Abstract
Rett syndrome is a neurodevelopmental disorder caused by loss of function mutations in the gene encoding the transcription factor methyl-CpG-binding protein 2 (MeCP2). One of its targets is the gene encoding brain-derived neurotrophic factor (bdnf). In vitro studies using cultured neurons have produced conflicting results with respect to the role of MeCP2 in BDNF expression. Acute intermittent hypoxia (AIH) induces plasticity in the respiratory system characterized by long-term facilitation of phrenic nerve amplitude. This paradigm induces an increase in BDNF protein. We hypothesized that AIH leads to augmentation of BDNF transcription in respiratory-related areas of the brainstem and that MeCP2 is necessary for this process. Wild-type and mecp2 null (mecp2−/y) mice were subjected to three 5-min episodes of exposure to 8% O2/4% CO2/88% N2, delivered at 5-min intervals. Normoxia control wild-type and mecp2 null mice were exposed to room air for the total length of time, i.e. 30 min. Following a recovery in room air, the pons and medulla were rapidly removed. Expression of BDNF protein and transcripts were determined by ELISA and quantitative PCR, respectively. AIH induced a significant increase in BDNF protein in the pons and medulla, and in mRNA transcript levels in the pons of wild-type animals. In contrast, there were no significant changes in either BDNF protein or transcripts in the pons or medulla of mice lacking Mecp2. The results indicate that Mecp2 is required for regulation of BDNF expression by acute intermittent hypoxia in vivo.
Keywords: medulla, pons, Rett syndrome
Rett syndrome (RTT) is a neurodevelopmental disorder caused by loss-of-function mutations in the X-linked gene encoding methyl-CpG-binding protein 2 (MeCP2; (Amir et al., 1999). Girls born with RTT show relatively normal development during their first 6–18 months of life, followed by sudden regression that can result in severe respiratory deficiencies among other problems (Chahrour and Zoghbi, 2007, Bird, 2008). Mecp2 protein is abundantly expressed in the brain in both neurons and glia (Ballas et al., 2009, Lioy et al., 2011a), and its levels increase progressively during the postnatal period of neuronal maturation, reaching mature levels around postnatal day (P) 35 in mice (Wang et al., 2006, Cohen and Greenberg, 2010, Cohen et al., 2011). In male mouse models that are null for Mecp2, the levels of brain-derived neurotrophic factor (BDNF), a neurotrophin essential for neuronal survival, differentiation, and synaptic plasticity, have been shown to be depleted relative to wild-type (WT) mice in a number of brain regions (Chang et al., 2006, Wang et al., 2006, Zhou et al., 2006, Ogier et al., 2007). Interestingly, BDNF levels in wild-type mice stay elevated during the perinatal period and decrease at around P35, coinciding with full expression of Mecp2 and with the onset of the pathological phenotype in null males, which are normal at birth and in juveniles (Chen et al., 2001, Guy et al., 2001). Thus, BDNF depletion in mecp2 null mutants may be caused by an inability to upregulate BDNF expression in the absence of Mecp2.
Mouse and rat BDNF is encoded by a complex gene that consists of eight 5′ untranslated exons all of which are individually spliced onto a common protein-coding 3′ exon. The neuronal activity-dependent regulation of bdnf specific promoter regions results in the spatial and temporal expression of specific bdnf transcripts, and thus control bdnf gene expression at several levels, including transcription, mRNA stability, translation, and sub-cellular distribution (Lauterborn et al., 1996, Liu et al., 2006, Aid et al., 2007). Newborn mice lacking functional bdnf (bdnf −/− mutants) exhibit severe respiratory disturbances (Erickson et al., 1996, Balkowiec and Katz 1998) that likely contribute to the lethality occurring shortly after birth. Indeed, BDNF has been shown to be required for survival of chemoafferent sensory neurons in the petrosal and nodose ganglia that innervate the carotid body chemoreceptors to provide sensory inputs to central respiratory circuits in the hindbrain (Hertzberg et al., 1994, Erickson et al., 1996, Katz, 2005). Moreover, BDNF is required for normal development of the central respiratory pattern generator (Balkowiec and Katz 1998). Together, these findings suggest that BDNF is required for the proper development and maintenance of the normal breathing behavior in mice. Significantly, mecp2-null mice show deficits in BDNF protein in the structures critical for respiration and autonomic control, including nodose ganglia and the brainstem (Chang et al., 2006, Wang et al., 2006, Zhou et al., 2006, Ogier et al., 2007).
