Abstract
Nuclear receptors and pioneer factors drive the development and progression of prostate cancer. In this disease, aggressive disease phenotypes and hormone therapy failures result from resurgent activity of androgen receptor (AR) and the upregulation of coactivator protein p300 and pioneer factors (e.g. GATA2 and FOXA1). Thus, a major current emphasis in the field is to identify mechanisms by which castrate-resistant AR activity and pioneer factor function can be combinatorially suppressed. Here we show that the turmeric spice isoflavone curcumin suppresses p300 and CBP occupancy at sites of AR function. Curcumin reduced the association of histone acetylation and pioneer factors, thereby suppressing AR residence and downstream target gene expression. Histone deacetylase inhibitors reversed the effects of curcumin on AR activity, further underscoring the impact of curcumin on altering the chromatin landscape. These functions precluded pioneer factor occupancy, leading ultimately to a suppression of ligand-dependent and ligand-independent AR residence on chromatin. Moreover, these functions were conserved even in cells with heightened pioneer factor activity, thus identifying a potential strategy to manage this subclass of tumors. Biological relevance was further identified using in vivo xenograft models mimicking disease progression. Curcumin cooperated in vivo with androgen deprivation as indicated by illustrated by a reduction in tumor growth and delay to the onset of castrate-resistant disease. Together, our results demonstrate the combinatorial impact of targeting AR and histone modification in prostate cancer, setting the stage for further development of curcumin as a novel agent to target AR signaling.
Keywords: Prostatic adenocarcinoma, hormone therapy, pioneer factors, CBP/p300, histone modification
Introduction
Steroid hormone receptors are critical targets for therapeutic intervention in hormone-dependent cancers, including tumors of the breast and prostate. While current strategies to suppress receptor activity entail use of agents that deplete ligand or compete for the ligand binding domains of steroid receptors (e.g. estrogen receptor (ER-α) in breast cancer and the androgen receptor (AR) in prostate cancer), tumor cells develop sophisticated mechanisms to bypass receptor-directed therapeutics (1, 2). In prostate cancer (PCa), a major mechanism of therapeutic failure and progression to advanced disease is inappropriate reactivation of AR (2). This stage of disease is referred to as castrate-resistant prostate cancer (CRPC), and a plethora of clinical and pre-clinical studies strongly support the contention that AR remains essential for growth and survival in CRPC (3, 4). Thus, development of novel treatments that can act in concert with AR-directed therapeutics would be of benefit.
Activated nuclear receptors function as ligand dependent transcription factors. Hence, receptor activity largely depends on access to binding sites on chromatin, facilitated in part by histone modifying enzymes (which directly promote a chromatin landscape favorable for transcriptional activation) and pioneer factors such as FOXA1 and GATA2 (which promote open chromatin structure, subsequent nuclear receptor binding, and resultant initiation of context-specific transcriptional programs) (5–7). Histone acetyl transferases (HAT) such as p300 and CBP promote AR-mediated transcription (8), and harbor pro-tumorigenic activity. Notably, human prostate tumors expressing high levels of p300 show aggressive phenotypes accompanied by increased proliferation and poor prognosis (9). p300 and CBP also promote transcription activity of selected pioneer factors (e.g. GATA2), which play critical roles in AR-dependent transcription (10), and are elevated in human disease ((1, 11). Resistance to treatment mediated by upregulation of pioneer factors is attributed, in part, through the ability to interact with AR and increase transcriptional activity (12, 13); consonantly, FOXA1 can promote CRPC development (13). Collectively, these observations suggest that disrupting pioneer factor binding and/or activity may be advantageous.
Here, using xenograft models mimicking androgen deprivation therapy (ADT) sensitive and CRPC prostate cancer, it is shown that the isoflavone curcumin suppresses both CBP/p300 activity and pioneer factor function, thereby attenuating both ligand dependent and castrate resistant AR activity. Remarkably, these effects were conserved in both ADT-sensitive and CRPC model systems. Substantial in vitro and in vivo analyses further demonstrate that curcumin cooperates with hormone therapy to suppress AR- dependent cell proliferation, tumor growth, and the transition to castration resistance. The findings presented herein suggest a new paradigm for nuclear receptor inhibition that may be relevant for a multitude of nuclear receptor-dependent cancers.
Materials and Methods
Cell Culture, reagents, and cell based assays
LNCaP, LAPC4, VCaP, C4-2, 22Rv1 cells were cultured in androgen ablative condition using charcoal dextran–treated (CDT) fetal bovine serum as described (14). Prostatic epithelial benign cells (BHPrE1) were cultured in presence of androgen as described (15). Curcumin (C7727), TSA (T8552) and DHT were obtained from Sigma-Aldrich. All experiments were performed with at least three independent biological replicates. Statistical significance was determined using Student’s t test*, P<0.05. Proliferation assays were performed as previously described (14). Cells cultured in androgen deprivation were transiently transfected using Lipofectin (Invitrogen) reagent and treated with vehicle or curcumin (2.5uM) for 24 hours. Plasmids encoding wild-type AR (pSG5-AR) and GATA2 construct have been previously described (11, 16). mRNA quantitation was performed as described.
Immunoblot
Cells were treated with vehicle or curcumin and subjected to SDS-PAGE for immunoblotting as previously described (14), using antisera described in the Supplement.
Chromatin Immunoprecipitation (ChIP) and q-PCR
Cells seeded in androgen deprivation for 72 hours were treated with ethanol or curcumin (8uM). For ADT-sensitive cells, DHT (1nM) was added 1 hour subsequent to curcumin treatment. At selected timepoints, cells were fixed and ChIP analyses were performed as described (14). Antibodies and primers used for ChIP are listed (Supplementary Table-1 and 2). For RNA analyses cells in absence of androgen were treated with either vehicle or curcumin (2.5uM) for 24 hours. Trizol reagent extracted RNA was used to generate cDNA using the superscript reverse transcription-PCR system (Invitrogen). Quantitative PCR was performed using Power SYBR-Green reagent (Applied Biosystems). Primer sequences are provided in Supplementary Table-1.
Xenograft analyses
Xenografts were developed as described (17). Mice were subjected to bilateral orchiectomy when tumors reached 200–250 mm3. Tumor-bearing animals were randomized into control group (receiving 100ul vehicle IP/48 hours) or a treatment group (receiving 50 mg/kg/d curcumin IP) (18). Tumors volumes were monitored weekly using calipers, and animals were followed for 5 weeks post-castration. BrdU analysis and immunoblotting was performed as described (17).
Results
Curcumin cooperates with hormone ablation to suppress cell growth and survival in vitro
Nuclear receptor-directed therapeutics typically entail agents that act through the ligand binding domain to modulate receptor signaling in disseminated PCa (19, 20). However, recurrent tumors arise through aberrant re-activation of the receptor (2, 14, 20–22). Thus, development of means to suppress receptor signaling could be of significant benefit. The isoflavone curcumin has been suggested in preclinical models of PCa to inhibit receptor function and decrease cell number, albeit using doses that may not be pharmacologically attainable (23). Here, these findings held true using physiologically attainable doses of curcumin (1–2.5µM, Supplementary Fig S1) (24). Since curcumin can suppress cell survival through multiple mechanisms, it was hypothesized that curcumin may act in concert with existing PCa therapeutics. As androgen deprivation therapy (ADT) is the first line of therapeutic intervention for non-organ confined tumors (2, 20), cells were subjected to hormone deprivation in the presence and absence of curcumin. As expected, all ADT-sensitive model systems exhibited a cytostatic effect upon hormone withdrawal used to mimic ADT. Remarkably, curcumin (1–2.5µM) augmented the effects of ADT (Fig.1A), and reduced cell number compared to androgen deprivation alone (Fig.1A). Consistently, high concentration curcumin (5µM) induced reduction in cell number (Supplemental Fig S1B). Moreover, BrdU incorporation assays showed that S-phase progression was significantly inhibited by curcumin with hormone withdrawal (Supplemental Fig. S2A), thus implicating curcumin as a potent inhibitor of both cell cycle and survival in PCa cells.
Figure 1. Curcumin cooperates with hormone ablation to suppress cell growth and survival in vitro.
