Abstract
Formins have conserved roles in cell polarity and cytokinesis and directly nucleate actin filament assembly through their FH2 domain. Here, we define the active region of the yeast formin Bni1 FH2 domain and show that it dimerizes. Mutations that disrupt dimerization abolish actin assembly activity, suggesting that dimers are the active state of FH2 domains. The Bni1 FH2 domain protects growing barbed ends of actin filaments from vast excesses of capping protein, suggesting that the dimer maintains a persistent association during elongation. This is not a species-specific mechanism, as the activities of purified mammalian formin mDia1 are identical to those of Bni1. Further, mDia1 partially complements BNI1 function in vivo, and expression of a dominant active mDia1 construct in yeast causes similar phenotypes to dominant active Bni1 constructs. In addition, we purified the Bni1-interacting half of the cell polarity factor Bud6 and found that it binds specifically to actin monomers and, like profilin, promotes rapid nucleotide exchange on actin. Bud6 and profilin show additive stimulatory effects on Bni1 activity and have a synthetic lethal genetic interaction in vivo. From these results, we propose a model in which Bni1 FH2 dimers nucleate and processively cap the elongating barbed end of the actin filament, and Bud6 and profilin generate a local flux of ATP-actin monomers to promote actin assembly.
INTRODUCTION
Formins, a conserved family of proteins defined by formin homology (FH) domains, are required for the establishment and maintenance of cell polarity and cytokinesis (reviewed in Frazier and Field, 1997; Evangelista et al., 2003; Wallar and Alberts, 2003). In the budding yeast Saccharomyces cerevisiae, two formin proteins (Bni1 and Bnr1) drive the assembly of actin cables and the cytokinetic ring (Evangelista et al., 2002; Sagot et al., 2002a; Tolliday et al., 2002). In fission yeast, the formin For3p is required for the formation of interphase actin cables (Feierbach and Chang, 2001) and together with the formin Cdc12p is required for the formation and proper constriction of the cytokinetic ring (Chang et al., 1997; Pelham and Chang, 2002). In animal cells, formins are similarly involved in the assembly of specialized F-actin-containing structures, such as stress fibers and the cytokinetic ring (Wasserman, 1998; Watanabe et al., 1999; Tominaga et al., 2000; Severson et al., 2002).
Several recent studies have shown that purified carboxyl terminal fragments of the budding yeast formin Bni1 and the fission yeast Cdc12p stimulate actin filament assembly in vitro (Pruyne et al., 2002; Sagot et al., 2002b; Kovar et al., 2003). Both of these formins associate with the barbed ends of actin filaments (Pruyne et al., 2002; Kovar et al., 2003; Pring et al., 2003); however, the mechanism of filament growth may differ. Studies on Bni1 concluded that it forms a specialized barbed end association, acting as a processive cap to allow filament elongation at the barbed end. Studies on Cdc12p concluded that it acts as a standard barbed end capping protein, limiting filament growth to the pointed end, until it is “gated” by profilin to allow growth at the barbed end. Many open questions about formin activities remain. What is the minimal active region of the FH2 domain of formins? What are its biophysical properties? How do formins maintain an association with a barbed end that is growing rapidly? How do formin-associated proteins affect formin activities? What are the activities of different mammalian formins, and are they more similar in function to Bni1 or Cdc12p?
The inherent actin nucleation function of Bni1 is mediated by its FH2 domain in vitro (Pruyne et al., 2002; Sagot et al., 2002b), but regions flanking the FH2 domain contribute to the assembly of polarized actin cables in vivo (Evangelista et al., 2002; Sagot et al., 2002a). The actin monomer binding protein profilin is required in vivo for the assembly of actin cables (Haarer et al., 1990; Wolven et al., 2000; Evangelista et al., 2002). Profilin stimulates formin-induced actin assembly in vitro, which requires its interactions with the FH1 domain and with actin monomers (Sagot et al., 2002b; Kovar et al., 2003; Pring et al., 2003).
Another potential regulator of the yeast formin Bni1 is Bud6/Aip3, first identified in a two-hybrid screen as an actin interacting protein (Amberg et al., 1995). Bud6 interacts by two-hybrid assay with a carboxyl terminal region of Bni1 (Evangelista et al., 1997; also see Figure 1) and has a similar in vivo localization to Bni1 (Amberg et al., 1997; Ozaki-Kuroda et al., 2001; Segal et al., 2002). Genetic evidence suggests that Bud6 has an important role in cell polarity and actin cable organization (Amberg et al., 1997; Glynn et al., 2001; Evangelista et al., 2002; Sagot et al., 2002a), but the biochemical activities of Bud6 have remained elusive, leaving its exact function unclear.
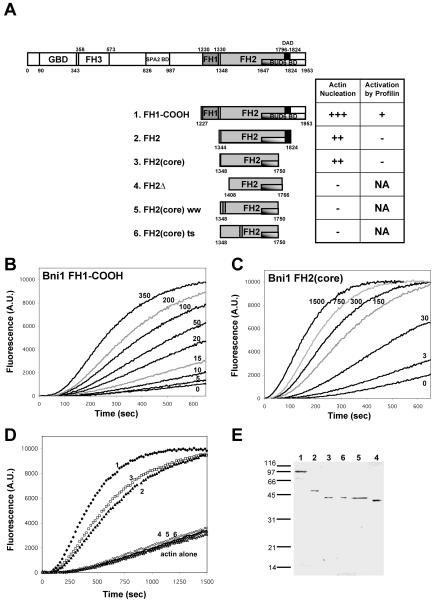
Figure 1.
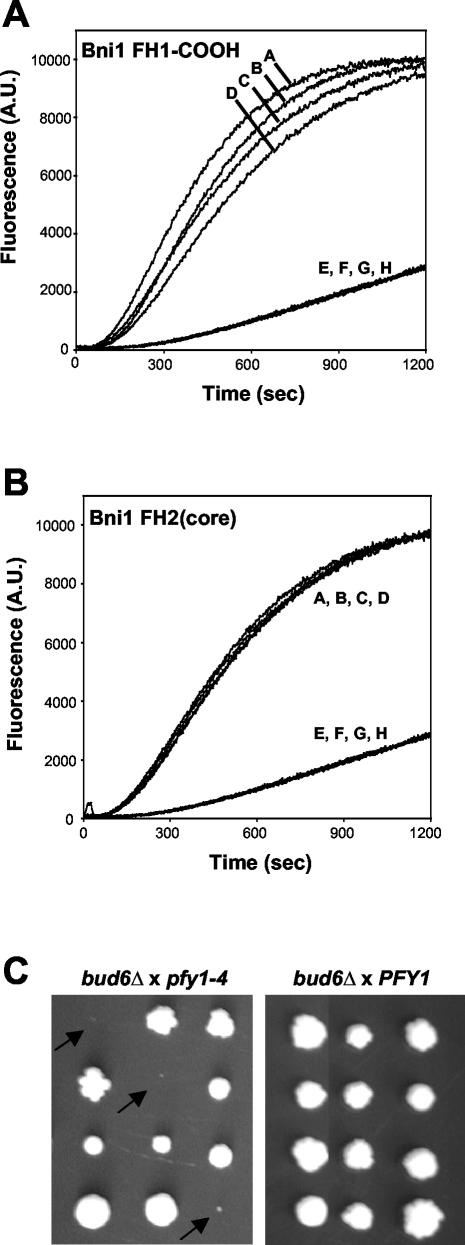
Mapping the core activity of the Bni1 FH2 domain. (A) Schematic diagram of Bni1 domain organization and Bni1 polypeptides tested. FH, formin homology; GBD, Rho GTPase binding domain; BD, binding domain; DAD, diaphanous autoregulatory domain; NA, not applicable. The bars in constructs 5 and 6 mark the residues mutated (W1363A and W1374A for construct 5; R1528A and R1530A for construct 6). (B and C) 2 μM actin (1% pyrene-labeled) was assembled in the presence of the indicated concentration (nM) of (B) Bni1 GST-FH1-COOH or (C) Bni1 FH2(core). (D) 2 μM actin (1% pyrene-labeled) was assembled in the presence of 100 nM of each Bni1 construct: FH1-COOH (curve 1: ♦); FH2 (curve 2: □); FH2(core) (curve 3: ▴); FH2(core) ts mutant (curve 6: ○); FH2(core) ww mutant (curve 5: ▵); FH2Δ (curve 4: ×). (E) Coomassie-stained SDS-PAGE gel showing purified Bni1 fragments (100-200 ng each). Lanes correspond to construct numbers in A.
