Abstract
The importance of inflammation pathways to the development of many human cancers prompted us to examine the associations between single-nucleotide polymorphisms (SNPs) in inflammation-related genes and risk of ovarian cancer. In a multi-site case-control study, we genotyped SNPs in a large panel of inflammatory genes in 930 epithelial ovarian cancer cases and 1,037 controls using a custom array and analyzed by logistic regression. SNPs with p<0.10 were evaluated among 3,143 cases and 2,102 controls from the Follow-up of Ovarian Cancer Genetic Association and Interaction Studies (FOCI) collaboration. Combined analysis revealed association with SNPs rs17561 and rs4848300 in the interleukin gene IL1A which varied by histologic subtype (heterogeneity p=0.03). For example, IL1A rs17561, which correlates with numerous inflammatory phenotypes, was associated with decreased risk of clear cell, mucinous, and endometrioid subtype, but not with the most common serous subtype. Genotype at rs1864414 in the arachidonate 5-lipoxygenase ALOX5 was also associated with decreased risk. Thus, inherited variation in IL1A and ALOX5 appears to affect ovarian cancer risk which, for IL1A, is limited to rarer subtypes. Given the importance of inflammation in tumorigenesis and growing evidence of subtype-specific features in ovarian cancer, functional investigations will be important to help clarify the importance of inherited variation related to inflammation in ovarian carcinogenesis.
Keywords: epidemiology, cytokine
INTRODUCTION
In 2011, there were 225,500 estimated new cases of ovarian cancer worldwide (1). High-risk mutations in BRCA1 and BRCA2 are responsible for most familial clusters of three or more cases; however, a substantial proportion of familial risk is unexplained (2). Part of this remaining risk is due to loci which confer moderate risk, such as common, low penetrance alleles identified in recent genome-wide association studies (3, 4). While characterization of recently confirmed genetic variants is critically important, the search for additional variants continues and includes comprehensive analysis of key candidate pathways.
Inflammation is a suspected initiator and promoter of ovarian carcinogenesis (5). Events which delay ovulation and the subsequent inflammatory response are associated with decreased risk of ovarian cancer, consistent with the hypothesis that increased lifetime ovulations create an inflammatory microenvironment promoting tumor growth and suppressing adaptive immunity (6). The Cancer Genome Atlas has described an immunoreactive subtype of high-grade serous disease characterized by expression of T-cell chemokine ligands CXCL11 and CXCL10 and the receptor CXCR3 (7), and over-expression of receptors of the inflammatory lipoxygenase pathway has been observed (8). Finally, reduced risk of ovarian cancer has been reported for a single-nucleotide polymorphism (SNP) in PTGS2, encoding prostaglandin endoperoxide synthase 2, or cyclooxygenase, which is thought to be responsible for the prostanoid biosynthesis involved in inflammation and mitogenesis (9).
Here, we hypothesize that inherited variants in inflammation-related genes are associated with risk of ovarian cancer and may have subtype-specific risks. Therefore, we examined SNPs in 27 inflammation-related genes in two case-control studies and sought replication in the studies of the Ovarian Cancer Association Consortium (OCAC).
MATERIALS AND METHODS
Study protocols were approved by the appropriate institutional review board or ethics panel, and all participants provided written informed consent.
Discovery Analysis
Participants included 930 epithelial ovarian cancer cases and 1,037 controls who were enrolled at the Mayo Clinic (MAY) and in the North Carolina Ovarian Cancer Study (NCO) (10) (Supplemental Table 1). TagSNPs for ALOX12, ALOX15, ALOX5, CCL11, CCL2, CCL3, CCR3, CRP, CXCL16, IL10, IL15RA, IL18, IL1A, IL1B, IL1RN, IL4R, IL6, IL6R, IL7R, IL8RA, IL8RB, IL9, NOS3, PTGS1, PTGS2, TLR2, and TNF were identified using HapMap CEU data (release 21a, ± 10 kb, MAF ≥ 0.05, r2 ≥ 0.8, Supplemental Table 2). These and non-synonymous SNPs (MAF ≥ 0.05) were genotyped using a custom Illumina GoldenGate™ BeadArray assay; SNPs with call rates below 90% were failed (10). Logistic regression estimated per-allele risk of ovarian cancer (odds ratios [OR] and 95% confidence intervals [95% CI]) adjusted for site, age, race, region of residence, body mass index, hormone therapy use, oral contraceptive use, parity, and age at first birth, in order to alleviate potential confounding effects.
