Abstract
Background
Total joint arthroplasty is widely performed in patients of all races with severe osteoarthritis. Prior studies have reported that African American patients tend to receive total joint arthroplasties in low-volume hospitals compared with Caucasian patients, suggesting potential racial disparity in the quality of arthroplasty care.
Questions/purposes
We asked whether (1) a hospital outcome measure of risk-adjusted mortality or complication rate within 90 days of primary TKA can be directly used to profile hospital quality of care, and (2) African Americans were more likely to receive TKAs at low-quality hospitals (or hospitals with higher risk-adjusted outcome rate) compared with Caucasian patients.
Patients and Methods
We developed a risk-adjusted, 90-day postoperative outcome measure to identify high-, intermediate-, and low-quality hospitals based on patient records in the Medicare Provider Analysis and Review files between July 1, 2002, and June 30, 2005 (the first cohort). We then analyzed a second cohort of African American and Caucasian patients receiving Medicare who underwent primary TKAs between July and December 2005 to determine the independent impact of race on admissions to high-, intermediate-, and low-quality hospitals.
Results
The risk-adjusted postoperative mortality/complication rate varied substantially across hospitals; hospitals can be meaningfully categorized into quality groups. In the second cohort of admissions, 8% of African American patients (n = 4894) versus 9.2% of Caucasian patients (n = 86,705) were treated in high-quality hospitals whereas 14.7% of African American patients versus 12.7% of Caucasians patients were treated in low-quality hospitals. After controlling for patient demographic, socioeconomic, geographic, and diagnostic characteristics, the odds ratio for admission to low-quality hospitals was 1.28 for African American patients compared with Caucasian patients (95% CI, 1.18–1.41).
Conclusions
Among elderly Medicare beneficiaries undergoing TKA, African American patients were more likely than Caucasian patients to be admitted to hospitals with higher risk-adjusted postoperative rates of complications or mortality. Future work is needed to address the residential, social, and referring factors that underlie this disparity and implications for outcomes of care.
Introduction
Total joint arthroplasty is widely performed to relieve pain and improve function for patients with advanced osteoarthritis [13, 14]. It is estimated the number of TKAs performed in the United States will increase from approximately 500,000 in 2005 to more than 3 million in 2030, and the number of THAs will increase to 600,000 in 2030 [26]. Notwithstanding the overall expansion in indications [17] and use of arthroplasty, data suggest persistently lower use among racial minorities [6, 18, 25, 38, 39].
Despite this well-documented disparity in use, less is known about the quality of arthroplasty care that racial minority patients receive. NonCaucasian patients reportedly tend to be treated in hospitals performing a lower volume of arthroplasties each year [28, 30]. Given the evidence of associations between lower procedure volume and inferior arthroplasty postoperative mortality and complications [19, 21, 29, 31], it has been assumed racial minorities might have reduced access to high-quality arthroplasty care. Nevertheless, volume per se is not a direct measure of quality [12], and prior studies [6, 28, 30, 38] have not directly assessed whether African Americans undergoing TKAs are more likely to have their surgery in lower-quality hospitals.
We therefore asked whether (1) a hospital outcome measure of risk-adjusted mortality or complication rate within 90 days of primary TKA developed using Medicare claims data can be directly used to profile hospital quality of care; and (2) African American patients were more likely to receive TKAs at low-quality hospitals (or hospitals with higher risk-adjusted outcome rate) compared with Caucasian patients.
Patients and Methods
The primary source of data was the Medicare Provider Analysis and Review (MedPAR) files between 2002 and 2005 [35]. The MedPAR claims contain information regarding each hospitalization for fee-for-service Medicare beneficiaries. Key data elements included patient demographics, primary and secondary diagnoses, and procedures recorded by the International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) codes, admission and discharge dates, date of death up to 3 years after discharge, and unique patient (encrypted) and hospital identifiers. Supplemental data included the 2005 American Hospital Association (AHA) annual survey data [2] to obtain hospital geographic information and the 2000 US Census file [40] to obtain patient socioeconomic measures (described later).
We used the MedPAR data to identify two separate cohorts of patients aged 65 years or older who underwent a primary TKA (ICD-9-CM procedure code 81.54): Cohort I, consisting of TKA admissions between July 1, 2002, and June 30, 2005, which were used to assess hospital quality based on risk-adjusted, 90-day postoperative adverse outcome rate (ie, to address the first study question); and Cohort II, consisting of TKA admissions between July 1, 2005, and December 31, 2005, which were used to answer the second question regarding the association of race with admissions to high-, intermediate-, and low-quality hospitals as profiled based on the sample in Cohort I.
In the analyses of Cohort I, we identified 635,439 TKA admissions to 3611 hospitals during a 3-year period. We retained only Caucasian and African American patients and excluded the 22,247 (3.5%) patients of other races or ethnicities owing to concerns regarding inaccurate race/ethnicity data in Medicare claims [6]. We further excluded the small number (< 1%) of admissions from emergency departments or transfers from other hospitals because these patients might be quite different from other patients having elective surgery with respect to treatment courses and postoperative mortality and complications. In addition, we expected hospital choice for these excluded patients was more likely to be dictated by their acuity rather than patient and provider preferences. The final sample (610,285 primary TKA admissions to 3101 hospitals) was used to estimate the risk-adjusted outcome for each hospital, as follows (Table 1).
