Abstract
Alcohol dependence and alcohol abuse represent major unmet medical needs. The zebrafish is considered to be a promising vertebrate species with which the effects of alcohol on brain function and behavior and the mechanisms underlying these effects may be studied. Alcohol is known to induce alterations in motor function as well as fear and anxiety. Here we present a recently developed fear paradigm in which we employ an animated (moving) image of a bird silhouette. We measure the effect of acute alcohol administration (dose range employed: 0.00 – 0.75 vol/vol percentage, bath exposure for 60 minutes) on the behavioral responses of zebrafish. We test these responses during a pre-stimulus, stimulus and post-stimulus period of the task using both a video-tracking and an observation based quantification method. The fear inducing stimulus was found to decrease the distance of the zebrafish from the bottom of the tank, to increase number of erratic movements, and to increase the number of jumps in alcohol exposed fish (versus control fish). Alcohol attenuated these fear responses in a dose dependent manner. In addition, alcohol decreased general activity at the highest dose, an effect that was independent of the presentation of the stimulus. We discuss the similarities and differences between observation and video-tracking based results and conclude that fear paradigms will be useful in revealing alcohol induced functional changes in the brain of zebrafish.
Keywords: alcoholism, anxiety, EtOH, fear, zebrafish
1. Introduction
Alcohol dependence and alcohol abuse are devastating diseases that represent major unmet medical needs [13]. A potentially fruitful way to facilitate the development of therapies is to investigate the mechanisms of the actions of alcohol in the brain of model organisms [26, 31]. The zebrafish has been proposed as an excellent research tool for this purpose because it represents an optimal compromise between system complexity (the zebrafish is a vertebrate species) and practical simplicity (zebrafish can be kept in large numbers in small tanks cheaply and have many other features that make them easier to work with than rodents) [17, 21]. Indeed, this species has been successfully employed in the analysis of the effects of alcohol administered acutely [21] and chronically [20]. The effect of early embryonic alcohol treatment leading to behavioral changes without gross morphological alterations has also been demonstrated and has started to be investigated using zebrafish [12]. Alcohol is known to act through a large number of molecular mechanisms and thus, not surprisingly, has also been found to affect a range of behavioral functions [2]. One of these functions is fear and anxiety (for recent reviews see 23, 33]. Acute alcohol exposure has been found to reduce anxiety (a behavioral state that is not directly induced by stimuli) and is also known to impair the behavioral responses to fear inducing stimuli [5, 10, 22].
In this paper we study fear responses, which we define as behavioral responses induced by aversive stimuli [15]. Although this definition may appear somewhat circular, extensive research published on zebrafish fear responses by now allow us to judge what stimuli may be considered aversive and what behavioral responses may be expected to be induced by such stimuli [1, 3, 4, 7, 11, 15, 24, 30, 34], a point we return to later in the discussion. Acute alcohol intoxication at higher doses is also known to impair motor function both in mammals and in fish [10, 21].
Acute alcohol effects have been shown to alter fear responses in zebrafish [8, 17, 19, 20, 21, 28 and references therein]. However, fully automated delivery of a stimulus that induces a robust fear response and at the same time allows automated quantification of the induced behavioral responses have been rarely achieved. Full automation of controlled and precisely timed stimulus delivery together with computerized quantification of behavioral responses allow multiple tests to be run in parallel and thus are crucial requirements for high throughput. High throughput is required for forward genetic screening and also for drug screening.
In the current paper, we investigate motor responses of zebrafish in a fear paradigm we recently developed [27]. We present the stimulus, a computer animated image of a bird silhouette, in the middle of the behavioral recording session and compare the effect of this visual stimulus across four acute alcohol dose groups, using both observation based and video-tracking generated behavioral measures. Our goal is to analyze how the appearance of the stimulus changes the behavior of our subjects and to investigate whether administration of alcohol alters the responses to the stimulus. Ultimately, we hope that this work will lead to the development of efficient behavioral phenotyping and screening applications in zebrafish with which the effects of novel anxiolytic drugs or mutations, altering fear responses and/or responses to alcohol, may be identified.
2. Materials and Methods
2.1 Animals and housing
Eighty-six (five month old) young adult wild-type zebrafish (Danio rerio) of the AB strain (approximately 50–50% males-females) were tested. The progenitors of our population were obtained from the Zebrafish International Research Centre (ZIRC) (Eugene, Oregon). All fish were laboratory-bred (the University of Toronto Mississauga (UTM) Vivarium) and were raised and maintained as described before [27]. Briefly, at 5 days post fertilization (dpf), the free swimming zebrafish fry were transferred to small rearing tanks where they were fed twice a day with Larval Artificial Plankton 100 (particle size below 100 μm, ZeiglerBros, Inc., Gardners, PA, USA). From the age of 15 dpf the zebrafish were fed nauplii of brine shrimp (Artemia salina) until they were four weeks old. Subsequently, all fish were given a 1:1 mixture of flake food (Tetramin Tropical fish flake food, Tetra Co, Melle, Germany) and powered spirulina (Jehmco Inc., Lambertville, NJ, USA). Adult zebrafish were housed in 2.8 L Plexi-glass tanks (approximately 15 fish per tank) that were part of a recirculating system (Aquaneering Inc., San Diego, CA, USA) with multi-stage filtration including a mechanical filter, a fluidized glass bed biological filter, activated carbon filter, and a UV light sterilizing unit. Ten percent of the water of the recirculating system rack was replaced with fresh system water (reverse osmosis de-ionized oxygenated water supplemented with 60 mg/L Instant Ocean Sea Salt, Big Al’s Pet Store, Mississauga, Ontario, Canada) each day. The water temperature was controlled by a thermostat and was kept at 27 °C. The light cycle was also controlled with fluorescent lights on the ceiling turned on at 08:00 h and off at 20:00 h.
2.2 Experimental design, test apparatus and procedure
Zebrafish, assigned to their particular treatment condition in a randomized manner, were exposed to one of four doses of acute alcohol treatment. The acute alcohol concentrations employed were: 0% (freshwater control), 0.25%, 0.5%, and 0.75% vol/vol percentage, and the sample sizes (n) were 20, 21, 22, 23, respectively. The exposure procedure followed those first describe by Gerlai et al [21]. Briefly, each fish was individually placed into a 500 ml exposure beaker containing the appropriate alcohol solution for 60 minutes, a period of time required for alcohol to reach stable and maximal levels in the brain of zebrafish [21, 17 and references therein]. Air pumped through air-stones provided oxygenation during the exposure. Immediately following the alcohol exposure, experimental fish were placed in the experimental tank and their behavior was recorded for 12 minutes.
