | THA | 21 | 23 | ||
| 24 | F/18 | I +II + III | III | Resection | Wide | Hemipelvic | Would healing disturbance | 21 | 23 | |
| 25 | F/29 | II + III | III | Resection + curettage | Intra-lesional | Hemipelvic | 18 | 24 | ||
| 26 | F/45 | II + III | III | Resection | Wide | Hemipelvic | Would healing disturbance | 18 | 22 | |
| 27 | M/27 | I + II | II | Curettage | Intra-lesional | THA | 20 | 26 |
The diagnosis of GCT was established based on the clinical information and imaging studies and confirmed by needle biopsy or open biopsy before surgery and pathology examination after surgery. The GCTs were staged radiographically according to the system of Campanacci [7]. Grade I indicates an intraosseous lesion; Grade II, an intraosseous lesion with cortical thinning and expansile borders; and Grade III, a lesion extending extraosseously forming a soft tissue mass. There were eight patients with Grade II lesions and 19 with Grade III lesions included in this series. The size of the tumors was estimated by measurement of its largest dimension from imaging studies. Median tumor size was 9.6 cm (range, 7–21 cm). According to the classification of pelvic tumors of Enneking and Dunham [16], tumor location and resection were classified as Type I (ilium), Type II (acetabulum), and Type III (pubis/ischium), and their combinations. The current series included Type II lesions in one patient, Type I + II in nine patients, Type II + III in 15 patients, and Type I + II + III in two patients. Patients were considered candidates for wide resection if they met all of the following criteria: (1) lesions extended into the neighboring soft tissue (Campanacci Grade III, aggressive); and (2) the medial and superior walls of the acetabulum were destroyed and there was no place left to hold the cement after curettage estimated by imaging studies.
All surgeries were performed by one surgeon (GW). The intralesional surgery group had two subtypes: one where curettage was planned and performed and the other where wide resection was planned but was not possible intraoperatively, resulting in partial resection and intralesional curettage. The intralesional group included the 13 patients treated with curettage and with partial resection and curettage. We performed curettage in eight patients with periacetabular lesions who underwent curettage, cementation, and THA. In the curettage group, a high-speed burr drill was used to remove the bony crest inside the tumor cavity after extensive curettage. We performed partial resection and curettage in five patients, including two young patients who underwent reconstruction by hip fusion and the other patients by modular pelvic prostheses. Fourteen patients underwent wide resection, including three who received wide resection and recycled tumor bone implantation with cemented THA (Fig. 1) and 11 patients who received wide resection and modular pelvic prosthetic reconstruction (Fig. 2). For the three patients who underwent wide resection and recycled tumor bone implantation, the resected tumor bone was immersed in preheated 10% saline at 65° for 20 minutes after eliminating the tumor tissue [9, 38, 40]. We did not use an embolization procedure because the tumor side of the common iliac artery was temporarily blocked with a vascular clamp to control bleeding in all patients during surgery. The common iliac artery was mobilized and encircled with nylon tape for temporary occlusion during removal of the tumor [19, 39]. Adjuvant therapy such as chemotherapy or radiotherapy was not used in 26 patients; one patient refused additional surgery for the recurrent tumor and was treated with radiotherapy.
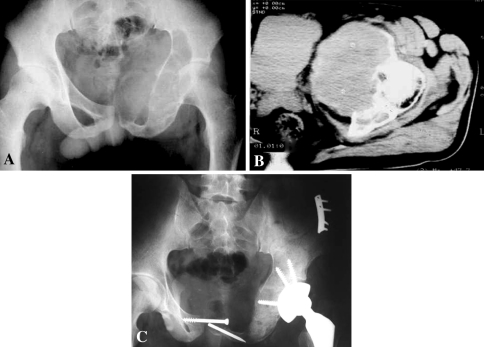
Fig. 1A–C.
(A) A plain film shows an osteolytic lesion in the left pubis, ischium, and periacetabular region of a 28-year-old female patient. This large lesion clearly involved the hip. (B) A CT scan shows the tumor involvement in the acetabulum where it produced a huge soft tissue mass. (C) The pelvic defect was reconstructed using recycled tumor bone implantation and a cemented THA was performed after tumor resection.