Table 1.
Characteristics of Cohort I and risk adjustment model for 90-day complication rate after primary TKA
| Characteristic | Patients in Cohort I (%) | Risk adjustment model | |||
|---|---|---|---|---|---|
| Total (n = 610,285) | Without complication (n = 573,062) | With complication (n = 37,223)* | Adjusted odds ratio | 95% confidence interval | |
| Age | |||||
| 65–69 years | 22.7 | 23.0 | 18.5 | † | † |
| 70–74 years | 34.6 | 34.7 | 32.5 | 1.14 | 1.11–1.18 |
| 75–79 years | 24.7 | 24.6 | 26.3 | 1.27 | 1.23–1.31 |
| 80–84 years | 13.6 | 13.4 | 16.7 | 1.44 | 1.39–1.50 |
| 85–89 years | 3.9 | 3.8 | 5.3 | 1.59 | 1.51–1.68 |
| ≥ 90 years | 0.5 | 0.5 | 0.7 | 1.75 | 1.54–2.02 |
| Race | |||||
| Caucasian | 94.7 | 94.8 | 93.0 | † | † |
| African American | 5.3 | 5.2 | 7.0 | † | † |
| Hospital percent of African American patients | 5.3 | 5.2 | 6.3 | 3.40 | 2.78–4.16 |
| Gender | |||||
| Male | 34.7 | 34.7 | 37.7 | † | † |
| Female | 65.3 | 65.3 | 62.3 | 0.89 | 0.81–0.91 |
| Comorbidity | |||||
| Congestive heart failure | 4.6 | 4.4 | 9.0 | 1.69 | 1.62–1.77 |
| Neurologic disease | 1.9 | 1.8 | 3.2 | 1.67 | 1.57–1.78 |
| Chronic renal failure | 0.8 | 0.7 | 2.2 | 2.35 | 2.17–2.54 |
| Chronic obstructive pulmonary disease | 12.4 | 12.2 | 15.9 | 1.20 | 1.16–1.24 |
| Arrhythmia | 12.8 | 12.5 | 17.4 | 1.23 | 1.19–1.26 |
| Liver disease | 0.4 | 0.4 | 0.7 | 1.49 | 1.29–1.71 |
| Weight loss | 0.2 | 0.1 | 0.5 | 2.44 | 1.95–3.06 |
| Valvular disorder | 4.8 | 4.7 | 6.3 | 1.08 | 1.03–1.13 |
| Hypothyroidism | 14.6 | 14.6 | 13.7 | 0.96 | 0.93–0.99 |
| Coagulopathy | 1.2 | 1.2 | 2.2 | 1.52 | 1.40–1.64 |
| Lymphoma | 0.2 | 0.2 | 0.3 | 1.45 | 1.20–1.75 |
| Pulmonary circulatory disease | 0.9 | 0.7 | 3.2 | 3.44 | 3.16–3.73 |
| Peripheral vascular disease | 2.0 | 1.9 | 2.8 | 1.29 | 1.21–1.38 |
| Fluid disorder | 6.8 | 6.6 | 9.4 | 1.26 | 1.21–1.30 |
| Paralysis | 0.1 | 0.1 | 0.2 | 2.61 | 2.07–3.29 |
| Psychosis | 0.5 | 0.4 | 0.7 | 1.43 | 1.25–1.63 |
| Metastatic cancer | 0.1 | 0.1 | 0.2 | 1.80 | 1.29–2.53 |
| High-risk condition | |||||
| Cancer with bone involvement | 0.1 | 0.1 | 0.3 | 1.77 | 1.26–2.49 |
| Femur fracture | 0.1 | 0.1 | 0.2 | 6.63 | 5.58–7.87 |
| Previous joint arthroplasty | 0.9 | 0.8 | 2.1 | 2.11 | 1.94–2.30 |
| Degenerative disease as indication for TKA | 2.3 | 2.3 | 2.7 | 1.94 | 1.51–2.47 |
| Knee infection as indication for TKA | 0.1 | 0.1 | 0.8 | 1.15 | 1.07–1.23 |
| Year | |||||
| 2002 | 13.9 | 14.2 | 10.8 | 0.66 | 0.64–0.69 |
| 2003 | 32.1 | 32.2 | 29.8 | 0.80 | 0.78–0.83 |
| 2004 | 34.9 | 34.8 | 37.1 | 0.91 | 0.88–0.94 |
| 2005 | 19.1 | 18.8 | 22.3 | † | † |
* p < 0.05 when compared with patients with complications in all characteristics; †reference group.