Acute intermittent hypoxia (AIH) is a well-studied paradigm for the induction of respiratory plasticity characterized by long-lasting increases in phrenic nerve amplitude (reviewed in (Dale-Nagle et al., 2010a, Dale-Nagle et al., 2010b)). This long-term facilitation is dependent on BDNF (Baker-Herman et al., 2004). These studies have shown that serotonin acting at 5-HT 2 receptors activates protein kinase C which, in turn, induces expression of BDNF protein. We reasoned that, if Mecp2 is essential for induction of BDNF expression, a physiological protocol, such as AIH, would fail to modulate BDNF in mice that lack Mecp2.
Neurons in both the pons and medulla are affected by hypoxia and modulated by BDNF. The lateral parabrachial nucleus in the pons contributes to changes in respiratory frequency during hypoxia (Song and Poon, 2009). Inhibitory synapses in the Kölliker-Fuse nucleus, also in the pons, are modulated by BDNF (Kron et al., 2007). Similarly, neurons in the medullary preBötzinger complex respond to hypoxia (reviewed in(Feldman et al., 2003)) and BDNF modulates the activity of these neurons (Thoby-Brisson et al., 2003).
The present study characterizes the expression of BDNF in two functionally distinct regions of the brainstem responsible for control of respiration, i.e. the pons and the medulla, in wild-type and mecp2−/y mice, using ELISA and quantitative PCR methodologies to determine protein and transcript levels, respectively.
Experimental procedures
Animals
The protocols used were approved by Institutional Animal Care and Use Committee of the Oregon Health and Science University, and were in agreement with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Studies were performed with B6.129P2(C)- mecp2tm1.1Bird (stock number 003890; Jackson Laboratory, Bar Harbor, ME) null mice and wild-type littermates. Mice were genotyped by PCR as previously described (Miralves et al., 2007). The strain was originally generated by insertion of loxP sites around mecp2 exons 3 and 4, followed by crossing homozygous “floxed” females with CMV Cre males (Guy et al., 2001).
Oxygen administration and tissue dissections
Mice were placed in a 2-liter clear Plexiglas chamber with access to food and water. The chamber was fitted with input and output ports for flow-through administration of respiratory gases, as previously described (Bissonnette and Knopp, 2008). To study the effects of acute intermittent hypoxia, animals were exposed to three 5-min episodes of hypoxia (8% O2/4% CO2/88% N2) each followed by a 5-min recovery period (room air). CO2 was added in order to prevent the time-dependent decline from the initial increase in minute ventilation (roll off) during exposure to hypoxia (Bissonnette and Knopp, 2006). In separate studies, respiratory parameters, frequency and tidal volume, were measured with body plethysmography as previously described (Bissonnette and Knopp, 2006). Following intermittent hypoxia, mice were returned to baseline conditions (normal air) for 60 min for the bdnf transcript studies and 180 min for the BDNF protein studies. Immediately following recovery, animals were euthanized (EuthasolR, approximately 0.1 ml/100 g body weight, intraperitoneally), and weighed. The brainstem with adjacent cervical spinal cord was removed from each animal and divided into pons, medulla and cervical spinal cord (C3–C5). Tissue samples were immediately placed in individual 1.5-ml microcentrifuge tubes. For bdnf transcripts by real-time PCR, each RNAse-free tube contained 1 mL of pre-chilled TRIzol and the tissue was immediately homogenized for RNA extraction at room temperature. For BDNF ELISA, each tube was siliconized (Sigmacote®; Sigma), pre-weighed and pre-chilled. The brain (without pons and medulla) was weighed immediately after the dissection. Pons and medulla samples that were processed for BDNF protein by ELISA were also weighed.
BDNF ELISA
The tissue-containing tubes were weighed, and 100 μl of pre-chilled lysis buffer [20 mM Tris buffer, pH 7.4, 137 mM NaCl, 1% Nonidet-P40, 10% glycerol, 1 mM phenylmethanesulfonyl fluoride (PMSF), 0.5 mM sodium vanadate, 10 μM aprotinin, 10 μM actinonin, and 100 μM leupeptin] was added to each tube, followed by mechanical grinding of the tissue with Kontes® Pellet Pestle® (Kimble-Chase, Vineland, NJ). Next, 400 μl of Block & Sample buffer (BDNF Emax™ ImmunoAssay System, Promega, Madison, WI) was added, and samples (total volume of 500 μl) were sonicated on ice using a microprobe sonicator (2 × 3.0 W, 5 sec each; Sonicator 3000, Misonix, Inc., Farmingdale, NY). The resulting crude lysate was transferred to an anti-BDNF-coated 96-well ELISA plate (100 μl per well; 3 wells per sample). BDNF ELISA was performed according to the manufacturer’s protocol (BDNF Emax™ ImmunoAssay System, Promega). BDNF levels were calculated from the standard curve prepared for each plate, using SOFTmax PROv.® 4.3 software (Molecular Devices). The standard curves were linear within the range used (0–500 pg/ml) and the quantities of BDNF in experimental samples were always within the linear range of the standard curve.