Here, we show that the Bni1 FH2 domain dimerizes, which has important implications for how formins maintain association with the growing end of an actin filament. Further, the FH2 dimers protect elongating barbed ends from the inhibitory effects of excess capping protein, demonstrating their persistent association with the growing filament ends. Activities similar to Bni1 were observed for mouse formin mDia1 in vitro and in vivo, demonstrating the strong evolutionary conservation of this mechanism. We also report a striking and unexpected parallel between the cellular functions of two Bni1-interacting proteins, Bud6 and profilin. The purified carboxyl terminal half of Bud6 (C-Bud6) binds to actin monomers and dramatically accelerates nucleotide exchange on actin. C-Bud6 and profilin additively stimulate Bni1-mediated actin assembly in vitro, and pfy1-4 and bud6Δ mutations are synthetic lethal in vivo. Thus, central to the formin mechanism in vivo is that two actin monomer binding proteins/actin nucleotide exchange factors interact on either side of the FH2 domain and stimulate formin activity.
MATERIALS AND METHODS
Yeast Strains, Media, and DNA Manipulations
Standard methods were used for DNA manipulations and for yeast growth, sporulation, and transformation (Rose et al., 1989). For testing mDia1 function in yeast cells (Figure 2), strains previously described were used: bni1-FH2#1 bnr1Δ (PY3744) and bni1Δ bnr1Δ carrying the plasmid 2μ URA3 BNI1 (PY4315) (Sagot et al., 2002b). These and the bud6Δ strain are isogenic to the wild-type BY4741 strain from Research Genetics (Invitrogen, Carlsbad, CA). The pfy1-4 yeast strain (DDY2008) has been described (Wolven et al., 2000).
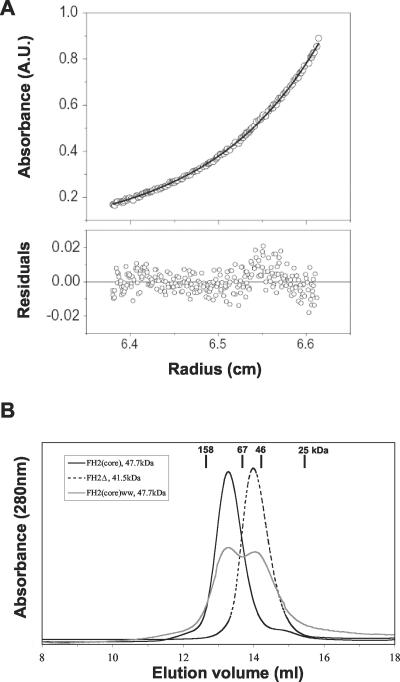
Figure 2.
Dimerization of Bni1 FH2 domain polypeptides. (A) Sedimentation equilibrium analysis of the Bni1 FH2(core) polypeptide (0.4 mg/ml) by analytical ultracentrifugation. The data points define a concentration distribution expected for a single species of 93,000 g/mol (solid black line), confirming the dimeric nature of the Bni1 FH2 domain. The bottom panel shows the distribution of the residuals for the single exponential fit. (B) Analytical gel filtration analysis of Bni1 polypeptides. Bni1 FH2(core) elutes at 13.3 ml, suggesting a molecular weight of 100 kDa (consistent with dimerization). Bni1 FH2Δ elutes at 14.0 ml, corresponding to a molecular weight of 50 kDa (consistent with a monomer). Bni1 FH2(core) ww elutes at two different peaks, one at a dimer position (13.3 ml) and one at a monomer position (14.0 ml).
For mDia1 expression in Escherichia coli and yeast, the coding sequence of mDia1, residues 739-1255 (FH2-COOH) or residues 553-1255 (FH1-COOH), were PCR amplified from pEGFP-mDia1 (a gift from T. Fujiwara). The PCR products were subcloned into either pET-GST-TEV (pET derivative for expression in E. coli, which has a TEV protease site inserted after GST) or a pRS415 derivative (a LEU2, CEN vector with an insertion of the yeast actin [ACT1] promoter; Christianson et al., 1992). For expression of Bni1 fragments in E. coli, the appropriate coding sequences were PCR amplified and subcloned into pET-GST-TEV. To generate the FH2(core) ww mutant, site-directed mutagenesis was performed using a PCR-based method to generate Trp1363(TGG) to Ala(GCG) and Trp1374(TGG) to Ala(GCC) substitutions. FH2(core) ts contains two mutations (R1528A and R1530A) previously described in Sagot et al. (2002a). For complementation analysis, we used pB1005 (URA3, 2μ) and pB1006 (LEU2, CEN). The latter, a generous gift from J. Pringle and M. Longtine, allows expression of full-length BNI1 under the control of its own promoter. pBNI1-mini (LEU2, CEN) encodes a fusion protein between protein A and amino acid residues 1215-1750 of Bni1 under the control of the ACT1 promoter. For expression of C-Bud6 in E. coli, the sequence encoding amino acids 489-788 of Bud6 was PCR amplified from genomic DNA and subcloned into pET-GST-TEV. All DNA constructs were verified by sequencing. Details of the constructs are available upon request.
Imaging of Actin Organization in Yeast Cells
Alexa-568-phalloidin staining and imaging of yeast cells was performed as described previously (Sagot et al., 2002a).
Protein Purification
Rabbit skeletal muscle actin, pyrene-labeled rabbit skeletal muscle actin, and human profilin were purchased from Cytoskeleton (Denver, CO). Yeast capping protein, cofilin, and profilin were purified by methods described (Amatruda and Cooper, 1992; Lappalainen and Drubin, 1997; Wolven et al., 2000). Mouse mDia1 and yeast Bni1 proteins were expressed as GST-fusion proteins in the E. coli strain BL21-Codon Plus (DE3)-RP (Stratagene, La Jolla, CA), which were grown at 37°C to log phase, then induced by addition of 0.2 mM IPTG, and grown for 16 h at 25°C. Cells were pelleted and resuspended in PBS buffer supplemented with a protease inhibitor mixture (final concentration of 1 mM PMSF and 0.5 μM each of antipain, leupeptin, pepstatin A, chymostatin, aprotinin) and then lysed by sonication. The lysate was cleared by centrifugation at 17,000 × g, and GST-fusion proteins were purified using glutathione-conjugated agarose beads (Sigma, St. Louis, MO). After washing beads into HEK buffer (20 mM HEPES, pH 7.5, 1 mM EDTA, 50 mM KCl), Bni1 or mDia1 fragments were released by digestion with recombinant TEV protease (Invitrogen), then further purified by size exclusion chromatography. Peak fractions were concentrated in a Microcon-10 device (Millipore, Billerica, MA). Cleavage of GST from the Bni1 construct FH1-COOH was inefficient, so we used the GST-fusion protein for biochemical assays. The presence of GST on this construct has been shown previously to have no effect on its activity (Pruyne et al., 2002).
The carboxyl terminal fragment of Bud6 (C-Bud6) was purified as a GST fusion protein by methods similar to above. After release from glutathione agarose by digestion with TEV protease, C-Bud6 was purified further on a Mono Q (5/5) anion exchange column (AP Biotech, Piscataway, NJ). Proteins were eluted from the column in HEK buffer and a linear gradient of KCl (0.1-0.5 M); C-Bud6 eluted at ∼250 mM KCl. Peak fractions were concentrated and exchanged into HEK buffer by multiple rounds of concentration and dilution in a Microcon-10 device.
Actin Filament Assembly and Elongation Assays
Actin filament assembly assays were performed essentially as described (Humphries et al., 2002). Briefly, 42 μl of monomeric actin (final concentration in reactions 2 μM, 1% pyrene-labeled) was mixed with 15 μl of HEK buffer or proteins in HEK buffer (Bni1, mDia1, profilin, and/or C-Bud6) and then mixed with 3 μl of 20× initiation mix (40 mM MgCl2, 10 mM ATP, 1 M KCl). Pyrene fluorescence was monitored at excitation 365 nm and emission 407 nm in a fluorescence spectrophotometer (Photon Technology International, Lawrenceville, NJ). In actin filament elongation assays, 35 μl preformed actin filaments (1 μM final) were mixed with 15 μl HEK buffer alone or premixed proteins in HEK buffer. The actin filaments were sheared by five passages through a 27-gauge needle and syringe. After 10 s, 20 μl of sheared filaments (333 nM final) were added to 40 μl actin monomers (0.5 μM final, 1% pyrene labeled) in F-buffer to initiate filament elongation (10 mM Tris, pH 7.5, 0.7 mM ATP, 0.2 mM CaCl2, 2 mM MgCl2, 50 mM KCl, 0.2 mM DTT). Pyrene fluorescence was monitored as above. The relative rates of actin assembly and elongation were determined from the slopes of the reaction curves during the early phase (first 200 s) of actin polymerization.
