Abstract
Background
Several reports have confirmed the ability of intraoperative periarticular injections to control pain after THA. However, these studies used differing combinations of analgesic agents and the contribution of each, including the local anesthetic agent, is uncertain. Understanding the independent effects of the various agents could assist in improved pain management after surgery.
Questions/purposes
We therefore determined the ability of intraoperative periarticular infiltration of levobupivacaine to (1) reduce postoperative pain, (2) reduce postoperative morphine requirements, and (3) reduce the incidence of nausea and urinary retention.
Patients and Methods
A double-blinded, randomized, placebo-controlled trial of patients undergoing primary THAs was performed. Patients were randomized to receive a periarticular infiltration of 150 mg levobupivacaine in 60 mL 0.9% saline (n = 45) or a placebo consisting of 60 mL 0.9% saline (n = 46). We obtained a short-form McGill pain score, visual analog scale (VAS), and morphine requirements via patient-controlled analgesia (PCA) as primary measures. Postoperative antiemetic requirements and need for catheterization for urinary retention were determined as secondary measures.
Results
Subjectively reported pain scores and the overall intensity scores were similar for both groups in the postoperative period. At the same time the mean morphine consumption was less in the levobupivacaine group, most notable in the first 12 hours after surgery: treatment group 11.5 mg vs control group 21.2 mg. We observed no differences in the frequency of postoperative nausea and vomiting or urinary retention.
Conclusions
Our observations suggest periarticular injection of levobupivacaine can supplement available postoperative analgesic techniques and reduce postoperative morphine requirements after THA.
Level of Evidence
Level I, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
THA is a major surgical procedure that can result in pain with moderate to severe intensity, consequently the use of opioid analgesia is common during the postoperative period [14]. Anesthesia should provide stable intraoperative conditions allowing patients to mobilize as rapidly and effectively as possible after surgery [11]. However, opioids have substantial side effects, including respiratory depression, hypotension, decreased gastrointestinal mobility, urinary retention, nausea, and vomiting [7, 9]. Improving patients’ analgesic control by intraoperative methods, such as peripheral nerve blocks [21], epidurals [5], and periarticular injections [4, 20] can reduce postoperative opioid requirements and thus susceptibility to the known side effects. Several studies have assessed the efficacy of an intraoperative periarticular injection during THA using multiple analgesic agents [4, 15, 20]. These multimodal techniques typically combine a local periarticular infiltration of local anesthetic, adrenaline, and an opioid to facilitate rehabilitation after hip arthroplasty.
Levobupivacaine is a long-acting local anesthetic in common use, and as a single enantiomer of bupivacaine (rather that a racemic mixture), it has a relatively low risk of cardiac and central nervous system side effects [6, 12]. However, as all previous studies used combinations of agents, it is unclear whether isolated use of periarticular infiltration of a local anesthetic agent at the time of surgery provides pain relief or reduces the frequency of complications.
We therefore determined the ability of intraoperative periarticular infiltration of levobupivacaine to (1) reduce postoperative pain, (2) reduce postoperative morphine requirements, and (3) reduce the incidence of nausea and urinary retention that classically are associated with opiate use.
Patients and Methods
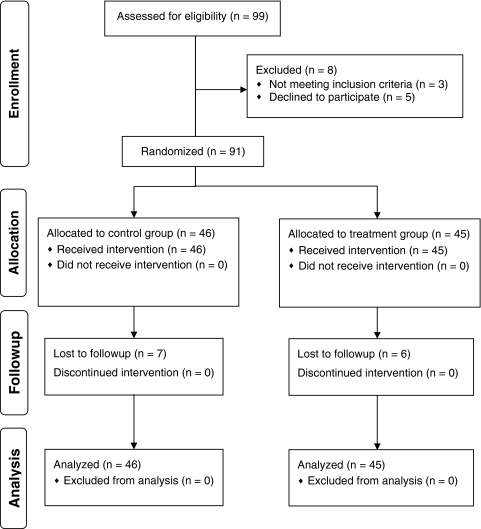
This study was a double-blinded, randomized, controlled trial of patients undergoing primary hip arthroplasty for osteoarthritis conducted between February 2009 and February 2010. Ninety-nine patients were assessed for eligibility, 8 of which were excluded. Patients with cognitive impairment, neurologic disorders, advanced liver or renal impairment, known ischemic heart disease, previous diagnosis of a chronic pain syndrome, opiate dependence, or any postoperative surgical or medical complications were excluded. All remaining 91 patients during this period were invited to participate. Local ethical board approval was obtained and the trial registered at ClinicalTrials.gov (NCT01106001).
