Abstract
Background
CD27 is a lymphocyte co-stimulatory molecule that regulates T, NK B, and plasma cell function, survival and differentiation. Based on its function and expression pattern we considered CD27 as a candidate gene in patients with hypogammaglobulinemia.
Objective
Description of the clinical and immunological phenotype of patients having genetic CD27 deficiency.
Methods
A molecular and extended immunological analysis was performed on two patients lacking CD27 expression.
Results
We identified two brothers with a homozygous mutation in CD27 leading to absence of CD27 expression. Both patients suffered from persistent symptomatic EBV viremia. The index patient was hypogammaglobulinemic and immunoglobulin replacement therapy was initiated. His brother developed aplastic anemia in the course of his EBV infection and died from fulminant Gram positive bacterial sepsis. Immunologically, lack of CD27 expression was associated with impaired T-cell dependent B-cell responses and T-cell dysfunction.
Conclusion
Our findings identify a role for CD27 in humans and suggest that this deficiency can explain particular cases of persistent symptomatic EBV viremia with hypogammaglobulinemia and impaired T cell dependent antibody generation.
Keywords: EBV, viremia, hypogammaglobulinemia, CD27, immunodeficiency, T cell, B cell, NK cell, phenotype
Introduction
CD27, a member of the TNF receptor family, is a transmembrane receptor which is widely used as a leukocyte differentiation marker for subsets of T, NK and B-cells(1–3). Importantly CD27 is recognized as marker for memory B cells, and is held to be of diagnostic/predictive value in common variable immunodeficiency (CVID). In CVID there can be decreased numbers of switched memory B cells (expressing surface IgA or IgG) which correlates with the presence of splenomegaly and granulomatous disease (4).
The function of human and murine CD27 has been studied in detail in vitro, and in vivo in mouse models(5;6). Ligation of CD27 by its unique ligand CD70 provides co-stimulatory signals for T, B and NK-cell activation. It furthermore enhances T-cell survival and effector function, NK-cell function, B-cell differentiation and plasma-cell function(7;8). In humans, an indispensable role of the co-stimulating signal provided by CD27–CD70 interaction towards immune function and disease susceptibility has not been formally proven. CD27 is a major differentiation/maturation marker for both NK cells(9) and B cells (10) . Based on its expression on memory B-cells and plasma cells, and the effect of CD27 ligation on in vitro B-cell function, CD27 has been proposed as a candidate gene in common variable immunodeficiency, but its expression on B, T and NK cells suggests that CD27 deficiency may result in a more combined type of immune deficiency.
Primary EBV infection is often asymptomatic in the immunocompetent host. In immunodeficient patients, however, primary EBV infection or secondary reactivation may result in persistent symptomatic EBV viremia, a clinical condition with a prolonged (>6 months) and distinct symptomatic phase with fever, lymphadenophathy and several other possible features such as hepatitis and pneumonia. Persistent symptomatic EBV viremia can be associated with lymphoma, lymphoproliferative disease, hemophagocyticlymphohistiocytosis (HLH) and aplastic anemia, but most typically goes into spontaneous remission(11).
EBV-specific immunity typically encompasses virus specific cellular and humoral immune responses, with T-cells being most important for long term control of disease. Several types of cellular immune deficiency may result in an abnormal course of EBV infection, including combined immune deficiencies (CID), X-linked lymphoproliferative disease (XLP)(12) , familial hemophagocytic lymphohistiocytosis (FHL)(13), and IL-2 inducible T cell kinase (ITK) deficiency (14). In the majority of persistent symptomatic EBV viremia cases however, a specific primary immune deficiency has not been identified.
We here describe two brothers with CD27 deficiency due to a homozygous mutation resulting in a premature stop codon in the gene encoding CD27. Clinically these patients presented as having persistent symptomatic EBV viremia with lethal aplastic anemia in one and hypogammaglobulinemia with impaired specific antibody function in the other. In the surviving patient, absence of CD27 was associated with an abnormal T-cell dependent B-cell response and disturbed T-cell function.
Methods
Evaluation of blood, bone marrow biopsy, vaccination responses and medical records were carried out after written informed consent was obtained in accordance with local medical ethics committee guidelines.
Case report
The index patient, a 21 year old male of Moroccan descent, was the third child of consanguineous parents (first cousins). At age 2 ½, he experienced fever, severe lymphadenopathy and hepato-splenomegaly lasting a total of 6 months. EBV seroconversion was noted for early antigen and viral capsid antigen but during follow up, no seroconversion for nuclear antigen (EBNA) was noted. Immunoglobulins were determined longitudinally and were initially increased (IgM 3.1g / L, IgG 15.9 g /L, IgA 1.9 g/L, see Figure E1 A in the Online Repository ). The peripheral blood lymphocyte compartment was pheno-typed regularly during the first one and a half year of follow up and changes in lymphocyte numbers showed signs compatible with viral infection (see Figure E1 A in the Online Repository). T cell proliferation assays showed strongly reduced mitogen and antigen specific responses the first 6 months after clinical presentation, and these responses gradually increased to subnormal and normal levels respectively, during the following year (data not shown). Clinical symptoms disappeared after 6 months and at the same time the patient became hypogammaglobulinemic (IgM 0.03 g / L, IgG 4.4 g /L, IgA 0.1 g/L). Immunoglobulin replacement therapy was initiated. Since receiving immunoglobulin prophylaxis, he has had an uneventful medical history: no abnormalities were noted in incidence, type or course of infections; vaccinations including live attenuated MMR were given without complications; there was normal growth and development; there were no additional hospitalizations; and the patient did not develop autoimmunity or cancer. EBV plasma load was monitored longitudinally in available samples with quantitative PCR and was detectable at low levels, but with an increased frequency compared to healthy controls (positive 4 out of 15 times, see Figure E1 B in the Online Repository); the patient was asymptomatic at these occasions. CD4 and CD8 T cells, NK cells and B cells were sorted from a reactive lymph node cell suspension (derived at age 3 years) and from PBMC (derived at age 21 years). In both cases EBV was detected in the purified B cell fraction (but compared to other reports in relative low amounts: viral loads were 3000 and 2050 viral copies /ug DNA respectively(15)).
Family history was notable for two childhood deaths (Figure 1A). An older sister died during infancy due to an unknown illness. She had otherwise had an uneventful medical history to our knowledge, although details were limited. An older brother was referred at age 3 with documented EBV-induced lymphadenopathy, fever, hepatosplenomegaly and EBV-associated uveitis, without evidence of malignancy. He had increasing EBV anti-VCA and EA titers but absence of EBNA seroconversion during follow up. At initial presentation, serum IgG was 10,4 g/L, IgM 2,7 g/L and IgA 0.4g/L); IgM normalized during a period of 8 months. Lymphocyte phenotyping 8 months after initial presentation showed 0.04 × 10E9/L CD20+ B cells, 0.16 ×109/L CD4+ T cells, 2.49×109 CD8+ T cells/L (CD4/CD8 ratio 0.064), and 1.38 ×109/L NK cells. Furthermore, mitogenic and antigenic T-cell responses were absent. After a period of 8 months with severe lymphadenopathy and recurrent episodes of fever, he developed aplastic anemia, and one month thereafter he died from fulminant Gram positive bacterial sepsis.