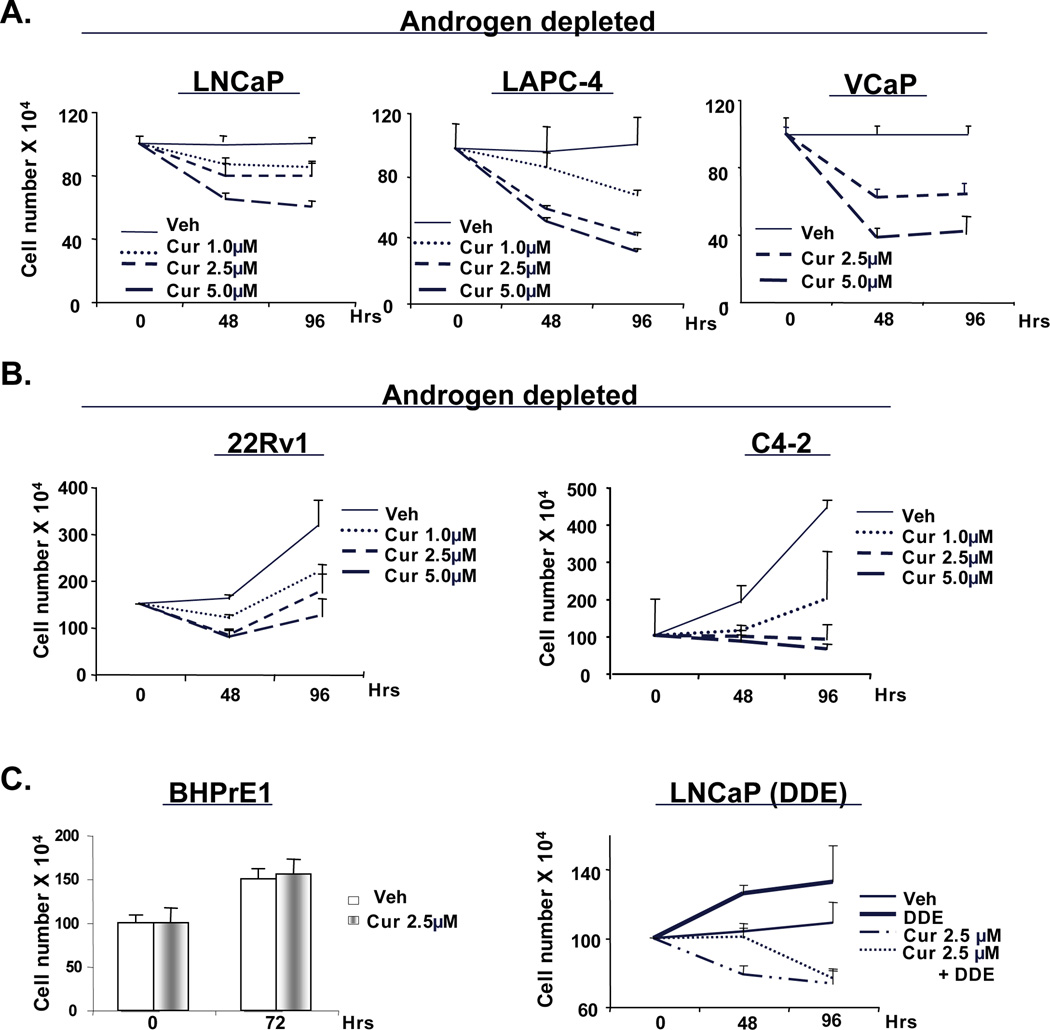
ADT-sensitive cells (LNCaP, LAPC-4 or VCaP) (A) or CRPC cells (C4-2 and 22Rv1) (B) were cultured in androgen deprivation condition for 24 hours before treating with vehicle or increasing concentrations of curcumin (1, 2.5, and 5µM). Cells were counted using trypan-blue exclusion. (C) Prostatic epithelial cells (BHPrE1, left panel) were cultured with vehicle or curcumin (2.5µM) and effect on cell number monitored. LNCaP cells (right panel) cultured under androgen deprivation were treated with DDE alone or in combination of curcumin, and cell number determined. P<0.05.
Next, the effect of curcumin on castrate resistant PCa (CRPC) cells was studied under conditions of androgen deprivation. As expected, CRPC cells sustained proliferative capacity under androgen deprivation, but curcumin (1µM) significantly increased the doubling time for CRPC cells (Fig.1B). Interestingly, both the CRPC and ADT-sensitive cells, enrichment of cleaved-PARP was observed only at high concentrations (Supplemental Fig. S2B), thus indicating that, lower doses curcumin (1–2.5µM) exhibit anti-proliferative effects, whereas higher doses (5–10µM) result in cell death. By contrast, combinations of curcumin with ADT did not significantly affect the survival of non-transformed prostatic epithelial cells (BHPrE1, Fig 1C, left panel), consistent with previous reports suggesting that curcumin shows little activity in non-neoplastic cells (25). Finally, since cells expressing selected tumor-derived mutants of AR (e.g. T877A) can bypass ADT therapy by utilizing endocrine disrupting compounds as agonists, the impact of curcumin on mutant AR was tested using cells treated with the endocrine disrupting compounds (DDE) (14). In cells cultured in the absence of androgen and stimulated with DDE (Fig. 1C, right panel), curcumin inhibited cell proliferation. Together, these data indicate that curcumin enhances the cellular response to ADT in both ADT-sensitive as well as castrate resistant cell types and suppress cell survival in concert with ADT.
Curcumin influences AR activity without affecting AR protein accumulation in the castrate-condition
To assess the means by which curcumin regulates castrate-resistant AR activity, the impact on AR accumulation was explored. Consistent with prior reports, the agent reduces AR protein levels in the presence of androgen (Supplementary Fig S1C) (26). However, under castrate conditions, curcumin did not alter AR mRNA (Supplementary Fig S3A) or AR protein levels in both ADT-sensitive and CRPC cells (Fig 2A). Prolonged curcumin exposure (48 and 96 hours) failed to alter AR protein levels in all cells tested, except in 22Rv1 cells (data not shown and Supplementary Fig S3B), wherein both full length-AR and an AR splice variant (AR-SV) were modestly reduced at high curcumin concentrations. These data indicate that low dose curcumin can suppress growth of PCa cells without affecting AR levels. Additionally, AR localization was not affected by curcumin treatment (Supplementary Fig S3, panel C and D).
Figure 2. Curcumin influences AR activity without affecting AR protein accumulation in the hormone ablated condition.
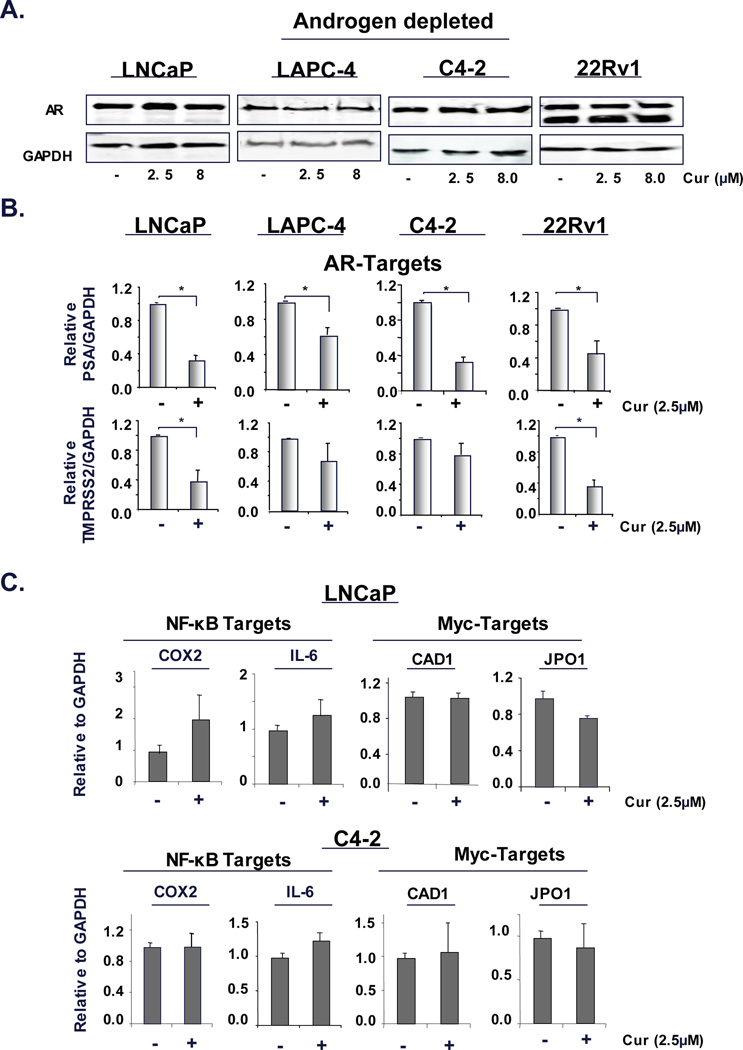
(A) ADT-sensitive cells (LNCaP and LAPC-4) and CRPC cells (C4-2 and 22Rv1) maintained under androgen deprivation were treated with curcumin (2.5 and 8µM) for 24 hours. Cells were then processed for immunoblotting (A) or mRNA isolation to determine expression of AR targets (PSA and TMPRSS2) (B) and NF-KB targets (COX2 and IL6) and Myc targets (CAD1 and JPO1) (C) relative to GAPDH. P<0.05.
