Abstract
The intracellularly stored GLUT4 glucose transporter is rapidly translocated to the cell surface upon insulin stimulation. Regulation of GLUT4 distribution is key for the maintenance of whole body glucose homeostasis. We find that GLUT4 is excluded from the plasma membrane of adipocytes by a dynamic retention/retrieval mechanism. Our kinetic studies indicate that GLUT4-containing vesicles continually bud and fuse with endosomes in the absence of insulin and that these GLUT4 vesicles are 5 times as likely to fuse with an endosome as with the plasma membrane. We hypothesize that this intracellular cycle of vesicle budding and fusion is an element of the active mechanism by which GLUT4 is retained. The GLUT4 trafficking pathway does not extensively overlap with that of furin, indicating that the trans-Golgi network, a compartment in which furin accumulates, is not a significant storage reservoir of GLUT4. An intact microtubule cytoskeleton is required for insulin-stimulated recruitment to the cell surface, although it is not required for the basal budding/fusion cycle. Nocodazole disruption of the microtubule cytoskeleton reduces the insulin-stimulated exocytosis of GLUT4, accounting for the reduced insulin-stimulated translocation of GLUT4 to the cell surface.
INTRODUCTION
An acute effect of insulin is to stimulate glucose uptake into muscle and fat cells by inducing the redistribution of the GLUT4 glucose transporter from intracellular compartments to the plasma membrane, a process that is key for disposal of dietary glucose (for a recent review, see Bryant et al., 2002). In the absence of insulin, GLUT4 is mostly excluded from the plasma membrane, and insulin reversibly stimulates the exocytosis of GLUT4-containing vesicles, thereby causing a net shift of GLUT4 to the cell surface.
The GLUT4 trafficking pathway is highly selective, and the only other protein known to traffic through the insulin-regulated pathway is IRAP, a transmembrane amino peptidase with an unknown biological function (Ross et al., 1997; Keller et al., 2002). In 3T3-L1 adipocytes, insulin induces a large increase (5-20-fold) in GLUT4 and IRAP on the surface, whereas surface expression of other membrane proteins is increased by only a small amount. For example, in 3T3-L1 adipocytes insulin increases the plasma membrane expression of the transferrin receptor (TR), a protein that traffics by the general recycling pathway, by at most twofold (Zeigerer et al., 2002). In the basal state, GLUT4 and IRAP are considerably more concentrated inside cells than are proteins of the general recycling pathway, with <5% of IRAP and GLUT4 on the surface compared with ∼40% of the TR. The mechanism by which insulin specifically stimulates exocytosis of GLUT4/IRAP-containing vesicles is unclear, although there is evidence that both the movement of these vesicles to, and fusion with, the plasma membrane are sites of regulation (Elmendorf et al., 1999; Inoue et al., 2003; Semiz et al., 2003).
Even though the kinetics of GLUT4 and IRAP trafficking are highly specialized, there is overlap between the compartments of the general recycling pathway and the GLUT4/IRAP pathway. In the basal state, approximately one-half of GLUT4 is localized to compartments of the general endocytic pathway (i.e., defined as those that are accessible to the TR) and the rest of intracellular GLUT4 and IRAP are in an intracellular compartment that is devoid of endocytic markers, late endosomal markers, and Golgi markers (Martin et al., 1996; Hah et al., 2002; Zeigerer et al., 2002). We refer to the latter pool of GLUT4 and IRAP as the “specialized” pool to distinguish it from the pool of GLUT4 and IRAP in the TR-containing endosomes (i.e., general endosomal compartments). The protein composition of this “specialized” pool has not been well characterized, and it is not known what other proteins accumulate in this compartment.
GLUT4 and IRAP are internalized from the plasma membrane through clathrin-coated pits, and therefore some overlap between the GLUT4-enriched compartments and TR-containing endosomes is expected (Robinson et al., 1992). However, the large amount of GLUT4 in endosomes in the absence of insulin indicates that endosomes play a role in the basal state retention of GLUT4. Supporting this hypothesis are the findings that accumulation of GLUT4 on the cell surface in the presence of insulin requires the mobilization of the endosomal pool of GLUT4 as well as the specialized, nonendosomal pool (Millar et al., 1999; Zeigerer et al., 2002). How GLUT4 is retained within endosomes and how the specialized pool of GLUT4 is created is not understood.
In this report, using quantitative optical methods, we find that GLUT4 in the basal state is efficiently excluded from the cell surface by a dynamic retrieval/retention mechanism. Intracellular GLUT4 traffics to the plasma membrane with a half-time of ∼230 min, and insulin reduces the half-time to ∼9 min. We also find that in the basal state GLUT4 rapidly cycles between a specialized compartment (devoid of TR) and endosomes that are accessible to the TR. The basal trafficking kinetics of this intracellular cycle are such that GLUT4-containing vesicles are ∼5 times as likely to fuse with endosomes as they are to fuse with the plasma membrane. We hypothesize that this rapid intracellular cycle of budding and fusion plays a role in the basal retention of GLUT4 and IRAP. The GLUT4/IRAP pathway does not overlap extensively with compartments that contain furin, thereby indicating that the GLUT4/IRAP pathway does not include late endosomes or the trans-Golgi network (TGN), two compartments known to contain furin (Thomas, 2002). An intact microtubule cytoskeleton is not required for basal retention or the steady-state distribution of GLUT4 between endosomes and the specialized compartment, although nocodazole disruption of microtubules does inhibit insulin-stimulated translocation.
MATERIALS AND METHODS
Ligands and Chemicals
Fluorescent antibodies were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA). All chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Mouse anti-hemagglutinin (HA) monoclonal antibody (mAb) (HA-11) was purified from ascites (Covance, Berkley, CA) by using a protein G affinity column (Amersham Pharmacia AB, Uppsala, Sweden). The concentration of anti-HA antibody required to saturate the HA-epitope of HA-GLUT4-green fluorescent protein (GFP) was determined for each preparation of antibody by measuring cell-associated anti-HA antibody in a 10-min pulse at 37°C (typically 50 μg/ml). Anti-HA was labeled with Cy3 (Biological Detection System, Pittsburgh, PA) per manufactures directions. Human Tf was purchased from Sigma-Aldrich, further purified by Sephacryl S-300 gel filtration, and conjugated to Cy3 per manufacture's instructions. Tf was conjugated to horseradish peroxidase (HRP) as described previously (Zeigerer et al., 2002). Anti-Tac antibody, 2A3A1H (American Type Culture Collection, Manassas, VA), was isolated from ascites by protein G purification and coupled to HRP or to Alexa488 as described for Tf. Insulin (170 nM) was used for all insulin stimulations.
Cell Culture
3T3-L1 cells were differentiated into adipocytes as described previously (Subtil et al., 2000) and transfected by electroporation 4 or 5 d after differentiation (Zeigerer et al., 2002). Cells were used early in differentiation, before accumulation of significant fat, because at this time they are fully insulin-responsive and are more amenable to electroporation and microscopy analysis than cells later in differentiation, which have accumulated more fat. Cells were electroporated at 180 V and 950 μF with 45 μg of plasmid DNA per ∼106 cells in a 1-cm cuvette (Bio-Rad, Hercules, CA) and plated onto coverslip bottom dishes. Cells were used for experiments one to 2 d after electroporation.
A chimera containing the cytoplasmic domain of IRAP substituted for the cytoplasmic domain of the human TR has been shown to traffic like GLUT4 (Johnson et al., 1998; Subtil et al., 2000). A GLUT4 construct engineered to contain an HA epitope on its first exofacial loop and a GFP on the carboxyl-cytoplasmic domain has been used to quantitatively measure the insulin-stimulated translocation of GLUT4 to the cell surface (Lampson et al., 2000; Zeigerer et al., 2002). The Tac-furin cDNA expression plasmid has been described previously (Bosshart et al., 1994).
Fluorescence Quantification
The images were collected on a DMIRB inverted microscope (Leica Microsystems, Deerfield, IL) with a cooled charge-coupled device 12-bit camera (Princeton Instruments, West Chester, PA). All images were acquired using a 40× 1.25 numerical aperture oil immersion objective. The camera is linear between 0 and 3600, therefore providing 3 orders of magnitude of dynamic range. Cells expressing HA-GLUT4-GFP were identified based on GFP fluorescence. Methods for quantification of the images have been described previously (Dunn et al., 1994; Lampson et al., 2000, 2001). The images were corrected for background by subtracting the fluorescence of cells not expressing HA-GLUT4-GFP. Background fluorescence was usually between 100 and 300. All images for an individual experiment were collected on the same day by using exposure times optimized individually for the Cy3 and GFP channels. The exposure times were chosen such that <5% of the image pixels were saturating (i.e., above the linear range of the camera) for the brightest sample. Approximately 20 cells per condition were collected in each experiment. MetaMorph (Universal Imaging, West Chester, PA) image processing software was used for quantification (Lampson et al., 2000, 2001; Zeigerer et al., 2002). The average Cy3 and GFP intensity per pixel was determined within each cell expressing HA-GLUT4-GFP. A correction for GLUT4 fluorescence was made by subtracting the Cy3 and GFP fluorescence per area collected from cells that did not express HA-GLUT4-GFP. The GFP fluorescence is used to normalize the Cy3 fluorescence for expression of HA-GLUT4-GFP per cell.