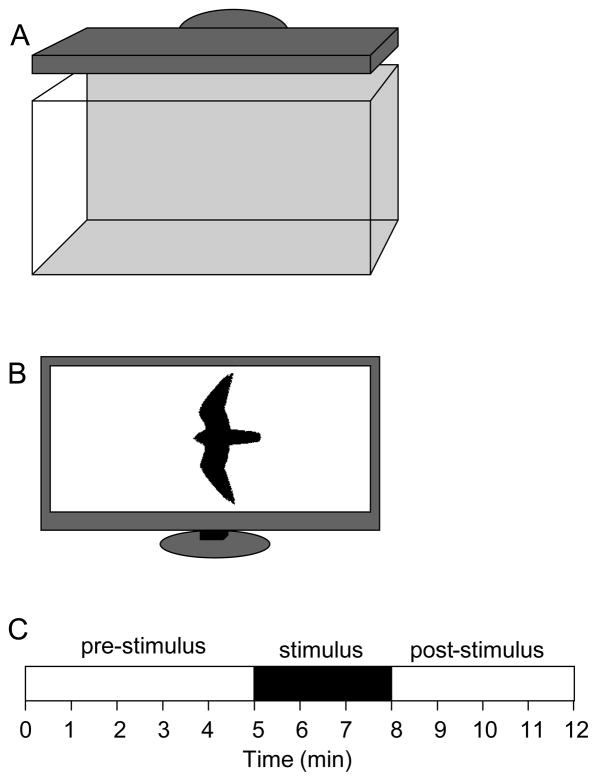
A 40 liter experimental tank (51cm×30cm×25cm, width × depth × height) was used to test each fish individually. In order to mimic the natural habitat of zebrafish and to increase visibility and contrast (required for event recording and video-tracking), a dark green plastic sheet was placed on the back side and the bottom of the test tank. The tank was illuminated from above by a flat panel LCD computer monitor (17 inch screen diameter, Samsung Syncmaster 732N) that also served as the stimulus delivery device (figure 1A). Although circadian activity dependent alcohol effects on fear responses have not been studied, to avoid potential circadian effects, we tested the fish only between the period which was at least 3 hours away from any light cycle changes, i.e. we started the behavioral recording 3 hours after the lights turned on at 11:00 h and stopped the experimental recording at 17:00h, three ours before they turned off. A digital hard disk video-camera (JVC Everio GZ-MG37U) was placed in front of the tank to record the subject’s behavior. The recordings were later replayed and analyzed using the Observer (version 5.0) software application and the videotracking software Ethovision XT (Noldus Information Technologies, Wageningen, The Netherlands). The computer monitor placed above the test tank facing downward was connected to a laptop computer (Dell Vostro 1000) that ran a custom software application (first described in [32]), which allowed the presentation of a computer-animated image, a moving black silhouette of a bird of prey (figure 1B). This stimulus was identical to what we employed recently [27]. In the latter paper we demonstrated this stimulus to effectively induce fear responses. However, it must be noted that we do not yet know whether zebrafish are responsive to particular bird shapes or even whether they differentiate bird-shaped stimuli from non-bird-shaped ones. The bird silhouette was 5 cm from beak to tail and had 10 cm wingspan. It moved across a white illuminated background on the computer monitor above the test tank with a speed of 14 cm/sec, i.e. the stimulus traversed the entire 50 cm long tank within 3.5 seconds. The stimulus was presented multiple times with 5 second inter-stimulus intervals during the stimulus period (which was 3 minutes long) and each time the direction of movement of the bird silhouette was changing randomly between left to right or right to left.
Fig 1.
The experimental set up (A), aversive stimulus employed (B) and the recording session timeline (C) are shown. Note that a flat panel LCD computer monitor was placed above the test tank facing down and this screen could present a moving black filled bird silhouette on a constant white background (shown on panel B). The recording session consisted of a 5 min pre-stimulus period during which no stimulus was shown, a 3 min stimulus period (during which the “bird flew” above the tank once in every 5 sec) and a 4 min post-stimulus period.
Animal behavior was monitored and analyzed for three separate intervals: 5 minute long pre-stimulus period, 3 minute long stimulus presentation, and 4 minute long post-stimulus presentation period (figure 1C). The computer monitor remained turned on showing a white background throughout the entire recording session, which provided a uniform illumination (except when the stimulus was shown).
2.3 Quantification of behavior
First, the video-recordings were analyzed using an observation based event recording method (with the Observer software) that allows the quantification of location of the fish as well as the measuring of fine motor and posture patterns [9]. The following behavioral parameters were quantified. The experimental tank was divided into three equal imaginary horizontal layers (upper, middle and bottom layer) during playback of the video-recordings and we measured the percent of time the test fish spent in each of these layers. These behavioral responses were quantified because zebrafish have been found to move away from the surface and spend increased amount of time on the bottom under aversive conditions [11, 25]. In addition to the horizontal lines, we also divided the tank to three imaginary vertical compartments, left, center and right. Together with the horizontal lines the vertical division gave 3×3 segments. The ambulation scored was used to measure the number of times the experimental fish crossed from one segment to another, which gave us an estimate of swimming activity. We quantified this behavior because fear inducing stimuli have been previously found to alter swimming activity in zebrafish [21]. Freezing, i.e. complete cessation of movement (during which only the eyes and the opercula may move) has also been shown to be associated with fear inducing stimuli. It is considered a fear reaction to aversive contexts [15]. Therefore, we measured the duration of time fish stayed immobile and we expressed this measure relative to observation period length (i.e. as percent of time). Erratic movement, or zigzagging [15, 21], has also been used as one of the most reliable responses induced by presentation of aversive stimuli, including sight of predators [1, 3, 18] or delivery of alarm substances [30, 34]. We quantified the percent of time fish performed this behavior. Jumping, similarly to erratic movement, is often seen as a direct and immediate response to the delivery of aversive stimuli. This behavior is when the fish, using mainly its caudal fin, accelerates quickly in a single leaping manner, after which it performs other behaviors. We counted the number of times fish jumped. The advantage of observation based methods is that they allow one to measure even complex motor responses that may be difficult to quantify using automated methods. Overall, however, the automated method of video-tracking is superior to the observation based quantification method because it can quantitatively measure the strength of behavioral responses. For example, using the Ethovision video-tracking software, we could precisely measure the total distance our fish swam as well as the average distance they were from the bottom of the tank precisely.
2.4 Statistical analysis
Data were analyzed using SPSS version 14 written for the PC. First, repeated measure two factorial ANOVA’s were conducted with Interval, the repeated measure factor, (3 levels: pre-stimulus, stimulus and post-stimulus interval) and alcohol concentration (with 4 levels), the between subject factor. In addition, the effect of sex as well as side of stimulus presentation was also analyzed. However, these main factors and the interaction between them and other main factors turned out to be non-significant therefore data were pooled for these factors. In case of significant alcohol concentration, and/or Interval × alcohol concentration interaction effects, the post hoc Tukey Honestly Significant Difference (HSD) multiple comparison test was employed separately for each interval. In case of significant interval effects, a repeated measure ANOVA was performed for each alcohol concentration separately for only the first two intervals (the pre-stimulus and the stimulus period) in order to investigate the effect of the presentation of the stimulus.
3. Results
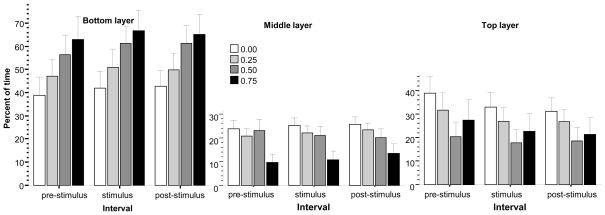
Zebrafish exposed to alcohol acutely appeared to spend increasing duration of time on the bottom of the experimental tank (left graph in Fig. 2), an observation confirmed by ANOVA, which showed a significant alcohol concentration effect (F(1, 75) = 2.488, p < 0.05), but no significant interval, or interval × alcohol concentration interaction. Tukey HSD post hoc test conducted separately for each interval, however, found no significant differences among concentration groups within any of the three intervals.