Since, curcumin yielded no effect on AR protein levels, yet cooperated with ADT to suppress cell growth (Fig 1), the impact of curcumin on AR activity was examined under castrate conditions. To accomplish this, cells were cultured in the absence of androgen, treated with 2.5µM curcumin and relative levels of AR target genes (PSA and TMPRSS2) were determined. ADT treatment alone significantly reduced AR target gene expression as expected (data not shown). As shown, curcumin further inhibited AR target gene expression in ADT-sensitive cells (LNCaP and LAPC-4, Fig 2B). As AR reactivation is known to be responsible for CRPC development, the impact of curcumin on AR activity in CRPC cells was assessed under castrate conditions. Curcumin curtailed AR target gene expression in CRPC cells (C4-2 and 22Rv1, Fig 2B). Moreover, curcumin inhibited gene expression of additional AR targets, especially important for AR-dependent G2/M progression in CRPC cells (UBE2C, CDK1 and CDC20) (13) (Supplementary Fig S4A). Consistent with previous studies linking these genes to AR regulation, the direct AR antagonist Casodex further suppressed gene expression under conditions of hormone ablation (Supplementary Fig S4B). These findings reveal new functions for curcumin, wherein this agent suppresses castrate resistant AR activity through mechanisms distinct from alteration of AR levels. Overall, these data indicate that curcumin impacts AR activity, without affecting AR expression in hormone depletion.
Previous research indicates that curcumin can also attenuate NF-κB activity. Consistent with the established connection between DHT and NF-κB (27), ADT alone significantly reduced NF-κB activity (data not shown); however, addition of curcumin did not further suppress NF-κB target gene expression (COX2 and IL-6) in ADT-sensitive cells or CRPC cells (Fig 2C). To further investigate the specificity of curcumin action on other transcriptional regulators known to foster pro-tumorigenic functions in prostate cancer, Myc target gene (CAD1 and JPO1) expression was assessed. Neither CAD1 nor JPO1 levels were altered by curcumin treatment alone (Fig 2C), indicating that under androgen depletion, pharmacologically attainable doses of curcumin (2.5µM) preferentially inhibit AR-mediated transcriptional regulation.
Curcumin alters on histone modification to suppress AR recruitment and activity
Chromatin binding of AR is known to regulate target gene induction via direct binding to regulatory loci. To determine if AR occupancy is affected by curcumin at the regulatory loci of genes with known PCa relevance, chromatin immunoprecipitation (ChIP) analysis was performed. For ADT-sensitive models, cells were initially steroid deprived, then stimulated with androgen. AR recruitment in steroid deprived cells was not consistently above background, consistent with the necessity of ligand in these cells to induce AR occupancy to levels reliably detected by ChIP assay (Supplementary Fig S4C). At the KLK3/PSA enhancer, DHT-induced AR recruitment was unaffected by curcumin at the earliest timepoints (1.75 hours post DHT, LNCaP), however, over time curcumin suppressed AR residence on chromatin (4 and 12 hours, Figure 3A). Similar effects were observed in the castrate setting (CRPC models), wherein the effects of curcumin on AR residence were observed 4 hours post DHT treatment at KLK3/PSA locus and continued over time (C4-2, Fig 3A, middle panel). Similarly, AR occupancy at enhancer regions of other castrate resistant model (22Rv1) was also reduced by curcumin (Fig 3A, right panel). At the TMPRSS2 locus, modest effects were observed at the earliest timepoints (LNCaP, Fig 3A, lower panel) but these effects were enhanced over time. In CRPC cells also, AR occupancy was significantly reduced at TMPRSS2 locus only at extended period (Fig 3A, middle and right panels). Together, these data suggest that within 4 hours, curcumin can alter AR occupancy at sites of transcriptional regulation and impact target gene expression (data not shown).
Figure 3. Curcumin suppresses histone acetylation and AR recruitment at AR-regulated loci.
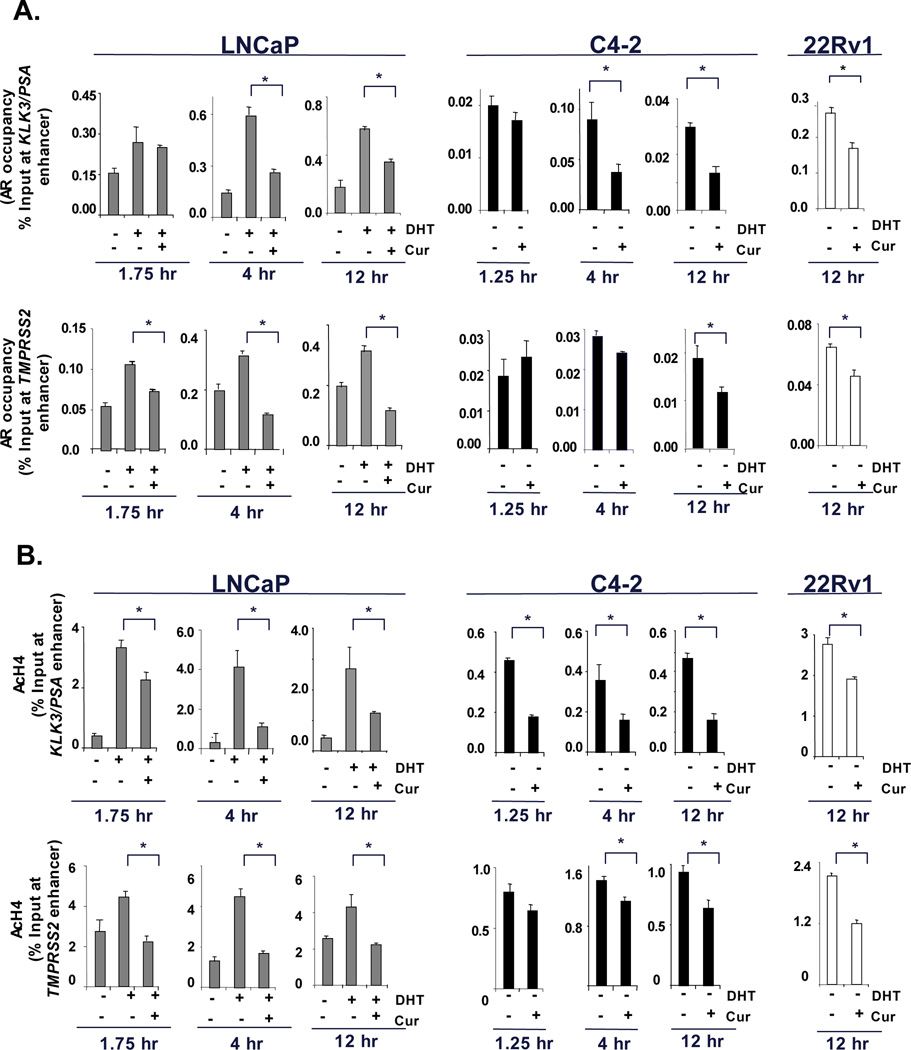
(A) ADT-sensitive cells (LNCaP) cultured under androgen deprivation were treated with DHT (1nM) alone or in combination with curcumin (8µM) for select timepoints (1.75 hours, 4 hours or 12 hours). Whereas, CRPC cells (C4-2 and 22Rv1) under androgen deprivation were treated with vehicle or curcumin (8µM) alone for selected timepoints (1.25 hours, 4 hours and 12 hours). The cells were processed for ChIP analysis to determine AR occupancy at the enhancer region of KLK3/PSA (upper panel) and TMPRSS2 (lower panel) (primer sequences, Supplementary Table-1). (B) Acetylated histone levels at KLK3/PSA (upper panel) or TMPRSS2 (lower panel) enhancer region were determined by ChIP analysis. P<0.05.