Using previously developed and tested ICD-9-CM algorithms [8], we defined a composite end point (the adverse outcome) of complications (identified using inpatient claims) and mortality within 90 days after TKA for each admission. The composite measure consisted of six separate postoperative outcomes within 90 days: sepsis, hemorrhage, pulmonary embolism, deep vein thrombosis, severe wound infection requiring readmission, and death. The ICD-9-CM codes for complications were reported in previous studies [8, 19, 21].
We determined predictors used to estimate the risk adjustment model according to the findings by Cram et al. [8] and our analyses, in which we performed bivariate tests and stepwise logistic regression models. Choice of predictors was made based on statistical criteria and clinical judgment. The final set of patient predictors for model estimation included age category (65–69, 70–74, 75–79, 80–84, 85–89, and ≥ 90 years); female gender (yes/no); selected comorbidity conditions defined according to the algorithm developed by Elixhauser et al. (such as congestive heart failure, chronic renal failure) [10]; additional high-risk conditions related to joint arthroplasty [19], including prior hip and knee arthroplasties, active joint infection, acute fracture, cancer with bone involvement, and degenerative diseases as indications for joint arthroplasty; and year dummies (2002, 2003, 2004, and 2005).
African American race was associated with mortality and complications after primary TKA according to previous studies [8, 17]. This is potentially problematic, as it is unclear whether this relationship is because patients of African American race pose greater risk and therefore experience higher postoperative mortality and complications after TKA or because African American patients are more likely to be admitted to poorer-quality hospitals. Thus, the inclusion of race might potentially overadjust hospital quality if African American patients tended to be admitted to lower-quality hospitals. However, failure to control for race might unfairly penalize hospitals that cared for large proportions of African American patients if in fact they are sicker. Therefore, the model included a hospital-level variable representing the proportion of African American patients admitted to the hospital as a marker of severity rather than access to care. To confirm the robustness of hospital profiling using the risk-adjusted outcome measure (ie, the first study question), we also conducted sensitivity analyses in which multivariable models did not control for percentage of African American patients admitted for TKAs and in which risk-adjusted outcomes were estimated based on Caucasian patients only.
To determine whether the hospital outcome measure could be used to profile hospital quality of care, we fit the risk adjustment model for Cohort I using the generalized estimating equation approach [43] with a binomial distribution and a logit link function of the response variable (whether a patient having a TKA experienced the adverse outcome). The statistical performance of this risk adjustment model was evaluated using the c-statistic [1], which summarizes the ability of the model to discriminate between patients who experienced the adverse outcome and those who did not. To account for the possible outcomes correlation between patients admitted to the same hospital, the model assumed a compound symmetric (exchangeable) correlation structure of the error term. Estimated coefficients of risk factors were used to predict the probability of having the adverse outcome develop for each patient. To calculate the risk-adjusted adverse outcome rate for each hospital, we first calculated the expected adverse outcome rate for each hospital (E) as the sum of the predicted probability of having the adverse outcome develop for all patients in the hospital divided by the number of patients having TKAs in the hospital. The observed adverse outcome rate for each hospital (O) then was calculated as the actual number of patients experiencing the outcome in the hospital divided by the number of patients having TKAs in the hospital. Finally, the risk-adjusted adverse outcome rate for each hospital was calculated as the ratio of the observed to expected adverse outcome rate (ie, O/E ratio) multiplied by the overall adverse outcome rate for all patients in the cohort [3]. This risk-adjusted outcome rate is comparable across hospitals [23].
To test the racial difference in access to high-, intermediate-, and low- quality hospitals (ie, study question 2), in Cohort II we initially identified 107,201 primary TKA admissions to 3612 hospitals between July 1 and December 31, 2005. Applying the same exclusion criteria described for Cohort I, we excluded 3880 (3.6%) nonCaucasian and nonAfrican American patients, and patients admitted from the emergency department or transferred from another hospital (< 1%). The retained sample then was linked to (1) the hospital risk-adjusted adverse outcome rate calculated as described previously (454 admissions could not be matched to a hospital risk-adjusted adverse outcome rate and were excluded); (2) the 2000 US Census file to obtain residence zip code-level education and median household income data (10,011 patients with missing or incorrect zip codes were excluded); and (3) the 2005 AHA annual survey data to obtain hospital zip code for calculating distance between patient residence and the admitting hospital (700 admissions were excluded owing to missing or incorrect hospital zip code). We calculated the distance between the zip code of each patient’s residence and the hospital where the surgery was performed using the linear arc distance between centroids [34]. The sample for final analyses in Cohort II included 91,599 admissions of Caucasian and African American patients receiving TKAs in 2842 hospitals (Table 2). African American patients (n = 4894) accounted for 5.3% of all admissions in Cohort II. African American patients undergoing TKAs also were more likely to be male, live in an urban area, travel a shorter distance for the procedure, and live in zip code areas with lower socioeconomic status (measured by education and income). Compared with the final analytic sample, excluded records attributable to missing information contained only slightly fewer African American patients (4.9% versus 5.3%) and did not differ substantially in other patient characteristics. We ranked hospitals treating patients having TKAs in Cohort II by the predefined, risk-adjusted 90-day postoperative adverse outcome rate and categorized hospitals into high-quality group (hospitals with an O/E ratio < 20th percentile), low-quality group (hospitals with O/E ratio ≥ 80th percentile), or intermediate-quality group (other hospitals). In sensitivity analyses, we used alternative cutoff points to define the high-quality (25th or 33rd percentile) and low-quality (75th or 67th percentile correspondingly) groups.
