Abstract
The best described function of the adaptor complex-1 (AP-1) is to participate in the budding of clathrin-coated vesicles from the trans-Golgi network and endosomes. Here, we show that AP-1 is also localized to phagocytic cups in murine macrophages as well as in Dictyostelium amoebae. AP-1 is recruited to phagosomal membranes at this early stage of phagosome formation and rapidly dissociates from maturing phagosomes. To establish the role of AP-1 in phagocytosis, we made used of Dictyostelium mutant cells (apm1- cells) disrupted for AP-1 medium chain. In this mutant, phagocytosis drops by 60%, indicating that AP-1 is necessary for efficient phagocytosis. Furthermore, phagocytosis in apm1- cells is more affected for large rather than small particles, and cells exhibiting incomplete engulfment are then often observed. This suggests that AP-1 could participate in the extension of the phagocytic cup. Interestingly, macropinocytosis, a process dedicated to fluid-phase endocytosis and related to phagocytosis, is also impaired in apm1- cells. In summary, our data suggest a new role of AP-1 at an early stage of phagosome and macropinosome formation.
INTRODUCTION
Phagocytosis involves the uptake of large particles (typically 1 μm in diameter) by phagocytic cells. In the human organism, phagocytic cells are responsible for the elimination of invasive microorganisms and thus participate in the immune defense. Phagocytosis is triggered by the recognition of particles by cell surface phagocyte receptors. This event results in the activation of signaling pathways, mainly depending on phosphatidyl inositol 3-kinase (Cox et al., 1999; Vieira et al., 2001) and syk tyrosine kinase (Durden and Liu, 1994; Greenberg et al., 1994; Cox et al., 1996) and leading to actin polymerization underneath the contact zone, pseudopodia extension, and particles sequestration in membrane-bound organelles called phagosomes. Phagosomes undergo a maturation process, which involves extensive recycling of proteins back to the cell surface and fusion with endocytic compartments. Whereas the fusion of endosomes with maturing phagosomes has been investigated in detail (for review, see Vieira et al., 2002), the mechanisms responsible for the transport of components into and out of phagosomes are still poorly understood.
Transport of proteins in the endocytic and biosynthetic pathways occurs via vesicles coated with specific proteins (Mellman, 1996; Rothman and Wieland, 1996). One of the best-characterized coat proteins is clathrin, which participates in multiple transport steps (Hirst and Robinson, 1998; Smith and Pearse, 1999). There is some evidence that clathrin associates with the phagocytic cup in mammalian cells, although its precise role remains unclear (Aggeler and Werb, 1982; Montesano et al., 1983; Clerc and Sansonetti, 1989). Because adaptor protein complexes (APs) are the main components of clathrin-coated vesicles, a specific AP complex is likely to be associated to clathrin on phagosomes. One early study on APs has reported that AP-2 adaptor complexes, specifically involved in endocytosis, are detected in purified phagosomes (Pitt et al., 1992). Since then, no other studies using more specific and reliable antibodies to AP subunits have confirmed this localization. To our knowledge, the role of other AP complexes in phagocytosis has never been studied.
The amoeba Dictyostelium discoideum is a professional phagocyte that can feed upon bacteria. This unicellular, eucaryotic organism has served as a paradigm for studying phagocytosis due to the development of genetic tools. The phagocytic mechanism at play in Dictyostelium is very similar to that described in mammalian cells (Cardelli, 2001; Rupper and Cardelli, 2001), particularly in the involvement of actin, and actin-binding regulatory proteins at the early stage of phagosome formation.
In this study, we report for the first time that clathrin-associated AP-1 complexes localize to the phagocytic cups of mammalian macrophages and Dictyostelium cells. Taking advantage of Dictyostelium mutant cells deficient in AP-1 medium chain subunit expression, we demonstrate that AP-1 is involved in an early step of phagocytosis and macropinocytosis, leading us to propose a new role for the AP-1 coat.
MATERIALS AND METHODS
Cell Culture, Plasmids, and Antibodies
D. discoideum strain DH1-10 (Cornillon et al., 2000), a subclone of DH1 (Caterina et al., 1994), was cultured up to a maximum density of 2 × 106 cells/ml at 22°C in HL5 medium.
Full-length (nucleotides 1-1287) and truncated (nucleotides 1-1233) apm1 cDNAs, encoding μ1 and μ1ΔCt, respectively, were amplified by PCR, sequenced (MWG-Biotech, Ebersberg, Germany) and cloned into the Dictyostelium expression vector pDXA-3C (Manstein et al., 1995).
Plasmid WF38 was a kind gift from P. Devreotes (Johns Hopkins Medical Institution, Baltimore, MD). This plasmid (Parent et al., 1998) contains the sequence coding for the pleckstrin homology domain of cytosolic regulator of adenyl cyclase (CRAC) fused to green fluorescent protein under the control of the actin promoter, for constitutive expression in Dictyostelium cells.
The polyclonal antibody to Dictyostelium μ1 was described previously (Lefkir et al., 2003). The rabbit polyclonal antibody to Dictyostelium cathepsin D was a kind gift from Dr. J. Garin (CEA, Grenoble, France). Monoclonal antibodies (mAbs) against coronin (176-2-5) and vacuolin B (221-1-1) have been characterized previously (Jenne et al., 1998; de Hostos, 1999). The mAb H161 is specific for a p80 cell surface marker (Ravanel et al., 2001). The rat anti-phagosome mAb M12A9, which cross-reacts with Dictyostelium γ-adaptin, has been reported previously (Morrissette et al., 1999) and characterized (Lefkir et al., 2003). Anti-mouse α- and γ-adaptin (A43920 and A36120, respectively) were from BD Transduction Laboratories (Lexington, KY).
Immunofluorescence Microscopy
Immunofluorescence microscopy of zymosan phagosomes in murine peritoneal macrophages and in Dictyostelium was performed as described previously (Ozinsky et al., 2000; Decave et al., 2002). Samples were analyzed using a Zeiss Axiovert microscope equipped with Bio-Rad confocal optics. Phagocytosis experiments in RBL-2H3 cells were performed as described previously (Massol et al., 1998). Briefly, cells were first incubated with IgE anti-DNP (dinitrophenyl) (1 μg/ml) for 1 h at 37°C, washed twice, and then incubated at 4°C for 15 min with tetramethylrhodamine B isothiocyanate (TRITC)-yeast coupled to DNP. After warming up at 37°C for 20 min, cells were fixed in 4% paraformaldehyde, permeabilized with saponin, and processed for immunofluorescence. To visualize phagocytic cups, Dictyostelium cells expressing the CRAC-green fluorescent protein (GFP) fusion protein were incubated with yeast cells for 15 min in HL5 medium and treated as described above. Dictyostelium and RBL-2H3 cells were visualized with a Zeiss confocal microscope (LSM510).
Adhesion of Cells to Substrate
The cell detachment assay was performed at 21°C in HL5 medium, according to Decave et al., 2002.
