Abstract
Background
The impact of hip arthroscopy on health-related quality of life (HRQoL) among younger patients with symptomatic femoroacetabular impingement (FAI) is unknown, but with increasing recognition of the condition there is likely to be increasing demand for arthroscopy.
Questions/purposes
We describe an approach to determine the incremental cost-effectiveness of hip arthroscopy compared with observation in patients with FAI; we also identified variables that influence its cost-effectiveness.
Patients and Methods
We constructed a Markov model including possible health states for 36-year-old patients with FAI using decision analysis software and compared two strategies: (1) observation and (2) hip arthroscopy, followed by THA with disease progression. We estimated the ratio of the incremental cost to the incremental benefit (reflected by HRQoL) of both strategies. We identified studies reporting Harris hip scores and complications after arthroscopy to estimate health state preferences and their probabilities. We performed sensitivity analyses on 30 input variables over a plausible range of estimates to determine the influence of uncertainty on the ICER with particular emphasis on the magnitude and duration of benefit.
Results
Among patients with FAI but no radiographic evidence of arthritis, the estimated ICER of hip arthroscopy was $21,700/QALY while the ICER for patients with preoperative arthritis was $79,500/QALY. Alteration of the natural history of arthritis by hip arthroscopy improved the ICER to $19,200/QALY and resulted in cost savings if THA was not performed until at least 16 years after arthroscopy.
Conclusions
Although limited by available data, our model suggests hip arthroscopy in patients with FAI without arthritis may result in a favorable ICER compared with other health interventions considered cost-effective. Further studies of hip arthroscopy are needed to determine the impact on quality of life, duration of symptomatic relief, and the effect on the need for subsequent THA.
Introduction
Femoroacetabular impingement (FAI) is a theorized causative factor in the pathophysiology of osteoarthritis (OA) resulting from abnormal shape of the femoral head and neck (cam-type) and/or the acetabulum (pincer-type) that results in repetitive microtrauma to the acetabular labrum and articular cartilage [7, 18, 23]. Hip arthroscopy has emerged as a minimally invasive approach to correct these bony abnormalities as well as their sequelae that may be precursors to OA. Specific techniques reported in the literature include femoral osteoplasty for cam-type FAI [3, 7, 18, 25], acetabular rim-trimming for pincer-type FAI [26, 40], labral débridement [8, 22, 29, 30, 33] and repair [24, 28], and osteochondral defect débridement and microfracture [18, 27, 36].
The efficacy of hip arthroscopy as a tool to prevent or delay the progression to OA and/or the need for THA among younger patients with FAI is unknown. In the longest followup available at a minimum of 10 years, Byrd and Jones reported improvement in the Harris hip score of at least 18 points among 15 of 18 patients without arthritis in contrast to seven of eight having conversion to THA in patients with prearthroscopy arthritis [8]. However, the study was conducted before the recognition of FAI and specific techniques designed to address it. More recent studies suggest improvements in the Harris hip score ranging from 9 to 27 points at 2- to 3-year followup with conversion to total hip in 0% to 9% of cases [21, 26]. However, these studies are limited by a small sample size, lack of a control group, and short-term followup.
Although these data suggest improvement in pain and function assessed by the Harris hip score, it is unclear if the magnitude and duration of benefit are substantial enough relative to the cost to warrant more widespread use of the procedure. We therefore believe it is important to estimate the cost-effectiveness using quantitative techniques to compare arthroscopy with other accepted surgical techniques. In addition, sensitivity analyses can be used to identify critical unknown variables that may help guide future clinical study.
A Markov process is one approach to modeling in decision analysis when empirically observed cost and quality of life are not available. The model approximates a disease process using a discrete set of health states. A hypothetical patient cohort transitions through the model, moving from one health state to another with each cycle based on probabilities estimated from the literature. As the cohort passes through the model, the costs and benefits are accumulated based on preset values for each health state.
A first-order Monte Carlo simulation is a related technique that can use the same model structure as the Markov model. It is distinguished by modeling individuals rather than a cohort, which allows for incorporation of individual histories such as a unique THA failure rate depending on the age at the index surgery. This is accomplished by running thousands or millions of “simulations” in which individuals pass through the model, each following a unique path rather than the entire cohort passing through simultaneously. The downside of Monte Carlo simulation is that a considerable amount of transparency is lost, making it more difficult to validate and debug. We therefore elected to rely primarily on Markov modeling techniques but used Monte Carlo simulation to test certain assumptions made for the Markov model.
The purpose of this study is to describe an approach 1) to estimate the incremental cost-per quality-adjusted life-year (QALY) of hip arthroscopy relative to observation using a Markov model with inputs derived from the available literature; and (2) use sensitivity analyses to identify variables with the strongest influence on the incremental cost-effectiveness ratio.
Patients and Methods
The study was designed according to the guidelines outlined by the Panel on Cost-effectiveness in Health and Medicine [35, 38]. A Markov model to estimate the cost and utility gained from hip arthroscopy was constructed using Treeage Pro 2009 (Treeage Software, Williamstown, MA). The cohort considered for analysis was a 36-year-old population with symptomatic FAI. This population was selected based on a weighted average of mean ages for patients in outcome studies identified [3, 7, 18, 23, 26]. The two strategies considered for comparison were (1) hip arthroscopy followed by THA in the event of progression to end-stage arthritis; and (2) observation followed by THA in the event of progression to end-stage arthritis. The primary outcome considered was the incremental cost-effectiveness ratio (ICER), which in this case is the net cost of hip arthroscopy divided by the net benefit (Fig. 1). Health benefits are expressed quantitatively using QALYs, the product of the utility and duration of a health state. Utility is a term from economics that expresses individual preference for a given health state and ranges from 0 (equivalent to death) to 1 (equivalent to perfect health). Utility is calculated by assessing individual’s willingness (or preference) to accept a tradeoff between the health state and death. One can equate this to the quality of life, as states with better quality of life are likely to be more preferable, but preference is unequivocally the most accurate definition for utility.