Figure 1. Absence of CD27 expression due to nonsense mutation in the CD27 gene.
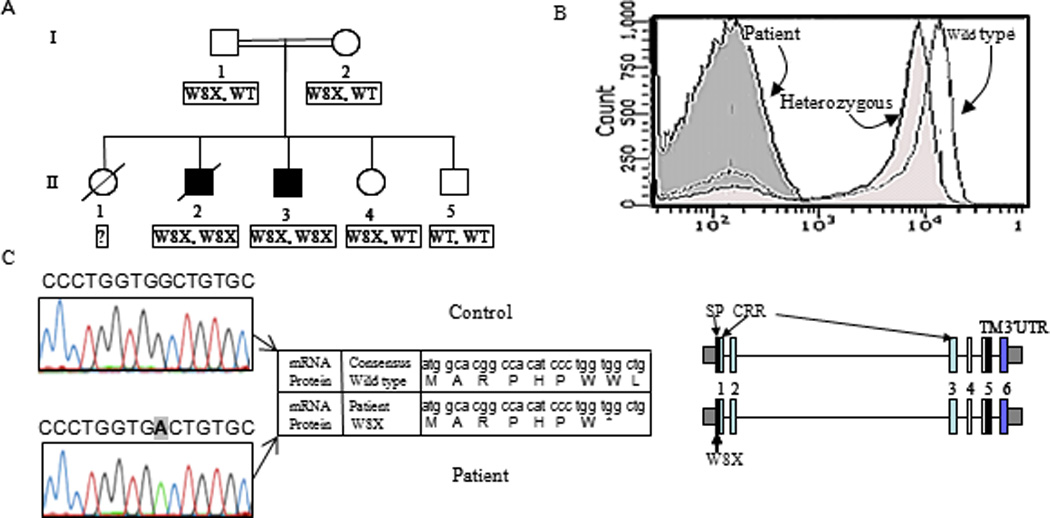
(A) Family pedigree (/ deceased persons, = consanguinity). (B) FACS analysis of CD27 membrane expression on PBMC. (C) Sequence analysis of the CD27 gene. The mutation (bold) identified in both patients in exon 1 results in a premature stop codon (X) in the signal peptide of the protein (NP_001233.1) as depicted in the protein sequence (superimposed on the gene sequence).
Genomic sequencing
Genomic DNA was isolated from PBMC or snap frozen lymph node tissue. Exon-specific M13-tagged primers were used to amplify all coding exons including flanking regions from the genes CD27 (NM_001242), SH2D1A (NM_002351), XIAP (NM_001167) and PRF1 (NM_001083116); primer sequences available upon request. PCR products were directly sequenced using M13 sequence primers and BigDye Terminator v1.1 cycle sequencing kit (Applied Biosystems, www.aplliedbiosystems.com) according to the manufacturer's protocol. Sequencing was performed on a 3130XL genetic analyzer (Applied Biosystems) and sequences were analysed using SeqScape v2.5 software (Applied Biosystems).
Flow cytometry
PBMC were isolated from whole blood using ficoll-hypaque density centrifugation. Antibody staining and FACS analysis were performed as described previously(6;16) (for the antibodies used in the analysis see Table 1 in the Online Repository). Custom conjugations of antibodies to Quantum (Q) dot nanocrystals (Invitrogen) were performed as previously described(17). For acquisition PMT voltages were based upon isotype negative controls and compensation upon positive controls using fluorphore-conjugated anti CD45 or Cytometer Setup and Tracking beads (CST, BD). Staining with recombinant CD70 was performed with day 3 culture supernatant of soluble Flag-TNC-CD70 transfected HEK cells(18). Anti Flag FITC (clone M2, Sigma- Aldrich, St. Louis, MO) was used as a second step to detect binding of the recombinant protein to the cell surface. CD4+CD3+ and CD8+CD3+ T lymphocytes within the lymphocytes were analyzed for their expression of CD45RA in combination with CCR7, CD62L, CD127, CD28 or FAS. The composition of the B-cell compartments was assessed in blood and bone marrow by flow cytometry(19;20).
Immunohistochemstry
Immunohistochemistry was performed on 4µm thick paraffin embedded sections from neutral buffered formaldehyde fixed tissue blocks. Immunohistochemical staining for CD3, CD20, CD79a, CD138, Kappa, Lambda, BCL2, and Mib-1 (Dakopatts, Glostrup, Denmark), CD5, CD23, CD27, and BCL6 (Novacastra, Newcastle upon Tyne, UK) was performed using a Bond-Max automated staining machine (Vision Biosystems, Norwell, MA) with the Bond polymer refine detection kit (Vision BioSystems, cat. no DS9800). As antigen retrieval for BCL6 and CD27 Epitope Retrieval Solution 2 (ERS-2; EDTA) was used, for all other reactions ERS-1 (citrate buffer) was used (Novacastra). Isotype controls were used throughout.
Functional assays
CD4+ cells were isolated using anti-CD4 coated magnetic beads (Miltenyi, Inc) according to the manufacturer’s instructions. For proliferation analysis 1.8×105/ml PBMC or isolated CD4+ were cultured for 3, 4 or 6 days in RH10 (RPMI1640 supplemented with L-Glutamate and 25mM HEPES (Invitrogen, Carlsbad, CA), containing 10% Human AB serum (Sanquin, Amsterdam, The Netherlands), Penicillin/Streptomycin (100 U/ml) (Invitrogen) and 6.0 × 10−5M β-mercaptoethanol (v/v) (Calbiochem, Merck, Darmstad, Germany)) and one of the following stimuli: phytohemagglutinin (PHA, 5 ug/ml, Wellcome, Beckenham, UK), Concanavalin A (ConA, 10 ug/ml, Calbiochem) (both 3 and 6 days), a cocktail of three anti CD2 mAbs (clone T11.1/1 T11.1/2 T11.1/3, Sanquin)(4 days), (PWM, 3.5 ug/ml, Sigma), tetanus toxoid ((TT) 70Lf/ml, Netherlands Vaccine Institute, Bilthoven, The Netherlands), Purified Protein Derivative((PPD), 13 ug/ml, Statens Serum Institute, Copenhagen, Denmark) and Candida albicans ((Cand. A.),7 ug/ml, Hal, Leiden, The Netherlands)( 6 days). 3H-Thymidine (1uCi /96well) was added 16 hours prior to harvesting. Backgound (media) only 3H-T incorporation was < 500 cpm. T cell co-stimulation via CD70 and CD8+ T cell polyfunctionality were tested as previously described(16;21).
For B cell differentiation assays 4×105 PBMC (at 2.5×105/ml) were cultured with either Staphylococcus aureus Cowan strain I (SAC, 40 units/ml) and IL-2 (50 units/ml) or pokeweed mitogen (PWM, 3.5 ug/ml, Sigma). After 7 days, cells were harvested, cytospins (105/sample) were prepared, air dried, and fixed with 95% EtOH/ 5% acetone and stained with FITC conjugated anti-Ig,IgM, IgA, IgG (SBA). Ten fields of 50 cells were analyzed by fluorescence microscopy and the fraction of plasmablasts were calculated.
