Abstract
The effect of ecdysteroid signaling on Drosophila female precopulatory behavior was investigated using two types of mutants with either globally reduced ecdysteroid availability or reduced expression of ecdysone receptors in fruitless neurons, known to control sexual behavior. While being courted by males, mutant females performed significantly less full ovipositor extrusion behavior to reject male copulation attempts. Ecdysteroid depleted females (ecdysoneless1) performed male-like courtship behaviors, including unilateral wing extension and song production with patterns very similar to male courtship song. These results support the hypothesis that ecdysteroids modulate female sexual behavior, perhaps acting as a regulator of sexual motivation, and as a component affecting the performance of sex specific behavior patterns.
Keywords: Full ovipositor extrusion, ecdysone, neuroactive, courtship, sex determination hierarchy
Introduction
In many animals, males and females cooperate in the performance of a courtship ritual prior to mating. In the courtship of the fruit fly Drosophila melanogaster, the male is the more overt participant. He communicates his sexual interest in his partner by orienting, following, singing, tapping, licking and attempting copulation (Bastock and Manning, 1955). Female Drosophila behave more passively and their contribution to courtship progress is not as well understood as that of the male (Ferveur, 2010). While being courted by a male, the female walks or runs about, apparently rejecting her suitor by various means including fully extruding her ovipositor (Connolly and Cook, 1973) and producing rejection sounds. After continued male attention, partial extrusion of the female’s ovipositor may signal her increased receptiveness to her suitor, as may the production of a pheromone droplet and subsequent distribution of its contents by grooming (LasBleiz et al., 2006). After sustained attention from the male, the female may allow the male to mount and copulation ensues.
Control of male courtship behavior has been shown to require the ecdysteroid hormone signaling system. Males deficient in either ecdysteroid level (Ganter et al., 2011) or the nuclear ecdysone receptor (EcR) (Ganter et al., 2007, Dalton et al., 2009) show elevated levels of male-male courtship. Derived from cholesterol mainly in the larval prothoracic gland, the ecdysteroids, especially 20-hydroxyecdysone, together with EcR comprise an essential developmental signal pathway (Riddiford, 1993). In the adult Drosophila female , ecdysteroids are produced in the ovary (Handler, 1982). Ecdysteroid signaling has been implicated in adult processes such as oogenesis (Carney and Bender, 2000), memory (Ishimoto et al., 2009) and sleep (Ishimoto and Kitamoto , 2010). In this report we show that the ecdysteroid system is involved in female precopulatory behavior as well. We studied females homozygous for ecdysoneless1, a temperature sensitive allele affecting ecdysteroid concentrations. This allele results in ecdysteroid levels that are sufficient for normal development at cooler temperatures such as 18°C, but ecdysteroid level can be reduced by increased temperature treatment (29°C) to about 13% of wild-type levels (Garen et al., 1977). We also used RNAi mediated knockdown of the EcR, targeted by the GAL-4/UAS system to identify the fruitless neurons as one cellular target of ecdysteroid signaling that influences sexual behavior.
Materials and Methods
Flies and conditions
Wild-type (Canton-S: FBst0000001), ecdysoneless1 / ecdysoneless1 (FBgn0000543; ecd[1] st[1] ca[1]) and UAS-EcR-RNAi (FBst0009327; w1118; P{UAS-EcR-RNAi}104) flies were obtained from the Bloomington Drosophila Stock Center. fruitless-Gal4 (Stockinger et al., 2005) was crossed with UAS-EcR-RNAi to achieve fruitless cell specific knockdown of all EcR isoforms. Flies were maintained on dextrose-yeast-cornmeal medium, 18°C (ecdysoneless1) or 24°C (others), 50–60% humidity, 12h light:12h dark. To ensure social naïveté in behavioral trials, all flies were isolated in individual vials as pharate adults.
Latency to mating
Socially naïve five-day-old ecdysoneless1 or wild-type females were treated at 29°C for 12 hours. Within 4 hours following subjective dawn, female were aspirated into a courtship chamber at 29°C with a socially naïve five-day-old wild-type male. The time until the pair began a copulation bout that lasted for more than one minute was measured, as was ovipositor extrusion and wing extension (described below) and observation was terminated after 30 minutes.
Ovipositor extrusion
Socially naïve five-day-old female ecdysoneless1 flies were transferred to 29°C. 12 hours later, within 4 hours following subjective dawn, test females and naïve wild-type male suitors of the same age were aspirated without anesthesia into 9mm diameter (0.27 cm3) assay chambers at 29°C, humidified by a small block of 1% agar gel separated from the assay chamber by a nylon mesh barrier. Video recording was initiated immediately. Mutant and wild-type experimental flies in rescue experiments were, for the six-day period between eclosion and assay, fed standard food supplemented with 10−4M 20-hydroxyecdysone (20E: Sigma), or control diet consisting of the standard food supplemented with solvent only (ethanol, 0.003% total). In preliminary studies, longer induction times (up to 96 hours) produced similar behavioral results, but rescue was less effective. Assays were recorded by video cameras placed in an incubator. Video recordings were analyzed by trained observers blind to the genotype and experimental treatment of the subjects. Stopwatches were used to measure the total time in a 30-minute assay period each subject spent performing full ovipositor extrusion, and other behaviors including male-like behaviors such as tapping, licking, mounting and unilateral wing extension. Full ovipositor extrusion index was calculated as the percentage of total duration of this behavior per second of singing performed by the male suitor, and means were compared by Student t-test. Female wing extension index was calculated as the percentage of total duration per second of unilateral wing extension during the precopulatory period. To analyze fru-GAL4/UAS-EcR-RNAi females, the same procedure was undertaken, except that females were reared and analyzed at 24°C.