Table 2.
Characteristics of Cohort II by race
| Characteristic | Patients in Cohort II (%) | |||
|---|---|---|---|---|
| Total (n = 91,599) | African American (n = 4894) | Caucasian (n = 86,705) | p Value* | |
| Hospital admission† | ||||
| High-quality hospital | 9.2 | 8.0 | 9.2 | < 0.001 |
| Low-quality hospital | 12.8 | 14.7 | 12.7 | |
| Intermediate-quality hospital | 78.0 | 77.3 | 78.1 | |
| Age | ||||
| 65–69 years | 19.7 | 26.9 | 19.3 | < 0.001 |
| 70–74 years | 36.1 | 37.2 | 36.0 | |
| 75–79 years | 25.7 | 22.1 | 25.9 | |
| 80–84 years | 14.3 | 10.8 | 14.5 | |
| 85–89 years | 3.9 | 2.6 | 4.0 | |
| ≥ 90 years | 0.4 | 0.5 | 0.4 | |
| Gender | ||||
| Male | 35.3 | 20.3 | 36.2 | < 0.001 |
| Female | 64.7 | 79.7 | 63.8 | |
| Residence location | ||||
| Rural | 8.0 | 3.7 | 8.3 | < 0.001 |
| Urban | 92.0 | 96.3 | 91.7 | |
| Median distance (miles) | 8.9 | 6.1 | 9.1 | < 0.001 |
| Zip code-level education (median of percent population with ≥ 12 years education) | 83.0 | 74.0 | 84.0 | < 0.001 |
| Median zip code-level income (US dollars) | 40,037 | 32,980 | 40,316 | < 0.001 |
| Comorbidity | ||||
| Congestive heart failure | 4.9 | 6.2 | 4.8 | < 0.001 |
| Neurologic disease | 2.0 | 1.9 | 2.0 | 0.408 |
| Chronic renal failure | 1.9 | 3.1 | 1.9 | < 0.001 |
| Chronic obstructive pulmonary disease | 13.4 | 15.6 | 13.3 | < 0.001 |
| Arrhythmia | 14.0 | 9.9 | 14.2 | < 0.001 |
| Liver disease | 0.4 | 0.5 | 0.4 | 0.441 |
| Weight loss | 0.2 | 0.3 | 0.2 | 0.113 |
| Valvular disorder | 5.2 | 3.5 | 5.3 | < 0.001 |
| Hypothyroidism | 15.9 | 9.3 | 16.2 | < 0.001 |
| Coagulopathy | 1.5 | 1.4 | 1.5 | 0.721 |
| Lymphoma | 0.3 | 0.1 | 0.3 | 0.070 |
| Pulmonary circulatory disease | 1.1 | 1.3 | 1.0 | 0.127 |
| Peripheral vascular disease | 2.2 | 2.6 | 2.2 | 0.048 |
| Fluid disorder | 8.1 | 9.4 | 8.0 | < 0.001 |
| Paralysis | 0.1 | 0.1 | 0.1 | 0.797 |
| Psychosis | 0.5 | 0.5 | 0.5 | 0.746 |
| Metastatic cancer | 0.1 | 0.1 | 0.1 | 0.161 |
| High-risk condition | 0.1 | 0.1 | 0.1 | |
| Cancer with bone involvement | 0.1 | 0.1 | 0.1 | 0.333 |
| Femur fracture | 0.6 | 0.6 | 0.6 | 0.970 |
| Previous joint arthroplasty | 0.5 | 0.5 | 0.5 | 0.955 |
| Degenerative disease as indication for TKA | 0.1 | 0.1 | 0.1 | 0.127 |
| Knee infection as indication for TKA | 2.3 | 1.8 | 2.3 | 0.018 |
* For comparisons between Caucasian and African American patients based on chi-square tests for categorical variables and nonparametric Wilcoxon tests for continuous variables; †high-quality hospitals were below the 20th percentile of the ranking of risk-adjusted complication rate; low-quality hospitals were above the 80th percentile of the ranking of risk-adjusted complication rate; intermediate-quality hospitals were in the middle.
The set of patient covariates that might have affected hospital choices [7, 8, 19, 28, 30] included age; gender; rural or urban residence of patient defined using rural-urban commuting area codes provided by the Rural Health Research Center [36]; distance between patient residence and the admitting hospital; zip code-level median household income and education attainment; selected comorbidity conditions defined according to Elixhauser et al. [10]; and additional high-risk conditions related to TKA as described before.