Scanning Electron Microscopy
For scanning electron microscopy, cells were fixed on plastic cell culture plates by using 2% glutaraldehyde in 200 mM phosphate buffer, pH 6, for 1 h. Cells were rinsed and postfixed in 1% osmium tetroxide in 100 mM phosphate buffer, pH 6, for 45 min. The fix solution was removed and cells were progressively dehydrated through an ethanol series of 30-100% ethanol followed by incubation into hexamethyl disilane. After air drying, the cells were sputter-coated in gold and viewed on a Hitachi S800 scanning electron microscope.
Fluid-Phase Endocytosis and Phagocytosis Assays
For fluid-phase endocytosis assays, cells were resuspended at 4 × 106 cells/ml in HL5 medium containing 2 mg/ml fluorescein isothiocyanate (FITC)-dextran (70 kDa; Molecular Probes, Eugene, OR) and incubated at 22°C. For each time point, 1-ml aliquots were transferred to 10 ml of ice-cold Soerensen buffer (SB; 0.017 M Na2HPO4/KH2PO4 pH 6.3). After two washes in cold SB and in 10 mM glycine buffer, pH 9.3, cells were lysed in 50 mM glycine buffer pH 9.3 containing 0.3% Triton X-100, and then lysates were cleared by quick centrifugation. Fluorescence was quantified using a spectrofluorometer (excitation and emission wavelengths 485 and 515 nm, respectively).
To observe the formation of macropinocytic cups, cells were grown on glass coverslips in HL5 medium and observed by phase contrast with a Zeiss Axiovert 100 microscope. Pictures were recorded every 6 s with a Hamamatsu Orca camera and analyzed using OpenLab 3 software.
Phagocytosis experiments in Dictyostelium were performed as described previously (Cornillon et al., 2000). Briefly, 105 cells were incubated for the indicated times in 1 ml of fresh HL5 medium containing either 1 μl of 1-μm-diameter fluoresbrite YG carboxylate microspheres (Polysciences, Warrington, PA) or 5 × 107 TRITC-labeled Klebsiella aerogenes or Escherichia coli bacteria. After washing twice in cold HL5 containing 0.1% sodium azide, cells were analyzed using a cell flow spectrofluorometer (FACSCalibur; BD Biosciences, San Jose, CA).
Isolation of Phagosomes
Isolation of phagosomes was essentially performed as described previously (Gotthardt et al., 2002). The following buffers were used: homogenization buffer (20 mM HEPES-KOH, pH 7.2, 0.25 M sucrose), membrane buffer (20 mM HEPES-KOH, pH 7.2, 20 mM KCl, 2.5 mM MgCl2, 1 mM dithiothreitol, 20 mM NaCl), storage buffer (25 mM HEPES-KOH, pH 7.2, 1.5 mM Mg-acetate, 1 mM NaHCO3, 1 μM CaCl2, 25 mM KCl, 1 mM ATP, 1 mM dithiothreitol, 100 mM sucrose)
Phagosomes at different stages of maturation were obtained after feeding with latex beads according to the following pulse-chase regime. Cells (109) were incubated with 2 × 1011 latex beads of 0.8 μm diameter (Sigma-Aldrich, St. Louis, MO), first on ice in 5 ml of SB/120 mM sorbitol, pH 8, and then in 100 ml of HL5 medium at room temperature in shaking culture (120 rpm) at a concentration of 107 cells/ml. Phagocytosis was stopped by the addition of 3 volumes of ice-cold SB/sorbitol and centrifugation. The chase was started by resuspending the cells in 100 ml of medium at room temperature to continue the process, and then stopped as described above for the pulse. Cells were homogenized by 8 passages through a ball bearing cell cracker. The homogenate was incubated with 10 mM ATP and 10 mM MgCl2 for 15 min before loading onto the sucrose step gradients. Gradients were centrifuged for 3 h at 100,000 × g in a Beckmann Coulter SW 28 rotor. The 10%/25% sucrose interface was collected, diluted in ∼30 ml of membrane buffer, and centrifuged for 1 h at 100,000 × g in the same rotor. The pellet was then resuspended in storage buffer and mixed with Laemmli buffer for SDS-PAGE. To normalize the phagosome concentrations between different samples, the volumes of storage buffer and Laemmli buffer were adjusted for each sample by calculating the number of latex beads trapped in phagosomes by measuring light scattering at 600 nm. Samples were analyzed by immunoblotting by using a chemiluminescence imager (LAS-1000; Fujifilm, Tokyo, Japan) and quantitation was carried out using Image Gauge version 3.0 (Fujifilm).
RESULTS
AP-1 Localizes to Nascent Phagosomes
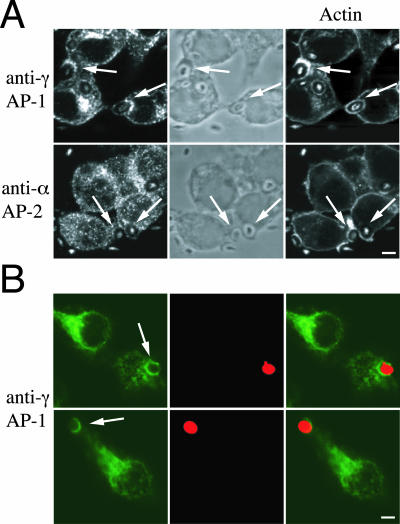
The presence of clathrin on membranes of forming phagosomes (Aggeler and Werb, 1982; Montesano et al., 1983; Clerc and Sansonetti, 1989; Castellano et al., 2000; Tse et al., 2003) suggested that clathrin-associated adaptor complexes could associate with phagosomes. To test this hypothesis, mouse peritoneal macrophages were incubated with zymosan particles and stained with anti-γ-adaptin (AP-1) or anti-α-adaptin (AP-2). In addition to perinuclear trans-Golgi network (TGN) membranes, γ-adaptin localized to phagosomes (staining around zymosan particles), whereas α-adaptin stained vesicles but not the phagocytic membrane (Figure 1A). Interestingly, γ-adaptin decorated phagosomes from the start of phagocytosis (together with actin), even before phagosome closure, and remained on the phagosomes even after actin dissociation (our unpublished data). The presence of γ-adaptin on phagosomes was not restricted to peritoneal macrophages but was also observed in phagocytic rat basophilic leukemia (RBL) cells (Figure 1B), a cellular model for Fc receptor-mediated phagocytosis (Pierini et al., 1996; Massol et al., 1998).
Figure 1.
AP-1 but not AP-2 localizes to phagosomes in mammalian macrophages. (A) Mouse macrophages were incubated with zymosan particles and stained with anti-γ-adaptin or anti-α-adaptin antibodies and incubated with phalloidin to detect actin. Cells were observed by phase-contrast (middle) and fluorescence microscopy. In addition to the TGN, γ-adaptin localized to phagosomes, whereas α-adaptin was excluded from phagocytic membranes. Arrows indicate phagocytic cups containing zymosan. (B) Phagocytic RBL-2H3 cells pretreated with IgE anti-DNP were incubated for 15min with TRITC-yeast cells coupled to DNP (red) and stained with the anti-γ-adaptin antibody (green). Double staining of yeast and γ-adaptin is shown on right panels. As expected, γ-adaptin localized to perinuclear TGN membranes but also to phagosomes of RBL cells. To exclude that γ-adaptin staining was due to the presence of Fc receptors on RBL plasma membranes, specificity controls were systematically performed (our unpublished data). Arrows indicate phagosomes. Bar, 10 μm.