Trafficking
Cells plated in coverslip bottom dishes were incubated in serum-free DMEM medium with 220 mM bicarbonate and 20 mM HEPES pH 7.4 (med 1) for 2 h at 37°C in 5% CO2/air. Translocation of HA-GLUT4-GFP was measured as described previously (Lampson et al., 2001). For experiments in which HA-GLUT4-GFP was prebound by the anti-HA antibody, cells were incubated with saturating concentration of antibody and 170 nM insulin for 60 min, and then returned to the basal state by washing twice with 150 mM NaCl, 20 mM HEPES, 1 mM CaCl2, 5 mM KCl, and 1 mM MgCl2, pH 7.2 (med 2), twice with 200 mM NaCl, 50 mM 2-(N-morpholino)ethanesulfonic acid, pH 5.0 (med 3), and two more times, 2 min each, with med 2, followed by a 2-h incubation in med 1 at 37°C. The amount of anti-HA antibody-bound HA-GLUT4-GFP on the cell surface was determined by incubating fixed cells with Cy3-labeled goat anti-mouse IgG. The total accumulation of anti-HA antibody inside the cell in a given period of time was determined by incubating saponin-permeablized cells with Cy3-labeled goat anti-mouse IgG.
Fluorescence Quenching and Epitope Ablation
To examine colocalization in the basal state, cells were incubated with 20 μg/ml HRP-Tf and the anti-HA antibody. After the appropriate incubation time, the cells were placed on ice and washed twice with cold med 2. The HRP-mediated 3,3′-diaminobenzidine (DAB) polymerization reaction was performed by incubating for 30 min on ice with a solution of 250 μg/ml DAB and 0.0025% H2O2 in med 2 in the dark. The cells were washed twice with cold med 2 and fixed with 3.7% formaldehyde. To determine the extent of epitope ablation, the cells were permeabilized with 0.025 μg/ml saponin and stained with Cy3-labeled goat anti-mouse. For fluorescence quenching, a Cy3-labeled anti-HA antibody is used in the first incubation. In this case, the DAB polymerization product quenches the Cy3 fluorescence. Maximum fluorescence quenching and maximum epitope ablation are both ∼80% (Lampson et al., 2001; Zeigerer et al., 2002). This is true for Tf quenching by Tf-HRP, Tac-Furin quenched by HRP-anti-Tac, and wheat germ quenching by wheat germ-HRP (Johnson et al., 2001; Lampson et al., 2001; Zeigerer et al., 2002). The remaining 20% of unquenchable fluorescence is therefore a characteristic of the assay and is not related to the intracellular compartments in which the quenching occurs, the HRP conjugate used, or the ligand being ablated. Therefore, the 80% fluorescence quenching and epitope ablation of anti-HA by HRP-Tf taken up by the IRAP-TR indicates that the chimera has access to all intracellular GLUT4 compartments.
A modification of the above-mentioned protocol was used to determine the effect of nocodazole on the distribution of GLUT4 between endosomes and the specialized pool in the presence and absence of insulin. The total pool of HA-GLUT4-GFP was bound during a 360-min incubation with anti-HA antibody. The cells were incubated with HRP-Tf ± 3 μM nocodazole for 30 min and then with HRP-Tf ± insulin ± nocodazole for an additional 30 min. The cells were chilled to 4°C and reacted with DAB/H2O2 as described above. The intact cells were incubated at 4°C with an unlabeled donkey anti-mouse IgG antibody to block the anti-HA antibody epitopes on the surface (i.e., surface HA-GLUT4-GFP). The cells were fixed, permeabilized, and stained with the same donkey anti-mouse IgG fluorescently labeled with Cy3. Only the epitopes on the intracellular anti-HA antibody are bound by the fluorescent donkey anti-mouse antibody, because the ones of the surface are already bound by the unlabeled antibody. Blocking the epitopes on the surface bound anti-HA antibody increases the sensitivity of examining the distribution of HA-GLUT4-GFP among intracellular pools in the presence of insulin.
Kinetic Modeling
SAAM II compartmental modeling software (SAAM II Institute, Seattle, WA) was used for simulations and fitting of the data (Barrett et al., 1998; Cobelli and Foster, 1998).
RESULTS
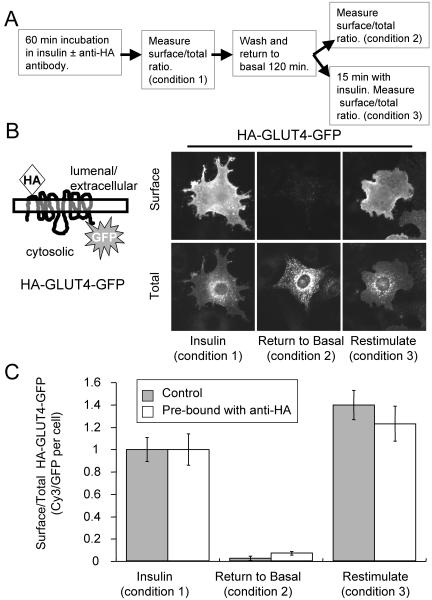
In this study, we used a GLUT4 construct, HA-GLUT4-GFP, that contains a single HA epitope engineered into the first exofacial loop and an eGFP fused to the carboxyl cytoplasmic domain of GLUT4 (Figure 1) (Lampson et al., 2000; Dawson et al., 2001). To establish that binding of the anti-HA mAb to its epitope does not alter trafficking of this construct, the behavior of HA-GLUT4-GFP prebound with antibody was determined and compared with that of control unbound HA-GLUT4-GFP. 3T3-L1 adipocytes, 4 d postdifferentiation, were electroporated with HA-GLUT4-GFP cDNA as described in MATERIALS AND METHODS. The cells, 24 h after electroporation, were incubated in serum-free medium supplemented with 170 nM insulin in the presence or absence of a saturating concentration of anti-HA antibody for 60 min. The HA epitope of HA-GLUT4-GFP was bound by the antibody as it cycled between the interior and cell surface. The cells were washed and incubated for 120 min in insulin-free medium to allow them to return to the basal state and then restimulated with insulin (Figure 1A). Surface HA-GLUT4-GFP prebound with anti-HA antibody was quantified by immunofluorescence (IF) with a Cy3-labeled goat anti-mouse IgG, whereas in control cells, HA-GLUT4-GFP (not prebound in these cells) on the surface was quantified by first binding anti-HA antibody to fixed cells followed by incubation with the Cy3-labeled goat anti-mouse IgG. Total HA-GLUT4-GFP was determined by measuring the GFP fluorescence per cell. The Cy3-to-GFP fluorescence ratio per cell accounts for differences in expression levels among the transiently expressing cells. HA-GLUT4-GFP prebound with anti-HA was sequestered intracellularly in the perinuclear region of cells in the absence of insulin, and it was rapidly redistributed to the surface when cells were treated with insulin (Figure 1B). Quantifying the distribution data demonstrates that HA-GLUT4-GFP prebound by anti-HA behaves like the unbound construct (Figure 1C), which establishes that the antibody can be used to monitor the trafficking of HA-GLUT4-GFP.
Figure 1.
Anti-HA antibody binding does not alter trafficking of HA-GLUT4-GFP. (A) Outline of the experiment protocol. The incubation with anti-HA antibody was performed in the presence of insulin because the recycling time of GLUT4 in the absence of insulin is very slow (Figure 2). (B) A cartoon of the HA-GLUT4-GFP construct. The distributions of HA-GLUT4-GFP prebound by anti-HA antibody in the presence of insulin, after removal of insulin, and after restimulation with insulin are shown. Surface HA-GLUT4-GFP was detected by indirect IF with a Cy3-labeled anti-mouse IgG antibody. The images are from representative cells for each condition. (C) Quantification of surface-to-total distribution of HA-GLUT4-GFP unbound and prebound with anti-HA-antibody. Prebound HA-GLUT4-GFP on the surface was measured in IF with a Cy3-goat anti-mouse IgG, and unbound HA-GLUT4-GFP (control cells) was measured by first binding anti-HA antibody to fixed cells followed by Cy3-Goat anti-mouse IgG. The results shown are the averages of 20 cells per condition ± SEM from a representative experiment. The data have been normalized to the surface expression level in the presence of insulin (condition 1). Insulin (170 nM) was used for stimulation.
GLUT4 Is Excluded from the Cell Surface by a Dynamic Retention/Retrieval Mechanism
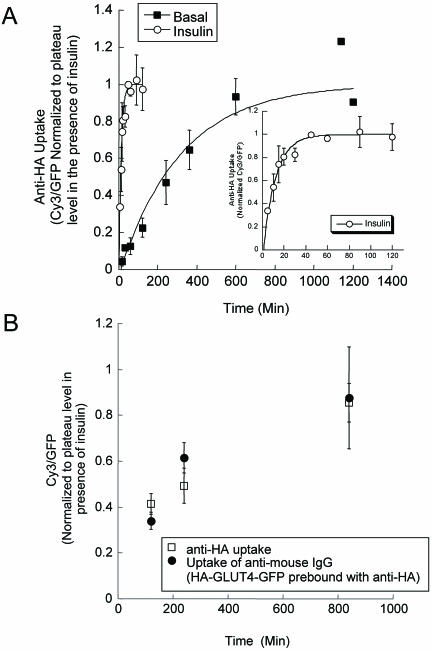
Most models for insulin-regulated trafficking propose that GLUT4 continually cycles between intracellular compartments and the cell surface in the absence and presence of insulin, although this has not been directly demonstrated in adipocytes. To measure the rate constant for HA-GLUT4-GFP exocytosis, adipocytes were incubated with a saturating concentration of anti-HA antibody for varying times, and the amount of cell-associated anti-HA antibody was determined by staining fixed, detergent-permeabilized cells with a Cy3-labeled anti-mouse secondary antibody (Figure 2A). The anti-HA antibody remains bound as the HA-GLUT4-GFP cycles through the cell (Zeigerer et al., 2002). Thus, the time-dependent increase in cell-associated anti-HA antibody is a measure of the movement of HA-GLUT4-GFP, not yet bound by anti-HA antibody, from inside the cells to the cell surface (i.e., exocytosis), and it is independent of the internalization rate constant (Ghosh et al., 1998). In the absence of insulin, the accumulation of anti-HA reached a plateau at ∼600 min, whereas in the presence of insulin the plateau was reached at ∼45 min. Based on single, first order processes, these correspond to exocytosis rate constants of 0.003 ± 0.0005 and 0.08 ± .006 min-1, respectively.