Attractiveness
Attractiveness assays were performed using test females decapitated with a razor blade under carbon dioxide anesthesia. Females that resumed standing after the procedure were introduced to a courtship chamber at 29°C (ecdysoneless1) or 24°C (fru-GAL4/UAS-EcR-RNAi) with a naïve wild-type male suitor. The total time the suitor spent performing unilateral wing extension, licking, mounting and attempting copulation was measured in a 10-minute period, and the total was divided by 600 seconds to produce a courtship index expressed as a percentage. Means were compared by Student t-test.
Motor activity
To measure motor activity (Figures 2, 4), the number of times each fly crossed the midline of the courtship chamber was counted during a 10-minute period without the introduction of a suitor, at 29°C (ecdysoneless1) or 24°C (fru-GAL4/UAS-EcR-RNAi). All means were compared by Student t-test (Microsoft Excel).
Figure 2.
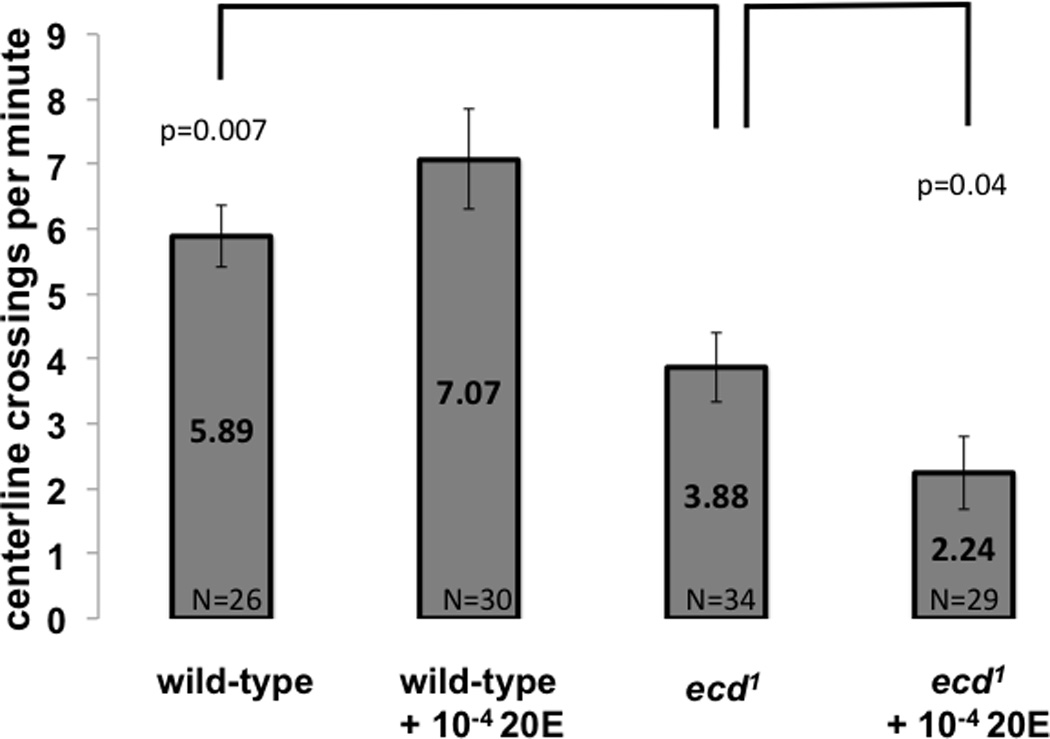
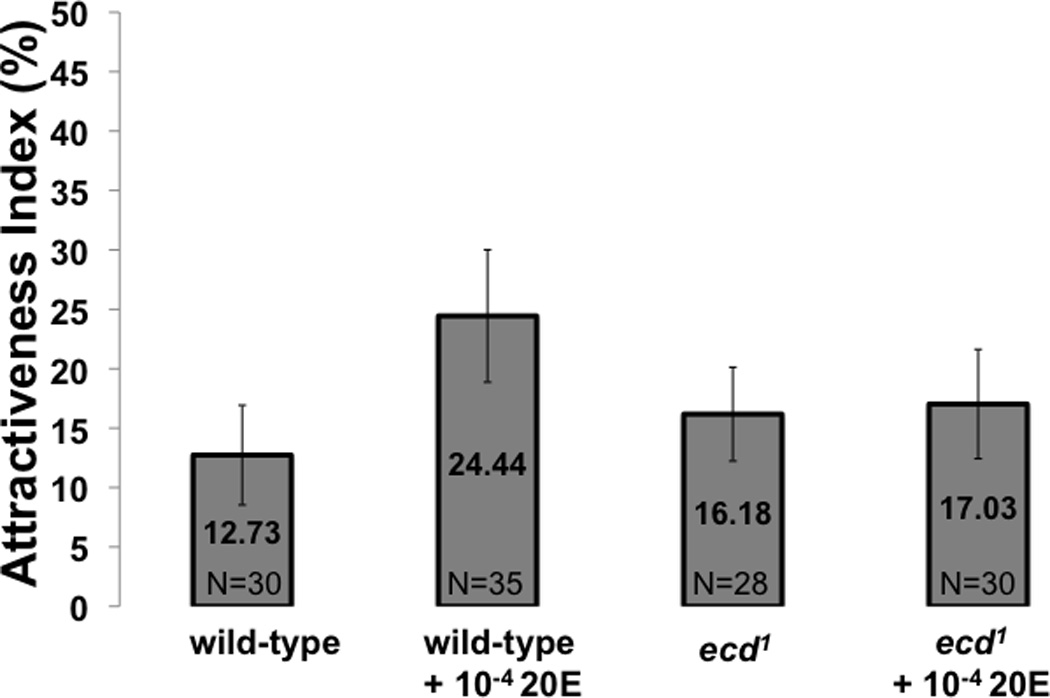
Figure 2A: Motor activity of ecdysoneless1 females. Ecdysone-depleted female flies are less active. The same ecdysoneless1 females treated at 29°C for 12 hours as in Figure 1 were analyzed for motor activity by measuring the frequency at which they cross an arbitrary line at the center of their otherwise empty chamber. ecdysoneless1 females were significantly less active than wild-type controls, and when their diet contained 10−4 M 20E, their activity was further reduced. Ecdysteroid supplementation of the diet of wild-type control females did not significantly affect motor activity. P values are from Student t-test. Whiskers indicate standard error of the mean.