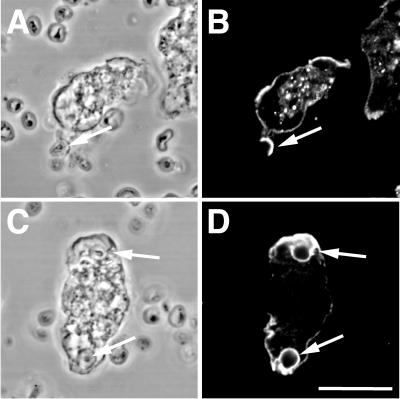
Next, we assessed whether this observation could be extended to another professional phagocytes amenable to genetic and biochemical studies, the amoeba D. discoideum. Immunofluorescence studies showed that the M12A9 antibody specific of Dictyostelium γ-adaptin (AP-1) (Morissette et al., 1999; Lefkir et al., 2003) labeled phagocytic cups as well as edges of the lamellae and also internal structures, which might correspond to the Dictyostelium Golgi apparatus of Dictyostelium cells (Figure 2, B and D).
Figure 2.
γ-adaptin is recruited to Dictyostelium phagosomes. Dictyostelium cells were fed on bacteria and stained with the M12A9 mAb (γ-adaptin, B and D). Cells were observed by phase-contrast (A and C) and fluorescence microscopy (B and D). M12A9 labeled phagosomes and the edges of actin-rich lamellae. Arrows indicate phagocytic cups containing zymosan particles. Bar, 10 μm.
Together, these results demonstrate for the first time that the AP-1 clathrin-associated complexes localizes on phagosomal membranes in three different model phagocytes.
AP-1 Association with Phagosomes Is Lost during Phagosome Maturation
The localization of γ-adaptin on nascent phagosomes suggested that AP-1 could play a role in early steps of phagocytosis both in Dictyostelium and mammalian cells. To determine the dynamics of AP-1 on phagosomes during phagosome maturation in Dictyostelium, phagosomes containing latex beads were isolated at different stages of maturation and analyzed for their content in AP-1. Biochemical characterization of isolated phagosomes indicated that the isolation procedure yielded very pure preparation of phagosomes with little contamination by membranes of the endosomal/lysomal pathway or from the endoplasmic reticulum and mitochondria as detected by immunoblotting experiments (Gotthardt et al., 2002).
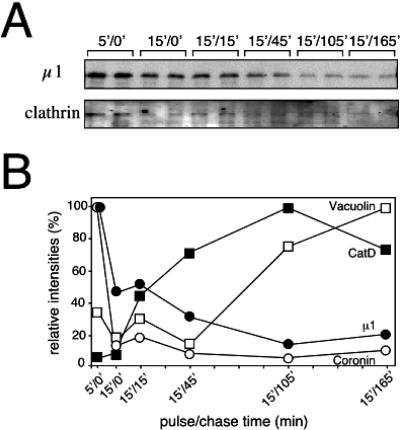
After a 5-min pulse with latex beads, μ1 (AP-1 medium chain) was detected on purified phagosomes, as was the actin-associated protein coronin (Figure 3). Because the purified phagosomes were devoid of associated vesicles and tubules (Gotthardt et al., 2002), this result demonstrated that AP-1 is truly associated with the phagosome membrane itself. About one-half of the μ1 signal disappeared after a 15-min chase and then slowly decreased with time as phagosome maturation progressed, as attested by the accumulation of the lysosomal enzyme CatD and vacuolin B, a marker of the postlysosomal compartment (Figure 3B). Interestingly, AP-1 and coronin showed similar dynamics as both proteins rapidly separated from phagosomes. When the same phagosomal preparations were tested for their contents in clathrin heavy chain, a weak signal after the progressive release of AP-1 was observed (Figure 3A). Therefore, AP-1 could be partially associated with clathrin on phagosomal membranes, although the biochemical procedure followed for phagosome purification could affect the integrity of clathrin-coated structures. Together, these biochemical data reinforced the possibility that AP-1 plays a role in phagosome biogenesis.
Figure 3.
Dynamics of AP-1 localization on purified phagosomes. (A) Phagosomes from different stages of maturation were obtained after feeding with latex beads (5- or 15-min pulse) followed by culture in HL5 medium for the indicated times (chase). Protein composition of the phagosomes was analyzed by immunoblotting with rabbit anti-μ1 and anti-clathrin heavy-chain sera as indicated. (B) Signals obtained above were quantified and expressed as percentage of signal obtained after a 5-min pulse with latex beads. μ1 (closed circles) separated from phagosomes at early time points as coronin (open circles). In contrast, the lysosomal hydrolase cathepsin D (closed squares) and the late endocytic marker vacuolin (open squares) accumulated in maturing phagosomes.
Phagocytosis Is Impaired in apm1- Cells
Because AP-1 is localized on Dictyostelium phagocytic cups, we took advantage of the genetic tools available in this organism to determine the role of the AP-1 coat complex in phagocytosis. We recently characterized the gene encoding the AP-1 medium chain subunit (apm1) (de Chassey et al., 2001). The targeted disruption of apm1 results in viable cells (apm1- cells) that show a specific defect in sorting and transport of lysosomal enzymes (Lefkir et al., 2003). In addition, apm1- cells fed upon bacteria show a slow growth rate that could reflect a defect in phagocytic uptake.
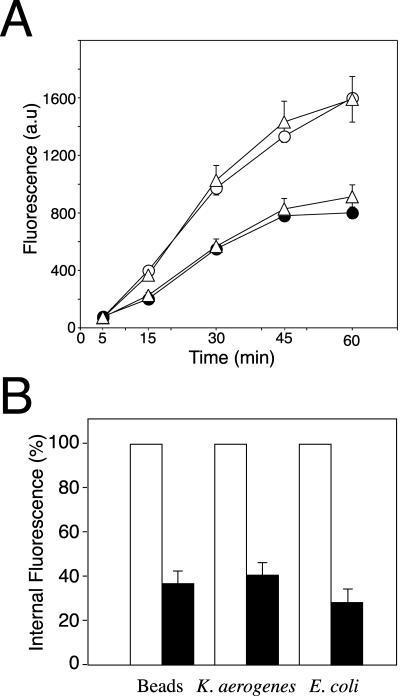
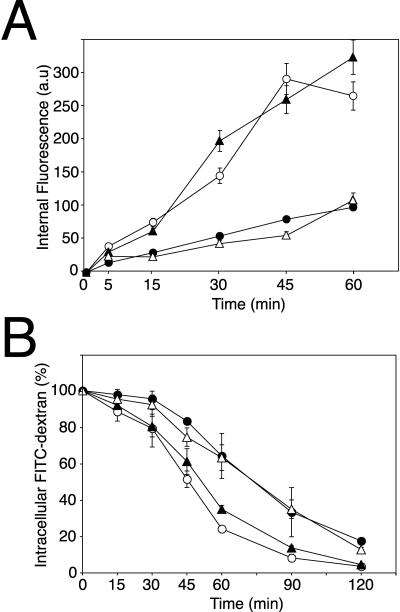
To evaluate the role of AP-1 in phagocytosis, apm1- mutant cells were incubated for different periods of time with fluorescent microspheres and the intracellular fluorescence was measured by flow cytometry. Compared with wild-type cells, apm1- cells showed a reduced uptake (Figure 4A) similar to that observed in the phagocytic mutant phg1 (Cornillon et al., 2000) (our unpublished data). Interestingly, whereas the stable transfection of native μ1 (apm1- + μ1) restored phagocytosis at a level comparable to wild-type cells (DH1), μ1 truncated by deletion of its 18 C-terminal residues expressed in mutant cells (apm1- + μ1ΔCt) is still impaired in phagocytosis (Figure 4A). The deleted domain in μ1ΔCt corresponds to a region in murine μ2 required for the interaction between μ chains and tyrosine-based signals (Owen and Evans, 1998). This truncated μ1 chain still associates with other AP-1 subunits, but the resulting AP-1 complexes are impaired in lysosomal enzyme targeting (Lefkir et al., 2003).