Figure 2.
Insulin increases the cycling rate of HA-GLUT4-GFP between the interior and cell surface. (A) The uptake of anti-HA antibody from the medium at 37°C in the presence and absence of insulin. Total cell associated anti-HA was measured by IF of permeabilized cells. The data are the average of three experiments ± SEM in which the Cy3/GFP ratio per cell for each experiment were normalized to the plateau level for the samples treated with insulin in that experiment. The curves are (Cy3/GFP)t = 1 - exp(-ke*t), where the (Cy3/GFP)t ratio is the fraction of HA-GLUT4-GFP bound by anti-HA antibody at time t, and ke is the exocytic rate constant. The inset panel is the data in the presence of insulin plotted on an expanded time scale. Insulin (170 nM) was used for stimulation. (B) HA-GLUT4-GFP prebound by anti-HA antibody (filled symbol) cycles to the cell surface in the basal state with similar kinetics to HA-GLUT4-GFP (open symbol), establishing that the basal retention is a dynamic process involving slow exocytosis and rapid retrieval. The data are from a single experiment (average values for 20 cells per time point ± SEM) and have been normalized to the plateau Cy3/GFP level when cells are incubated with anti-HA for 60 min in the presence of insulin (A).
Previous studies have argued that the predominant effect of insulin is on the exocytic rate of GLUT4 (Jhun et al., 1992; Czech and Buxton, 1993; Yang and Holman, 1993). The insulin-stimulated ∼26-fold increase in GLUT4 exocytosis that we measured would result in an ∼13-fold increase in the fraction of total GLUT4 that is on the surface if there is no change in GLUT4 internalization. We measured a 16 ± 3-fold increase in surface (Figure 1C), and consequently, the change in GLUT4 exocytosis is sufficient to account for the magnitude of insulin-stimulated GLUT4 translocation, providing additional evidence that the majority of the GLUT4 redistribution can be accounted for by increased exocytosis. (The fold change in the fraction of total GLUT4 on the surface is not the same as the fold change in exocytosis rate because at steady state: [GLUT4Surface]*[kInternalization] = [GLUT4Internal]*[kExocytosis], which when written as the fraction of total GLUT4 on the surface is [GLUT4Surface]/[GLUT4Total] = 1/[1 + (kInternalization/kExocytosis)]).
The observation that the same plateau levels were reached in the presence and absence of insulin demonstrates that the major effect of insulin is to redistribute a continually cycling pool of HA-GLUT4-GFP. The time-dependent increases in HA-GLUT4-GFP bound by anti-HA are fit by a single exponential (r = 0.973 and 0.994, in the absence and presence of insulin, respectively), indicating that in both conditions GLUT4 traffics back to the cell surface mainly as a single kinetic pool.
To confirm that the increase in Cy3 signal in the basal state is due to the continued cycling of HA-GLUT4-GFP between the plasma membrane and intracellular compartments, rather than the transport of newly synthesized HA-GLUT4-GFP from the biosynthetic system to the plasma membrane, HA-GLUT4-GFP was prebound with anti-HA antibody during a 60-min incubation at 37°C in the presence of insulin. The cells were washed free of insulin, returned to the basal state, and incubated at 37°C with a saturating amount of Cy3-labeled goat anti-mouse IgG in the medium. As the anti-HA bound HA-GLUT4-GFP traffics to the surface, the goat anti-mouse IgG antibody in the medium will bind the anti-HA antibody. In this protocol, only HA-GLUT4-GFP prebound by anti-HA antibody during the first incubation is detected, and therefore newly synthesized HA-GLUT4-GFP delivered to the cell surface during this incubation will not contribute to the signal. Both unbound and prebound HA-GLUT4-GFP trafficked to the surface in the basal state at similar rates, demonstrating that HA-GLUT4-GFP is in equilibrium with the cell surface in the absence of insulin (Figure 2B).
GLUT4 Rapidly Cycles between the Specialized Compartment and Endosomes in the Basal State
In the basal state, GLUT4 is distributed between intracellular compartments that contain TR, a marker for general endosomes, and a specialized, postendosomal compartment(s) that excludes the TR (Martin et al., 1996; Zeigerer et al., 2002). We have quantified the distribution of HA-GLUT4-GFP among these pools by using a fluorescence quenching method (Zeigerer et al., 2002). Briefly, a Cy3-labeled anti-HA antibody internalized from the medium is localized to the lumen of compartments containing HA-GLUT4-GFP, and a horseradish peroxidase-transferrin conjugate (HRP-Tf) is delivered to the lumen of endosomes via the TR, or to endosomes and the specialized pool of GLUT4 by a chimera between IRAP and TR (Zeigerer et al., 2002). This chimera contains the cytoplasmic domain of IRAP fused to the transmembrane and extracellular domains of the human TR. This chimera traffics through the insulin-regulated pathway in adipocytes, and it can be used to deliver HRP-Tf to the lumen of GLUT4 compartments (Subtil et al., 2000; Zeigerer et al., 2002). The fraction of HA-GLUT4-GFP in the HRP-Tf-containing compartments is determined by incubating cells with DAB and H2O2 on ice. Fluorescence is quenched in the compartments containing the HRP-catalyzed DAB polymerization product. The Cy3-anti-HA fluorescence was maximally quenched when HRP-Tf was internalized by binding to the IRAP-TR chimera (Zeigerer et al., 2002). Approximately 50% of the Cy3-anti-HA fluorescence accessible to the chimera was also accessible to the TR (i.e., quenched when HRP-Tf was internalized by the TR), demonstrating that 50% of HA-GLUT4-GFP is in TR-containing endosomes in the basal state (Zeigerer et al., 2002). The dense HRP-catalyzed DAB polymerization product also destroys (“ablates”) epitopes; and an alternative approach to determine colocalization is to assay for the destruction of epitopes on the anti-HA antibody prebound to HA-GLUT4-GFP (Martin et al., 1996). Using the epitope ablation method, we also found that GLUT4 is equally distributed between endosomes and the specialized compartment in the basal state (49.9% ± 3, n = 5; in the specialized pool).
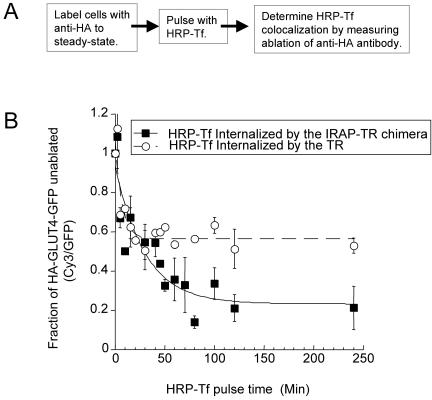
To examine transport between endosomes and the specialized pool of GLUT4, cells expressing HA-GLUT4-GFP and the TR or the IRAP-TR chimera were incubated with anti-HA antibody under conditions in which all the HA-GLUT4-GFP was bound by antibody. Cells in the basal state were pulsed with HRP-Tf, and the codistribution of the TR or the chimera with HA-GLUT4-GFP was determined by measuring ablation of epitopes on the anti-HA antibody (Figure 3). HRP-Tf internalized by the TR gained access to the endosomal pool of HA-GLUT4-GFP with a half-time of ∼5 min. The maximum level of epitope ablation remained constant during prolonged incubation with HRP-Tf of incubation, documenting that the TR is efficiently excluded from the postendosomal, specialized GLUT4 compartment.
Figure 3.
The IRAP-TR rapidly moves from the cell surface to the specialized compartment in the basal state. (A) Flow diagram of experiment shown in B. (B) IRAP-TR is rapidly transported from the cell surface to both the TR-containing endosomes and the specialized GLUT4/IRAP compartment. Cells coexpressing HA-GLUT4-GFP and the TR or the IRAP-TR chimera were incubated with unlabeled anti-HA antibody to label the complete cycling pool. The cells were pulse labeled with HRP-Tf, and at the indicated times, cells were incubated with DAB/H2O2 at 4°C. The amount of anti-HA epitope ablation was determined by staining saponin-permeabilized cells with Cy3-labeled anti-mouse IgG. The data are the averages of five experiments ± SEM. In each experiment the average Cy3/GFP ratio of 20 cells per time point were normalized to the Cy3/GFP ratio of cells that were not incubated with HRP-Tf (time 0). The curves are (Cy3/GFP)t = A*exp(-ke*t) + C, where A is the fraction of anti-HA antibody that can be ablated, ke is the rate constant for the epitope ablation, and C is the plateau level, which reflects the fraction of HA epitopes that are not ablated. The (Cy3/GFP)t value is the fraction of epitopes unablated at time t.