Figure 2B: Attractiveness of decapitated females. The non-behavioral attractiveness of mutant and wild-type females was assessed by measuring the level of courtship they elicit following decapitation. The time wild-type male suitors spent performing unilateral wing extension, licking, tapping, and attempting copulation was measured in a 10-minute period and an attractiveness index was expressed as duration of courtship divided by total time. No significant differences were observed. Whiskers indicate standard error of the mean.
Figure 4.
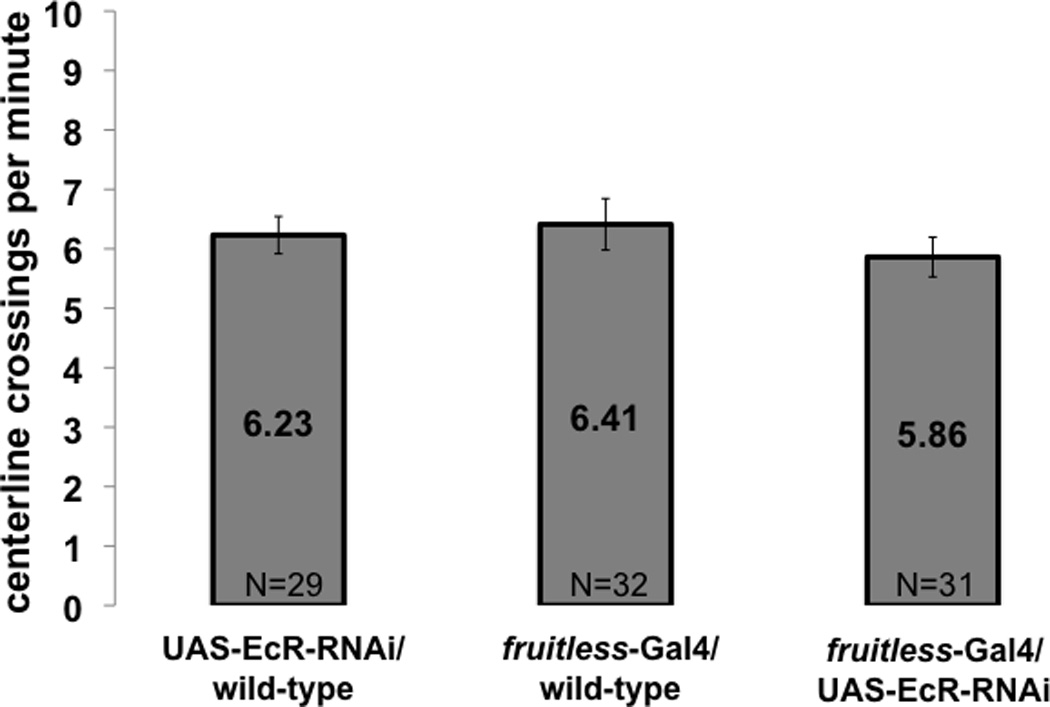
Motor activity of EcR mutant females. The same female flies as in Figure 3, with reduced EcR in fruitless neurons, were analyzed for motor activity by measuring the frequency at which they cross an arbitrary line at the center of their otherwise empty chamber. Females with fruitless-specific reduction of EcR level are as active as controls. P values are from Student t-test. Whiskers indicate standard error of the mean.
Acoustic analysis
Courtship songs were continuously recorded during 20-minute interactions of one wild-type male with one ecdysoneless1 female at 27–29 °C (Fig 5). Acoustic signals of males and females were recorded with a microphone (Bruel & Kjaer Type 4165), amplified (Bruel & Kjaer Type 2619 and 5935) and directly digitized. The software Audacity 1.3.12beta (http://audacity.sourceforge.net) was used for data acquisition and analysis. Frequency spectra of pulse songs and sine songs were determined by Fourier transformation with a 2048 width Hanning window.
Figure 5.