Figure 4.
Phagocytosis is defective in μ1 null cells. (A) Time course of TRITC-microspheres uptake. Control (open circles), apm1- (closed circles), apm1- + μ1ΔCt (open triangles), and apm1- + μ1 (closed triangles) were incubated with TRITC-microspheres for the indicated times. Uptake was measured by fluorescence-activated cell sorting analysis and results expressed as fluorescence arbitrary units (a.u). Stable transfection of native μ1 but not μ1ΔCt chains restored normal phagocytic capacities to apm1- cells. (B) μ1 null cells show phagocytic uptake defects. Control (open boxes) and mutant cells (closed boxes) were incubated for 20 min with TRITC-microspheres, TRITC-labeled K. aerogenes or DH5α bacteria and intracellular fluorescence was measured by flow cytometry. Results are expressed as the percentage of intracellular fluorescence of wild-type cells. In comparison to control cells, μ1 null cells showed an uptake reduction of 60% for all tested particles.
This result demonstrates that AP-1 is important for the phagocytic process. Notably, the μ1ΔCt construct did not rescue the phagocytic defect of apm1- cells, suggesting that fully functional μ1 chains with intact sorting capacities are required for efficient phagocytosis.
To determine whether the phagocytic defect in apm1- cells was dependent on the nature of the internalized particle, cells were incubated with latex beads, K. aerogenes, or E. coli bacteria. A 60% reduction in uptake was observed for all particles tested (Figure 4B). These results demonstrate that the AP-1 adaptor complex is required for efficient phagocytosis of latex beads and bacteria.
Cell Surface Adhesion Is Not Affected in apm1- Cells
An early step in phagocytosis is the adhesion of particles to the plasma membrane. As reported for several mutants defective in phagocytosis, the phagocytic defect in apm1- cells could result from defective cellular adhesion. To test this possibility, adhesion was assayed by measuring the hydrodynamic stress necessary to detach cells from a glass plate (Decave et al., 2002). Although the shear flow of culture medium required for detaching 50% of the adhesion mutant phg1 (Cornillon et al., 2000) was <0.03 Pa, kinetics was similar for wild-type and apm1- cells (0.6 ± 0.1 and 0.5 ± 0.2 Pa, respectively). Thus, AP-1 disruption does not lead to a significant defect in cellular adhesion. It is therefore unlikely that the phagocytic defect in apm1- cells is due to a defect in adhesion to internalized particles.
Sorting of an Endosomal Marker Is Not Affected in apm1- Cells
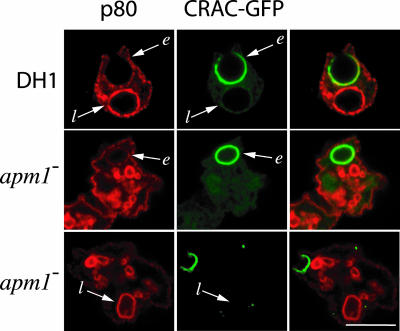
The p80 protein was characterized previously as a marker of the endocytic pathway (Ravanel et al., 2001). Indeed, p80 is detected at the cell surface (at a very low concentration), in early endocytic compartments (at a low concentration), and also in late endocytic compartments (at a high concentration) in both DH1 and apm1- cells (Lefkir et al., 2003). During the formation of phagosomes, p80 is found in the phagocytic cup at a concentration equivalent to that found at the plasma membrane. As phagosomes mature and fuse with endocytic compartments, they acquire high levels of p80 protein (Ravanel et al., 2001). To assess the role of AP-1 in membrane sorting in the phagocytic pathway, we analyzed p80 localization during phagocytosis in cells expressing CRAC-GFP, a convenient marker for newly formed phagosomes. The CRAC-GFP protein binds on the cytosolic face of forming phagosomes and dissociates a few minutes after the closure of the phagosome (Parent et al., 1998; Ravanel et al., 2001). In apm1- mutant cells, we observed a pattern identical to that seen in wild-type cells: p80 is incorporated in nascent phagosomes (phagocytic cups, CRACGFP positive), whereas mature phagosomes devoid of CRAC-GFP showed high amounts of p80 both in wild-type and apm1- cells (Figure 5). Therefore, AP-1 is apparently not involved in membrane sorting of p80 in the phagocytic pathway.
Figure 5.
Sorting of a cell surface protein during phagosome maturation. In axenic growth condition, p80 localized at the plasma membrane and in late endocytic compartments both in DH1 and apm1- cells. After 15 min of incubation with yeast, the localization of p80 (red staining) during phagocytosis in cells expressing CRACGFP (green staining), a marker for newly formed phagosomes, was analyzed. p80 was detected on the phagocytic cup (CRAC-GFP structures, designated e) of DH1 and apm1- cells at a level similar to that observed at the plasma membrane. Mature phagosomes devoid of CRAC-GFP (designated l) showed high amounts of p80 in both cell types. Double staining of p80 and CRAC-GFP is shown on right panels. Arrows indicate phagosomes. Bar, 10 μm.