Fig 2.
Percent of time spent by zebrafish in the bottom (left most graph), middle (middle graph) and in the top (right most graph) layer of the experimental tank. Mean ± S.E.M. are shown. The data are expressed for three intervals separately, the pre-stimulus period (the first interval during which no stimulus was shown), the stimulus period (during which the moving black bird silhouette was presented multiple times), and the post-stimulus period (during which no stimulus was presented). The shading of the bars corresponds to the alcohol dose used with darker shades indicating higher concentrations (see legends). For details of the results of statistical analyses see Results.
Variance Analysis of the duration of time spent in the middle layer of the experimental tank (Fig. 2, middle graph) showed a significant alcohol concentration effect (F(1, 75) = 2.986, p < 0.05), but found the effect of interval and the alcohol concentration × interval interaction to be non-significant. Tukey HSD post hoc tests conducted separately for each interval revealed that the control group was significantly (p < 0.05) different from the highest dose group during the pre-stimulus and stimulus periods, but for the post-stimulus period this difference was found non-significant. Other group differences were also non-significant.
Analysis of the duration of time spent in the top layer of the experimental tank (Fig. 2, panel on right) revealed a significant interval effect (F(2, 150) = 9.865, p < 0.01) but the effect of alcohol concentration and the alcohol concentration × interval interaction were not significant. Multiple comparison post hoc tests are not appropriate for repeated measures variables and thus we conducted pair-wise Bonferroni corrected comparisons of intervals for each dose group separately using univariate repeated measures ANOVAs (with interval as the only factor with 2 levels). These ANOVAs revealed significant differences between the pre-stimulus and stimulus intervals for the freshwater control and the lowest (0.25% alcohol) dose groups (F(1, 18) > 5.11, p < 0.05), but found other interval differences non-significant. This latter finding suggests that the stimulus was effective in the control and the 0.25% alcohol concentration groups while higher doses of alcohol (0.50 and 0.75%) blunted the stimulus induced change.
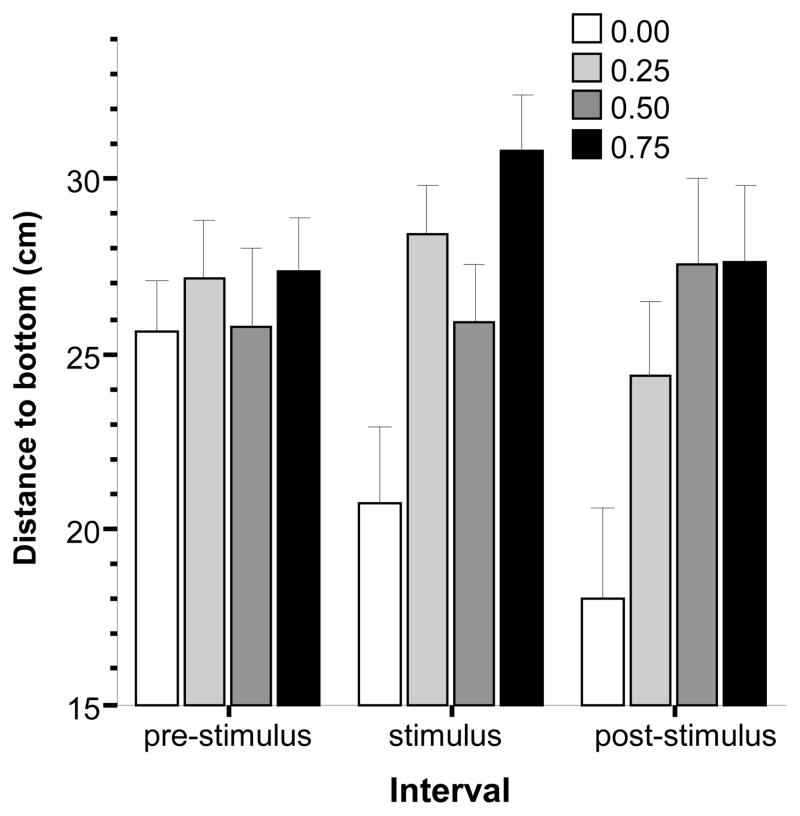
Subsequently, we analyzed the videotracking quantified measure, distance from bottom (Fig. 3). Variance Analysis revealed a significant alcohol concentration effect (F(1, 75) = 3.438, p < 0.05), a significant interval effect (F(2, 150) = 3.141, p < 0.05) and significant alcohol concentration × interval interaction (F(6,150) = 3.237, p < 0.01). To further analyze these effects we conducted three separate Tukey HSD post hoc comparisons (one for each interval). These analyses revealed that while fish showed no alcohol dose related differences during the pre-stimulus period (p > 0.05), in response to the stimulus (i.e. during the stimulus period) the control group significantly (p < 0.05) reduced their distance from the bottom (i.e. stayed closer to the bottom) as compared to the 0.25% and 0.75% concentration groups, while other differences were found non significant. For the post-stimulus interval, the multiple comparison analysis found the control group to be significantly (p < 0.05) below the 0.05% and the 0.75% concentration groups (other group differences were non significant, p > 0.05), i.e. fish from these latter two groups swam further from the bottom. Briefly, these results suggest that alcohol blunted the escape to the bottom in zebrafish.
Fig 3.
Distance to the bottom is reduced by the presentation of the stimulus and this effect is blunted by acute alcohol administration. Mean ± S.E.M. are shown. The data are expressed for three intervals separately, the pre-stimulus period (the first interval during which no stimulus was shown), the stimulus period (during which the moving black bird silhouette was presented multiple times), and the post-stimulus period (during which no stimulus was presented). The shading of the bars corresponds to the alcohol dose used with darker shades indicating higher concentrations (see legends). For details of the results of statistical analyses see Results.