Since transcriptionally active regions at chromatin are marked by increased acetylation of histones, and curcumin is known to affect HAT proteins, the impact of curcumin on histone modifications was studied. In contrast to the delayed effects on AR occupancy, curcumin treatment significantly reduced histone H4 acetylation at the KLK3/PSA enhancer with rapid kinetics (1.75 hrs) (Fig 4B, left panel), and these effects were enhanced over time (4 and 12 hours). Similarly, in CRPC cells, from the shortest timepoint (1.25 hours), curcumin significantly inhibited histone acetylation at the AR regulatory region at the KLK3/PSA enhancer (C4-2, Fig 3B, middle panel); and sustained the effects for longer periods (4 and 12 hours). Other CRPC cells (22Rv1) also showed similar reduction in acetyl-H4 at the KLK3/PSA enhancer. A second AR target gene TMPRSS2 also showed significant reduction in histone-acetylation upon curcumin treatment (1.75 hours, LNCaP, Fig 3B, lower panel) and continued to repress acetylation after extended periods (4 and 12 hours). Even in CRPC cells, histone H4-acetylation was significantly reduced at TMPRSS2 locus from the earliest timepoint (C4-2, 1.25 hours) and continued for extended periods. Other models of CRPC (22Rv1) also showed reduced histone-acetylation at TMPRSS2 region. Investigation of another AR target gene locus (FKBP5) revealed similar effects (Supplementary Fig S5A), and the effects observed were conserved with lower doses of curcumin (2.5µM) (Supplementary Fig S5B). These observations suggested that curcumin significantly impacts histone acetylation, and that this event precedes observed changes in AR occupancy.
Figure 4. Curcumin impinges on histone modifying enzymes to suppress AR activity.
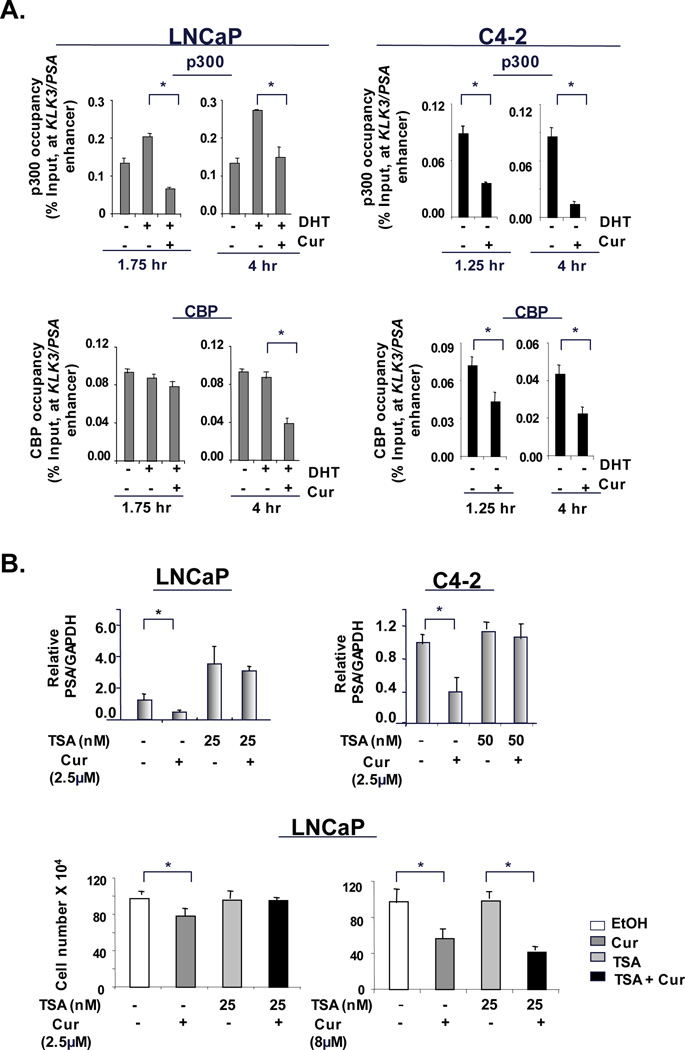
(A). ChIP analysis for p300 and CBP recruitment was performed as described in Figure 3. (B) ADT-sensitive cells (LNCaP) and CRPC cells (C4-2) under androgen deprivation were treated with TSA alone or in combination with curcumin (2.5µM) for 24 hours. Relative KLK3/PSA mRNA expression was analyzed by q-PCR. Using same treatments with curcumin (2.5 and 8.0µM) cell survival analysis was performed at 48 hours. P<0.05.
Curcumin impinges on histone modifying enzymes to suppress AR activity
Key components of the AR transcriptional complex include histone acetyl transferases p300 and CBP, and ChIP analyses herein revealed that curcumin treatment significantly suppressed p300 recruitment (KLK3/PSA, Fig 4A, left panel). Extended curcumin exposure resulted in significant reduction in recruitment of both p300 and CBP proteins at KLK3/PSA enhancer, thus indicating that curcumin can ultimately impinge on both cofactors. Interestingly, in CRPC cells curcumin significantly inhibited recruitment of these proteins at PSA locus from the earliest timepoint (Fig 4A, right panel) and sustained these effects on both proteins over extended timepoints. Interestingly, no changes in p300 or CBP protein levels were observed at the earliest timepoint in the conditions used for ChIP analyses (Supplementary Fig S6A, LNCaP, left panel), thus indicating that curcumin primarily affects recruitment without altering protein levels; longer exposure to curcumin did reduce p300 and CBP protein expression in both ADT-sensitive and CRPC cells (Supplementary Fig S6A, right panels). No changes were observed in histone-methylation in both ADT-sensitive and CRPC cells (Supplementary Fig S6B) at the time when CBP/p300 occupancy and histone acetylation was suppressed. Interestingly, histone acetylation was also inhibited at the promoter regions of genes not affected by curcumin (IL-6 and COX2, Supplementary Fig S6C) indicating that the effect of curcumin on higher acetylation status is not always sufficient to alter gene expression. Thus, while these data indicated that a primary effect of curcumin is to suppress CBP/p300 occupancy at chromatin, the consequence of this function of curcumin required further consideration.
To functionally challenge the impact of curcumin mediated histone alterations, cells were treated with the HDAC inhibitor, trichostatin (TSA), to result in net gain of acetylated histones. Consistent with previous reports (28), TSA treatment alone increased PSA mRNA levels in ADT-sensitive cells (Fig 4B, left panel) but not in CRPC cells. However, in both cell types, TSA reversed curcumin-mediated inhibition of PSA mRNA (Fig 4B). Similar effects were also observed with another HDAC inhibitor SAHA (data not shown). TSA reversed the ability of curcumin to inhibit cell survival (Fig 4B, left panel) but these effects were nullified by curcumin (8µM) (Fig 4B, right panel). These collective findings suggest that the ability of curcumin to suppress AR activity is dependent on alteration of histone acetylation. These effects appear to be specific to curcumin, as other isoflavone with known inhibitory effects on PCa and AR activity, Indole-3-Carbinol (I3C) (29, 30), failed to show additive effects on AR activity, recruitment, or histone-acetylation under androgen depletion (Supplementary Fig S7A and Fig S7B).
Curcumin suppresses occupancy of pioneering factors at enhancers of AR target genes
While early response to curcumin under castrate conditions involves displacement of CBP/p300 and reduced histone-acetylation at sites of AR activity, the observation that AR occupancy was ultimately reduced indicated that other events likely cooperate with the effects of curcumin on CBP/p300. Recent findings demonstrated that AR function is influenced by prior recruitment of “pioneer factors” (GATA2 and FOXA1), that act as placeholders for AR recruitment. Interestingly, several reports indicate that transcriptional activity of pioneering factors is influenced by CBP or p300 function (10, 31). Conversely, GATA2 is necessary for AR recruitment (13) whereas, FOXA1 cooperates with AR function to stimulate expression of G2/M phase genes (5, 12, 13). Notably, mRNA levels of UBE2C, CDC20 and CDK1 were suppressed by curcumin in both ADT-sensitive (Supplementary Fig S4A) and CRPC cells (Supplementary Fig S4B), thus indicating that curcumin may impinge on pioneer factor function. ChIP analyses showed that GATA2 recruitment was not consistently altered at AR target gene loci in the early timepoints (1.75 hours, Fig 5A and Supplementary Fig S5A, right panel). In CRPC cells (C4-2 and 22Rv1) no significant displacement of GATA2 was observed at shortest timepoint (1.25 hours) (C4-2, KLK3/PSA and TMPRSS2, Fig 5A, middle panel). However, by 4 hours a significant reduction of GATA2 occupancy in ADT-sensitive cells was consistently observed (Fig 5A and Supplementary Fig S5A, right panel) and sustained at the 12 hour timepoints. Similar analysis of FOXA1 indicated that although FOXA1 occupancy was significantly inhibited at KLK3/PSA regulatory region (Fig 5B) in the ADT-sensitive (LNCaP) and CRPC cells; FOXA1 recruitment was not affected consistently at TMPRSS2 in CRPC cells. Protein levels of pioneer factors were not affected by curcumin (Supplemental Fig S8A), indicating that consonant to p300/CBP, curcumin appears to modulate GATA2 residence at sites of AR activity. As pioneer factors are critical for AR-dependent transcription of genes of PCa relevance, it was therefore not surprising that Pol-II recruitment was ultimately suppressed (Supplementary Fig S8B).