Preliminary data included 48 patients with 23 in the treatment group and 25 in the control group. A post hoc power analysis based on the VAS results indicated the need to include at least 42 patients in each study group to achieve 80% power; the minimally detectable change for VAS was 1.3 [1], standard deviation (σ) was 2.1, and alpha (α) was 0.05. Accounting for dropouts, an additional 50 patients were assessed for eligibility (Fig. 1). Five patients refused to participate, one had Parkinson’s disease, and another patient was excluded postoperatively owing to acute pulmonary complications which resolved but precluded accurate compliance with the patient-derived outcome measures.
Fig. 1.
The CONSORT flow diagram is shown.
The demographics and preoperative pain scores according to the WOMAC index scores were similar in both groups (Table 1).
Table 1.
Patient demographics and preoperative WOMAC scores for the control and treatment groups
| Demographic | Control | Treatment | p Value |
|---|---|---|---|
| Number | 46 | 45 | |
| Average age (SD) | 54.3 (15.4) | 57.4 (11.03) | 0.423* |
| Average weight (kg) | 83.8 (14.6) | 79.1(15.1) | 0.104* |
| Gender (M:F) | 28:17 | 23:22 | 0.25+ |
| Average preoperative WOMAC score (SD) | 16.9 (8.9) | 18.7 (9.32) | 0.66※ |
* Mann-Whitney U test; +Chi square test; ※Student’s t-test.
After providing informed consent, patients were randomized by sealed opaque envelope into two groups by the research coordinator (DB). To minimize confounding factors the surgeries were performed by the senior author (KJM) and a posterior approach was used in all cases. Temazepam (10 mg by mouth) was given before the patient was taken to the operating room. Preoperative analgesia consisted of intravenous paracetamol (1 g) and per rectum diclofenac (100 mg) at induction. All patients received a spinal anesthetic using 15 mg bupivacaine and no indwelling spinal catheters were used. One hundred fifty milligrams of levobupivacaine in 60 mL 0.9% saline was injected intraoperatively through the medial and anterior capsular spaces in the region of the obturator and femoral nerves and also around the short external rotators and gluteus maximus in the region of the inferior and superior gluteal nerves. This was infiltrated after insertion of the acetabular component and before insertion of the femoral stem. Ten milliliters then was infiltrated around the tensor fascia lata and subcutaneously before closing the wound. The placebo group received 60 mL 0.9% saline injected in the same manner. The surgeon was blinded to the method of treatment until it was necessary to administer the injection.
Both groups received opioid analgesia via a PCA device for the initial 48 hours after surgery before conversion to a standard postoperative regime of paracetamol (1 g, by mouth, four times a day), diclofenac (75 mg, by mouth, twice a day), and regular oral opioids (OxyContin™; Purdue Pharma LP, Stamford, CT, USA) (10 mg, twice a day) (Table 2).
Table 2.
Analgesic regimen used for THA
| Preoperative | Intraoperative | Postoperative Days 0–2 | Postoperative days > 2 |
|---|---|---|---|
| Temazepam 10 mg, orally | Levobupivacaine 150 mg | Paracetamol 1 g, 4 times daily, orally | Paracetamol 1 g, 4 times daily, orally |
| Paracetamol 1 g, intravenous | Diclofenac 75 mg, twice daily, orally | Diclofenac 75 mg, twice daily, orally | |
| Diclofenac 100 mg, per rectum | Patient controlled analgesia | OxyContin 10 mg, twice daily, orally |
Rehabilitation was started the day after surgery with all patients mobilized full weightbearing with the assistance of crutches. An abduction pillow was used during the hospital stay.