Analytical Ultracentrifugation and Gel Filtration Analyses
Sedimentation equilibration was performed in a Beckman XLA analytical ultracentrifuge using an An-Ti60 rotor and 6-channel cells. Samples (110 μl; 0.2, 0.4, 0.7 mg/ml) were centrifuged at 10,000 and 13,000 rpm for 24 h at 4°C in HND buffer (20 mM HEPES, pH 7.5, 200 mM NaCl, 2 mM DTT). Absorbance scans were obtained at 280 nm. The calculated partial specific volume for FH2(core) was 0.7299 ml/g and buffer density was 1.007. Concentration profiles were analyzed by SEDFIT85 (Lebowitz et al., 2002).
Gel filtration analysis was performed using a Superdex 200 (10/30) column (AP Biotechnology) equilibrated in HND buffer. Elution peaks were monitored by absorbance at 280 nm and verified by SDS-PAGE of column fractions. The size standards used were aldolase (158 kDa), albumin (67 kDa), focal adhesion kinase FERM fragment (46 kDa), and chymotrypsinogen A (25 kDa).
Actin Monomer Binding and Nucleotide Exchange Assays
To measure rates of nucleotide exchange on actin monomers, 57.2 μl G-buffer (10 mM Tris, pH 7.5, 0.2 mM CaCl2, 0.2 mM DTT, no ATP) containing actin (2.5 μM final) was mixed with 10 μl HEK buffer or proteins in HEK buffer (profilin, cofilin, or C-Bud6) and added to 2.8 μl etheno-ATP (50 μM final). The reaction was monitored at 25°Cina fluorescence spectrophotometer as above. Nucleotide exchange rates were determined from the initial, linear slopes of the curves. For native gel electrophoresis, C-Bud6 and actin (each 10 μM final) were mixed for 15 min and then fractionated on 7.5% native gels as described (Safer, 1989).
RESULTS
Defining the Active Region of the Bni1 FH2 Domain
Based on the sequence alignment of multiple formins, the boundaries of the Bni1 FH2 domain have been defined previously as amino acid residues 1348-1824, and this region alone induces actin assembly in vitro (Pruyne et al., 2002). To better define the active portion of the FH2 domain, we expressed a large series of constructs spanning residues 1327-1953, some of which are shown in Figure 1A. The shortest soluble construct with actin assembly-promoting activity was Bni1 FH2(core) (residues 1348-1750). This fragment had equivalent actin nucleation activity to that of a longer 1344-1824 FH2 domain (Figure 1D, curves 2 and 3). As previously shown for the 1348-1824 FH2 domain (Pruyne et al., 2002), FH2(core) displayed a slightly lower actin nucleation activity than FH1-COOH (compare Figure 1B and 1C; also see Figure 1D). The mutations R1528A and R1530A, which confer thermosensitivity to Bni1 for actin cable formation in vivo (Sagot et al., 2002a), abolished actin nucleation activity of the FH2(core) (Figure 1D, curve 6). This demonstrates that the biochemical activities of the FH2 domain correlate with its in vivo functions.
Analysis of FH2(core) and other Bni1 constructs using a panel of proteases revealed a chymotrypsin cleavage site at Leu1407 (our unpublished results), which led us to generate the FH2Δ fragment (1408-1766). This truncation abolished actin nucleation activity (Figure 1D, curve 4). Sequence alignments across species showed that this 60 amino acid region (1348-1408) contains two highly conserved tryptophan residues located on the carboxyl-terminal side of the FH1 domain (residues 1363 and 1374 in Bni1). Tryptophan to alanine substitutions at these two sites (W1363A/W1374A) to generate the construct FH2(core) ww abolished actin nucleation activity (Figure 1D, curve 5). These data define residues 1348-1750 as a minimal active region of the FH2 domain and indicate that the sequence 1348-1408, and the two conserved tryptophan residues, play crucial roles in actin assembly.
Active Bni1 FH2 Domain Polypeptides Dimerize
In the course of purifying Bni1 FH2(core), we noted that it eluted from a gel filtration column as a single symmetrical peak at approximately the volume expected for a dimer. We therefore measured its molar mass by analytical ultracentrifugation. Sedimentation equilibrium analysis of FH2(core) yielded a mass of 93,000 g/mol, very close to the mass expected for an FH2(core) dimer (95,400 g/mol) based on its primary sequence (Figure 2A). Using analytical gel filtration, we compared the elution profiles of FH2(core), FH2Δ, and FH2(core) ww. In contrast to the active FH2(core) dimers, the inactive FH2Δ construct behaved as a monomer by gel filtration (Figure 2B). Furthermore, the inactive FH2(core) ww polypeptide was impaired for dimerization; its migration as a mixed population suggests that this mutant forms an unstable dimer. These data demonstrate that residues 1348-1407 are critical for dimerization of the FH2 domain and that W1363 and W1374 are required for stable dimerization. There is also a correlation between ability to form stable dimers and ability to promote actin assembly. Taken together, these data suggest that the dimer is the functional state of formins. This conclusion is supported by our observations that a Bni1 fragment similar to the FH2(core) polypeptide crystallizes as a dimer (Y.X., I.S., J.M., B.G., D.P. and M.E., unpublished results).
Bni1 Dimers Processively Cap the Growing Barbed Ends of Actin Filaments and Block the Inhibitory Effects of Excess Capping Protein
Bni1 associates with the barbed ends of actin filaments with an apparent Kd of 20 nM and reduces only modestly the rate of barbed end elongation (Pruyne et al., 2002; Pring et al., 2003). The addition of higher concentrations of Bni1 (600 nM) fails to reduce further the rate of elongation, suggesting Bni1 forms a processive cap that maintains association with the barbed end during filament elongation. As an additional test of this mechanism, we assayed the ability of Bni1 to associate with growing barbed ends of filaments in the presence of capping protein, which terminates barbed end elongation. We reasoned that if Bni1 maintains a strong and persistent association with barbed ends during filament growth, it should be able to protect ends from the inhibitory effects of excess capping protein.
First, we measured the rate of actin filament elongation at barbed ends upon addition of 0.5 μM monomeric actin to preformed actin filament seeds (free barbed-end concentration ∼0.3 nM). As expected, yeast capping protein inhibited elongation in a dose-dependent manner (Figure 3A). Half maximal inhibition occurred at 20 nM capping protein, defining this as the apparent Kd for association with the barbed ends of filaments, and 500 nM capping protein abolished elongation (Figure 3B). The affinity of yeast capping protein for actin filament barbed ends has not previously been determined, and our data show that it is over an order of magnitude weaker than mammalian CapZ isoforms (Caldwell et al., 1989; Schafer et al., 1996). These differences in affinity may reflect varying requirements for barbed end dynamic behavior in different organisms and cell types.
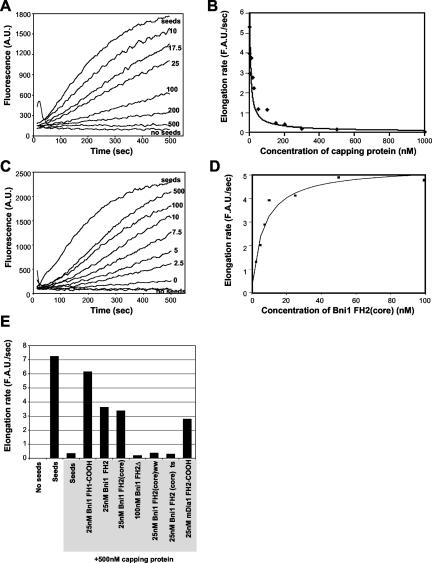
Figure 3.
Bni1 protects the barbed ends of actin filaments from capping protein. (A) Monomeric actin (0.5 μM; 1% pyrene labeled) was polymerized at the barbed ends of mechanically sheared actin filament seeds (333 nM) in the presence of the indicated concentrations (nM) of yeast capping protein. Complete inhibition of actin filament elongation was reached at 500 nM capping protein. (B) The data from A were graphed as rate of filament elongation vs. concentration of capping protein. Half-maximal inhibition of elongation occurred at 20 nM capping protein. (C) Bni1 FH2(core) protects filament barbed ends from 500 nM capping protein in a dose-responsive manner. Actin monomers, 0.5 μM, were assembled as above on actin filament seeds sheared in the presence of 500 nM capping protein and the indicated concentrations (nM) of Bni1 FH2(core). The unlabeled curve at the bottom, paralleling the “no seed” curve, demonstrates that 500 nM FH2(core) has no effect on the rate of assembly of 0.5 μM actin in the absence of filament seeds. (D) The rates of filament elongation from C were plotted vs. the concentration of Bni1 FH2(core). Half-maximal protection occurred at 7 nM Bni1 FH2(core). (E) Rates of filament elongation, calculated as in C, for different Bni1 and mDia1 constructs in the presence of 500 nM capping protein. Shaded labels indicate reactions that contain 500 nM capping protein.