Figure 5. Curcumin suppresses occupancy of pioneering factors at AR target gene enhancers.
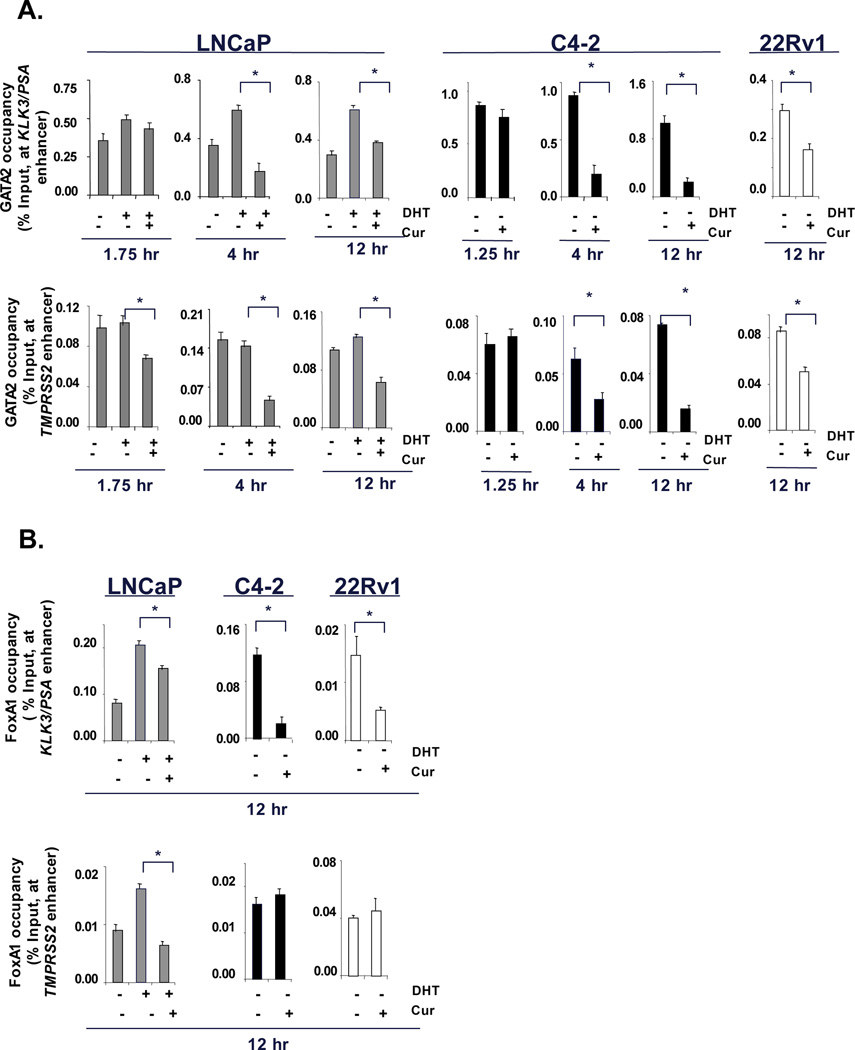
(A) ChIP analysis for pioneer factor occupancy at AR target gene enhancer region was carried out as described in Fig 3. (B) Similar analyse were carried out for FOXA1 (12 hours) in both ADT-sensitive and CRPC cells. P<0.05.
Curcumin suppresses GATA2 mediated, castrate-resistant AR activity
The effect of curcumin on GATA2 occupancy at sites of AR activity are of potentially strong clinical interest, as tumors showing GATA2 overexpression are associated with poor prognosis (11). Moreover, GATA2 binding at the promoter region of the AR gene can enhance overall production of AR, an event known to be associated with CRPC development (11). To determine whether curcumin can inhibit castrate resistance induced by either pioneering factors or AR itself, AR or GATA2 was transiently transfected in ADT-sensitive cells and the impact on AR activity assessed. As expected, PSA mRNA induction was observed under castrate conditions upon AR, as well as after ectopic GATA2 re-expression, but curcumin treatment significantly inhibited PSA induction under both conditions (Fig 6A). Similarly, in CRPC cells curcumin significantly inhibited both AR, and GATA2 mediated enhanced PSA expression (Fig 6B). These findings further underscore the potential importance of curcumin as a means to dampen GATA2-mediated AR activity in select tumors.
Figure 6. Curcumin suppresses GATA2 mediated, castrate-resistant AR activity.
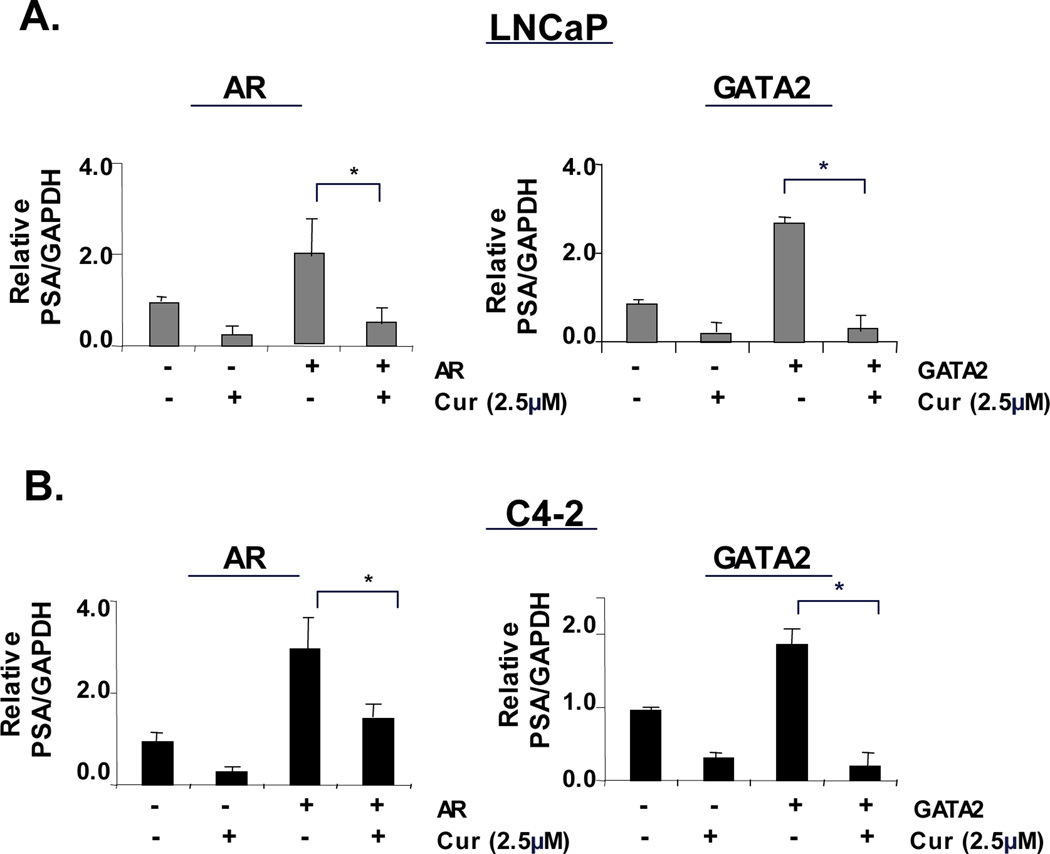
ADT-sensitive cells (LNCaP) (A) and CRPC cells (C4-2) (B) transiently transfected with expression plasmids encoding AR (left panel) or GATA2 (right panel) or empty vector, were treated with vehicle or curcumin (2.5µM) under androgen deprivation. After 24 hours, RNA was isolated for qualitative analysis of PSA mRNA expression. P<0.05.