Replication Analysis
Participants were self-reported white women including 3,143 invasive epithelial ovarian cancer cases and 2,102 controls enrolled in studies at Brigham and Women’s Hospital (BWH), the NCI Ovarian Case-Control Study in Poland (POL), the Tampa Bay Ovarian Cancer Study (TBO), the Familial Ovarian Tumor Study (TOR), and a British collaboration (UK) including the UK Ovarian Cancer Population Study, Studies of Epidemiology and Risk Factors in Cancer Heredity - Ovarian Cancer, the UK Familial Ovarian Cancer Registry, and the Welcome Trust Case Control Consortium (11) (Supplemental Table 3). SNPs with p-value < 0.10 in the discovery analysis described above were assessed using Illumina 317k or 610-Quad arrays, with harmonization of alleles and imputation to HapMap v 26 (www.sph.umich.edu/csg/abecasis/MACH/) as part of the Follow-up of Ovarian Cancer Genetic Association and Interaction Studies (FOCI) collaboration. Log-additive logistic regression models estimated risk of ovarian cancer adjusted for study site using direct genotype calls or imputed allele dosage values. Combined analysis of discovery and replication data was restricted to self-reported white participants, adjusted for study site, and included tests for heterogeneity between study phases. For SNPs highlighted in combined analysis, we performed regression including multiple SNPs per gene, and we estimated risks by histological subtype using polytomous regression to test for heterogeneity. Finally, among 6,253 combined participants with available age data, we ran analyses with and without age adjustment to assess potential confounding effects. P-values were not adjusted for multiple testing.
RESULTS
Estimation of ovarian cancer risks associated with minor alleles of SNPs in inflammation-related genes began with analysis of 162 successfully genotyped SNPs in approximately 900 epithelial ovarian cancer cases and 1,000 controls described in Supplemental Table 1. Twenty-one SNPs (in ALOX15, ALOX5, CCL2, CRP, IL18, IL1A, IL1B, IL6, NOS3, and PTGS1) were associated with risk of ovarian cancer with per-allele p’s < 0.10. Two SNPs were associated at p < 0.01: ALOX5 (arachidonate 5-lipoxygenase) rs1864414 (OR 0.77, 95% CI 0.64–0.92, p=0.004) and NOS3 (nitric oxide synthase 3) rs743507 (OR 0.79, 95% CI 0.68–0.92, p=0.003). Analyses within ALOX12, CCL11, CCL3, CCR3, CXCL16, IL10, IL15RA, IL1RN, IL4R, IL6R, IL7R, IL8RA, IL8RB, IL9, PTGS2, TLR2, and TNF did not suggest associations between genotype and risk of ovarian cancer (p’s > 0.10). Complete results are provided in Supplemental Table 4.
Twenty-one SNPs with p < 0.10 in discovery analysis were evaluated in an additional approximate 3,100 cases and 2,100 controls described in Supplemental Table 3, and, to enable combined analysis, discovery data were re-analyzed excluding self-reported non-whites and adjusted for only study site. These results and estimates of pairwise linkage disequilibrium (r2) are shown in Table 1. Of 21 SNPs in 10 genes, those in IL1A (interleukin 1, alpha) and ALOX5 (arachidonate 5-lipoxygenase) were associated at p-value < 0.01 in combined analysis.
Table 1.