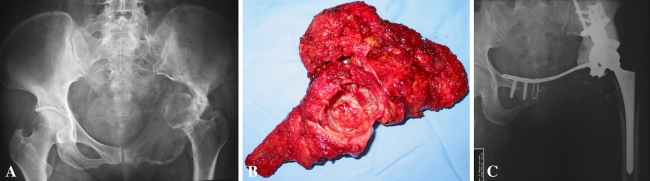
Fig. 2A–C.
(A) The image of the hip of a 32-year-old man whose main complaint was left hip area pain for 6 months that was more severe over the previous 3 days is shown. Osteolytic lesions can be seen in the left pubis, ischium, and periacetabular region on the plain film. The femoral head was dislocated and protruded into the pelvic cavity. (B) The tumor was resected wide, including the whole pubis and ischium, and the superior cut line was at the normal bone above the acetabulum. (C) A hemipelvic prosthesis was used for reconstruction of the defect after tumor resection. The prosthesis was fixed at the residual ilium, and bone cement was placed around the periacetabular prosthesis to reinforce the stability. The pubic plate was fixed between the acetabular and contralateral pubis.
In one patient who presented with multiple benign lung metastases confirmed by biopsy with the assistance of thoracoscopy at the time of surgery, the number and size of the lung foci after the tumor resection were reduced and the patient remained stable with no progression of lung foci at the last followup.
We obtained followup information during regular examinations and imaging of the patients every 3 months, and questionnaires were sent to the patients. At each visit, we assessed complications and local disease control. Local recurrence was established by evidence of new bone involvement assessed by radiographs or CT. If we suspected a tumor on radiographs or CT, we performed a biopsy. At the last followup, we obtained MSTS 93 scores [17]. The MSTS 93 score measures patient activity, including pain, function, emotional acceptance, supports, walking ability, and gait. Each of these six variables was assessed on a five-point scale, giving a maximum score of 30 points. A major complication was defined as one that necessitated additional surgical procedures. A minor complication was defined as a problem that necessitated nonoperative management.
Results
Four of the 13 patients who had intralesional surgery had local recurrences 10 to 17 months after surgery whereas none of the 14 patients treated with wide resection had recurrences during the followup period. Of the four patients with recurrences, one initially had a partial resection and curettage and the other three underwent curettage and cementation. One of these four patients who had previously undergone hip fusion refused additional surgery after the local recurrence and received radiation therapy; the other three patients underwent wide resection after recurrence. The patient treated with radiation was free of disease 113 months after the latest treatment. The other three patients were free of disease 6, 22, and 103 months after the latest treatment.
The mean MSTS functional score at last followup was 23 (range, 16–29) for all patients. The mean functional score was 24 for the 13 patients who underwent intralesional surgery and 22 for the 14 patients treated with wide resection.
Nine of the 27 patients had complications (Table 2). One minor and one major complication occurred in the group of patients who underwent intralesional surgery and one minor and six major complications occurred in the group of patients who underwent wide resection. Of these nine patients, seven underwent additional surgery to treat the complications. One patient with a deep infection eventually had the prosthesis removed because of uncontrolled infection after wound débridement and drainage. Five patients with wound healing problems were treated by débridement with eventual healing between 3 and 4 weeks. Dislocation of the hip occurred in one patient with hemipelvic prosthetic reconstruction and an open reduction was performed in this patient, with stability achieved by the 2-month followup. One patient had nonunion of the recycled tumor bone and remaining ilium junction; the nonunion was not treated further because no infection or fracture occurred and the patient could walk with crutches at the last followup. One patient had a distal deep vein thrombosis and was treated with anticoagulant drugs for 6 months with resolution.
Table 2.
Complications and outcomes at the last followup
| Patient number | Initial treatment group | Complication | Retreatment | Outcome | Followup (months) |
|---|---|---|---|---|---|
| 3 | Wide | Delayed infection | Prosthesis removal | Infection controlled | 52 |
| 6 | Wide | Wound healing disturbance | Débridement | Healing | 47 |
| 8 | Wide | Bone nonunion | No | Bone nonunion | 75 |
| 11 | Intralesional | Thrombosis | Anticoagulant drugs | Complete DVT resolution | 119 |
| 13 | Wide | Dislocation | Open reduction | Stability achieved | 44 |
| 15 | Intralesional | Wound healing disturbance | Débridement | Healing | 41 |
| 18 | Wide | Wound healing disturbance | Débridement | Healing | 24 |
| 24 | Wide | Wound healing disturbance | Débridement | Healing | 21 |
| 27 | Wide | Wound healing disturbance | Débridement | Healing | 18 |
DVT = deep vein thrombosis.