RNA extraction and real-time PCR
Total RNA was extracted using TRIzol (Invitrogen) according to the manufacturer’s instructions, followed by re-suspension in 10 mM Tris pH 8.5 and storage at −80°C until use. To make cDNA, 1 μg of total RNA was reverse transcribed (20 μl) using Tetro cDNA synthesis kit (Bioline, Taunton, MA). Quantitative PCR was performed in triplicates following the MIQE guidelines (Bustin et al., 2009) in a final volume of 10 μl [2 μl of cDNA (diluted 1:4), 3 μM of each forward and reverse primers, and Sensimix Plus SYBR master mix (Quantace, Taunton, MA)]. The amplification reaction was carried out using a MX3000P real-time PCR system (Stratagene; Cedar Creek, TX). All primer sequences (see below) were designed using Primer3 online software and synthesized by Integrated DNA Technologies (Coralville, IA). To verify primer specificity, DNA products were amplified by PCR using Taq polymerase (Invitrogen, Carlsbad, CA). Single PCR products were gel-purified using QIAquick Gel Extraction Kit (Qiagen, Valencia, CA), sequenced (OHSU DNA sequencing facilities) and quantified spectrophotometrically. Real-time PCR was performed with DNA standard dilutions ranging from 10 pg to 10 ag. The amplification data were collected continuously using the software supplied with the MX3000P real-time PCR system. Cq values for standard curves were between 10- and 30, with Cq values for samples falling within this range. Quantitative PCR efficiencies were all above 97%. The primer sequences for the BDNF-protein-coding region were 5′-ctcccccttttaactgaagagaa -3′ (forward) and 5′-attcacgctctccagagtcc -3′ (reverse), with an amplicon size of 188 bp. Data normalization was carried out against the endogenous reference gene transcript hypoxanthine phosphoribosyltransferase 1 (HPRT, forward: 5′-caggccagactttgttggat-3, reverse: 5′-ggctggcctataggctcatag-3′; PCR product of 287 bp in size). Genbank accession numbers are NM001048142.1 (BDNF protein-coding region), and NM013556.2 (HPRT).
Data analysis
To analyze differences in body and brain weight, as well as age of the animals, an unpaired t-test was used to detect significant differences between wild-type and mecp2−/y mutants. For bdnf expression patterns, transcript levels obtained from real-time PCR measurements were normalized relative to hprt levels for each sample (average of bdnf triplicates divided by average of hprt triplicates). To compare the expression of BDNF protein and transcripts in the pons and in the medulla, t-test was used when the data were normally distributed and Mann-Whitney rank sum test when distribution was not normal. Results are presented as mean ± S.E.M. Differences were considered significant when p-value was below 0.05.
Results
Animal body and brain weights
Knockout mouse models with disrupted Mecp2 function result in a phenotype that mimics many key clinical features of the Rett syndrome, including an early normal life followed by regression in development that have, among other consequences, irregular breathing and respiratory distress (Chen et al., 2001, Guy et al., 2001). Consistent with the data reported previously (Guy et al., 2001), the overall body and brain of mecp2−/y mice were significantly smaller in both size and weight compared to their WT littermates (Table 1). The average body weight of WT mice was 3.36 g greater than mecp2−/y mice (95% CI: 2.247 – 4.479). Similarly, the average weight of WT brain (without the pons and medulla) was 0.042 g greater than the average weight of mecp2−/y brain (95% CI: 0.031 – 0.053). Importantly, the observed differences cannot be accounted for by differences in developmental stage, as the average age of animals was not significantly different between the two groups (44.4 ± 0.64 days for WT, n=36, and 45.3 ± 0.82 days for mecp2−/y mice, n=32; 95% CI: 43.05–45.66 and 43.64–46.99, respectively, p=0.355).
Table 1.