SNPs in Combined Analysis and Risk of Ovarian Cancer
| Gene | rsid | r2 to next | Discovery (829 cases, 941 controls) |
Replication (3,143 cases, 2,102 controls) |
Combined (3,972 cases, 3,043 controls) |
|||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | OR (95%CI) | p | |||
| ALOX15 | rs1076039 | 0.006 | 1.13 (0.94–1.37) | 0.20 | 1.00 (0.89–1.12) | 0.98 | 1.03 (0.94–1.14) | 0.51 |
| rs2664593 | --- | 1.12 (0.95–1.32) | 0.18 | 1.03 (0.92–1.16) | 0.60 | 1.06 (0.96–1.17) | 0.22 | |
|
| ||||||||
| ALOX5 | rs1864414 | 0.651 | 0.75 (0.62–0.90) | 0.002 | 0.91 (0.81–1.01) | 0.08 | 0.86 (0.78–0.95) | 0.002 |
| rs745986 | --- | 0.84 (0.72–1.00) | 0.05 | 0.91 (0.82–1.01) | 0.07 | 0.89 (0.82–0.97) | 0.01 | |
|
| ||||||||
| CCL2 | rs11652585 | 0.993 | 0.75 (0.58–0.96) | 0.02 | 1.09 (0.93–1.27) | 0.28 | 0.98 (0.86–1.12) | 0.76 |
| rs8068314 | --- | 0.74 (0.58–0.95) | 0.02 | 1.09 (0.93–1.27) | 0.29 | 0.98 (0.86–1.11) | 0.72 | |
|
| ||||||||
| CRP | rs1800947 | --- | 1.27 (0.97–1.66) | 0.09 | 0.98 (0.82–1.18) | 0.84 | 1.06 (0.91–1.24) | 0.43 |
|
| ||||||||
| IL18 | rs4937113 | 0.266 | 0.87 (0.76–1.00) | 0.04 | 1.06 (0.97–1.15) | 0.22 | 1.00 (0.93–1.07) | 0.94 |
| rs2043055 | --- | 1.12 (0.98–1.29) | 0.10 | 1.01 (0.93–1.10) | 0.78 | 1.04 (0.97–1.12) | 0.28 | |
|
| ||||||||
| IL1A | rs17561 | 0.990 | 0.87 (0.75–1.00) | 0.06 | 0.91 (0.83–0.99) | 0.03 | 0.90 (0.83–0.97) | 0.005 |
| rs4848300 | 0.300 | 0.87 (0.75–1.00) | 0.05 | 0.91 (0.83–0.99) | 0.04 | 0.90 (0.83–0.97) | 0.005 | |
| rs3783516 | 0.993 | 1.12 (0.98–1.28) | 0.08 | 1.09 (1.00–1.19) | 0.04 | 1.10 (1.03–1.18) | 0.01 | |
| rs2856838 | --- | 1.13 (0.99–1.28) | 0.08 | 1.09 (1.00–1.19) | 0.04 | 1.10 (1.03–1.18) | 0.01 | |
|
| ||||||||
| IL1B | rs7596684 | --- | 0.87 (0.75–1.02) | 0.09 | 0.92 (0.83–1.02) | 0.10 | 0.90 (0.83–0.99) | 0.02 |
|
| ||||||||
| IL6 | rs10242595 | --- | 0.86 (0.74–1.00) | 0.05 | 1.03 (0.94–1.13) | 0.53 | 0.98 (0.91–1.06) | 0.62 |
|
| ||||||||
| NOS3 | rs1800783 | 0.848 | 0.81 (0.66–0.99) | 0.04 | 1.01 (0.92–1.11) | 0.82 | 0.97 (0.90–1.06) | 0.52 |
| rs743507 | --- | 0.81 (0.69–0.94) | 0.01 | 0.97 (0.88–1.06) | 0.49 | 0.92 (0.85–1.00) | 0.04 | |
|
| ||||||||
| PTGS1 | rs4837963 | 0.049 | 1.23 (0.99–1.53) | 0.06 | 0.97 (0.85–1.11) | 0.67 | 1.03 (0.93–1.16) | 0.55 |
| rs10306135 | 0.634 | 1.14 (0.95–1.36) | 0.16 | 1.09 (0.96–1.24) | 0.19 | 1.11 (1.00–1.23) | 0.06 | |
| rs2282169 | 0.085 | 1.21 (1.02–1.43) | 0.03 | 1.06 (0.94–1.18) | 0.34 | 1.10 (1.00–1.21) | 0.04 | |
| rs10513402 | --- | 1.26 (1.01–1.56) | 0.04 | 0.97 (0.85–1.10) | 0.63 | 1.04 (0.93–1.16) | 0.53 | |
Adjusted for study site; self-reported whites only; bold indicates p<0.01.