HRP-Tf internalized by the IRAP-TR chimera gained access to intracellular HA-GLUT4-GFP with a half-time of ∼20 min (Figure 3). Intracellular HA-GLUT4-GFP equilibrates with the cell surface with a half-time of ∼230 min (Figure 2), and therefore the observation that the chimera reaches all the intracellular compartments that contain HA-GLUT4-GFP ∼10 times faster than GLUT4 equilibrates with the cell surface establishes that the IRAP-TR chimera and HA-GLUT4-GFP mix intracellularly (i.e., without first traveling to the cell surface). Although these data establish mixing, the 20-min half-time does not necessarily correlate with the rate of movement of IRAP-TR from endosomes because a few molecules of HRP may be sufficient to ablate a compartment.
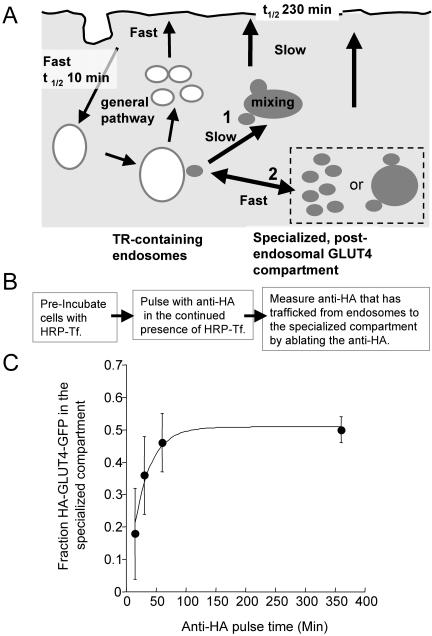
Two models could account for the extensive mixing along the insulin-regulated trafficking pathway (Figure 4A). In one model (pathway 1), traffic from endosomes to the specialized compartment is unidirectional. In this case, the specialized compartment(s) must be fusion-fission competent to account for the rapid mixing documented in Figure 3. Unidirectional traffic is incompatible with the specialized compartment being a reservoir of preformed GLUT4 vesicles. Alternatively, traffic between endosomes and the specialized compartment could be bidirectional (pathway 2), which would allow the endosomal and specialized pools to rapidly mix without first moving to the cell surface. The bidirectional model is compatible with the specialized compartment being fusion-fission competent (as in the unidirectional model) or a reservoir of GLUT4 vesicles. In the latter case, the mixing would occur as GLUT4 vesicles refuse with endosomes.
Figure 4.
There is rapid bidirectional transport between endosomes and the specialized GLUT4/IRAP compartment. (A) Cartoon of the two models. GLUT4 equilibrates with the cell surface with a half-time of 230 min. If there is unidirectional traffic from endosomes to the specialized compartment(s), then the specialized compartment must be a stable compartment to which GLUT4 vesicles fuse and from which vesicles bud (pathway 1). In this model, exit of GLUT4 from endosomes is necessarily slow to achieve the near equal distribution between endosomes and the specialized compartment. The bidirectional model (pathway 2) is compatible with the specialized compartment(s) being fusion-fission competent (as in the unidirectional model) or a reservoir of GLUT4 vesicles. In the latter case, the mixing would occur as GLUT4 vesicles refuse with endosomes. In this model transport from endosomes can be rapid. (B) Flow diagram of experiment shown in C. (C) GLUT4 equilibrates between TR-containing endosomes and the specialized compartment with a half-time of ∼20 min. Adipocytes coexpressing HA-GLUT4-GFP and the TR or the IRAP-TR chimera were incubated with HRP-Tf for 4 h to load the endosomes, or endosomes and the specialized compartment, respectively. The cells were pulsed in the continued presence of HRP-Tf with anti-HA and at the times specified the codistribution of the anti-HA antibody and Tf-HRP were determined as in Figure 3. The fraction of HA-GLUT4-GFP in the specialized compartment at each time point is shown. The data are the averages ± SEM from four independent experiments. The curve is (Cy3/GFP)t = A0 - (A0*exp(-k*t)), where A0 is the fraction of HA-GLUT4-GFP in the specialized compartment at steady state and k is the rate constant for equilibration between endosomes and the specialized pool. The (Cy3/GFP)t value is the fraction of HA-GLUT4-GFP in the specialized compartment at time t.
These two models can be distinguished by examining GLUT4 trafficking from endosomes. In the unidirectional model, equilibration of GLUT4 between the specialized compartment and endosomes requires transport from the specialized pool to the plasma membrane followed by internalization into endosomes. GLUT4 internalization from the cell surface is rapid (t1/2 ∼10 min; e.g., Tengholm et al., 2003) compared with GLUT4 exocytosis (t1/2 ∼230 min). Therefore, the unidirectional model predicts that GLUT4 moves from the specialized compartment to endosomes with half-time approximately equal to the 230-min half-time for movement to the plasma membrane. To achieve the near equal distribution of GLUT4 between endosomes and the specialized pool, the rate of exit from the endosomes must be similar to the rate of movement from the specialized pool to the cell surface. Thus, this model requires that GLUT4 is transported from endosomes at a slow rate. In the bidirectional model, the transport from endosomes could be rapid because equilibration occurs directly between endosomes and the specialized compartment without involving slow transport to the cell surface. Therefore, if HA-GLUT4-GFP exits endosomes rapidly, then transport between the specialized compartment and endosomes must be bidirectional.
To examine traffic from the endosomes, HRP-Tf was localized to endosomes in cells expressing the HA-GLUT4-GFP and the TR, and to endosomes and the specialized compartments in cells expressing the HA-GLUT4-GFP and the IRAP-TR chimera. The cells were pulsed with anti-HA antibody in the continued presence of HRP-Tf for varying periods of time. At the specified times the cells were incubated with DAB/H2O2 on ice. The amount of unablated cell-associated anti-HA antibody was determined. An equal distribution of GLUT4 between endosomes and the specialized compartment was reached within 60 min, which is too rapid to be compatible with unidirectional traffic from endosomes to the specialized compartment. In that model, a steady-state distribution would not be reached until ∼600 min, the time required to fill the specialized compartment with HA-GLUT4-GFP internalized from the cell surface (Figure 4B). These studies support a model in which GLUT4 rapidly cycles between endosomes and the specialized compartment in the absence of insulin, with GLUT4-containing vesicles preferentially fusing with endosomes relative to the plasma membrane (as depicted in pathway 2, Figure 4B). We propose that restricting GLUT4 to this intracellular cycle of vesicle formation and fusion actively depletes the cell surface of GLUT4.
The GLUT4/IRAP Specialized Trafficking Pathway Does Not Overlap with the Furin Trafficking Pathway
We next sought to characterize trafficking of GLUT4 that has exited TR-containing endosomes. It has recently been shown that syntaxin 6 and 16 overlap with GLUT4, indicating that upon leaving the TR-containing endosomes, GLUT4 traffics through the TGN (Perera et al., 2003; Shewan et al., 2003). However, TGN38, a commonly used marker for the TGN, does not significantly colocalize with GLUT4 that has exited TR-containing endosomes, arguing against localization of GLUT4 to the TGN (Livingstone et al., 1996). Furin, like TGN38, continually cycles among the cell surface, endosomes, and the TGN (Thomas, 2002). However, furin traffics to the TGN by a different pathway than TGN38. Furin traffics from sorting endosomes to late endosomes and then to the TGN, whereas TGN38 traffics from sorting endosomes to the recycling compartment and then to the TGN (Mallet and Maxfield, 1999). Furin is concentrated intracellularly by cycling between endosomes and the TGN. To determine whether the GLUT4 pathway overlaps with the furin pathway, we used HRP fluorescence quenching in adipocytes expressing a Tac-furin chimera. The Tac-furin chimera, which has been used as a reporter of furin traffic, contains the cytoplasmic and transmembrane domains of furin fused to the extracellular domain of the interleukin-2 receptor alpha chain (Bosshart et al., 1994; Voorhees et al., 1995; Mallet and Maxfield, 1999).
To determine the overlap between the TR recycling pathway and the furin pathway, adipocytes coexpressing Tacfurin and the TR were incubated with a Alexa488-labeled anti-Tac mAb and HRP-Tf for 5 h. During this incubation, Tac-furin is bound by the antibody as it cycles between the interior and surface of cells (Mallet and Maxfield, 1999). The quenching of the fluorescence of the anti-Tac antibody by the HRP/DAB polymerization product was determined (Figure 5A). Approximately 20% of the anti-Tac fluorescence was in compartments accessible to the TR (i.e., 20% quenched). This small amount of overlap is consistent with studies in fibroblasts that have shown that Tac-furin mostly accumulates in late endosomes and the TGN, two compartments that are not frequented by the TR (Bosshart et al., 1994; Mallet and Maxfield, 1999). In fibroblasts, it has been shown that furin overlaps with the TR in sorting endosomes, and therefore it is likely that in the adipocytes the 20% overlap between furin and TR is also restricted to sorting endosome.
Figure 5.