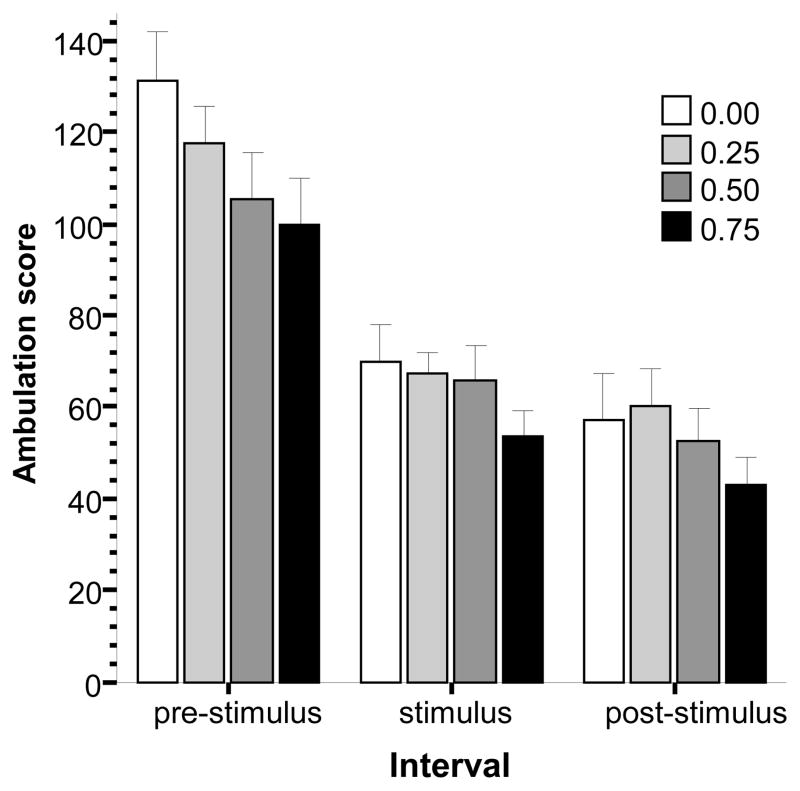
Variance analysis of the observation based activity count, the ambulation score (Fig. 4), showed a significant interval effect (F(2, 150) = 161.600, p < 0.001) but the effect of alcohol concentration and the alcohol concentration × interval interaction term was non-significant. To further analyze the interval effect and because multiple post hoc comparisons are not appropriate for repeated measure designs we conducted repeated measures ANOVAs with interval as factor with two levels (to compare the three intervals in a pairwise manner) separately for each alcohol concentration group. This analysis revealed that the ambulation score of fish of all concentration groups significantly decreased from the pre-stimulus period to the stimulus period and the difference between the pre-stimulus period and the post-stimulus period was also significant (F(1, 21) > 34.378, p < 0.001) but the difference between the stimulus and post-stimulus period performance was non-significant for all concentration groups. It is notable that ANOVA has been found insensitive to find interaction terms significant (Wahslten, 1990) and thus we also conducted an analysis in which we compared the different concentration groups for each interval separately (one way ANOVA followed by Tukey HSD test). These analyses, however, confirmed the overall repeated measure ANOVA results and found no significant concentration effect for any interval. Taken together, these results suggest that alcohol did not affect the gross locomotor behavior of zebrafish at the concentrations employed, however, the aversive stimulus did: locomotion was reduced during and after the presentation of the bird silhouette.
Fig 4.
Locomotory activity, quantified as the number of times fish crossed the lines of a 3 × 3 virtual grid (the ambulation score) is reduced by the presentation of the stimulus. Mean ± S.E.M. are shown. The data are expressed for three intervals separately, the pre-stimulus period (the first interval during which no stimulus was shown), the stimulus period (during which the moving black bird silhouette was presented multiple times), and the post-stimulus period (during which no stimulus was presented. The shading of the bars corresponds to the alcohol dose used with darker shades indicating higher concentrations (see legends). Note the apparent, but non-significant, alcohol concentration dependent linear trend, especially during the pre-stimulus interval, towards reduced activity. For details of the results of statistical analyses see Results.
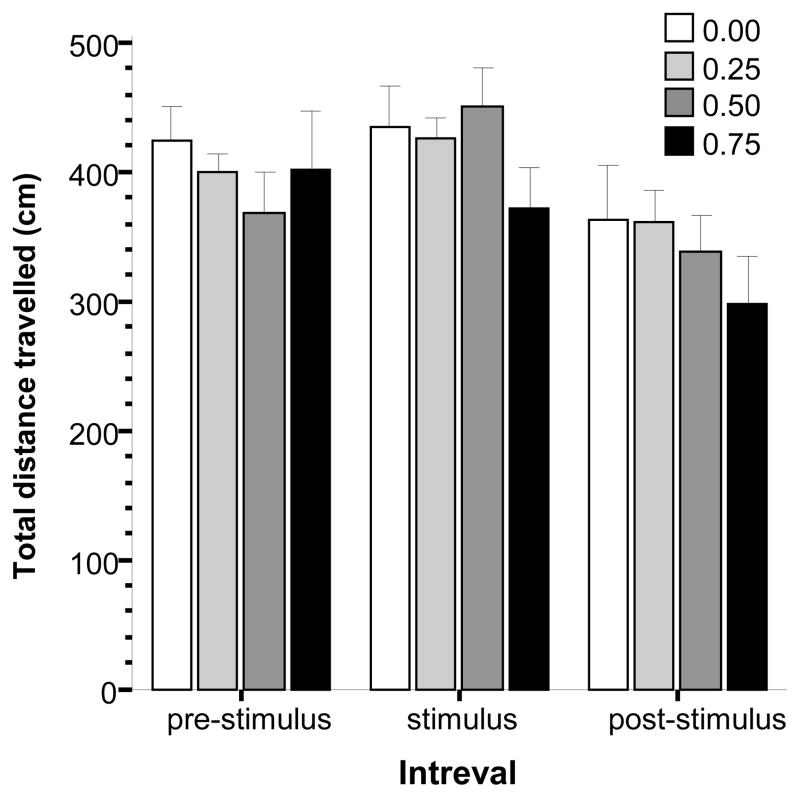
We obtained another activity measure, total distance travelled (Fig. 5), but in contrast to the ambulation score, this measure was quantified using video-tracking. ANOVA found a significant interval effect (F(2, 150) = 17.821, p < 0.001), but detected no significant alcohol concentration effect or alcohol concentration × interval interaction. To further investigate the interval effect, we conducted repeated measure ANOVAs with interval as the only factor (with two levels) separately for each concentration group. This analysis revealed no significant difference between the pre-stimulus and stimulus periods for the control group (F(1, 21) = 0.27, p > 0.60) and for the highest dose group (0.75%), but found the difference to be marginally significant for the 0.25% alcohol group (F(1, 20) = 4.017, p = 0.059) and significant for the 0.50% group (F(1, 21) = 6.318, p < 0.05), results that suggest a stimulus induced hyperactivity in the intermediate alcohol dose groups. Comparison of the total distance traveled during the stimulus and the post-stimulus periods showed that the distance decreased when the stimulus was turned off in all concentration groups (F(1, 20–22) > 8.800, p < 0.01).
Fig 5.
The total distance traveled by zebrafish as quantified by videotracking during the pre-stimulus, stimulus presentation and post-stimulus presentation intervals. Mean ± S.E.M. are shown. Note that during the pre-stimulus period, the first interval, no stimulus was shown, during the stimulus period a moving black bird silhouette was presented multiple times, and during the post-stimulus period again no stimulus was presented. The shading of the bars corresponds to the alcohol dose used with darker shades indicating higher concentrations (see legends). For details of the results of statistical analyses see Results.