This assay monitors the rate of actin filament growth specifically at the barbed ends, as indicated by three lines of evidence. First, capping protein abolishes filament elongation in the assay. Because capping protein terminates elongation specifically at filament barbed ends, we conclude that under these conditions polymerization is limited to barbed ends. Second, addition of 5 μM profilin had no effect on the rate of filament elongation (our unpublished results). Because profilin binds to actin monomers and prohibits their addition to the pointed ends of filaments (Pollard and Cooper, 1984), this means that pointed end elongation does not make a detectable contribution in the assay. Third, the total actin concentration in the elongation reactions is 0.8 μM, and the critical concentration for growth of actin filament pointed ends is 0.5-0.8 μM (Pollard and Cooper, 1986; Rickard and Sheterline, 1986; Pollard et al., 2000). These three points clearly demonstrate that pointed end growth is not being measured.
Next, we tested the ability of Bni1 to protect filament ends from capping protein by premixing filament seeds with a combination of 500 nM capping protein and varying concentrations of Bni1 FH2(core) and then adding actin monomers to initiate elongation. As shown in Figure 3, C and D, Bni1 FH2(core) rescued barbed end elongation in a dose-dependent manner, demonstrating that the formin protects filament ends from capping protein while allowing rapid elongation. The rate of elongation of FH2(core)-capped filaments was only slightly reduced compared with uncapped filaments in agreement with previous reports (Pruyne et al., 2002; Pring et al., 2003). In a control experiment, 500 nM FH2(core) did not promote filament assembly in the absence of actin filament seeds (Figure 3C), indicating that in this assay, the effects of FH2(core) are specific to elongation and cannot be attributed to nucleation of new filaments.
The half maximal effect for protecting filament ends from 500 nM capping protein was 7 nM Bni1 FH2(core) (Figure 3D). This indicates a very strong association of the FH2 domain with the barbed end, significantly greater than capping protein, which may provide a key part of the mechanism in cells that allows elongation of particular actin structures (e.g., yeast cables) in the presence of capping protein. These data provide complementary support for the proposed mechanism of Pruyne et al. (2002), that Bni1 maintains a persistent association with the barbed end during filament growth. As shown in Figure 3E, all of the Bni1 polypeptides that were active for actin nucleation also were active in protecting barbed ends of filaments from capping protein, and polypeptides that were defective in actin nucleation did not show barbed end protection activity. Thus, there is a close correlation between the activities in Bni1 required for actin assembly and the ability of Bni1 to associate with the growing barbed end.
Bud6 Binds to Actin Monomers and Accelerates Nucleotide Exchange on Actin
In previous studies, we and others have shown that profilin binds to the FH1 domain of Bni1 and stimulates Bni1-induced actin assembly in vitro (Sagot et al., 2002b; Pring et al., 2003). Profilin is also required for formation and maintenance of formin-dependent actin cables in vivo (Haarer et al., 1990; Wolven et al., 2000; Evangelista et al., 2002), suggesting that the cellular mechanism of formin-induced actin assembly involves regulation by formin-associated proteins. Here, we addressed the role of another Bni1-interacting protein, Bud6. Full-length Bud6 expressed in E. coli was insoluble and did not refold following urea denaturation and dialysis into aqueous buffers. However, we were able to purify a soluble carboxyl terminal fragment of Bud6 (C-Bud6; residues 489-788) that encompasses the domains interacting with actin and Bni1 by two-hybrid assay (Figure 4A; Amberg et al., 1995; Evangelista et al., 1997).
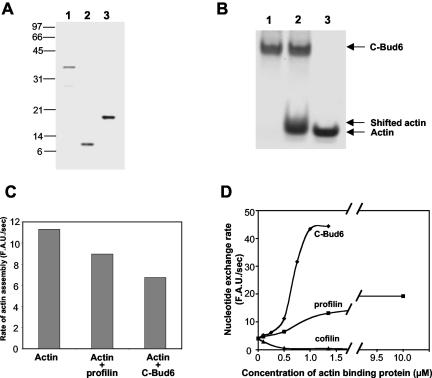
Figure 4.
The carboxyl terminus of Bud6/Aip3 binds to actin monomers and promotes nucleotide exchange. (A) SDS-PAGE gel stained with Coomassie blue showing purified proteins use for these experiments: lane 1, 100 ng C-Bud6 (residues 489-788); lane 2, 100 ng yeast profilin; lane 3, 200 ng yeast cofilin. (B) Coomassie-stained native gel showing the interaction of C-Bud6 with actin monomers: lane 1, 10 μM C-Bud6; lane 2, 10 μM C-Bud6 and 10 μM actin monomers; lane 3, 10 μM actin monomers. (C) Actin monomer binding proteins profilin and C-Bud6 reduce the rate of actin assembly. The rates of actin assembly (2 μM actin, 1% pyrene labeled) alone and in the presence of 1.5 μM profilin or 1.5 μM C-Bud6 were compared during the early linear phase of polymerization. (D) Rate of nucleotide exchange on 2.5 μM actin monomers in the presence of varying concentrations of C-Bud6, profilin, or cofilin. The rate of nucleotide exchange was determined from the change in etheno-ATP fluorescence upon binding to actin (see MATERIALS AND METHODS).
Bud6 was identified as an actin interacting protein by the two hybrid assay, but it has not previously been shown whether it has biochemical affinity for actin, and if so, whether it prefers actin monomers or filaments. C-Bud6 showed no affinity for actin filaments in cosedimentation assays (our unpublished results), but interacted with actin monomers in three independent assays. First, C-Bud6 retarded the migration of monomeric actin in native gels (Figure 4B), causing a modest but reproducible shift (see lane 2, Figure 4B). This result is consistent with a dynamic association between C-Bud6 and actin monomers under these conditions, similar to the reported profilin-actin interaction (Mockrin and Korn, 1980). Second, 1.5 μM C-Bud6 inhibited the maximal rate of 2 μM actin assembly in spontaneous actin assembly reactions and did so more efficiently than 1.5 μM yeast profilin (Figure 4C). This activity is common to many actin monomer-binding proteins and reflects their ability to suppress spontaneous actin nucleation by interfering with G-actin associations required to form polymerization intermediates. Third, and perhaps most significantly, C-Bud6 affected the rate of nucleotide exchange on actin monomers (Figure 4D). Profilin and CAP (cyclase associated protein) are the only two other actin monomer binding proteins previously shown to promote nucleotide exchange on actin (Eads et al., 1998; Wolven et al., 2000; Moriyama and Yahara, 2002). Many other actin monomer-binding proteins inhibit nucleotide exchange on actin (e.g., cofilin and twinfilin; Lappalainen et al., 1997; Goode et al., 1998). As expected, cofilin inhibited nucleotide exchange and profilin promoted nucleotide exchange on actin. C-Bud6 increased the rate of nucleotide exchange approximately three times more efficiently than profilin. The observation that profilin and Bud6 each have this unique activity and each associate with Bni1 suggests that their cellular functions may be highly related.
Bud6 and Profilin Additively Stimulate Bni1-mediated Actin Assembly and Share a Genetically Overlapping Function
Next, we tested the ability of C-Bud6 to affect Bni1-induced actin assembly. Because Bud6 interacts by two-hybrid assay with the carboxyl terminus of Bni1, residues 1647-1954 (Evangelista et al., 1997), we tested the effects of C-Bud6 on Bni1 FH1-COOH, which contains the Bud6 binding domain (see Figure 1A). C-Bud6 reproducibly stimulated the actin assembly activity of 25 nM FH1-COOH, with a maximal effect at 50 nM C-Bud6 (Figure 5A). At higher concentrations of C-Bud6, the inhibitory effect from actin monomer sequestering reduced the overall rate of actin assembly in a formin-independent manner. Yeast profilin (200 nM) also stimulated actin assembly mediated by Bni1 FH1-COOH, consistent with previous reports (Sagot et al., 2002b; Pring et al., 2003). As shown in curve A (Figure 5A), 200 nM profilin and 50 nM C-Bud6 together reproducibly stimulated the activity of Bni1 FH1-COOH in an additive manner, and importantly, at these concentrations they did not affect the rate of assembly of actin alone (no Bni1 present). Further, C-Bud6 and profilin had no activity on Bni1 FH2(core), which lacks the FH1 domain (profilin binding site) and a portion of the Bud6-binding domain (Figure 5B). Therefore, the ability of C-Bud6 and profilin to stimulate Bni1 activity requires the presence of their known binding sites on Bni1.