Fig. 1.
The incremental cost-effectiveness ratio (ICER) is the ratio of the net benefits and the net costs. QALY = quality-adjusted life-year.
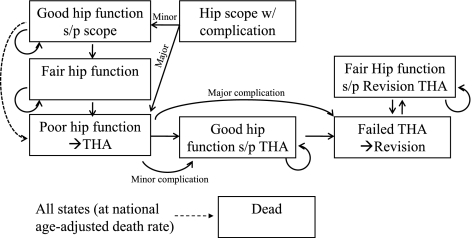
A total of eight discrete health states were included in the model (Fig. 2). In the observation group, all patients begin with fair hip function, which is defined as the state of hip pain and function in untreated symptomatic FAI. Poor hip function represents the state of hip pain and function equivalent to end-stage arthritis, and these patients were predicted to undergo THA. Good hip function is defined as the state of hip pain and function that occurs after the treatment of symptomatic FAI with hip arthroscopy. We estimated these patients progress to poor hip function at a rate of 3.3% per year. This estimate was derived from a study by Bardakos and Villar, who observed progression of arthritis in one-third of patients with symptomatic FAI treated nonoperatively over 10 years [4]. To explore the impact of hip arthroscopy among patients with more advanced arthritis, a higher rate of progression resulting in the need for THA in 90% of cases in 10 years was used, similar to a subgroup of patients with more advanced arthritis who underwent hip arthroscopy in a study by Byrd and Jones [9]. In addition, we presumed hip arthroscopy in the presence of arthritis would result in a smaller improvement in hip pain and function and therefore result in lower postoperative utility than patients without arthritis (Table 1).
Fig. 2.
The flow-state diagram demonstrates the discrete health states considered after hip arthroscopy. Patients begin with either good hip function or a complication, which can be major or minor. After a specified duration, they progress to fair hip function. The dashed line denotes the two scenarios considered in which hip arthroscopy alters the progression of arthritis or provides symptomatic relief only. In the latter scenario, patients progress from good hip function after arthroscopy to poor hip function at the same rate as patients with fair hip function. In contrast, if hip arthroscopy alters the progression of arthritis, patients only progress to poor hip function after the progression from good to fair hip function. In the observation model, all patients begin in the “fair hip function” state.
Table 1.
All variables used in model with low and high estimates
| Input variable | Estimate | Low | High | Reference |
|---|---|---|---|---|
| Duration of hip arthroscopy benefit | 3 years | 1 | 10 | [4, 8, 21, 26, 29] |
| Probability of arthroscopy complication | .015 | 0.005 | 0.05 | [11, 13, 23, 34] |
| Annual probability of death | Variable by age | – | – | 2005 US Life Tables |
| Probability of death after revision THA | 0.008 | 0.006 | 0.01 | [3, 43] |
| Probability of death after primary THA | 0.003 | 0.002 | 0.004 | [3, 43] |
| Annual probability of primary THA failure | Variable by age | 0.003 | 0.025 | [14, 18] |
| Probability of deep infection after THA | .007 | 0.005 | 0.01 | [18, 37] |
| Probability of major complication after arthroscopy | 0.1 | 0.05 | 0.3 | [23] |
| Probability of major complication from revision THA | 0.04 | 0.03 | 0.05 | [43] |
| Probability of major complication after THA | 0.021 | 0.016 | 0.026 | [43] |
| Probability of minor complication after revision THA | 0.117 | 0.088 | 0.146 | [43] |
| Probability of minor complication after THA | 0.067 | 0.05 | 0.084 | [43] |
| Probability of osteoarthritis progression | 0.033 | 0.033 | 0.23 | [5] |
| Annual probability of revision THA failure | 0.046 | 0.034 | 0.058 | [2] |
| Cost of hip arthroscopy | $11,850 | 8900 | 14800 | UCSF Data |
| Cost of primary THA | $24,200 | 18150 | 30250 | UCSF Costing Database |
| Cost of revision THA | $34,700 | 26000 | 43375 | UCSF Costing Database |
| Cost of revision THA for infection | $53,300 | 40000 | 66600 | [38] |
| Cost of a minor complication of THA | $10,000 | 7500 | 12500 | [1] |
| Utility after primary THA | 0.9 | 0.675 | 1.0 | [16] |
| Utility after revision THA | 0.81 | 0.6075 | 1.0 | [16] |
| Utility of poor hip function | 0.5 | 0.375 | 0.625 | [4, 8, 12, 21, 26, 29, 38] |
| Utility of good hip function if baseline arthritis | 0.84 | 0.63 | 1.0 | [12, 29, 38] |
| Utility of good hip function | 0.94 | 0.705 | 1.0 | [4, 8, 12, 21, 26, 29, 38] |
| Utility of fair hip function | 0.75 | 0.56 | 0.94 | [4, 8, 12, 21, 26, 29, 38] |
| Disutility of primary THA | −0.1 | −0.2 | 0 | [15, 38] |
| Disutility of revision THA | −0.12 | −0.3 | 0 | [15, 38] |
| Disutility of minor complication of THA | −0.05 | −0.1 | 0 | No literature to support |
| disutility of a minor arthroscopy complication | −0.05 | −0.1 | 0 | No literature to support |
| disutility of hip arthroscopy | −0.05 | −0.1 | 0 | No literature to support |
In the hip arthroscopy treatment arm, two scenarios were considered: (1) hip arthroscopy provides symptomatic relief only and has no impact on the progression of arthritis; and (2) hip arthroscopy alters the natural history and results in delay in the progression of arthritis. To make a conservative estimate, the benefit of arthroscopy was estimated to last 3 years, which is equivalent to the longest followup currently available for arthroscopic treatment of FAI lesions. The effect of a longer duration of benefit was then explored using sensitivity analysis.
