Abstract
Glucokinase catalyzes the ATP-dependent phosphorylation of glucose, a chemical transformation that represents the rate-limiting step of glycolytic metabolism in the liver and pancreas. Glucokinase is a central regulator of glucose homeostasis as evidenced by its association with two disease states, maturity onset diabetes of the young (MODY) and persistent hyperinsulinemia of infancy (PHHI). Mammalian glucokinase is subject to homotropic allosteric regulation by glucose — the steady-state velocity of glucose 6-phosphate production is not hyperbolic, but instead displays a sigmoidal response to increasing glucose concentrations. The positive cooperativity displayed by glucokinase is intriguing since the enzyme functions as a monomer under physiological conditions and contains only a single binding site for glucose. Despite the existence of several models of kinetic cooperativity in monomeric enzymes, a consensus has yet to be reached regarding the mechanism of allosteric regulation in glucokinase. Experimental evidence collected over the last 45 years by a number of investigators supports a link between cooperativity and slow conformational reorganizations of the glucokinase scaffold. In this review, we summarize advances in our understanding of glucokinase allosteric regulation resulting from recent X-ray crystallographic, pre-equilibrium kinetic and high-resolution nuclear magnetic resonance investigations. We conclude with a brief discussion of unanswered questions regarding the mechanistic basis of kinetic cooperativity in mammalian glucokinase.
Keywords: Mammalian glucokinase, Monomeric kinetic cooperativity, Maturity-onset diabetes of the young, Diabetes mellitus, Hyperinsulinemia of infancy, Mnemonic model, Ligand-induced slow transition model, Enzyme functional dynamics
Introduction
The ATP-dependent phosphorylation of glucose represents the first step of glycolytic metabolism. In mammals, four isozymes are responsible for catalyzing this chemical transformation [1, 2]. Three of these enzymes, hexokinases I–III, are primarily localized within the brain, muscle and erthyrocytes [3–6]. The fourth enzyme, glucokinase (hexokinase IV), is predominantly produced in the liver and the pancreatic β-cells [7, 8], although lower levels have been detected in the hypothalamus, enteroendocrine cells, gonadotropes and the pituitary gland [9–12]. Hexokinase isozymes I–III are referred to as “low Km” isoforms because of their high sensitivity for glucose — their Km values range from 30–300 µM [13–16]. In contrast, the kinetic response of glucokinase does not reach a half-maximal value until glucose concentrations approach 8 mM [17–19]. Notably, this value approximates the mean physiological glucose concentration in the blood system. The phosphorylation of glucose by glucokinase is the rate-limiting step of glucose metabolism in the liver and pancreas [20, 21]. As such, glucokinase plays a central role in regulating glycolytic metabolism in these tissue types.
Glucokinase activity in pancreatic β-cells is responsible for maintaining glucose homeostasis throughout the body [21]. This is achieved through a cascade of cellular events that links the rate of glucose 6-phosphate production to the release of insulin into the bloodstream. This process begins when glucose enters the β-cells via the GLUT family of transporters, where it is phosphorylated by glucokinase. Continuation of the glycolytic pathway produces an increase in the ATP/ADP ratio, which stimulates closure of the ATP-responsive K+ channels in the β-cell plasma membrane. Upon channel closure, the plasma membrane becomes depolarized allowing an influx of Ca2+ into the cell. Combined with intracellular Ca2+ pools, these ions promote fusion of insulin-containing secretory granules with the plasma membrane. In adipose and muscle cells, the resulting release of insulin acts to stimulate glucose uptake and metabolism by triggering the translocation of the glucose transporter GLUT4 from intracellular storage vesicles to the plasma membrane. In the liver, insulin stimulates glucokinase-mediated glucose utilization and storage as glycogen. Together, these processes act to decrease blood glucose concentrations [21–23]. The role of glucokinase in glucose homeostasis is emphasized by several disease states associated with glucokinase dysfunction.
Disease States associated with Glucokinase Dysfunction
To date, more than 600 disease-associated mutations have been identified in the glucokinase loci [24, 25]. A vast majority of these substitutions result in impairment of enzyme activity, which manifests itself either as maturity-onset diabetes of the young (MODY) or a more severe condition known as permanent neonatal diabetes mellitus (PNDM). Mutations associated with MODY and PNDM are spread throughout all 10 exons of the coding sequence and do not cluster to a particular region. Functional characterization of MODY and PNDM-associated variants reveals that these enzymes typically display a reduced rate of glucose phosphorylation at physiologically relevant glucose concentrations. Some inactive variants also appear to be less stable than the wild-type enzyme, while others appear to have an altered affinity for known interacting partners including the glucokinase regulatory protein (GKRP). An in vivo decrease in glucokinase activity translates into a decrease in β-cell glucose usage, which leads to hyperglycemia [26–35].
Gain-of-function glucokinase mutations have been identified in patients that suffer from persistent hypoglycemic hyperinsulinemia of infancy (PHHI). PHHI is a condition in which patients experience an oversecretion of insulin under hypoglycemic conditions. In extreme cases this condition leads to death within the first six months of life [36]. Although fewer in number than MODY or PNDM mutations, PHHI-associated glucokinase variants display an increased rate of glucose phosphorylation at physiological glucose concentrations. Functional characterization indicates that most PHHI-associated variant enzymes also display a reduced level of positive cooperativity toward substrate glucose. Interestingly, many of the PHHI-associated substitutions co-localize to a common region of the glucokinase scaffold, which is distant from the glucose binding site (vide infra) [36–46]. Genetic selection experiments in a bacterial model system, however, have identified several activating substitutions that lie outside of this putative allosteric site [47]. Included among these are distal activating substitutions such as N180D and M197V. Notably, the identification of M197 as an activating location was subsequently confirmed as a disease-associated glucokinase variant, when it was identified in a patient suffering from hyperinsulinemia [48].
Matschinsky et al. have used the kinetic properties of glucokinase variants to derive a mathematical model that predicts the threshold for glucose stimulated insulin release (GSIR) in normal humans, as well as in patients suffering from various glycemic diseases [45]. According to this model, insulin release is triggered when the rate of glucose phosphorylation by glucokinase achieves ~25% of its maximal value, which occurs at a basal blood glucose concentration of 5 mM. Inactive glucokinase variants dampen glucose 6-phosphate synthesis rates, causing a shift in the GSIR threshold toward higher glucose concentrations. The mathematical model predicts a threshold for GSIR in MODY patients near 7 mM, a value that compares favorably with the clinical data that report blood glucose concentrations of 6.8–7.3 mM. In contrast, activated glucokinase variants, such as those found in PHHI patients, display a shift in the GSIR threshold to lower blood glucose concentrations. In these patients, the threshold varies between 1.4 and 3.1 mM [49].
Synthetic Activators of Glucokinase
While screening a library of more than 120,000 synthetic compounds for molecules that disrupt the interaction of glucokinase and GKRP, Grimsby and coworkers identified a small molecule that effectively stimulates glucokinase activity [50]. This and related molecules mimic the kinetic effects of natural, PHHI-associated mutations by increasing the glucose sensitivity of the enzyme. The structure of human glucokinase in complex with the activator, which will be described in greater detail later, demonstrates that this molecule binds to the same location as many of the naturally occurring PHHI-associated substitutions described above. The overlap of the allosteric binding site and the activating mutations suggests that a common mechanism of action may explain both effects. In vivo, synthetic activators produce a 6-fold increase in glucose phosphorylation rates and stimulate insulin secretion from β-cells, thereby rapidly reducing blood glucose levels in mouse models. Glucokinase activators hold great promise as potential diabetes therapeutics. Indeed, at least one such molecule has advanced to phase II clinical trials as an anti-diabetic agent, although these studies were halted for undisclosed reasons [51]. A plethora of structurally unique synthetic glucokinase activators have surfaced over the last 5 years, attesting to the promise and allure of such compounds. Several excellent reviews are available on the topic of small-molecule allosteric activators of human glucokinase, so these molecules will not be discussed further [51–56].