Figure 5.
C-Bud6 and profilin enhance Bni1-induced actin assembly in an additive manner. (A) Actin (2 μM, 1% pyrene labeled) was assembled in the presence of 25 nM FH1-COOH (curve D), 25 nM FH1-COOH + 200 nM profilin (curve C), 25 nM FH1-COOH + 50 nM C-Bud6 (curve B), or 25 nM FH1-COOH + 200 nM profilin + 50 nM C-Bud6 (curve A). Control curves (E-H) show assembly of 2 μM actin alone, 2 μM actin + 200 nM profilin, 2 μM actin + 50 nM C-Bud6, and 2 μM actin + 200 nM profilin + 50 nM C-Bud6, respectively. (B) Actin was assembled as in A, in the presence of 100 nM FH2(core) (curve A), 100 nM FH2(core) + 200 nM profilin (curve B), 100 nM FH2(core) + 50 nM C-Bud6 (curve C), 100 nM FH2(core) + 200 nM profilin + 50 nM C-Bud6 (curve D), 200 nM profilin (curve E), 50 nM C-Bud6 (curve F), 200 nM profilin + 50 nM C-Bud6 (curve G), and actin alone (curve H). (C) Genetic dissection of parental diploid strains resulting from two crosses: PFY1 × bud6Δ and pfy1-4 × bud6Δ. Parental strains were sporulated and tetrads were dissected. Growth of the resulting haploid colonies after 3 days of growth at 25°C on rich medium is shown for representative tetrads. Double mutant pfy1-4 bud6Δ cells showed little or no colony growth (arrows).
Given the similarity in biochemical activities between Bud6 and profilin, we tested their genetic interactions in vivo. A bud6Δ strain was crossed to a strain bearing a thermosensitive allele of the yeast profilin gene (pfy1-4) with defects in actin monomer binding and nucleotide exchange (Wolven et al., 2000). The bud6Δ pfy1-4 double mutant cells displayed a severe additive growth defect at 25°C (Figure 5C); 22 tetrads were dissected, and 27 of 27 of the resulting double mutants showed this phenotype. These genetic data support our biochemical data suggesting that profilin and Bud6 act together to promote formin-dependent actin assembly.
The Mammalian Formin mDia1 Promotes Actin Assembly by a Mechanism Similar to Bni1 and Partially Complements Loss of BNI1 Function In Vivo
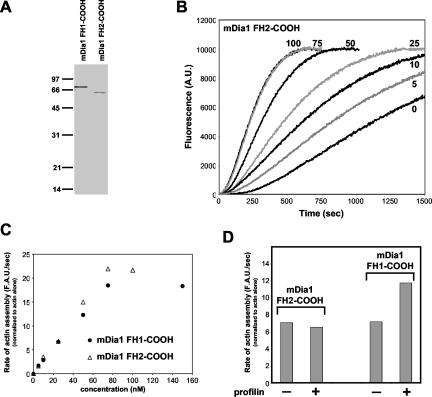
Recent studies have shown that the budding yeast formin Bni1 (Pruyne et al., 2002; Sagot et al., 2002b) and the Schizosaccharomyces pombe formin Cdc12p (Kovar et al., 2003) directly nucleate actin assembly, but by apparently different mechanisms. To address whether mammalian formins have activity similar to either Bni1 or Cdc12p, we purified two fragments of the Diaphanous-related mouse formin mDia1 (Figure 6A). The fragment FH1-COOH (amino acids 553-1255) includes the FH1, FH2, and carboxyl-terminal domains. The fragment FH2-COOH (amino acids 739-1255) includes the FH2 and carboxyl-terminal domains. As shown in Figure 6, B and C, both fragments of mDia1 stimulated the polymerization of mammalian actin in a dose-responsive manner. As previously demonstrated for yeast profilin with Bni1 (Sagot et al., 2002b; Pring et al., 2003), mammalian profilin enhanced the rate of actin polymerization induced by mDia1, and this acceleration required the mDia1 FH1 domain (Figure 6D). Further, mDia1 displayed the same ability to protect growing barbed ends of filaments from termination by excess capping protein (Figure 3E). These data suggest that formin-mediated actin assembly is a highly conserved function.
Figure 6.
mDia1 promotes actin assembly and is activated by profilin. (A) Coomassie-stained SDS-PAGE gel showing purified mDia1 fragments. Lane 1: mDia1 FH1-COOH (200 ng). Lane 2: mDia1 FH2-COOH (200 ng). (B) 2 μM actin (1% pyrene-labeled) was assembled in the presence of the indicated concentrations (nM) of mDia1 FH2-COOH. (C) Rate of actin assembly plotted vs. concentration of mDia1 FH1-COOH or mDia1 FH2-COOH. Rates of actin assembly were calculated from the slopes of the assembly curves during the initial rapid phase of growth and standardized against rate of assembly of actin alone. (D) Rate of actin assembly mediated by 25 nM mDia1 FH1-COOH or mDia1 FH2-COOH in the presence and absence of 300 nM human profilin. Rates were normalized as above to the rate of assembly of actin alone.
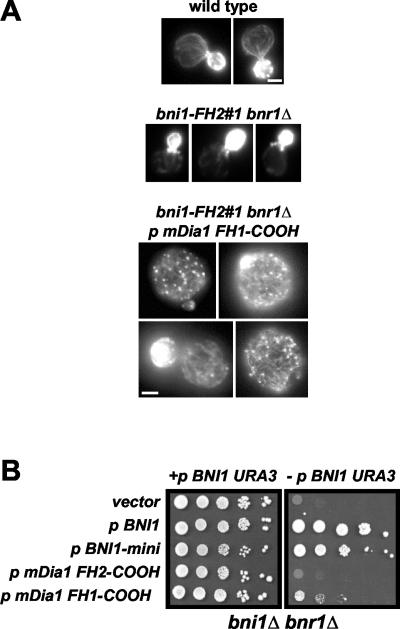
To investigate conservation of formin activity in vivo, we tested whether mDia1 could promote actin filament assembly in yeast. Previously, it has been shown that yeast cells expressing dominant active Bni1 fragments accumulate excess actin cables, resulting in enlarged, depolarized cells (Evangelista et al., 2002; Sagot et al., 2002a). Similarly, the expression of carboxyl terminal constructs of mDia1 in mammalian cells promotes the assembly of excessive filamentous actin (Copeland and Treisman, 2002). To test mDia1 activity in yeast, we expressed the two mDia1 fragments under the control of a constitutive promoter (the yeast ACT1 promoter) in a formin temperature-sensitive mutant yeast strain (bni1-FH2#1 bnr1Δ) (Sagot et al., 2002a). When grown at the permissive temperature, expression of mDia1 FH1-COOH promoted the assembly of numerous filamentous actin structures (Figure 7A, lower panel). Similar to previous in vivo domain analysis on Bni1 (Evangelista et al., 2002; Sagot et al., 2002a), we found that the FH1 domain of mDia1 was required for these effects. In three independent experiments (n > 250 cells), 40 ± 1% of the cells expressing mDia1 FH1-COOH displayed this phenotype, compared with <1% of cells transformed with a control vector (middle panel). These data show that mDia1 can promote the assembly of supernumerary F-actin structures in vivo, consistent with its biochemical activities described above. Further, expression of the FH1-COOH, but not the FH2-COOH, fragment of mDia1 partially rescued the lethality of a strain deleted for the two yeast formin genes, BNI1 and BNR1 (Figure 7B).
Figure 7.
Effects of expressing mouse formin mDia1 in yeast cells. (A) mDia1 FH1-COOH promotes actin cable formation in yeast. Panels show Alexa-phalloidin staining for wild-type cells (top panel), bni1-FH2#1 bnr1Δ cells carrying a control vector (middle panel: note that actin cables are fainter and less numerous than in wild-type cells), and bni1-FH2#1 bnr1Δ cells expressing mDia1 FH1-COOH (bottom panel). All cells were grown at the permissive temperature of 24°C. Bar, 2 μm. (B) Expression of mDia1 FH1-COOH partially rescues the lethality of a bni1Δ bnr1Δ strain. Left: serial dilutions of bni1Δ bnr1Δ cells spotted on selective medium, where both the 2μ BNI1 URA3 plasmid and the LEU2 plasmids designated on the left are maintained. Right: the same cells spotted onto medium containing 5-fluoroorotic acid to select against the 2μ BNI1 URA3 plasmid. From top to bottom, cells are transformed with a LEU2 control vector, a plasmid expressing full-length BNI1, a plasmid expressing Bni1-mini (amino acid residues 1215-1750; Sagot et al., 2002b), a plasmid expressing mDia1 FH2-COOH, and a plasmid expressing mDia1 FH1-COOH.