The model used a 1-year cycle length over a lifetime horizon. Age-adjusted death rates were used based on 2005 US life tables. A 3% discount rate was used for analysis, per the recommendations of the US Panel on Cost-Effectiveness in Health and Medicine [16, 35, 38], and the impact of a higher discount rate was explored in sensitivity analysis. A first-order Monte Carlo simulation was used to incorporate the impact of an increasing rate of THA failure based on the age at the time of the initial surgery. It was also used to explore the impact of a delay in the decision to undergo THA after developing end-stage arthritis. This was accomplished by allowing the individuals in the simulation to remain in the poor health state without undergoing THA for up to 20 years. With each simulation the delay was chosen at random from a uniform distribution from 0 to 20. For each scenario, 100,000 microsimulations were performed to reach a stable solution.
A comprehensive review of the literature was performed to identify studies reporting the Harris hip score [17] after arthroscopy for FAI (Table 2) [3, 7, 18, 23, 26]. The modified Harris hip score [6], which includes only pain and function (91 points) and excludes the ROM and deformity components (9 points), was reported in most studies. These scores were adjusted to the 100-point scale by assuming a full score for the ROM and deformity components, ie, adding 9 points. Utilities (the individual patient preference for a health state) were derived from this “corrected” Harris hip score by linear extrapolation of a previously described algorithm by Chang et al. [11]. The midpoint of each range in Harris hip score was plotted with the corresponding utility, and a linear fit was performed. Using this algorithm as well as a weighted average of the Harris hip scores (Table 2) yielded an estimated utility before and after hip arthroscopy of 0.75 and 0.94, respectively. The postoperative utility for patients with arthritis was based on the study by Phillipon et al., who demonstrated a smaller improvement in the Harris hip score of 10 points for patients with joint space less than 2 mm [26]. Using the aforementioned algorithm, this translated to a postoperative utility of 0.84.
Table 2.
Studies reporting Harris hip score after hip arthroscopy for FAI
| Study | Number of hips | Preoperative HHS* | Postoperative HHS* | Conversion to THA (number) (%) | Followup (months) |
|---|---|---|---|---|---|
| Haviv et al. [18] | 170 | 79.7 | 95.1 | 2 (1.2%) | 22 |
| Byrd and Jones [8] | 207 | 74 | 94 | 3 (1.4%) | 16 |
| Philippon et al. [28] | 112 | 67 | 93 | 10 (8.9%) | 28 |
| Bardakos and Villar [4] | 24 | 68 | 92 | 0 (0%) | 12 |
| Larson and Giveans [24] | 100 | 70 | 92 | 3 (3%) | 10 |
* Adjusted to total score of 100; FAI = femoroacetabular impingement; HHS = Harris hip score.
Disutilities are transient decreases in utility resulting from undesirable events such as the period of pain and decreased function after major surgery [19]. A prior study applied a disutility of 0.1 to primary THA, 0.12 to revision THA, and 0.2 for revision as a result of infection with the assumption that a disutility of 0.1 is equivalent to 5 weeks of poor health [12]. For our model we applied the aforementioned disutilities to THA as well as a disutility of 0.05 to hip arthroscopy.
Major complications of hip arthroscopy such as permanent nerve injury [10], femoral neck fracture [32], and avascular necrosis [31] have been reported inconsistently across studies [20]. A review by Ilizaliturri estimated an overall complication rate of 1.5% with the majority being transient neuropraxias [20]. We included a 1.5% rate of complication and estimated 90% were transient and associated with a small disutility, whereas 10% were important and would result in poor hip function and the need for THA.
The inputs for the THA component of the model were also derived from the literature. The utility after primary and revision THA in the model was 0.9 and 0.81, respectively [13]. The annual failure rate of primary THA was predicted to increase with age beginning at 2.5% per year under age 50 years and decreasing linearly to 0.3% per year over age 75 years. This was derived from the 2008 report of the Swedish Hip Arthroplasty Register, which demonstrated survivorship of 65% and 95% at 17 years in these age groups, respectively [15]. Complications requiring readmission but not reoperation such as thromboembolic disease and superficial infection were considered minor, whereas major complications were those requiring reoperation. Major and minor complication rates of 2.1% and 6.7%, respectively, were derived from a study by Zhan et al. [39]. Of the major complications, a subset of 0.7% [15] was considered secondary to deep infection and therefore associated with additional cost and disutility [5, 34]. Revision THA was associated with an annual failure rate of 4.6% [2], a major complication rate of 4%, and a minor complication rate of 11.7% [39].
Direct costs of hip arthroscopy for FAI were estimated from a review of the 10 most recent cases performed at our institution in 2010 US dollars. To estimate hospital costs from billed charges, a cost-to-charge ratio was calculated based on payments received relative to billed charges for the professional fees. This ratio was then applied to the total charges including facility and anesthesia fees for each procedure. The mean cost computed using this methodology was $11,850. Indirect costs of clinic visits, lost work, and productivity were not considered in the model because we assumed these costs were insignificant relative to the cost of surgery and likely to be similar between the two groups. However, we tested this assumption using sensitivity analysis by varying the cost of hip arthroscopy and nonoperative care and assessing the impact on the ICER.