For NK cell cytotoxicity, NK cells were negatively selected from PBMC (Miltenyi NK cell isolation kit-II) and were added in different ratios to chromium labeled K562, 721.221, or Raji cells, or Raji cells with Rituximab and chromium release was measured after 4 hours as previously described(22).
Vaccination studies
The index patient was vaccinated once with 23 valent (polysaccharide) pneumococcal vaccine (Pneumovax, Merck Sharp & Dohme, Darmstadt, Germany), with TT conjugated Men C vaccine (NeissVac, Baxter, Utrecht, The Netherlands), and twice (12 weeks apart) with the human diploid-cell rabies vaccine (Institut Pasteur Merieux, Merck Sharp & Dohme) according to standard protocols. During infancy, he was vaccinated three times with Tetanus Toxoid vaccine (Tetanusvaccin, Netherlands Vaccine Institute, Bilthoven, The Netherlands), followed by 2 booster vaccinations in childhood, as part of the standard Dutch national vaccination program.
Quantification of serum antibody levels
IgM, IgG, IgA and IgG subclasses were quantified by nephelometry on an Image 800 nephelometer (Beckmann Coulter). Specific antibody levels against vaccination antigens were measured by ELISA(23). Blood samples for antigen-specific antibody determination were drawn at the indicated time points post-vaccination. Reference sera for Rabies and TT were used secondary local standards that were calibrated on international standards supplied by NIBSC, www.nibsc.ac.uk. The response to both antigens was compared with the response of 20 healthy adults.
Detection of plasma viral EBV load
EBV plasma load was measured as described previously(24).
Analysis of somatic hypermutation
Hypermutation of immunoglobulin genes was analysed as described(25). In short, VH3-Cγ fragments were amplified from randomly primed cDNA and cloned in a pGEM-T Easy Vector (Easy Vector System II, Promega, San Luis, CA). Fifteen individual clones were sequenced (ABI Prism 3100 Genetic analyser, Applied Biosystems), analysed using BioEdit Version 5.0.9 and aligned to the germ line with the highest homology within the region from framework region (FR) 1 to FR3, as deposited in http://www.ncbi.nlm.nih.gov/igblast. This database contains defined functional V genes. The percentage homology to the germ line VH gene segment was determined from the beginning of FR1 to the end of FR3.
Results
Patient identification and genetic diagnosis
Lymphocyte subset analysis was performed in a patient with a confirmed but unexplained diagnosis of CVID and persistent symptomatic EBV viremia. In this patient, no CD27 positive T or memory B-cells were detected, despite the presence of normal percentages of naive (CD45RA+) T-cells and switched IgG and IgA positive B-cells (subpopulations normally expressing CD27). Immunohistochemistry (results not shown) and extended flow cytometric analysis with additional anti-CD27 monoclonal antibodies and a recombinant ligand (CD70) protein confirmed the absence of CD27 expression on all lymphocytes (Figure 1B), in the presence of normal lymphocyte subsets distributions, suggesting a genetic defect in CD27. Mutation analysis in the index patient and in the deceased brother revealed a homozygous mutation (c.24G>A) in the gene encoding CD27, resulting in a premature stop codon (W8X, Figure 1 C). Both parents and an unaffected brother were asymptomatic heterozygous carriers (Figure 1A). To rule out other known genetic causes of EBV susceptibility, we analyzed both the SH2D1A- and XIAP genes (which can result in XLP) as well as the PRF1 gene (which can cause HLH) in the index patient and his mother; no mutations were found.
To better elucidate whether this patients’ CD27 deficiency could explain his clinical course of persistent symptomatic EBV viremia and hypogammaglobulinemia, we evaluated the phenotype and function of patient lymphocytes in vitro and in vivo.
B-cell compartment
At age 21, the patient had normal B-cell counts, and although he was receiving IgG replacement therapy, decreased serum IgA and IgM values (0.14 g / L (normal 5–95% range: 0.70 – 4.0) and 0.31 g / L (normal 2–95% range: 0.40 – 2.3) respectively) were documented. FACS analysis of early and mature B-cell compartments in bone marrow (data not shown) and blood (Figure 2A) revealed normal numbers and percentages of all stages of B-cell development, including surface IgA and IgG positive B-cells. The percentage of bone marrow plasma cells, however, was slightly reduced. In vitro induction of B-cells using the T-cell independent stimuli S. Aureus Cowan I antigen and interleukin-2(26) resulted in normal plasmablast formation, however none were detected using T-cell dependent stimulation with pokeweed mitogen (PWM, Figure 3A). These results reflected the in vivo responses as vaccination with pneumococcal-polysaccharide and meningococcus-C-conjugated vaccines resulted in normal levels of specific antibody production (IgG - Figure 3B and IgM - data not shown). In contrast, repeated protein antigen vaccinations (Rabies) and Tetanus Toxoid (TT) booster vaccinations resulted in severely impaired antibody production, suggesting a defect in T-cell dependent B-cell responses (Figure 3C).
Figure 2. Phenotypic analysis of patient B-cells.
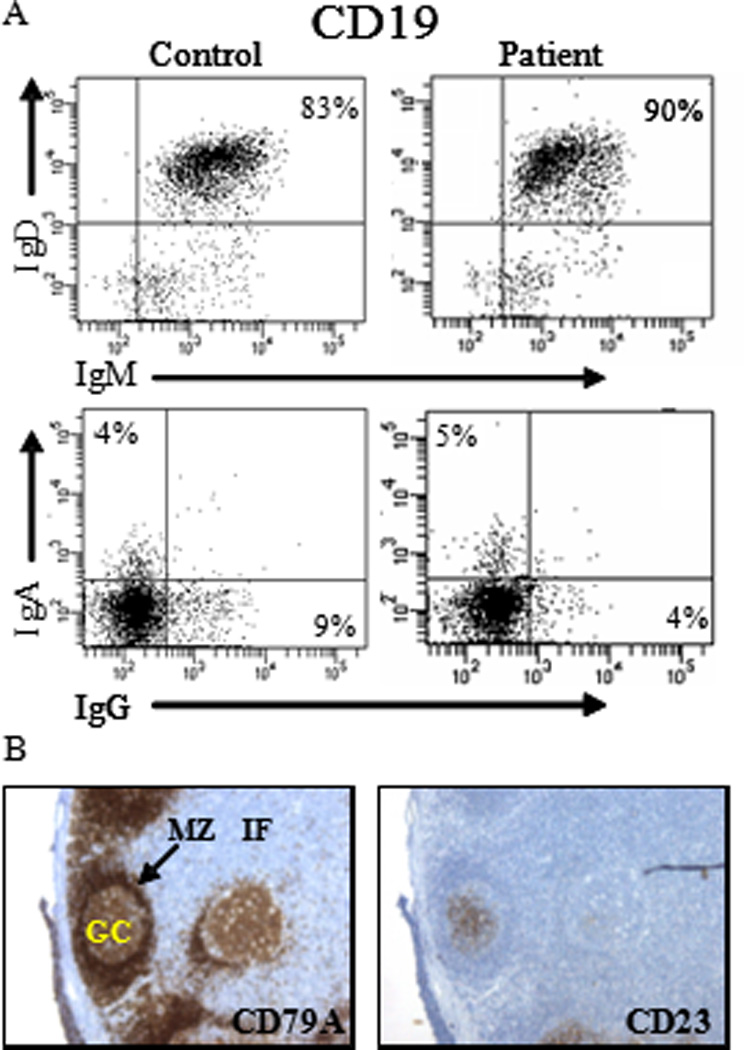
(A) The presence of different B-cell subsets was analyzed by FACS. Within the CD19+ B-cells, the fraction of naive (IgM+, IgD+), or memory (IgA+ or IgG+) B-cells was determined. (B) The presence of germinal centers (GC) was determined by staining tissue sections with anti CD79a and CD23 mAb. Mantle zone (MZ) and interfolliculair area (IF).