In recognition of the highly subjective nature of and inherent difficulties in quantifying pain [13], all patients were assessed preoperatively using the WOMAC index [17]. To assess for differences in subjective pain between the treatment and control groups, the short form of the McGill Pain Questionnaire (SF-MPQ) [18] was administered at baseline and at 8, 24, 48, and 72 hours postoperatively. This contains 11 questions referring to the sensory dimension of the pain experience and four related to the affective dimension. Each descriptor is ranked on a four-point intensity scale (0 = none, 1 = mild, 2 = moderate, 3 = severe). The pain rating index and pain intensity score of the standard McGill Pain Questionnaire and VAS also were included. The VAS is reportedly versatile, reliable, and valid for assessing acute pain [8]. We recorded postoperative morphine consumption every 12 hours for each patient up to 48 hours postoperatively. The clinical charts were reviewed on a daily basis to identify the presence of complications that could be attributed to morphine excess: specifically, nausea and vomiting and urinary retention.
All data were checked for errors and normality. To confirm the two groups were comparable preoperatively, Pearson chi square was used to analyze categorical variables, whereas, depending on the assumption of normality, the Wilcoxon-Mann-Whitney test or Student’s t-test was used for continuous variables. The incidence of morphine-related complications also was analyzed using the same techniques. The data returned by each patient form a nested repeated measure where the longitudinal scores are nested in each patient. These types of data can be analyzed using repeated measures analysis of variance (RM)-ANOVA. Three well-known problems with ANOVA are the sphericity assumption, sampling hierarchy, and the requirement for complete data sets. In this study, the patients sometimes were unable to provide a hip score and this leads to a missed observation. There were seven missed observations in the control group and six in the treatment group. In RM-ANOVA missing data are not allowed, and if a subject misses an observation, then all data from that subject have to be discarded. We therefore used a statistical method that can cope with missing data, namely, hierarchical regression or multilevel modeling [10, 16, 19]. Because multilevel modeling is capable of handling missing data, all available data are used for analysis. The data collected from the McGill pain questionnaires and the total morphine consumption were analyzed using this method. The statistical analyses were performed using Minitab® and JMP (SAS Institute, Research Triangle Park, NC, USA) software packages.
Results
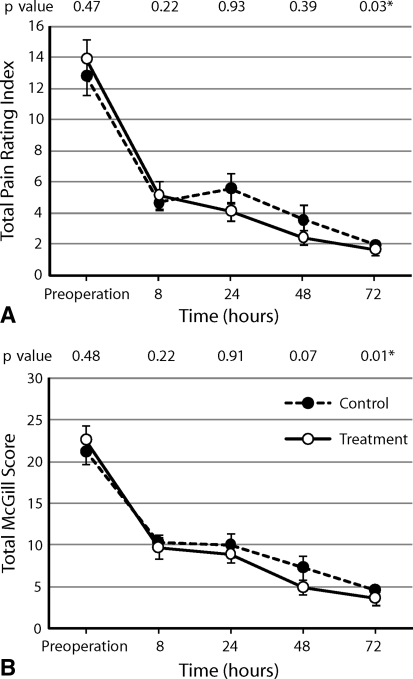
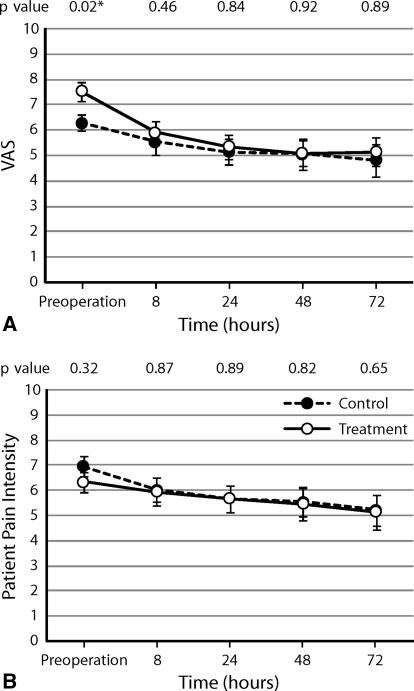
Subjectively reported pain scores referring to the sensory and affective dimensions of pain were similar (p = 0.731) in the postoperative period between the two groups (Fig. 2). At the same time, there were no differences in the overall intensity scores (VAS pain scores or patient pain intensity score) (Fig. 3).