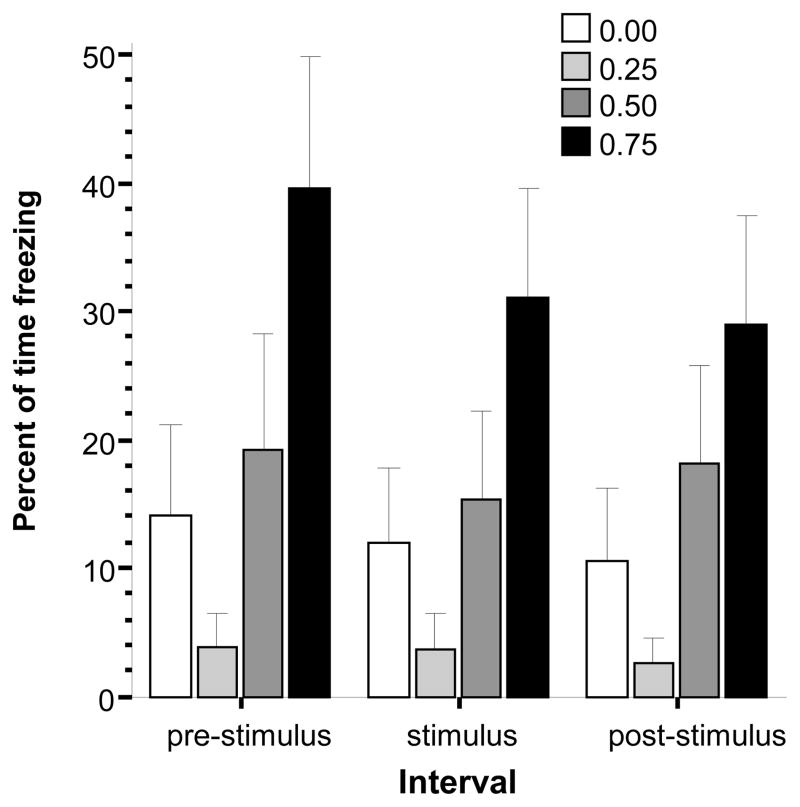
Percent of time freezing (Fig. 6) showed a U-shaped dose response curve, whereby the 0.25% alcohol concentration induced a decrease and the highest dose (0.75%) induced an increase of freezing. These observations were confirmed by ANOVA, which detected a significant alcohol concentration effect (F(1, 75) = 3.440, p < 0.05), an interval effect (F(2, 150) = 7.753, p < 0.01) and also found a significant interaction between these factors (F(6,150) = 2.447, p < 0.05). To further investigate this interaction, we conducted one-way non-repeated measure ANOVAs with alcohol concentration as the factor separately for the three intervals followed by Tukey HSD tests. These analyses showed that for all three intervals the 0.25% group performed significantly (p < 0.05) less freezing as compared to the 0.75% group, differences between other groups were found non-significant (p > 0.05). These results confirm a stimulus independent decrease of freezing in the 0.25% dose group, which may be due to generalized hyperactivity.
Fig 6.
Percent of time freezing is significantly reduced by acute exposure to 0.25% alcohol in a stimulus presentation independent manner. Mean ± S.E.M. are shown. Note that during the pre-stimulus period, the first interval, no stimulus was shown, during the stimulus period a moving black bird silhouette was presented multiple times, and during the post-stimulus period again no stimulus was presented. The shading of the bars corresponds to the alcohol dose used with darker shades indicating higher concentrations (see legends). For details of the results of statistical analyses see Results.
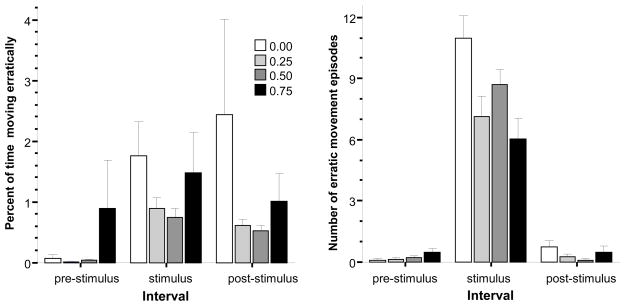
Erratic movement, a sign of fear in zebrafish [15] occurs fast and sometimes its duration and other times its frequency (number of occurrences) are analyzed. Here we report on both of these measures (Fig. 7). Analysis of the percent of time moving erratically, revealed a significant interval effect (F(2, 174) = 6.943, p < 0.01), but the effect of alcohol concentration and the interaction between these factors were both non-significant. Given the insensitivity of ANOVA to detect interaction effects [38] and the suggestive pattern of results shown on Fig. 7 (left graph), we conducted four separate repeated measure ANOVAs, one for each alcohol concentration level, to investigate the change of behavior between the pre-stimulus and stimulus presentation periods. The results of this analysis showed that the stimulus had a significant effect on fish that received no alcohol (freshwater control, F(1, 18) = 9.756, p < 0.01), and also on fish that received 0.25% alcohol (F(1, 19) = 26.331, p < 0.001) and 0.50% alcohol (F(1, 19) = 28.915, p < 0.001). Fish in the 0.75% alcohol treatment group, however, showed no significant stimulus induced change.
Fig 7.
The percent of time (left graph) and frequency (right graph) of erratic movement (zig-zagging) exhibited during the pre-stimulus, stimulus presentation, and post-stimulus periods. Mean ± S.E.M. are shown. Note that during the pre-stimulus period, the first interval, no stimulus was shown, during the stimulus period a moving black bird silhouette was presented multiple times, and during the post-stimulus period again no stimulus was presented. The shading of the bars corresponds to the alcohol dose used with darker shades indicating higher concentrations (see legends). For details of the results of statistical analyses see Results.
The frequency of erratic movement episodes showed a different pattern of results (Fig. 7, graph on the right): a robust increase in the number of erratic movement episodes only during the stimulus period. ANOVA confirmed this observation and found a significant interval effect (ANOVA F(2, 174) = 252.000, p < 0.001), revealed a significant alcohol concentration effect (F(3, 87) = 4.507, P < 0.01), and a significant interaction between these two factors (F(6, 174) = 4.720, p < 0.001). To further explore the significant alcohol effect and alcohol interval interaction we compared the four alcohol concentration groups during each interval. ANOVA found no difference among groups of fish acutely exposed to different alcohol concentrations during the pre-stimulus period and during the post-stimulus period, but demonstrated a significant difference for the stimulus period (F(3, 87) = 4.857, p < 0.01). Post hoc Tukey HSD test showed that the 0.25% and 0.75% alcohol groups had significantly (p < 0.05) increased number of erratic movement episodes as compared to the freshwater control group during the stimulus presentation period and other group differences were non-significant (p > 0.05). The results suggest that alcohol counteracted the effect of the aversive stimulus and at least in the 0.25% and 0.75% significantly reduced the stimulus’ fear inducing effect.