We performed bivariate and multivariable analyses to compare patient characteristics and hospital quality between Caucasian and African American patients. In bivariate analyses, we used chi square tests for comparison of categorical variables and nonparametric Wilcoxon tests for comparison of continuous variables. In multivariable analysis, separate multinomial logistic models were estimated to test the independent impact of race (the key independent variable) on admissions to high-, intermediate-, and low-quality hospitals alternatively defined above (the dependent variable, intermediate-quality hospitals as the reference group), after controlling for the patient covariates described above. All analyses were performed with SAS® (Version 9.2; SAS Institute Inc, Cary, NC, USA).
Results
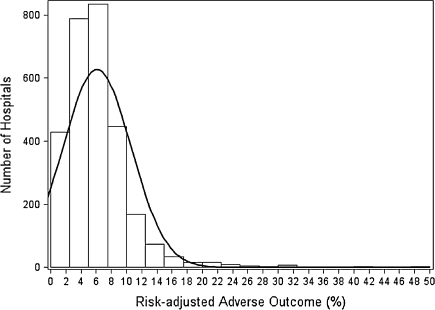
In the analyses on Cohort I to develop a hospital risk-adjusted, 90-day postoperative adverse outcome measure for primary TKA, we found that patients experiencing postoperative adverse outcomes tended to be female, older, African Americans, and to have more comorbidities and high-risk conditions (Table 1). The c-statistic for the risk adjustment model was 0.70, which is comparable to those in validated models of short-term inpatient mortality based on Medicare claims [23, 24], and suggests appropriate statistical performance of our estimated model. The risk-adjusted postoperative mortality/complication rate varied substantially across hospitals (Fig. 1), which allows us to categorize hospitals into different quality groups. In the sensitivity analyses of the risk-adjustment model excluding the hospitals’ percentage of African Americans admitted for TKAs and the model based on Caucasian patients only, the results were close to the results in the main analyses.
Fig. 1.
The distribution of hospital risk-adjusted 90-day postoperative adverse outcome rate for primary TKA is shown.
In the analyses on Cohort II, African American patients undergoing TKAs were less likely to be admitted to high-quality hospitals (8% versus 9.2%) but more likely to be admitted to low-quality hospitals (14.7% versus 12.7%) compared with Caucasian patients. In multivariable analyses controlling for patient covariates (Table 3), African American race was not a predicator of admissions to high-quality hospitals (adjusted odds ratio [AOR] = 1.07; 95% CI, 0.93–1.24; p = 0.34) but was associated with admissions to low-quality hospitals (AOR = 1.28; 95% CI 1.18–1.41; p < 0.001). We found similar results when high-, intermediate-, and low-quality hospitals were defined using alternative cutoff points.
Table 3.
Admission to high- and low-quality hospitals for African American patients compared with Caucasian patients*
| Hospitals | Adjusted odds ratio | 95% confidence interval | p Value |
|---|---|---|---|
| High-quality hospitals | |||
| < 20th percentile | 1.07 | 0.93–1.24 | 0.34 |
| < 25th percentile | 0.82 | 0.73–0.92 | < 0.0001 |
| < 33rd percentile | 0.89 | 0.82–0.97 | 0.009 |
| Low-quality hospitals | |||
| > 80th percentile | 1.28 | 1.18–1.41 | < 0.0001 |
| > 75th percentile | 1.19 | 1.10–1.29 | < 0.0001 |
| > 67th percentile | 1.02 | 0.96–1.10 | 0.46 |
* Separate multinomial logistic regression models adjusted for age, gender, rural/urban residence, travel distance, education, household income, comorbidities, and high-risk conditions related to TKA (Table 2).
Discussion
The last two decades have seen dramatic expansions in clinical indications and actual use of joint arthroplasties [17, 26]. As such, the quality of arthroplasties received by patient groups is of increasing interest to policymakers and the public [4, 5, 41]. For instance, in a pay-for-performance demonstration project, the Centers for Medicare and Medicaid Services (CMS) publicly reported three hospital quality measures for arthroplasty [41]. Although several studies suggest racial minority patients are less likely to undergo arthroplasties [6, 18, 38, 39], little is known about the quality of arthroplasty care that African Americans receive relative to Caucasians. In this study we asked whether (1) a hospital outcome measure of risk-adjusted mortality or complication rate within 90 days of primary TKA developed using Medicare claims data can be directly used to profile hospital quality of care; and (2) African American patients were more likely to undergo a TKA at a low-quality hospital (or hospitals with higher risk-adjusted outcome rate) compared with Caucasian patients.