Body and brain weights of wild-type and mecp2-deficient mice
| Wild-type | n | mecp2−/y (n=32) | n | p value | |
|---|---|---|---|---|---|
| Body (g) | 20.908 ± 0.312 | 36 | 17.546 ± 0.477 | 32 | <0.0001* |
| Brain (g)a | 0.357 ± 0.004 | 36 | 0.315 ± 0.004 | 32 | <0.0001* |
| Pons (g) | 0.029 ± 0.001 | 13 | 0.023 ± 0.001 | 11 | <0.0001* |
| Medulla (g) | 0.024 ± 0.001 | 13 | 0.020 ± 0.001 | 11 | 0.005* |
without pons and medulla.
Significant (unpaired t-test).
In order to establish that the hypoxia protocol reliably affects respiratory parameters, we examined the protocol in a separate group of mice. In WT mice hypoxia increased respiratory frequency from 271 ± 8 to 305 ± 6 breaths per minute and tidal volume from 108 ± 1.8 to 137 ± 6.1μl. In mecp2 null animals, respiratory frequency increased from 259 ± 10 to 294 ± 14 breaths per minute, whereas tidal volume from 86 ± 8.2 to 114 ± 12.5μl.
Effect of acute intermittent hypoxia on BDNF protein expression
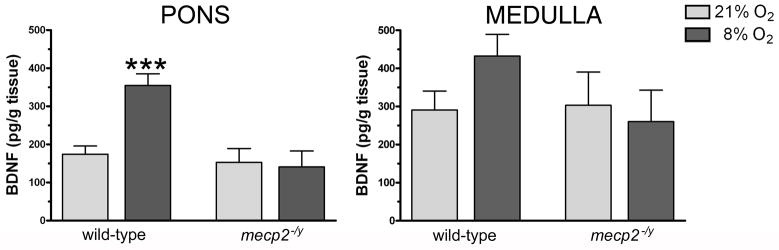
The differences between WT and mecp2−/y animals were revealed by the AIH challenge, which resulted in a dramatic increase in BDNF protein levels in the pons of WT mice (p=<0.001, t-test) and also in the medulla (p<0.05, Mann-Whitney). In contrast, AIH failed to induce changes in BDNF protein in either the pons or medulla of mecp2 null mice (Fig. 1). There was a considerable variability in baseline BDNF protein levels within both brain regions among individual litters (Table 2). Therefore, we separately analyzed effects of AIH on BDNF expression within littermates. In WT pons, AIH increased BDNF protein in all 6 litters that totaled 8 comparisons (85.8 to 266.75% above normoxia). In mecp2 null mutants, however, BDNF protein in the pons decreased in two matched littermates (28.6 and 69.0% of the normoxic value), was unchanged in two, and increased in one pair (30.5% above normoxia). Similarly in the WT medulla, AIH caused an increase in BDNF in 6 littermate pairs (90.0 to 165.7% above normoxia), was unchanged in one, and decreased in one (40.3% of normoxia). BDNF protein in mecp2 null medulla decreased in three matched littermates (9.3, 14.4 and 41.0% of normoxia), was unchanged in one, and increased in one (116.2% above normoxia). We attempted to measure BDNF protein in the cervical spinal cord, but the levels were below the delectability limit of the assay (data not shown).
Figure 1. Changes in BDNF protein in the brainstem of wild-type and mecp2−/y mice in response to acute intermittent hypoxia.
Levels of BDNF protein in the pons and medulla of wild-type (WT) and mecp2 null (mecp2−/y) mice exposed to normoxia (21% O2, light gray bars) or acute intermittent hypoxia (8% O2, black bars, please see Experimental Procedures for details of the acute intermittent hypoxia protocol). In the pons (left panel), BDNF protein levels were significantly increased in response to intermittent hypoxia in WT mice (p=<0.001; t-test), while no hypoxia-induced changes were detected in mecp2 null mutants. In the medulla (right panel), intermittent hypoxia elevated BDNF protein levels in WT (p=0.022; Mann-Whitney rank sum test) but had no effect in mecp2−/y mice. The number of samples (normoxia/hypoxia): WT pons (10/11), mecp2−/y pons (7/6), WT medulla (10/11), mecp2−/y medulla (7/6). Results are shown as mean ± S.E.M.
Table 2.
BDNF protein levels in pons and medulla of littermates
| Mouse strain | Litter | PONS | MEDULLA | ||
|---|---|---|---|---|---|
| 21% O2 | 8% O2 | 21% O2 | 8% O2 | ||
| Wild type | A | 206 226 |
420 | 225 235 |
420 |
| B | 174 | 486 | 234 | 519 | |
| C | 158 | 301 486 |
150 | 285 354 |
|
| D | 117 | 429 | 284 | 307 | |
| E | 171 | 424 | 258 | 154 | |
| F | 59 102 |
210 | 172 242 |
457 | |
|
| |||||
| mecp2−/y | G | 84 | 26 73 |
195 | 115 157 |
| H | 322 | 230 | 713 | 646 | |
| I | 74 | 75 | 80 | 174 | |
| J | 222 | 280 | 338 | 320 | |
BDNF protein is presented in pg/g of tissue. Some litters had two animals of the same genotype per treatment.