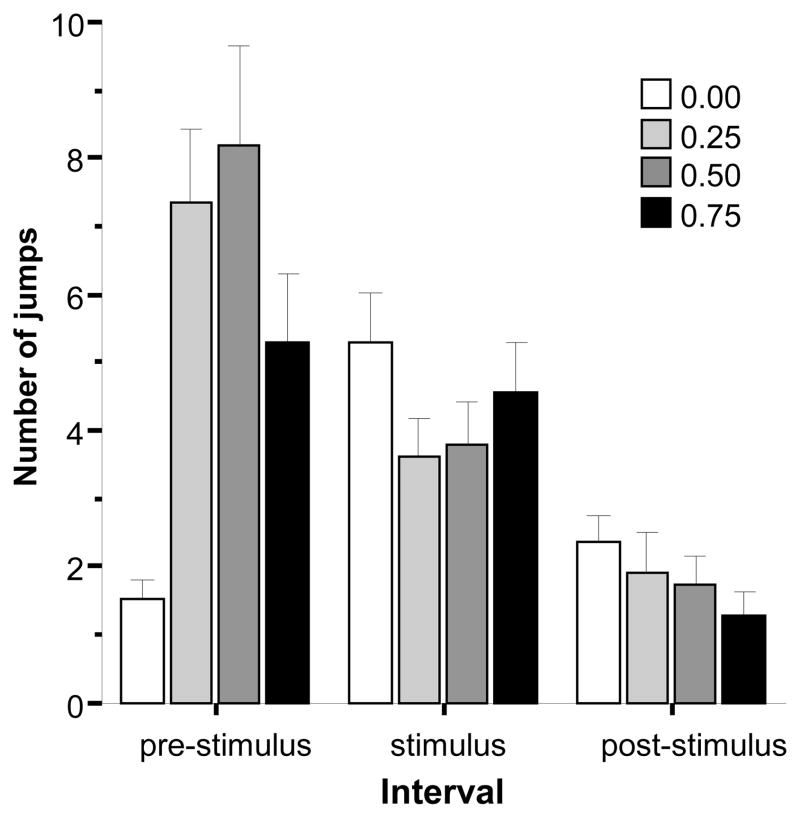
The frequency of jumps performed during the three intervals of the behavioural recording session is shown in Fig. 8. It appears that intermediate doses of alcohol increased jumping frequency during the pre-stimulus period but blunted the effect of the stimulus (reduced the number of jumps) during the stimulus period. ANOVA confirmed a significant interval effect (F(2, 174) = 36.473, p < 0.001) as well as the significant interaction between interval and alcohol concentration (F(6, 174) = 10.403, p < 0.001), but found the alcohol concentration to have no significant effect. Comparison of the alcohol groups by interval confirmed that during the pre-stimulus period the control fish performed significantly fewer jumps as compared to all alcohol treated groups (ANOVA F3, 87) = 7.962, p < 0.001; Tukey HSD, p < 0.05) while other group differences were non-significant (Tukey HSD p > 0.05). However, significant alcohol concentration effect was not found for the stimulus and the post-stimulus intervals. These results suggest that intermediate doses of acute alcohol enhanced the reactivity of experimental zebrafish to the novel test tank leading to increased number of jumps performed during the first few minutes of being in the tank (pre-stimulus period), and the bird silhouette presented from above during the stimulus period reduced this hyperactivity to a level indistinguishable from that of the control fish.
Fig 8.
The frequency of jumps exhibited during the pre-stimulus, stimulus presentation, and post-stimulus periods. Mean ± S.E.M. are shown. Note that during the pre-stimulus period, the first interval, no stimulus was shown, during the stimulus period a moving black bird silhouette was presented multiple times, and during the post-stimulus period again no stimulus was presented. The shading of the bars corresponds to the alcohol dose used with darker shades indicating higher concentrations (see legends). For details of the results of statistical analyses see Results.
4. Discussion
High throughput is an important pre-requisite for zebrafish screens. The current paper employed a stimulus delivery paradigm in which fear responses could be induced and quantified using computer automated methods. The fear responses were blunted by acute alcohol administration. Thus, our results suggest that alcohol induced behavioral changes in fear may be tested in a high throughput manner with the use of zebrafish.
We investigated alcohol induced alterations of fear responses to a consistent computer generated stimulus, a moving silhouette of a bird presented on top of the experimental tank. Given their high fitness value, fear reactions are expected to be robust and reliably induced behavioral responses in zebrafish [15]. As such, they may be appropriate tools for discovering changes in brain function induced by a variety of methods. Alcohol is known to affect anxiety and fear responses and thus fear paradigms may serve as efficient methods with which the effects of alcohol and changes induced by mutations or drugs altering these effects may be revealed. Acute alcohol exposure has been shown to lead to behavioral alterations in fear related paradigms in zebrafish [6, 14, 19, 20, 21, 28, 36]. Many of these paradigms, however, utilized the light vs. dark paradigm, which, although may appear fairly simple, has significant controversies surrounding it [6, 21, 29, 37]. For example, it is unclear as of today whether zebrafish avoid less illuminated areas or in fact prefer them. Also, the uncertainty extends to whether they avoid or prefer areas that have dark bottoms or substrates and whether they avoid areas that are covered by solid (non-transparent) walls or actually prefer hiding in such places (for more detailed review and discussion of this topic see [6, 15, 37]. In addition, it has been shown that the actual level of illumination (which is almost never quantified) as well as the level of hunger and/or the presence or absence of certain olfactory cues can all alter light dark preference responses [37]. Given that several of such features of the light dark test remain debatable, at this point it is difficult to identify an optimal light dark task in which the anxiolytic properties of acute alcohol treatment may be evaluated. Another seemingly simple task in which fear reactions and/or the effects of acute alcohol treatment has been evaluated is the novel open tank. Novelty has been shown to induce fear responses in a variety of species including zebrafish [11, 21, 25], but novelty is not an easy fear inducing stimulus to work with. For example, it has no clear onset or offset and it has many components that are difficult to control, including handling of the subjects with the inherent variability involved in the process, which may in turn lead to inconsistent results. Similarly, the use of live predatory fish as stimulus [3] or the natural [34] or synthetic alarm substance of zebrafish [30] may not be appropriate for high throughput screening applications due to the fact that the live stimulus fish cannot be controlled consistently and odor cues are difficult to be turned on or off at will. Given these problems and the fact that the diurnal zebrafish may use vision as their primary modality, it was suggested that synthetic animated (moving) images may be appropriate for inducing fear responses [18]. These images may be turned on and off at will allowing not only precise spatial but also temporal control of their presentation. Analysis of the effects of a sympatric predator, and its animated image, however, revealed some controversial results, indicating that stimulus response depends not only upon its features but also on the context, for example the size of the experimental tank in which it is delivered [1, 18]. To address some of the above issues, we recently compared a series of fear inducing visual stimuli and showed that zebrafish have a stimulus specific and complex fear repertoire [27]. Also importantly, we identified aversive visual stimuli that appeared to induce the most robust and consistent reactions. The bird silhouette presented from above the test tank was one of these stimuli [27]. Our current results confirm this previous finding. At this point, we cannot claim that the shape of the stimulus is important, i.e. that zebrafish actually “recognize” the silhouette as that of a fishing bird, one of the potentially most dangerous types of sympatric predators for zebrafish [35]. It is possible that any large moving objects above the tank would elicit similar reactions. However, as we have shown before, the location of this stimulus, (shown from above in the current study) is crucial since the same stimulus elicited a significantly less robust and a different set of fear reactions when presented on the side of the test tank [27].
A strong reaction induced by the bird silhouette above the tank was found to be escaping to the bottom [27]. In the current study both the observation-based and the videotracking quantified data suggested that zebrafish spent more time near or swam closer to the bottom after acute exposure to alcohol, but only the videotracking results revealed that swimming to the bottom was induced by stimulus presentation and that alcohol counteracted this response. It is notable that discretized variables (such as time spent in particular areas in the tank) lead to loss of information as compared to continuously varying variables (such as the distance to bottom). Briefly, the more precise nature of the automated videotracking method, which allowed recording of the actual distance from the bottom, may explain the discrepancy between the videotracking and observation-based quantification methods. Nevertheless, the alcohol dependent reduction of the effect of the fear inducing stimulus found in the current study reflects the known anxiolytic effect of acutely administered alcohol.