This study has several limitations. First, our analyses were limited to elderly Medicare patients. However, approximately 2/3 of primary TKAs were performed in patients 65 years and older [6], and most of the growth in TKA volume has occurred among the elderly [9]. Second, our claims-based risk adjustment model might not capture all important clinical risk factors and thus the risk-adjusted adverse outcome measure might not be fully risk adjusted [15]. However, our model predicted the adverse outcome very well in comparison to other published models [23, 24], and similarly developed risk-adjusted outcome measures based on Medicare claims agreed to a substantial degree with the measures developed based on medical records containing more extensive clinical information [23, 24]. Therefore, our measure for each hospital based on the O/E ratio should reflect largely the quality of hospital care rather than the effects of preexisting patient characteristics [8, 20]. Third, the second part of our analysis for racial disparities in hospital admissions adjusted for income and education attainment at zip code levels (instead of for each patient); there may be unmeasured socioeconomic components associated with African American patients that can lead to their increased likelihood of admission to lower-quality hospitals. However, evidence suggests that the aggregate level socioeconomic measures offer a valid approach to overcoming the absence of socioeconomic data in medical claims [22].
We first developed the risk-adjusted, 90-day postoperative adverse outcome measure after primary TKA to profile hospital quality of care. There is ongoing debate regarding how to best measure and profile hospital quality using alternative structural, process-of-care, and outcome indicators [4, 15, 33, 42]. The three arthroplasty process measures published by the CMS included prophylactic antibiotic use within 1 hour before surgical incision, appropriate choice of antibiotics, and discontinuation of prophylactic antibiotics within 24 hours after surgery [41]. These measures convey important quality information to the public but take only a narrow perspective on the overall quality of perioperative arthroplasty care, ie, antibiotic administration. Furthermore, one study reported these process measures did not have adequate ability to discriminate among hospitals and correlated weakly with important clinical outcomes [4]. Although primary TKA is relatively safe with short-term mortality less than 1%, our analyses found, as previous studies did [8, 19–21], that major complications occur in a proportion of patients undergoing the procedure (approximately 6% on average in our study). In addition, adverse outcome rates varied considerably across hospitals (with a range of 0% to 50% in our study), thereby providing meaningful classification of hospital performance in arthroplasties. Finally, our risk adjustment model predicted the adverse outcome reasonably well, which assured our ability to ‘level the playing field’ for hospital classifications [16]; several studies in other areas of inpatient care suggest risk-adjusted outcomes similarly developed using Medicare claims data provide highly valid classifications of hospital performance [23, 24].
Racial variations in the pattern of admissions to high-, intermediate-, and low-quality hospitals for joint arthroplasty have not been studied. Losina et al. [28, 30] reported that racial minorities and patients of lower socioeconomic status undergoing joint arthroplasties tended to be treated in hospitals performing low volumes of arthroplasties. This suggests potential racial and socioeconomic disparities in access to high-quality arthroplasty care given the evidence that hospital volume of arthroplasty procedures correlates with well-defined and risk-adjusted postoperative outcomes [19, 21, 31] and, as such, can be seen as a proxy measure of hospital quality [12]. Nevertheless, the observed volume-outcome relationship in arthroplasty might be partially explained by unobserved hospital and patient characteristics [12]. For instance, the degree of specialization in orthopaedic care might contribute to quality and outcomes improvement above and beyond the effect of volume. It has been documented that orthopaedic specialty hospitals have lower postoperative complication rates for joint arthroplasty relative to general hospitals even after controlling for differences in patient volume [8]. Small specialty centers might have the ability to provide arthroplasty care with better outcomes than large general hospitals [19]. Therefore, our finding that African Americans were more likely than Caucasian patients to receive primary TKAs in hospitals with higher risk-adjusted postoperative mortality/complication rates provides direct evidence of racial disparities in access to high-quality arthroplasty care. As suggested by Losina et al. [28, 30] and others [27, 32, 37], the potential racial disparity we observed in access to high-quality hospitals for joint arthroplasty might be explained by multiple factors. For example, African American patients are more likely to come from poor neighborhoods with less hospital-care resources, which might predict their increased likelihood of admissions to poor-quality hospitals [30]. In addition, residential racial segregation might lead to segregated access to inpatient care whereby African American patients tend to be clustered in hospitals with poor financial resources [11] and poor quality and healthcare outcomes [37]. Furthermore, physicians who first treated and referred the patient for TKA might further play an important role in the choices of orthopaedic surgeons and hospitals, thus explaining our findings of racial disparities in access to high-quality hospitals [32]. Therefore, more in-depth studies are needed to explore these neighborhood, social, and physician-referring factors to better understand and address the issues of unequal access and quality of arthroplasties.
Our study of Medicare beneficiaries in the nation reveals that among patients undergoing primary TKAs, African American patients were more likely than Caucasian patients to be treated in hospitals with higher risk-adjusted postoperative adverse outcome rate. The potential racial disparity in access to high-quality arthroplasty care is a major issue, especially as arthroplasties have and continue to be more widely performed in Caucasian and nonCaucasian patients with severe osteoarthritis. Future work is needed to address the residential, social, and clinical referring factors that underlie this disparity.
Acknowledgment
We thank Carrie L. Franciscus for database maintenance.