Fig. 2A–B.
There was no difference between the control and treatment groups in (A) total pain rating index (p = 0.731) or (B) total McGill score (p = 0.494) as determined by the RM-ANOVA. Values are expressed as averages with standard error bars. Individual p values at each time were calculated using the Mann-Whitney U-test.
Fig. 3A–B.
There was no difference between the control and treatment groups in (A) VAS score (p = 0.463) or (B) pain intensity score (p = 0.814) as determined by the RM-ANOVA, suggesting equivalent efficacy in postoperative analgesic control. Values are expressed as averages with standard error bars. Individual p values at each time were calculated using the Mann-Whitney U-test.
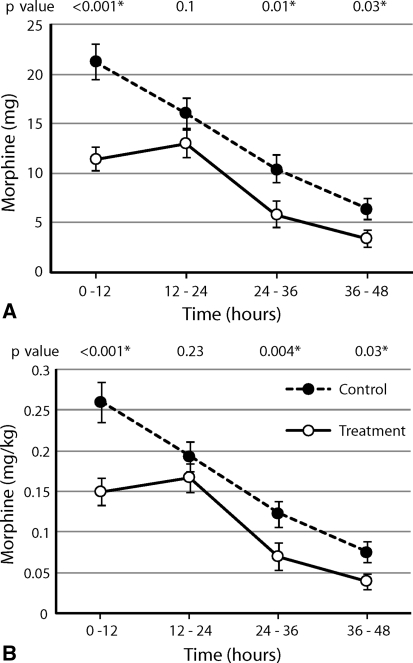
After surgery we observed reduced (p < 0.001) PCA-delivered morphine consumption in the levobupivacaine group (Fig. 4). This was most marked during the first 12 hours after surgery (treatment group, 11.46 mg [SD, 8.03]; control group, 21.23 mg [SD, 12.12]).
Fig. 4A–B.
There was a difference between treatment and control groups in average opioid use via PCA measured in (A) milligrams (p < 0.001) and (B) milligrams per kilogram (p < 0.001) during 12-hour periods postoperatively. Values are expressed as averages with standard error bars. Individual p values at each time were calculated using the Mann-Whitney U-test.
There were three incidences of urinary retention in the control group during the postoperative period, and only one in treatment group (p = 0.317). In addition we found no difference between the two groups regarding antiemetic use (p = 0.221). On average the control group took more antiemetics than the treatment group: 1.6 (SD, 1.45) compared with 1 (SD, 0.9).
Discussion
Many approaches exist for managing postoperative pain after THA. The aims of each remain the same: to minimize pain while avoiding the side effects of excess administration of analgesics and not delaying commencement of the rehabilitative phase. In this study, we aimed to assess the effects of intraoperative periarticular infiltration of levobupivacaine given in isolation on subjectively reported pain using an established scale, total rescue morphine requirement in the postoperative period, and the incidence of potential morphine-related complications.
We note several limitations of our study. First, this is a one-surgeon series. Although this guarantees a consistent method of infiltration, it might limit applicability of the results to others who might use different approaches to the hip or infiltrate the tissues in a different way. Surgeons inevitably will infiltrate different regions and understanding the anatomy of hip innervation is necessary [2]. Second, our power analysis was based on preliminary VAS pain scores; the study was underpowered regarding the secondary outcomes (ie, postoperative antiemetic requirement or need for catheterization for urinary retention), although we noted several trends. Third, the nature of pain makes objective measurement impossible. Valid and reliable assessment of pain is essential for clinical trials and effective pain management [3]. The pain scales used in the study are useful for clinically assessing how intensely patients are feeling pain and for monitoring the effectiveness of treatments at different times.