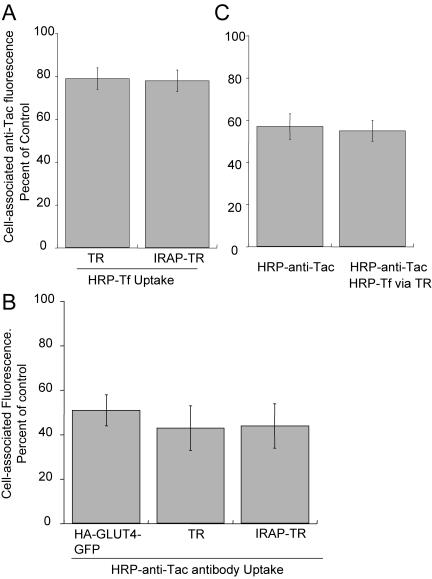
Overlap of the insulin-regulated pathway with the furin pathway is restricted to TR-containing endosomes. (A) IRAP-TR, like TR, has access to only 20% of the Alexa488-anti-Tac fluorescence internalized by Tac-furin. Cells coexpressing Tacfurin and the TR or the IRAP-TR chimera were incubated with an Alexa488-labeled anti-Tac-antibody and HRP-Tf for 5 h. The cells were chilled to 4°C and treated with DAB/H2O2. The Alexa488 fluorescence in cells incubated with HRP-Tf is plotted as a percentage of the Alexa488 fluorescence in cells that were not incubated with HRP-Tf. The data are from a representative experiment and are the averages ± SEM of 25 cells per condition. (B) Fifty percent of the TR, the IRAP-TR chimera, and HA-GLUT4-GFP are in intracellular compartments accessible to Tac-furin. Cells expressing Tac-furin and the TR, IRAP-TR chimera, or HA-GLUT4-GFP were incubated with an HRP-anti-Tac antibody and Cy3-Tf (in cells expressing TR or IRAP-TR) or Cy3-anti-HA-antibody (in cells expressing HA-GLUT4-GFP) for 6 h. The Cy3 fluorescence is plotted as a percentage of the Cy3 fluorescence in cells that were not incubated with HRP-anti-Tac antibody. The data are the averages ± SEM of four independent experiments. (C) Tacfurin and HA-GLUT4-GFP overlap in TR endosomes. Cells expressing Tac-furin, TR and HA-GLUT4-GFP were incubated for 6.5 h in medium containing saturating concentrations of Cy3-anti-HA and HRP-anti-Tac antibodies with or without HRP-Tf. The cells were chilled to 4°C and treated with DAB/H2O2. The data are plotted as the percentage of Cy3 fluorescence in cells that were not incubated with either HRP-anti-Tac or HRP-Tf. The Cy3 fluorescence was divided by GFP per cell to correct for expression of HA-GLUT4-GFP. The data shown are the averages ± SEM (at least 20 cells per condition) from a representative experiment.
To examine the extent of overlap between the GLUT4/IRAP and the furin pathways, cells expressing the IRAP-TR chimera and Tac-furin were examined. As was the case for the TR, ∼20% of the fluorescence of the anti-Tac antibody was quenched by HRP-Tf taken up by the IRAP-TR chimera (Figure 5A). Thus, the GLUT4/IRAP pathway has no more overlap with the Tac-furin pathway than does the TR. This suggests that GLUT4 overlaps with furin within TR-containing endosomes and that it does not accumulate in other compartments enriched in furin (e.g., late endosomes and aspects of the TGN).
As an alternative to examine the codistribution of furin and the GLUT4/IRAP pathway, we used an HRP-anti-Tac antibody conjugate to quench Cy3-anti-HA internalized by HA-GLUT4-GFP or Cy3-Tf internalized by the TR or the IRAP-TR chimera. We found that HRP-anti-Tac quenched 80% of fluorescence of a fluorescently labeled anti-Tac internalized by 3T3-L1 adipocytes expressing Tac-furin construct. This is the same maximum quenching we measure for HRP-Tf quenching of fluorescent-Tf, indicating that 80% maximum quenching is a characteristic of the method and that it is not related to the HRP conjugate, the ligand being quenched, or the compartment in which the quenching occurs. HRP-anti-Tac quenched ∼50% of the Tf internalized by the TR and the IRAP-TR as well as anti-HA internalized by HA-GLUT4-GFP (Figure 5B). The findings that HRP-anti-Tac quenched no more HA-GLUT4-GFP than TR indicate that furin does not significantly overlap with GLUT4 outside of the TR-containing endosomes.
The incomplete quenching of TR by HRP-anti-Tac is consistent with the overlap between furin and the TR being restricted to sorting endosomes (i.e., furin does not traffic through the recycling compartment; Mallet and Maxfield, 1999). These data indicate that the GLUT4/IRAP and furin pathways overlap in TR-containing endosomes and that ∼50% of the TR is in endosomes that do not contain GLUT4, demonstrating that that GLUT4 does not have access to all of the endosomal compartments along the recycling pathway.
To confirm that the colocalization of furin and GLUT4 is in TR-containing endosomes, the quenching of Cy3-anti-HA prebound to HA-GLUT4-GFP, by cointernalized HRP-anti-Tac and HRP-Tf was determined (Figure 5C). In cells expressing the TR, Tac-furin and HA-GLUT4-GFP, the degree of anti-HA Cy3-fluorescence quenching was similar when HRP-anti-Tac was taken up alone as when HRP-Tf and HRP-anti-Tac were cointernalized. These data indicate that the overlap between TR, GLUT4 and furin is in endosomes and thereby provide additional evidence that late endosomes and the TGN, two compartments enriched in furin, are not a significant component of the specialized GLUT4 compartment.
The Microtubule Cytoskeleton Is Required for Insulin-Stimulated Translocation of GLUT4 But Not for Basal Cycling between the Specialized Compartment and Endosomes
Microtubules are known to play a role in the targeted vesicular transport; however, their involvement in insulin-stimulated translocation of GLUT4 is controversial. Some previous studies found that insulin-stimulated glucose uptake and redistribution of GLUT4 to the surface requires an intact microtubule cytoskeleton (Emoto et al., 2001; Olson et al., 2001; Patki et al., 2001), whereas in other studies an intact cytoskeleton was not required for GLUT4 translocation (Molero et al., 2001; Shigematsu et al., 2002).
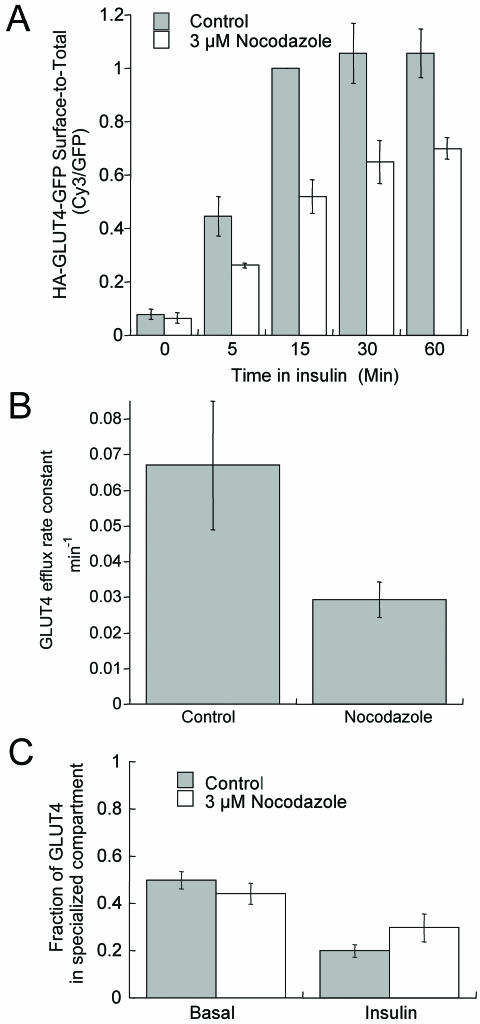
The identification of the intracellular retention cycle provides an additional site for possible microtubule involvement and regulation. To address the possible role of microtubules in the retention cycle, we examined the effect of nocodazole on surface GLUT4 levels in the stimulated and unstimulated states. We found that 3 μM nocodazole, which depolymerized microtubules, did not affect the amount of GLUT4 on the surface in the basal state, indicating that microtubules are not required for retention, at least during the time period examined in these experiments (total of 60 min in presence of nocodazole) (Figure 6A). However, 3 μM nocodazole reduced the amount of GLUT4 on the surface in the presence of insulin at steady state by ∼40%, and it increased the time to reach the new steady-state distribution (Figure 6A). In control cells, it takes ∼15 min for the amount of GLUT4 on the surface to plateau (i.e., reach new steady state), and nocodazole increased the time to reach a new steady-state level to between 15 and 30 min. Both these effects of nocodazole could be accounted for by an effect on GLUT4 exocytosis.
Figure 6.
Nocodazole disruption of microtubules partially inhibits translocation of GLUT4 without affecting basal retention. (A) Insulin-stimulated recruitment of GLUT4 to the cell surface was inhibited by nocodazole. Cells were incubated with 3 μM nocodazole for 30 min at 37°C and then treated with 170 nM insulin and nocodazole for the times indicated. Control cells were similarly treated except nocodazole was not included in the incubations. Surface HA-GLUT4-GFP was measured by IF. To allow for direct comparison among experiments, the data from each experiment were normalized to the control 15-min insulin-stimulated surface-to-total value determined in that experiment. The data shown are the averages of at least four independent measurements ± SEM, except for the 60-min time point, which is the average of two experiments ± SD. Nocodazole (3 μM) depolymerized the microtubule cytoskeleton as determined by IF in fixed cells. (B) Insulin-stimulated exocytosis of GLUT4 was inhibited by nocodazole. Cells were incubated with 3 μM nocodazole for 30 min, followed by a 15-min incubation with 170 nM insulin and 3 μM nocodazole. After this incubation antibody was added to the cells in the continued presence of nocodazole and insulin, and the accumulation of anti-HA antibody was measured as described in Figure 2. The efflux rate constants for exocytosis, measured as described in Figure 2, are presented. Control cells were similarly treated except nocodazole was not included in the incubations. The data are the average efflux rate constants ± SEM calculated in three separate experiments. (C) Nocodazole does not alter distribution of GLUT4 between endosomes and the specialized compartment. Cells expressing HA-GLUT4-GFP and TR or IRAP-TR were preincubated with anti-HA antibody and with or without HRP-Tf for 390 min at 37°C in medium without insulin. Half of the samples received 3 μM nocodazole for the final 30 min of incubation. Cells were then incubated for an additional 30 min with or without HRP-Tf ± insulin ± 3 μM nocodazole. Colocalization of intracellular HA-GLUT4-GFP with TR and IRAP-TR was determined as described in MATERIALS AND METHODS. The data are the averages ± SEM of five independent measurements.