DISCUSSION
FH2 Dimers Nucleate Actin Assembly and Processively Cap Growing Barbed Ends of Filaments
Bni1 nucleates the initial formation of an actin filament by a mechanism that appears to involve stabilization of otherwise short-lived actin dimers, intermediates in actin polymerization (Pring et al., 2003). After nucleation, formins have been proposed to remain associated with the barbed ends of filaments as they elongate (Pruyne et al., 2002; Pring et al., 2003). A similar mechanism of “riding” the fast-growing ends of actin filaments also has been suggested for Ena/VASP proteins (Bear et al., 2002). However, this model has presented a paradox, because it is difficult to imagine how a protein remains bound to a filament end that is rapidly elongating (insertion of 4-5 actin subunits per second in our assays in Figure 3). Our data shed new light on the mechanism underlying this activity in two important ways. First, we demonstrate that formins protect the barbed ends of growing actin filaments from the inhibitory effects of excess capping protein, which provides new experimental support for the model that Bni1 maintains a persistent (or processive) association with the barbed end during growth. Bni1 and mDia1 both have this activity, which means that this is a conserved formin function. In fact, formins (or possibly Ena/VASP proteins) may underlie a previously reported activity in neutrophil extracts that protects growing barbed ends of filaments from capping protein (Huang et al., 1999). This ability to associate with and protect the growing barbed end of an actin filament from capping protein may be crucial in vivo for generating specific actin structures, such as yeast actin cables that appear to be free of capping protein (Amatruda and Cooper, 1992). Second, we show that Bni1 dimerizes and that ability to dimerize correlates with ability to nucleate actin filament assembly and to protect growing barbed ends from capping protein. Dimerization of the FH2 domain may help to explain how persistent barbed end association is maintained. Each half of the dimer (each FH2 domain) may be capable of making independent contacts with actin subunits exposed at the barbed end of the filament. This would permit new actin subunit insertion while allowing the formin dimer to alternate contacts and thus, walk up the growing filament end (see model below).
The activities of Bni1 and mDia1 differ significantly from those recently reported for the S. pombe formin Cdc12p (Kovar et al., 2003). These authors concluded that formins cap actin filament barbed ends in the absence of profilin, preventing all barbed end growth until gated by profilin to allow barbed end growth. In contrast, we have demonstrated that both Bni1 and mDia1 direct barbed end elongation in the absence of profilin. Further, the addition of profilin does not affect the rate of elongation in the presence of these formins (our unpublished results). These activities are supported by another recent study, which shows that mDia1 activities are similar to those of Bni1 (Li and Higgs, 2003). We do not believe that the differences in activities between Cdc12p and other formins (Bni1 and mDia1) are due to construct variation, because we have purified Cdc12p constructs and observed activities similar to those reported by Kovar and colleagues (J.M., B.G., A. Yonetani, and F. Chang, unpublished results). Thus, Bni1 and mDia1 behave distinctly from Cdc12p in the absence of profilin in vitro. What remains uncertain is the physiological relevance of the differences (profilin-gated vs. nongated). The simplest prediction is that all of the formins discussed above should allow barbed end elongation in vivo, because profilin-bound actin monomers are abundant in most eukaryotic cells. Therefore, unless profilin accessibility is limited under specific cellular conditions or in particular subcellular locations, all of these formins may have similar activities in vivo (i.e., promote barbed end assembly of actin filaments).
Regulation of Bni1 Activities by Profilin and Bud6
A central aspect of formin function in vivo is the association of multiple interacting proteins to build large protein complexes that regulate cell polarity and cytokinesis (reviewed in Pruyne and Bretscher, 2000). The known Bni1-associated proteins include profilin, Bud6, eF1α, Spa2, and the Rho-family GTPases Rho1 and Cdc42 (Kohno et al., 1996; Evangelista et al., 1997; Imamura et al., 1997; Fujiwara et al., 1998; Umikawa et al., 1998), and the in vivo mechanism of formin-induced actin assembly likely involves the coordinated activities of these factors. Diaphanous-related formins, such as Bni1 and mDia1, are proposed to exist in an autoinhibited conformation until released by binding of Rho GTPases to the amino terminal Rho-binding domain (RBD; see Figure 1; Watanabe et al., 1999; Alberts, 2001; Palazzo et al., 2001; Li and Higgs, 2003). In budding yeast, this regulation is controlled by distinct Rho signaling pathways acting on Bni1 and Bnr1 at different stages of bud morphogenesis and in response to cell stress (Dong et al., 2003). Rho GTPase activation is thought to release the carboxyl terminus, containing the FH1, FH2, and COOH domains, which stimulate actin assembly. The functions of the carboxy terminus (FH1-COOH) are likely subject to coordinated regulation by multiple associated proteins. We and others have shown that profilin, which binds the FH1 domain, stimulates the actin assembly-promoting activity of yeast formins (Sagot et al., 2002b; Kovar et al., 2003; Pring et al., 2003). Here we have extended this analysis to mammalian formins, showing that human profilin stimulates mDia1-induced actin assembly.
In addition, we defined the regulatory role of the formin-associated protein Bud6, which has an established role in cell polarity. Although Bud6 bears no sequence identity to profilin, the carboxyl terminal half of Bud6 (C-Bud6) has similar biochemical activities to profilin, dramatically accelerating nucleotide exchange on actin and enhancing Bni1-induced actin filament assembly. This similar activity suggests that Bud6, like profilin (Schutt et al., 1993), may bind to actin monomers at their barbed ends, promoting nucleotide exchange on actin and leaving free the pointed ends of the monomers for addition to filament barbed ends. Further, the stimulatory effects of Bud6 on Bni1 are additive with those of profilin, which binds to sequences located on the opposite side of the FH2 domain. These activities are consistent with the synthetic lethal genetic interaction that we observed between bud6Δ and pfy1-4. Taken together, these data suggest that Bud6 and profilin have related functions in vivo in stimulating formin-induced actin assembly. This is supported by reports that bud6 and pfy1 mutants have similar defects in the formation and organization of formin-nucleated actin cables (Haarer et al., 1990; Amberg et al., 1997; Wolven et al., 2000; Glynn et al., 2001; Evangelista et al., 2002; Sagot et al., 2002a).
A Model for Processive Capping by Formins during Actin Filament Elongation
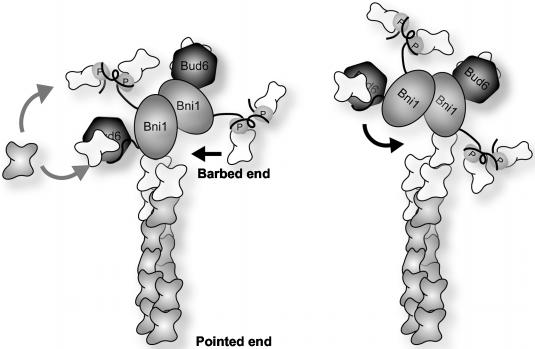
On the basis of our data showing 1) dimerization of the Bni1 FH2 domain, 2) persistent and protective association at the growing barbed ends of actin filaments, and 3) additive stimulation of Bni1 activity by profilin and Bud6, we propose a mechanism for processive capping by formins during filament elongation (Figure 8). At any given time, there are two actin subunits exposed at the barbed end of an uncapped actin filament, one protruding and one recessed. In our model, one of the formins in a dimer contacts the protruding actin subunit, and new actin subunit insertion occurs at the recessed site (left frame in model). This leads to binding of the other formin in the dimer to the newly inserted actin subunit, which is now the protruding end. This event may in turn trigger dissociation of the first formin, exposing the next free site for actin subunit insertion (right frame in model). In this manner, we hypothesize that the formin dimer walks or teeter-totters up the barbed end of the actin filament, maintaining a stable association while the filament end while allowing actin subunit insertion and filament growth. Key to this mechanism is that the FH2 domain forms a stable dimer, which allows it to maintain steady association with the filament end via alternating contacts of two linked FH2 domains. Bud6 and profilin bind to sequences flanking the FH2 domain and to actin monomers, promoting rapid nucleotide exchange on actin. They facilitate formin-mediated actin filament assembly by generating a local flux of ATP-actin and/or by hand feeding ATP-actin monomers to the open sites at the filament end.
Figure 8.
A model for how Bni1 and possibly other formins processively cap the elongating barbed end of an actin filament (see DISCUSSION for details). FH2 domains are depicted as shaded ovals and are labeled Bni1. FH1 domains are shown as black lines that bind to profilin (labeled P). The carboxyl terminal regions of Bni1 are shown as black lines that bind to Bud6 (darkly shaded hexagons). ADP-actin subunits are shaded, and ATP-actin subunits are white.