Functional Attributes of Mammalian Glucokinase
The role of glucokinase as the body’s principle glucose sensor arises, in large part, from its unique kinetic features. The steady-state velocity of the glucokinase-catalyzed reaction is not hyperbolic, but instead displays a sigmoidal dependence upon increasing glucose concentrations. This kinetic behavior is a hallmark of allosteric regulation. The kinetic cooperativity observed in mammalian glucokinase can be described by the Hill equation (Figure 1) [57]. According to this formalism, the value of the Hill coefficient (h) measures the degree of cooperativity. When the value of h=1, the system is classified as non-cooperative, when h<1, the system is negatively cooperative (substrate association reduces the reaction velocity) and when h>1, the system is positively cooperative (substrate association increases the reaction velocity). A Hill coefficient of 1.7 is sufficient to describe the positive cooperativity of glucokinase for substrate glucose. The midpoint of the glucokinase kinetic response (K0.5) is 8 mM, which approximates the normal physiological concentration of glucose in the blood. Notably, sigmoidal kinetics is only observed with variable glucose concentrations; the reaction rate adheres to Michaelis-Menten kinetics with respect to the concentration of MgATP2− [58]. The positive cooperativity observed as a function of glucose concentration is intriguing since glucokinase functions exclusively as a monomer [59]. Much of the early work on mammalian glucokinase was centered on establishing the validity of the sigmoidal kinetic response and investigating the oligomeric state of the enzyme. The monomeric nature of glucokinase was firmly established by Cardenas and coworkers who used size-exclusion chromatography to demonstrate that the enzyme does not oligomerize in the presence of any known ligand, including glucose and ATP [59].
Figure 1.
Steady-state kinetic assays of the wild-type human glucokinase fitted to the Hill equation. The data are plotted as a function of glucose concentration to indicate the positive cooperativity in the rate of glucose-6-phosphate formation as a function of glucose concentration. The sigmoidal response is most noticeable at low glucose concentrations (inset).
Although called glucokinase, the mammalian enzyme accepts a variety of hexose substrates. The specificity of the human enzyme, reflected by the second order rate constant kcat/K0.5, demonstrates the following preference of substrates: glucose = mannose > deoxyglucose > fructose = glucosamine [60]. The kcat/K0.5 values for these substrates, in units of M−1 s−1, are 11000, 12000, 3000, 490, and 330, respectively. Based on carbohydrate structural similarities, Xu et al. suggest that the 1’-OH is critical for sugar binding and phosphorylation [60]. The K0.5 values of methyl-α-glucopyranose and methyl-β-glucopyranose are >1 M. In addition, 5-anhydroglucitol does not appear to be a substrate for the human enzyme. The orientation and hydrogen bonding capabilities of the 3’-OH and 4’-OH also appear to be critical based on the fact that allose and galactose are not substrates. Flexibility in the 2’-OH position was observed via the ability of glucokinase to phosphorylate mannose, 2’-deoxyglucose, and glucosamine. Using intrinsic protein fluorescence, Zelent et al. found the following preference of equilibrium binding to the enzyme: N-acetyl-D-glucosamine > D-glucose > D-mannose > D-mannoheptulose > 2’-deoxy-D-glucose ≫ L-glucose [61]. These studies indicate that glucokinase displays broad sugar specificity, similar to other hexokinases. Unlike the other mammalian isozymes, however, glucokinase is not susceptible to feedback inhibition by product glucose-6-phosphate [19].
Models of Kinetic Cooperativity in Monomeric Enzymes
Classical models of cooperativity have largely attempted to conceptualize and mathematically describe communications that occur between independent ligand binding sites. These binding sites use conformational changes to communicate their relative degrees of occupancy, thus allowing one ligand association event to perturb subsequent binding events [62, 63]. The results of this communication network can either be observed in equilibrium affinities, as in the case of O2 association with hemoglobin [64], or in the kinetics of substrate transformation, as in the case of allosteric regulatory enzymes such as aspartate transcarbamoylase [65]. A unique form of enzyme allostery occurs in monomeric enzymes that contain only one substrate binding site. The lack of multiple binding sites, and the observation that cooperativity is observed exclusively in the rates of substrate transformation, make this type of allosteric regulation conceptually distinct. Therefore different theories are required to understand kinetic cooperativity in monomeric enzymes. Mammalian glucokinase has emerged as a model system for understanding this type of cooperative response.
The observation of slight positive cooperativity in the rates of glucose-6-phosphate synthesis by mammalian glucokinase triggered the formulation of several general mechanisms of monomeric allostery. Two of the most frequently encountered proposals are the mnemonic model [66] and the ligand-induced slow transition model (LIST) (Figure 2) [67]. Ricard, Meunier and Buc first formulated the mnemonic model in an effort to explain the ability of monomeric enzymes to display non-hyperbolic kinetics. Their model was based upon earlier conceptual work by Rabin and Frieden, who postulated that the conformation of an enzyme following product release could be different from the initial enzyme state [68, 69]. As applied to the glucokinase reaction, the mnemonic model involves the oscillation between two distinct enzyme species – a low-affinity state (E*) and a high-affinity state (E) (Figure 2A). The catalytic cycle begins when glucose binds to the low-affinity state and induces a conformational change to the high-affinity state (E). This conformational transition is slow, presumably because the conversion involves significant structural rearrangements. Upon reaching the high-affinity state, glucokinase rapidly binds MgATP2− to generate the ternary complex (E·G·MgATP2−). Catalysis occurs from this high-affinity state, as does product release. If substrate glucose is abundant, the high-affinity state can rapidly undergo a second round of catalysis without formation of the slowly realized, low-affinity conformation (E*). In contrast, if glucose concentrations are low, the enzyme slowly relaxes to the low-affinity conformation (E*) before another molecule of glucose has time to bind. In this manner, the concentration of glucose dictates whether the catalytic pathway includes or excludes the formation of the thermodynamically favored E* state.
Figure 2.
Schematic representations of the mnemonic (A) and ligand-induced slow transition (LIST) (B) models. Red arrows indicate the slow conformational changes postulated to cause non-hyperbolic kinetics.
Evidence in support of the mnemonic model of glucokinase kinetic cooperativity comes from several sources. Simulations of rate constants demonstrate that deviations from linearity in reciprocal plots ([E0]/v versus 1/[S]) can be observed using the constraints of the mnemonic model. Richard et al. were the first to simulate this deviation and to apply the predictions to explain the cooperative response of wheat germ hexokinase L1 [70–72]. These authors determined that the extent of cooperativity does not depend on the equilibrium constant between the conformations, but instead upon the kon values describing substrate association. Pollard-Knight et al. tested the prediction of the mnemonic model that glucose cooperativity depends on the ability of MgATP2− to react rapidly with the binary glucokinase-glucose complex [73]. As expected, they found a loss in cooperativity when a poor nucleotide substrate (Mg ITP2−) replaced MgATP2−. Steady-state kinetic measurements and isotope exchange studies, which are consistent with an ordered binding of substrates, have also been taken as support for the mnemonic model [74–77].
The ligand-induced slow transition (LIST) model developed by Neet provides a more general description of kinetic cooperativity. Similar to the mnemonic model, the LIST mechanism postulates the existence of two distinct enzyme conformations, E and E* (Figure 2B). In the LIST model, however, a pre-existing equilibrium exists between these two enzyme species in the absence of substrate. The two conformations possess different affinities for substrate glucose, and the equilibrium between these states is controlled by the concentration of glucose. In the LIST mechanism, emphasis is placed upon: (1) the existence of conformational changes that occur more slowly than kcat, (2) the non-equilibration of substrate binding due to these slow conformational changes, and (3) the variation in the preferred catalytic route with fluctuations in glucose concentrations. In the LIST mechanism two separate catalytic cycles are possible, each of which involves a different enzyme state. By contrast, the mnemonic model postulates only one catalytic cycle, because the high-affinity state is realized only after glucose binds.
The observation of a Hill coefficient lower than 1 under subsaturating MgATP2− concentrations represents the most compelling evidence for the requirement of two catalytic cycles, as postulated by the LIST mechanism [78, 79]. Simulations conducted by Neet and Ainslie also demonstrate several cases in which appropriate choices of microscopic rate constants can produce not only positive and negative cooperativity, but also account for the observed bursts or lags present in the approach to the steady-state regime. The LIST mechanism is also consistent with the effects of various glucokinase inhibitors including N-acetylglucosamine [80] and palmitoyl-CoA [81, 82].
