Abstract
Background
Surgical treatment for degenerative conditions of the hip, knee, and spine has an impact on overall healthcare spending. Surgical rates have increased dramatically and considerable regional variation has been observed. The reasons behind these increasing rates and variation across regions have not been well elucidated.
Questions/purposes
We therefore identified demographic (D), social structure (SS), health belief (HB), personal (PR) and community resources (CR), and medical need (MN) factors that drive rates of hip, knee, and spine surgery.
Methods
We conducted a systematic review to include all observational, population-based studies that compared surgical rates with potential drivers (D, SS, HB, PR, CR, MN). We searched PubMed combining key words focusing on (1) disease and procedure; (2) study methodology; and (3) explanatory models. Independent investigators selected potentially eligible studies from abstract review and abstracted methodological and outcome data. From an initial search of 256 articles, we found 37 to be potentially eligible (kappa 0.86) but only 28 met all our inclusion criteria.
Results
Age, nonminority, insurance coverage, and surgeon enthusiasm all increased surgical rates. Rates of arthroplasty were higher for females with higher education, income, obesity, rurality, willingness to consider surgery, and prevalence of disease, whereas spinal rates increased with male gender, lower income, and the availability of advanced imaging.
Conclusions
Regional variation in these procedures exists because they are examples of preference-sensitive care. With strategies that may affect change in factors that are potentially modifiable by behavior or resources, extreme variation in rates may be reduced.
Introduction
Orthopaedic procedures have allowed surgeons to improve the lives of patients with degenerative musculoskeletal conditions by reducing pain and restoring function. THA and TKA are well-recognized examples of successful interventions in degenerative joint diseases [9, 15, 20, 33, 38, 45, 49, 50]. In degenerative disease of the lumbar spine (DDLS), recent studies have shown that surgical decompression and fusion can improve pain and function for specific indications [55, 56].
The success of these surgical interventions coupled with increased demands of a growing aging population has produced a commensurate increase in the use rates [6, 8, 42, 53]. In the past decade, we have seen a rise in the use of these procedures. From 1992 to 2001, rates of THA increased 34% to 2.9 per 1000 Medicare enrollees, TKA increased 40% to 5.7 per 1000 enrollees, and spinal surgery increased 53% in the Medicare population to 4.3 per 1000 enrollees [54]. In addition, as technology has advanced, the individual implant costs have increased and now comprise a considerable amount of healthcare spending. Hospital Medicare payments for joint arthroplasty have increased nearly 22% since 1993 and the US hip and knee market for implants and devices was estimated at $6.4 billion in 2009 [41]. The US spinal implant market is now $6.8 billion, 30 times larger than it was in 1994 [40]. The annual cost of treating musculoskeletal health conditions from 2002–2004 was $510 billion, equivalent to 4.6% of the US gross domestic product (GDP) with indirect costs totaling nearly $850 billion (7.7% of GDP) [3].
Although use rates and costs have increased in recent years, many reports document use rates across geographic regions suggesting that where you live may determine the likelihood of undergoing surgery [7, 14, 17, 30–32, 35, 37, 44, 53, 54, 61]. For example, there is a reported nearly 20-fold difference in rates of spinal fusion across US counties [54]. The systematic component of variance (SCV) measures the variation in rates adjusting for random variation within regions, which is stable across a range of rates and population sizes. The SCV for TKA, THA, and spinal surgery in 2000–2001 was 55.0, 67.2, and 93.6, respectively, whereas in comparison, the SCV for hip fracture repair during the same period was only 13.8 [54].
This phenomenon, termed small-area variation, is better explained by differences in factors other than disease prevalence or resource availability, mainly physician uncertainty or enthusiasm [10, 51, 54, 57, 58]. Several studies have examined individual factors that influence surgical rates, namely race, income, education, disease, and the use of diagnostic imaging [13, 18, 28, 29, 35, 46]. However, few have considered multiple factors collectively for individual conditions such as surgeon attitude, patient preference, socioeconomic status, and race, making the appreciation of the relative importance of one factor over another problematic [7, 30, 47]. The combination of high costs and considerable variation warrant further examination into reasons behind the disparate nature of their use.
Andersen’s Behavioral Model of Health Services Use provides a comprehensive framework for explaining patient behavior in the use of health services whereby behavior is primarily influenced by patients’ predisposing characteristics (demographics, social structure, and health beliefs), enabling resources (personal and community), and need (perceived and evaluated) [5].
The purpose of our study was to identify the factors influencing use rates of total joint arthroplasty (TJA), namely THA and TKA, and lumbar spinal surgery (LSS) for DDLS. Specifically, we aimed to determine the influence of demographics (D), social structure (SS), health beliefs (HB), personal resources (PR), community resources (CR), and medical need (MN) on rates of THA, TKA, and DDLS surgery using this conceptual model. We presumed (D) age and gender; (SS) income, education, and race/ethnicity; (PR) insurance status; and (MN) rates of degenerative disease influence rates of surgery, whereas (HB) health beliefs and (CR) community resources play a smaller role.
Search Strategy and Criteria
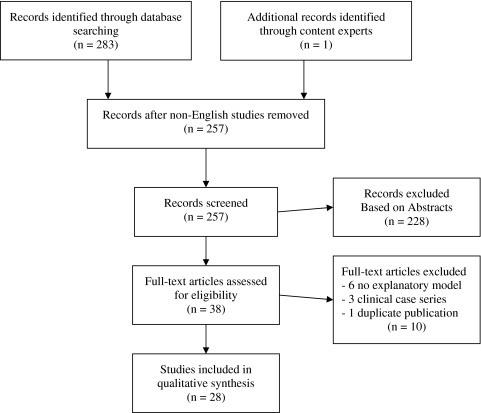
With the assistance of a medical librarian, we searched PubMed for all English language journals to identify relevant articles up to September 16, 2010, and contacted content experts for additional studies. Conceptually, we combined three main themes (disease and procedure; study methodology; and explanatory models) (see Appendix A). These strategies yielded 283 references (Fig. 1). We included all population-based observational studies (ie, administrative claims data or nationwide surveys) with a primary outcome of use rates of hip or knee arthroplasty or lumbar spinal surgery for degenerative conditions. We excluded 27 studies in languages other than English.
Fig. 1.
This flow diagram illustrates the number of articles selected from search and the number remaining after sequential exclusion criteria applied.
Two investigators (CDR, PDK) independently selected potentially eligible studies from a review of the abstracts. Any disagreements were resolved by a third investigator (SSB). We measured the overall agreement using Cohen’s kappa statistic [34].
Potentially eligible articles were retrieved in full and blinded to author and institution. Two investigators (NNB, RG) then applied our inclusion and exclusion criteria to all potentially eligible articles. Disagreements were resolved by a third investigator (SSB). Data on study methodology and results were abstracted using a standardized form for all included studies and we excluded those that did not have multivariate or bivariate statistical explanatory models of use (Appendix B).