Our data emphasize a functional distinction between the CapZ family of capping proteins and the formins Bni1 and mDia1. Kovar et al. (2003) hypothesized that formins may fold like capping protein to terminate barbed end growth, based on the activities of Cdc12p and suggested sequence homology between capping protein and formin FH2 domains. However, our alignments show no significant sequence homology between formin FH2 domains and capping protein. Further, our recently solved crystal structure of the Bni1 FH2 domain reveals a unique structure that bears no resemblance to that of capping protein (Yamashita et al., 2003), but instead is a flexibly tethered dimer, consistent with our model (Y.Z., I.S., J.M., B.G., D.P., and M.E., unpublished results).
One key future challenge is to define the activities and regulation of formins from diverse species in the context of larger regulatory complexes. We have demonstrated that an FH2 domain-containing fragment of the mammalian formin mDia1, like Bni1, nucleates actin filament assembly and processively caps the barbed ends of filaments during elongation. Further, mDia1 partially complements yeast formin function in vivo. We speculate that many of the mechanisms for regulating formin-dependent actin assembly also may be conserved. Most formin homologues contain a profilin-binding FH1 domain, located on the amino terminal side of the FH2 domain. Many formins also contain a domain on the carboxyl terminal side of the FH2 domain, but the function(s) of these sequences are less well understood. Here we defined a regulatory role for Bud6, which binds to the carboxyl terminal extension of Bni1. Bud6 homologues are described in other fungi, but no clear functional homologue has been identified in animals. Interestingly, database searches here and in a previous study (Glynn et al., 2001) identified a region of homology (22% identity, 46% similarity >236 amino acids) between residues 517-753 of Bud6 and residues 501-734 of human p160 ROCK serine/threonine kinase (see Supplementary Material). Moreover, ROCK kinase has been linked to formin activity in vivo (Nakano et al., 1999; Watanabe et al., 1999). The vertebrate formin Daam1, which has an important role in Wnt signaling, interacts through its carboxyl terminal extension with Disheveled, which binds to actin (Torres and Nelson, 2000; Habas et al., 2001; Capelluto et al., 2002). Thus, it will be important to determine if these and other formin binding partners have functions related to Bud6.
Acknowledgments
We are grateful to S. Bear, P. Carvalho, Y. Li, and S. Swalley for support and technical assistance in these studies and to F. Chang, A. Goodman, and A. Rodal for critical reading of the manuscript. M.E. is a recipient of a Scholar award from the Leukemia and Lymphoma Society of America. D.P. was supported by the National Institutes of Health (NIH) Grant GM61345 and the Leukemia and Lymphoma Society of America. B.L.G. was supported by the Pew Scholars program, a Basil O'Conner grant from the March of Dimes, and the NIH Grant GM63691.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-08-0621. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-08-0621.
References
- Alberts, A. (2001). Identification of a carboxyl-terminal diaphanous-related formin homology protein autoregulatory domain. J. Biol. Chem. 276, 2824-2830. [DOI] [PubMed] [Google Scholar]
- Amatruda, J.F., and Cooper, J.A. (1992). Purification, characterization, and immunofluorescence localization of Saccharomyces cerevisiae capping protein. J. Cell Biol. 117, 1067-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amberg, D.C., Basart, E., and Botstein, D. (1995). Defining protein interactions with yeast actin in vivo. Nat. Struct. Biol. 2, 28-35. [DOI] [PubMed] [Google Scholar]
- Amberg, D.C., Zahner, J.E., Mulholland, J.M., Pringle, J.R., and Botstein, D. (1997). Aip3p/Bud6p, a yeast actin-interacting protein that is involved in morphogenesis and the selection of bipolar budding sites. Mol. Biol. Cell. 8, 729-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear, J.E. et al. (2002). Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109, 509-521. [DOI] [PubMed] [Google Scholar]
- Caldwell, J.E., Heiss, S.G., Mermall, V., and Cooper, J.A. (1989). Effects of CapZ, an actin capping protein of muscle, on the polymerization of actin. Biochemistry 28, 8506-8514. [DOI] [PubMed] [Google Scholar]
- Capelluto, D.G., Kutateladze, T.G., Habas, R., Finkielstein, C.V., He, X., and Overduin, M. (2002). The DIX domain targets dishevelled to actin stress fibres and vesicular membranes. Nature 419, 726-729. [DOI] [PubMed] [Google Scholar]
- Chang, F., Drubin, D., and Nurse, P. (1997). cdc12p, a protein required for cytokinesis in fission yeast, is a component of the cell division ring and interacts with profilin. J. Cell Biol. 137, 169-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson, T.W., Sikorski, R.S., Dante, M., Shero, J.H., and Hieter, P. (1992). Multifunctional yeast high-copy-number shuttle vectors. Gene 110, 119-122. [DOI] [PubMed] [Google Scholar]
- Copeland, J.W., and Treisman, R. (2002). The diaphanous-related formin mDia1 controls serum response factor activity through its effects on actin polymerization. Mol. Biol. Cell. 13, 4088-4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong, Y., Pruyne, D., and Bretscher, A. (2003). Formin-dependent actin assembly is regulated by distinct modes of Rho signaling in yeast. J. Cell Biol. 161, 1081-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eads, J.C., Mahoney, N.M., Vorobiev, S., Bresnick, A.R., Wen, K.K., Rubenstein, P.A., Haarer, B.K., and Almo, S.C. (1998). Structure determination and characterization of Saccharomyces cerevisiae profilin. Biochemistry 37, 11171-11181. [DOI] [PubMed] [Google Scholar]
- Evangelista, M., Blundell, K., Longtine, M.S., Chow, C.J., Adames, N., Pringle, J.R., Peter, M., and Boone, C. (1997). Bni1p, a yeast formin linking Cdc42p and the actin cytoskeleton during polarized morphogenesis. Science 276, 118-122. [DOI] [PubMed] [Google Scholar]
- Evangelista, M., Pruyne, D., Amberg, D.C., Boone, C., and Bretscher, A. (2002). Formins direct Arp2/3-independent actin filament assembly to polarize cell growth in yeast. Nat. Cell Biol. 4, 32-34. [DOI] [PubMed] [Google Scholar]
- Evangelista, M., Zigmond, S., and Boone, C. (2003). Formins: signaling effectors for assembly and polarization of actin filaments. J. Cell Sci. 116, 2603-2611. [DOI] [PubMed] [Google Scholar]
- Feierbach, B., and Chang, F. (2001). Roles of the fission yeast formin for3p in cell polarity, actin cable formation and symmetric cell division. Curr. Biol. 11, 1656-1665. [DOI] [PubMed] [Google Scholar]
- Frazier, J.A., and Field, C.M. (1997). Actin cytoskeleton: are FH proteins local organizers? Curr. Biol. 7, R414-R417. [DOI] [PubMed] [Google Scholar]
- Fujiwara, T., Tanaka, K., Mino, A., Kikyo, M., Takahashi, K., Shimizu, K., and Takai, Y. (1998). Rho1p-Bni1p-Spa2p interactions: Implications in localization of Bni1p at the bud site and regulation of the actin cytoskeleton in Saccharomyces cerevisiae. Mol. Biol. Cell 9, 1221-1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn, J.M., Lustig, R.J., Berlin, A., and Chang, F. (2001). Role of bud6p and tea1p in the interaction between actin and microtubules for the establishment of cell polarity in fission yeast. Curr. Biol. 11, 836-845. [DOI] [PubMed] [Google Scholar]
- Goode, B.L., Drubin, D.G., and Lappalainen, P. (1998). Regulation of the cortical actin cytoskeleton in budding yeast by twinfilin, a ubiquitous actin monomersequestering protein. J. Cell Biol. 142, 723-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haarer, B.K., Lillie, S.H., Adams, A.E. M., Magdolen, V., Bandlow, W., and Brown, S.S. (1990). Purification of profilin from Saccharomyces cerevisiae and analysis of profilin-deficient cells. J. Cell Biol. 110, 105-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas, R., Kato, Y., and He, X. (2001). Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell 107, 843-854. [DOI] [PubMed] [Google Scholar]