To more closely examine the effects of nocodazole, we measured the steady-state insulin-stimulated efflux of GLUT4 by using the method described in Figure 3 (Figure 6B). The insulin-stimulated steady-state efflux rate constant is reduced by ∼60%, which accounts for the magnitude of inhibition of insulin-stimulated redistribution (Figure 6A). These data show that insulin-stimulated GLUT4 exocytosis is slowed in the absence of an intact microtubule cytoskeleton. The observation that the change in translocation is accounted for by the reduced efflux rate constant argues against nocodazole effecting GLUT4 internalization
We next determined the effect of nocodazole on the distribution of GLUT4 between endosomes and the specialized pool (Figure 6C). Nocodazole does not alter the basal intracellular distribution of HA-GLUT4-GFP between these pools, consistent with nocodazole not affecting the amount of GLUT4 on the surface in the basal state. In control cells, insulin induces a shift of the distribution of intracellular GLUT4 from ∼50% in endosomes to ∼80% in endosomes, due to the more rapid trafficking of GLUT4 in the presence of insulin (Figure 6C). Despite the fact that insulin-stimulated GLUT4 efflux is slowed by nocodazole, the intracellular distribution of GLUT4 between endosomes and the specialized compartment was shifted toward endosomes, just as it is in insulin-treated control cells. Although in these experiments there was a trend toward a greater fraction of HA-GLUT4-GFP in the specialized pool in the presence of nocodazole and insulin than in insulin alone, the difference was not statistically significant.
DISCUSSION
Here, we demonstrate that GLUT4 is in equilibrium with the cell surface in the basal state. Previous studies have demonstrated that GLUT4 traffics between the interior and surface of cells in the presence of insulin, but trafficking of GLUT4 to the cell surface in the absence of insulin had not been directly quantified (Jhun et al., 1992; Holman et al., 1994; Palacios et al., 2001). Our data provide direct evidence for a model in which GLUT4 is dynamically excluded from the plasma membrane by a retention/retrieval mechanism (Figure 7; Foster et al., 2001; Bryant et al., 2002).
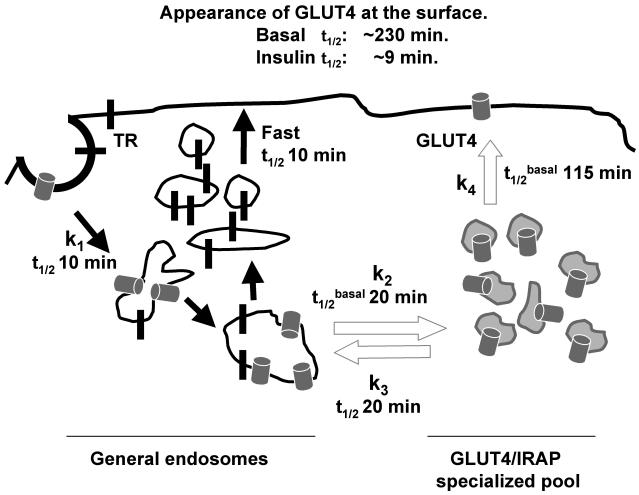
Figure 7.
Model for the dynamic exclusion of GLUT4 from the plasma membrane by a retention-retrieval mechanism. In the basal state, GLUT4 vesicles preferentially fuse with endosomes relative to the plasma membrane, thereby depleting the cell surface of GLUT4. Any GLUT4 that is introduced into the plasma membrane is rapidly retrieved by clathrin-mediated endocytosis back to endosomes. Insulin increases the exocytic rate of GLUT4 and thereby induces an increase of GLUT4 in the plasma membrane. Compartments of the insulin-regulated pathway are in gray. The half-time for appearance of GLUT4 at the cell surface in the presence and absence of insulin were directly measured, as was the equilibration time between endosomes and the specialized compartment in the basal state. For kinetic modeling experiments, the following parameters were used: a half-time of GLUT4 internalization of 10 min (Tengholm et al., 2003), and a steady-state distribution of GLUT4 in the basal state of 4% in the plasma membrane and 48% each in endosomes and the specialized pool. A value of 4% on the surface was calculated from % GLUT4Surface = 1/(1 + (kInternalziation/kExocytosis)), with kInternalziation = 0.07 min-1 (Tengholm et al., 2003) and kExocytosis = 0.003 min-1. The values for k2 in insulin and k4 in basal and insulin conditions were determined using SAAM II compartmentalization modeling software (Barrett et al., 1998; Cobelli and Foster, 1998). See text for discussion of the model.
We define two intracellular pools of GLUT4 based on accessibility to TR: the endosomal pool, which is accessible to TR; and the specialized pool, which is not. We refer to the latter as a specialized pool to distinguish it from the pool of GLUT4 that colocalizes with TR in general endosomes. A number of lines of evidence support the existence of the specialized pool of GLUT4 in fat cells (e.g., Martin et al., 1996; Palacios et al., 2001; Kupriyanova et al., 2002). We propose that GLUT4 and IRAP are actively and continually sorted from endosomes to the specialized compartment and that the TR is excluded from this pathway. The determinants that target GLUT4 and IRAP to the specialized compartment have been partially identified, although the machinery that recognizes this information is yet to be discovered (Verhey et al., 1995; Subtil et al., 2000; Palacios et al., 2001; Shewan et al., 2003).
We currently do not understand how the TR is efficiently segregated from this pathway. The iterative nature of this process may account for the high efficiency with which TR is sorted from the specialized pool of GLUT4. In our studies of adipocytes transfected with the human TR, we do not see a correlation between the level of expression of the TR and the size of the GLUT4/IRAP-specialized compartment (Zeigerer et al., 2002). Therefore, the failure to detect TR in the GLUT4-containing vesicles is not related to the amount of TR expressed in cells, at least over an ∼20-fold range of expression, consistent with an active sorting mechanism.
Morphological and biochemical studies indicate that GLUT4 is mainly found in vesicular structures (e.g., Slot et al., 1991; Martin et al., 2000; Ramm et al., 2000; Kupriyanova et al., 2002), suggesting that the specialized compartment is a pool of GLUT4/IRAP-enriched vesicles (Figure 7). There is tremendous interest in characterizing this postendosomal reservoir of GLUT4. We found that the overlap of the GLUT4/IRAP pathway with the furin pathway is restricted to TR-containing endosomes, which argues against the TGN being a major intracellular reservoir of GLUT4 because furin accumulates in the TGN. Others have also found little overlap between GLUT4 and markers of the TGN, including TGN38 (Martin et al., 1994; Palacios et al., 2001). However, there are data supporting a role of the TGN in GLUT4 trafficking. It has recently been proposed that IRAP continually cycles through the TGN (Shewan et al., 2003). In addition, the observations that the cation dependent mannose 6-phosphate receptor and the clathrin adaptin protein AP-1, both of which partially localize to the TGN, were found on perinuclear GLUT4 vesicles suggests a role for the TGN in GLUT4 trafficking (Martin et al., 2000; Ramm et al., 2000). Thus, it is possible that GLUT4 moves through a subdomain of the TGN that is not frequented by TGN38 or furin. The GLUT4 pathway is complex, and it may transit a number of intracellular compartments as it cycles through the cells and additional studies are required to further identify these compartments. Regardless, the lack of overlap between the GLUT4 and furin pathways emphasizes the specialized nature of postendosomal GLUT4 trafficking.
Defining GLUT4 trafficking in the basal state is key for understanding how insulin regulates GLUT4 distribution. Here, we find that HA-GLUT4-GFP equilibrates between the specialized vesicle pool and endosomes more rapidly than it returns to the cell surface. We measure a half-time for equilibration of GLUT4 between endosomes and the specialized pool of 20 min (Figure 4) and a half-time of 230 min for GLUT4 to equilibrate with the plasma membrane (Figure 2). These data demonstrate that GLUT4 vesicles can either fuse with the plasma membrane or with endosomes and that in the basal state these vesicles preferentially fuse with endosomes. These data support a model in which GLUT4 is retained by the dynamic intracellular cycling of GLUT4 between the specialized pool and endosomes.
The 230-min half-time we measure for equilibration of intracellular GLUT4 with the cell surface (Figure 2) reflects both the rates of transport of GLUT4 to the plasma membrane and of direct return to endosomes (k4 and k3, Figure 7). If we use a half-time of 20 min for equilibration between endosomes and the specialized compartment, a half-time of 230 min for equilibration with the cell surface, and the constraint that GLUT4 is equally distributed between endosomes and the specialized pool, a half-time of 115 min for k4 is derived from modeling the basal-state data in Figure 2. Thus, on the average a GLUT4-containing vesicle formed from an endosome is ∼5 times as likely to refuse with an endosome as it is to fuse with the plasma membrane (i.e., ratio of k4 to k3). We propose that this intracellular budding/fusion cycle is part of the active mechanism by which GLUT4 is excluded from the plasma membrane. There are a number of possible mechanisms by which GLUT4-containing vesicles could be preferentially targeted for fusion with endosomes in the basal state, and our current data do not address this issue. Regardless, the fact that GLUT4 rapidly cycles among intracellular compartments has important ramifications for understanding of the retention mechanism and the effect of insulin on GLUT4 traffic at a molecular level.