Our initial search produced 256 articles and we identified one additional article from content experts. Of those, we found 38 abstracts to be potentially eligible (kappa 0.86). We excluded six studies not including a predictor model explaining differential rates and three with an arbitrarily selected cohort. After blinded review, 29 met our inclusion criteria. Two studies were similar analyses based on the same cohort [30, 31] where one measured outcomes as arthroplasty rates [30] and the other on ratios of provision relative to need, which was excluded. Twenty-eight studies were included in our analysis with various characteristics [2, 7, 8, 11, 13, 14, 17–19, 22, 25–27, 29–32, 35–37, 39, 44, 46, 47, 52–54, 60, 61] (Table 1). Twenty-six of the articles arose from English-speaking countries (US, Canada, England, and Australia) [7, 8, 11, 13, 14, 17–19, 22, 25–27, 30–32, 35, 36, 39, 44, 46, 47, 52–54, 60, 61] and 22 were from fee-for-service healthcare systems [2, 7, 8, 13, 14, 18, 19, 22, 25–27, 32, 35, 39, 44, 45, 47, 52–54, 60, 61]. Sixteen of the articles explained the use of surgery using a multivariate model (two to 13 variables) [7, 13, 17–19, 22, 25, 27, 30–32, 36, 37, 44, 47, 53, 61], whereas 12 articles looked at univariate analyses to explain variation in surgical rates. Twenty articles were derived from population-based claims data, in which three used additional survey data in their explanatory model [2, 7, 8, 11, 13, 14, 17, 22, 26, 29–32, 35–37, 44, 53, 54, 60, 61]. Eight articles were derived from survey data primarily.
Table 1.
Study characteristics
| Author (publication year) | Setting | Health system | Years | Primary source | Procedures | Multivariate model (number of factors)* | Standardization method |
|---|---|---|---|---|---|---|---|
| Agabiti (2007) | Italy | FFS | 1997–2000 | Claims | THA | N (3) | Age-gender-city |
| Bederman (2011) | Ontario, Canada | FFS | 2002–2006 | Claims | LSS | Y (12) | Age-gender |
| Bederman (2009) | Ontario, Canada | FFS | 1995–2001 | Claims | LSS | N (2) | None |
| Cookson (2007) | England | NHS | 1991, 2001 | Claims | THA | N (1) | Age-gender-ward |
| Coyte (1997) | Ontario, Canada | FFS | 1984–1990 | Claims | TKA | Y (3) | Age-gender-year |
| Coyte (1996) | Ontario, Canada | FFS | 1984–1990 | Claims | TKA | N (3) | Age-gender |
| Dixon (2006) | England | NHS | 2000 | Claims | THA, TKA | Y (6) | Age |
| Dunlop (2008) | USA | FFS | 1998–2004 | Surveys | THA, TKA | Y (9) | Age-race |
| Dunlop (2003) | USA | FFS | 1993, 1995 | Surveys | TJA | Y (5) | None |
| Friedman (1995) | USA | FFS | 1980–1987 | Claims | THA | Y (7) | None |
| Hanchate (2008) | USA | FFS | 1994–2004 | Surveys | TKA | Y (9) | None |
| Harris (2009) | NSW, Australia | FFS | 1997–2006 | Claims | THA, TKA, LSS | N (1) | None |
| Hawker (2006) | Ontario, Canada | FFS | 1996–1998 | Surveys | THA, TKA | Y (12) | None |
| Jarvholm (2008) | Sweden | NHS | 1987–1999 | Claims | THA, TKA | N (1) | Age-BMI |
| Judge (2009) | England | NHS | 2002 | Claims | THA, TKA | Y (11) | Age-gender-ward |
| Katz (1996) | USA | FFS | 1985–1990 | Claims | TKA | Y (3) | Age |
| Lurie (2003) | USA | FFS | 1996–1997 | Claims | LSS | N (1) | None |
| Majeed (2002) | England | NHS | 1997–1998 | Claims | THA | Y (2) | Age-gender |
| Makela (2010) | Finland | Public | 1998–2005 | Claims | THA | Y (7) | Age-gender |
| McWilliams (2009) | USA | FFS | 1996–2005 | Surveys | TJA | N (1) | None |
| Peterson (1992) | USA | FFS | 1988 | Claims | THA, TKA | Y (2) | Age |
| Skinner (2006) | USA | FFS | 2000 | Surveys | TKA | N (3) | Age-gender-race |
| Steel (2008) | USA | FFS | 2000–2004 | Surveys | THA, TKA | Y (10) | None |
| Wang (2009) | Victoria, Australia | FFS | 2001–2005 | Surveys | THA, TKA | N (5) | None |
| Weinstein (2004) | USA | FFS | 2000–2001 | Claims | THA, TKA, LSS | Y (3) | Age-gender-race |
| Weinstein (2006) | USA | FFS | 1992–2003 | Claims | LSS | N (2) | Age-gender-race |
| Wilson (1994) | USA | FFS | 1980–1988 | Claims | TKA | N (2) | Age |
| Wright (1999) | Ontario, Canada | FFS | 1984–1990 | Claims | TKA | Y (4) | Age-gender |
FFS = fee-for-service; NHS = National Health System; TJA = total joint arthroplasty; LSS = lumbar spinal surgery; N = no; Y = yes; BMI = body mass index.
* Number of factors included in analysis.
Results
Arthroplasty rates were associated with demographic (D) predictors, age and gender (Table 2). Age followed an inverted U-shaped distribution (peak age 60s–70s). Higher rates were found for female gender.
Table 2.
Demographic predictors of surgical rates
| Predictor | Procedure | Reference | Categories | Effect size | 95% Confidence interval | p | Comment |
|---|---|---|---|---|---|---|---|
| Age | TJA | 18 | 65+ to 51–64 | HR, 2.34 | (1.23–4.29) | ||
| TJA | 19 | 70–79 to 80+ | OR, 1.69 | (1.12–2.56) | |||
| TJA | 27 | 63–68 to 62– | HR, 1.47 | (1.03–2.10) | 0.001 | Inverted U-shaped | |
| TJA | 47 | 65–74 to 60–64 | OR, 1.06 | (0.64–1.76) | 0.81 | Inverted U-shaped | |
| THA | 2 | 65–74 to 75+ | RR, 2.2–2.7 | N/R | N/R | Income quintiles | |
| THA | 17 | 65–84 | r, 0.72 | 0.045 | Inverted U-shaped | ||
| THA | 30 | 70–74 to 50–54 | RR, 6.92 | (6.55–7.31) | 0.001 | Inverted U-shaped | |
| THA | 52 | per year | HR, 1.07 | (1.06–1.09) | 0.001 | ||
| TKA | 13 | 75–79 to 54– | OR, 106.2 | < 0.001 | Inverted U-shaped | ||
| TKA | 17 | 65–84 | r, 0.48 | 0.23 | Inverted U-shaped | ||
| TKA | 25 | 47–64 to 65+ | OR, 0.72 | (0.52–1.01) | |||
| TKA | 30 | 75–79 to 50–54 | RR, 14.95 | (13.99–15.98) | 0.001 | Inverted U-shaped | |
| TKA | 32 | 75–79 to 65–69 | OR, 1.41 | ||||
| TKA | 52 | per year | HR, 1.08 | (1.07–1.10) | 0.001 | ||
| TKA | 61 | %pop > 75y | β, 115.6 | < 0.001 | Linear regression parameter | ||
| LSS | 7 | 70–74 to 80+ | IRR, 2.17 | (1.95–2.42) | < 0.001 | Inverted U-shaped | |
| Gender | TJA | 18 | F to M | HR, 1.16 | (0.88–1.41) | ||
| TJA | 27 | F to M | HR, 1.09 | (0.81–1.45) | 0.57 | ||
| TJA | 47 | F to M | OR, 0.97 | (0.61–1.54) | 0.91 | ||
| THA | 2 | F to M | RR, 1.5–1.6 | N/R | Income quintiles | ||
| THA | 30 | F to M | RR, 1.30 | (1.28–1.33) | |||
| THA | 52 | F to M | HR, 1.06 | (0.88–1.28) | 0.55 | ||
| TKA | 13 | F to M | OR, 1.331 | < 0.001 | |||
| TKA | 25 | F to M | OR, 1.26 | (1.08–1.48) | |||
| TKA | 30 | F to M | RR, 1.10 | (1.08–1.12) | |||
| TKA | 32 | F to M | OR, 1.95 | ||||
| TKA | 46 | F to M | OR, 1.32 | (1.30–1.33) | <0.001 | ||
| TKA | 52 | F to M | HR, 1.08 | (0.90–1.29) | 0.42 | ||
| TKA | 60 | F to M | RR, 1.37 | For whites | |||
| TKA | 60 | F to M | RR, 3.03 | For blacks | |||
| LSS | 7 | F to M | IRR, 0.84 | (0.79–0.89) | < 0.001 |
TJA = total joint arthroplasty; HR, hazard ratio; OR, odds ratio; RR, rate ratio; r = correlation coefficient; β = regression parameter; IRR = incidence rate ratio; N/R = not reported.