Direct costs of primary and revision THA were estimated from a costing database at our institution to be $24,200 and $34,700 (Table 1). The cost of a minor complication was estimated to be $10,000 based on the cost of hospitalization for pulmonary embolism from HCUPnet, the Healthcare Cost and Utilization Project online database [1, 37]. Major complications resulted in revision hip arthroplasty and were associated with the same cost and disutility, except in the subset of patients with deep infection, who faced an additional cost of $18,600 [34].
One-way sensitivity analysis was performed to identify variables with the strongest influence on the ICER. This technique calculates the ICER across a range of plausible input values for a single variable. Threshold analysis is an extension of one-way sensitivity analysis that seeks to identify the value of a given variable where the preferred strategy shifts from one treatment to another. This was used to identify the duration of benefit that yielded an ICER less than $50,000/QALY and cost savings, if achievable. Two-way sensitivity analysis, which explores the preferred strategy across ranges of two variables, was used to assess uncertainty in the duration and magnitude of benefit simultaneously. Because there are a large number of uncertain variables, tornado diagrams were used to perform one-way sensitivity analysis on every variable in the model. These are then plotted together on the same scale of the ICER to determine which variables have the most impact. From these data, the variables that resulted in a change in the ICER of greater than $1000/QALY were included in a multivariate sensitivity analysis known as a probabilistic sensitivity analysis (PSA) or second-order Monte Carlo simulation. This method uses probability distributions (Table 3) rather than point estimates for select inputs to explore the impact of uncertainty in multiple parameters simultaneously. Ten thousand iterations of the model were performed for the PSA in each case drawing unique values from the distributions of the variables with the greatest influence over the ICER. The results were then plotted on cost-effectiveness acceptability curves, which show the likelihood of choosing each strategy as a function of the willingness to pay.
Table 3.
Distributions used in probabilistic sensitivity analysis
| Variable | Distribution type | Parameters |
|---|---|---|
| Duration of hip arthroscopy benefit | Triangle | Minimum = 1, likeliest = 3, maximum = 5 |
| Cost of hip arthroscopy | Gamma | Mean = 11850, SD = 3000 |
| Change in utility from hip arthroscopy | Beta | Mean = 0.19, SD = 0.05 |
| Cost of nonoperative care | Uniform | Mean = 250, range = 0 to 500 |
| Disutility of hip arthroscopy | Uniform | Mean = −0.05, range = 0 to −0.1 |
Results
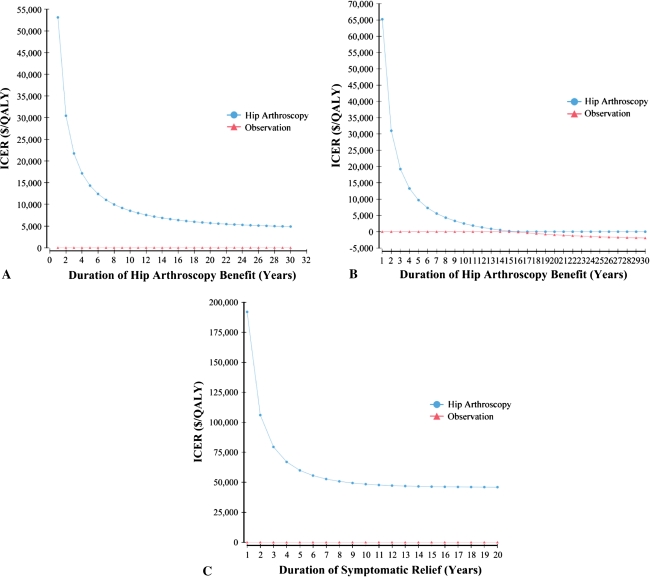
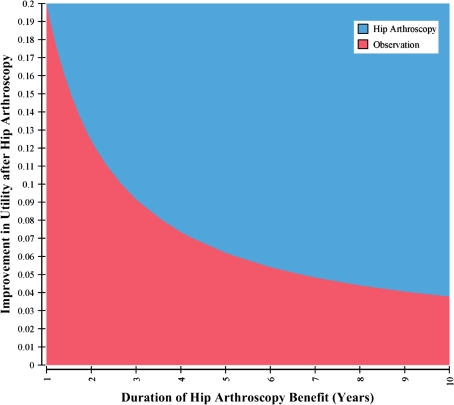
Using the conservative estimate of only 3 years of symptomatic relief with no impact on the rate of progression to end-stage arthritis, the estimated ICER was $21,700/QALY. The threshold for the duration of benefit resulting in an ICER less than $50,000/QALY was 1.1 years; that is, if the duration of benefit is greater than 13 months, the procedure has an ICER less than $50,000/QALY (Fig. 3A). If hip arthroscopy delays the progression of OA for the same 3-year period, the ICER decreases to $19,200/QALY. If the progression is delayed by greater than 5 years, it becomes highly cost-effective (ICER less than $10,000/QALY) and after 16 years, it becomes cost-saving (Fig. 3B). If only symptomatic relief is achieved, the procedure is never cost-saving regardless of the duration of benefit, but the ICER is highly favorable (ICER less than $10,000/QALY) if symptoms are improved for greater than 8 years. For patients with preoperative arthritis and therefore lower postoperative utility and a higher rate of progression to poor hip function, the ICER was $79,500/QALY. The threshold for duration of benefit resulting in an ICER less than $50,000/QALY is 8 years (Fig. 3C).
Fig. 3A–C.
The ICER is shown as a function of the duration of benefit for (A) symptomatic relief only with no baseline arthritis; (B) delay in progression of arthritis with no baseline arthritis; and (C) symptomatic relief with baseline arthritis. ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life-year.