We found periarticular infiltration of levobupivacaine in isolation resulted in similar subjectively reported pain scores during the postoperative period compared with scores reported by those in the placebo group. Busch et al. reported that periarticular infiltration of multimodal analgesic agents has a positive effect on subjective pain reporting after THA with lower subjective scores reported by patients while in the postanesthesia care unit after surgery [4] (Table 3). Parvataneni et al. also noted that patients receiving periarticular infiltration of a multimodal injection had lower reported scores than those receiving placebo and morphine via PCA on Days 1 to 3 after surgery although this difference had disappeared by Day 4 [20]. Lee et al. reported lower VAS pain scores on Days 1 to 4 in patients treated with periarticular injection at the time of surgery compared with a group treated with a conventional pain management regime after THA [15]. This is in contrast to our study where we found only equivalence in subjective reporting of pain. This may be attributable to our use of one anesthetic agent (levobupivacaine) in isolation suggesting that there is some benefit or synergistic effect by combining a couple or several agents. Alternatively the different scoring system we used (short-form McGill Pain Questionnaire) might have had some influence although we also analyzed a VAS score as the others did.
Table 3.
Main findings in randomized controlled trials assessing the efficacy of periarticular injections
| Study | Number of patients | Periarticular injection | Outcome measures | Findings |
|---|---|---|---|---|
| Lee et al. [15] | 60 | Morphine HCL 5 mg; methylprednisolone acetate 40 mg; ropivacaine 6.8 mg; normal saline – to dilute to 90 mL volume | 100 mm VAS pain score; analgesic use; rehabilitation milestones; complications (nausea, vomiting, surgical site infection) | Significantly lower VAS pain scores in injected group Days 1–4, but not on Day 5; similar oral analgesic requirements throughout Similar hospital stays; similar operative time; earlier straight leg raise in treatment group |
| Parvataneni et al. [20] | 71 | Bupivacaine (0.5%) 200–400 mg; morphine sulfate 0.4–1.0 mL; epinephrine (1:1000) 0.3 mL; methylprednisolone acetate 40 mg; cefuroxime (10 mL) 750 mg; normal saline 22 mL | Mean pain scores; time to straight leg raise; mean hospital stay; discharge destination | Lower mean pain scores in injected group on Days 1–3 after surgery; greater satisfaction scores during hospital stay for injection group; overall less narcotic use in injection group during hospital stay; shorter hospital stay by 1 day in the injected group and earlier straight leg raise; more discharges to home instead of to a rehabilitation facility (70% versus 50%) |
| Busch et al. [4] | 64 | Ropivacaine HCl 400 mg; ketorolac 30 mg; epinephrine (1:1000) 0.6 mL; morphine 5 mg; saline – to make 100 mL volume | PCA-based morphine use; VAS pain score (at rest and activity); VAS satisfaction score; length of surgery; length of hospital stay; incidence of wound complications; incidence of ropivacaine toxicity; Ultrasound scan for lower limb DVT at Day 5 | Less PCA use in injected group at 6 and 24 hours postsurgery; lower VAS scores recorded for pain at activity in the postanesthesia care unit; no significantly different findings for other measures; one patient in injected group had a DVT 5 days after surgery |
| Current study | 91 | 150 mg of levobupivacaine; normal saline to make total volume of 60 mL | McGill pain score; VAS pain score; PCA delivered morphine use; incidence of postoperative nausea and vomiting and urinary retention | Equivalent VAS pain scores; reduced PCA requirement in treatment group; no significant difference between groups for postoperative nausea and vomiting or urinary retention |
VAS = visual analog scale; PCA = patient-controlled anesthesia; DVT = deep vein thrombosis.
The main benefit of periarticular infiltration of levobupivacaine we found was that it resulted in a reduced requirement of morphine use via PCA after surgery. A reduced postoperative opiate requirement was reported in other randomized controlled trials assessing periarticular regimes (Table 3). Similar to the our study, Busch et al. found that use of a multiagent periarticular injection at THA resulted in less use of PCA-delivered morphine during the first 6 (11.1 versus 19.6 mg) to 24 hours (28.5 versus 43.3 mg) after surgery compared with the control [4]. The almost 50% reduction in PCA use during the first 6 hours is similar to the reduction we found during the first 12 hours. Parvataneni et al. reported reduced total use of narcotic analgesia among patients administered a multiagent periarticular injection after THA when compared with patients not given the injection [20]. However, they did not use PCA-delivered analgesia making direct comparison difficult.