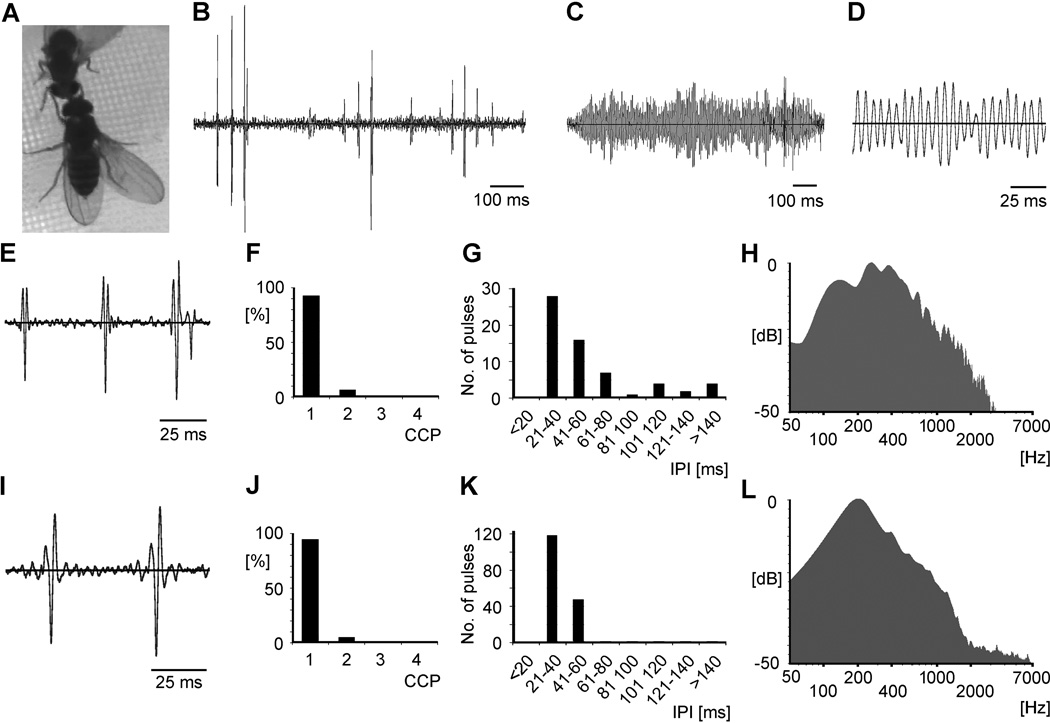
ecdysoneless1 female and wild-type male song patterns. A ecdysoneless1 female producing sound by vibration of one extended wing. B Sequence of pulses from an ecdysoneless1 female. C, D Sequence and enlarged fraction of sine song from an ecdysoneless1 female. E–H Pulse song patterns of ecdysoneless1 females. E Three pulses. F Oscillation cycles per pulse (CPP), mean 1.07, N= 75. G Inter pulse intervals (IPI), mean 59.82 ± 46.93 ms, N= 62. H Power spectrum with dominant frequency 256 Hz. I–L Pulse song patterns of wild-type males. I Two pulses. J CPP, mean 1.05, N= 177. K Inter pulse intervals (IPI), mean 38.16 ± 8.75 ms, N= 168. L Power spectrum with dominant frequency 208 Hz.
Results
Adult ecdysoneless1 females depleted of ecdysteroid perform significantly less full ovipositor extrusion to reject male mating attempts (Fig 1A), compared to wild-type. Wild-type females initially respond to male courtship with frequent full extrusion of their ovipositors, spending an average of about 20 seconds with their ovipositors extruded for every 100 seconds of male singing. In contrast, mutant females, while capable of doing so (Fig 1B), rarely fully extruded their ovipositor during courtship, spending only an average of about 3 seconds with their ovipositors fully extruded for every 100 seconds of male singing (Fig 1A, compare columns 1 and 3). When 20-hydroxyecdysone (20E) was added to the ecdysoneless1 females’ diet (10−4 M) prior to the courtship assay, the level of full ovipositor extrusion was significantly increased (Fig 1A, compare columns 3 and 4), although not to the level seen in wild-type females. While not rising to the level of statistical significance, we noticed a nominal increase in full ovipositor extrusion in the wild-type females fed 20E as well (column 2). In an experiment comparing latency to mating (data now shown), 95% of wild-type females mated within 30 minutes, while only 75% of steroid-depleted females did so. Among those mutant females failing to mate within 30 minutes, the average ovipositor extrusion index was 3.73% (+/− 5.53), and wing extension index, a behavioral element typical of male courtship, was 1.52% (+/−3.16). Among those mutant females that did mate in the 30-minute observation period, 67% mated in less than five minutes with an average ovipositor extrusion index of 0.91%(+/−1.24) and wing extension index of 0.18%(+/−0.38).
Figure 1.
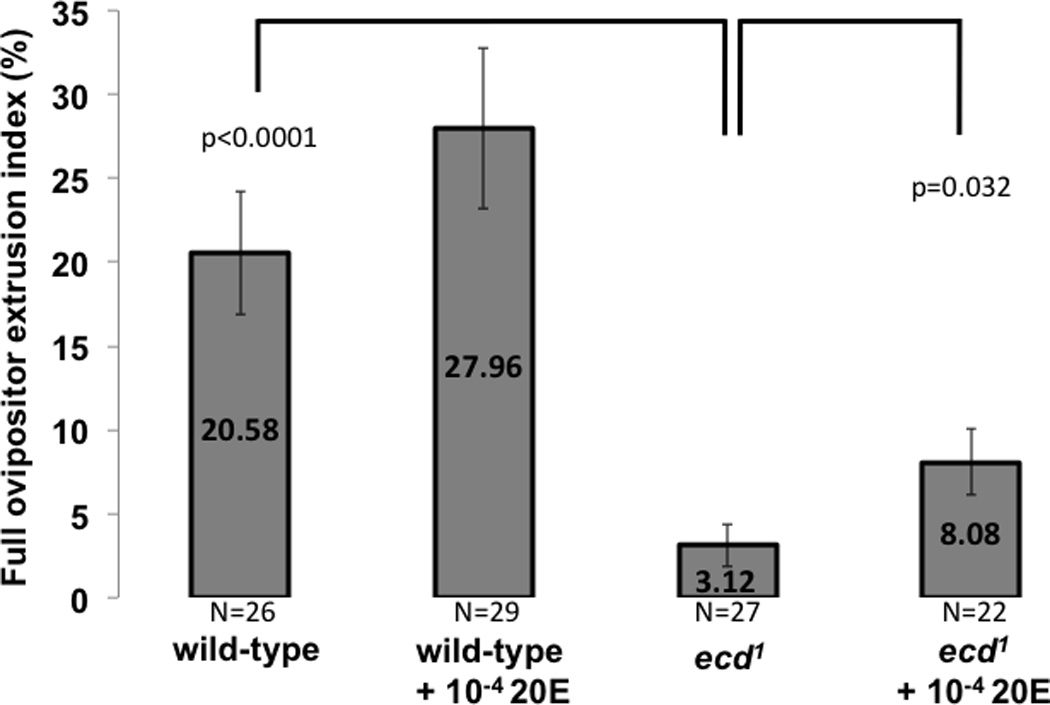

Figure 1A: ecdysoneless1 full ovipositor extrusion index. Ecdysteroid depleted female flies perform significantly less full ovipositor extrusion than controls. The descriptions underneath each histogram bar describe treatment condition of female flies. Socially naïve five-day-old ecdysoneless1 females treated at 29°C for 12 hours to reduce ecdysteroid levels respond to wild-type male courtship with significantly less performance of full ovipositor extrusion than similarly treated wild-type (Canton-S) control females. ecdysoneless1 females fed diet containing 10−4 M 20E during exposure to 29°C performed full ovipositor extrusion significantly more than those fed normal diet. P values are from Student t-test. Whiskers indicate standard error of the mean.