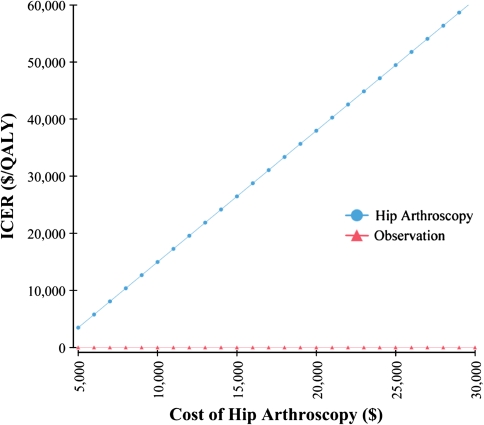
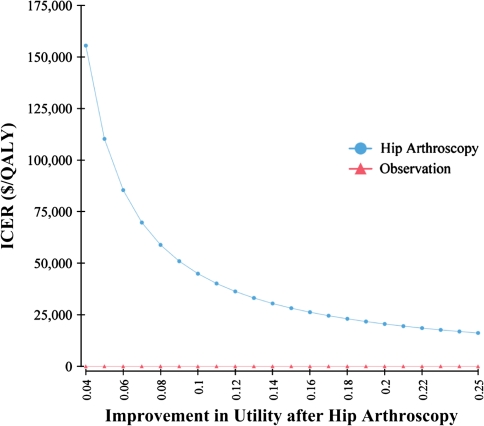
In addition to the duration of hip arthroscopy benefit and the presence of arthritis preoperatively, the ICER was most sensitive to the cost of hip arthroscopy and the improvement in utility postoperatively. The threshold for the cost of hip arthroscopy resulting in an ICER of $50,000/QALY was $27,300 (Fig. 4). The threshold for the utility after hip arthroscopy resulting in an ICER of $50,000/QALY is 0.84, equivalent to a change in utility of 0.09 assuming a baseline utility of 0.75 (Fig. 5). The influence of improvement in utility after hip arthroscopy and duration of benefit on the ICER were assessed simultaneously in two-way sensitivity analysis (Fig. 6). To achieve an ICER of $50,000 with duration of benefit of 1 year, the utility would have to improve by 0.2. In contrast, for a duration of benefit of 10 years, the utility would have to improve by only 0.04 to achieve an ICER less than $50,000/QALY.
Fig. 4.
The incremental cost-effectiveness ratio as a function of the cost of hip arthroscopy is shown in a one-way sensitivity analysis. ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life-year.
Fig. 5.
The incremental cost-effectiveness ratio as a function of the utility after hip arthroscopy is shown in a one-way sensitivity analysis. ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life-year.
Fig. 6.
Two-way sensitivity analysis indicates the preferred strategy as a function of both the magnitude and duration of benefit using a willingness to pay of $50,000/quality-adjusted life-year.
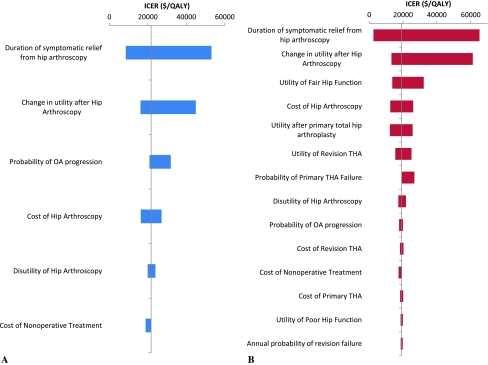
Tornado diagrams demonstrated that the ICER was robust within a large range of cost and outcome of THA (Fig. 7). Although all variables were tested (Table 1), for clarity only those that resulted in a change in the ICER of greater than $1000/QALY over a plausible range of estimates are included in the diagrams. Variables pertaining to the cost and benefits of THA such as the cost, utility, and rate of failure and other complications had minimal effect on the ICER of hip arthroscopy in the case of symptomatic relief alone. In the scenario in which hip arthroscopy delays the progression of arthritis, there was a trend toward lower ICER with lower utility, higher failure rate, and higher cost of primary and revision THA. The utility of fair hip function was also important in this scenario because if the hip function of symptomatic FAI is extremely poor, then delaying THA can actually be a detriment. None of these variables, however, had sufficient impact on the ICER to alter conclusions. A first-order Monte Carlo simulation was performed to more accurately model the failure rate after THA and incorporate the decision to delay THA after developing end-stage arthritis. These changes had a minimal impact in the scenario of symptomatic relief only. In the case of a delay in the progression of arthritis, the ICER decreased to $18,000/QALY if there was no delay in undergoing THA with end-stage arthritis and $14,600/QALY if the waiting period ranged from 0 to 20 years at random.
Fig. 7A–B.
Tornado diagrams show the range of incremental cost-effectiveness ratios resulting from one-way sensitivity analysis of multiple variables on the same scale for the case of (A) no arthritis at baseline and (B) arthritis at baseline. Variables that resulted in a range of less than $1000/quality-adjusted life-year were not included. ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life-year.