Discussion
A pelvic GCT is rare, accounting for only 1.5% to 6.1% of GCTs of the bone [31, 35, 44]. Pelvic GCTs are usually large by the time they are found because of the complexity of the anatomic site. There is no standard treatment procedure for a GCT involving the acetabular bone. The treatment options include radiation therapy [11, 12], intralesional curettage with or without adjunctive techniques [5, 14, 23, 25, 30, 34], and wide resection [5, 14, 25, 30, 34]. It is difficult for surgeons to achieve balance between the local control and surgical morbidity in patients with pelvic GCTs. We therefore compared intralesional excision and wide resection in terms of local control, function, and complications.
Readers should be aware of the limitations of our study. First, because pelvic GCTs are rare, we can report only on a small number of patients and therefore cannot make any definitive statements regarding the differences in recurrences and complications between intralesional surgery and wide resection. However, our data are consistent with a lower recurrence rate in patients with wide resection. Second, treatment was not randomized between different patient groups that might not comparable. Nevertheless this comparative study made the advantages and disadvantages of different treatment methods more clear which might be helpful for surgeons in the decision-making process. Third, we limited patients to those initially treated at our center. The findings therefore might not be generalizable. Fourth, the minimum followup is short and additional local recurrences might occur with longer followup. Nevertheless, 70% of local recurrences occur within 2 years [6].
Few publications have specifically addressed pelvic GCTs [5, 14, 23, 25, 30, 34, 37]. The local recurrence rate can be as much as 43% for therapeutic options other than wide resection [5, 14, 23, 25, 30, 34]. Donati et al. [14] reported that two of five patients with pelvic GCTs had local recurrence develop after intralesional surgery. Leggon et al. [25] reported 17 cases of GCTs of the pelvis and sacrum and performed a literature review of pelvic lesions treated with surgery, which revealed 41% of patients who had surgery with an intralesional margin had a local recurrence whereas 0% of patients who had surgery with a wide margin had a local recurrence (Table 3). In our study, none of the patients who underwent wide resection had a local recurrence, whereas four of 13 patients who underwent intralesional surgery had a local recurrence. Wide resection can minimize the recurrence rate because adjacent muscles can be resected with the tumor to ensure an adequate resection margin. Considering the local aggressiveness of GCTs, initial surgical treatment is important because recurrence of the tumor often makes it unresectable. If the medial and superior walls are destroyed by the tumor, there is no place to hold the cement after curettage. In this situation, we believe wide resection is a reasonable choice. Tumors with extensive cortical destruction and a large soft tissue mass are indications for wide resection to ensure achieving a safe margin.
Table 3.
Comparison of local control of various treatment studies
| Study | Number of patients | Methods of surgical treatment | Adjuvant procedures | Local recurrence rate (%) | Mean followup (months) |
|---|---|---|---|---|---|
| Kattapuran et al. [23] | 7 | Intralesional surgery (curettage in two patients and excision-curettage in five) | None | 43 | 36 |
| Sanjay et al. [34] | 17 | Intralesional surgery in 15 patients (curettage in six and excision-curettage in nine); wide resection in two patients | Phenol; radiation | 17.6 (20% for intralesional surgery; 0% for wide resection) | 226 |
| Osaka & Toriyama [30] | 5 | Intralesional surgery in one patient; wide resection in four patients | None | 0 | 163 |
| Donati et al. [14] | 10 | Intralesional surgery in five patients; wide resection in five patients | Drill burring; phenol; ethanol; radiation; bone cement | 20 (40% for intralesional surgery; 0% for wide resection) | 102 |
| Balke et al. [5] | 19 | Intralesional surgery in 16 patients (curettage in nine and excision-curettage in seven); wide resection in three patients | Drill burring; cryosurgery; radiation; bone cement; arterial embolization | 5.3 (6.3% for intralesional surgery; 0% for wide resection) | 49 |
| Leggon et al. [25] | 6 | Intralesional margins in two patients (curettage in two); wide margins in four patients | Radiation | 16.7 (20% for intralesional margins; 0% for wide margins) | 102 |
| Current study | 27 | Intralesional surgery in 13 patients (curettage in eight and excision-curettage in five); wide resection in 14 patients | Drill burring | 14.8 (30.8% for intralesional surgery; 0% for wide resection) | 50 |