Figure 3. Functional analysis of the index patients B-cells.
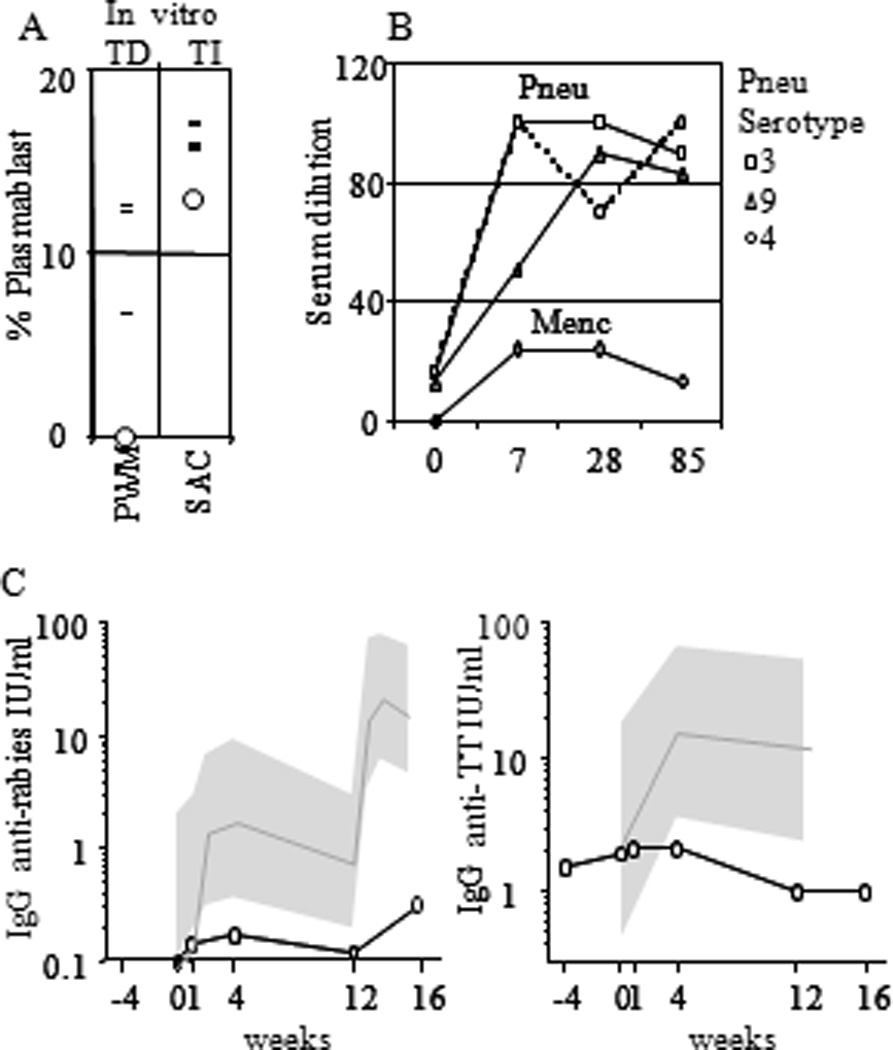
(A) PBMC were tested for their capacity to differentiate into plasmablasts after T-cell independent (SAC/IL2) or T-cell dependent stimulation (TD, PWM). The fraction of plasmablasts was determined based on morphology and intracellular Ig staining on day 7. Index patient (Ο), controls (−). (B) Vaccination response of the index patient to polysaccharide vaccine (Pneumovax 23), conjugated polysaccharide Men-C (NeissVac). (C) Vaccination response of the index patient to protein antigen (diploid-cell rabies vaccine). The mean of healthy controls (black line) with the 95% confidence intervals (gray region).
Since CD27 and its ligand are expressed in germinal centers (GCs)(27;28), we next tested whether disturbed GC formation could explain the impaired T-cell dependent B-cell responses. Immunohistochemistry on a reactive lymph node, obtained for diagnostic purposes from the index patient in infancy during lymphadenopathy showed a lymph-node architecture with a large T-cell zone, primary follicles and GCs (Figure 2B). Most GC were small but had a normal constitution. Large numbers of (CD138+) plasma cells were scattered in the lymph node. Analysis of somatic hypermutation (SHM), a process occurring in GC, showed mutation frequencies in the VH-Cγ transcripts of peripheral blood (PB) B-cells within the normal range (data not shown) comparable to those found in unaffected protein driven secondary immune responses(29). Thus, while follicles were formed and SHM occurred, the relative small size of secondary follicles supported the observation of impaired T-cell dependent responses.
NK-cell compartment
Given the critical role of NK-cells in defense against herpes viruses(30) and the fact that CD27 is an important marker for NK-cell development(1), NK-cells were analyzed phenotypically and functionally. NK-cells were present in normal numbers in peripheral blood, and appeared to be fully differentiated (see Figure E2 in the Online Repository). Importantly, the cytotoxic function of NK-cells was also normal, as PBMC or highly enriched PB NK-cells lysed MHC class-I deficient erythroleukemia cells and EBV-transformed B-cells, and were functional in antibody dependent-cell mediated cytotoxicity (data not shown and Figure 4). FACS analysis on reactive lymph nodes showed normal (1.5%) and increased (16%) frequencies of NK-cell numbers in the index patient and the deceased brother, respectively.
Figure 4. Analysis of the index patients NK cells.