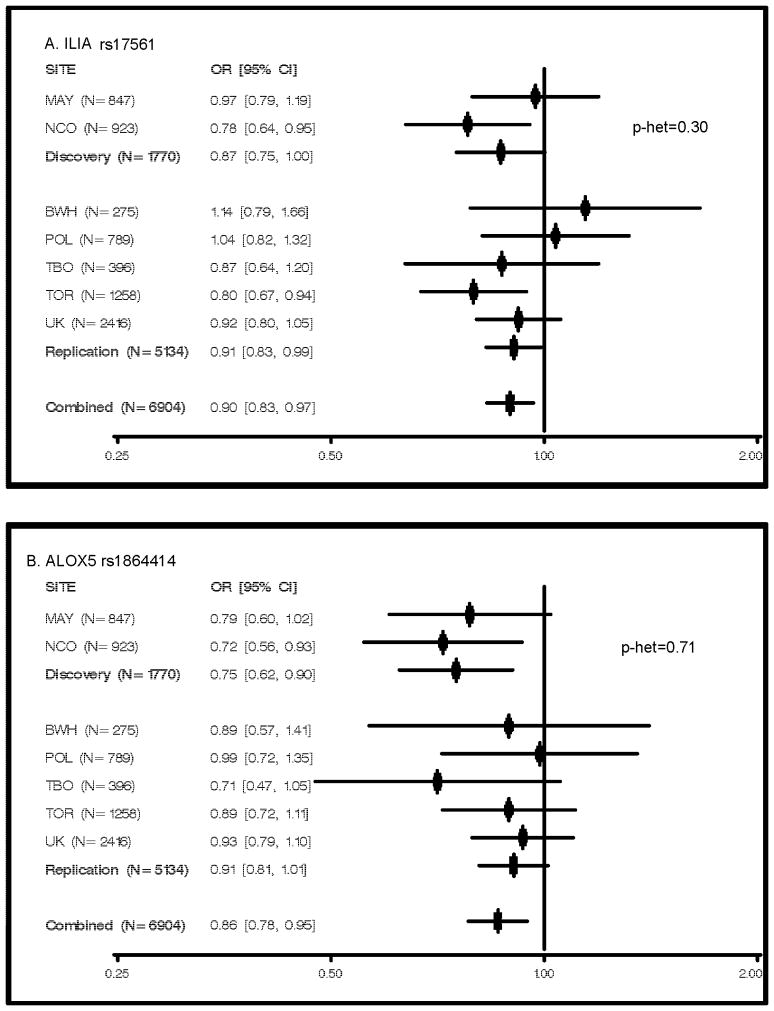
Two highly-correlated SNPs in IL1A (rs17561 and rs4848300, r2=0.990) yielded essentially identical results, showing associations with decreased risk of ovarian cancer in combined analyses (OR 0.90, 95% CI 0.83–0.97, p=0.005). Replication analysis yielded p’s < 0.05 (Table 1), and results were consistent between discovery and replication phases (heterogeneity p=0.30; Figure 1). IL1A rs17561 (MAF=0.31) is a missense change (A114S), and rs4848300 (MAF=0.32) resides 3,586 bp from the 3′ end of the gene. As other loosely-correlated IL1A SNPs (r2=0.300) were also associated with ovarian cancer risk (rs3783516 and rs2856838, r2=0.993, OR 1.10, 95% CI 1.03–1.18, p=0.01), we attempted logistic regression including all four IL1A SNPs in the combined dataset. However, modeling was uninformative due to convergence issues and unstable parameter estimates.
Figure 1. Per-allele Risks of Ovarian Cancer by Study Site.
Odds ratios and 95% confidence intervals adjusted for study site (where combined) based on analysis of self-reported whites only are plotted; N represents cases and controls combines; p-het based on heterogeneity test of discovery versus replication participants; Mayo Clinic (MAY) and North Carolina Ovarian Cancer Study (NCO); Brigham and Women’s Hospital (BWH); NCI Ovarian Case-Control Study in Poland (POL); Tampa Bay Ovarian Cancer Study (TBO); the Familial Ovarian Tumour Study (TOR); the UK Ovarian Cancer Population Study, the UK Studies of Epidemiology and Risk Factors in Cancer Heredity Ovarian Cancer Study, the UK Familial Ovarian Cancer Registry, and the Wellcome Trust Case Control Consortium (UK).