General locomotory activity has been shown to be impaired (reduced) by acute alcohol in a dose dependent manner in the AB zebrafish strain [19]. The same acute doses have been shown to elicit an inverted U-shaped dose response with intermediate alcohol concentration treated fish showing hyper- while the highest (1%) alcohol concentration treated fish hypo-activity in some genetically heterogeneous outbred stocks of zebrafish [19, 21]. Analysis of the number of times fish crossed from one segment to another (the manually quantified ambulation score) showed no significant differences among the alcohol dose groups, the apparently linear dose dependent decline of locomotor activity shown on Fig. 4 is in line with what has been demonstrated for AB fish before [19]. It is also notable that in this previous study the highest concentration of alcohol applied was 1% (vol/vol), which was above the highest dose of the current study (0.75%). The reduction of overall activity across intervals we observed is also notable. It may be due to two separate factors that we cannot distinguish at this point: 1), habituation to the test environment (time dependent reduction of activity), or 2), increased passivity elicited by the aversive stimulus.
The analysis of activity levels as quantified by videotracking showed a different set of results. Perhaps the most interesting aspect of this analysis was the finding that hyperactivity was induced by an intermediate concentration of alcohol (0.50%) when the fear inducing stimulus was presented. Similar hyper-reactivity to a fear stimulus (a predator model moved manually) was shown in zebrafish with intermediate alcohol doses before [21]. This result signifies the complex mode of action of alcohol. Acute alcohol may blunt some, but enhance other fear reactions depending not only on type of administration regimen (e.g. acute vs. chronic), concentration, and strain of zebrafish used, but also on the actual behaviors measured. The discrepancy between the results of the activity measures obtained by the observation-based and videotracking methods is also important to discuss in this context. Notably, the ambulation score as quantified here reflects large scale locomotory activity. For example, it misses activity performed by the fish within segments. In contrast, the videotracking analysis does not distinguish large scale and small scale movements, it quantifies all activity. Previously, we have shown that antipredatory escape reactions may be associated with thrashing away from the stimulus, i.e. back and forth swimming against the glass wall of the test tank or by swimming on the area of the tank furthest away from the stimulus presented e.g. [1]. This response may be missed by measuring ambulation score (the movements may be occurring within one segment), but not by the videotracking analysis. It is possible that the increased activity shown by videotracking for the 0.50% alcohol concentration group is due to such smaller scale escape related movements and not to generally increased locomotor activity.
Freezing, i.e. the complete absence of movement, is often considered a typical and natural fear reaction in fish [21] and many other species including rodents [16]. It is assumed that freezing allows the prey to efficiently evade hunting predators that may not see, hear, or otherwise detect the motionless prey, e.g. [5]. In the current study, however, the fear stimulus did not affect the amount of freezing performed. Notably, freezing as a fear reaction has been found context dependent [5]. For example, freezing may manifest more robustly if hiding places are provided. The current test tank was barren, no hiding places were provided, and the distance from the surface (the location of presentation of the stimulus) was also relatively small, factors that may have biased our experimental fish against performing freezing. Freezing may occur also as a result of motor dysfunction and this may explain the enhanced freezing levels seen across all intervals in the highest concentration group. Last, hyperactivity (reduced freezing) was also reported after acute exposure to intermediate concentrations of alcohol in zebrafish [21], a finding that is in line with our results obtained for the 0.25% alcohol treated fish of the current study.
Erratic movement, although usually infrequently performed by zebrafish, has been found consistently exhibited in fear inducing paradigms [3, 11, 18, 34]. Similarly, in the current study, the number of erratic movement episodes was found to be robustly increased by the presentation of the bird silhouette. Also importantly, alcohol was found to significantly blunt the effect of the aversive stimulus and to reduce the number of erratic movement episodes elicited by this stimulus, an effect we attribute to the anxiolytic properties of this substance. The duration of erratic movement turned out to be a less reliable measure of fear induced by the stimulus and of the anxiolytic effects of alcohol, possibly due to higher inter-individual variability. We propose that initiation of erratic movement, and hence its frequency, better reflects the level of fear induced whereas the duration of this behavior may depend upon several factors that can vary stochastically. For example, within the confines of the small experimental tank a fish swimming erratically may bump into the glass walls and depending on the force of the physical contact and its frequency the fish may continue or arrest the behavior. Given that zig-zagging (erratic movement) is a rather uncoordinated movement, collisions with the solid glass wall may happen in a highly unpredictable, and thus variable, manner leading to varying duration of time for which the behavior is continued.
The frequency of jumping was found increased during the pre-stimulus period by intermediate alcohol doses. Given that during this period the aversive bird silhouette was not delivered, this alcohol induced behavioral change may be regarded as generalized hyperactivity [11, 25]. It is also notable that zebrafish in the control group performed increased number of jumps in response to the bird stimulus compared to their baseline level during the pre-stimulus period, but fish treated with intermediate alcohol doses reduced their jumping frequency during the presentation of the bird silhouette, again pointing towards the ability of acute alcohol exposure to blunt responses to aversive stimuli.
In summary, acute alcohol administration led to a number of behavioral changes that demonstrated the anxiolytic properties of this substance when administered acutely. The results also confirmed our previous conclusions [21, 27] that the fear response repertoire of zebrafish is complex and context dependent and that alcohol affects numerous aspects of these responses in a dose dependent manner. This conclusion is also in line with the experimental demonstration of context dependent sensitization to alcohol shown recently in zebrafish by Blaser et al [8].
Last, it is notable that the stimulus presentation and recording methods employed in the current study were computerized and thus allow scaling up, i.e. high throughput testing. The stimulus presentation required hardware and software components that are publically available and cheap, facilitating the running of multiple setups and thus making scaling up for screening purposes cost effective. Perhaps the only component that may be somewhat expensive is the video-tracking system that at this point may not be optimal for monitoring larger number of experimental tanks. Nevertheless, our findings suggest that fear paradigms using adult zebrafish will have utility in screening for drug and mutation induced changes in the effects of alcohol. We hope that ultimately such paradigms will facilitate our understanding of the mechanisms of the actions of alcohol on fear responses of zebrafish and, given the translational relevance of this species, ultimately those of human alcoholism and alcohol abuse as well.
RESEARCH HIGHLIGHTS.