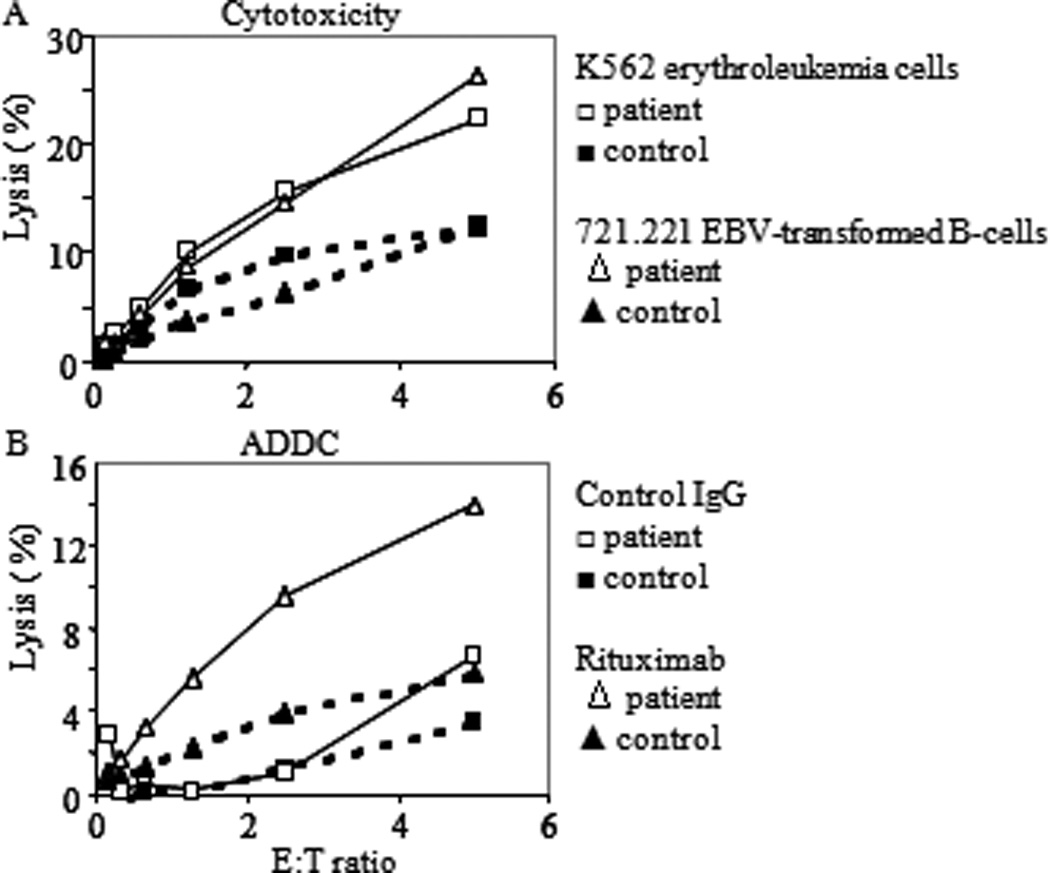
(A) Cytotoxic activity of highly enriched negatively selected ex-vivo PBMC NK-cells against the indicated targets. (B) Antibody-dependent-cellular cytotoxicity of Raji cells induced by the addition of Rituximab relative to added control IgG.
T-cell compartment
CD27 was first recognized as a co-stimulatory molecule on human T-cells and thus we evaluated the ability of patient T-cells to respond to activation. Overall, in vitro proliferative responses of patient T-cells were reduced in response to mitogens that strongly depend on CD27 (CD2 and PWM, Figure 4A and Figure E3 in the Online Repository). Proliferative responses against a variety of recall antigens could be detected ex vivo and after vaccination with TT the specific proliferative response was increased (data not shown). To determine if there was an impact of CD27 deficiency upon T cell maturation or specific T-cell subsets, extended phenotypic and functional analyses were performed. NKT cells and γδ T cells were present in similar numbers and fractions of T cells as in healthy controls. γδ T cells produced similar amounts of T-bet and IFN gamma (after PMA ionomycin stimulation, data not shown).This limited analysis argues against a role for CD27 in γδ T development. For both CD4+ and CD8+ T-cells, the phenotypic subset distribution, as defined by CCR7, CD127, CD45RA(31) (Figure 5B, upper) as well as cytokine (IL-2, IFN-γ and TNF-α) production(32), after polyclonal stimulation (see Figure E4 in the Online Repository) was comparable to healthy controls. Ex vivo analysis of T-bet expression showed comparable percentages and levels in the patients and control subsets of CD4 and CD8 T cells (data not shown), and HLA-A2 restricted EBV-specific CD8+ T-cells were readily detected and amounted to 0.41% of memory CD8+ T-cells. These cells de-granulated, produced IFN-γ, TNF-α and MIP1-a and proliferated (see Figure E4 in Repository) after EBV specific stimulation in vitro. Autologous EBV transformed B-cells were lysed by patient EBV specific T-cell lines (data not shown). Despite a normal differentiation phenotype, however, the frequency of IL-2 producing EBV-specific CD8 T-cells was reduced compared to healthy controls. (Figure 4B, lower)(32). This suggests that despite there being normal T-cell differentiation and maturation the ability to maintain physiologically relevant recall in the CD8+ T cell population was deficient.
Figure 5. Analysis of the index patients T-cells.
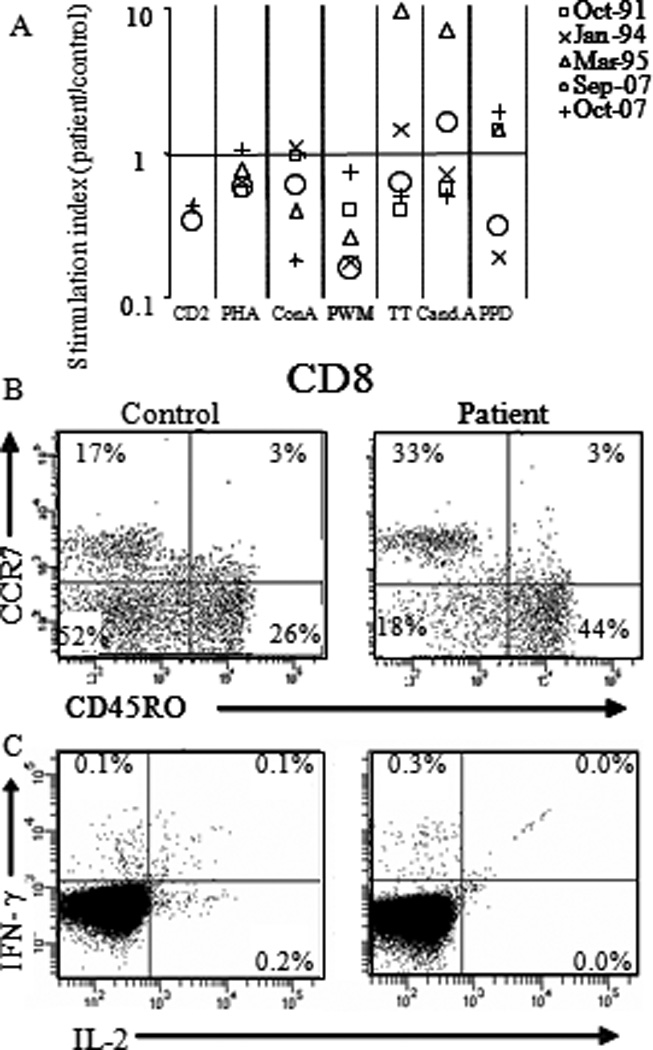
(A) Proliferation assays with PBMC or CD4+ T-cells and the indicated stimuli. Results are presented as stimulation index (index patient CPM/ healthy control CPM). (B) Subsets analysis of T-cells(48) in PBMC by FACS. Within CD8+CD3+ T-cells the fraction of naive (CCR7+, CD45ROneg), central memory (CCR7+, CD45RO+), effector memory(CCR7neg, CD45RO+) and effector cells (CCR7neg, CD45RO+) were determined. (C) Intracellular accumulation of IL-2 and IFN-γ in EBV-specific CD3+CD8+ T-cells after stimulation with HLA-A2 restricted EBV peptides pools.