Footnotes
One of the authors (PC) was supported by a K23 career development award (RR01997201) from the National Center for Research Resources at the National Institutes of Health and the Robert Wood Johnson Physician Faculty Scholars Program. One or more of the authors (PC) also was supported by R01 HL085347-01A1 from the National Heart, Lung, and Blood Institute at the National Institutes of Health. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Agresti A. Categorical Data Analysis. 2. Hoboken, NJ: John Wiley & Sons Inc; 2002. [Google Scholar]
- 2.American Hospital Association. AHA Data and Directories. Available at http://www.aha.org/aha/resource-center/Statistics-and-Studies/data-and-directories.html. Accessed August 17, 2011.
- 3.Ash AS, Shwartz M, Pekoz EA. Comparing outcomes across providers. In: Iezzoni LI, editor. Risk Adjustment for Measuring Health Care Outcomes. Chicago IL: Health Administration Press; 2003. pp. 297–334. [Google Scholar]
- 4.Bhattacharyya T, Freiberg AA, Mehta P, Katz JN, Ferris T. Measuring the report card: the validity of pay-for-performance metrics in orthopedic surgery. Health Aff (Millwood) 2009;28:526–532. doi: 10.1377/hlthaff.28.2.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhattacharyya T, Mehta P, Freiberg AA. Hospital characteristics associated with success in a pay-for-performance program in orthopaedic surgery. J Bone Joint Surg Am. 2008;90:1240–1243. doi: 10.2106/JBJS.G.01172. [DOI] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Racial disparities in total knee replacement among Medicare enrollees—United States, 2000–2006. MMWR Morb Mortal Wkly Rep. 2009;58:133–138. [PubMed] [Google Scholar]
- 7.Cram P, Vaughan-Sarrazin MS, Rosenthal GE. Hospital characteristics and patient populations served by physician owned and non physician owned orthopedic specialty hospitals. BMC Health Serv Res. 2007;7:155. doi: 10.1186/1472-6963-7-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cram P, Vaughan-Sarrazin MS, Wolf B, Katz JN, Rosenthal GE. A comparison of total hip and knee replacement in specialty and general hospitals. J Bone Joint Surg Am. 2007;89:1675–1684. doi: 10.2106/JBJS.F.00873. [DOI] [PubMed] [Google Scholar]
- 9.DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. National Health Statistics Reports. US Department of Health and Human Services. Centers for Disease Control and Prevention. National Center for Health Statistics. Available at: http://www.cdc.gov/nchs/data/nhsr/nhsr005.pdf. Accessed August 2, 2011.
- 10.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Gaskin DJ, Hadley J. Population characteristics of markets of safety-net and non-safety-net hospitals. J Urban Health. 1999;76:351–370. doi: 10.1007/BF02345673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Halm EA, Lee C, Chassin MR. Is volume related to outcome in health care? A systematic review and methodologic critique of the literature. Ann Intern Med. 2002;137:511–520. doi: 10.7326/0003-4819-137-6-200209170-00012. [DOI] [PubMed] [Google Scholar]
- 13.Harris WH, Sledge CB. Total hip and total knee replacement (1) N Engl J Med. 1990;323:725–731. doi: 10.1056/NEJM199009133231106. [DOI] [PubMed] [Google Scholar]
- 14.Harris WH, Sledge CB. Total hip and total knee replacement (2) N Engl J Med. 1990;323:801–807. doi: 10.1056/NEJM199009203231206. [DOI] [PubMed] [Google Scholar]
- 15.Iezzoni LI. The risks of risk adjustment. JAMA. 1997;278:1600–1607. doi: 10.1001/jama.278.19.1600. [DOI] [PubMed] [Google Scholar]
- 16.Iezzoni LI. Risk Adjustment for Measuring Health Care Outcomes. Chicago, IL: Health Administration Press; 2003. [Google Scholar]
- 17.Jain NB, Higgins LD, Ozumba D, Guller U, Cronin M, Pietrobon R, Katz JN. Trends in epidemiology of knee arthroplasty in the United States, 1990–2000. Arthritis Rheum. 2005;52:3928–3933. doi: 10.1002/art.21420. [DOI] [PubMed] [Google Scholar]
- 18.Jha AK, Fisher ES, Li Z, Oray EJ, Epstein AM. Racial trends in the use of major procedures among the elderly. N Engl J Med. 2005;353:683–691. doi: 10.1056/NEJMsa050672. [DOI] [PubMed] [Google Scholar]
- 19.Katz JN, Barrett J, Mahomed NN, Baron JA, Wright RJ, Losina E. Association between hospital and surgeon procedure volume and the outcomes of total knee replacement. J Bone Joint Surg Am. 2004;86:1909–1916. doi: 10.1302/0301-620X.86B7.14358. [DOI] [PubMed] [Google Scholar]
- 20.Katz JN, Bierbaum BE, Losina E. Case mix and outcomes of total knee replacement in orthopaedic specialty hospitals. Med Care. 2008;46:476–480. doi: 10.1097/MLR.0b013e31816c43c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katz JN, Losina E, Barrett J, Phillips CB, Mahomed NN, Lew RA, Guadagnoli E, Harris WH, Poss R, Baron JA. Association between hospital and surgeon procedure volume and outcomes of total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2001;83:1622–1629. doi: 10.1302/0301-620X.83B3.10487. [DOI] [PubMed] [Google Scholar]