Postsecondary education, higher income, obesity, nonminority race/ethnicity, and rural residence (SS) were associated with higher rates of TJA, THA, and TKA (Table 3).
Table 3.
Social support predictors of surgical rates
| Predictor | Procedure | Reference | Categories | Effect size | 95% Confidence Interval | p | Comment |
|---|---|---|---|---|---|---|---|
| Education | TJA | 18 | < 12 years compared with 12 years or more | HR, 0.79 | (0.55–1.02) | ||
| TJA | 27 | Postsecondary | HR, 1.54 | (1.08–2.20) | 0.02 | ||
| TJA | 47 | OR, 1.54 | (1.00–2.38) | 0.048 | |||
| THA | 22 | OR, 1.55 | 0.01 | ||||
| THA | 52 | Postsecondary | HR, 1.37 | (1.33–1.66) | 0.001 | ||
| TKA | 25 | Postsecondary | OR, 1.37 | (1.10–1.72) | |||
| TKA | 52 | Postsecondary | HR, 0.98 | (0.81–1.18) | 0.81 | ||
| LSS | 7 | Postsecondary | IRR, 0.85 | (0.46–1.57) | 0.6 | ||
| Employment | TJA | 27 | HR, 1.09 | (0.56–2.12) | 0.81 | ||
| TJA | 47 | OR, 1.29 | (0.74–2.25) | 0.374 | |||
| THA | 17 | r, 0.49 | 0.215 | ||||
| THA | 29 | Floor layers to white collar | RR, 1.58 | (0.93–2.68) | |||
| TKA | 17 | Manual labor | r, 0.21 | 0.622 | |||
| TKA | 25 | OR, 1.17 | (0.95–1.44) | ||||
| TKA | 29 | Floor layers to white collar | RR, 4.72 | (1.8–12.3) | |||
| Income | TJA/LSS | 53 | lower | N/R | Correlation analysis | ||
| TJA | 18 | Quartiles | HR, 0.58 | (0.40–0.77) | Lower income to higher income | ||
| TJA | 27 | 40 k+ to 20 k− | HR, 0.61 | (0.34–1.10) | 0.021 | ||
| TJA | 47 | Tertiles | OR, 1.24 | (0.76–2.04) | 0.927 | ||
| THA | 2 | Quintiles | RR, 1.15 | (1.05–1.23) | 0.002 | ||
| THA | 22 | OR, 0.78 | NS | ||||
| TKA | 25 | $20,000+ to $5000− | OR, 1.54 | (1.19–1.96) | |||
| TKA | 46 | Median (zip) | OR, 1.19 | (1.17–1.22) | |||
| LSS | 7 | Per $10,000 | IRR, 0.89 | (0.83–0.96) | 0.002 | ||
| Social Needs | THA | 11 | Dichotomous | RR, 0.79 | (0.76–0.81) | N/R | Composite score (unemployment, overcrowding, noncar/home ownership) |
| THA | 17 | Quartiles | r, −0.45 | 0.26 | Quartiles of deprivation | ||
| THA | 30 | Quintiles | RR, 0.94 | (0.90–0.99) | 0.036 | Composite score (income, employment, health, education, skills/training, housing/services, crime, environment) | |
| THA | 36 | Acute needs index | r, −0.17 | 0.17 | Composite measure of need | ||
| THA | 36 | GMS cash limited index | r, −0.12 | 0.33 | Composite measure of need | ||
| TKA | 17 | Quartiles | r, −0.07 | 0.866 | Quartiles of deprivation | ||
| TKA | 30 | Quintiles | RR, 1.05 | (1.00–1.10) | 0.006 | Composite score (income, employment, health, education, skills/training, housing/services, crime, environment) | |
| Obesity | TJA | 18 | HR, 1.67 | (1.35–1.97) | |||
| TJA | 27 | HR, 1.48 | (1.03–2.12) | 0.004 | |||
| TJA | 47 | OR, 1.32 | (0.88–2.00) | 0.184 | |||
| THA | 52 | HR, 1.05 | (1.03–1.07) | 0.001 | |||
| TKA | 25 | OR, 2.61 | (2.15–3.17) | ||||
| TKA | 52 | HR, 1.13 | (1.12–1.15) | 0.001 | |||
| Race/ethnicity | TJA | 18 | Black | HR, 0.4 | (0.19–0.58) | ||
| TJA | 18 | Hispanic | HR, 0.87 | (0.16–2.10) | |||
| TJA | 19 | Non-white | OR, 0.63 | (0.40–1.00) | 0.05 | ||
| TJA | 27 | Non-white | HR, 0.96 | (0.50–1.88) | 0.9 | ||
| TJA | 47 | Black | OR, 0.34 | (0.17–0.66) | 0.002 | ||
| THA | 17 | Ethnicity | r, −0.38 | 0.354 | |||
| THA | 30 | Black | RR, 0.98 | (0.91–1.05) | |||
| THA | 52 | Italy/Greece versus UK/Australia | HR, 0.35 | (0.26–0.47) | 0.001 | Country of birth | |
| TKA | 17 | Ethnicity | r, −0.17 | 0.695 | |||
| TKA | 25 | Nonwhite | OR, 0.73 | (0.59–0.89) | 0.002 | ||
| TKA | 32 | Black | OR, 0.4 | N/R | For men | ||
| TKA | 32 | Black | OR, 0.86 | N/R | For women | ||
| TKA | 46 | Non-white | OR, 0.62 | (0.60–0.63) | < 0.001 | ||
| TKA | 52 | Italy/Greece versus UK/Australia | HR, 0.31 | (0.24–0.40) | 0.001 | Country of birth | |
| TKA | 60 | Black | RR, 0.31 | (0.17–0.28) | For men | ||
| TKA | 60 | Black | RR, 0.69 | (0.66–0.72) | For women | ||
| LSS | 7 | Nonofficial language | IRR, 0.89 | (0.83–0.95) | < 0.001 | ||
| Rural | TJA/LSS | 53 | Higher | N/R | Correlation | ||
| TJA | 27 | HR, 1.01 | (0.79–1.30) | 0.91 | |||
| THA | 17 | r, 0.64 | 0.085 | ||||
| THA | 30 | RR, 1.05 | (1.01–1.10) | 0.008 | |||
| THA | 44 | r, 0.50 | < 0.001 | ||||
| TKA | 17 | r, 0.76 | 0.028 | ||||
| TKA | 30 | RR, 0.99 | (0.95–1.03) | 0.66 | |||
| TKA | 44 | r, 0.46 | 0.001 | ||||
| Social support | TJA | 27 | Lives alone | HR, 0.56 | (0.28–1.12) | 0.079 | |
| TJA | 47 | Married | OR, 1.43 | (0.87–2.34) | 0.155 | ||
| TJA | 47 | Grandchild care | OR, 1.15 | (0.73–1.80) | 0.557 |
TJA = total joint arthroplasty; HR = hazard ratio; OR = odds ratio; RR = rate ratio; IRR = incidence rate ratio; r = correlation coefficient; N/R = not reported; NS = not significant.