Despite a statistically significant reduction in opiate use during the first 12-hours after surgery, this reduction was small and not sustained to the same degree beyond 12 hours, unlike the longer benefit reported by Busch et al. [4]. It is plausible that the multimodal injection that Busch et al. used resulted in a synergistic effect between agents and this resulted in lasting pain control and likely greater clinical benefit. Nonetheless our study showed that levobupivacaine results in a 50% reduction in opiate requirement during the first 12 hours after surgery, and additional study incorporating these results might be able to improve the efficacy of multimodal periarticular injections. Despite the reduction in PCA use, we did not observe a reduction in side effects attributable to opiate use including nausea, vomiting, and urinary retention. Lee et al. also reported no difference between injected and noninjected groups for postoperative nausea and vomiting [15]. Possible reasons for failure to find a difference with respect to potential side effects is the relatively small difference in opiate consumption between the two groups and failure of this difference to be sustained beyond 12 hours. An alternative possibility for not finding a difference is that the potential complications related to morphine use are more commonly associated with the use of spinal morphine [22], an approach to pain control not used in this study. We acknowledge that failure to find a reduction in opiate-related side effects limits the clinical importance of the reduction in postoperative opiate use in the treatment group. Consideration could be given to the use of periarticular infiltration of levobupivacaine however, where it is known that a patient is opiate intolerant.
It is unclear from the literature which agents or aspects of such multimodal regimens influence patient outcomes the most. Variable agents as noted have been used and the benefits of infiltrating additional agents such as morphine or epinephrine are uncertain. It is plausible however, that multiple agents might confer a synergistic effect resulting in less total drug administration and therefore less systemic toxicity than if one agent is used in isolation. We did not see any evidence of toxicity attributable to levobupivacaine in our patients although we did not specifically measure levels. Busch et al. noted that ropivacaine levels after a multiagent injection stayed well below toxic levels [4]. As already noted, a synergistic effect may be one explanation for the longer reduction in opiate requirement reported by Busch et al. [4]. Likewise Lee et al. reported their use of an injection containing morphine, ropivacaine, and methylprednisolone diluted in normal saline and found no increased risk for the patient [15].
Intraoperative periarticular infiltration of levobupivacaine in isolation reduces postoperative opiate consumption as a rescue analgesia without sacrificing overall pain control. Additional studies to maximize this effect can determine what combinations of agents might further reduce or potentially eliminate the need for postoperative opioid use and improve analgesic control.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at Cappagh National Orthopaedic Hospital.