Curcumin reduces castrate resistant tumor growth in vivo
Given the potent effects of curcumin as a means to suppress GATA2 and p300/CBP dependent AR activity, in vivo efficacy of curcumin was assessed. For these studies, immunocompromised mice harboring established human tumor xenografts were castrated (to mimic ADT) and randomized into cohorts treated with curcumin or control. As shown in Figure 7A, post-castration ADT-sensitive tumors showed delayed growth kinetics. Tumor volume as well as tumor wet-weight analyses indicated that curcumin induced significant growth retardation (Fig. 7A). Similarly for the aggressive CRPC xenograft tumors (22Rv1), curcumin treatment significantly reduced tumor growth kinetics and tumor mass (Fig 7B). These data demonstrate for the first time that curcumin not only hampers the transition of ADT-sensitive disease to castration-resistance, but is also effective in blocking the growth of established CRPC tumors in vivo.
Figure 7. Curcumin inhibits castrate resistant tumor growth and the transition to castration resistance in vivo.
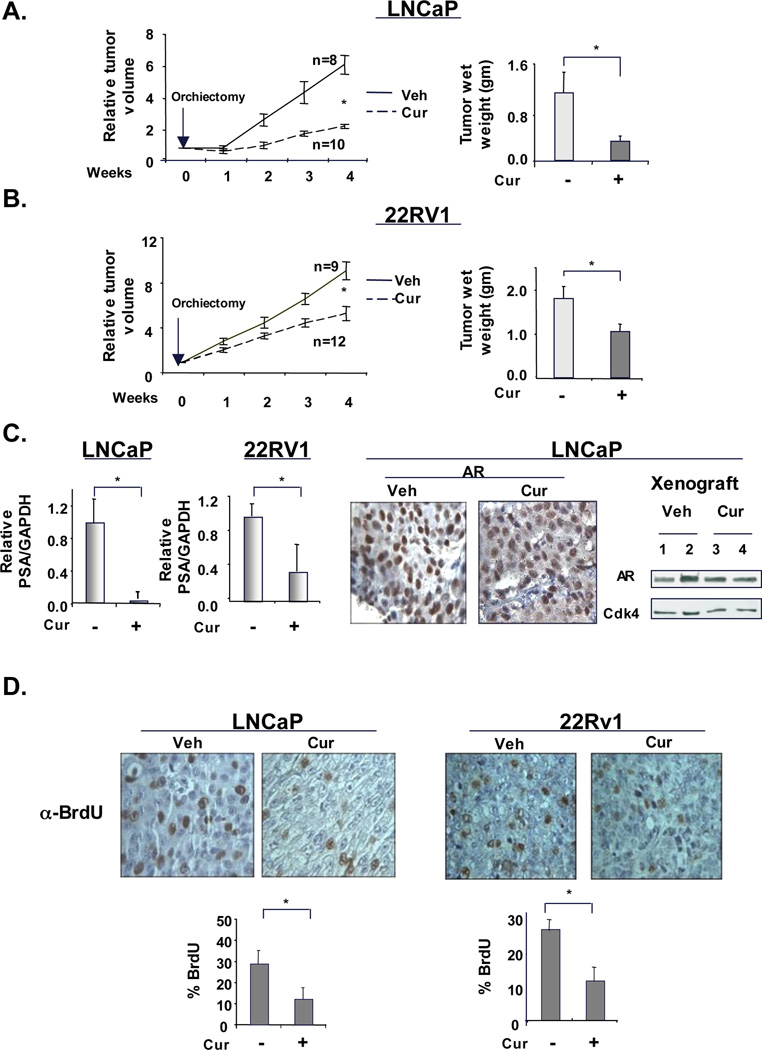
ADT-sensitive cells (LNCaP) (A) or CRPC cells (22Rv1) (B) were injected subcutaneously for xenograft studies. Once tumors reached a volume of 200–250mm3, mice were castrated to mimic hormone depletion. From the day of castration, mice were injected with vehicle or curcumin (50 mg/kg/d) intraperitoneally every 48 hours, for four weeks. At the end of the five weeks period, tumor volume (left panel) and tumor weight (right panel) was measured. (C) All tumor samples were processed for RNA isolation and relative PSA transcript was determined by q-PCR. AR expression was studied using both immunohistochemistry (middle panel) and immunoblot (right panel). (D) Mice were injected with BrdU before sacrifice and tumors were processed for immunohistochemical analysis. P<0.05.
To determine the impact of curcumin on AR activity, AR target gene expression was analyzed. As shown, KLK3/PSA and TMPRSS2 mRNA levels were reduced in both tumor types by curcumin (Fig 7C and supplementary Fig S9A). Serum-PSA quantification further confirmed that curcumin inhibits AR activity in vivo (data not shown). Immunohistochemistry (IHC) and immunoblotting of tumor tissues showed that, similar to in vitro data, curcumin exposure did not alter AR expression under conditions of androgen deprivation (Fig 7C, right panels). However, congruent with the in vitro data, in presence of constitutively active AR splice variants, curcumin inhibited mRNA and protein expression of full length-AR as well as AR-SV (Supplemental Fig S9B and S9C).
Curcumin significantly inhibited the in vivo cell proliferation of both ADT-sensitive and CRPC tumors, as tested by BrdU incorporation (Fig. 7D). Detection of apoptotic cells using caspase-3 detection as well as TUNEL assay failed to show any significant changes in xenograft tissues (data not shown). These data indicated that curcumin largely inhibits tumor cell proliferation and suggest that a combination of curcumin with currently used ADT treatment may suppress ligand- dependent as well as castrate-resistant AR activity, thus providing a novel means to target hormone receptor signaling in CRPC.
Discussion
Resurgent AR activity is the underlying cause of therapeutic failure and progression to the incurable castrate-resistant state (2, 14, 20–22). Here, it was demonstrated that curcumin cooperates with androgen-deprivation to suppress AR activity on chromatin. Functional analyses revealed that a primary and critical action of curcumin is to inhibit CBP/p300 recruitment to sites of AR activity and displace pioneer factors governing AR recruitment, thus suppressing AR-dependent cell survival. These functions of curcumin were conserved in ADT-sensitive cells as well as in CRPC model systems. Consequently, curcumin could act in concert with androgen deprivation to delay the transition to castration resistance in ADT-sensitive tumors and was able to slow the growth of castrate resistant tumors. These collective findings identify curcumin as a novel means to thwart p300/CBP and GATA2-dependent AR activity and resultant tumor growth.
Curcumin effectively suppressed CBP/p300 recruitment and acetylation of histones as a primary response and thus manifest the effects through alteration of the chromatin environment. Curcumin has been suggested to alter CBP/p300 auto-acetylation activity required for chromatin recruitment (albeit at high concentration, 100µM)(32), and inhibit p300 HAT function (33). Specifically, curcumin forms covalent bonds with p300 proteins, thus inhibiting the catalytic activity required for acetylation function (33). Moreover, HDAC inhibitors reversed the effects of curcumin on AR-target gene expression, thus indicating that curcumin mediated effects on histone acetylation are necessary for curcumin action. To our knowledge, the present study is the first to demonstrate that: 1) curcumin-mediated changes in CBP/p300 activity are attainable at physiologically attainable concentrations, and 2) inhibition of CBP/p300 function by curcumin ultimately impinges on recruitment of pioneer factors and AR (34, 35). These findings are of potentially strong clinical relevance, as CBP/p300 support ligand-independent AR activity (22) and progression to CRPC (8). As a result, recent attempt have been made to therapeutically target CBP/p300 for prostate cancer (36). Moreover, p300/CBP function is also pivotal for transcriptional function of the pioneer factor GATA2 (10, 37). Concordantly, curcumin resulted in reduced occupancy of GATA2 to regulatory loci. These observations are of relevance, as GATA2 is a major effector of AR activity and is associated with poor prognosis in advanced prostate cancer (13, 38). While curcumin dramatically altered GATA2 occupancy in both ADT-sensitive and CRPC cells, these effects were delayed as compared to the reduction in CBP/p300 occupancy (Fig 4, 5A and Fig 6A), further implicating the role of CBP/p300 in GATA2 displacement. To date, the present study provides one of the first reports to identify achievable means for suppressing GATA2 function in prostate cancer. Moreover, curcumin reduced expression of G2/M genes regulated by thus suggesting a positive outcome for different stages of PCa. While the ability of curcumin to suppress pioneer factor activity was unexpected, it suggests that combinatorial targeting of CBP/p300 HAT function, and AR activity could be of clinical benefit.