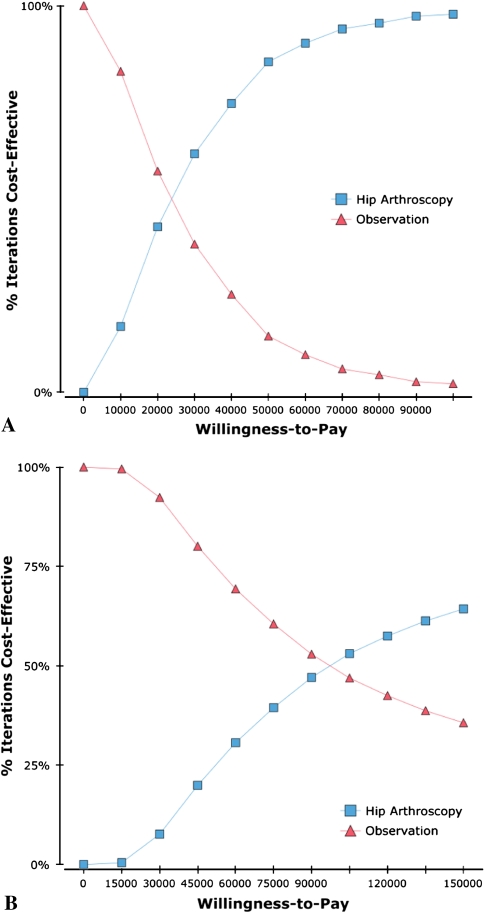
In the probabilistic sensitivity analysis, the ICER was less than $50,000/QALY in 85% of trials and less than $100,000/QALY in 97% of trials among patients without arthritis. In contrast, with preoperative arthritis, hip arthroscopy results in an ICER less than $50,000/QALY and $100,000/QALY in 23% and 51% of trials, respectively. The PSA is demonstrated graphically with the cost-effectiveness acceptability curves, which are a graphic representation of the probability of preferring a strategy as a function of willingness to pay (Fig. 8).
Fig. 8A–B.
The diagrams show cost-effectiveness acceptability curves for patients with a low rate of progression to arthritis (A) and a high rate of progression to arthritis (B).
Discussion
There is a growing number of studies reporting the use of hip arthroscopy to address FAI, and with high prevalence and increasing recognition of the condition, there is likely to be increasing demand for the procedure. Although available evidence is limited, we describe an approach to determine the incremental cost-effectiveness of hip arthroscopy compared with observation in patients with FAI. As a secondary goal, we sought to identify sensitive variables that influence the cost-effectiveness of hip arthroscopy in this setting.
There are several important limitations of this study. First, the accuracy of estimates from any model is dependent on the quality of the inputs, which are in turn limited by the available literature. Specifically, the rate of progression of arthritis among patients with both treated and untreated FAI is unknown. It may have many determinants such as age, activity level, and degree of deformity that are not incorporated into the model. We would argue, however, that decisions regarding use of the procedure must be made regardless of the quality of evidence, and cost-effectiveness allows a quantitative synthesis of information, particularly in the case in which uncertainty exists that is preferable to a nonsystematic approach. Furthermore, important information regarding the relevant clinical questions for further clinical study can be elucidated. Second, we used a simplified model with discrete health states, which does not reflect the spectrum of disease and symptoms encountered in clinical practice. We believed our model provided a reasonable balance of accurately modeling reality without unnecessary complexity. Utilities could not be directly assessed for each health state by either direct administration of an empiric measure such as the standard gamble or by preference-weighted measures of health-related quality of life. Third, we used linear interpolation of a previously reported method of converting from Harris hip score to utility, but this has never been validated. This is an important limitation because our study identified a high degree of sensitivity to the postoperative utility. Fourth, we aimed to perform the study from the societal standpoint but were not able to obtain estimates of the indirect costs of hip arthroscopy. We did not believe this would be a major influence relative to the direct cost of surgery, particularly considering the differential between the hip arthroscopy group and the observation group is likely to be even smaller. We therefore believed direct costs could accurately serve as a surrogate for societal costs.
Our analysis indicates that if hip arthroscopy provides an improvement in quality of life equivalent to a change in utility of 0.19 for greater than 13 months, the procedure has favorable cost-effectiveness regardless of its impact on the need for THA. For patients with baseline arthritis, a smaller improvement in quality of life and higher likelihood of progression of arthritis have been demonstrated in prior studies [9, 14]. As a result, hip arthroscopy is unlikely to be a cost-effective strategy in this setting. If a substantial delay in the need for THA among these patients were possible by performing hip arthroscopy, the cost-effectiveness would improve substantially, but there is no current evidence to support this idea.
One of the major goals of surgery to address FAI is to alter the natural history of arthritis and prevent the need for THA. In our model, a delay in the progression of arthritis of greater than 5 years resulted in a highly favorable cost-effectiveness ratio, whereas a delay greater than 16 years resulted in cost savings. Whether hip arthroscopy has an effect on the progression of arthritis remains an unanswered question. In our review of the literature, several studies with less than 2 years followup showed rates of conversion to THA of 0% to 3%. A study by Philippon, in contrast, showed a rate of conversion to THA of 9% at 28 months [26]. Although not a valid statistical comparison, this is actually in excess of the rate of THA among a group of nonoperatively treated patients with FAI observed by Bardakos and Villar for 10 years [4]. Studies with a nonoperatively treated control group followed for a longer period of time are needed to address this question directly.
The use of arthroscopy in patients with radiographic evidence of arthritis preoperatively is controversial. Our results supported the idea that joint-preserving surgery among these patients is less effective than in patients with a lower likelihood of disease progression. If a period of symptomatic relief with improved quality of life can be achieved, our model suggests arthroscopic intervention in these patients may have moderate-to-low cost-effectiveness with an ICER between $50,000 and $100,000 per QALY. The majority of outcomes studies to date have excluded patients with Tönnis Grade 2 or 3 arthritis, making an accurate estimation of the outcomes among these patients difficult. However, our estimate of an improvement in utility of 0.09 maintained for 3 years may be optimistic at best.