Both the mnemonic and the LIST models attribute cooperativity to slow conformational changes promoted by glucose association; however, mechanisms that do not involve slow structural rearrangements have also been proposed. One such mechanism postulates that random addition of substrates, which could create multiple pathways for substrate binding, could produce cooperativity if formation of one of these binary complexes is slower than kcat [83]. It is well established that the glucokinase mechanism is ordered. Glucose always binds first, which excludes the possibility that a random addition of substrates accounts for the observed kinetic cooperativity. A different theoretical mechanism for cooperativity involves the formation of a non-productive ternary complex between enzyme, glucose and ADP. If the association of glucose to the binary enzyme-ADP complex stimulates nucleotide release at a rate faster than would occur in the absence of glucose, cooperativity could result [84]. This mechanism has not found support in the currently available experimental data.
Structural Bases for Allosteric Transitions in Glucokinase
The first structural data for glucokinase was reported in 2004 by Kamata and coworkers, who determined the 2.3 Å resolution crystal structure of the human enzyme in complex with glucose and a synthetic activator [85]. These investigators also successfully determined the structure of unliganded glucokinase, albeit at a much lower resolution of 3.4 Å. Since that initial report, ten additional crystal structures of human glucokinase have been reported [86–94]. With these structures, data is presently available for several complexes formed along the reaction coordinate, including the binary enzyme-glucose complex, as well as the ternary complex formed between enzyme, glucose and a non-hydrolyzable ATP analog. Structures are also available with six structurally distinct activators bound to the allosteric site. The structures reveal a very similar fold and active site architecture shared by glucokinase and other hexokinase isozymes. The principal structural difference for glucokinase compared to the other isozymes stems from the unliganded structure, which suggests that glucose-induced conformational changes are largest for glucokinase. This difference is postulated to provide the structural basis for its unique allosteric properties.
A comparison of the unliganded (PDB code 1V4T) and glucose-bound (PDB code 1V4S) structures suggests that glucose induces a large-scale structural rearrangement in the glucokinase scaffold. The large domain appears to remain largely static during glucose binding, however the small domain undergoes a 99° rotation toward the large domain. Overall, this hinge bending motion forces the enzyme to adopt a more compact, globular structure whereas the unliganded conformation is more extended. During this domain closure, the C-terminal α-13 helix moves from a solvent exposed orientation to a more internal location. In particular, the α-13 helix is sequestered by a mobile loop consisting of residues 63–72, which appears to constrain the mobility of the helix in the glucose-bound state. This loop, which constitutes one surface of the activator binding site also adopts a strikingly different structure in the absence of ligand. The unliganded state lacks a noticeable binding site for either glucose or the allosteric activator. A second loop region containing residues 157–179, which harbors two side chains that form interactions with bound glucose, is disorganized and undetectable in the absence of ligand. Together, these differences suggest that the association of either activator or glucose with the enzyme involves the disruption and reformation of many interactions.
The available structural data provides important insights into the mechanism by which the association of allosteric activating molecules is communicated more than 16Å to the glucose binding site. Communication between the allosteric site and the active site involves a network of amino acids in the small domain that are located in three distinct secondary structural elements, the α-5 helix containing residues 204–215, the C-terminal α-13 helix consisting of residues 443–465, and an extended loop containing residues 151–179 (Figure 3). One face of the activator binding site is constructed from residues in the α-5 helix including Met-210, Ile-211, Tyr-214 and Tyr-215. Two active site residues, including the critical active site base Asp-205, are found at the amino terminal end of the α-5 helix (Figure 3A). Thus, the presence of activator is expected to directly impact glucose association by influencing the position of the α-5 helix. Three residues in the α-13 helix, Val-452, Val-455 and Ala-456, form another face of the activator binding site. Two of these residues, Val-452 and Ala-456 form hydrophobic interactions with the side chain of Ile-159, which is located in the β-hairpin turn on the 151–179 loop (Figure 3B). One can postulate that interactions between Val-452, Ala-456 and Ile-159 are stabilized in the presence of activator. This stabilization is communicated to the glucose binding site by the fact that two additional loop residues, Thr-168 and Lys-169, form hydrogen bonds with the O1 and O6 atoms of bound glucose. It is also possible that the loop is stabilized by direct interactions between Ile-159 and the thiazole ring of bound activator. Evidence in support of these modes of communication includes the observation that single amino acid replacements of Val-455, Ala-456, Ile-211, Tyr-214 and Tyr-215 stimulate catalysis, reduce cooperativity and produce kinetic effects similar to that observed with activators [47, 56].
Figure 3.
Proposed pathways of communication between the glucose binding site and the allosteric activator binding site. (A) α-5 helix (yellow) contains the active site Asp-205 (yellow sticks), which H-bonds with glucose (green sticks), as well as Tyr-215 (yellow sticks), which forms hydrophobic interaction with the allosteric activator (green sticks). (B) The loop comprised of residues 151–179 (yellow) forms H-bonds with glucose via Lys-169 (yellow sticks) and communicates this interaction to the α-13 helix (red) via the interaction between Ile-159 (yellow spheres) and Ala-452 (red spheres). Alternatively, the loop could communicate directly to the allosteric activator via the interaction of Ile-159 (yellow spheres) with the thiazole ring of the activator molecule (green sticks). H-bonds are represented as dashed black lines. Image was generated using PDB entry 1V4S [85] and PyMol 1.1 [108].
It is important to note that the structural alterations that accompany ligand binding described above are based upon a single crystallographic structure of the unliganded enzyme. This is notable because the structural alterations inferred via a simple comparison between the unliganded and various ligand-bound structures are questionable given recent experimental results. Of particular interest in this respect is the position of the α-13 helix. In the presence of glucose, the α-13 helix is sequestered into an internal location within the smaller domain, in part by the loop containing residues 63–72 (Figure 4). In the unliganded structure, the α-13 helix is shorter by four residues and has been fully released from its internal location to a more-solvent exposed position, where it does not interact with residues 63–72. Experiments in which the α-13 helix has been increased in length by appending a poly-alanine tail of up to 10 residues indicates that this mutational alteration has no impact upon the kinetic features of the enzyme [95]. Moreover, fusion of the 25-kDa glutathione transferase domain to the C-terminus results in unaltered kinetic constants [96]. It is difficult to reconcile these experimental results with the model for glucose-induced conformational changes that emerges from a comparison of the crystal structures. In particular, it is difficult to envision how the α-13 helix could transition between sequestered and free positions when a large number of additional amino acids are appended to the C-terminus (Figure 4). Given the high degree of similarity among the liganded structures, combined with the singularity and rather low resolution of the unliganded structure, it remains to be determined whether the structure reflected in PDB 1V4T represents a viable, on-pathway conformational state.
Figure 4.
Conformational changes experienced by the C-terminal α-13 helix upon glucose binding. (A) The α-13 helix (red) is solvent exposed and released from the connecting loop (yellow) in the unliganded state. (B) In the presence of glucose and an allosteric activator (not shown), the α-13 helix is constrained by the connecting loop. Experiments indicate that fusion of additional residues at the C-terminus has no effect upon steady-state kinetic properties, an observation that is difficult to reconcile with this structural data. Image was generated using PDB entry 1V4T, 1V4S [85] and PyMol 1.1 [108].
Conformational Changes Detected by Transient Kinetic Methods
The structural data provided by X-ray crystallographic analysis of glucokinase is valuable in elucidating details of the rearrangements accompanying glucose association. Nevertheless, it does not provide information regarding the rates of conformational reorganizations promoted by glucose. In an attempt to understand the kinetics of the glucose induced conformational changes in more depth, a variety of rapid mixing experiments have been conducted. Each of these studies took advantage of the fact that glucokinase experiences a measurable increase in intrinsic tryptophan fluorescence intensity upon glucose association. Below, we briefly describe the results of four independent stopped-flow investigations of glucose binding to glucokinase.