Together, the in vitro and in vivo data presented suggested a complex hierarchy for curcumin, wherein the agent suppresses early events required for AR function (i.e. histone acetylation and pioneer factor binding), thus resulting in loss of AR function, critical for tumor growth and progression to castrate resistance. Although previous reports indicate that curcumin inhibits both AR gene and protein expression in PCa cells (validated in, Fig S1C), under castrate conditions no changes were detected in AR expression (Fig 2 and Fig S3) (with an exception of cells expressing AR-splice variants, wherein differential AR regulation is reported). Interestingly, androgen itself affects AR regulation by increasing protein expression and stabilizing AR protein. Also, androgen-bound AR can either induce or inhibit AR mRNA expression in prostate cancer. Therefore the changes in culture condition (i.e. androgen status) may explain the difference in AR regulation by curcumin. Nonetheless, while effects of curcumin on relative AR levels appear to be variant depending on context and cell type, the inhibitor effect on AR activity is conserved across these model systems.
Overall, the study likely has implications far beyond PCa, since CBP/p300 and pioneer factor activity is important in multiple nuclear receptor-dependent malignancies (39–41). For example, CBP promotes treatment resistance by facilitating ligand independent activation of ER-α and the oncogenic coactivator AIB1 in breast cancer (41, 42). Similarly, GATA2 and FOXA1 function as pioneer factors for multiple nuclear receptors and are associated with poor prognosis (1, 43–45). In tumors wherein CBP function is known to be necessary for GATA2 activity (37), curcumin may prove promising therapeutic agent. Conversely, in tumors wherein GATA2 function is repressed by HDAC3 (46), curcumin mediated-CBP repression may increase response to HDAC3-targeted therapies. Similarly, FOXA1, a putative oncogene is essential in breast cancer initiation, and has been speculated to be a potential therapeutic target for both ERα positive and ERα negative apocrine breast tumors (1, 47). Since curcumin significantly reduced both GATA2 and FOXA1 residence on chromatin, it is enticing to speculate that this agent may demonstrate heightened anti-cancer activities in tumors with high FOXA1 and/or GATA.
While the data herein focused on the significant effects of curcumin on resultant AR activity, involvement of other pathways were also studied (Akt, NF-κB and Myc), but no significant changes were observed, thus indicating that under androgen depletion, curcumin mediated inhibition of CBP/p300 predominantly affects AR regulatory functions. This discrepancy in susceptibility of transcription factor function to curcumin could be due to changes in curcumin concentration used here, or due to dependency of certain loci on pioneering factors, or CBP/p300 compensation by other factors (e.g. GCN5, PCAF).
Finally, the translational relevance of the findings should be further addressed, since this agent increases the efficacy of AR-targeted strategies, and delays the onset of CRPC. Interestingly, curcumin has been assessed in multiple clinical trials (60–62) and showed little toxicity. Moreover, curcumin effectively curtailed serum-PSA expression in a clinical trial involving patients with increased PSA (but no prostate cancer) (48). In the present pre-clinical study, it is noteworthy that curcumin was effective in models that achieved ligand-independent AR activity through heightened pioneer factor expression (GATA2, Fig 6), in tumors that sustain castration-resistant phenotypes through expression of constitutively active AR splice variants (22Rv1), and in model systems wherein the means by which castration-resistance was attained remains scantly defined (C4-2). Based on these findings, it is reasonable to hypothesize that curcumin may cooperate with the second generation of AR antagonists (MDV3100 or EPI-001) (49, 50).
In summary, the present study demonstrates that curcumin impinges on CBP/p300 and pioneer factor function, thus altering the chromatin landscape so as to act in concert with hormone deprivation. Most critically, these effects were sufficient to delay the onset of tumor progression and provided novel means to control advanced tumor growth in vivo. These studies lay the foundation for further development of agents that combinatorially target factors requisite for nuclear receptor activity on chromatin and hold significant implications for the management of hormone dependent cancers.
Supplementary Material
Acknowledgement
This study was supported by grants awarded to K.E.K. from NIH (ES016675-10, and CA099996 CA116777), and by the Prostate Cancer Foundation. Additional funding was provided to S. Shah (DOD: PC080697). We thank Clay Comstock, Randy Schrecengost, Sucharitha Balasubramaniam, Michael Augello, and Matt Schiewer for their critical feedback and ongoing discussions.
References
- 1.Hurtado A, Holmes KA, Ross-Innes CS, Schmidt D, Carroll JS. FOXA1 is a key determinant of estrogen receptor function and endocrine response. Nat Genet. 43(1):27–33. doi: 10.1038/ng.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knudsen KE, Scher HI. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin Cancer Res. 2009;15(15):4792–4798. doi: 10.1158/1078-0432.CCR-08-2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yuan X, Balk SP. Mechanisms mediating androgen receptor reactivation after castration. Urol Oncol. 2009;27(1):36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knudsen KE, Penning TM. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol Metab. 21(5):315–324. doi: 10.1016/j.tem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lupien M, Brown M. Cistromics of hormone-dependent cancer. Endocr Relat Cancer. 2009;16(2):381–389. doi: 10.1677/ERC-09-0038. [DOI] [PubMed] [Google Scholar]
- 6.Lupien M, Eeckhoute J, Meyer CA, et al. FoxA1 translates epigenetic signatures into enhancer-driven lineage-specific transcription. Cell. 2008;132(6):958–970. doi: 10.1016/j.cell.2008.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carroll JS, Liu XS, Brodsky AS, et al. Chromosome-wide mapping of estrogen receptor binding reveals long-range regulation requiring the forkhead protein FoxA1. Cell. 2005;122(1):33–43. doi: 10.1016/j.cell.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Heemers HV, Debes JD, Tindall DJ. The role of the transcriptional coactivator p300 in prostate cancer progression. Adv Exp Med Biol. 2008;617:535–540. doi: 10.1007/978-0-387-69080-3_54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Debes JD, Sebo TJ, Lohse CM, Murphy LM, Haugen DA, Tindall DJ. p300 in prostate cancer proliferation and progression. Cancer Res. 2003;63(22):7638–7640. [PubMed] [Google Scholar]
- 10.Hayakawa F, Towatari M, Ozawa Y, Tomita A, Privalsky ML, Saito H. Functional regulation of GATA-2 by acetylation. J Leukoc Biol. 2004;75(3):529–540. doi: 10.1189/jlb.0603389. [DOI] [PubMed] [Google Scholar]
- 11.Bohm M, Locke WJ, Sutherland RL, Kench JG, Henshall SM. A role for GATA-2 in transition to an aggressive phenotype in prostate cancer through modulation of key androgen-regulated genes. Oncogene. 2009;28(43):3847–3856. doi: 10.1038/onc.2009.243. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Li W, Liu XS, et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell. 2007;27(3):380–392. doi: 10.1016/j.molcel.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Q, Li W, Zhang Y, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138(2):245–256. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah S, Hess-Wilson JK, Webb S, et al. 2,2-bis(4-chlorophenyl)-1,1-dichloroethylene stimulates androgen independence in prostate cancer cells through combinatorial activation of mutant androgen receptor and mitogen-activated protein kinase pathways. Mol Cancer Res. 2008;6(9):1507–1520. doi: 10.1158/1541-7786.MCR-07-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang M, Strand DW, Fernandez S, et al. Functional remodeling of benign human prostatic tissues in vivo by spontaneously immortalized progenitor and intermediate cells. Stem Cells. 28(2):344–356. doi: 10.1002/stem.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tong Q, Tsai J, Tan G, Dalgin G, Hotamisligil GS. Interaction between GATA and the C/EBP family of transcription factors is critical in GATA-mediated suppression of adipocyte differentiation. Mol Cell Biol. 2005;25(2):706–715. doi: 10.1128/MCB.25.2.706-715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wetherill YB, Fisher NL, Staubach A, Danielsen M, de Vere White RW, Knudsen KE. Xenoestrogen action in prostate cancer: pleiotropic effects dependent on androgen receptor status. Cancer Res. 2005;65(1):54–65. [PubMed] [Google Scholar]