Effect of acute intermittent hypoxia on bdnf transcript expression
In light of the effects of AIH on BDNF protein expression, we conducted quantitative reverse-transcriptase PCR to determine whether the changes in bdnf expression occur at the transcriptional level. Following reverse transcription of total RNA, bdnf transcripts for the protein-coding region were analyzed relative to a reference gene. Quantitative gene expression data are often normalized to the expression levels of reference genes, with the inherent assumption that their expression remains constant. However, recent studies examining the modulation of the common reference gene encoding glyceraldehyde-3 –phosphate dehydrogenase (GAPDH) by hypoxia showed that its mRNA expression levels were increased by 43% under 3% O2 conditions (Bémeur et al., 2004). Therefore, the gene encoding hypoxanthine-guanidine phosphoribosyltransferase (HPRT) was used as the reference gene. The hprt gene is constitutively expressed at low levels throughout the nervous system, which makes it suitable as an endogenous mRNA control in real-time PCR for highly sensitive quantification of low copy or rare mRNAs (Steen et al., 1990, Pernas-Alonso et al., 1999). We tested the effects of AIH on hprt expression in our system and detected no changes in its expression when the two oxygen conditions were compared (unpaired t-test, p=0.389 for wild type mice, p=0.776 for mecp2 mutant mice).
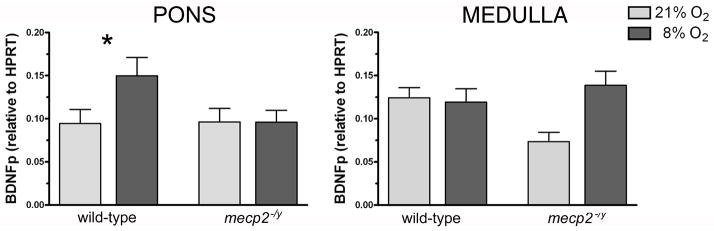
Consistent with our BDNF protein data, exposure to AIH resulted in an increase in bdnf mRNA levels in the pons of WT mice (p=0.040, Mann-Whitney) but not in mecp2−/y mutants (Fig. 2). In the medulla, bdnf transcripts were not affected in either strain. The difference in expression in the medulla of mecp2 null mice, even though it approached significance, was not significant (p=0.079, Mann-Whitney).
Figure 2. Changes in BDNF mRNA expression in the brainstem of wild-type and mecp2−/y mice in response to acute intermittent hypoxia.
Levels of the protein-coding region of the bdnf gene transcript in the pons and medulla of wild-type (WT) and mecp2 null (mecp2−/y) mice exposed to normoxia (21% O2, light gray bars) or acute intermittent hypoxia (8% O2, dark gray bars, please see Experimental Procedures for details of the acute intermittent hypoxia protocol). In the pons (left panel), bdnf mRNA levels increased in response to acute intermittent hypoxia in WT mice (p=0.040; Mann-Whitney) while no hypoxia-induced changes were detected in mecp2-deficient mice. In the medulla (right panel), intermittent hypoxia did not have any effect on BDNF transcript levels in WT mice. The change in mecp2 null mice is not significant (p=0.079; Mann-Whitney). Quantitative PCR data are mean values of independent experiments performed in triplicates, bdnf transcript levels are calculated relative to the reference gene hprt. Number of samples (normoxia/hypoxia): WT pons (13/13), mecp2−/y pons (9/10), WT medulla (14/13), mecp2−/y medulla (9/10). Results are shown as mean ± S.E.M.
Discussion
The current study shows, to our knowledge, for the first time that acute intermittent hypoxia (AIH) results in a dramatic upregulation of BDNF protein in the pons and, to a lesser extent, in the medulla of WT mice. The same hypoxia exposure protocol has previously been shown to increase BDNF in the cervical spinal cord in rat (Baker-Herman et al., 2004), but we were unable to reliably measure protein or mRNA levels in the smaller spinal cord sample size of mice. The twofold increase in BDNF protein that we observed in WT mouse pons after AIH (Fig. 1) exceeds that seen in the cervical spinal cord of rat (~50% increase;(Baker-Herman et al., 2004). The effect of AIH on BDNF protein in the pons and medulla has not been examined before in the mecp2 null mice. Previous studies have reported depressed levels of BDNF protein and bdnf non-coding transcripts in mecp2 null males at P35 (Chang et al., 2006, Wang et al., 2006, Zhou et al., 2006, Ogier et al., 2007, Lioy et al., 2011b) that were not observed in this report. The discrepancies may be due to differences in the age of animals with which these studies were performed, and/or the region of the nervous system that was examined.