- 22.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–710. doi: 10.2105/AJPH.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krumholz HM, Wang Y, Mattera JA, Han LF, Ingber MJ, Roman S, Normand SL. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with an acute myocardial infarction. Circulation. 2006;113:1683–1692. doi: 10.1161/CIRCULATIONAHA.105.611186. [DOI] [PubMed] [Google Scholar]
- 24.Krumholz HM, Wang Y, Mattera JA, Han LF, Ingber MJ, Roman S, Normand SL. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–1701. doi: 10.1161/CIRCULATIONAHA.105.611194. [DOI] [PubMed] [Google Scholar]
- 25.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487–1497. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 26.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Glance LG, Cai X, Mukamel DB. Are patients with coexisting mental disorders more likely to receive CABG surgery from low-quality cardiac surgeons? The experience in New York State. Med Care. 2007;45:587–593. doi: 10.1097/MLR.0b013e31803d3b54. [DOI] [PubMed] [Google Scholar]
- 28.Losina E, Barrett J, Baron JA, Levy M, Phillips CB, Katz JN. Utilization of low-volume hospitals for total hip replacement. Arthritis Rheum. 2004;51:836–842. doi: 10.1002/art.20700. [DOI] [PubMed] [Google Scholar]
- 29.Losina E, Barrett J, Mahomed NN, Baron JA, Katz JN. Early failures of total hip replacement: effect of surgeon volume. Arthritis Rheum. 2004;50:1338–1343. doi: 10.1002/art.20148. [DOI] [PubMed] [Google Scholar]
- 30.Losina E, Wright EA, Kessler CL, Barrett JA, Fossel AH, Creel AH, Mahomed NN, Baron JA, Katz JN. Neighborhoods matter: use of hospitals with worse outcomes following total knee replacement by patients from vulnerable populations. Arch Intern Med. 2007;167:182–187. doi: 10.1001/archinte.167.2.182. [DOI] [PubMed] [Google Scholar]
- 31.Manley M, Ong K, Lau E, Kurtz SM. Total knee arthroplasty survivorship in the United States Medicare population: effect of hospital and surgeon procedure volume. J Arthroplasty. 2009;24:1061–1067. doi: 10.1016/j.arth.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Mukamel DB, Weimer DL, Mushlin AI. Referrals to high-quality cardiac surgeons: patients’ race and characteristics of their physicians. Health Serv Res. 2006;41:1276–1295. doi: 10.1111/j.1475-6773.2006.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peterson ED, Roe MT, Mulgund J, DeLong ER, Lytle BL, Brindis RG, Smith SC, Jr, Pollack CV, Jr, Newby LK, Harrington RA, Gibler WB, Ohman EM. Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA. 2006;295:1912–1920. doi: 10.1001/jama.295.16.1912. [DOI] [PubMed] [Google Scholar]
- 34.Phibbs CS, Luft HS. Correlation of travel time on roads versus straight line distance. Med Care Res Rev. 1995;52:532–542. doi: 10.1177/107755879505200406. [DOI] [PubMed] [Google Scholar]
- 35.Research Data Assistance Center. Medicare documentation. Available at http://www.resdac.org/ddvh/index.asp. Accessed August 17, 2011.
- 36.Rural Health Research Center. RUCA Data version 2.0. Available at: http://depts.washington.edu/uwruca/ruca-data.php. Accessed August 2, 2011.
- 37.Sarrazin MV, Campbell M, Rosenthal GE. Racial differences in hospital use after acute myocardial infarction: does residential segregation play a role? Health Aff (Millwood) 2009;28:w368–w378. doi: 10.1377/hlthaff.28.2.w368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Skinner J, Weinstein JN, Sporer SM, Wennberg JE. Racial, ethnic, and geographic disparities in rates of knee arthroplasty among Medicare patients. N Engl J Med. 2003;349:1350–1359. doi: 10.1056/NEJMsa021569. [DOI] [PubMed] [Google Scholar]
- 39.Suarez-Almazor ME, Souchek J, Kelly PA, O’Malley K, Byrne M, Richardson M, Pak C. Ethnic variation in knee replacement: patient preferences or uninformed disparity? Arch Intern Med. 2005;165:1117–1124. doi: 10.1001/archinte.165.10.1117. [DOI] [PubMed] [Google Scholar]
- 40.US Census Bureau. Census 2000. Available at http://www.census.gov/main/www/cen2000.html. Accessed August 17, 2011.
- 41.US Department of Health and Human Services. Hospital Compare. Available at: www.hospitalcompare.hhs.gov. Accessed May 17, 2011.
- 42.Werner RM, Bradlow ET. Relationship between Medicare’s hospital compare performance measures and mortality rates. JAMA. 2006;296:2694–2702. doi: 10.1001/jama.296.22.2694. [DOI] [PubMed] [Google Scholar]
- 43.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. doi: 10.2307/2531248. [DOI] [PubMed] [Google Scholar]