Live cell imaging studies have documented that the GLUT4 vesicles are motile in the basal state, although we do not know whether those dynamics correlate with the cycle of budding and fusion that we have documented (Patki et al., 2001). For example, it is possible that the GLUT4 vesicles formed from endosomes do not move any appreciable distance from the endosomes, which may explain why it has been difficult to morphologically characterize the specialized GLUT4 compartment by using fluorescence microscopy.
We previously proposed that insulin directly regulates movement of GLUT4 to the plasma membrane from both endosomes and from the specialized compartment (Zeigerer et al., 2002). That proposal was based on the hypothesis that GLUT4 traffic between endosomes and the specialized compartment was unidirectional. Although we now revise that model to include bidirectional traffic between endosomes and the specialized compartment, our data still support the proposal that insulin affects movement of GLUT4 from endosomes. This conclusion is based on the observations that in the basal state we find that GLUT4 moves from endosomes with a half-time of ∼20 min (Figure 3), which is slower than the ∼9-min exocytosis half-time we measure for GLUT4 in the presence of insulin (Figure 2). Exit of GLUT4 from endosomes in the presence of insulin cannot be slower than its appearance at the cell surface; therefore, insulin must increase the transport of GLUT4 from endosomes. Insulin must also increase delivery of GLUT4 from the specialized pool to the plasma membrane (k4, Figure 7). Our data support the hypothesis that insulin affects GLUT4 traffic from endosomes and from the specialized pool; they do not, however, address questions concerning the mechanism by which insulin modulates GLUT4 redistribution. There is evidence that both transport of GLUT4 vesicles to the cell surface and fusion of these vesicles with the plasma membrane is regulated (Inoue et al., 2003; Semiz et al., 2003). Both of these changes would contribute to the increased appearance of GLUT4 on the cell surface in the presence of insulin.
Insulin may mobilize GLUT4 vesicles by a cytoskeleton-dependent mechanism. Live cell imaging studies have shown that GLUT4 moves along microtubules and that the frequency of GLUT4 vesicle movement is increased with insulin (Patki et al., 2001; Semiz et al., 2003). We found that 3 μM nocodazole, a concentration that depolymerizes microtubules and does not directly block the ability of GLUT4 to transport glucose (Molero et al., 2001), reduced the amount of GLUT4 on the surface of cells in the presence of insulin by twofold. Nocodazole had no effect on the basal surface expression of GLUT4, indicating that the role of microtubules is specific to the insulin-stimulated state. Insulin-stimulated exocytosis of GLUT4 is inhibited by nocodazole, accounting for the reduced steady-state translocation. We do not understand why our results differ from the previous studies that found similar concentrations of nocodazole had no effect on GLUT4 translocation (Molero et al., 2001; Shigematsu et al., 2002).
We found that nocodazole had no effect on the intracellular distribution of GLUT4 between endosomes and the specialized compartment in the basal state or in the presence of insulin. No change in the basal state distribution is consistent with our findings that nocodazole did not alter the amount of GLUT4 on the surface in the absence of insulin, indicating that the basal cycle of GLUT4 vesicle formation and fusion with endosomes does not require an intact microtubule cytoskeleton.
In control cells, the fraction of GLUT4 in endosomes increases with insulin treatment (Figure 6). These data are consistent with our proposal that in the presence of insulin the rate-limiting step in return of GLUT4 to the plasma membrane is movement from endosomes. We anticipated that nocodazole would alter the insulin-induced redistribution of GLUT4 between endosomes and the specialized pool because insulin-dependent recruitment of GLUT4 to the surface was inhibited by nocodazole. For example, if the sole effect of nocodazole on GLUT4 traffic was to inhibit movement from the specialized compartment to the cell surface, then in the presence of insulin nocodazole should promote GLUT4 accumulation in the specialized vesicles relative to control cells (i.e., reduce k4insulin without changing other GLUT4 trafficking parameters, Figure 7). We did not observe a consistent effect of nocodazole on GLUT4 distribution. These findings suggest that the effects of nocodazole are not limited to k4insulin. One interpretation of these data is that insulin mobilizes GLUT4 from both endosomes and the specialized pool in a microtubule-dependent process, which is consistent with the finding that the frequency of movement (i.e., GLUT4 mobilization) but not the rate of transport of GLUT4 vesicles along microtubules is increased by insulin (Semiz et al., 2003). More specific reagents need to be used to rigorously address the role microtubule-based movement in insulin-stimulated GLUT4 translocation, because microtubule disruption will have multiple and potentially confounding effects. It has recently been shown that kinesin KIF5B is involved in GLUT4 translocation (Semiz et al., 2003), and it is of interest to determine whether disruption of KIF5B alters the intracellular distribution of GLUT4.
There is precedent for the retention of membrane proteins based on an intracellular cycle. In yeast Saccharomyces cerevisiae, an intracellular reservoir of chitin synthase III is maintained by a transport between early endosomes and the TGN (Ziman et al., 1998; Valdivia et al., 2002). Chitin synthase III is targeted to the plasma membrane at the site of the mother-bud junction. Once the daughter cell reaches full size, the synthase is internalized and stored intracellularly for use during the next round of cell division. Thus, the amount of chitin synthase III on the cell surface is regulated based on cycling between two intracellular compartments. The conceptual similarities between chitin synthase III and the model we proposes for GLUT4 retention extends the previously noted similarities between these two regulated endocytic membrane trafficking processes (Ziman et al., 1998).
Retention by a dynamic cycle of vesicle formation and fusion with endosomes may be a general mechanism for the regulated expression of proteins on the plasma membrane. For example, a number of features of the regulated expression of the aquaporin-2 water channel on the surface kidney cells are similar to that of insulin regulation of GLUT4 surface expression, and it may be that aquaporin-2 is retained by a similar intracellular trafficking cycle (Brown, 2003).
Acknowledgments
We thank Fred Maxfield and Nadine Wertheim for useful comments and Daniel Chuang for expert technical assistance. The work was supported by National Institutes of Health grants DK52852 and DK57689 to T.E.M.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-07-0517. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-07-0517.
Abbreviations used: HRP, horseradish peroxidase; IF, immunofluorescence; Tac, interleukin receptor α chain; Tf, transferrin; TR, transferrin receptor; TGN, trans-Golgi network.
References
- Barrett, P., Bell, B., Cobelli, C., Golde, H., Schumitzky, A., Vicini, P., and Foster, D. (1998). SAAM II: simulation, analysis, and modeling software for tracer and pharmacokinetic studies. Metabolism 47, 484-492. [DOI] [PubMed] [Google Scholar]
- Bosshart, H., Humphrey, J., Deignan, E., Davidson, J., Drazba, J., Yuan, L., Oorschot, V., Peters, P.J., and Bonifacino, J.S. (1994). The cytoplasmic domain mediates localization of furin to the trans-Golgi network en route to the endosomal/lysosomal system. J. Cell Biol. 126, 1157-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D. (2003). The ins and outs of aquaporin-2 trafficking. Am. J. Physiol. Renal. Physiol. 284, F893-F901. [DOI] [PubMed] [Google Scholar]
- Bryant, N., Govers, R., and James, D. (2002). Regulated transport of the glucose transporter GLUT4. Nat. Rev. Mol. Cell. Biol. 3, p267-277. [DOI] [PubMed] [Google Scholar]
- Cobelli, C., and Foster, D. (1998). Compartmental models: theory and practice using the SAAM II software system. Adv. Exp. Med. Biol. 445, 79-101. [DOI] [PubMed] [Google Scholar]
- Czech, M.P., and Buxton, J.M. (1993). Insulin action on the internalization of the GLUT4 glucose transporter in isolated rat adipocytes. J. Biol. Chem. 268, 9187-9190. [PubMed] [Google Scholar]
- Dawson, K., Aviles-Hernandez, A., Cushman, S., and Malide, D. (2001). Insulin-regulated trafficking of dual-labeled glucose transporter 4 in primary rat adipose cells. Biochem. Biophys. Res. Commun. 287, 445-454. [DOI] [PubMed] [Google Scholar]
- Dunn, K., Mayor, S., Myers, J., and Maxfield, F. (1994). Applications of ratio fluorescence microscopy in the study of cell physiology. FASEB J. 8, 573-582. [DOI] [PubMed] [Google Scholar]
- Elmendorf, J., Boeglin, D., and Pessin, J. (1999). Temporal separation of insulin-stimulated GLUT4/IRAP vesicle plasma membrane docking and fusion in 3T3L1 adipocytes. J. Biol. Chem. 274, p37357-37361. [DOI] [PubMed] [Google Scholar]
- Emoto, M., Langille, S.E., and Czech, M.P. (2001). A role for kinesin in insulin-stimulated GLUT4 glucose transporter translocation in 3T3-L1 Adipocytes. J. Biol. Chem. 276, 10677-10682. [DOI] [PubMed] [Google Scholar]
- Foster, L., Li, D., Randhawa, V., and Klip, A. (2001). Insulin accelerates inter-endosomal GLUT4 traffic via phosphatidylinositol 3-kinase and protein kinase B. J. Biol. Chem. 276, 44212-44221. [DOI] [PubMed] [Google Scholar]