During the past few years, various adjuvant modalities have been used to decrease the local recurrence rate after curettage. The adjuvant modalities included the use of cytotoxic agents such as phenol [34], zinc chloride [45], ethanol [14], and physical adjuvants such as polymethylmethacrylate [5, 14], cryosurgery [5], and a high-speed burr drill [5, 14]. We used a high-speed burr drill to deal with any residual GCT cells that may have remained on the surface of the curettage cavity. We did not use nitrogen or other chemicals because we rarely had a complete bone shell and wanted to avoid the chemical injury to surrounding tissues from leaking adjuvants. The role of radiation for treating pelvic GCTs is controversial. The review of the literature of pelvic and sacral GCTs by Leggon et al. [25] revealed that local recurrence rates were similar with curettage alone and curettage combined with radiation therapy. Increasing the radiation dosage may not decrease the local recurrence rate but could increase surgical complications and the sarcoma transformation risk [25]. The sarcoma transformation rate after radiation ranges from 11% to 25% in the pelvic region [25, 34]. Considering the risk of development of a sarcoma, 26 of the patients in our study did not undergo radiation therapy; only one patient who refused additional surgery for the recurrent tumor received radiation therapy.
Compared with intralesional excision, however, function of the periacetabular resection appears worse. Many approaches can be used to reconstruct the defect after acetabular bone resection: iliofemoral arthrodesis, ischiofemoral arthrodesis, arthroplasty, and saddle prosthesis. Arthroplasty achieves the best function if the internal cortex of the acetabulum is continuous and the integrity of the pelvic ring remains intact after tumor curettage. Arthrodesis after hip resection was seldom used in our patients because it limits ambulation and there is a high risk of nonunion. The mean functional scores for patients treated with intralesional excision were higher than those of patients who had wide resection. For GCTs involving the periacetabular region, hemipelvic prostheses have been used to reconstruct the integrity of the pelvis [43]. There are various reconstructive methods for acetabular bone, but we prefer to use a modular hemipelvic prosthesis based on our experience [20]. The modular hemipelvic prosthesis was designed by our department and produced by a local company (ChunLi Co, Beijing, China). The length of the prosthesis can be adjusted based on the defect after tumor resection. The prosthesis can be fixed to the remaining ilium after Type II + III resection and to the sacrum after Type I + II resection. The results of prosthetic reconstruction show favorable hip function. Most of the patients could resume their routine activities within 3 months after surgery.
Wide wound exposure, extensive soft tissue stripping, local hematoma formation, and poor skin flap blood supply are adverse factors affecting wound healing. As reported in the literature, the overall infection rate in several series varied from 12% to 47% after internal hemipelvectomy [1–3, 10]. We had one major complication in the group undergoing intralesional excision whereas six of the 14 patients who underwent wide resection had major complications. Facing the goal of better local control, a certain number of complications is acceptable. Protection of the skin flap, soft tissue closure without tension, adequate drainage, and careful wound care should be emphasized during postoperative care.
There have been some recent reports of bisphosphonate [4, 42] and denosumab [41] for treatment of GCTs of bone. The rationale for bisphosphonate treatment is that these compounds reduce osteoclast numbers and inhibit osteoclastic destruction [4]. Denosumab is a fully human monoclonal antibody that specifically inhibits RANKL, thereby inhibiting osteoclast-mediated bone resorption [41]. Balke et al. [4] studied 25 patients with bone GCTs who were treated with bisphosphonate and found that the disease was stabilized in most cases that had been refractory to conventional treatment, and most inoperable sacral and pelvic tumors did not increase in size. Lung metastases did not increase in size or number after the treatment. Thomas et al. [41] reported their results of denosumab treatment in 35 patients with bone GCTs and found 30 of 35 patients had a tumor response assessed by histology or radiology. As was reported [4, 41, 42], bisphosphonate and denosumab could be used for treatment of recurrent or metastatic disease or for primary inoperable pelvic and sacral tumors.