A fear paradigm using an animated aversive visual stimulus tested the effects of acute alcohol exposure in zebrafish
The stimulus induced a diving response in zebrafish
Alcohol reduced the stimulus induced diving response
The stimulus increased the frequency of erratic movement of zebrafish
Alcohol reduced the stimulus induced increase of erratic movement
Acknowledgments
This study was supported by an R01 grant to RG from NIH/NIAAA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahmed O, Seguin D, Gerlai R. An automated predator avoidance task in zebrafish. Behav Brain Res. 2011;216:166–171. doi: 10.1016/j.bbr.2010.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfonso-Loeches S, Guerri C. Molecular and behavioral aspects of the actions of alcohol on the adult and developing brain. Crit Rev Clin Lab Sci. 2011;48:19–47. doi: 10.3109/10408363.2011.580567. [DOI] [PubMed] [Google Scholar]
- 3.Bass SLS, Gerlai R. Zebrafish (Danio rerio) responds differentially to stimulus fish: The effects of sympatric and allopatric predators and harmless fish. Behav Brain Res. 2008;186:107–117. doi: 10.1016/j.bbr.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 4.Bencan Z, Sledge D, Levin ED. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacol Biochem Behav. 2009;94:75–80. doi: 10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchard RJ, Magee L, Veniegas R, Blanchard DC. Alcohol and anxiety: ethopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry. 1993;17:171–182. doi: 10.1016/0278-5846(93)90041-p. [DOI] [PubMed] [Google Scholar]
- 6.Blaser RE, Peñalosa YM. Stimuli affecting zebrafish (Danio rerio) behavior in the light/dark preference test. Physiol Behav. 2011;104:831–837. doi: 10.1016/j.physbeh.2011.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Blaser RE, Chadwick L, McGinnis GC. Behavioral measures of anxiety in zebrafish (Danio rerio) Behav Brain Res. 2010;208:56–62. doi: 10.1016/j.bbr.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 8.Blaser RE, Koid A, Poliner RM. Context-dependent sensitization to ethanol in zebrafish (Danio rerio) Pharmacol Biochem Behav. 2010b;95:278–284. doi: 10.1016/j.pbb.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Blaser R, Gerlai R. Behav phenotyping in Zebrafish: comparison of three behavioral quantification methods. Behavior Res Methods. 2006;38:456–469. doi: 10.3758/bf03192800. [DOI] [PubMed] [Google Scholar]
- 10.Da Silva GE, Vendruscolo LF, Takahashi RN. Effects of ethanol on locomotor and anxiety-like behaviors and the acquisition of ethanol intake in Lewis and spontaneously hypertensive rats. Life Sci. 2005;77:693–706. doi: 10.1016/j.lfs.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 11.Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes Y, Gerlai R. Long-term behavioral changes in response to early developmental exposure to ethanol in zebrafish. Alcohol Clin Exp Res. 2009;33:601–609. doi: 10.1111/j.1530-0277.2008.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardner TJ, Kosten TR. Therapeutic options and challenges for substances of abuse. Dialogues Clin Neurosci. 2007;9:431–445. doi: 10.31887/DCNS.2007.9.4/tgardner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebauer DL, Pagnussat N, Piato AL, Schaefer IC, Bonan CD, Lara DR. Effects of anxiolytics in zebrafish: similarities and differences between benzodiazepines, buspirone and ethanol. Pharmacol Biochem Behav. 2011;99:480–486. doi: 10.1016/j.pbb.2011.04.021. [DOI] [PubMed] [Google Scholar]
- 15.Gerlai R. Zebrafish antipredatory responses: a future for translational research? Behav Brain Res. 2010;207:223–231. doi: 10.1016/j.bbr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gerlai R. Contextual learning and cue association in fear conditioning in mice: a strain comparison and a lesion study. Behav Brain Res. 1998;95:191–203. doi: 10.1016/s0166-4328(97)00144-7. [DOI] [PubMed] [Google Scholar]
- 17.Gerlai R, Chatterjee D, Pereira T, Sawashima T, Krishnannair R. Acute and Chronic alcohol dose: population differences in behavior and neurochemistry of zebrafish. Genes, Brain Behav. 2009;8:586–599. doi: 10.1111/j.1601-183X.2009.00488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerlai R, Fernandes Y, Pereira T. Zebrafish (Danio rerio) responds to the animated image of a predator: towards the development of an automated aversive task. Behav Brain Res. 2009b;201:318–324. doi: 10.1016/j.bbr.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerlai R, Prajapati S, Ahmad F. Differences in acute alcohol induced behavioral responses among zebrafish populations. Alcohol Clin Exp Res. 2008;32:1763–1773. doi: 10.1111/j.1530-0277.2008.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gerlai R, Lee V, Blaser R. Effects of acute and chronic ethanol exposure on the behavior of adult zebrafish (Danio rerio) Pharmacol Biochem Behav. 2006;85:752–761. doi: 10.1016/j.pbb.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerlai R, Lahav M, Guo S, Rosenthal A. Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacol Biochem Behav. 2000;67:773–782. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- 22.Gilman JM, Ramchandani VA, Davis MB, Bjork JM, Hommer DW. Why we like to drink: a functional magnetic resonance imaging study of the rewarding and anxiolytic effects of alcohol. J Neurosci. 2008;28:4583–4591. doi: 10.1523/JNEUROSCI.0086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levin ED. Zebrafish assessment of cognitive improvement and anxiolysis: filling the gap between in vitro and rodent models for drug development. Rev Neurosci. 2011;22:75–84. doi: 10.1515/RNS.2011.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levin ED, Bencan Z, Cerutti DT. Anxiolytic effects of nicotine in zebrafish. Physiol Behav. 2007;90:54–58. doi: 10.1016/j.physbeh.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 26.Lovinger DM, Crabbe JC. Laboratory models of alcoholism: treatment target identification and insight into mechanisms. Nat Neurosci. 2005;8:1471–1480. doi: 10.1038/nn1581. [DOI] [PubMed] [Google Scholar]
- 27.Luca RM, Gerlai R. In search of optimal fear inducing stimuli: differential behavioral responses to computer animated images in zebrafish. Behav Brain Res. 2011;226:66–76. doi: 10.1016/j.bbr.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mathur P, Guo S. Differences of acute versus chronic ethanol exposure on anxiety-like behavioral responses in zebrafish. Behav Brain Res. 2011;219:234–239. doi: 10.1016/j.bbr.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maximino C, Marques de Brito T, Dias CA, Gouveia A, Jr, Morato S. Scototaxis as anxiety-like behavior in fish. Nat Protoc. 2010;5:209–216. doi: 10.1038/nprot.2009.225. [DOI] [PubMed] [Google Scholar]
- 30.Parra KV, Adrian JC, Jr, Gerlai R. The synthetic substance hypoxanthine 3-N-oxide elicits alarm reactions in zebrafish (Danio rerio) Behav Brain Res. 2009;205:336–341. doi: 10.1016/j.bbr.2009.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips T. Animal models for the genetic study of human alcohol phenotypes. Alcohol Res Health. 2002;26:202–207. [PMC free article] [PubMed] [Google Scholar]
- 32.Saverino C, Gerlai R. The social zebrafish: behavioral responses to conspecific, heterospecific, and computer animated fish. Behav Brain Res. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silberman Y, Bajo M, Chappell AM, Christian DT, Cruz M, Diaz MR, et al. Neurobiological mechanisms contributing to alcohol-stress-anxiety interactions. Alcohol. 2009;43:509–519. doi: 10.1016/j.alcohol.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Speedie N, Gerlai R. Alarm substance induced behavioral responses in zebrafish (Danio rerio) Behav Brain Res. 2008;188:168–177. doi: 10.1016/j.bbr.2007.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spence R, Fatema MK, Reichard M, Huqk KA, Wahab MA, Ahmed ZF, et al. The distribution and habitat preferences of the zebrafish in Bangladesh. J Biol. 2006;69:1435–1448. [Google Scholar]
- 36.Steenbergen PJ, Richardson MK, Champagne DL. Patterns of avoidance behaviours in the light/dark preference test in young juvenile zebrafish: a pharmacological study. Behav Brain Res. 2011;222:15–25. doi: 10.1016/j.bbr.2011.03.025. [DOI] [PubMed] [Google Scholar]
- 37.Stephenson JF, Whitlock KE, Partridge JC. Zebrafish preference for light or dark is dependent on ambient light levels and olfactory stimulation. Zebrafish. 2011;8:17–22. doi: 10.1089/zeb.2010.0671. [DOI] [PubMed] [Google Scholar]
- 38.Wahlsten D. Insensitivity of the analysis of variance to heredity-environment interaction. Behav Brain Sci. 1990;13:109–161. [Google Scholar]