Conflicting mechanisms for the role of Mecp2 in BDNF upregulation have been reported. Membrane depolarization of neurons leads to a calcium-dependent phosphorylation of Mecp2, (Zhou et al., 2006), and binding of this phosphorylated form to methylated DNA is impaired (Chen et al., 2003), which predicts that expression of bdnf transcripts would increase. An increase in bdnf -IV, formerly known as bdnf -III, however, was the same in WT and mecp2 null neurons subjected to depolarizing concentrations of KCl (please see figure 3 in(Chen et al., 2003).The latter studies were performed in embryonic day 18 rat cortical neurons, cultured for 5 days. In contrast, Klein et al. (2007), using postnatal neurons, found that membrane depolarization induced expression of miR-132 which, in turn, reduced Mecp2 protein. These authors proposed a homeostatic feedback loop mechanism in which neuronal activity increases the levels of miR-132 that, in turn, decreases Mecp2 (Klein et al., 2007). To close the loop, Mecp2 increases bdnf transcript IV in rat, whereas BDNF regulates miR-132. The studies by Klein and colleagues (2007) employed mature neurons that likely expressed higher amounts of both Mecp2 and miR-132. Here we show, using a physiological protocol known to increase BDNF protein in respiratory areas of the brain, that in the pons and medulla, BDNF protein levels significantly increase in WT mice, but not in mecp2 mutants. These experiments demonstrate an essential role for Mecp2 in regulating BDNF expression. The observation that AIH increased the protein-coding transcript of bdnf in the pons of WT animals but not in the medulla raises the possibility that the BDNF protein is transported caudally after transcription in the pons. Previous studies indicate that BDNF plays a critical role in development and plasticity of the cardiorespiratory control system (Erickson et al., 1996, Balkowiec and Katz 1998, Bouvier et al., 2008, Martin et al., 2009, Clark et al., 2011). The present study shows that hypoxia-induced upregulation of BDNF expression in the areas of the brain responsible for the respiratory control is compromised in mecp2 mutants. Our data suggest that BDNF expression stimulated by other forms of neuronal activity in these brain areas of mecp2 mutants may also be compromised and, as a consequence, impact BDNF-dependent processes.
In summary, our results suggest that Mecp2 is required for regulation of BDNF expression in the respiratory circuits of the brainstem. Disruption of the Mecp2 function is likely to have serious consequences for the development and plasticity of neuronal connections within the respiratory circuits and hypoxic ventilatory responses. Subjects with Rett syndrome incur in episodes of hypoxia (Southall et al., 1988). The failure of these episodes to induce increases in brainstem BDNF protein may contribute to respiratory instability.
Acknowledgments
The authors would like to thank Huiya Hsieh for expert technical assistance. This work was supported by a grant from the National Heart, Lung, and Blood Institute of the National Institutes of Health (HL076113) to A.B.; March of Dimes research grant Fy06-314 and International Rett Syndrome Foundation grants 0802 and 2705 to J.M.B.