Treatment of GCTs involving the periacetabular region remains a challenge. The major issues are the resection methods to reduce local recurrence and reconstruction techniques to maintain hip function after tumor resection. Even with a higher complication rate with wide resection and prosthetic reconstruction, we believe the lower local recurrence rate makes wide resection a reasonable option for selected patients. The balance between recurrence of a benign (although locally aggressive) tumor and higher complications with wide resection should be discussed thoroughly with the patient during the decision-making process.
Acknowledgment
We thank Yanchun She for assistance with data collection.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Aboulafia AJ, Buch R, Mathews J, Li W, Malawer MM. Reconstruction using the saddle prosthesis following excision of primary and metastatic periacetabular tumors. Clin Orthop Relat Res. 1995;314:203–213. [PubMed] [Google Scholar]
- 2.Abudu A, Grimer RJ, Cannon SR, Carter SR, Sneath RS. Reconstruction of the hemipelvis after the excision of malignant tumours: complications and functional outcome of prostheses. J Bone Joint Surg Br. 1997;79:773–779. doi: 10.1302/0301-620X.79B5.6749. [DOI] [PubMed] [Google Scholar]
- 3.Apffelstaedt JP, Driscoll DL, Karakousis CP. Partial and complete internal hemipelvectomy: complications and long-term follow-up. J Am Coll Surg. 1995;181:43–48. [PubMed] [Google Scholar]
- 4.Balke M, Campanacci L, Gebert C, Picci P, Gibbons M, Taylor R, Hogendoorn P, Kroep J, Wass J, Athanasou N. Bisphosphonate treatment of aggressive primary, recurrent and metastatic giant cell tumour of bone. BMC Cancer. 2010;10:462. doi: 10.1186/1471-2407-10-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balke M, Streitbuerger A, Budny T, Henrichs M, Gosheger G, Hardes J. Treatment and outcome of giant cell tumors of the pelvis. Acta Orthop. 2009;80:590–596. doi: 10.3109/17453670903350123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blake SM, Gie GA. Large pelvic giant cell tumor: a case report and a review of current treatment modalities. J Arthroplasty. 2004;19:1050–1054. doi: 10.1016/s0883-5403(04)00350-x. [DOI] [PubMed] [Google Scholar]
- 7.Campanacci M. Giant cell tumor. In: Campanacci M, Enneking WF, editors. Bone and Soft Tissue Tumors: Clinical Features, Imaging, Pathology and Treatment. 2. New York, NY: Springer-Verlag; 1999. pp. 99–136. [Google Scholar]
- 8.Campanacci M, Baldini N, Boriani S, Sudanese A. Giant-cell tumor of bone. J Bone Joint Surg Am. 1987;69:106–114. [PubMed] [Google Scholar]
- 9.Campanacci M, Laus M. Local recurrence after amputation for osteosarcoma. J Bone Joint Surg Br. 1980;62:201–207. doi: 10.1302/0301-620X.62B2.6928851. [DOI] [PubMed] [Google Scholar]
- 10.Capanna R, Horn JR, Guernelli N, Briccoli A, Ruggieri P, Biagini R, Bettelli G, Campanacci M. Complications of pelvic resections. Arch Orthop Trauma Surg. 1987;106:71–77. doi: 10.1007/BF00435417. [DOI] [PubMed] [Google Scholar]
- 11.Caudell JJ, Ballo MT, Zagars GK, Lewis VO, Weber KL, Lin PP, Marco RA, El-Naggar AK, Benjamin RS, Yasko AW. Radiotherapy in the management of giant cell tumor of bone. Int J Radiat Oncol Biol Phys. 2003;57:158–165. doi: 10.1016/S0360-3016(03)00416-4. [DOI] [PubMed] [Google Scholar]