Figure 1B: Full ovipositor extrusion. A female Drosophila initially responds to male courtship by fully extruding her ovipositor (arrow). This action has been suggested to represent the female’s initial rejection of her suitor’s advances (Lableiz et al., 2006). An ecdysoneless1 female is depicted.
To assess the general fitness of mutant females, their motor activity was measured in the same courtship chambers by counting the number of times each female crossed the midline while alone in the chamber (Fig 2A). ecdysoneless1 females were found to be less active than wild-type females, showing a roughly 30% lower centerline crossing rate. This mild hypoactivity became more pronounced when the female ecdysoneless1 were fed 20E, but the same treatment did not significantly change motor activity of wild-type females.
Since ecdysteroid-related alterations to pheromone profiles have been observed by others (Wicker and Jallon, 1995), and since it is possible that such alterations might affect our assays of female precopulatory behavior by altering suitor attraction to experimental females, we measured the attractiveness of decapitated ecdysteroid-depleted females to wild-type male suitors. Thus we were able to measure the level of attractiveness of a female in the absence of her own behavior. We also used decapitated wild-type females as targets in courtship assays with wild-type males, and measured the time the male spent performing any elements of the standard male courtship sequence, including singing, tapping, licking and attempting copulation. We found no significant differences in the attractiveness of ecdysone-depleted females compared to that of wild-type females, or females fed 20E during the ecdysone depleting temperature elevation period (Fig 2B).
Because ecdysone signaling in fruitless expressing neurons has been shown to affect courtship behavior in the male (Dalton et al., 2009), precopulatory behavior of females with EcR levels reduced by RNAi in fruitless neurons was analyzed. In the presence of wild-type males, such females showed significant reduction in full ovipositor extrusion, compared to controls (Fig 3). While these fruitless neuron specific EcR mutant females recapitulated the ovipositor phenotype noticed in ecdysoneless1 females subjected to global ecdysteroid deficiency, motor activity, measured as the number of centerline crossings of females alone in courtship assay chambers, was unaffected by this more specific manipulation (Fig 4).
Figure 3.
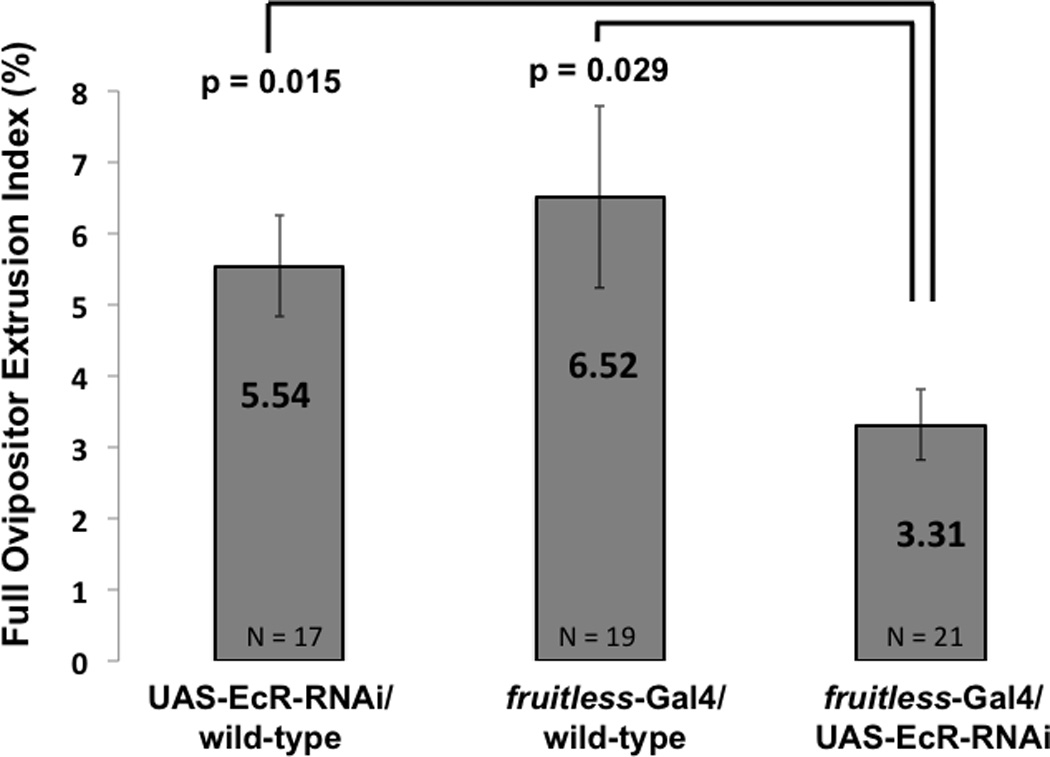
Ecdysone receptor mutant full ovipositor extrusion index. Female fruitless-Gal4/UAS-EcR-RNAi flies in which EcR was reduced by RNA interference specifically in fruitless neurons perform significantly less full ovipositor extrusion than controls. P values are from Student t-test. Whiskers indicate standard error of the mean.