Abbreviations
- AIH
acute intermittent hypoxia
- BDNF
brain-derived neurotrophic factor
- MeCP2 or Mecp2
methyl-CpG binding protein 2
- WT
wild-type
References
- Aid T, Kazantseva A, Piirsoo M, Palm K, Timmusk T. Mouse and rat BDNF gene structure and expression revisited. Journal of Neuroscience Research. 2007;85:525–535. doi: 10.1002/jnr.21139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Baker-Herman TL, Fuller DD, Bavis RW, Zabka AG, Golder FJ, Doperalski NJ, Johnson RA, Watters JJ, Mitchell GS. BDNF is necessary and sufficient for spinal respiratory plasticity following intermittent hypoxia. Nature neuroscience. 2004;7:48–55. doi: 10.1038/nn1166. [DOI] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Brain-derived neurotrophic factor is required for normal development of the central respiratory rhythm in mice. J Physiol. 1998;510:527–533. doi: 10.1111/j.1469-7793.1998.527bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballas N, Lioy DT, Grunseich C, Mandel G. Non–cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nature Neuroscience. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bémeur C, Ste-Marie L, Desjardins P, Hazell AS, Vachon L, Butterworth R, Montgomery J. Decreased β-actin mRNA expression in hyperglycemic focal cerebral ischemia in the rat. Neuroscience Letters. 2004;357:211–214. doi: 10.1016/j.neulet.2003.12.081. [DOI] [PubMed] [Google Scholar]
- Bird A. The methyl-CpG-binding protein MeCP2 and neurological disease. Biochem Soc Trans. 2008;36 (Pt 4):575–583. doi: 10.1042/BST0360575. [DOI] [PubMed] [Google Scholar]
- Bissonnette JM, Knopp SJ. Separate Respiratory Phenotypes in Methyl-CpG-Binding Protein 2 (Mecp2) Deficient Mice. Pediatr Res. 2006;59:513–518. doi: 10.1203/01.pdr.0000203157.31924.4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonnette JM, Knopp SJ. Effect of inspired oxygen on periodic breathing in methyl-CpG-binding protein 2 (Mecp2) deficient mice. J Appl physiol. 2008;198:198–204. doi: 10.1152/japplphysiol.00843.2007. [DOI] [PubMed] [Google Scholar]
- Bouvier J, Autran S, Dehorter N, Katz DM, Champagnat J, Fortin G, Thoby-Brisson M. Brain-derived neurotrophic factor enhances fetal respiratory rhythm frequency in the mouse preBötzinger complex in vitro. European Journal of Neuroscience. 2008;28:510–520. doi: 10.1111/j.1460-9568.2008.06345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CL. The MIQE guidelines: Minimum Information for publication of Quantitative Real-Time PCR Experiments. Clinical Chemistry. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- Chahrour M, Zoghbi HY. The story of Rett syndrome: from clinic to neurobiology. Neuron. 2007;56:422–437. doi: 10.1016/j.neuron.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–348. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nature Genetics. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- Chen WG, Ghang Q, Lin Y, Meissner A, West AE, Griffith EC, Jaenisch R, Greenberg ME. Derepression of BDNF transcription involves calcium-dependent phosphorylation of Mecp2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- Clark CG, Hasser EM, Kunze DL, Katz DM, Kline DD. Endogenous Brain-Derived Neurotrophic Factor in the Nucleus Tractus Solitarius Tonically Regulates Synaptic and Autonomic Function. J Neurosci. 2011;31:12318–12329. doi: 10.1523/JNEUROSCI.0746-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Gabel H, Hemberg M, Hutchinson A, Sadacca L, Ebert D, Harmin D, Greenberg R, Verdine V, Zhou Z, Wetsel W, West A, Greenberg M. Genome-wide activity-dependent MeCP2 phosphorylation regulates nervous system development and function. Neuron. 2011;72:72–85. doi: 10.1016/j.neuron.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Greenberg ME. A Bird’s-Eye View of MeCP2 Binding. Molecular Cell. 2010;37:451–452. doi: 10.1016/j.molcel.2010.02.006. [DOI] [PubMed] [Google Scholar]
- Dale-Nagle E, Hoffman M, MacFarlane P, Satriotomo I, Lovett-Barr M, Vinit S, Mitchell G. Spinal plasticity following intermittent hypoxia: implications for spinal injury. Ann N Y Acad Sci. 2010b;1198:252–259. doi: 10.1111/j.1749-6632.2010.05499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale-Nagle EA, Hoffman MS, MacFarlane PM, Mitchell GS. Multiple Pathways to Long-lasting Phrenic Motor Facilitation. Adv Exp Med Biol. 2010a;669:225–230. doi: 10.1007/978-1-4419-5692-7_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JT, Conover JC, Borday V, Champagnat J, Barbacid M, Yancopoulos G, Katz DM. Mice lacking brain-derived neurotrophic factor exhibit visceral sensory neuron losses distinct from mice lacking NT4 and display a severe developmental deficit in control of breathing. J Neurosci. 1996;16:5361–5371. doi: 10.1523/JNEUROSCI.16-17-05361.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman J, Mitchell G, Nattie E. Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nature Genetics. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- Hertzberg T, Fan G, Finley J, Erickson J, Katz D. BDNF supports mammalian chemoafferent neurons in vitro and following peripheral target removal in vivo. Developmental Biology. 1994;166:801–811. doi: 10.1006/dbio.1994.1358. [DOI] [PubMed] [Google Scholar]