Discussion
We describe two cases of CD27 deficiency that presented with persistent symptomatic EBV viremia after primary infection with EBV at young age. In the one case where more detailed immunologic evaluation was possible, the immunologic phenotype shared similarities with that of CVID in that there was hypogammaglobulinemia and partially impaired specific antibody function. Overall our findings demonstrate that CD27 deficiency may be associated with a distinct immunological phenotype. One of disturbed T-cell dependent B-cell function and subtle, but likely clinically relevant, abnormalities in T-cell function. CD27 deficiency does not seem to influence NK cell or γδ T development or function, despite it being an important lineage-specific marker. The clinical relevance of CD27 deficiency is likely to vary as reflected by the clinical courses in the two patients presented here, one eventually recovering (and currently healthy, although receiving immunoglobulin replacement therapy), and the other succumbing from a presumed complication of persistent symptomatic EBV viremia (i.e. aplastic anemia).
Several studies have provided evidence for decreased ADDC, decreased NK-cell activity(33), and lack of NKT cells or EBV-specific CTLs (14;34) during persistent symptomatic EBV viremia (35). The strongly reduced numbers of NKT cells in SAP-, ITK- and in a large number of XIAP-deficient patients points towards a role of this cell type in control of EBV infection(14;34;36;37). The successful treatment of patients with persistent symptomatic EBV viremia using EBV-specific CTL infusions further emphasizes the importance of virus specific CTL for EBV clearance (38). Importantly our patient had normal NK cells and normal numbers of NKT cells, but demonstrated a subtle CTL abnormality. Investigation of the index patient’s immunity at adult age showed poly-functional EBV specific CD8+ T-cells, and killing of autologous EBV transformed B-cells by patients EBV T-cell lines. In line with this EBV-specific immunity, repeated measurements of plasma EBV DNA showed periodic low-but detectable viral loads (range <50–234 copies /ml). When compared to the prevalence of EBV DNA in the plasma of healthy controls (no detectable load in 300 healthy controls, R. Schuurman, personal communication), the levels detected in the patient are higher than expected. This finding could be a feature of a higher incidence of B cell activation as a result of (sub-clinical) infections due to the patients lowered immunoglobulin levels. In keeping with the periodic EBV viremia, sorted patient B cells obtained during viremic periods, as well as during resolution, also demonstrated cell-associated viral loads. This is likely an indication of abnormal EBV-specific immunity.
In mouse models, absence of CD27 does not dramatically change in vivo immunity(5;39), but does affect distinct aspects of CD8 T-cell responses(5;40;41). In the index patient, naive, memory, memory effector and terminally differentiated T-cells and gamma delta T-and NKT cells were present within the normal range, and memory CD4+ and CD8+ T-cell responses were detected. Thus, on the surface the immune elements required for anti-EBV responses would seem to have been intact. The ex vivo fraction of IL-2 producing EBV-specific CD8+ however, was decreased. In line with this observation, absence of CD27 in mice results in decreased frequencies of IL-2 producing, but not IFN-γ producing, CD8 memory cells(42). In mice, NKT dependent induction of the CD70 ligand on DC(43) enhances CD8 T cell activation and lack of CD27 also results in a decreased number of CD8 effector cells at the site of viral infection(44). Given the importance of CTL for control of EBV infection, the phenotype of CD27 −/− mice is compatible with a subtle, yet important role for CD27 in CTL induction and function which could result in the EBV-related abnormalities observed in our patients. Finally, it is conceivable that the role of CD27 deficiency is more pronounced during the initiation of the cellular immune response, which could preclude identifying additional abnormalities as we were only able to investigate EBV specific immunity at adult age in the surviving patient.
Phenotypic and functional analyses did not reveal abnormalities of peripheral blood NK-cells. Analysis of reactive lymph nodes showed normal (1,5%) to increased (16%) percentages of NK-cells in lymph nodes of the index patient and his diseased brother, respectively, which suggests that NK-cells were participating in the response against EBV. Recently, CD27 has been identified as a marker that distinguishes both human and murine developmental NK cell subsets(1) and is expressed almost exclusively on immature NK-cells. This suggests that CD27 is involved in NK-cell maturation into mature cytolytic cells. CD27 also effects NK cell cytolytic capacity as CD27 deficient murine NK cells showed reduced cytotoxicity(45), albeit only under activating conditions. Our analysis however showed no abnormalities in the composition of NK-cell compartment, effectively ruling out a requirement for CD27 in the development of human NK-cells. Since EBV infection induces IFN production(46), which can prime NK cells for CD27 dependent- and other increases in cytolytic capacity(45), it is still possible that the lack of a defect in the patient could represent a cytokine-compensated phenotype. While this may be possible, the diverse expression of inhibitory and activating receptors on the patients NK cells, however, strongly suggest that the interaction between CD70 and CD27 is dispensable for human NK cell differentiation, maturation and cytotoxic function.
In humans, CD27 is an important co-stimulator of plasma cell differentiation. The index patient became hypogammaglobulinemic at the time of recovery of lymphadenopathy and based on his immunoglobulin profile and vaccination responses could even be classified as having CVID(47), although the abnormal course of EBV infection suggests sub-optimal cellular immunity. In this patient, CD27 deficiency did not interfere with B-cell development in the bone marrow, and B-cell numbers and subset composition were normal in peripheral blood. The presence of a normal frequency of IgG+ B-cells carrying hypermutated IgH genes and structurally normal germinal centers of the index patient implied that CD27 deficiency does not interfere with Ig-isotype switching and somatic hypermutation. Despite the absence of abnormalities in the cellular composition of the B-cell compartment, however, abnormal T-cell dependent immune responses in vivo (rabies vaccine) and in vitro (pokeweed mitogen) were observed. In contrast, T-cell independent responses against SAC in vitro and Pneumococcal and Meningococcal polysaccharides in vivo were effectively induced. This suggests that CD27 deficiency impairs post-primary GC, terminal B-cell differentiation possibly by disturbing the balance between induction of memory B-cells and plasma cells. The decreased numbers of plasma cells in the BM but increased numbers of plasmablasts in the lymph node shows that CD27 is dispensable for plasma cell differentiation, but suggest a novel role for CD27 in regulating plasmablast survival and/or migration. Together the data of our index patient suggest that CD27 function in humans is not essential for any specific stage of the adaptive immune response, as is observed in CD27KO mice. The functions of certain TNF receptor family member functions, like these of e.g. CD27, 4-1bb and OX-40, appear to be largely overlapping. Fine-tuning of immune responses, via highly restricted expression of their respective ligands, appears to be their role. That CD27 deficiency seems to reveal itself during EBV infection might reflect its heightened potential during EBV infection due to the large amounts of EBV induced CD70 expression.
In conclusion, we describe the clinical and immunological phenotype of two brothers with CD27 deficiency presenting with persistent symptomatic EBV viremia. Our data suggest that CD27 deficiency is a new molecularly-defined primary immunodeficiency disease associated with persistent symptomatic EBV viremia, hypogammaglobulinemia and impairment in specific-antibody function resulting from disturbed CD8+ T-cell and T-cell dependent B-cell responses.
Clinical implications.
In patients with EBV viremia and/or disturbed T cell dependent humoral immunity, CD27 expression should be tested in consideration of genetic deficiency.
Acknowledgements
We like to thank H. Wajant (University Hospital Wuerzburg, Germany) for the kind gift of the CD70 expression vector, C. van Noessel (Academic Medical Center) for advice on the immunohistochemistry, M. Hillen (University Medical Center Utrecht) for preparation of the recombinant soluble CD70, and A. Hersperger (University of Pennsylvania) for conducting EBV-specific CD8 experiments.