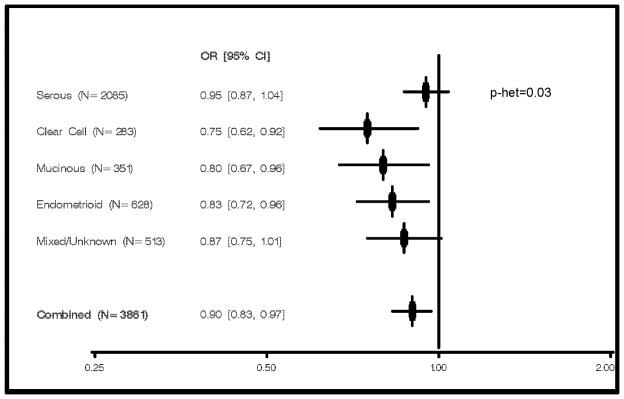
Of particular interest, analysis of IL1A rs17561 by histological subtype revealed evidence of heterogeneity (p=0.03, Figure 2). Specifically, an inverse association was present for risk of clear cell subtype (p=0.004), mucinous subtype (p=0.02), and endometrioid subtype (p=0.01), yet absent for risk of the more common (54%) serous subtype (p=0.26). Consistently, the risk estimate for tumors with mixed or unknown histology was intermediate to those for rarer subtypes and serous subtype (OR 0.87, 95% CI 0.75–1.01, Figure 2).
Figure 2. Per-allele Risks of Ovarian Cancer by Histological Subtype, IL1A rs17561.
Odds ratios and 95% confidence intervals adjusted for study site based on analysis of self-reported whites only are plotted (N cases by subtype are displayed, N=3.043 controls); p-het based on heterogeneity test using polytomous regression.
In combined analysis, the estimated ovarian cancer risk associated with minor alleles at ALOX5 rs1864414 (MAF=0.18, intron 1), one of the strongest initial discovery set signals, was 0.86 (95% CI 0.79–0.95, p=0.002). Results were consistent between discovery and replication datasets (heterogeneity p=0.71), and, with the exception of POL, risk estimates were less than 1.0 in each study (Figure 1). Because a correlated ALOX5 SNP was also associated with ovarian cancer risk (rs745986, r2=0.651, OR 0.89, 95% CI 0.82–0.97, p=0.01), we included both SNPs in regression modeling. Results suggested that rs1864414 was driving the association (rs1864414 OR 0.88, 95% CI 0.75–1.03, p=0.009; rs745986 OR 0.98, 95% CI 0.85–1.14, p=0.11). No heterogeneity by histological subtype was observed (p=0.74)
As shown in Table 1, combined analyses at IL1B (interleukin 1, beta) rs7596684 and PTGS1 (prostaglandin-endoperoxide synthase 1) rs2282169 suggested association with ovarian cancer risk (p=0.02 and p=0.04, respectively). Results were null or inconsistent with study phases at SNPs in ALOX15, CCL2, CRP, IL18, and IL6. Among women with available age information, combined analyses adjusting additionally for age yielded similar results at all SNPs.
DISCUSSION
Chronic inflammation has demonstrated an important role in cancer (12); tumor microenvironments of numerous cancers, including ovarian, have shown high-levels of inflammatory proteins, such as cytokines, and aberrations in interleukin 1 alpha, interleukin 6, and tumor necrosis factor alpha (13). Thus, examinations of genetic variants in these pathways may shed light on inflammation-related carcinogenesis. In the largest study to date, we examined the relationship between SNPs in inflammation-related genes and risk of ovarian cancer using a two-study discovery analysis followed by examination of five studies participating in the Ovarian Cancer Association Consortium. We found that minor alleles at rs17561 and rs4848300 in IL1A and rs1864414 in ALOX5 were consistently inversely associated with risk of ovarian cancer, and, intriguingly, that the IL1A associations were limited to rarer histological subtypes.
Interleukin 1 alpha is a cytokine which is involved in numerous immune responses and inflammatory processes; it is produced by monocytes and macrophages and released in response to cell injury for the induction of apoptosis. A recent analysis of 4,552 SNPs in cytokines reported that IL1A rs17561 is one of only eight SNPs leading to a predicted intolerable amino acid change, that it is by far the most widely studied cytokine SNP, and that minor alleles here have been “strongly” associated with risk of numerous diseases involving an inflammatory response such as allergic rhinitis, atopy, nasal polyposis, malaria, gingival hyper-inflammatory response, systemic sclerosis, severity of periodontitis, antibody responses to periodontal microbiota (14) and, more recently, ankylosing spondylitis (15). While we are the first to report an association with ovarian cancer risk, a meta-analysis of prospective breast cancer studies has reported increased risk with IL1A rs17561 (p=0.008) as well as modification of the dose-response relationship between cumulative personal diagnostic radiation and breast cancer risk (interaction p=0.004) (16). Another study of breast cancer risk found that IL1A was the most significant of 232 inflammation genes in gene-level and haplotype analyses (p=4 × 10−4) (17). Of note, a prior ovarian cancer study of approximately 300 participants in total found no association between risk and other SNPs in IL1A; they did not examine rs17561 or rs4848300 and were underpowered to detect associations of the type we observed (18).