References
- 1.Bird SB, Dickson EW. Clinically significant changes in pain along the visual analog scale. Ann Emerg Med. 2001;38:639–643. doi: 10.1067/mem.2001.118012. [DOI] [PubMed] [Google Scholar]
- 2.Birnbaum K, Prescher A, Hepler S, Heller KD. The sensory innervation of the hip joint: an anatomical study. Surg Radiol Anat. 1997;19:371–375. doi: 10.1007/BF01628504. [DOI] [PubMed] [Google Scholar]
- 3.Breivik H, Borchgrevink PC, Allen SM, Rosseland LA, Romundstad L, Hals EK, Kvarstein G, Stubhaug A. Assessment of pain. Br J Anaesth. 2008;101:17–24. doi: 10.1093/bja/aen103. [DOI] [PubMed] [Google Scholar]
- 4.Busch CA, Whitehouse MR, Shore BJ, MacDonald SJ, McCalden RW, Bourne RD. The efficacy of periarticular multimodal drug infiltration in total hip arthroplasty. Clin Orthop Relat Res. 2010;468:2152–2159. doi: 10.1007/s11999-009-1198-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi P, Bhandari M, Scott J, Douketis J. Epidural analgesia for pain relief following hip or knee replacement. Cochrane Database Syst Rev. 2003;3:CD003071. doi: 10.1002/14651858.CD003071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foster RH, Markham A. Levobupivacaine: a review of its pharmacology and use as a local anaesthetic. Drugs. 2000;59:551–579. doi: 10.2165/00003495-200059030-00013. [DOI] [PubMed] [Google Scholar]
- 7.Frater RA, Moores MA, Parry P, Hanning CD. Analgesia-induced respiratory depression: comparison of meptazinol and morphine in the postoperative period. Br J Anaesth. 1989;63:260–265. doi: 10.1093/bja/63.3.260. [DOI] [PubMed] [Google Scholar]
- 8.Gallagher EJ, Bijur PE, Latimer C, Silver W. Reliability and validity of a visual analog scale for acute abdominal pain in the ED. Am J Emerg Med. 2002;20:287–290. doi: 10.1053/ajem.2002.33778. [DOI] [PubMed] [Google Scholar]
- 9.Gedney JA, Liu EH. Side-effects of epidural infusions of opioid bupivacaine mixtures. Anaesthesia. 1998;53:1148–1155. doi: 10.1046/j.1365-2044.1998.00636.x. [DOI] [PubMed] [Google Scholar]
- 10.Goldstein H, Browne W, Rasbash J. Multilevel modelling of medical data. Stat Med. 2002;21:3291–3315. doi: 10.1002/sim.1264. [DOI] [PubMed] [Google Scholar]
- 11.Grant CR, Checketts MR. Analgesia for primary hip and knee arthroplasty: the role of regional anaesthesia. Contin Educ Anaesth Crit Care Pain. 2008;8:56–61. doi: 10.1093/bjaceaccp/mkn007. [DOI] [Google Scholar]
- 12.Gristwood RW, Greaves JL. Levobupivacaine: a new safer long acting local anaesthetic agent. Expert Opin Investig Drugs. 1999;8:861–876. doi: 10.1517/13543784.8.6.861. [DOI] [PubMed] [Google Scholar]
- 13.Gwilym SE, Pollard TC, Carr AJ. Understanding pain in osteoarthritis. J Bone Joint Surg Br. 2008;90:280–287. doi: 10.1302/0301-620X.90B3.20167. [DOI] [PubMed] [Google Scholar]
- 14.Horlocker TT. Pain management in total joint arthroplasty: a historical review. Orthopedics. 2010;33(9 suppl):14–19. doi: 10.3928/01477447-20100722-65. [DOI] [PubMed] [Google Scholar]
- 15.Lee KJ, Min BW, Bae KC, Cho CH, Kwon DH. Efficacy of multimodal pain control protocol in the setting of total hip arthroplasty. Clin Orthop Surg. 2009;1:155–160. doi: 10.4055/cios.2009.1.3.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leyland A, Groenewegen PP. Multilevel modelling and public health policy. Scand J Public Health. 2003;31:267–274. doi: 10.1080/14034940210165028. [DOI] [PubMed] [Google Scholar]
- 17.McConnell S, Kolopack P, Davis AM. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC): a review of its utility and measurement properties. Arthritis Rheum. 2001;45:453–461. doi: 10.1002/1529-0131(200110)45:5<453::AID-ART365>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 18.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–197. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 19.O’Connell AA, McCoach DB. Applications of hierarchical linear models for evaluations of health interventions: demystifying the methods and interpretations of multilevel models. Eval Health Prof. 2004;27:119–151. doi: 10.1177/0163278704264049. [DOI] [PubMed] [Google Scholar]
- 20.Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty. 2007;22(6 suppl 2):33–38. doi: 10.1016/j.arth.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 21.Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg. 1998;87:88–92. doi: 10.1097/00000539-199807000-00019. [DOI] [PubMed] [Google Scholar]
- 22.Souron V, Delaunay L, Schifrine P. Intrathecal morphine provides better postoperative analgesia than psoas. Can J Anaesth. 2003;50:574–579. doi: 10.1007/BF03018643. [DOI] [PubMed] [Google Scholar]