In addition to longer-term studies with a control group, it is important that future studies administer outcome instruments that allow calculation of quality of life in addition to hip function. Preference-weighted survey instruments that allow calculation of utility such as the EQ-5D, SF-6D, or Health Utilities Index can be administered quickly and would allow more accurate estimation of the magnitude of benefit for use in decision analysis. To our knowledge, none of these instruments has been administered to patients with FAI before or after surgical intervention.
In conclusion, we have used a model-based approach to estimate the ICER of hip arthroscopy to address symptomatic FAI. Our model, which is limited by the poor quality of available evidence, suggests that among patients without radiographic evidence of arthritis, hip arthroscopy may produce a favorable cost-effectiveness ratio relative to other health interventions that are considered cost-effective. Although it is a purely hypothetical scenario based on current literature, the model supports the idea that if hip arthroscopy can prevent or delay the development of arthritis, the procedure may be highly cost-effective or even cost-saving. In contrast, our model suggests patients with FAI in the setting of arthritis receive a relatively small incremental benefit compared with the cost of hip arthroscopy, which negatively impacts the estimated cost-effectiveness. Although our study does not allow us to confidently determine the cost-effectiveness of hip arthroscopy, our findings indicate future studies should collect cost data and assess quality of life using preference-weighted generic instruments for both operative and nonoperative care at long-term followup. In addition, cohort studies are needed to determine the natural history of FAI, in particular the rate of arthritis and subsequent total hip arthroplasty compared with unaffected individuals and the impact of hip arthroscopy on the progression of disease.
Acknowledgments
We thank Eula McKinney and Skye Ramos for their assistance in preparing the manuscript.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
This work was performed at the University of California, San Francisco Medical Center, San Francisco, CA, USA.
References
- 1.Agency for Health Care Research and Quality. HCUPnet: A tool for identifying, tracking, and analyzing national hospital statistics. Healthcare Cost and Utilization Project (HCUP). Available at: http://hcupnet.ahrq.gov. Accessed December 23, 2010.
- 2.Australian Orthopaedic Association. National Joint Replacement Registry. Annual Report. Adelaide: AOA; 2009.
- 3.Bardakos NV, Vasconcelos JC, Villar RN. Early outcome of hip arthroscopy for femoroacetabular impingement: the role of femoral osteoplasty in symptomatic improvement. J Bone Joint Surg Br. 2008;90:1570–1575. doi: 10.1302/0301-620X.90B12.21012. [DOI] [PubMed] [Google Scholar]
- 4.Bardakos NV, Villar RN. Predictors of progression of osteoarthritis in femoroacetabular impingement: a radiological study with a minimum of ten years follow-up. J Bone Joint Surg Br. 2009;91:162–169. doi: 10.1302/0301-620X.91B2.21137. [DOI] [PubMed] [Google Scholar]
- 5.Bozic KJ, Ries MD. The impact of infection after total hip arthroplasty on hospital and surgeon resource utilization. J Bone Joint Surg Am. 2005;87:1746–1751. doi: 10.2106/JBJS.D.02937. [DOI] [PubMed] [Google Scholar]
- 6.Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 2-year follow-up. Arthroscopy. 2000;16:578–587. doi: 10.1053/jars.2000.7683. [DOI] [PubMed] [Google Scholar]
- 7.Byrd JW, Jones KS. Arthroscopic femoroplasty in the management of cam-type femoroacetabular impingement. Clin Orthop Relat Res. 2009;467:739–746. doi: 10.1007/s11999-008-0659-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrd JW, Jones KS. Hip arthroscopy for labral pathology: prospective analysis with 10-year follow-up. Arthroscopy. 2009;25:365–368. doi: 10.1016/j.arthro.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Byrd JW, Jones KS. Prospective analysis of hip arthroscopy with 10-year followup. Clin Orthop Relat Res. 2010;468:741–746. doi: 10.1007/s11999-009-0841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Byrd JWT. Operative Hip Arthroscopy. 2nd ed. New York: Springer; 2005:xvi.
- 11.Chang RW, Pellisier JM, Hazen GB. A cost-effectiveness analysis of total hip arthroplasty for osteoarthritis of the hip. JAMA. 1996;275:858–865. doi: 10.1001/jama.1996.03530350040032. [DOI] [PubMed] [Google Scholar]
- 12.Cummins JS, Tomek IM, Kantor SR, Furnes O, Engesaeter LB, Finlayson SR. Cost-effectiveness of antibiotic-impregnated bone cement used in primary total hip arthroplasty. J Bone Joint Surg Am. 2009;91:634–641. doi: 10.2106/JBJS.G.01029. [DOI] [PubMed] [Google Scholar]
- 13.Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty. A qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86:963–974. doi: 10.2106/00004623-200405000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Farjo LA, Glick JM, Sampson TG. Hip arthroscopy for acetabular labral tears. Arthroscopy. 1999;15:132–137. doi: 10.1053/ar.1999.v15.015013. [DOI] [PubMed] [Google Scholar]
- 15.Garellick G, Karrholm J, Rogmark C, Herberts P. Swedish Hip Arthroplasty Register: Annual Report. Goteborg: Sahlgrenska University Hospital, Department of Orthopaedics; 2008. [Google Scholar]