HB was considered in a single study of TJA [27]. The willingness of patients to consider surgery was positively associated (hazard ratio [HR], 3.2; 95% confidence interval [CI], 2.46–4.16], p < 0.001) with rates of TJA.
Insurance (PR) was assessed in three studies on TJA [18, 19, 39]. One study [19] found that Medicare coverage only (without supplemental insurance) was associated with lower rates of TJA (odds ratio [OR], 0.45; 95% CI, [0.22–0.90]). A second study [39] found patients without prior insurance had higher rates of TJA (OR, 0.45; 95% CI, [0.22–0.90], p < 0.006) once under Medicare coverage compared with those continuously insured. Another study [18] found having no insurance coverage was not associated with rates of TJA (HR, 0.86; 95% CI, 0.32–1.81]). One Australian study [26] found rates of surgery in the private compared with public system were higher (rate ratio, 1.1 for THA, 1.2 for TKA); however, no statistical inference was provided. One other study on TKA [25] found uninsured patients had lower rates of surgery (OR, 0.61; 95% CI, 0.40–0.92]) compared with those holding private insurance.
CR was assessed with multiple predictors relating to the surgeon, other physicians, the hospital system, and imaging resources (Table 4). Increased surgeon supply was associated with higher rates of THA but not TKA. The propensity of surgeons to recommend surgery (“enthusiasm”) was associated with higher TKA rates. Higher supply of nonsurgeons, more female physicians, and more specialized nonsurgical physicians were all associated with lower rates of arthroplasty.
Table 4.
Community resource predictors of surgical rates
| Predictor | Procedure | Reference | Categories | Effect size | 95% Confidence interval | p | Comment |
|---|---|---|---|---|---|---|---|
| Surgeon supply | TJA/LSS | 53 | higher | N/R | Correlation | ||
| THA | 22 | OR, 1.52 | 0.01 | ||||
| THA | 30 | Quintiles | RR, 1.06 | (1.00–1.12) | 0.07 | ||
| THA | 44 | r, 0.06 | 0.7 | ||||
| TKA | 14 | r, −0.16 | 0.26 | ||||
| TKA | 30 | Quintiles | RR, 1.05 | (0.99–1.11) | 0.023 | ||
| TKA | 44 | r, 0.09 | 0.1 | ||||
| LSS | 7 | IRR, 0.98 | (0.96–1.01) | 0.2 | |||
| LSS | 54 | R-sq, 0.02 | |||||
| Surgeon Volume | LSS | 8 | High volume | OR, 2.9 | (2.5–3.2) | < 0.001 | Fusions to decompressions |
| Surgeon specialty | LSS | 8 | Orthopaedists | OR, 12.46 | (10.6–14.6) | < 0.001 | Fusions to decompressions |
| LSS | 54 | Orthopaedists | R-sq, 0.03 | ||||
| Surgeon attitudes | TKA | 61 | Enthusiasm | β, 6.7 | < 0.001 | Propensity of surgeons to operate | |
| TKA | 61 | Outcome perception | β, 0.14 | 0.08 | Perception of treatment outcomes | ||
| LSS | 7 | Enthusiasm | IRR, 1.26 | (1.05–1.51) | 0.013 | Propensity of surgeons to recommend surgery | |
| MD supply | THA | 22 | MD supply | OR, 0.68 | N/R | ||
| THA | 30 | Anes supply | RR, 0.9 | (0.86–0.95) | 0.001 | Highest to lowest quintile | |
| THA | 30 | MD supply | RR, 0.79 | (0.73–0.85) | 0.001 | Highest to lowest quintile | |
| THA | 37 | Anes supply | none | ||||
| TKA | 14 | PMR supply | r, −0.42 | 0.002 | |||
| TKA | 14 | Rheum supply | r, −0.25 | 0.08 | |||
| TKA | 14 | PCP supply | r, −0.10 | 0.47 | |||
| TKA | 30 | Anes supply | RR, 0.92 | (0.87–0.96) | 0.001 | Highest to lowest quintile | |
| TKA | 30 | MD supply | RR, 0.91 | (0.86–0.97) | 0.001 | Highest to lowest quintile | |
| LSS | 7 | PCP supply | IRR, 1.08 | (0.99–1.18) | 0.08 | ||
| MD factors | THA | 36 | PCP Trainers (%) | r, −0.24 | 0.05 | ||
| THA | 36 | Child Health GPs (%) | r, −0.36 | 0.003 | |||
| TKA | 61 | Female (%) | β, −6.5 | 0.02 | Linear regression parameter estimate | ||
| TKA | 61 | NA-trained (%) | β, −4.1 | 0.002 | Linear regression parameter estimate | ||
| LSS | 7 | Enthusiasm | IRR, 1.14 | (0.96–1.34) | 0.13 | Propensity of referring MDs to refer for surgery | |
| Hospital factors | THA | 17 | Hospital supply | r, −0.66 | 0.073 | Number of centers offering TJA | |
| THA | 22 | Hospital volume | OR, 2.54 | 0.01 | |||
| THA | 30 | Bed occupancy | RR, 0.98 | (0.93–1.03) | 0.12 | Highest to lowest quintile | |
| THA | 30 | Hosp Volume | RR, 1.11 | (1.05–1.18) | 0.005 | Highest to lowest quintile | |
| TKA | 17 | Hospital supply | r, −0.80 | 0.017 | Number of centers offering TJA | ||
| TKA | 30 | Bed occupancy | RR, 1.06 | (1.00–1.12) | 0.33 | Highest to lowest quintile | |
| TKA | 30 | Hosp Volume | RR, 1.09 | (1.03–1.16) | 0.001 | Highest to lowest quintile | |
| Hospital type | THA | 22 | Government Hosp | OR, 0.90 | N/R | ||
| THA | 22 | Insurance | OR, 2.46 | 0.01 | Private Insurance charges (%) | ||
| THA | 22 | Teaching | OR, 0.85 | N/R | |||
| THA | 30 | Teaching | RR, 0.97 | (0.93–1.01) | |||
| TKA | 30 | Teaching | RR, 0.9 | (0.86–0.95) | |||
| TKA | 61 | Teaching beds (%) | β, 1.2 | 0.04 | Linear regression parameter estimate | ||
| OR supply | THA | 30 | Day-case OR supply | RR, 0.93 | (0.88–0.99) | 0.12 | Highest to lowest quintile |
| THA | 30 | OR supply | RR, 1.11 | (1.03–1.20) | 0.005 | Highest to lowest quintile | |
| TKA | 30 | Day-case OR supply | RR, 1.11 | (1.04–1.18) | 0.011 | Highest to lowest quintile | |
| TKA | 30 | OR supply | RR, 0.96 | (0.91–1.01) | 0.018 | Highest to lowest quintile | |
| Medical costs | THA | 22 | MD Fees | OR, 0.10 | 0.05 | ||
| THA | 37 | Care expenses | increase | 0.001 | Need-adjusted expenses of specialized care | ||
| Imaging | LSS | 7 | MRI Scanners | IRR, 1.3 | (1.09–1.57) | 0.004 | |
| LSS | 35 | CT/MRI rates | R2, 0.22 | r, 0.47 | 0.001 |
TJA = total joint arthroplasty; LSS = lumbar spine surgery; HR = hazard ratio; OR = odds ratio; RR = rate ratio; r = correlation coefficient; β = regression parameter; IRR = incidence rate ratio; N/R = not reported; MDs = referring physicians, ORs = operating rooms; Anes = anesthesiologists; PMR = physical medicine and rehabilitation; Rheum = rheumatologists; PCP = primary care physicians; NA-trained = North-American-trained.