- Katz DM. Regulation of respiratory neuron development by neurotrophic and transcriptional signalling mechanisms. Respir Physiol Neurobiol. 2005;149:99–109. doi: 10.1016/j.resp.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Klein ME, Lioy DT, Ma L, Impey S, Mandel G, Goodman RH. Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nature neuroscience. 2007;10:1513–1514. doi: 10.1038/nn2010. [DOI] [PubMed] [Google Scholar]
- Kron M, Zhang W, Dutschmann M. Developmental changes in the BDNF-induced modulation of inhibitory synaptic transmission in the Kolliker-Fuse nucleus of rat. Eur j neurosci. 2007;26:3449–3457. doi: 10.1111/j.1460-9568.2007.05960.x. [DOI] [PubMed] [Google Scholar]
- Lauterborn JC, Rivera S, Stinis CT, Hayes VY, Isackson PJ, Gall CM. Differential Effects of Protein Synthesis Inhibition on the Activity-Dependent Expression of BDNF Transcripts: Evidence for Immediate-Early Gene Responses from Specific Promoters. J Neurosci. 1996;16:7428–7436. doi: 10.1523/JNEUROSCI.16-23-07428.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N, Mandel G. A role for glia in the progression of Rett’s syndrome. nature. 2011a;475:496–502. doi: 10.1038/nature10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lioy DT, Wu WW, Bissonnette JM. Autonomic dysfunction with mutations in the gene that encodes methyl-CpG-binding protein 2: Insights into Rett syndrome. Autonomic Neuroscience. 2011b;161:55–62. doi: 10.1016/j.autneu.2011.01.006. [DOI] [PubMed] [Google Scholar]
- Liu Q-R, Lu L, Zhu X-G, Gong J-P, Shaham Y, Uhl GR. Rodent BDNF genes, novel promoters, novel splice variants, and regulation by cocaine. Brain Research. 2006;1067:1–12. doi: 10.1016/j.brainres.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Martin J, Jenkins V, Hsieh H, Balkowiec A. Brain-derived neurotrophic factor in arterial baroreceptor pathways: implications for activity-dependent plasticity at baroafferent synapses. Journal of Neurochemistry. 2009;108:450–464. doi: 10.1111/j.1471-4159.2008.05781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralves J, Magdeleine E, Joly E. Design of an improved set of oligonucleotide primers for genotyping MeCP2tm1.1Bird KO mice by PCR. Molecular neurodegeneration. 2007;2:16. doi: 10.1186/1750-1326-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogier M, Wang H, Hong EJ, Wang Q, Greenberg ME, Katz DM. Brain-Derived Neurotrophic Factor Expression and Respiratory Function Improve after Ampakine Treatment in a Mouse Model of Rett Syndrome. J Neurosci. 2007;27:10912–10917. doi: 10.1523/JNEUROSCI.1869-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernas-Alonso R, Morelli F, di Porzio U, Perrone C, Carla Multiplex semi-quantitative reverse transcriptase-polymerase chain reaction of low abundance neuronal mRNAs. Brain Research Protocols. 1999;4:395–406. doi: 10.1016/s1385-299x(99)00045-8. [DOI] [PubMed] [Google Scholar]
- Song G, Poon C. Lateral parabrachial nucleus mediates shortening of expiration during hypoxia. Respir Physiol Neurobiol. 2009;165:1–8. doi: 10.1016/j.resp.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southall D, Kerr A, Tirosh E, Amos P, Lang M, Stephenson J. Hyperventilation in the awake state: potentially treatable component of Rett syndrome. Arch dis child. 1988;63:1039–1048. doi: 10.1136/adc.63.9.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steen A-M, Luthman H, Hellgren D, Lambert B. Levels of hypoxanthine phosphoribosyltransferase RNA in human cells. Experimental Cell Research. 1990;186:236–244. doi: 10.1016/0014-4827(90)90301-p. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Cauli B, Champagnat J, Fortin G, Katz D. Expression of functional tyrosine kinase B receptors by rhythmically active respiratory neurons in the pre-Botzinger complex of neonatal mice. J Neurosci. 2003;23:7685–7689. doi: 10.1523/JNEUROSCI.23-20-07685.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Chan S-a, Ogier M, Hellard D, Wang Q, Smith C, Katz DM. Dysregulation of brain-derived neurotrophic factor expression and neurosecretory function in Mecp2 null mice. J Neurosci. 2006;26:10911–10915. doi: 10.1523/JNEUROSCI.1810-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Hong EJ, Cohen S, Zhao W-n, Henry Ho H-y, Schmaidt L, Chen WG, Lin Y, Savner E, Griffith EC, Hu L, Steen J, AJ, Weitz CJ, Greenberg ME. Brain-specific phosphorylation of MeCP2 regulates activity-dependent bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]