AP-1 Is Required for Membrane Extension around Particles
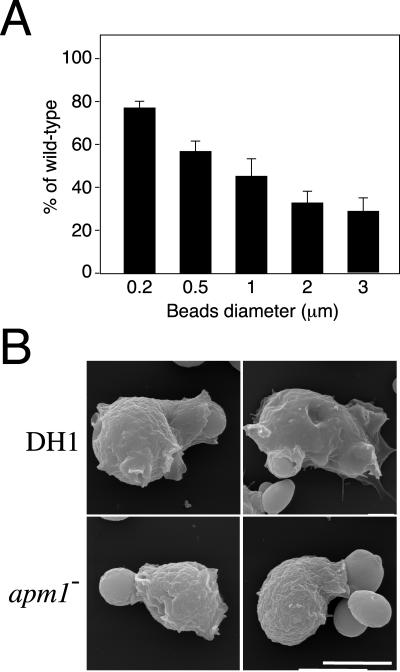
On particle recognition, signaling events lead to pseudopodia elongation and particles sequestration in these membrane extensions. The early recruitment of AP-1 on forming phagosomes suggested that this coat protein complex could participate in the formation of nascent phagosomes from the plasma membrane. To determine whether μ1 deletion interfered with this membrane extension process, apm1- cells were incubated with beads of increasing diameter. Indeed, ingestion of large particles requires larger amount of membranes than with small particles, and a defect in the process of membrane delivery to the phagocytic area should result in a phagocytic defect proportional to the size of the particle. As observed in Figure 6A, phagocytosis in apm1- cells was less efficient for large than small particles, with a maximal defect observed for the largest particles tested (3 μm in diameter). Interestingly, scanning electron microscopy revealed that after incubation with yeast cells (approximatively 3 μm in diameter), apm1- cells frequently showed incompletely engulfed particles, suggesting that engulfment is slowed down in these mutant cells (Figure 6B). However, further confocal microscopy analysis of apm1- cells expressing CRAC-GFP ruled out any blockade of phagocytosis at this stage (Figure 5; our unpublished data). Together, these results suggest that AP-1 participate in the membrane extension around particles required for particle engulfment.
Figure 6.
Role of AP-1 in membrane delivery during phagocytosis. (A) μ1 null cells show phagocytic uptake defects proportional to the size of particles. Control and mutant cells were incubated for 20 min with FITC-microspheres of different diameter, and intracellular fluorescence was measured by flow cytometry. Results are expressed as percentage of intracellular fluorescence of wild-type cells. (B) DH1 and apm1- cells were incubated with yeast, fixed, and processed for scanning electron microscopy. In contrast to DH1 cells, incomplete engulfment of particles was often observed in apm1- cells. Bars, 7.5 μm.
Fluid-Phase Uptake Is Affected in apm1- Cells
In Dictyostelium, macropinocytosis accounts for most of fluid-phase uptake (Hacker et al., 1997). Because the early stages of macropinocytosis and phagocytosis share some similarities (Cardelli, 2001; Rupper and Cardelli, 2001), apm1 disruption could also impair macropinocytosis. To test this hypothesis, cells were incubated for different periods with the fluid-phase marker FITC-dextran and intracellular fluorescence was measured by fluorimetry (Figure 7A). After 30 min, the rate of fluid-phase endocytosis was significantly reduced in mutant cells compared with control cells (0.9 μl for 4 × 106 cells/min and 2.5 μl for 4 × 106 cells/min, respectively), a defect comparable with the defect observed for phagocytosis of particles. Whereas the stable transfection of native μ1 (apm1- + μ1) restored the uptake at a level comparable with wild-type cells (DH1), μ1 truncated by deletion of its 18 C-terminal residues expressed in mutant cells (apm1- + μ1ΔCt) did not complement the uptake defect (Figure 7A), thus suggesting that μ1 sorting capacities are required for efficient macropinocytosis. This slow accumulation of FITC-dextran in apm1- cells was not due to a faster rate of exocytosis (Figure 7B). Conversely, apm1- and apm1- + μ1ΔCt cells showed a slight reduction in exocytosis of internalized FITC-dextran. Therefore, the fluid-phase endocytosis defect observed in apm1- cells suggests that AP-1 is implicated in the pinocytic process.
Figure 7.
Fluid-phase endocytosis is deficient in μ1 null cells. (A) Fluid-phase endocytosis is defective in μ1 mutants. Control (open circles), apm1- (closed circles), apm1- + μ1ΔCt (open triangles), and apm1- + μ1 (closed triangles) were incubated for different periods of time with the fluid-phase marker, FITC-dextran, and the amount of FITC-dextran internalized per 4 × 106 cells was plotted. apm1- and apm1- + μ1ΔCt cells showed a reduced rate of endocytosis compared with control and apm1- + μ1 cells. (B) Exocytosis is slightly reduced in μ1 null cells. Control (open circles), apm1- (closed circles), apm1- + μ1ΔCt (open triangles), and apm1- + μ1 (closed triangles) were allowed to internalized FITC-dextran for 2 h to load all endocytic compartments. After incubation in fresh HL5 medium for the indicated time, the fluorescence remaining into the cells was quantified and expressed as the percentage of the total fluorescence endocytosed.
DISCUSSION
In this study, we report for the first time that a clathrin-associated complex plays a functional role in phagocytosis and macropinocytosis, a process dedicated to fluid-phase endocytosis and related to phagocytosis. Taking advantage of genetic tools available in Dictyostelium, we demonstrate that the deletion of one single AP subunit, μ1, results in major defects in particle and fluid-phase uptake. In addition, we establish that the μ1 Ct domain, which was shown previously to interact with tyrosine-based sorting signals, is essential for these two uptake processes, suggesting that the AP-1 sorting function is important for both phagocytosis and macropinocytosis. The evidence presented here suggests that AP-1 is implicated in an early step shared between both phagocytosis and macropinocytosis and leads to membrane extension around particles.
AP-1 Is Required for Efficient Phagocytosis
Several electron microscopy studies on macrophages have reported the enrichment of nascent phagosomal membranes with large flat clathrin patches, which disappear after 30 min (Aggeler and Werb, 1982; Montesano et al., 1983; Clerc and Sansonetti, 1989). Clathrin-coated pits have been also observed on phagosomes upon engagement of Fc receptors in phagocytic RBL-2H3 cells (Castellano et al., 2000). We now further extend these observations by showing that major components of clathrin coats, the adaptor protein complexes, are also present in forming phagosomes. Our study provides evidence for the specific distribution of the AP-1 clathrin-adaptor complex on the phagocytic cup of mouse peritoneal macrophages and macrophagic RBL-2H3 cells with dynamics comparable with that of clathrin recruitment. Remarkably, AP-2, whose function is restricted to the endocytosis of cell surface integral proteins, is not detected on nascent phagosomes, suggesting that phagocytosis and endocytosis make use of distinct sets of AP complexes. This result is in contradiction with previous work showing that AP-2 is present on purified phagosomes (Pitt et al., 1992), although the presence of AP-2 could be due to contamination of phagosome preparations with plasma membranes or to specificity problems of the anti-AP-2 antibody used in that study. The reason for this discrepancy will have to be further investigated.
The recruitment of AP-1 to the phagocytic cup is the most unexpected finding of our work. However, the text-book notion that AP-1 is only present on the trans-Golgi apparatus has been revisited over the past few years. For instance, AP-1 has been localized on endosomes and implicated both in the transport of the transferrin receptor from apical-to-basolateral membranes in epithelial cells (Futter et al., 1998) and the recycling of the low-density lipoprotein receptor and the transferrin receptor to the basolateral membrane (Gan et al., 2002). Thus, AP-1 could function in more than one location, although how a single vesicular coat could play multiple roles is not understood yet. Another puzzling observation we made is that AP-1 is specifically recruited to forming phagosomes and excluded from the plasma membrane. Further studies in our laboratory will be aimed at understanding the molecular mechanisms responsible for this specific and transient AP-1 recruitment on forming phagosomes.