A history of degenerative osteoarthritis (OA) and the presence of physical limitations (MN) were both associated with higher TJA rates (Table 5). Higher rates of THA were seen with higher prevalence of OA, whereas physical limitations were related to higher TKA rates.
Table 5.
Medical need predictors of surgical rates
| Predictor | Procedure | Reference | Categories | Effect size | 95% Confidence interval | p | Comment |
|---|---|---|---|---|---|---|---|
| Degenerative | TJA | 18 | Previous OA | HR, 6.03 | (4.29–9.26) | ||
| OA | TJA | 19 | History of OA | OR, 9.0 | (5.41–15.0) | ||
| TJA | 19 | Previous TJA | OR, 12.6 | (9.07–17.5) | |||
| TJA | 27 | OA | HR, 1.6 | (1.25–2.05) | 0.001 | ||
| TJA | 47 | OA | OR, 2.18 | (0.52–9.15) | 0.29 | ||
| THA | 37 | THA (OA)/THA(other) | Increase | 0.001 | |||
| TKA | 13 | Previous OA | OR, 0.93 | 0.35 | |||
| LSS | 7 | Back pain | IRR, 0.81 | (0.55–1.17) | 0.26 | ||
| Disability | TJA | 18 | OA-related limitation | HR, 2.36 | (2.02–2.79) | ||
| TJA | 19 | ADL limitations | OR, 3.32 | (2.26–4.86) | |||
| TJA | 19 | physical limitations | OR, 2.02 | (1.40–2.92) | |||
| TJA | 27 | SF-36 score 67+ to 25− | HR, 1.44 | (0.99–2.10) | 0.004 | ||
| TJA | 27 | WOMAC score 54 + to 27− | HR, 2.17 | (1.49–3.16) | 0.001 | ||
| TJA | 47 | Diff walking | OR, 1.37 | (0.91–2.07) | 0.13 | ||
| THA | 37 | Permanent disability | Decrease | 0.001 | |||
| TKA | 25 | Physical limitations | OR, 3.05 | (2.51–3.69) | Stooping or crouching |
TJA = total joint arthroplasty; LSS = lumbar spine surgery; OA = osteoarthritis; ADL = activities of daily living; HR = hazard ratio; OR = odds ratio; IRR = incidence rate ratio.
Rates of LSS from one study [7] were associated with male gender and older age (Table 2). Age followed an inverted U-shaped distribution (highest age 60s–70s).
Lower income and more prevalent knowledge of official languages (SS) were associated with higher rates of LSS (Table 3).
HB was evaluated in a single study of LSS [7]. Those authors found no association between surgical rates and the propensity of patients to consider surgery (incidence rate ratio, 1.04; 95% CI, 0.95–1.13], p < 0.4).
One study [26] found LSS rates in the private system (PR) were four times higher than that in the public; however, no statistical inference was provided.
The propensity of surgeons to recommend surgery (“enthusiasm”) but not the surgeon supply (CR) was associated with higher LSS rates (Table 4). Orthopaedic surgeons compared with neurosurgeons and higher over lower volume surgeons were more likely to perform fusions over decompressions. Availability of MRI scanners and higher rates of CT/MRI imaging were also associated with higher LSS rates.
Prevalence of back pain (MN) was not associated with higher rates of LSS (Table 5).
Discussion
Increasing surgical rates and high variation in hip, knee, and spine surgery have been implicated in the escalating costs of health care. Driving factors behind these rates have not been well understood. We aimed to identify the main factors (D, SS, HB, PR, CR, and MN) that drive rates of TJA and LSS.
We recognize limitations to the literature and our study. First, studies that consider the factors influencing surgical rates must come from observational population-based cohorts. Our systematic review was limited to observational studies in which patients were included on population-based criteria like registries, administrative claims data, or national surveys rather than observational clinical trials in which patients are enrolled if they seek care. Second, like all systematic reviews, the quality of the review is limited by the quality of the individual studies and the search. Third, while we used a comprehensive search strategy, we searched only PubMed. A different strategy with other databases may have yielded a different search. Fourth, administrative population-based studies are disadvantaged by their data, usually designed for reimbursement and not for research purposes. However, they are strengthened by their large patient numbers and remain the most practical source for studying relationships that cannot occur at a direct patient level (eg, surgeon/hospital supply). Additionally, these ecologic relationships (in which data are aggregated over groups rather than used at an individual level) studied here do not link an individual patient’s chance of getting surgery with some individual factor; rather, they suggest a relationship at a population or regional level. Finally, data obtained from nationwide surveys may not be representative of the population as a whole. However, the relationships from survey data can be analyzed at the level of the individual patient. Experimental health services research such as randomizing hospitals could, in theory, better evaluate these relationships. However, it would be impossible to conduct for many factors (eg, surgeon enthusiasm, surgeon volume). This study design remains the best source of information from which to draw conclusions.
From this study, we found age was related to rates of TJA and LSS and followed an inverted U-shaped distribution. Like most degenerative diseases, prevalence increases with age, but higher comorbidity limits the surgical options for older patients. Females had higher rates of TJA, whereas males had higher rates of LSS. Females have a higher prevalence of arthritis as well as back pain [4, 48]. Despite more back pain in females, males accounted for 56% of healthcare visits [4].