Epithelial ovarian cancer has several histologic subtypes with characteristic molecular signatures, cytogenetic features, oncologic signaling pathways, and clinical behavior. Nonetheless, for maximal sample size and smaller numbers of tests, molecular epidemiologists have traditionally combined cases and performed subtype analyses only on SNPs associated with risk overall, as we did here. However, our IL1A findings demonstrate that, if multiple rarer subtypes share a common genetic etiology, associations with rarer subtypes (rather than the most common and most studied serous subtype) may drive the result among all cases. These results underscore the need for additional candidate gene or genome-wide studies of clear cell, mucinous, and endometrioid ovarian cancer.
Arachidonate 5-lipoxygenase is a member of the lipoxygenase gene family assisting with conversion of arachidonic acid to leukotrienes, which are important mediators of a number of inflammatory and allergic conditions; it is expressed in the ovarian cancer (8). Interestingly, we previously reported an association with poorer survival and ALOX5 rs2115819 and rs12264801 among the MAY cases studied here (serous HR 1.54, 95% CI 1.18–2.02, p=0.002; HR 1.31, 95% CI 1.02–1.69, p=0.04, respectively) (19). Even though rs1864414 was not associated with ovarian cancer survival (p=0.23) (19), we evaluated whether the minor allele may be under-represented among cases studied here leading to a false inverse association with risk. We saw no association with rs1864414 genotype and time to enrollment and no difference in risk estimates by mean time of enrollment, and therefore don’t expect that the inverse association reported here is due to survival bias.
In addition to a large sample size (7,212 participants), strengths of this report include inclusion of numerous genes (most comprehensive to date), use of the tagSNP approach, and independent analyses of discovery and replication datasets. The latter issue requires some consideration. Because false discovery is a concern in SNP association studies, we sought replication of initial results in a large independent dataset. Some argue for the routine analysis of the likelihood of false positive results. With one commonly used method (20), the IL1A and ALOX5 associations are “noteworthy” (probability of a false positive result < 0.2) only if the prior probability of an association was 0.1 or greater. Of course, choice of the appropriate value for prior probability is a matter of great debate. Some argue that in a discovery-replication study, prior probabilities of association is high, given that significant results were seen in discovery analysis; others argue that in a candidate gene study, biological plausibility is increased. Regardless, additional replication is the key to revealing the “truth” of these associations.
Limitations of our analyses include lack of genotypic coverage of uncommon variants, assessment of epistasis or gene-environment interactions, and multi-SNP IL1A analysis. Additional genotyping or next generation sequencing efforts are needed to target additional variants, and, due to the need for greater statistical power, larger samples sizes are needed to address uncommon variants as well as interactions. A larger study would also enable simultaneous examination of multiple SNPs in IL1A and the related chromosome 2 cytokine gene cluster. We also note that the boundaries of biological pathways, such as inflammation, continue to evolve; thus expansion of genes from this set of 27 genes will be informative in future efforts. Finally, incorporation of genotype-phenotype studies considering quantification of tumor immune cells, tumor mRNA or protein expression, or inflammatory serum biomarkers into studies of inflammation SNPs will enhance these projects as well.
In conclusion, SNPs in ALOX5 and IL1A appear to harbor common inherited variants associated with modest differences in risk of ovarian cancer. Efforts to expand current data on inflammation and this deadly disease are a high priority.