- 12.Chakravarti A, Spiro IJ, Hug EB, Mankin HJ, Efird JT, Suit HD. Megavoltage radiation therapy for axial and inoperable giant-cell tumor of bone. J Bone Joint Surg Am. 1999;81:1566–1573. doi: 10.2106/00004623-199911000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Dahlin DC, Cupps RE, Johnson EW., Jr Giant-cell tumor: a study of 195 cases. Cancer. 1970;25:1061–1070. doi: 10.1002/1097-0142(197005)25:5<1061::AID-CNCR2820250509>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 14.Donati D, Wafa H, Di Bella C, Colangeli M, Colangeli S, Bertoni F. Management of pelvic giant cell tumours involving the acetabular bone. Acta Orthop Belg. 2008;74:773–778. [PubMed] [Google Scholar]
- 15.Eckardt JJ, Grogan TJ. Giant cell tumor of bone. Clin Orthop Relat Res. 1986;204:45–58. [PubMed] [Google Scholar]
- 16.Enneking WF, Dunham WK. Resection and reconstruction for primary neoplasms involving the innominate bone. J Bone Joint Surg Am. 1978;60:731–746. [PubMed] [Google Scholar]
- 17.Enneking WF, Dunham WK, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed] [Google Scholar]
- 18.Gradinger R, Rechl H, Hipp E. Pelvic osteosarcoma: resection, reconstruction, local control, and survival statistics. Clin Orthop Relat Res. 1991;270:149–158. [PubMed] [Google Scholar]
- 19.Guo W, Ji T, Tang X, Yang Y. Outcome of conservative surgery for giant cell tumor of the sacrum. Spine (Phila Pa 1976). 2009;10:1025–1031. doi: 10.1097/BRS.0b013e31819d4127. [DOI] [PubMed] [Google Scholar]
- 20.Guo W, Li D, Tang X, Yang Y, Ji T. Reconstruction with modular hemipelvic prostheses for periacetabular tumor. Clin Orthop Relat Res. 2007;461:180–188. doi: 10.1097/BLO.0b013e31806165d5. [DOI] [PubMed] [Google Scholar]
- 21.Hillmann A, Hoffmann C, Gosheger G, Rodl R, Winkelmann W, Ozaki T. Tumors of the pelvis: complications after reconstruction. Arch Orthop Trauma Surg. 2003;123:340–344. doi: 10.1007/s00402-003-0543-7. [DOI] [PubMed] [Google Scholar]
- 22.Johnson KA, Riley LH., Jr Giant cell tumor of bone: an evaluation of 24 cases treated at the Johns Hopkins Hospital between 1925 and 1955. Clin Orthop Relat Res. 1969;62:187–191. doi: 10.1097/00003086-196901000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Kattapuram AS, O’Donnell RJ, Huszar M, Rosenberg AE, Kattapuram SV, Mankin HJ. Surgical management of innominate giant cell tumor. Clin Orthop Relat Res. 1996;329:281–287. doi: 10.1097/00003086-199608000-00035. [DOI] [PubMed] [Google Scholar]
- 24.Kay RM, Eckardt JJ, Seeger LL, Mirra JM, Hak DJ. Pulmonary metastasis of benign giant cell tumor of bone: six histologically confirmed cases, including one of spontaneous regression. Clin Orthop Relat Res. 1994;302:219–230. [PubMed] [Google Scholar]
- 25.Leggon RE, Zlotecki R, Reith J, Scarborough MT. Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin Orthop Relat Res. 2004;423:196–207. doi: 10.1097/01.blo.0000128643.38390.07. [DOI] [PubMed] [Google Scholar]
- 26.Malawer MM, Bickels J, Meller I, Buch RG, Henshaw RM, Kollender Y. Cryosurgery in the treatment of giant cell tumor: a long-term followup study. Clin Orthop Relat Res. 1999;359:176–188. doi: 10.1097/00003086-199902000-00019. [DOI] [PubMed] [Google Scholar]
- 27.McDonald DJ, Sim FH, McLeod RA, Dahlin DC. Giant-cell tumor of bone. J Bone Joint Surg Am. 1986;68:235–242. [PubMed] [Google Scholar]
- 28.Nilsonne U, Kreicbergs A, Olsson E, Stark A. Function after pelvic tumour resection involving the acetabular ring. Int Orthop. 1982;6:27–33. doi: 10.1007/BF00267812. [DOI] [PubMed] [Google Scholar]
- 29.Oda Y, Miura H, Tsuneyoshi M, Iwamoto Y. Giant cell tumor of bone: oncological and functional results of long-term follow-up. Jpn J Clin Oncol. 1998;28:323–328. doi: 10.1093/jjco/28.5.323. [DOI] [PubMed] [Google Scholar]