- 18.Chadalapaka G, Jutooru I, Burghardt R, Safe S. Drugs that Target Specificity Proteins Downregulate Epidermal Growth Factor Receptor in Bladder Cancer Cells. Mol Cancer Res. doi: 10.1158/1541-7786.MCR-09-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buchan NC, Goldenberg SL. Intermittent androgen suppression for prostate cancer. Nat Rev Urol. 7(10):552–560. doi: 10.1038/nrurol.2010.141. [DOI] [PubMed] [Google Scholar]
- 20.Chen CD, Welsbie DS, Tran C, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 21.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Debes JD, Comuzzi B, Schmidt LJ, Dehm SM, Culig Z, Tindall DJ. p300 regulates androgen receptor-independent expression of prostate-specific antigen in prostate cancer cells treated chronically with interleukin-6. Cancer Res. 2005;65(13):5965–5973. doi: 10.1158/0008-5472.CAN-04-2837. [DOI] [PubMed] [Google Scholar]
- 23.Zhang HN, Yu CX, Zhang PJ, et al. Curcumin downregulates homeobox gene NKX3.1 in prostate cancer cell LNCaP. Acta Pharmacol Sin. 2007;28(3):423–430. doi: 10.1111/j.1745-7254.2007.00501.x. [DOI] [PubMed] [Google Scholar]
- 24.Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21(4B):2895–2900. [PubMed] [Google Scholar]
- 25.Syng-Ai C, Kumari AL, Khar A. Effect of curcumin on normal and tumor cells: role of glutathione and bcl-2. Mol Cancer Ther. 2004;3(9):1101–1108. [PubMed] [Google Scholar]
- 26.Tsui KH, Feng TH, Lin CM, Chang PL, Juang HH. Curcumin blocks the activation of androgen and interlukin-6 on prostate-specific antigen expression in human prostatic carcinoma cells. J Androl. 2008;29(6):661–668. doi: 10.2164/jandrol.108.004911. [DOI] [PubMed] [Google Scholar]
- 27.Osterlund KL, Handa RJ, Gonzales RJ. Dihydrotestosterone alters cyclooxygenase-2 levels in human coronary artery smooth muscle cells. Am J Physiol Endocrinol Metab. 298(4):E838–E845. doi: 10.1152/ajpendo.00693.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petre CE, Wetherill YB, Danielsen M, Knudsen KE. Cyclin D1: mechanism and consequence of androgen receptor co-repressor activity. J Biol Chem. 2002;277(3):2207–2215. doi: 10.1074/jbc.M106399200. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, Hsu BAJ, Kinseth BAM, Bjeldanes LF, Firestone GL. Indole-3-carbinol induces a G1 cell cycle arrest and inhibits prostate-specific antigen production in human LNCaP prostate carcinoma cells. Cancer. 2003;98(11):2511–2520. doi: 10.1002/cncr.11844. [DOI] [PubMed] [Google Scholar]
- 30.Wang TT, Schoene NW, Milner JA, Kim YS. Broccoli-derived phytochemicals indole-3- carbinol and 3,3'-diindolylmethane exerts concentration-dependent pleiotropic effects on prostate cancer cells: Comparison with other cancer preventive phytochemicals. Mol Carcinog. doi: 10.1002/mc.20774. [DOI] [PubMed] [Google Scholar]
- 31.Zhang C, Wang L, Wu D, et al. Definition of a FoxA1 Cistrome that is Crucial for G1-S Phase Cell- Cycle Transit in Castration-Resistant Prostate Cancer. Cancer Res. doi: 10.1158/0008-5472.CAN-11-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Black JC, Choi JE, Lombardo SR, Carey M. A mechanism for coordinating chromatin modification and preinitiation complex assembly. Mol Cell. 2006;23(6):809–818. doi: 10.1016/j.molcel.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 33.Marcu MG, Jung YJ, Lee S, et al. Curcumin is an inhibitor of p300 histone acetylatransferase. Med Chem. 2006;2(2):169–174. doi: 10.2174/157340606776056133. [DOI] [PubMed] [Google Scholar]
- 34.Li HL, Liu C, de Couto G, et al. Curcumin prevents and reverses murine cardiac hypertrophy. J Clin Invest. 2008;118(3):879–893. doi: 10.1172/JCI32865. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Balasubramanyam K, Varier RA, Altaf M, et al. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004;279(49):51163–51171. doi: 10.1074/jbc.M409024200. [DOI] [PubMed] [Google Scholar]
- 36.Santer FR, Hoschele PP, Oh SJ, et al. Inhibition of the Acetyltransferases p300 and CBP Reveals a Targetable Function for p300 in the Survival and Invasion Pathways of Prostate Cancer Cell Lines. Mol Cancer Ther. doi: 10.1158/1535-7163.MCT-11-0182. [DOI] [PubMed] [Google Scholar]
- 37.Jiang H, Liu L, Yang S, Tomomi T, Toru N. CREB-binding proteins (CBP) as a transcriptional coactivator of GATA-2. Sci China C Life Sci. 2008;51(3):191–198. doi: 10.1007/s11427-008-0038-4. [DOI] [PubMed] [Google Scholar]
- 38.Cirillo LA, Lin FR, Cuesta I, Friedman D, Jarnik M, Zaret KS. Opening of compacted chromatin by early developmental transcription factors HNF3 (FoxA) and GATA-4. Mol Cell. 2002;9(2):279–289. doi: 10.1016/s1097-2765(02)00459-8. [DOI] [PubMed] [Google Scholar]
- 39.Acevedo ML, Kraus WL. Mediator and p300/CBP-steroid receptor coactivator complexes have distinct roles, but function synergistically, during estrogen receptor alpha-dependent transcription with chromatin templates. Mol Cell Biol. 2003;23(1):335–348. doi: 10.1128/MCB.23.1.335-348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Luca A, Severino A, De Paolis P, et al. p300/cAMP-response-element-binding-protein ('CREB')-binding protein (CBP) modulates co-operation between myocyte enhancer factor 2A (MEF2A) and thyroid hormone receptor-retinoid X receptor. Biochem J. 2003;369(Pt 3):477–484. doi: 10.1042/BJ20020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamei Y, Xu L, Heinzel T, et al. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85(3):403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 42.Shang Y, Hu X, DiRenzo J, Lazar MA, Brown M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell. 2000;103(6):843–852. doi: 10.1016/s0092-8674(00)00188-4. [DOI] [PubMed] [Google Scholar]
- 43.Tsai FY, Orkin SH. Transcription factor GATA-2 is required for proliferation/survival of early hematopoietic cells and mast cell formation, but not for erythroid and myeloid terminal differentiation. Blood. 1997;89(10):3636–3643. [PubMed] [Google Scholar]
- 44.Anttonen M, Unkila-Kallio L, Leminen A, Butzow R, Heikinheimo M. High GATA-4 expression associates with aggressive behavior, whereas low anti-Mullerian hormone expression associates with growth potential of ovarian granulosa cell tumors. J Clin Endocrinol Metab. 2005;90(12):6529–6535. doi: 10.1210/jc.2005-0921. [DOI] [PubMed] [Google Scholar]
- 45.Sebastian S, Takayama K, Shozu M, Bulun SE. Cloning and characterization of a novel endothelial promoter of the human CYP19 (aromatase P450) gene that is up-regulated in breast cancer tissue. Mol Endocrinol. 2002;16(10):2243–2254. doi: 10.1210/me.2002-0123. [DOI] [PubMed] [Google Scholar]
- 46.Ozawa Y, Towatari M, Tsuzuki S, et al. Histone deacetylase 3 associates with and represses the transcription factor GATA-2. Blood. 2001;98(7):2116–2123. doi: 10.1182/blood.v98.7.2116. [DOI] [PubMed] [Google Scholar]
- 47.Robinson JL, Macarthur S, Ross-Innes CS, et al. Androgen receptor driven transcription in molecular apocrine breast cancer is mediated by FoxA1. Embo J. 30(15):3019–3027. doi: 10.1038/emboj.2011.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ide H, Tokiwa S, Sakamaki K, et al. Combined inhibitory effects of soy isoflavones and curcumin on the production of prostate-specific antigen. Prostate. 70(10):1127–1133. doi: 10.1002/pros.21147. [DOI] [PubMed] [Google Scholar]
- 49.Scher HI, Beer TM, Higano CS, et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1-2 study. Lancet. 375(9724):1437–1446. doi: 10.1016/S0140-6736(10)60172-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