Neet and coworkers conducted the first fluorescence based investigation of the kinetics of glucose binding to glucokinase in 1990 using non-recombinant rat liver enzyme [97]. These investigators observed a hyperbolic response of protein fluorescence intensity as a function of increasing glucose concentrations in the presence of 5% or 30% glycerol. This observation was the first experimental demonstration that the enzyme undergoes a slow conformational reorganization upon glucose binding. The kinetic data was interpreted on the basis of a two-step mechanism involving a pre-equilibrium step and a subsequent conformational change that was characterized by a half-life of several minutes and an isomerization equilibrium constant of ~ 30 [97] (Figure 5A). Due to instrumental limitations, the authors could not observe changes in fluorescence intensity that occurred within the first few seconds after mixing. These results, along with other kinetic data, were interpreted on the basis of the ligand-induced slow transition model of kinetic cooperativity in monomeric glucokinase [97, 98].
Figure 5.
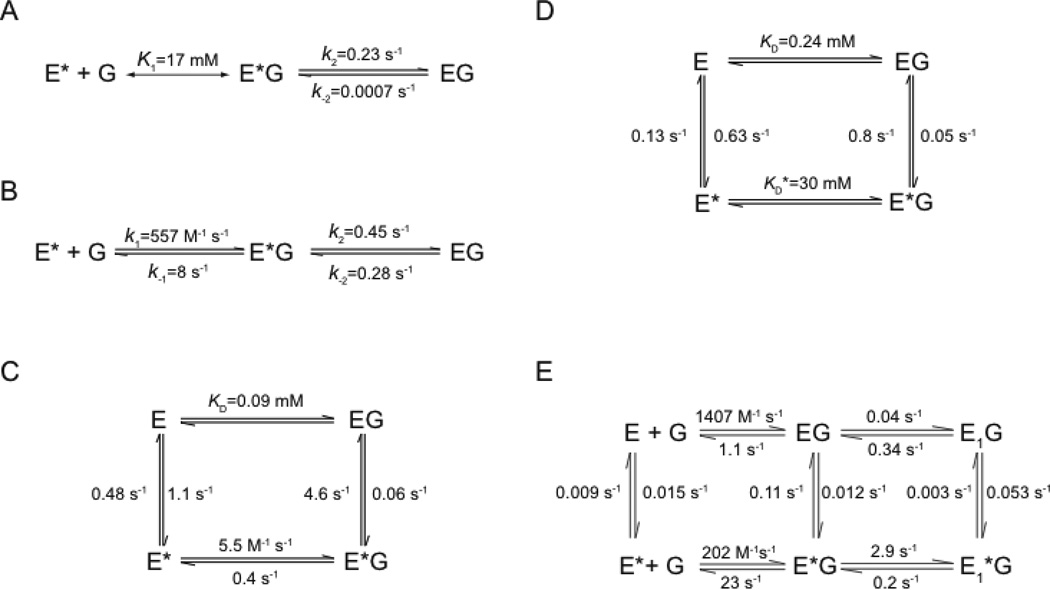
Kinetic mechanisms used to describe glucose association with mammalian glucokinase resulting from various transient state binding studies. (A) Kinetic scheme proposed by Neet and coworkers involving the fast formation of a binary enzyme-glucose complex, followed by a slow reversible isomerization [98]. (B) Kinetic model for glucose binding to human pancreatic glucoskinase, as proposed by Heredia et al., based on KIMSIM simulations [99]. (C) The Kim model, which includes a preexisting equilibrium to describe the transient-state glucose binding traces [100]. The values are obtained from the simulated dependence of kobs with increase glucose concentrations. (D) The Antoine et al. model, which includes a preexisting equilibrium to describe the transient state glucose binding traces. The kinetic values shown are obtained from the simulated dependence of kobs3 with increasing glucose concentration [101]. (E) The Larion model, which resulted from global fitting of rate and amplitude data of glucose binding traces at high enzyme concentrations. The calculated KD for the high-affinity state, E, is 782 µM. The calculated KD for the low-affinity state, E*, is 114 mM [102].
Sixteen years after the initial rapid mixing studies of Neet and coworkers, a group of investigators from Pfizer revisited the transient kinetics of glucose binding to glucokinase [99]. This study utilized recombinant human pancreatic glucokinase and a sensitive stopped-flow spectrophotometer to observe protein-based fluorescence changes in the millisecond to second time regime. Similar to previous observations, Heredia and colleagues detected a biphasic response in the change of intrinsic protein fluorescence as a function of glucose concentrations. The kinetic pathway for glucose binding was postulated to involve a reversible pre-equilibrium glucose binding step that was characterized by an equilibrium constant of 69 M−1, followed by a slower conformational isomerization step characterized by forward and reverse rate constants of 0.45 s−1 and 0.28 s−1, respectively (Figure 5B). This conformational change is slower than catalytic turnover, thus providing an explanation for the sigmoidal response observed in the steady-state regime. Kinetic simulation of the data, in combination with the structural data of Kamata and coworkers, was taken in support of the mnemonic mechanism. The stopped-flow kinetic traces depicted in Heredia’s manuscript are noteworthy, however, by the apparent absence of data obtained at time ranges longer than 10 seconds. Prior work by Neet had clearly demonstrated that glucokinase fluorescence produces an observable event between 30 and 300 seconds. This fact suggests that the 2006 results were interpreted using an incomplete data set.
In 2007, investigators from Bristol-Myers Squibb performed their own transient-state kinetic analysis of glucose binding to recombinant human liver glucokinase [100]. In this study, time-dependent traces of intrinsic fluorescence could be fit to a single exponential function that demonstrated a biphasic dependence upon glucose concentrations. Attempts to simulate the glucose binding traces resulted in a model involving a pre-existing equilibrium between two distinct enzyme conformations, E* and E, the ratio of which depends upon glucose concentrations (Figure 5C). The two enzyme species have different affinities for glucose, and kinetic modeling placed values for the glucose dissociation constants of the E* and E states at 0.17 mM and 73 mM respectively. Simulations also estimated that the relative population of the E* and E conformations, in the absence of glucose, was 20% and 80% respectively. The results of these studies were consistent with the ligand-induced slow transition model, rather than the mnemonic mechanism.
Two additional studies extend the information available from the 2006 and 2007 pre-equilibrium measurements. Antoine and colleagues found that transient-state glucose binding traces require several hundred seconds to reach equilibrium, confirming the initial observations of Neet from 1990 [101]. Fitting of the entire glucose binding traces required an expression that included the sum of four exponential terms, demonstrating the complexity of the binding process. Notably, the phase with the highest amplitude exhibited the same biphasic behavior as that previously observed by the Bristol-Myers Squibb group [100]. Progress curve analysis led Antoine to conclude that glucokinase is characterized by at least two slowly interconverting forms of the enzyme with a relative population ratio similar to that previously proposed [100]. The relative dissociation constants for substrate glucose displayed by these discrete conformations were modeled as having values of 0.2 mM and 30 mM respectively (Figure 5D). In a subsequent study, the observations of Antoine and coworkers were verified by similar transient-state kinetic studies involving high concentrations of enzyme [102]. Four kinetically distinguishable events were detected upon mixing glucose and recombinant pancreatic glucokinase. Global fit analysis using both rate and amplitude data suggested a minimal kinetic model of glucokinase cooperativity involving at least two unliganded conformations, and four discrete enzyme-glucose binary complexes (Figure 5E). These data were also consistent with the postulate that glucokinase conformational heterogeneity exists both in the absence and presence of bound glucose.
Several notes regarding the totality of transient-state data described above should be made. First, no information has been presented for steps following catalysis, such as product release. Second, the enzyme has three tryptophan residues, which differentially contribute to the change in fluorescence observed upon glucose binding. The presence of multiple fluorophores, which may report on distinct modes of protein structural reorganization, complicates efforts to interpret transient-state glucose binding data. In principle, the system could be made less complicated by removal of one or more tryptophan residues via site-directed mutagenesis, however such substitutions impact the kinetic attributes of the enzyme, including both the degree of cooperativity and the turnover number [61]. It is clear from the transient-state data that the binding process is complex. Thus, a full description of glucokinase dynamics upon glucose association will require the combination of several methods.