Funding:
J.S. Orange: NIH AI-067946
M. Betts: NIH RO1 AI076066
Abbreviations
- Cand. A.
Candida Albicans
- CCR
Cysteine rich regions
- EBV
Epstein Barr virus
- EBNA
Epstein Barr Nuclear Antigen
- EA
Early antigen
- FACS
Fluorescence-activated cell sorting
- PHA
Phytohaemagglutinin
- PWM
Poke Weed Mitogen
- PPD
Purified Protein Derivative
- SAC
S. Aureus Cowan I antigen
- SP
Signal peptide
- TM
Transmembrane domain
- TT
Tetanus toxoid
- UTR
Untranslated region
- VCA
Viral capsid antigen
- XLP
X-linked lympho-proliferative disease
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors declare that they have no conflict of interest.
References
- 1.Chiossone L, Chaix J, Fuseri N, Roth C, Vivier E, Walzer T. Maturation of mouse NK cells is a 4-stage developmental program. Blood. 2009;113(22):5488–5496. doi: 10.1182/blood-2008-10-187179. [DOI] [PubMed] [Google Scholar]
- 2.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186(9):1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188(9):1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111(1):77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 5.Hendriks J, Gravestein LA, Tesselaar K, van Lier RA, Schumacher TN, Borst J. CD27 is required for generation and long-term maintenance of T cell immunity. Nat Immunol. 2000;1(5):433–440. doi: 10.1038/80877. [DOI] [PubMed] [Google Scholar]
- 6.Tesselaar K, Arens R, van Schijndel GM, Baars PA, van d V, Borst J, et al. Lethal T cell immunodeficiency induced by chronic costimulation via CD27-CD70 interactions. Nat Immunol. 2003;4(1):49–54. doi: 10.1038/ni869. [DOI] [PubMed] [Google Scholar]
- 7.Borst J, Hendriks J, Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol. 2005;17(3):275–281. doi: 10.1016/j.coi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Agematsu K, Hokibara S, Nagumo H, Shinozaki K, Yamada S, Komiyama A. Plasma cell generation from B-lymphocytes via CD27/CD70 interaction. Leuk Lymphoma. 1999;35(3–4):219–225. doi: 10.3109/10428199909145724. [DOI] [PubMed] [Google Scholar]
- 9.Vossen MT, Matmati M, Hertoghs KM, Baars PA, Gent MR, Leclercq G, et al. CD27 defines phenotypically and functionally different human NK cell subsets. J Immunol. 2008;180(6):3739–3745. doi: 10.4049/jimmunol.180.6.3739. [DOI] [PubMed] [Google Scholar]
- 10.Klein U, Rajewsky K, Kuppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells. J Exp Med. 1998;188(9):1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams H, Crawford DH. Epstein-Barr virus: the impact of scientific advances on clinical practice. Blood. 2006;107(3):862–869. doi: 10.1182/blood-2005-07-2702. [DOI] [PubMed] [Google Scholar]
- 12.Dupre L, Andolfi G, Tangye SG, Clementi R, Locatelli F, Arico M, et al. SAP controls the cytolytic activity of CD8+ T cells against EBV-infected cells. Blood. 2005;105(11):4383–4389. doi: 10.1182/blood-2004-08-3269. [DOI] [PubMed] [Google Scholar]
- 13.Filipovich AH. Hemophagocytic lymphohistiocytosis and other hemophagocytic disorders. Immunol Allergy Clin North Am. 2008;28(2):293–313. doi: 10.1016/j.iac.2008.01.010. viii. [DOI] [PubMed] [Google Scholar]
- 14.Huck K, Feyen O, Niehues T, Ruschendorf F, Hubner N, Laws HJ, et al. Girls homozygous for an IL-2-inducible T cell kinase mutation that leads to protein deficiency develop fatal EBV-associated lymphoproliferation. J Clin Invest. 2009;119(5):1350–1358. doi: 10.1172/JCI37901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calattini S, Sereti I, Scheinberg P, Kimura H, Childs RW, Cohen JI. Detection of EBV genomes in plasmablasts/plasma cells and non-B cells in the blood of most patients with EBV lymphoproliferative disorders by using Immuno-FISH. Blood. 2010;116(22):4546–4559. doi: 10.1182/blood-2010-05-285452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Makedonas G, Hutnick N, Haney D, Amick AC, Gardner J, Cosma G, et al. Perforin and IL-2 upregulation define qualitative differences among highly functional virus-specific human CD8 T cells. PLoS Pathog. 2010;6(3):e1000798. doi: 10.1371/journal.ppat.1000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chattopadhyay PK, Price DA, Harper TF, Betts MR, Yu J, Gostick E, et al. Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry. Nat Med. 2006;12(8):972–977. doi: 10.1038/nm1371. [DOI] [PubMed] [Google Scholar]
- 18.Wyzgol A, Muller N, Fick A, Munkel S, Grigoleit GU, Pfizenmaier K, et al. Trimer stabilization, oligomerization, and antibody-mediated cell surface immobilization improve the activity of soluble trimers of CD27L, CD40L, 41BBL, and glucocorticoid-induced TNF receptor ligand. J Immunol. 2009;183(3):1851–1861. doi: 10.4049/jimmunol.0802597. [DOI] [PubMed] [Google Scholar]
- 19.van GR, van Tilburg CM, Nibbelke EE, Otto SA, Gaiser JF, Janssens-Korpela PL, et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin Immunol. 2009;133(1):95–107. doi: 10.1016/j.clim.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 20.van Lochem EG, van dV V, Wind HK, te Marvelde JG, Westerdaal NA, van Dongen JJ. Immunophenotypic differentiation patterns of normal hematopoiesis in human bone marrow: reference patterns for age-related changes and disease-induced shifts. Cytometry B Clin Cytom. 2004;60(1):1–13. doi: 10.1002/cyto.b.20008. [DOI] [PubMed] [Google Scholar]
- 21.Tesselaar K, Gravestein LA, van Schijndel GM, Borst J, van Lier RA. Characterization of murine CD70, the ligand of the TNF receptor family member CD27. J Immunol. 1997;159(10):4959–4965. [PubMed] [Google Scholar]
- 22.Orange JS, Roy-Ghanta S, Mace EM, Maru S, Rak GD, Sanborn KB, et al. IL-2 induces a WAVE2-dependent pathway for actin reorganization that enables WASp-independent human NK cell function. J Clin Invest. 2011;121(4):1535–1548. doi: 10.1172/JCI44862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brinkman DM, Jol-van der Zijde CM, ten Dam MM, Vossen JM, Osterhaus AD, Kroon FP, et al. Vaccination with rabies to study the humoral and cellular immune response to a T-cell dependent neoantigen in man. J Clin Immunol. 2003;23(6):528–538. doi: 10.1023/b:joci.0000010429.36461.6b. [DOI] [PubMed] [Google Scholar]
- 24.van Esser JW, Niesters HG, Thijsen SF, Meijer E, Osterhaus AD, Wolthers KC, et al. Molecular quantification of viral load in plasma allows for fast and accurate prediction of response to therapy of Epstein-Barr virus-associated lymphoproliferative disease after allogeneic stem cell transplantation. Br J Haematol. 2001;113(3):814–821. doi: 10.1046/j.1365-2141.2001.02789.x. [DOI] [PubMed] [Google Scholar]