While full ovipositor extrusion rejection behavior was reduced in female ecdysteroid signaling mutants, the performance of male-like courtship behaviors, not observed in wild-type females, was increased. 62.5% of ecdysoneless1 females oriented toward their male suitors and tapped them, mounted them by approaching the males’ anterior ends and climbing onto their heads, spending an average total of 24.76 seconds in a 30-minute assay period performing such behaviors. 47% of ecdysoneless1 females extended one wing into the typical position for sound production, with an average total duration of 8.97 seconds in a 30-minute assay period. Ecdysteroid-depleted female unilateral wing extension was always directed toward a courting male (Fig 5A). ecdysoneless1 females displaying any of these behaviors towards other females were not observed (N=50, not shown).
Sound recordings revealed that by vibrating a laterally extended wing, mutant females produced sounds that resembled wild-type male courtship song. Female song included both sine (Fig. 5C, D) and pulse components (Fig. 5B, E). Sine songs were rarely produced (only three recordings from two different females) and consisted of regular oscillations with major frequency components of 158 Hz, 189 Hz and 195 Hz. Dominant frequencies of wild-type male sine songs are 140–170 Hz (von Schilcher, 1976). Female pulse sequences contained up to 13 individual pulses (Fig. 5E). Most of these pulses (95%) consisted of one cycle (Fig. 5F, average cycles per pulse: 1.07), occurred at quite variable intervals of 20–80 ms (Fig. 5G, average inter pulse interval (IPI) was 59.82 ± 46.93 ms) and had major frequency components ranging from 204 Hz to 268 Hz (Fig. 5H shows one example). Pulse songs of wild-type males recorded during the same male-female interactions contained up to 45 individual pulses (Fig. 5I). Like in ecdysoneless1 females, most pulses (98%) consisted of one oscillation cycle (Fig. 5J, average cycles per pulse: 1.05), which occurred at more regular and on average shorter IPIs (Fig. 5K, average 38.16 ± 8.75) and contained dominant frequencies ranging from 167 Hz to 232 Hz (Fig. 5L shows one example).
Discussion
In previous studies, when ecdysteroid signaling levels were reduced in male EcR mutants (Ganter et al., 2007; Dalton et al., 2009) or male ecdysoneless1 mutants (Ganter et al., 2011), male flies showed significantly elevated male-male attraction including all typical elements of female-directed courtship except copulation. This suggests that in male fruit flies, ecdysteroid signaling modulates courtship behavior. In this study, we report evidence that ecdysteroid signaling influences female fruit fly precopulatory behavior as well.
In courtship between wild-type males and ecdysteroid-depleted ecdysoneless1 mutant females, we observed significantly less performance of a female precopulatory behavior called full ovipositor extrusion (Fig 1B). This action has been described as a rejection behavior (Connolly and Cook, 1973; Lasbleiz et al., 2006), as it appears to block copulation attempts by the male, and is performed by females during early stages of courtship. If full ovipositor extrusion indicates unwillingness to copulate, then perhaps reduction of this behavior is related to an increase in sexual motivation and receptivity. A comparable increase in sexual motivation is evident in previous studies of the ecdysoneless1 male, which shows an elevated courtship level in the presence of both male and female targets (Ganter et al., 2011). In this way, the evidence supports the hypothesis that in both sexes, ecdysteroid deficiency positively affects sexual motivation. The role of steroid hormones in the modulation of sexual behavior in vertebrates has been well established (McCarthy and Ball, 2008; Henley et al., 2011).
When ecdysoneless1 females were fed exogenous 20E prior to assay, their level of full ovipositor extrusion increased significantly, although not to levels observed in the wild-type. This rescue effect supports the notion that it is specifically the ecdysteroid deficiency feature, and no other feature of this mutant, that underlies the observed alterations in behavior. A similar increase in full ovipositor extrusion behavior was also noted in wild-type females fed exogenous 20E. Although this increase was not statistically significant, the nominal increment is consistent with the idea that higher ecdysteroid levels increase the likelihood of any female fly to perform this precopulatory behavior. The observed failure to completely rescue the ecdysoneless1 female to wild-type levels of full ovipositor extrusion may be due to poor availability of exogenously supplied ecdysteroid at its site of action. Higher levels of dietary 20E did not ameliorate this situation (10−3 M, not shown).