Our results provide several lines of evidence suggesting that in addition to their localization on phagosomes, AP-1 coat complexes play also an important role in phagocytosis. First, γ-adaptin, as well as μ1, specifically localize to Dictyostelium phagosomes and this localization disappears concomitantly with phagosome maturation. Second, deletion of the apm1 gene results in a reduced uptake for all particles tested, whereas adhesion is not affected in mutant cells. Finally, the extension of membrane around large particles is affected in apm1- cells.
Do clathrin and adaptor proteins also participate in phagocytosis in other professional phagocytes? In mammalian cells, clathrin has been implicated in the phagocytic process. Liposome-mediated delivery of antibodies to clathrin was shown to inhibit alveolar macrophage phagocytosis (Perry et al., 1999). Furthermore, the presence on phagosomes of molecules associated to the clathrin machinery and their role in phagocytosis has been previously reported, reinforcing the role of clathrin mediated events in phagocytosis. Hence dynamin 2, which is a GTPase thought to participate in the scission of clathrin-coated structures, was found to be enriched on early phagosomes and dynamin dominant-negative forms inhibited phagocytosis (Gold et al., 1999). Time-lapse studies of RAW 264.7 macrophages expressing EGFP-dynamin 2 further established that dynamin 2 encircles particles during engulfment and that upon completion of phagocytosis, EGFP-dynamin 2 dissociates from phagosomes (Di et al., 2003). Also, amphiphysin II m, an isoform of amphiphysin II that recruits dynamin, was localized on early phagosomes and mutant forms also inhibited phagocytosis (Gold et al., 2000). More recently, Grinstein and colleagues have reported opposite effects of clathrin and dynamin I mutants on phagocytosis in RAW 264.7 cells (Tse et al., 2003); therefore, the purpose of clathrin on nascent phagosomes is still unclear.
Recently, gene knockdown experiments in Drosophila S2 cells, by using double-stranded RNA interference, have shown that clathrin, AP-1 and the nonclathrin coat COP1 are involved in phagocytosis of bacteria (Ramet et al., 2002). Thus, RNA interference-reduced expression of COP1 subunits led to a severe inhibition of phagocytosis (between 73 and 81%, depending on the targeted subunit and the bacterial strain used in the assay), whereas inhibition of clathrin, Hsp70, or the β1-AP-1 subunit only resulted in a moderate decrease in uptake (∼30%).
In mammalian cells, evidence also suggests that the coat protein complex COPI is recruited to phagosomes and is involved in the selective retrieval of the transferrin receptor (TfR) to the cell surface (Botelho et al., 2000; Beron et al., 2001; Hackam et al., 2001). Interestingly, impairing COPI function only partially inhibits TfR removal from phagosomes and the recycling of other receptors to the cell surface seems COPI independent. These observations have led to the proposal that protein recycling to the plasma membrane could be mediated by COPI but also by an alternative mechanism. Our results in Dictyostelium suggest that this COPI-independent pathway could rely on AP-1. Indeed, phagosome maturation occurs concomitantly with the disappearance of AP-1 from phagosomal membranes (Figure 3), as expected for a coat required for vesicle budding from phagosomes. In addition, apm1- cells show only a partial defect in phagocytosis, implicating that an AP-1 independent mechanism (e.g., COPI mediated) could contribute to this residual phagocytic activity. The distinct roles of COPI and AP-1 in phagocytosis will have to be further assessed in Dictyostelium.
μ1 Deletion Also Affects Fluid-Phase Endocytosis
In Dictyostelium, macropinocytosis is responsible for most fluid-phase uptake (Hacker et al., 1997). The actin cytoskeleton and actin-associated proteins, such as coronin, play an essential role in this process, especially for the formation of membrane ruffles that protrude from the cell surface and engulf the fluid phase (de Hostos, 1999). Interestingly, there is a close relationship between the first steps of macropinosome and phagocytic cup formation, because the presence of coronin, for instance, is essential for both processes (Hacker et al., 1997) and several proteins are shared for their regulation (Cardelli, 2001; Rupper and Cardelli, 2001). Because apm1- cells show similar defects (60% reduction) in these two processes, AP-1 could participate in an early step shared between theses two uptake mechanisms. Noteworthy, Clathrin heavy-chain null mutant cells (chc-) are also severely impaired in fluid-phase endocytosis (Ruscetti et al., 1994). Together with our results, this confirms a role for clathrin and adaptor proteins in macropinocytosis. A clathrin- and actin-independent mechanism of fluid-phase uptake, distinct from macropinocytosis, was recently identified (Neuhaus et al., 2002). This pathway could explain the partial inhibition of fluid-phase uptake observed both in chc- and apm1- cells.
Is AP-1 Involved in Membrane Delivery to Forming Phagosomes and Macropinosomes?
The formation of phagosomes requires the mobilization of a large amount of membranes and still there is no loss of surface area of phagocytes, suggesting that membranes from intracellular pools are added to forming phagosomes (Booth et al., 2001). Focal exocytosis of membrane enriched in the VAMP3, a v-SNARE found on recycling vesicles, has been reported at the phagocytic cups of chinese hamster ovary cells stably expressing FcγRIIA receptors (Bajno et al., 2000). The role of recycling endosomes in membrane delivery is also documented for macrophages in which the GTPase rab11, implicated in transferrin recycling from endosomes, is also detected in nascent phagosomes (Cox et al., 2000). Recently, focal exocytosis was shown to depend on Dynamin 2 and amphiphysin II (Di et al., 2003). Another membrane reservoir, the endoplasmic reticulum, has been also described for mouse macrophages (Gagnon et al., 2002).
One possible explanation for the localization of AP-1 on phagosomes could be that this decoration results from the fusion of TGN-derived AP-1-positive tubulo-vesicular structures with the phagocytic cup, bringing membranes or other components required for phagosome formation. Thus, AP-1 could facilitate phagosome formation by recruiting factors implicated in cytoskeleton reorganization or membrane remodeling upon particle engulfment. This possibility is strengthened by recent live cell microscopy studies on YFP-tagged AP-1 expressed in human fibroblast cells (Huang et al., 2001; Waguri et al., 2003). In contrast to AP-2, AP-1 is not released upon vesicle budding from the TGN but instead stays on tubulo-vesicular structures rapidly moving along microtubular tracks toward the cell periphery and eventually fusing with peripheral AP-1-positive structures (Huang et al., 2001; Waguri et al., 2003). According to this last hypothesis, the phagocytic defects in apm1- cells would result from the inability of such cells to provide membranes at the site of the forming phagosome. The fact that the uptake of large particles is less efficient in apm1- cells and that incomplete engulfment is observed confirm this prediction.
In conclusion, the inactivation of μ1-AP-1 subunit in Dictyostelium suggests new functions for this clathrin-associated adaptor complex. Additional biochemical and genetic studies in Dictyostelium will be required to further dissect the function of AP-1 coats in the phagocytic and macropinocytic processes.