- 30.Osaka S, Toriyama S. Surgical treatment of giant cell tumors of the pelvis. Clin Orthop Relat Res. 1987;222:123–131. [PubMed] [Google Scholar]
- 31.Reid R, Banerjee SS, Sciot R. Giant cell tumour. In: Fletcher CDM, Unni KK, Mertens F, editors. Pathology and Genetics of Tumors of Soft Tissue and Bone. Lyon, France: IARC Press; 2002. pp. 309–313. [Google Scholar]
- 32.Rock MG, Sim FH, Unni KK, Witrak GA, Frassica FJ, Schray MF, Beabout JW, Dahlin DC. Secondary malignant giant-cell tumor of bone: clinicopathological assessment of nineteen patients. J Bone Joint Surg Am. 1986;68:1073–1079. [PubMed] [Google Scholar]
- 33.Sanerkin NG. Malignancy, aggressiveness, and recurrence in giant cell tumor of bone. Cancer. 1980;46:1641–1649. doi: 10.1002/1097-0142(19801001)46:7<1641::AID-CNCR2820460725>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 34.Sanjay BK, Frassica FJ, Frassica DA, Unni KK, McLeod RA, Sim FH. Treatment of giant-cell tumor of the pelvis. J Bone Joint Surg Am. 1993;75:1466–1475. doi: 10.2106/00004623-199310000-00007. [DOI] [PubMed] [Google Scholar]
- 35.Schajowicz F. Giant cell tumor. In: Schajowicz F, Sundaram M, Gitelis S, McDonald DJ, editors. Tumors and Tumorlike Lesions of Bone. 2. New York, NY: Springer-Verlag; 1996. pp. 257–295. [Google Scholar]
- 36.Seider MJ, Rich TA, Ayala AG, Murray JA. Giant cell tumors of bone: treatment with radiation therapy. Radiology. 1986;161:537–540. doi: 10.1148/radiology.161.2.3763928. [DOI] [PubMed] [Google Scholar]
- 37.Shankman S, Greenspan A, Klein MJ, Lewis MM. Giant cell tumor of the ischium: a report of two cases and review of the literature. Skeletal Radiol. 1988;17:46–51. doi: 10.1007/BF00361455. [DOI] [PubMed] [Google Scholar]
- 38.Singh VA, Nagalingam J, Saad M, Pailoor J. Which is the best method of sterilization of tumour bone for reimplantation? A biomechanical and histopathological study. Biomed Eng Online. 2010;9:48. doi: 10.1186/1475-925X-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang X, Guo W, Yang R, Tang S, Ji T. Risk factors for blood loss during sacral tumor resection. Clin Orthop Relat Res. 2009;467:1599–1604. doi: 10.1007/s11999-008-0483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang XD, Guo W, Yang RL, Yang Y, Ji T. Limb salvage surgery for osteosarcoma around the knee in children and adolescent patients][in Chinese. Zhonghua Wai Ke Za Zhi. 2007;45:669–672. [PubMed] [Google Scholar]
- 41.Thomas D, Henshaw R, Skubitz K, Chawla S, Staddon A, Blay JY, Roudier M, Smith J, Ye Z, Sohn W, Dansey R, Jun S. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11:275–280. doi: 10.1016/S1470-2045(10)70010-3. [DOI] [PubMed] [Google Scholar]
- 42.Tse LF, Wong KC, Kumta SM, Huang L, Chow TC, Griffith JF. Bisphosphonates reduce local recurrence in extremity giant cell tumor of bone: a case-control study. Bone. 2008;42:68–73. doi: 10.1016/j.bone.2007.08.038. [DOI] [PubMed] [Google Scholar]
- 43.Uchida A, Myoui A, Araki N, Yoshikawa H, Ueda T, Aoki Y. Prosthetic reconstruction for periacetabular malignant tumors. Clin Orthop Relat Res. 1996;326:238–245. doi: 10.1097/00003086-199605000-00029. [DOI] [PubMed] [Google Scholar]
- 44.Unni KK. Giant cell tumor. In: Unni KK, Dahlin DC, editors. Dahlin’s Bone Tumors: General Aspects and Data on 11, 087 Cases. 5. Philadelphia, PA: Lippincott-Raven; 1996. pp. 263–289. [Google Scholar]
- 45.Zhen W, Yaotian H, Songjian L, Ge L, Qingliang W. Giant-cell tumour of bone. The long-term results of treatment by curettage and bone graft. J Bone Joint Surg Br. 2004;86:212–216. doi: 10.1302/0301-620X.86B2.14362. [DOI] [PubMed] [Google Scholar]