While it might be expected that because of their low performance of rejection behavior, steroid-depleted females would mate more promptly than wild-type, we did not find that to be the case. While 50% of mutant females mated in less than 5 minutes, 25% failed to mate within the 30-minute observation period. The fast-mating group had the lowest ovipositor extrusion index (0.91%) and wing-extension frequency (0.98%), and the group failing to copulate had the highest (3.73% and 1.52%, respectively). We propose that while reduced rejection behavior may serve to reduce copulation latency, the appearance of ectopic male-like behaviors may serve to increase it, even to the point at which mating fails entirely. We propose that while rejection behavior may be reduced in ecdysteroid-depleted females, which would lead to faster mating, the production of male behaviors may in fact interfere with courtship progression by either interrupting female arousal or confusing the male suitor, or both.
In the male, one site of ecdysteroid action appears to be the behavior-controlling neurons that express the sex-specific transcript of the fruitless gene, since in previous studies disruption of the expression of the nuclear ecdysone receptor EcR specifically in these neurons recapitulated the high male-male courtship phenotype of ecdysoneless1 mutants (Dalton et al., 2009). fruitless is a member of the Drosophila sex determination hierarchy, and is thought to control the sex of the nervous system (Gailey and Hall, 1989; Siwicki and Kravitz, 2009). This study shows that the fruitless expressing neurons of female flies, like those of males, apparently also require EcR to modulate precopulatory behavior. When EcR level is reduced in fruitless neurons by the cell-specific expression of an RNAi allele of EcR, females perform significantly less full ovipositor extrusion than control genotypes (Fig 3). The fruitless-specific expression pattern produced in females of the fruitless-Gal4 genotype used in this study is strikingly similar to that of males (Stockinger et al., 2005). Male flies’ fruitless expressing neurons produce a male-specific fruitless protein. However, due to differential splicing, female flies are not known to produce any sex specific fruitless protein, although they express other fruitless products responsible for some undefined functions that affect general vitality (Gailey and Hall, 1989; Anand et al., 2001). Nevertheless, we show that EcR deficiency in the fruitless neurons of females leads to altered sexual behavior including a decrease in precopulatory full ovipositor extrusion that may indicate increased sexual motivation.
Some insight as to the mechanism of ecdysteroid modulation of the sexual behavior related fruitless neurons might be gained by considering another, more dramatic alteration of sexual behavior in the steroid depleted ecdysoneless1 female. In addition to their reduced rejection behavior, about one half of ecdysoneless1 females analyzed displayed a group of actions that are similar to male courtship behavior. They oriented toward, followed, tapped, and mounted their male suitors, and performed unilateral wing extension and vibration. These ecdysteroid-depleted females thus show an increase in sexual motivation by both a reduction in the typical female rejection behavior, and an addition of behaviors typical of high male sexual interest. Abdomen bending, normally employed by males during courtship and copulation, was not observed. The acoustic signals produced by these females were very similar to wild type male courtship song. While ecdysoneless1 female songs were comparatively brief, they included both sine and pulse components produced by males. Major song characteristics such as dominant frequency of sine and pulse songs, numbers of oscillations per pulse and the predominant duration of interpulse intervals (IPI) were quite similar to male courtship songs, whereas the number of pulses within each pulse sequence was lower and irregular and average duration of IPIs was increased. Increased IPIs were also observed in a study of von Philipsborn et al. (2011) who stimulated both male and female song production by thermal activation of TrpA1 channels in particular subsets of fruitless-expressing neurons, suggesting that the thoracic song pattern generating circuits produce slower pulse rhythms in females. In a similar study by Clyne and Miesenböck (2008) both typical sine and pulse songs were stimulated by photoactivation of fruitless-expressing neurons in both male and female headless flies. Both studies demonstrated that the thoracic neuronal circuitry for the generation of sine and pulse song patterns is expressed and functional in Drosophila females. However, wild-type females do not produce courtship songs, suggesting that the neural commands for song initiation (e.g. descending command neurons or appropriate coupling to sensory systems) are absent in females. Sounds that are produced by wild-type females include grooming sounds, occasional pulses generated during female- and male-directed aggression and buzzes to reject male mating attempts (Paillete et al., 1991; our own recordings). All these signals are clearly distinguishable from sine and pulse courtship songs, which are absent from the natural behavior of wild-type females.
Whereas wild-type D. melanogaster females do not sing, other mutant females of this species (Demir and Dickson, 2005) and wild-type females of other Drosophila species (Satokangas et al., 1994) have been reported to sing. Misexpression of the male-specific isoform of fruitless in an otherwise normal D. melanogaster female fly allows her to perform many elements of male reproductive behavior including courtship singing, showing that manipulation of the sex of the nervous system can lead to a corresponding change in sex-specific behaviors (Demir and Dickson, 2005; Manoli et al., 2005). The fact that ecdysteroid depleted female flies sing during interactions with courting males, but not with females, suggests that ecdysteroids play a role in shaping the sex specificity of the sensory integration or motor output elements of courtship behavior. Perhaps ecdysteroids achieve such control by interacting with the sex determination hierarchy or by influencing the excitability of certain subsets of the neurons involved in controlling sexual behavior. Reduction of ecdysteroid signaling levels in females, or males (Ganter et al., 2011), does not alter behavioral patterns involved in courtship but rather alters the initiation of those patterns in response to courtship target-derived olfactory, gustatory and visual stimuli (Koganezawa et al., 2010; Pan et al., 2011). This result joins the growing body of evidence suggesting that ecdysteroids have a role in regulating adult behavioral processes, for example, in memory and in sleep (Ishimoto et al., 2009; Ishimoto and Kitamoto, 2010).