- Huang, M., Yang, C., Schafer, D.A., Cooper, J.A., Higgs, H.N., and Zigmond, S.H. (1999). Cdc42-induced actin filaments are protected from capping protein. Curr. Biol. 9, 979-982. [DOI] [PubMed] [Google Scholar]
- Humphries, C.L., Balcer, H.I., D'Agostino, J.L., Winsor, B., Drubin, D.G., Barnes, G., Andrews, B.J., and Goode, B.L. (2002). Direct regulation of Arp2/3 complex activity and function by the actin binding protein coronin. J. Cell Biol. 159, 993-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura, H., Tanaka, K., Hihara, T., Umikawa, M., Kamei, T., Takahashi, K., Sasaki, T., and Takai, Y. (1997). Bni1p and Bnr1p: downstream targets of the Rho family small G-proteins which interact with profilin and regulate actin cytoskeleton in Saccharomyces cerevisiae. EMBO J. 16, 2745-2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno, H. et al. (1996). Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J. 15, 6060-6068. [PMC free article] [PubMed] [Google Scholar]
- Kovar, D.R., Kuhn, J.R., Tichy, A.L., and Pollard, T.D. (2003). The fission yeast cytokinesis formin Cdc12p is a barbed end actin filament capping protein gated by profilin. J. Cell Biol. 161, 875-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen, P., and Drubin, D.G. (1997). Cofilin promotes rapid actin filament turnover in vivo. Nature 388, 78-82. [DOI] [PubMed] [Google Scholar]
- Lappalainen, P., Federov, E.V., Federov, A.A., Almo, S.C., and Drubin, D.G. (1997). Essential functions and actin-binding surfaces of yeast cofilin revealed by systematic mutagenesis. EMBO J. 16, 5520-5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz, J., Lewis, M.S., and Schuck, P. (2002). Modern analytical ultracentrifugation in protein science: a tutorial review. Protein Sci. 11, 2067-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F., and Higgs, H.N. (2003). The mouse formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr. Biol. 13, 1335-1340. [DOI] [PubMed] [Google Scholar]
- Mockrin, S.C., and Korn, E.D. (1980). Acanthamoeba profilin interacts with G-actin to increase the rate of exchange of actin-bound adenosine 5′-triphosphate. Biochemistry 19, 5359-5562. [DOI] [PubMed] [Google Scholar]
- Moriyama, K., and Yahara, I.. (2002). Human CAP1 is a key factor in the recycling of cofilin and actin for rapid actin turnover. J. Cell Sci. 115, 1591-1601. [DOI] [PubMed] [Google Scholar]
- Nakano, K., Takaishi, K., Kodama, A., Mammoto, A., Shiozaki, H., Monden, M., and Takai, Y. (1999). Distinct actions and cooperative roles of ROCK and mDia in Rho small G protein-induced reorganization of the actin cytoskeleton in Madin-Darby canine kidney cells. Mol. Biol. Cell. 10, 2481-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki-Kuroda, K., Yamamoto, Y., Nohara, H., Kinoshita, M., Fujiwara, T., Irie, K., and Takai, Y. (2001). Dynamic localization and function of Bni1p at the sites of directed growth in Saccharomyces cerevisiae. Mol. Biol. Cell 21, 827-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palazzo, A.F., Cook, T.A., Alberts, A.S., and Gundersen, G.G. (2001). mDia mediates Rho-regulated formation and orientation of stable microtubules. Nat. Cell Biol. 3, 723-729. [DOI] [PubMed] [Google Scholar]
- Pelham, R.J., and Chang, F. (2002). Actin dynamics in the contractile ring during cytokinesis in fission yeast. Nature 419, 82-86. [DOI] [PubMed] [Google Scholar]
- Pollard, T.D., Blanchoin, L., and Mullins, R.D. (2000). Molecular mechanisms controlling actin filament dynamics in nonmuscle cells. Annu. Rev. Biophys. Biomol. Struct. 29, 545-576. [DOI] [PubMed] [Google Scholar]
- Pollard, T.D., and Cooper, J.A. (1984). Quantitative analysis of the effect of Acanthamoeba profilin on actin filament nucleation and elongation. Biochemistry 23, 6631-6641. [DOI] [PubMed] [Google Scholar]
- Pollard, T.D., and Cooper, J.A. (1986). Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu. Rev. Biochem. 55, 987-1035. [DOI] [PubMed] [Google Scholar]
- Pring, M., Evangelista, M., Boone, C., Yang, C., and Zigmond, S.H. (2003). Mechanism of formin-induced nucleation of actin filaments. Biochemistry 42, 486-496. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., and Bretscher, A. (2000). Polarization of cell growth in yeast. J. Cell Sci. 113, 571-585. [DOI] [PubMed] [Google Scholar]
- Pruyne, D., Evangelista, M., Yang, C., Bi, E., Zigmond, S., Bretscher, A., and Boone, C. (2002). Role of formins in actin assembly: nucleation and barbed end association. Science 297, 612-615. [DOI] [PubMed] [Google Scholar]
- Rickard, J.E., and Sheterline, P. (1986). Cytoplasmic concentrations of inorganic phosphate affect the critical concentration for assembly of actin in the presence of cytochalasin D or ADP. J. Mol. Biol. 191, 273-280. [DOI] [PubMed] [Google Scholar]
- Rose, M.D., Winston, F., and Hieter, P. (1989). Methods in Yeast Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Safer, D. (1989). An electrophoretic procedure for detecting proteins that bind actin monomers. Anal. Biochem. 178, 32-37. [DOI] [PubMed] [Google Scholar]
- Sagot, I., Klee, S.K., and Pellman, D. (2002a). Yeast formins regulate cell polarity by controlling the assembly of actin cables. Nat. Cell Biol. 4, 42-50. [DOI] [PubMed] [Google Scholar]
- Sagot, I., Rodal, A.A., Moseley, J., Goode, B.L., and Pellman, D. (2002b). An actin nucleation mechanism mediated by Bni1 and profilin. Nat. Cell Biol. 4, 626-631. [DOI] [PubMed] [Google Scholar]
- Schafer, D.A., Jennings, P.B., and Cooper, J.A. (1996). Dynamics of capping protein and actin assembly in vitro: uncapping barbed ends by polyphosphoinositides. J. Cell Biol. 135, 169-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutt, C.E., Myslik, J.C., Rozycki, M.D., Goonesekere, N.C., and Lindberg, U. (1993). The structure of crystalline profilin-beta-actin. Nature 365, 810-816. [DOI] [PubMed] [Google Scholar]
- Segal, M., Bloom, K., and Reed, S.I. (2002). Kar9p-independent microtubule capture at Bud6p cortical sites primes spindle polarity before bud emergence in Saccharomyces cerevisiae. Mol. Biol. Cell. 13, 4141-4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson, A.F., Baillie, D.L., and Bowerman, B. (2002). A formin homology protein and a profilin are required for cytokinesis and Arp2/3-independent assembly of cortical Microfilaments in C. elegans. Curr. Biol. 12, 2066-2075. [DOI] [PubMed] [Google Scholar]
- Tolliday, N., VerPlank, L., and Li, R. (2002). Rho1 directs formin-mediated actin ring assembly during budding yeast cytokinesis. Curr. Biol. 12, 1864-1870. [DOI] [PubMed] [Google Scholar]
- Tominaga, T., Sahai, E., Chardin, P., McCormick, F., Courtneidge, S.A., and Alberts, A.S. (2000). Diaphanous-related formins bridge Rho GTPase and Src tyrosine kinase signaling. Mol. Cell. 5, 13-25. [DOI] [PubMed] [Google Scholar]
- Torres, M.A., and Nelson, W.J.. (2000). Colocalization and redistribution of dishevelled and actin during Wnt-induced mesenchymal morphogenesis. J. Cell Biol. 149, 1433-1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umikawa, M., Tanaka, K., Kamei, T., Shimizu, K., Imamura, H., Sasaki, T., and Takai, Y. (1998). Interaction of Rho1p target Bni1p with F-actin-binding elongation factor 1alpha: implication in Rho1p-regulated reorganization of the actin cytoskeleton in Saccharomyces cerevisiae. Oncogene 16, 2011-2016. [DOI] [PubMed] [Google Scholar]
- Wallar, B.J., and Alberts, A.S. (2003). The formins: active scaffolds that remodel the cytoskeleton. Trends Cell Biol. 13, 435-446. [DOI] [PubMed] [Google Scholar]
- Wasserman, S. (1998). FH proteins as cytoskeletal organizers. Trends Cell Biol. 8, 111-115. [DOI] [PubMed] [Google Scholar]
- Watanabe, N., Kato, T., Fujita, A., Ishizaki, T., and Narumiya, S. (1999). Cooperation between mDia1 and ROCK in Rho-induced actin reorganization. Nat. Cell Biol. 1, 136-143. [DOI] [PubMed] [Google Scholar]
- Wolven, A.K., Belmont, L.D., Mahoney, N.M., Almo, S.C., and Drubin, D.G. (2000). In vivo importance of actin nucleotide exchange catalyzed by profilin. J. Cell Biol. 150, 895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita, A., Maeda, K., and Maeda, Y. (2003). Crystal structure of CapZ: structural basis for actin filament barbed end capping. EMBO J. 22, 1529-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]