- 16.Gold M. Panel on cost-effectiveness in health and medicine. Med Care. 1996;34(Suppl):DS197–DS199. [PubMed] [Google Scholar]
- 17.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty. An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed] [Google Scholar]
- 18.Haviv B, Singh PJ, Takla A, O’Donnell J. Arthroscopic femoral osteochondroplasty for cam lesions with isolated acetabular chondral damage. J Bone Joint Surg Br. 2010;92:629–633. doi: 10.1302/0301-620X.92B5.23667. [DOI] [PubMed] [Google Scholar]
- 19.Hunink M. Decision Making in Health and Medicine. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- 20.Ilizaliturri VM., Jr Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop Relat Res. 2009;467:760–768. doi: 10.1007/s11999-008-0618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilizaliturri VM, Jr, Orozco-Rodriguez L, Acosta-Rodríguez E, Camacho-Galindo J. Arthroscopic treatment of cam-type femoroacetabular impingement: preliminary report at 2 years minimum follow-up. J Arthroplasty. 2008;23:226–234. doi: 10.1016/j.arth.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 22.Kamath AF, Componovo R, Baldwin K, Israelite CL, Nelson CL. Hip arthroscopy for labral tears: review of clinical outcomes with 4.8-year mean follow-up. Am J Sports Med. 2009;37:1721–1727. doi: 10.1177/0363546509333078. [DOI] [PubMed] [Google Scholar]
- 23.Larson CM, Giveans MR. Arthroscopic management of femoroacetabular impingement: early outcomes measures. Arthroscopy. 2008;24:540–546. doi: 10.1016/j.arthro.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 24.Larson CM, Giveans MR. Arthroscopic débridement versus refixation of the acetabular labrum associated with femoroacetabular impingement. Arthroscopy. 2009;25:369–376. doi: 10.1016/j.arthro.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Lincoln M, Johnston K, Muldoon M, Santore R. Combined arthroscopic and modified open approach for cam femoroacetabular impingement: a preliminary experience. Arthroscopy. 2009;25:392–399. doi: 10.1016/j.arthro.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Philippon MJ, Briggs KK, Yen YM, Kuppersmith DA. Outcomes following hip arthroscopy for femoroacetabular impingement with associated chondrolabral dysfunction: minimum two-year follow-up. J Bone Joint Surg Br. 2009;91:16–23. doi: 10.1302/0301-620X.91B1.21329. [DOI] [PubMed] [Google Scholar]
- 27.Philippon MJ, Schenker ML, Briggs KK, Maxwell RB. Can microfracture produce repair tissue in acetabular chondral defects? Arthroscopy. 2008;24:46–50. doi: 10.1016/j.arthro.2007.07.027. [DOI] [PubMed] [Google Scholar]
- 28.Philippon MJ, Weiss DR, Kuppersmith DA, Briggs KK, Hay CJ. Arthroscopic labral repair and treatment of femoroacetabular impingement in professional hockey players. Am J Sports Med. 2010;38:99–104. doi: 10.1177/0363546509346393. [DOI] [PubMed] [Google Scholar]
- 29.Potter BK, Freedman BA, Andersen RC, Bojescul JA, Kuklo TR, Murphy KP. Correlation of Short Form-36 and disability status with outcomes of arthroscopic acetabular labral débridement. Am J Sports Med. 2005;33:864–870. doi: 10.1177/0363546504270567. [DOI] [PubMed] [Google Scholar]
- 30.Robertson WJ, Kadrmas WR, Kelly BT. Arthroscopic management of labral tears in the hip: a systematic review of the literature. Clin Orthop Relat Res. 2007;455:88–92. doi: 10.1097/BLO.0b013e31802c7e0f. [DOI] [PubMed] [Google Scholar]
- 31.Sampson TG. Complications of hip arthroscopy. Clin Sports Med. 2001;20:831–835. doi: 10.1016/S0278-5919(05)70288-X. [DOI] [PubMed] [Google Scholar]
- 32.Sampson TG. Arthroscopic treatment of femoroacetabular impingement. Techniques in Orthopaedics. 2005;20:56–62. doi: 10.1097/01.bto.0000153635.24366.b5. [DOI] [Google Scholar]
- 33.Santori N, Villar RN. Acetabular labral tears: result of arthroscopic partial limbectomy. Arthroscopy. 2000;16:11–15. doi: 10.1016/S0749-8063(00)90121-X. [DOI] [PubMed] [Google Scholar]
- 34.Sharifi E, Sharifi H, Morshed S, Bozic K, Diab M. Cost-effectiveness analysis of periacetabular osteotomy. J Bone Joint Surg Am. 2008;90:1447–1456. doi: 10.2106/JBJS.G.00730. [DOI] [PubMed] [Google Scholar]
- 35.Siegel JE, Weinstein MC, Russell LB, Gold MR. Recommendations for reporting cost-effectiveness analyses. Panel on Cost-Effectiveness in Health and Medicine. JAMA. 1996;276:1339–1341. doi: 10.1001/jama.1996.03540160061034. [DOI] [PubMed] [Google Scholar]
- 36.Singh PJ, O’Donnell JM. The outcome of hip arthroscopy in Australian football league players: a review of 27 hips. Arthroscopy. 2010;26:743–749. doi: 10.1016/j.arthro.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 37.Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Eff Clin Pract. 2002;5:143–151. [PubMed] [Google Scholar]
- 38.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the panel on cost-effectiveness in health and medicine. JAMA. 1996;276:1253–1258. doi: 10.1001/jama.1996.03540150055031. [DOI] [PubMed] [Google Scholar]
- 39.Zhan C, Kaczmarek R, Loyo-Berrios N, Sangl J. Bright RA Incidence and short-term outcomes of primary and revision hip replacement in the United States. J Bone Joint Surg Am. 2007;89:526–533. doi: 10.2106/JBJS.F.00952. [DOI] [PubMed] [Google Scholar]
- 40.Zumstein M, Hahn F, Sukthankar A, Sussmann PS, Dora C. How accurately can the acetabular rim be trimmed in hip arthroscopy for pincer-type femoral acetabular impingement: a cadaveric investigation. Arthroscopy. 2009;25:164–168. doi: 10.1016/j.arthro.2008.09.016. [DOI] [PubMed] [Google Scholar]