Glucokinase Functional Dynamics from High-Resolution NMR
Current experimental evidence supports the view that glucokinase cooperativity relies upon slow conformational changes that prevent glucose binding from reaching equilibrium during the catalytic cycle. Steady-state kinetic assays reveal a kcat value of 60 s−1 for enzymatic turnover, suggesting that conformational changes linked to cooperativity must occur with a half-life ≥ 12 msec. Recent advances in high-resolution NMR make this method ideally suited for investigating protein dynamics in this time regime [103]. In particular, the development of multidimensional pulse sequences that probe magnetization exchange and relaxation processes are expected to be most important in measuring the rates of slow conformational reorganizations.
The only application of high-resolution NMR to investigate glucokinase functional dynamics appeared in 2010 [104]. In this study, the 1H-15N TROSY NMR spectrum of uniformly 15N-labeled recombinant human pancreatic glucokinase was collected in the presence and absence of glucose. In the absence of ligand the spectrum revealed a substantial degree of cross-peak overlap and an unexpected degree of heterogeneity in linewidths and peak intensities. These spectral features were conserved across a range of acquisition temperatures and a range of protein concentrations. A comparison of these spectral characteristics with those observed in the 1H-15N TROSY NMR spectrum of similarly sized, monomeric arginine kinase indicated that the unusual NMR behavior of glucokinase was not solely the consequence of its largeness. Addition of glucose to the enzyme increased the quality of the 1H-15N TROSY spectrum, but did not eliminate the heterogeneity in peak intensities or cross peak overlap. Subsequent studies suggest that the concentration of glucose used in these studies may not be sufficient to fully remove contributions from the unliganded enzyme from the spectrum (Larion et al., unpublished results). Nevertheless, the observations were consistent with the postulate that glucokinase is a highly flexible, mobile enzyme.
In separate experiments, these same investigators used NMR to detect the exchange of amide protons with deuterated solvent. The collection of successive, time-dependent 1H-15N HSQC spectra demonstrated that roughly 75% of the amide proton signal was lost during the first two hours of exchange at room temperature and neutral pH. The remaining signal experienced a much slower exchange. The presence of glucose had little effect on the rate of the slow exchanging population, however the effect of glucose on the fast exchanging protons could not be assessed give the rapid time scale of the exchange. The H/D exchange results were consistent with the observations of the 1H-15N TROSY experiments. A substantial fraction of the polypeptide backbone is highly solvent exposed in at least one conformational state, suggesting that the enzyme is quite dynamic both in the presence and absence of bound glucose.
In an attempt to detect specific conformational states, site-specific labels were incorporated into both the backbone and side chain atoms of glucokinase. The 1H-15N HSQC spectrum of glucokinase containing 15N tryptophan side chain labels showed a single broad peak that contains contributions from all three glucokinase tryptophan residues. Notably, the chemical shift of this signal Iies in a region where disordered tryptophan side chains are expected to reside. Upon the addition of glucose, three discrete cross-peaks can be observed in the 1H-15N HSQC spectrum. Subsequent assignment of these peaks using site-directed mutagenesis indicates that the peaks corresponding to Trp-167 and Trp-99 display additional shoulders, which could be attributable to a minor conformation (Larion et al., unpublished results). Both of these residues are located in the smaller domain, which appears to experience the most significant structural alterations upon glucose binding based upon crystallographic data. The appearance of multiple tryptophan side chain signals suggests that glucokinase samples multiple glucose-bound conformations on a relatively slow time scale. Similar conclusions were drawn from the appearance and behavior of 15N isoleucine backbone labels that were incorporated at seventeen discrete locations in the glucokinase polypeptide.
In total, the results of high-resolution NMR suggest that the unliganded enzyme is highly dynamic and conformationally heterogeneous. Upon glucose association, the heterogeneity decreases, but is not completely eliminated. It is clear that ligand binding quenches dynamics and induces a more structured state. In particular, the ability of glucose to modulate enzyme functional dynamics is likely key to the kinetic cooperativity observed with this substrate. Residue specific assignments, combined with a more thorough structural characterization of the remaining complexes formed along the reaction coordinate, is likely to provide additional insight into the dynamic behavior of glucokinase. In addition, the application of several distinct relaxation methods offers the possibility of measuring the rates of slow conformational exchange that are thought to provide cooperativity. For example, ZZ-exchange spectroscopy can be used to monitor the exchange of longitudinal magnetization between two states [105]. Rates of exchange are quantified by the variation in cross-peak intensity that is generated and observed over a variety of mixing times. Similarly, CPMG relaxation spectroscopy can monitor transverse magnetization relaxation in a two-state system, affording information about the interconversion rate, the relative population of the exchanging species and the chemical shift difference between the two states [105]. Both of the methods could prove useful in elucidating further details of glucokinase conformational changes.
Conclusions
Nearly 50 years has passed since the allosteric behavior of mammalian glucokinase was first recognized. Glucokinase is the first, and perhaps the best-studied, example of a cooperative monomeric enzyme with a single-ligand binding. Indeed, very few cases of monomeric single ligand-binding site enzymes have been described in the literature. Nevertheless, much progress has been made, both in developing models for monomeric cooperativity and in applying those models to experimental investigations of the kinetics of glucose association. Based on the collection of data presently available, a variety of explanations for the manifestation of kinetic cooperativity in monomeric glucokinase have been removed from consideration. Included among the eliminated possibilities are the presence of a second cryptic glucose binding site [106], a random order of substrate addition, and the formation of a non-productive glucose-ADP ternary complex that promotes rapid recycling of the enzyme. Current models hold that glucokinase cooperativity is exclusively associated with the conformational reorganization that is experienced upon glucose association. Moreover, these structural changes are substantial and appear to proceed more slowly than is typically experienced in a number of other well-studied enzyme systems.
In closing, it is worthwhile to highlight data that is lacking. Despite transient-state and NMR investigations, the extent of conformational heterogeneity in the unliganded state of glucokinase is unclear. Does the enzyme sample a small number of discrete states or does an ensemble of conformations more accurately describe the unliganded species? If the later is true, then the models for kinetic cooperativity outlined above will need to be modified, since it will not be possible to describe the energetic landscape of the enzyme with an equilibrium constant the governs the interconversion of two discrete states. Even if NMR relaxation methods succeed in measuring exchange, these approaches are limited by the assumption that exchange is occurring between two discrete states. Given this fact, alternate, more sensitive spectroscopic approaches may be needed to precisely map the conformational ensemble and energetic landscape of the enzyme. In addition, these spectroscopic methods could be useful in investigating the extent to which the unliganded structure reflected in PDB 1V4T represents a catalytically relevant conformational state.
Uncertainly also surrounds the extent to which the unliganded state is structurally disordered. Is it possible that a portion of the glucokinase scaffold lacks substantial secondary or tertiary structure prior to association with glucose? Many of the models of allostery, including the classic MWC and KNF models, employ a traditional view of protein structure. Recent investigations of protein folding have resulted in the emergence of a new view of polypeptide structure, which suggests that describing protein conformational landscapes in terms of one or a few discrete states may be an oversimplification. The introduction of increased levels of disorganization into a structural model affords striking deviations from the expected outcomes of single-state models such as the Michaelis-Menten formalism. Indeed, there is a growing interest in the study of intrinsically disordered proteins, which lack a well-defined three-dimensional fold in isolated solution, but which fold upon encountering their biological target [107]. The extent to which such considerations could be relevant to the generation of sigmoidal steady-state kinetics in monomeric enzymes such as glucokinase is presently unknown, but worthy of additional investigation.
Finally, although much attention has been paid to the glucose-binding component of the catalytic cycle – and rightfully so – it is unclear which step (or steps) of the catalytic cycle is described by kcat. Kinetic studies using single-turnover experiments should provide insight into the rates of both the chemical step as well as data regarding the order and rates of product release. These experiments and others are well within the reach of contemporary biochemistry. Clearly, the final picture of glucokinase allosteric regulation will require the merging of data from multiple experimental approaches. Given the critical physiological role of glucokinase in regulating glucose homeostasis and controlling the rate of insulin secretion from β-cells, obtaining a detailed understanding of the mechanism of glucokinase cooperativity remains an important objective.
Acknowledgement
Work in the corresponding authors’ laboratory is supported by NIH/NIDDK R01DK081358.