Arthroplasty rates were higher for patients with higher education and income, nonminority race/ethnicity, obesity, and rural residence (SS). For LSS, lower income and nonminority were both associated with higher surgical rates. Disparities are now being reported with increasing frequency in musculoskeletal health and other disciplines of health care [24, 43]. Although there may certainly be altered disease prevalence based on environmental factors and cultural acceptance of limitations associated with the impairments from degenerative musculoskeletal conditions among ethnic minorities, culturally competent care and increasing the diversity among providers may be strategies to reduce this potential access disparity.
Patient willingness to consider surgery (HB) was associated with higher rates of TKA but not LSS. Surgery for degenerative conditions of the hip, knee, and spine is an example of preference-sensitive care [59]. Wennberg described three categories of care, namely, effective, preference-sensitive, and supply-sensitive. Effective care includes treatments that are supported by strong evidence (ie, beta-blockers for myocardial infarction). Supply-sensitive care is delivered according to the resources available (ie, nonpalliative treatments at end-of-life care) [21]. Preference-sensitive includes the choice of different treatments, each with different risks and benefits. Because THA, TKA, and LSS are indicated to diminish pain and improve quality of life at the discretion of the patient, these procedures fall into this category. In other words, similar disease severity (from the surgeon’s perspective) may be interpreted differently by patients based on their values, beliefs, and assessment of risk and benefit. Therefore, there exists no “true rate” and regional variation is to be expected to some extent. The main driver of this type of care lies with the patient and informed decision-making is the solution. Shared decision-making can play a large role in better informing patients about their condition and expectations after treatment; however, their direct relationship to surgical rates is less clear [1, 16].
Private/supplemental insurance (PR) was associated with higher surgical rates. In fee-for-service health systems, insurance coverage for discretionary procedures plays a critical role. With a renewed interest in universal coverage in the United States, it is yet unclear how this will affect overall surgical rates.
Surgeon supply was associated with increased TJA rates (CR). For TJA and LSS, surgeon “enthusiasm” was related to increased surgical rates. The influence of provider enthusiasm was first hypothesized by Chassin [10]. Conventional wisdom, at that time, suggested inappropriateness and clinical uncertainty were the main influences on regional variation. Chassin showed that geographic differences in use of health services may be caused by differences in the “enthusiasm” of physicians for particular services. Higher supply and specialization of nonsurgeons was associated with lower arthroplasty rates but not LSS. LSS was increased with higher rates and availability of advanced imaging. MRI use illustrates the problem of Granger causality, which exists when two associated factors are driven by a third process [23]. For example, economic growth or expansion may result in both an increase in medical resources and use simultaneously that may erroneously suggest that an increase in resources causes an increase in use [12]. Thus, the relationships between diagnostic and surgical use may be misleading.
Prevalence of OA or physical limitations (MN) was associated with higher rates of arthroplasty; however, back pain was not related to rates of LSS. The selection of a patient with a degenerative spinal disorder who would benefit from surgery is less straightforward than a patient with hip or knee OA. This diagnostic challenge may account for the lack of association with disease prevalence.
This systematic review identified a variety of factors that influence differential use of surgery for degenerative conditions of the hip, knee, and spine beyond. Potentially modifiable factors, from a policy perspective, include surgeon enthusiasm, patient health beliefs, resource allocation, and insurance coverage.
Regional variation in these procedures exists because they are highly sensitive to surgeon enthusiasm and preferences of patients informed by their social structure and medical need [58]. With strategies that may affect change in factors that are potentially modifiable by behavior or resources, extreme variation in rates may be reduced.
Acknowledgments
We thank Linda Murphy and Bob Johnson from the Grunigen Medical Library at the UC Irvine Medical Center for assistance with our bibliographic search.
Appendix 1. Search strategy
Performed September 16, 2010
(Physician’s Practice Patterns/utilization [mh] OR Health Services Research [mh] OR Health Services Accessibility [mh] OR Health Services Needs and Demand [mh] OR Hospitalization [mh] OR Residence Characteristics [mh] OR Socioeconomic Factors [mh] OR Databases, Factual [mh] OR Population Surveillance [mh] OR Small-Area Analysis [mh] OR Health Status Disparities [mh] OR Population Surveillance[mh] OR registries [mh] OR Medicare [mh] OR Medicaid [mh]) OR “population-based” OR “population based” OR register OR “procedure volume” OR “surgical rate*” OR “population based” OR “population-based” OR “Medicare” OR “administrative database” OR “claims database” OR “area variation” OR “geographic variation” OR “regional variation” OR “small area analysis” OR “procedure volume” OR “frequency of use”
[742359 references]
AND
Arthroplasty, Replacement[mh] OR Hip Prosthesis[mh] OR Spinal fusion[mh] OR Lumbar Vertebrae/surgery[mh] OR Spinal diseases/surgery[mh] OR joint prosthesis[mh]) OR “hip replacement” OR “knee replacement” OR “spine surgery” OR ((“low back” OR “spine” OR “hip” OR “knee”) and “degenerative” and (“surgery” or “surgical”)) OR “spinal fusion” OR “arthroplasty” OR “pedicle screw” OR “pedicle screws”
[86761 references]
AND
(“determinants” OR “influence” OR “driven” OR “drivers” OR “explanation” OR “explained” OR “correlated”)
[1232560 references]
COMBINE 1 and 2 and 3
[283 references]
LIMIT: English
[256 references]
Appendix 2




Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Adam JA, Khaw FM, Thomson RG, Gregg PJ, Llewellyn-Thomas HA. Patient decision aids in joint replacement surgery: a literature review and an opinion survey of consultant orthopaedic surgeons. Ann R Coll Surg Engl. 2008;90:198–207. doi: 10.1308/003588408X285748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agabiti N, Picciotto S, Cesaroni G, Bisanti L, Forastiere F, Onorati R, Pacelli B, Pandolfi P, Russo A, Spadea T, Perucci CA, Italian Study Group on Inequalities in Health Care The influence of socioeconomic status on utilization and outcomes of elective total hip replacement: a multicity population-based longitudinal study. Int J Qual Health Care. 2007;19:37–44. doi: 10.1093/intqhc/mzl065. [DOI] [PubMed] [Google Scholar]