Supplementary Material
Acknowledgments
This work was supported by the National Cancer Institute (R01-CA86888, R01-CA122443, R01-CA106414, R01-CA76016, R01-CA114343, P50-CA136393, and U19-CA148112), the Ovarian Cancer Research Fund, and the Mayo Foundation.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ramus SJ, Harrington PA, Pye C, DiCioccio RA, Cox MJ, Garlinghouse-Jones K, et al. Contribution of BRCA1 and BRCA2 mutations to inherited ovarian cancer. Hum Mutat. 2007;28:1207–15. doi: 10.1002/humu.20599. [DOI] [PubMed] [Google Scholar]
- 3.Bolton KL, Tyrer J, Song H, Ramus SJ, Notaridou M, Jones C, et al. Common variants at 19p13 are associated with susceptibility to ovarian cancer. Nat Genet. 2010;42:880–4. doi: 10.1038/ng.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goode EL, Chenevix-Trench G, Song H, Ramus SJ, Notaridou M, Lawrenson K, et al. A genome-wide association study identifies susceptibility loci for ovarian cancer at 2q31 and 8q24. Nat Genet. 2010;42:874–9. doi: 10.1038/ng.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rutkowski MJ, Sughrue ME, Kane AJ, Mills SA, Parsa AT. Cancer and the complement cascade. Mol Cancer Res. 2010;8:1453–65. doi: 10.1158/1541-7786.MCR-10-0225. [DOI] [PubMed] [Google Scholar]
- 6.Cramer DW, Finn OJ. Epidemiologic perspective on immune-surveillance in cancer. Curr Opin Immunol. 2011;23:265–71. doi: 10.1016/j.coi.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocconi RP, Kirby TO, Seitz RS, Beck R, Straughn JM, Jr, Alvarez RD, et al. Lipoxygenase pathway receptor expression in ovarian cancer. Reprod Sci. 2008;15:321–6. doi: 10.1177/1933719108316390. [DOI] [PubMed] [Google Scholar]
- 9.Lurie G, Terry K, Wilkens L, Thompson P, McDuffie K, Carney M, et al. Pooled analysis of the association of PTGS2 rs5275 polymorphism and NSAID use with invasive ovarian carcinoma risk. Cancer Causes Control. 2010;21:1731–41. doi: 10.1007/s10552-010-9602-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.White KL, Vierkant RA, Phelan CM, Fridley BL, Anderson S, Knutson KL, et al. Polymorphisms in NF-kappaB inhibitors and risk of epithelial ovarian cancer. BMC Cancer. 2009;9:170. doi: 10.1186/1471-2407-9-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Permuth-Wey J, Kim D, Tsai YY, Lin HY, Chen YA, Barnholtz-Sloan J, et al. LIN28B polymorphisms influence susceptibility to epithelial ovarian cancer. Cancer Res. 2011;71:3896–903. doi: 10.1158/0008-5472.CAN-10-4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 13.Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis. 2011;70 (Suppl 1):i104–8. doi: 10.1136/ard.2010.140145. [DOI] [PubMed] [Google Scholar]
- 14.Shen J, Deininger PL, Zhao H. Applications of computational algorithm tools to identify functional SNPs in cytokine genes. Cytokine. 2006;35:62–6. doi: 10.1016/j.cyto.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Sims AM, Timms AE, Bruges-Armas J, Burgos-Vargas R, Chou CT, Doan T, et al. Prospective meta-analysis of interleukin 1 gene complex polymorphisms confirms associations with ankylosing spondylitis. Ann Rheum Dis. 2008;67:1305–9. doi: 10.1136/ard.2007.081364. [DOI] [PubMed] [Google Scholar]
- 16.Sigurdson AJ, Bhatti P, Doody MM, Hauptmann M, Bowen L, Simon SL, et al. Polymorphisms in apoptosis- and proliferation-related genes, ionizing radiation exposure, and risk of breast cancer among U.S. Radiologic Technologists. Cancer Epidemiol Biomarkers Prev. 2007;16:2000–7. doi: 10.1158/1055-9965.EPI-07-0282. [DOI] [PubMed] [Google Scholar]
- 17.Han W, Kang SY, Kang D, Park SK, Lee JY, Kim H, et al. Multiplex genotyping of 1107 SNPs from 232 candidate genes identified an association between IL1A polymorphism and breast cancer risk. Oncol Rep. 2010;23:763–9. [PubMed] [Google Scholar]
- 18.Hefler LA, Ludwig E, Lebrecht A, Zeillinger R, Tong-Cacsire D, Koelbl H, et al. Polymorphisms of the interleukin-1 gene cluster and ovarian cancer. J Soc Gynecol Investig. 2002;9:386–90. [PubMed] [Google Scholar]
- 19.Goode EL, Maurer MJ, Sellers TA, Phelan CM, Kalli KR, Fridley BL, et al. Inherited determinants of ovarian cancer survival. Clin Cancer Res. 2010;16:995–1007. doi: 10.1158/1078-0432.CCR-09-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst. 2004;96:434–42. doi: 10.1093/jnci/djh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.