Abbreviations
- MODY
maturity-onset diabetes of the young
- PNDM
permanent neonatal diabetes mellitus
- GKRP
glucokinase regulatory protein
- PHHI
persistent hypoglycemic hyperinsulinemia of infancy
- GSIR
glucose stimulated insulin release
- h
Hill coefficient
- LIST
ligand-induced slow transition
- NMR
nuclear magnetic resonance
- TROSY
transverse relaxation optimized spectroscopy
- HSQC
heteronuclear single-quantum correlation spectroscopy
- CPMG
Carr-Purcell-Meiboom-Gill
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.González C, Ureta T, Sánchez R, Niemeyer H. Biochem. Biophys. Res. Commun. 1964;16:347–352. doi: 10.1016/0006-291x(64)90038-5. [DOI] [PubMed] [Google Scholar]
- 2.Katzen HM, Schimke RT. Proc. Natl. Acad. Sci. USA. 1965;54:1218–1225. doi: 10.1073/pnas.54.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilson JE. Rev. Physiol. Biochem. Pharmacol. 1995;126:65–198. doi: 10.1007/BFb0049776. [DOI] [PubMed] [Google Scholar]
- 4.Wilson JE. J. Exp. Biol. 2003;206:2049–2057. doi: 10.1242/jeb.00241. [DOI] [PubMed] [Google Scholar]
- 5.Sui D, Wilson JE. Arch. Biochem. Biophys. 1997;345:111–125. doi: 10.1006/abbi.1997.0241. [DOI] [PubMed] [Google Scholar]
- 6.Stocchi V, Magnani M, Novelli G, Dachà M, Fornaini G. Arch. Biochem. Biophys. 1983;226:365–376. doi: 10.1016/0003-9861(83)90303-x. [DOI] [PubMed] [Google Scholar]
- 7.Walker DG, Rao S. Biochem. J. 1964;90:360–368. doi: 10.1042/bj0900360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matschinsky FM, Ellerman JE. J. Biol. Chem. 1968;243:2730–2736. [PubMed] [Google Scholar]
- 9.Jetton TL, Liang Y, Pettepher CC, Zimmerman EC, Cox FG, Horvath K, Matschinsky FM, Magnuson MA. J. Biol. Chem. 1994;269:3641–3654. [PubMed] [Google Scholar]
- 10.Zelent D, Golson ML, Koeberlein B, Quintens R, van Lommel L, Buettger C, Weik-Collins H, Taub R, Grimsby J, Schuit F, Kaestner KH, Matschinsky FM. Diabetes. 2006;55:1923–1929. doi: 10.2337/db06-0151. [DOI] [PubMed] [Google Scholar]
- 11.Sorenson RL, Stout EL, Brelje TC, Jetton TL, Matschinsky FM. J. Histochem. Cytochem. 2007;55:555–566. doi: 10.1369/jhc.6A7117.2007. [DOI] [PubMed] [Google Scholar]
- 12.Kang L, Dunn-Meynell A, Routh VH, Gaspers LD, Nagata Y, Nishimura T, Eiki J, Zhang BB, Levin BE. Diabetes. 2006;55:412–420. doi: 10.2337/diabetes.55.02.06.db05-1229. [DOI] [PubMed] [Google Scholar]
- 13.Traut T. In: Allosteric Regulatory Enzymes. Springel, editor. 2007. pp. 157–186. [Google Scholar]
- 14.Aleshin AE, Malfois M, Liu X, Kim CS, Fromm HJ, Honzatko RB, Koch MH, Svergun DI. Biochemistry. 1999;38:8359–8366. doi: 10.1021/bi990523n. [DOI] [PubMed] [Google Scholar]
- 15.Ardehali H, Yano Y, Printz RL, Koch S, Whitesell RR, May JM, Granner DK. J. Biol. Chem. 1996;26:1849–1852. doi: 10.1074/jbc.271.4.1849. [DOI] [PubMed] [Google Scholar]
- 16.Palma F, Agostini D, Mason P, Dachà M, Piccoli G, Biagiarelli B, Fiorani M, Stocchi V. Mol. Cell. Biochem. 1996;155:23–29. doi: 10.1007/BF00714329. [DOI] [PubMed] [Google Scholar]
- 17.DiPietro DL, Sharma C, Weinhouse S. Biochemistry. 1962;1:455–462. doi: 10.1021/bi00909a014. [DOI] [PubMed] [Google Scholar]
- 18.Walker DG. Biochem. J. 1962;84:118. [Google Scholar]
- 19.Salas M, Sols A. J. Biol. Chem. 1963;238 PC 1175. [PubMed] [Google Scholar]
- 20.Ferre T, Riu E, Bosch F, Valera A. FASEB J. 1996;10:1213–1218. doi: 10.1096/fasebj.10.10.8751724. [DOI] [PubMed] [Google Scholar]
- 21.Matschinsky FM. Diabetes. 1990;39:647–652. doi: 10.2337/diab.39.6.647. [DOI] [PubMed] [Google Scholar]
- 22.Meglasson MD, Matschinsky FM. Am. J. Physiol. Endocrinol. Metab. 1984;246:E1–E13. doi: 10.1152/ajpendo.1984.246.1.E1. [DOI] [PubMed] [Google Scholar]
- 23.Al-Hasani H, Tschöp MH, Cushman SW. Mol. Interv. 2003;3:367–374. doi: 10.1124/mi.3.7.367. [DOI] [PubMed] [Google Scholar]
- 24.Golyn A. Hum. Mutat. 2003;22:353–362. doi: 10.1002/humu.10277. [DOI] [PubMed] [Google Scholar]
- 25.Osbak KK, Colclough K, Saint-Martin C, Beer NL, Bellanné-Chantelot C, Ellard S, Gloyn AL. Hum. Mutat. 2009;30:1512–1526. doi: 10.1002/humu.21110. [DOI] [PubMed] [Google Scholar]
- 26.Froguel P, Vaxillaire M, Sun F, Velho G, Zouali H, Butel MO, Lesage S, Vionnet N, Clement K, Fougerousse F, Tanizawa Y, Weissenbach J, Beckmann JS, Lathrop GM, Passa Ph, Permutt MA, Cohen D. Nature. 1992;356:162–164. doi: 10.1038/356162a0. [DOI] [PubMed] [Google Scholar]
- 27.Vionnet N, Stoffel M, Takeda J, Yasuda K, Bell GI, Zouali H, Lesage S, Velho G, Iris F, Passa P, Froguel Ph, Cohen D. Nature. 1992;356:721–722. doi: 10.1038/356721a0. [DOI] [PubMed] [Google Scholar]
- 28.Gidh-Jain M, Takeda J, Xu LZ, Lange AJ, Vionnet N, Stoffel M, Froguel P, Velho G, Sun F, Cohen D, Patel P, Lo Y-MD, Hattersley AT, Luthman H, Wedell A, St. Charles R, Harrison RW, Weber IT, Bell GI, Pilkis SJ. Proc. Natl. Acad. Sci. USA. 1993;90:1932–1936. doi: 10.1073/pnas.90.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kesavan P, Wang L, Davis E, Cuesta A, Sweet I, Niswender K, Magnuson MA, Matschinsky FM. Biochem. J. 1997;322:57–63. doi: 10.1042/bj3220057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Njølstad PR, Søvik O, Cuesta-Muñoz A, Bjørkhaug L, Massa O, Barbetti F, Undlien DE, Shiota C, Magnuson MA, Molven A, Matschinsky FM, Bell GI. N. Engl. J. Med. 2001;344:1588–1592. doi: 10.1056/NEJM200105243442104. [DOI] [PubMed] [Google Scholar]
- 31.Njølstad PR, Sagen JV, Bjørkhaug L, Odili S, Shehadeh N, Bakry D, Sarici SU, Alpay F, Molnes J, Molven A, Søvik O, Matschinsky FM. Diabetes. 2003;52:2854–2860. doi: 10.2337/diabetes.52.11.2854. [DOI] [PubMed] [Google Scholar]
- 32.Miller SP, Anand GR, Karschnia EJ, Bell GI, LaPorte DC, Langen AJ. Diabetes. 1999;48:1645–1651. doi: 10.2337/diabetes.48.8.1645. [DOI] [PubMed] [Google Scholar]