- 3.The Burden of Musculoskeletal Diseases in the United States: Prevalence, Societal and Economic Cost. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008. [Google Scholar]
- 4.United States Bone and Joint Decade: The Burden of Musculoskeletal Diseases in the United States. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2008. [Google Scholar]
- 5.Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behavior. 1995;36:1–10. doi: 10.2307/2137284. [DOI] [PubMed] [Google Scholar]
- 6.Appleby J, Raleigh V, Frosini F, Bevan G, Gao H, Lyscom T. Variations in Health Care: The Good, the Bad and the Inexplicable. London: The King’s Fund; 2011. [Google Scholar]
- 7.Bederman SS, Coyte PC, Kreder HJ, Mahomed NN, McIsaac WJ, Wright JG. Who’s in the driver’s seat? The influence of patient and physician enthusiasm on regional variation in degenerative lumbar spinal surgery: a population-based study. Spine (Phila Pa 1976) 2011;36:481–489. doi: 10.1097/BRS.0b013e3181d25e6f. [DOI] [PubMed] [Google Scholar]
- 8.Bederman SS, Kreder HJ, Weller I, Finkelstein JA, Ford MH, Yee AJ. The who, what and when of surgery for the degenerative lumbar spine: a population-based study of surgeon factors, surgical procedures, recent trends and reoperation rates. Can J Surg. 2009;52:283–290. [PMC free article] [PubMed] [Google Scholar]
- 9.Berry DJ, Harmsen WS, Cabanela ME, Morrey BF. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am. 2002;84:171–177. doi: 10.2106/00004623-200202000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Chassin MR. Explaining geographic variations: the enthusiasm hypothesis. Med Care. 1993;31:YS37–YS44. doi: 10.1097/00005650-199305001-00006. [DOI] [PubMed] [Google Scholar]
- 11.Cookson R, Dusheiko M, Hardman G. Socioeconomic inequality in small area use of elective total hip replacement in the English National Health Service in 1991 and 2001. J Health Serv Res Policy. 2007;12(Suppl 1):510–517. doi: 10.1258/135581907780318365. [DOI] [PubMed] [Google Scholar]
- 12.Cooper RA, Getzen TE, Laud P. Economic expansion is a major determinant of physician supply and utilization. Health Serv Res. 2003;38:675–696. doi: 10.1111/1475-6773.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyte P, Wang PP, Hawker G, Wright JG. The relationship between variations in knee replacement utilization rates and the reported prevalence of arthritis in Ontario, Canada. J Rheumatol. 1997;24:2403–2412. [PubMed] [Google Scholar]
- 14.Coyte PC, Hawker G, Wright JG. Variations in knee replacement utilization rates and the supply of health professionals in Ontario, Canada. J Rheumatol. 1996;23:1214–1220. [PubMed] [Google Scholar]
- 15.D’Antonio JA, Capello WN, Manley MT, Geesink R. Hydroxyapatite femoral stems for total hip arthroplasty: 10- to 13-year followup. Clin Orthop Relat Res. 2001;393:101–111. doi: 10.1097/00003086-200112000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Deyo RA, Cherkin DC, Weinstein J, Howe J, Ciol M, Mulley AG., Jr Involving patients in clinical decisions: impact of an interactive video program on use of back surgery. Med Care. 2000;38:959–969. doi: 10.1097/00005650-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Dixon T, Shaw ME, Dieppe PA. Analysis of regional variation in hip and knee joint replacement rates in England using Hospital Episodes Statistics. Public Health. 2006;120:83–90. doi: 10.1016/j.puhe.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 18.Dunlop DD, Manheim LM, Song J, Sohn MW, Feinglass JM, Chang HJ, Chang RW. Age and racial/ethnic disparities in arthritis-related hip and knee surgeries. Med Care. 2008;46:200–208. doi: 10.1097/MLR.0b013e31815cecd8. [DOI] [PubMed] [Google Scholar]
- 19.Dunlop DD, Song J, Manheim LM, Chang RW. Racial disparities in joint replacement use among older adults. Med Care. 2003;41:288–298. doi: 10.1097/01.MLR.0000044908.25275.E1. [DOI] [PubMed] [Google Scholar]
- 20.Engh CA, Jr, Claus AM, Hopper RH, Jr, Engh CA. Long-term results using the anatomic medullary locking hip prosthesis. Clin Orthop Relat Res. 2001;393:137–146. doi: 10.1097/00003086-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 21.Fisher ES, Wennberg JE. Health care quality, geographic variations, and the challenge of supply-sensitive care. Perspect Biol Med. 2003;46:69–79. doi: 10.1353/pbm.2003.0004. [DOI] [PubMed] [Google Scholar]
- 22.Friedman B, Elixhauser A. The changing distribution of a major surgical procedure across hospitals: were supply shifts and disequilibrium important? Health Econ. 1995;4:301–314. doi: 10.1002/hec.4730040406. [DOI] [PubMed] [Google Scholar]
- 23.Granger CWJ. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37:424–438. doi: 10.2307/1912791. [DOI] [Google Scholar]
- 24.Groman R, Ginsberg J, American College of Physicians Racial and ethnic disparities in health care: a position paper of the American College of Physicians. Ann Intern Med. 2004;141:226–232. doi: 10.7326/0003-4819-141-3-200408030-00015. [DOI] [PubMed] [Google Scholar]
- 25.Hanchate AD, Zhang Y, Felson DT, Ash AS. Exploring the determinants of racial and ethnic disparities in total knee arthroplasty: health insurance, income, and assets. Med Care. 2008;46:481–488. doi: 10.1097/MLR.0b013e3181621e9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris IA, Dao AT. Trends of spinal fusion surgery in Australia: 1997 to 2006. ANZ J Surg. 2009;79:783–788. doi: 10.1111/j.1445-2197.2009.05095.x. [DOI] [PubMed] [Google Scholar]
- 27.Hawker GA, Guan J, Croxford R, Coyte PC, Glazier RH, Harvey BJ, Wright JG, Williams JI, Badley EM. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54:3212–3220. doi: 10.1002/art.22146. [DOI] [PubMed] [Google Scholar]
- 28.Hawker GA, Wright JG, Glazier RH, Coyte PC, Harvey B, Williams JI, Badley EM. The effect of education and income on need and willingness to undergo total joint arthroplasty. Arthritis Rheum. 2002;46:3331–3339. doi: 10.1002/art.10682. [DOI] [PubMed] [Google Scholar]
- 29.Jarvholm B, From C, Lewold S, Malchau H, Vingard E. Incidence of surgically treated osteoarthritis in the hip and knee in male construction workers. Occup Environ Med. 2008;65:275–278. doi: 10.1136/oem.2007.033365. [DOI] [PubMed] [Google Scholar]
- 30.Judge A, Welton NJ, Sandhu J, Ben-Shlomo Y. Geographical variation in the provision of elective primary hip and knee replacement: the role of socio-demographic, hospital and distance variables. J Public Health (Oxf) 2009;31:413–422. doi: 10.1093/pubmed/fdp061. [DOI] [PubMed] [Google Scholar]