Acknowledgments
We thank B. Hoflack (Max-Planck-Institute of Molecular Cell Biology and Genetics, Dresden, Germany) and P. Rousselle (Institut de Biologie et Chemie des Protéines, Lyons, France) for helpful discussions and encouragement. This work was supported by grants from the Association pour la Recherche sur le Cancer, the Fondation pour la Recherche Médicale, and the “Emergence” program of the Région Rhône-Alpes. P.C.'s laboratory is funded by a START Fellowship of the Fonds National Suisse dela Recherche Scientifique and a grant from the Fondation Gabriella Giorgi-Cavaglieri. The work by D.G. and T.S. was supported by the Max-Planck Society.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-06-0365. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-06-0365.
References
- Aggeler, J., and Werb, Z. (1982). Initial events during phagocytosis by macrophages viewed from outside and inside the cell: membrane-particle interactions and clathrin. J. Cell Biol. 94, 613-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajno, L., Peng, X.R., Schreiber, A.D., Moore, H.P., Trimble, W.S., and Grinstein, S. (2000). Focal exocytosis of VAMP3-containing vesicles at sites of phagosome formation. J. Cell Biol. 149, 697-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beron, W., Mayorga, L.S., Colombo, M.I., and Stahl, P.D. (2001). Recruitment of coat-protein-complex proteins on to phagosomal membranes is regulated by a brefeldin A-sensitive ADP-ribosylation factor. Biochem. J. 355, 409-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth, J.W., Trimble, W.S., and Grinstein, S. (2001). Membrane dynamics in phagocytosis. Semin. Immunol. 13, 357-364. [DOI] [PubMed] [Google Scholar]
- Botelho, R.J., Hackam, D.J., Schreiber, A.D., and Grinstein, S. (2000). Role of COPI in phagosome maturation. J. Biol. Chem. 275, 15717-15727. [DOI] [PubMed] [Google Scholar]
- Cardelli, J. (2001). Phagocytosis and macropinocytosis in Dictyostelium: phosphoinositide-based processes, biochemically distinct. Traffic 2, 311-320. [DOI] [PubMed] [Google Scholar]
- Castellano, F., Montcourrier, P., and Chavrier, P. (2000). Membrane recruitment of Rac1 triggers phagocytosis. J. Cell Sci. 113, 2955-2961. [DOI] [PubMed] [Google Scholar]
- Caterina, M.J., Milne, J.L., and Devreotes, P.N. (1994). Mutation of the third intracellular loop of the cAMP receptor, cAR1, of Dictyostelium yields mutants impaired in multiple signaling pathways. J. Biol. Chem. 269, 1523-1532. [PubMed] [Google Scholar]
- Clerc, P.L., and Sansonetti, P.J. (1989). Evidence for clathrin mobilization during directed phagocytosis of Shigella flexneri by HEp2 cells. Microb. Pathog. 7, 329-336. [DOI] [PubMed] [Google Scholar]
- Cornillon, S., Pech, E., Benghezal, M., Ravanel, K., Gaynor, E., Letourneur, F., Bruckert, F., and Cosson, P. (2000). Phg1p is a nine-transmembrane protein superfamily member involved in Dictyostelium adhesion and phagocytosis. J. Biol. Chem. 275, 34287-34292. [DOI] [PubMed] [Google Scholar]
- Cox, D., Chang, P., Kurosaki, T., and Greenberg, S. (1996). Syk tyrosine kinase is required for immunoreceptor tyrosine activation motif-dependent actin assembly. J. Biol. Chem. 271, 16597-16602. [DOI] [PubMed] [Google Scholar]
- Cox, D., Lee, D.J., Dale, B.M., Calafat, J., and Greenberg, S. (2000). A Rab11-containing rapidly recycling compartment in macrophages that promotes phagocytosis. Proc. Natl. Acad. Sci. USA 97, 680-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, D., Tseng, C.C., Bjekic, G., and Greenberg, S. (1999). A requirement for phosphatidylinositol 3-kinase in pseudopod extension. J. Biol. Chem. 274, 1240-1247. [DOI] [PubMed] [Google Scholar]
- de Chassey, B., Dubois, A., Lefkir, Y., and Letourneur, F. (2001). Identification of clathrin-adaptor medium chains in Dictyostelium discoideum: differential expression during development. Gene 262, 115-122. [DOI] [PubMed] [Google Scholar]
- de Hostos, E.L. (1999). The coronin family of actin-associated proteins. Trends Cell Biol. 9, 345-350. [DOI] [PubMed] [Google Scholar]
- Decave, E., Garrivier, D., Brechet, Y., Bruckert, F., and Fourcade, B. (2002). Peeling process in living cell movement under shear flow. Physiol. Rev. Lett. 89, 108101. [DOI] [PubMed] [Google Scholar]
- Di, A., Nelson, D.J., Bindolias, V., Brown, M.E., Libonao, F., and Palfrey, H.C. (2003). Dynamin regulates focal exocytosis in phagocytosing macrophages. Mol. Biol. Cell 14, 2016-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durden, D.L., and Liu, Y.B. (1994). Protein-tyrosine kinase p72syk in Fc gamma RI receptor signaling. Blood 84, 2102-2108. [PubMed] [Google Scholar]
- Futter, C.E., Gibson, A., Allchin, E.H., Maxwell, S., Ruddock, L.J., Odorizzi, G., Domingo, D., Trowbridge, I.S., and Hopkins, C.R. (1998). In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules. J. Cell Biol. 141, 611-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon, E., Duclos, S., Rondeau, C., Chevet, E., Cameron, P.H., Steele-Mortimer, O., Paiement, J., Bergeron, J.J., and Desjardins, M. (2002). Endoplasmic reticulum-mediated phagocytosis is a mechanism of entry into macrophages. Cell 110, 119-131. [DOI] [PubMed] [Google Scholar]
- Gan, Y., McGraw, T.E., and Rodriguez-Boulan, E. (2002). The epithelial-specific adaptor AP1B mediates post-endocytic recycling to the basolateral membrane. Nat. Cell Biol. 4, 605-609. [DOI] [PubMed] [Google Scholar]
- Gold, E.S., Morrissette, N.S., Underhill, D.M., Guo, J., Bassetti, M., and Aderem, A. (2000). Amphiphysin IIm, a novel amphiphysin II isoform, is required for macrophage phagocytosis. Immunity 12, 285-292. [DOI] [PubMed] [Google Scholar]
- Gold, E.S., Underhill, D.M., Morrissette, N.S., Guo, J., McNiven, M.A., and Aderem, A. (1999). Dynamin 2 is required for phagocytosis in macrophages. J. Exp. Med. 190, 1849-1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotthardt, D., Warnatz, H.J., Henschel, O., Bruckert, F., Schleicher, M., and Soldati, T. (2002). High-resolution dissection of phagosome maturation reveals distinct membrane trafficking phases. Mol. Biol. Cell 13, 3508-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg, S., Chang, P., and Silverstein, S.C. (1994). Tyrosine phosphorylation of the gamma subunit of Fc gamma receptors, p72syk, and paxillin during Fc receptor-mediated phagocytosis in macrophages. J. Biol. Chem. 269, 3897-3902. [PubMed] [Google Scholar]