- Ghosh, R.N., Mallet, W.G., Soe, T.T., McGraw, T.E., and Maxfield, F.R. (1998). An endocytosed TGN38 chimeric protein is delivered to the TGN after trafficking through the endocytic recycling compartment in CHO cells. J. Cell Biol. 142, 923-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hah, J., Ryu, J., Lee, W., Kim, B., Lachaal, M., Spangler, R., and Jung, C. (2002). Transient changes in four GLUT4 compartments in rat adipocytes during the transition, insulin-stimulated to basal: implications for the GLUT4 trafficking pathway. Biochemistry 41, 14364-14371. [DOI] [PubMed] [Google Scholar]
- Holman, G.D., Lo Leggio, L., and Cushman, S.W. (1994). Insulin-stimulated GLUT4 glucose transporter recycling. A problem in membrane protein subcellular trafficking through multiple pools. J. Biol. Chem. 269, 17516-17524. [PubMed] [Google Scholar]
- Inoue, M., Chang, L., Hwang, J., Chiang, S., and Saltiel, A. (2003). The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature 422, 629-633. [DOI] [PubMed] [Google Scholar]
- Jhun, B.H., Rampal, A.L., Liu, H., Lachaal, M., and Jung, C.Y. (1992). Effects of insulin on steady state kinetics of GLUT4 subcellular distribution in rat adipocytes. Evidence of constitutive GLUT4 recycling. J. Biol. Chem. 267, 17710-17715. [PubMed] [Google Scholar]
- Johnson, A., Lampson, M., and McGraw, T. (2001). A di-leucine sequence and a cluster of acidic amino acids are required for dynamic retention in the endosomal recycling compartment of fibroblasts. Mol. Biol. Cell 12, 367-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, A.O., Subtil, A., Petrush, R., Kobylarz, K., Keller, S.R., and McGraw, T.E. (1998). Identification of an insulin-responsive, slow endocytic recycling mechanism in Chinese hamster ovary cells. J. Biol. Chem. 273, 17968-17977. [DOI] [PubMed] [Google Scholar]
- Keller, S., Davis, A., and Clairmont, K. (2002). Mice deficient in the insulin-regulated membrane aminopeptidase show substantial decreases in glucose transporter GLUT4 levels but maintain normal glucose homeostasis. J. Biol. Chem. 277, 17677-17686. [DOI] [PubMed] [Google Scholar]
- Kupriyanova, T., Kandror, V., and Kandror, K. (2002). Isolation and characterization of the two major intracellular Glut4 storage compartments. J. Biol. Chem. 277, 9133-9138. [DOI] [PubMed] [Google Scholar]
- Lampson, M., Racz, A., Cushman, S., and McGraw, T. (2000). Demonstration of insulin-responsive trafficking of GLUT4 and vpTR in fibroblasts. J. Cell Sci. 113, 4065-4076. [DOI] [PubMed] [Google Scholar]
- Lampson, M., Schmoranzer, J., Zeigerer, A., Simon, S., and McGraw, T. (2001). Insulin-regulated release from the endosomal recycling compartment is regulated by budding of specialized vesicles. Mol. Biol. Cell 12, 3489-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone, C., James, D.E., Rice, J.E., Hanpeter, D., and Gould, G.W. (1996). Compartment ablation analysis of the insulin-responsive glucose transporter (GLUT4) in 3T3-L1 adipocytes. Biochem. J. 315, 487-495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet, W.G., and Maxfield, F.R. (1999). Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways. J. Cell Biol. 146, 345-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, S., Ramm, G., Lyttle, C., Meerloo, T., Stoorvogel, W., and James, D. (2000). Biogenesis of insulin-responsive GLUT4 vesicles is independent of brefeldin A-sensitive trafficking. Traffic 1, 652-660. [DOI] [PubMed] [Google Scholar]
- Martin, S., Reaves, B., Banting, G., and Gould, G.W. (1994). Analysis of the co-localization of the insulin-responsive glucose transporter (GLUT4) and the trans Golgi network marker TGN38 within 3T3-L1 adipocytes. Biochem. J. 300, 743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, S., Tellam, J., Livingstone, C., Slot, J.W., Gould, G.W., and James, D.E. (1996). The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulin-sensitive cells. J. Cell Biol. 134, 625-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar, C.A., Shewan, A., Hickson, G.R.X., James, D.E., and Gould, G.W. (1999). Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3-L1 adipocytes. Mol. Biol. Cell 10, 3675-3688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molero, J., Whitehead, J., Meerloo, T., and James, D. (2001). Nocodazole inhibits insulin-stimulated glucose transport in 3T3-L1 adipocytes via a microtubule-independent mechanism. J. Biol. Chem. 276, 43829-43835. [DOI] [PubMed] [Google Scholar]
- Olson, A., Trumbly, A., and Gibson, G. (2001). Insulin-mediated GLUT4 translocation is dependent on the microtubule network. J. Biol. Chem. 276, 10706-10714. [DOI] [PubMed] [Google Scholar]
- Palacios, S., Lalioti, V., Martinez-Arca, S., Chattopadhyay, S., and Sandoval, I. (2001). Recycling of the insulin-sensitive glucose transporter GLUT4. Access of surface internalized GLUT4 molecules to the perinuclear storage compartment is mediated by the Phe5-Gln6-Gln7-Ile8 motif. J. Biol. Chem. 276, 3371-3383. [DOI] [PubMed] [Google Scholar]
- Patki, V., Buxton, J., Chawla, A., Lifshitz, L., Fogarty, K., Carrington, W., Tuft, R., and Corvera, S. (2001). Insulin action on GLUT4 traffic visualized in single 3T3-l1 adipocytes by using ultra-fast microscopy. Mol. Biol. Cell 12, 129-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera, H., Clarke, M., Morris, N., Hong, W., Chamberlain, L., and Gould, G. (2003). Syntaxin 6 regulates Glut4 trafficking in 3T3-L1 adipocytes [In Process Citation]. Mol. Biol. Cell 14, 2946-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm, G., Slot, J., James, D., and Stoorvogel, W. (2000). Insulin recruits GLUT4 from specialized VAMP2-carrying vesicles as well as from the dynamic endosomal/trans-Golgi network in rat adipocytes. Mol. Biol. Cell 11, 4079-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, L.J., Pang, S., Harris, D.S., Heuser, J., and James, D.E. (1992). Translocation of the glucose transporter (GLUT4) to the cell surface in permeabilized 3T3-L1 adipocytes: effects of ATP insulin, and GTP gamma S and localization of GLUT4 to clathrin lattices. J. Cell. Biol. 117, 1181-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, S.A., Herbst, J.J., Keller, S.R., and Lienhard, G.E. (1997). Trafficking kinetics of the insulin-regulated membrane aminopeptidase in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 239, 247-251. [DOI] [PubMed] [Google Scholar]
- Semiz, S., Park, J., Nicoloro, S., Furcinitti, P., Zhang, C., Chawla, A., Leszyk, J., and Czech, M. (2003). Conventional kinesin KIF5B mediates insulin-stimulated GLUT4 movements on microtubules [In Process Citation]. EMBO J. 22, 2387-2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan, A.M., van Dam, E.M., Martin, S., Luen, T.B., Hong, W., Bryant, N.J., and James, D.E. (2003). GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in syntaxins 6 and 16 but not TGN38, involvement of an acidic targeting motif. Mol. Biol. Cell 14, 973-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigematsu, S., Khan, A., Kanzaki, M., and Pessin, J. (2002). Intracellular insulin-responsive glucose transporter (GLUT4) distribution but not insulin-stimulated GLUT4 exocytosis and recycling are microtubule dependent. Mol. Endocrinol. 16, 1060-1068. [DOI] [PubMed] [Google Scholar]
- Slot, J.W., Geuze, H.J., Gigengack, S., Lienhard, G.E., and James, D.E. (1991). Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J. Cell. Biol. 113, 123-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil, A., Lampson, M.A., Keller, S.R., and McGraw, T.E. (2000). Characterization of the insulin-regulated endocytic recycling mechanism in 3T3-L1 adipocytes using a novel reporter molecule. J. Biol. Chem. 275, 4787-4795. [DOI] [PubMed] [Google Scholar]
- Tengholm, A., Teruel, M., and Meyer, T. (2003). Single cell imaging of PI3K activity and glucose transporter insertion into the plasma membrane by dual color evanescent wave microscopy. Sci. STKE 2003, PL4. [DOI] [PubMed] [Google Scholar]
- Thomas, G. (2002). Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell. Biol. 3, 753-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia, R., Baggott, D., Chuang, J., and Schekman, R. (2002). The yeast clathrin adaptor protein complex 1 is required for the efficient retention of a subset of late Golgi membrane proteins. Dev. Cell. 2, 283-294. [DOI] [PubMed] [Google Scholar]
- Verhey, K.J., Yeh, J.I., and Birnbaum, M.J. (1995). Distinct signals in the GLUT4 glucose transporter for internalization and for targeting to an insulin-responsive compartment. J. Cell. Biol. 130, 1071-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees, P., Deignan, E., van Donselaar, E., Humphrey, J., Marks, M.S., Peters, P.J., and Bonifacino, J.S. (1995). An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO. J. 14, 4961-4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, J., and Holman, G.D. (1993). Comparison of GLUT4 and GLUT1 subcellular trafficking in basal and insulin-stimulated 3T3-L1 cells. J. Biol. Chem. 268, 4600-4603. [PubMed] [Google Scholar]
- Zeigerer, A., Lampson, M.A., Karylowski, O., Sabatini, D.D., Adesnik, M., Ren, M., and McGraw, T.E. (2002). GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps. Mol. Biol. Cell. 13, 2421-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman, M., Chuang, J., Tsung, M., Hamamoto, S., and Schekman, R. (1998). Chs6p-dependent anterograde transport of Chs3p from the chitosome to the plasma membrane in Saccharomyces cerevisiae. Mol. Biol. Cell 9, 1565-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]