- 33.Liang Y, Kesavan P, Wang LQ, Niswender K, Tanizawa Y, Permutt MA, Magnuson MA, Matschinsky FM. Biochem. J. 1995;309:167–173. doi: 10.1042/bj3090167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sagen VJ, Odili S, Bjørkhaug L, Zelent D, Buettger C, Kwagh J, Stanley C, Dahl-Jørgensen K, de Beaufort C, Bell GI, Han Y, Grimsby J, Taub R, Molven A, Søvik O, Njølstad PR, Matschinsky FM. Diabetes. 2006;55:1713–1722. doi: 10.2337/db05-1513. [DOI] [PubMed] [Google Scholar]
- 35.Galan M, Vincent O, Roncero I, Azriel S, Boix-Pallares P, Delgano-Alvarez E, Diaz-Cadorniga F, Blazquez E, Navas MA. Biochem. J. 2006;393:389–396. doi: 10.1042/BJ20051137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cuesta-Muñoz AL, Huopio H, Otonkoski T, Gomez-Zumaquero JM, Näntö-Salonen K, Rahier J, López-Enriquez S, García-Gimeno MA, Sanz P, Soriguer FC, Laakso M. Diabetes. 2004;53:2164–2168. doi: 10.2337/diabetes.53.8.2164. [DOI] [PubMed] [Google Scholar]
- 37.Glaser B, Kesavan P, Heyman M, Davis E, Cuesta A, Buchs A, Stanley CA, Thornton PS, Permutt MA, Matschinsky FM, Herold KC. N. Engl. J. Med. 1998;338:226–230. doi: 10.1056/NEJM199801223380404. [DOI] [PubMed] [Google Scholar]
- 38.Golyn A. Hum. Mutat. 1998;22:353–362. doi: 10.1002/humu.10277. [DOI] [PubMed] [Google Scholar]
- 39.Glaser B, Thornton PS, Otonkoski T, Junien C. Arch. Dis. Child. 2000;82:79–86. doi: 10.1136/fn.82.2.F79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cuesta A, Huopio H, Gomez J, Garcia A, Sanz P, Soriguer FC, Laakso M. Diabetes. 2003;52:A249. [Google Scholar]
- 41.Meissner H, Cobo-Vuilleumier N, Maringa M, Guez-Bada PD, A-Gimero MAG, Castro-Santiago MJ, Aledo JC, De Fonseca FR, Weber J, Sanz P, Cuesta-Munoz AL. Diabetologia. 2008;51:285. [Google Scholar]
- 42.Christesen HBT, Tribble ND, Molven A, Siddiqui J, Sandal T, Brusgaard K, Ellard S, Njølstad PR, Alm J, Jacobsen BB, Hussain K, Gloyn AL. Eur. J. Endocrinol. 2008;159:27–34. doi: 10.1530/EJE-08-0203. [DOI] [PubMed] [Google Scholar]
- 43.Wabitsch M, Lahr G, Van de Bunt M, Marchant C, Lindner M, Von Puttkamer J, Fenneberg A, Debatin KM, Klein R, Ellard S, Clark A, Gloyn AL. Diabetic Med. 2007;24:1393–1399. doi: 10.1111/j.1464-5491.2007.02285.x. [DOI] [PubMed] [Google Scholar]
- 44.Gloyn AL, Noordam K, Willemsen MAAP, Ellard S, Lam WWK, Campbell IW, Midgley P, Shiota C, Buettger C, Magnuson MA, Matschinsky FM, Hattersley AT. Diabetes. 2003;52:2433–2440. doi: 10.2337/diabetes.52.9.2433. [DOI] [PubMed] [Google Scholar]
- 45.Davis EA, Cuesta-Munoz AL, Raoul M, Buettger C, Sweet I, Moates M, Magnuson MA, Matschinsky FM. Diabetologia. 1999;42:1175–1186. doi: 10.1007/s001250051289. [DOI] [PubMed] [Google Scholar]
- 46.Gloyn AL, Odili S, Buettger C, Njølstad PR, Shiota C, Magnuson MA, Matschinsky FM. In: Glucokinase and Glycemic Disease: From Basics to Novel Therapeutics. Matschinsky FM, Magnuson MA, editors. Basel: Karger; 2004. pp. 92–109. [Google Scholar]
- 47.Pal P, Miller BG. Biochemistry. 2009;48:814–816. doi: 10.1021/bi802142q. [DOI] [PubMed] [Google Scholar]
- 48.Sayed S, Langdon DR, Odili S, Chen P, Buettger C, Schiffman AB, Suchi M, Taub R, Grimsby J, Matschinsky FM, Stanley CA. Diabetes. 2009;58:1419–1427. doi: 10.2337/db08-1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zelent D, Najafi H, Odili S, Buettger C, Weik-Collins H, Li C, Doliba N, Grimsby J, Matschinsky FM. Biochem. Soc. Trans. 2005;33:306–310. doi: 10.1042/BST0330306. [DOI] [PubMed] [Google Scholar]
- 50.Grimsby J, Sarabu R, Corbett WL, Haynes NE, Bizzarro FT, Coffey JW, Guertin KR, Hilliard DW, Kester RF, Mahaney PE, Marcus L, Qi L, Spence CL, Tengi J, Magnuson MA, Chu CA, Dvorozniak MT, Matschinsky FM, Grippo JF. Science. 2003;301:370–373. doi: 10.1126/science.1084073. [DOI] [PubMed] [Google Scholar]
- 51.Matschinsky FM, Zelent B, Doliba N, Li C, Vanderkooi JM, Naji A, Sarabu R, Grimsby J. Diabetes Care. 2011;34:S236–S243. doi: 10.2337/dc11-s236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarabu R, Grimsby J. Curr. Opin. Drug Discov. Devel. 2005;8:631–637. [PubMed] [Google Scholar]
- 53.Guertin KR, Grimsby J. Curr. Med. Chem. 2006;13:1839–1843. doi: 10.2174/092986706777452551. [DOI] [PubMed] [Google Scholar]
- 54.Johnson D, Shepherd RM, Gill D, Gorman T, Smith DM, Dunne MJ. Biochem. Soc. Trans. 2007;35:1208–1210. doi: 10.1042/BST0351208. [DOI] [PubMed] [Google Scholar]
- 55.Kumari V, Li C. Curr. Chem. Genom. 2008;2:76–89. doi: 10.2174/1875397300802010076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matschinsky FM. Nat. Rev. Drug Discov. 2009;8:399–416. doi: 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- 57.Hill AV. J. Physiol. (Lond) 1910;40:iv–vii. [Google Scholar]
- 58.Holroyde MJ, Allen MB, Storer AC, Warsy AS, Chesher JME, Trayer JP, Cornish-Bowden A, Walker DG. Biochem. J. 1976;153:163–373. doi: 10.1042/bj1530363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cardenas M, Rabajille E, Niemeyer H. Arch. Biochem. Biophys. 1978;190:142–148. doi: 10.1016/0003-9861(78)90261-8. [DOI] [PubMed] [Google Scholar]
- 60.Xu LZ, Zhang W, Webers IT, Harrison RW, Gidh-Jain M, Pilkis SJ. Biochemistry. 1995;34:6083–6092. doi: 10.1021/bi00018a011. [DOI] [PubMed] [Google Scholar]
- 61.Zelent B, Odili S, Buettger C, Shiota C, Grimsby J, Taub R, Magnuson MA, Vanderkooia JM, Matschinsky FM. Biochem. J. 2008;413:269–280. doi: 10.1042/BJ20071718. [DOI] [PubMed] [Google Scholar]
- 62.Monod J, Wyman J, Changeux J-P. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 63.Koshland DE, Jr, Némethy G, Filmer D. Biochemistry. 1966;5:365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- 64.Wyman J. Cold Spring Harb. Symp. Quant. Biol. 1963;28:483–489. [Google Scholar]
- 65.Kantrowitz ER, Lipscomb WN. Science. 1988;241:669–674. doi: 10.1126/science.3041592. [DOI] [PubMed] [Google Scholar]
- 66.Richard J, Meunier J-C, Buc J. Eur. J. Biochem. 1974;49:195–208. doi: 10.1111/j.1432-1033.1974.tb03825.x. [DOI] [PubMed] [Google Scholar]
- 67.Ainslie GR, Jr, Shill JP, Neet KE. J. Biol. Chem. 1972;247:7088–7096. [PubMed] [Google Scholar]