- Hackam, D.J., Botelho, R.J., Sjolin, C., Rotstein, O.D., Robinson, J.M., Schreiber, A.D., and Grinstein, S. (2001). Indirect role for COPI in the completion of FCgamma receptor-mediated phagocytosis. J. Biol. Chem. 276, 18200-18208. [DOI] [PubMed] [Google Scholar]
- Hacker, U., Albrecht, R., and Maniak, M. (1997). Fluid-phase uptake by macropinocytosis in Dictyostelium. J. Cell Sci. 110, 105-112. [DOI] [PubMed] [Google Scholar]
- Hirst, J., and Robinson, M.S. (1998). Clathrin and adaptors. Biochim. Biophys. Acta 1404, 173-193. [DOI] [PubMed] [Google Scholar]
- Huang, F., Nesterov, A., Carter, R.E., and Sorkin, A. (2001). Trafficking of yellow-fluorescent-protein-tagged μ1 subunit of clathrin adaptor AP-1 complex in living cells. Traffic 2, 345-357. [DOI] [PubMed] [Google Scholar]
- Jenne, N., Rauchenberger, R., Hacker, U., Kast, T., and Maniak, M. (1998). Targeted gene disruption reveals a role for vacuolin B in the late endocytic pathway and exocytosis. J. Cell Sci. 111, 61-70. [DOI] [PubMed] [Google Scholar]
- Lefkir, Y., de Chassey, B., Dubois, A., Bogdanovic, A., Brady, R.J., Destaing, O., Bruckert, F., O'Halloran, T.J., Cosson, P., and Letourneur, F. (2003). The AP-1 clathrin-adaptor is required for lysosomal enzymes sorting and biogenesis of the contractile vacuole complex in Dictyostelium cells. Mol. Biol. Cell 14, 1835-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manstein, D.J., Schuster, H.P., Morandini, P., and Hunt, D.M. (1995). Cloning vectors for the production of proteins in Dictyostelium discoideum. Gene 162, 129-134. [DOI] [PubMed] [Google Scholar]
- Massol, P., Montcourrier, P., Guillemot, J.C., and Chavrier, P. (1998). Fc receptor-mediated phagocytosis requires CDC42 and Rac1. EMBO J 17, 6219-6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman, I. (1996). Endocytosis and molecular sorting. Annu. Rev. Cell Dev. Biol. 12, 575-625. [DOI] [PubMed] [Google Scholar]
- Montesano, R., Mossaz, A., Vassalli, P., and Orci, L. (1983). Specialization of the macrophage plasma membrane at sites of interaction with opsonized erythrocytes. J. Cell Biol. 96, 1227-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morissette, N.S., Gold, E.S., Guo, J., Hamerman, J.A., Ozinsky, A., Bedian, V., and Aderem, A.A. (1999). Isolation and characterization of monoclonal antibodies directed against novel components of macrophage phagosomes. J. Cell Sci. 112, 4705-4713. [DOI] [PubMed] [Google Scholar]
- Neuhaus, E.M., Almers, W., and Soldati, T. (2002). Morphology and dynamics of the endocytic pathway in Dictyostelium discoideum. Mol. Biol. Cell 13, 1390-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen, D.J., and Evans, P.R. (1998). A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282, 1327-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozinsky, A., Underhill, D.M., Fontenot, J.D., Hajjar, A.M., Smith, K.D., Wilson, C.B., Schroeder, L., and Aderem, A. (2000). The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc. Natl. Acad. Sci. USA 97, 13766-13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent, C.A., Blacklock, B.J., Froehlich, W.M., Murphy, D.B., and Devreotes, P.N. (1998). G protein signaling events are activated at the leading edge of chemotactic cells. Cell 95, 81-91. [DOI] [PubMed] [Google Scholar]
- Perry, D.G., Daugherty, G.L., and Martin, W.J., 2nd. (1999). Clathrin-coated pit-associated proteins are required for alveolar macrophage phagocytosis. J. Immunol. 162, 380-386. [PubMed] [Google Scholar]
- Pierini, L., Holowka, D., and Baird, B. (1996). Fc epsilon RI-mediated association of 6-micron beads with RBL-2H3 mast cells results in exclusion of signaling proteins from the forming phagosome and abrogation of normal downstream signaling. J. Cell Biol. 134, 1427-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt, A., Mayorga, L.S., Stahl, P.D., and Schwartz, A.L. (1992). Alterations in the protein composition of maturing phagosomes. J. Clin. Investig. 90, 1978-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramet, M., Manfruelli, P., Pearson, A., Mathey-Prevot, B., and Ezekowitz, R.A. (2002). Functional genomic analysis of phagocytosis and identification of a Drosophila receptor for E. coli. Nature 416, 644-648. [DOI] [PubMed] [Google Scholar]
- Ravanel, K., de Chassey, B., Cornillon, S., Benghezal, M., Zulianello, L., Gebbie, L., Letourneur, F., and Cosson, P. (2001). Membrane sorting in the endocytic and phagocytic pathway of Dictyostelium discoideum. Eur. J. Cell Biol. 80, 754-764. [DOI] [PubMed] [Google Scholar]
- Rothman, J.E., and Wieland, F.T. (1996). Protein sorting by transport vesicles. Science 272, 227-234. [DOI] [PubMed] [Google Scholar]
- Rupper, A., and Cardelli, J. (2001). Regulation of phagocytosis and endophagosomal trafficking pathways in Dictyostelium discoideum. Biochim. Biophys. Acta 1525, 205-216. [DOI] [PubMed] [Google Scholar]
- Ruscetti, T., Cardelli, J.A., Niswonger, M.L., and O'Halloran, T.J. (1994). Clathrin heavy chain functions in sorting and secretion of lysosomal enzymes in Dictyostelium discoideum. J. Cell Biol. 126, 343-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, C.J., and Pearse, B.M. (1999). Clathrin: anatomy of a coat protein. Trends Cell Biol. 9, 335-338. [DOI] [PubMed] [Google Scholar]
- Tse, S.M., Furuya, W., Gold, E., Schreiber, A.D., Sandvig, K., Inman, R.D., and Grinstein, S. (2003). Differential role of actin, clathrin, and dynamin in Fc gamma receptor-mediated endocytosis and phagocytosis. J. Biol. Chem. 278, 3331-3338. [DOI] [PubMed] [Google Scholar]
- Vieira, O.V., Botelho, R.J., and Grinstein, S. (2002). Phagosome maturation: aging gracefully. Biochem. J. 366, 689-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira, O.V., Botelho, R.J., Rameh, L., Brachmann, S.M., Matsuo, T., Davidson, H.W., Schreiber, A., Backer, J.M., Cantley, L.C., and Grinstein, S. (2001). Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J. Cell Biol. 155, 19-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waguri, S., Dewitte, F., Le Borgne, R., Rouille, Y., Uchiyama, Y., Dubremetz, J.F., and Hoflack, B. (2003). Visualization of TGN to endosome trafficking through fluorescently labeled MPR and AP-1 in living cells. Mol. Biol. Cell 14, 142-155. [DOI] [PMC free article] [PubMed] [Google Scholar]