- 31.Judge A, Welton NJ, Sandhu J, Ben-Shlomo Y. Equity in access to total joint replacement of the hip and knee in England: cross sectional study. BMJ. 2010;341:c4092. doi: 10.1136/bmj.c4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Katz BP, Freund DA, Heck DA, Dittus RS, Paul JE, Wright J, Coyte P, Holleman E, Hawker G. Demographic variation in the rate of knee replacement: a multi-year analysis. Health Serv Res. 1996;31:125–140. [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly MA, Clarke HD. Long-term results of posterior cruciate-substituting total knee arthroplasty. Clin Orthop Relat Res. 2002;404:51–57. doi: 10.1097/00003086-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 35.Lurie JD, Birkmeyer NJ, Weinstein JN. Rates of advanced spinal imaging and spine surgery. Spine (Phila Pa 1976) 2003;28:616–620. doi: 10.1097/01.BRS.0000049927.37696.DC. [DOI] [PubMed] [Google Scholar]
- 36.Majeed A, Eliahoo J, Bardsley M, Morgan D, Bindman AB. Variation in coronary artery bypass grafting, angioplasty, cataract surgery, and hip replacement rates among primary care groups in London: association with population and practice characteristics. J Public Health Med. 2002;24:21–26. doi: 10.1093/pubmed/24.1.21. [DOI] [PubMed] [Google Scholar]
- 37.Makela KT, Peltola M, Hakkinen U, Remes V. Geographical variation in incidence of primary total hip arthroplasty: a population-based analysis of 34, 642 replacements. Arch Orthop Trauma Surg. 2010;130:633–639. doi: 10.1007/s00402-009-0919-4. [DOI] [PubMed] [Google Scholar]
- 38.Maloney WJ, Schmalzried T, Harris WH. Analysis of long-term cemented total hip arthroplasty retrievals. Clin Orthop Relat Res. 2002;405:70–78. doi: 10.1097/00003086-200212000-00009. [DOI] [PubMed] [Google Scholar]
- 39.McWilliams JM, Meara E, Zaslavsky AM, Ayanian JZ. Medicare spending for previously uninsured adults. Ann Intern Med. 2009;151:757–766. doi: 10.7326/0003-4819-151-11-200912010-00149. [DOI] [PubMed] [Google Scholar]
- 40.Mendenhall S. 2010 spinal industry update. Orthopedic Network News. 2010;21:3–6. [Google Scholar]
- 41.Mendenhall S. 2011 CMS payment update. Orthopedic Network News. 2010;21:22–23. [Google Scholar]
- 42.Naylor CD. Variations in selected surgical procedures and medical diagnoses by year and region. In: Goel V, Williams JI, Anderson GM, Blackstein-Hirsch P, Fooks C, Naylor D, editors. Patterns of Health Care in Ontario. The ICES Practice Atlas. Ottawa: Canadian Medical Association; 1996. pp. 51–146. [Google Scholar]
- 43.Nelson CL. Disparities in orthopaedic surgical intervention. J Am Acad Orthop Surg. 2007;15(Suppl 1):S13–S17. doi: 10.5435/00124635-200700001-00005. [DOI] [PubMed] [Google Scholar]
- 44.Peterson MG, Hollenberg JP, Szatrowski TP, Johanson NA, Mancuso CA, Charlson ME. Geographic variations in the rates of elective total hip and knee arthroplasties among Medicare beneficiaries in the United States. J Bone Joint Surg Am. 1992;74:1530–1539. [PubMed] [Google Scholar]
- 45.Ritter MA, Herbst SA, Keating EM, Faris PM, Meding JB. Long-term survival analysis of a posterior cruciate-retaining total condylar total knee arthroplasty. Clin Orthop Relat Res. 1994;309:136–145. [PubMed] [Google Scholar]
- 46.Skinner J, Zhou W, Weinstein J. The influence of income and race on total knee arthroplasty in the United States. J Bone Joint Surg Am. 2006;88:2159–2166. doi: 10.2106/JBJS.E.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Steel N, Clark A, Lang IA, Wallace RB, Melzer D. Racial disparities in receipt of hip and knee joint replacements are not explained by need: the Health and Retirement Study 1998–2004. J Gerontol A Biol Sci Med Sci. 2008;63:629–634. doi: 10.1093/gerona/63.6.629. [DOI] [PubMed] [Google Scholar]
- 48.Theis KA, Helmick CG, Hootman JM. Arthritis burden and impact are greater among US women than men: intervention opportunities. J Womens Health (Larchmt) 2007;16:441–453. doi: 10.1089/jwh.2007.371. [DOI] [PubMed] [Google Scholar]
- 49.Vessely MB, Whaley AL, Harmsen WS, Schleck CD, Berry DJ. The Chitranjan Ranawat Award: Long-term survivorship and failure modes of 1000 cemented condylar total knee arthroplasties. Clin Orthop Relat Res. 2006;452:28–34. doi: 10.1097/01.blo.0000229356.81749.11. [DOI] [PubMed] [Google Scholar]
- 50.Vince KG, Insall JN, Kelly MA. The total condylar prosthesis. 10- to 12-year results of a cemented knee replacement. J Bone Joint Surg Br. 1989;71:793–797. doi: 10.1302/0301-620X.71B5.2584249. [DOI] [PubMed] [Google Scholar]
- 51.Volinn E, Mayer J, Diehr P, Koevering D, Connell FA, Loeser JD. Small area analysis of surgery for low-back pain. Spine. 1992;17:575–581. doi: 10.1097/00007632-199205000-00017. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y, Simpson JA, Wluka AE, Urquhart DM, English DR, Giles GG, Graves S, Cicuttini FM. Reduced rates of primary joint replacement for osteoarthritis in Italian and Greek migrants to Australia: the Melbourne Collaborative Cohort Study. Arthritis Res Ther. 2009;11:R86. doi: 10.1186/ar2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinstein JN, Bronner KK, Morgan TS, Wennberg JE. Trends and geographic variations in major surgery for degenerative diseases of the hip, knee, and spine. Health Aff (Millwood). 2004;Suppl Web Exclusives:VAR81–VAR89. [DOI] [PubMed]
- 54.Weinstein JN, Lurie JD, Olson PR, Bronner KK, Fisher ES. United States’ trends and regional variations in lumbar spine surgery: 1992–2003. Spine (Phila Pa 1976) 2006;31:2707–2714. doi: 10.1097/01.brs.0000248132.15231.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weinstein JN, Lurie JD, Tosteson TD, Hanscom B, Tosteson AN, Blood EA, Birkmeyer NJ, Hilibrand AS, Herkowitz H, Cammisa FP, Albert TJ, Emery SE, Lenke LG, Abdu WA, Longley M, Errico TJ, Hu SS. Surgical versus nonsurgical treatment for lumbar degenerative spondylolisthesis. N Engl J Med. 2007;356:2257–2270. doi: 10.1056/NEJMoa070302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weinstein JN, Tosteson TD, Lurie JD, Tosteson AN, Blood E, Hanscom B, Herkowitz H, Cammisa F, Albert T, Boden SD, Hilibrand A, Goldberg H, Berven S, An H, SPORT Investigators Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358:794–810. doi: 10.1056/NEJMoa0707136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wennberg JE, Gittelsohn A. Health care delivery in Maine I: patterns of use of common surgical procedures. J Maine Med Assoc. 1975;66:123–130. [PubMed] [Google Scholar]
- 58.Wennberg JE, Gittelsohn A. Variations in medical care among small areas. Sci Am. 1982;246:120–134. doi: 10.1038/scientificamerican0482-120. [DOI] [PubMed] [Google Scholar]
- 59.Wennberg JE, Peters PG Jr. Unwarranted variations in the quality of health care: can the law help medicine provide a remedy/remedies? Spec Law Dig Health Care Law. 2004:9–25. [PubMed]
- 60.Wilson MG, May DS, Kelly JJ. Racial differences in the use of total knee arthroplasty for osteoarthritis among older Americans. Ethn Dis. 1994;4:57–67. [PubMed] [Google Scholar]
- 61.Wright JG, Hawker GA, Bombardier C, Croxford R, Dittus RS, Freund DA, Coyte PC. Physician enthusiasm as an explanation for area variation in the utilization of knee replacement surgery. Med Care. 1999;37:946–956. doi: 10.1097/00005650-199909000-00010. [DOI] [PubMed] [Google Scholar]