- 68.Rabin BR. Biochem. J. 1967;102:22c–23c. doi: 10.1042/bj1020022c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Frieden C. J. Bid. Chem. 1970;245:5788–5799. [PubMed] [Google Scholar]
- 70.Ricard J, Buc J, Meunier J-C. Eur. J. Biochem. 1977;80:581–592. doi: 10.1111/j.1432-1033.1977.tb11915.x. [DOI] [PubMed] [Google Scholar]
- 71.Buc J, Ricard J, Meunier J-C. Eur. J. Biochem. 1977;80:593–601. doi: 10.1111/j.1432-1033.1977.tb11916.x. [DOI] [PubMed] [Google Scholar]
- 72.Meunier J-C, Buc J, Ricard J. Eur. J. Biochem. 1979;97:573–583. doi: 10.1111/j.1432-1033.1979.tb13146.x. [DOI] [PubMed] [Google Scholar]
- 73.Pollard-Knight D, Cornish-Bowden A. Biochem J. 1987;245:625–629. doi: 10.1042/bj2450625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Storer AC, Cornish-Bowden A. Biochem. J. 1977;165:61–69. doi: 10.1042/bj1650061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Niemeyer H, Cardenas ML, Rabajille E, Ureta T, Clark-Turri L, Pefnaranda J. Enzyme. 1975;20:321–333. doi: 10.1159/000458957. [DOI] [PubMed] [Google Scholar]
- 76.Cornish-Bowden A, Storer AC. Biochem J. 1986;240:293–296. doi: 10.1042/bj2400293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gregoriou M, Trayer IP, Cornish-Bowden A. Biochemistry. 1981;20:499–506. doi: 10.1021/bi00506a009. [DOI] [PubMed] [Google Scholar]
- 78.Neet KE. In: Contemporary Enzyme Kinetics and Mechanism. Purich DL, editor. New York: Academic Press; 1983. pp. 267–320. 1983. [Google Scholar]
- 79.Neet KE, Ainslie GR., Jr Methods Enzymol. 1980;64:192–226. doi: 10.1016/s0076-6879(80)64010-5. [DOI] [PubMed] [Google Scholar]
- 80.Cardenas ML, Rabajille E, Niemeyer H. Eur. J. Biochem. 1984;145:163–171. doi: 10.1111/j.1432-1033.1984.tb08536.x. [DOI] [PubMed] [Google Scholar]
- 81.Tippett PS, Neet KE. J. Biol. Chem. 1982;257:12846–12852. [PubMed] [Google Scholar]
- 82.Tippett PS, Neet KE. J. Biol. Chem. 1982;257:12839–12845. [PubMed] [Google Scholar]
- 83.Pettersson G. Biochem. J. 1886;233:347–350. doi: 10.1042/bj2330347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cornish-Bowden A, Storer AC. Biochem. J. 1986;240:293–296. doi: 10.1042/bj2400293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kamata K, Mitsuya M, Nishimura T, Eiki J, Nagata Y. Structure. 2004;12:429–439. doi: 10.1016/j.str.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 86.Petit P, Gluais L, Lagarde A, Vuillard L, Boutin JA, Ferry G. Midwest Center for Structural Genomics (MCSG) [Google Scholar]
- 87.Nishimura T, Iino T, Mitsuya M, Bamba H, Watanabe H, Tsukahara D, Kamata K, Sasaki K, Ohyama S, Hosaka H, Futamura M, Nagata Y, Eiki J. Bioorg. Med. Chem. Lett. 2009;19:1357–1360. doi: 10.1016/j.bmcl.2009.01.053. [DOI] [PubMed] [Google Scholar]
- 88.Mitsuya M, Kamata K, Bamba M, Watanabe H, Sasaki Y, Sasaki K, Ohyama S, Hosaka H, Nagata Y, Eiki J, Nishimura T. Bioorg. Med. Chem. Lett. 2009;19:2718–2721. doi: 10.1016/j.bmcl.2009.03.137. [DOI] [PubMed] [Google Scholar]
- 89.Bebernitz GR, Beaulieu V, Dale BA, Deacon R, Duttaroy A, Gao J, Grondine MS, Gupta RC, Kakmak M, Kavana M, Kirman LC, Liang J, Maniara WM, Munshi S, Nadkarni SS, Schuster HF, Stams T, St Denny I, Taslimi PM, Vash B, Caplan SL. J. Med. Chem. 2009;52:6142–6152. doi: 10.1021/jm900839k. [DOI] [PubMed] [Google Scholar]
- 90.Takahashi K, Hashimoto N, Nakama C, Kamata K, Sasaki K, Yoshimoto R, Ohyama S, Hosaka H, Maruki H, Nagata Y, Eiki J, Nishimura T. Bioorg. Med. Chem. 2009;17:7042–7051. doi: 10.1016/j.bmc.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 91.Petit P, Lagarde A, Boutin JA, Ferry G, Vuillard L. Midwest Center for Structural Genomics (MCSG) 2010 [Google Scholar]
- 92.Petit P, Lagarde A, Boutin JA, Ferry G, Vuillard L. Midwest Center for Structural Genomics (MCSG) 2010 [Google Scholar]
- 93.Petit P, Lagarde A, Boutin JA, Ferry G, Vuillard L. Midwest Center for Structural Genomics (MCSG) 2010 [Google Scholar]
- 94.Liu Q, Shen Y, Liu S, Weng J, Liu J. FEBS. Lett. 2011;585:1175–1179. doi: 10.1016/j.febslet.2011.03.026. 2011. [DOI] [PubMed] [Google Scholar]
- 95.Larion M, Miller BG. Biochemistry. 2009;48:6157–6165. doi: 10.1021/bi9007534. [DOI] [PubMed] [Google Scholar]
- 96.Liang Y, Kesavan P, Wang L-Q, Niswender K, Tanizawa Y, Permutt M, Magnuson MA, Matschinsky FM. Biochem. J. 1995;309:167–173. doi: 10.1042/bj3090167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin SX, Neet KE. J. Biol. Chem. 1990;265:9670–9675. [PubMed] [Google Scholar]
- 98.Neet KE, Keenan RP, Tippett PS. Biochemistry. 1990;29:770–777. doi: 10.1021/bi00455a026. [DOI] [PubMed] [Google Scholar]
- 99.Heredia VV, Thomson J, Nettleton D, Sun S. Biochemistry. 2006;45:7553–7562. doi: 10.1021/bi060253q. [DOI] [PubMed] [Google Scholar]
- 100.Kim YB, Kalinowski SS, Marcinkeviciene J. Biochemistry. 2007;46:1423–1431. doi: 10.1021/bi0617308. [DOI] [PubMed] [Google Scholar]
- 101.Antoine M, Boutin JA, Ferry G. Biochemistry. 2009;48:5466–5482. doi: 10.1021/bi900374c. [DOI] [PubMed] [Google Scholar]
- 102.Larion M, Miller BG. Biochemistry. 2010;49:8902–8911. doi: 10.1021/bi1008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Boehr DD, Dyson HJ, Wright PE. Chem. Rev. 2006;106:3055–3079. doi: 10.1021/cr050312q. [DOI] [PubMed] [Google Scholar]
- 104.Larion M, Salinas RK, Bruschweiler-Li L, Brüschweiler R, Miller BG. Biochemistry. 2010;49:7969–7971. doi: 10.1021/bi101098f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cavanagh J, Fairbrother WJ, Palmer AG, III, Rance M, Skelton NJ. Protein NMR Spectroscopy: Principles and Practice. second edition. Elsevier: Academic Press; 2007. pp. 702–724. [Google Scholar]
- 106.Agius L, Stubbs M. Biochem. J. 2000;346:413–421. [PMC free article] [PubMed] [Google Scholar]
- 107.Dyson HJ, Wright PE. Nat. Rev. Mol. Cell. Biol. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 108.DeLano WL. The PyMOL Molecular Graphics System. San Carlos, CA, USA: DeLano Scientific; 2002. http://www.pymol.org. [Google Scholar]