- 25.Eurelings M, Notermans NC, Lokhorst HM, van KB, Jacobs BC, Wokke JH, et al. Immunoglobulin gene analysis in polyneuropathy associated with IgM monoclonal gammopathy. J Neuroimmunol. 2006;175(1–2):152–159. doi: 10.1016/j.jneuroim.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Kobata T, Jacquot S, Kozlowski S, Agematsu K, Schlossman SF, Morimoto C. CD27-CD70 interactions regulate B-cell activation by T cells. Proc Natl Acad Sci U S A. 1995;92(24):11249–11253. doi: 10.1073/pnas.92.24.11249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lens SM, de JR, Hooibrink B, Koopman G, Pals ST, van Oers MH, et al. Phenotype and function of human B cells expressing CD70 (CD27 ligand) Eur J Immunol. 1996;26(12):2964–2971. doi: 10.1002/eji.1830261223. [DOI] [PubMed] [Google Scholar]
- 28.Jung J, Choe J, Li L, Choi YS. Regulation of CD27 expression in the course of germinal center B cell differentiation: the pivotal role of IL-10. Eur J Immunol. 2000;30(8):2437–2443. doi: 10.1002/1521-4141(2000)30:8<2437::AID-IMMU2437>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 29.Klein U, Kuppers R, Rajewsky K. Evidence for a large compartment of IgM-expressing memory B cells in humans. Blood. 1997;89(4):1288–1298. [PubMed] [Google Scholar]
- 30.Orange JS. Human natural killer cell deficiencies and susceptibility to infection. Microbes Infect. 2002;4(15):1545–1558. doi: 10.1016/s1286-4579(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 31.Koch S, Larbi A, Derhovanessian E, Ozcelik D, Naumova E, Pawelec G. Multiparameter flow cytometric analysis of CD4 and CD8 T cell subsets in young and old people. Immun Ageing. 2008;5:6. doi: 10.1186/1742-4933-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Makedonas G, Hutnick N, Haney D, Amick AC, Gardner J, Cosma G, et al. Perforin and IL-2 upregulation define qualitative differences among highly functional virus-specific human CD8 T cells. PLoS Pathog. 2010;6(3):e1000798. doi: 10.1371/journal.ppat.1000798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joncas J, Monczak Y, Ghibu F, Alfieri C, Bonin A, Ahronheim G, et al. Brief report: killer cell defect and persistent immunological abnormalities in two patients with chronic active Epstein-Barr virus infection. J Med Virol. 1989;28(2):110–117. doi: 10.1002/jmv.1890280211. [DOI] [PubMed] [Google Scholar]
- 34.Rigaud S, Fondaneche MC, Lambert N, Pasquier B, Mateo V, Soulas P, et al. XIAP deficiency in humans causes an X-linked lymphoproliferative syndrome. Nature. 2006;444(7115):110–114. doi: 10.1038/nature05257. [DOI] [PubMed] [Google Scholar]
- 35.Okano M, Matsumoto S, Osato T, Sakiyama Y, Thiele GM, Purtilo DT. Severe chronic active Epstein-Barr virus infection syndrome. Clin Microbiol Rev. 1991;4(1):129–135. doi: 10.1128/cmr.4.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marsh RA, Madden L, Kitchen BJ, Mody R, McClimon B, Jordan MB, et al. XIAP deficiency: a unique primary immunodeficiency best classified as X-linked familial hemophagocytic lymphohistiocytosis and not as X-linked lymphoproliferative disease. Blood. 2010;116(7):1079–1082. doi: 10.1182/blood-2010-01-256099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rezaei N, Mahmoudi E, Aghamohammadi A, Das R, Nichols KE. X-linked lymphoproliferative syndrome: a genetic condition typified by the triad of infection, immunodeficiency and lymphoma. Br J Haematol. 2011;152(1):13–30. doi: 10.1111/j.1365-2141.2010.08442.x. [DOI] [PubMed] [Google Scholar]
- 38.Savoldo B, Huls MH, Liu Z, Okamura T, Volk HD, Reinke P, et al. Autologous Epstein-Barr virus (EBV)-specific cytotoxic T cells for the treatment of persistent active EBV infection. Blood. 2002;100(12):4059–4066. doi: 10.1182/blood-2002-01-0039. [DOI] [PubMed] [Google Scholar]
- 39.Xiao Y, Hendriks J, Langerak P, Jacobs H, Borst J. CD27 is acquired by primed B cells at the centroblast stage and promotes germinal center formation. J Immunol. 2004;172(12):7432–7441. doi: 10.4049/jimmunol.172.12.7432. [DOI] [PubMed] [Google Scholar]
- 40.Xiao Y, Peperzak V, Keller AM, Borst J. CD27 instructs CD4+ T cells to provide help for the memory CD8+ T cell response after protein immunization. J Immunol. 2008;181(2):1071–1082. doi: 10.4049/jimmunol.181.2.1071. [DOI] [PubMed] [Google Scholar]
- 41.Peperzak V, Xiao Y, Veraar EA, Borst J. CD27 sustains survival of CTLs in virus-infected nonlymphoid tissue in mice by inducing autocrine IL-2 production. J Clin Invest. 2010;120(1):168–178. doi: 10.1172/JCI40178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matter MS, Claus C, Ochsenbein AF. CD4+ T cell help improves CD8+ T cell memory by retained CD27 expression. Eur J Immunol. 2008;38(7):1847–1856. doi: 10.1002/eji.200737824. [DOI] [PubMed] [Google Scholar]
- 43.Taraban VY, Martin S, Attfield KE, Glennie MJ, Elliott T, Elewaut D, et al. Invariant NKT cells promote CD8+ cytotoxic T cell responses by inducing CD70 expression on dendritic cells. J Immunol. 2008;180(7):4615–4620. doi: 10.4049/jimmunol.180.7.4615. [DOI] [PubMed] [Google Scholar]
- 44.Peperzak V, Xiao Y, Veraar EA, Borst J. CD27 sustains survival of CTLs in virus-infected nonlymphoid tissue in mice by inducing autocrine IL-2 production. J Clin Invest. 2010;120(1):168–178. doi: 10.1172/JCI40178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De C V, Taveirne S, Delforche M, De SM, Vandekerckhove B, Taghon T, et al. CD27-deficient mice show normal NK-cell differentiation but impaired function upon stimulation. Immunol Cell Biol. 2011 doi: 10.1038/icb.2010.171. [DOI] [PubMed] [Google Scholar]
- 46.Quan TE, Roman RM, Rudenga BJ, Holers VM, Craft JE. Epstein-Barr virus promotes interferon-alpha production by plasmacytoid dendritic cells. Arthritis Rheum. 2010;62(6):1693–1701. doi: 10.1002/art.27408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wehr C, Kivioja T, Schmitt C, Ferry B, Witte T, Eren E, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111(1):77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 48.Sallusto F, Langenkamp A, Geginat J, Lanzavecchia A. Functional subsets of memory T cells identified by CCR7 expression. Curr Top Microbiol Immunol. 2000;251:167–171. doi: 10.1007/978-3-642-57276-0_21. [DOI] [PubMed] [Google Scholar]