In contrast with ecdysoneless1 females, we did not observe any EcR mutant females singing or performing any other male-like behaviors and their locomotor activity was not reduced, although full ovipositor extrusion was reduced in both mutants. This indicates a behavioral role for EcR in neurons other than those expressing the fruitless-specific EcR RNAi allele in these EcR mutant females or perhaps the involvement of non-neuronal tissues. Alternatively the ecdysteroid-sensitive neurons that control singing in the female could respond to ecdysteroid via a different receptor, like the dopamine sensitive G-protein coupled membrane receptor DopEcR (Srivastava et al., 2005), and are hence unaffected by nuclear EcR deficiency. It is also possible that the reduction in ecdysteroid signaling achieved in the ecdysoneless1 mutant female is more profound than that achieved in the EcR mutant, and only at such low levels of ecdysteroid signaling is singing allowed to occur.
Ecdysteroid levels are known to affect the profiles of cuticular hydrocarbon pheromones. In one study, ecdysoneless1 females showed a reduction in aphrodisiac diene levels (Wicker and Jallon, 1995). Because a pheromonal effect of steroid deficiency must be considered in the interpretation of the behavior of ecdysoneless1 females, we measured female attractiveness in a behavioral assay. When freshly-decapitated females were used as objects in courtship assays with wild-type males, no difference was observed in the attractiveness of ecdysteroid-depleted females, compared to wild-type females, or to females fed 20E (Fig 2B). We conclude that ecdysteroid-dependent changes in female precopulatory behavior are not the result of any ecdysteroid-dependent changes in female pheromone expression that influence female attractiveness and hence the intensity of male courtship. To the same point, alteration of the cuticular hydrocarbon profile is not expected in females that are mutant for EcR specifically in fruitless neurons. The altered behavior of both mutants is therefore likely to be entirely the result of the female’s specifically perturbed nervous system, and not a result of altered pheromonal profile and corresponding changes in the perception of the mutant female by male suitors.
The motor activity level of female flies in these experiments appears to show some correlation to ecdysteroid levels (Fig 2A). Ecdysteroid depleted females show a significant reduction in motor activity, measured by centerline crossings, compared to wild-type. This could be the result of a specific effect of ecdysteroid depletion on spontaneous motor activity. Reduced adult female movement becomes more dramatic with the addition of exogenous 20E to the female ecdysoneless1 flies’ food, whereas it has no significant effect on the wild-type. Reduced locomotion and alteration of other physiological parameters in ecdysoneless1 larvae were noticed by Li and coworkers (2001) and since upper threshold concentrations have been shown to exist for ecdysteroids (Cherbas et al., 1981; Peel and Milner, 1992; Champlin and Truman, 1998a and b), it may be that supplying exogenous 20E at this concentration has the same effect on motor activity as further depleting ecdysteroid levels. Ecdysteroid levels may indeed have an effect on overall adult female locomotion in this assay, but ecdysteroid-related changes in activity level are not correlated with ecdysteroid-related performance of full ovipositor extrusion. This can be seen more clearly when ecdysteroid signaling is intact everywhere in the fly except the fruitless expressing cells of the nervous system. In females lacking EcR in the fruitless neurons, full ovipositor extrusion is reduced while motor activity is not (compare figures 2 and 4). Activity levels are the same for EcR mutants as they are for controls including wild-type. Therefore it appears that overall motor suppression is not the cause of the reduced full ovipositor extrusion seen in these mutants.
Taken together, the results described here support the hypothesis that one role of ecdysteroids in the adult female Drosophila is as a neuroactive modulator of sexual behavior.
Research Highlights.
Ecdysteroid depleted female flies sing to male suitors
Ecdysteroid depleted female flies sing songs acoustically similar to those of wild-type males
Ecdysteroid depleted females flies show reduced rejection behavior that can be reversed by ecdysone feeding
Acknowledgements
The authors thank R. Weston for technical assistance. This work was supported by grants from the National Institutes of Health (NIGMS R15 GM080713-01 and 3R15GM080713-01A1S1 to GG), and by the Dean's and Provost's offices of the College of Arts and Sciences, University of New England, and these funding sources had no scientific involvement. Fly stocks were provided by the Bloomington Drosophila Stock Center at Indiana University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
GK Ganter, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
JB Desilets, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
JA Davis-Heim, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
AE Panaitiu, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
M Sweezy, Department of Pharmaceutical Sciences, School of Pharmacy, Saint Joseph College, West Hartford, Connecticut, 06117, USA.
J Sungail, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
LCH Tan, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
AM Adams, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
EA Fisher, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
JRM O’Brien, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
KM Kincaid, Department of Biology, College of Arts and Sciences, University of New England, Biddeford, Maine, 04005, USA.
R Heinrich, Department of Cellular Neurobiology, Institute for Zoology, Georg-August-University, Göttingen, Germany.
Works Cited
- Anand A, Villella A, Ryner LC, Carlo T, Goodwin SF, Song H-J, Gailey DA, Morales A, Hall JC, Baker BS, Taylor BJ. Molecular genetic dissection of the sex-specific and vital functions of the Drosophila melanogaster sex determination gene fruitless. Genetics. 2001;158:1569–1595. doi: 10.1093/genetics/158.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastock M, Manning A. The courtship of Drosophila melanogaster. Behaviour. 1955;8:85–111. [Google Scholar]
- Carney GE, Bender M. The Drosophila ecdysone receptor (EcR) gene is required maternally for normal oogenesis. Genetics. 2000;154(3):1203–1211. doi: 10.1093/genetics/154.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champlin DT, Truman JW. Ecdysteroids govern two phases of eye development during metamorphosis of the moth, Manduca sexta. Development. 1998a;125(11):2009–2018. doi: 10.1242/dev.125.11.2009. [DOI] [PubMed] [Google Scholar]
- Champlin DT, Truman JW. Ecdysteroid control of cell proliferation during optic lobe neurogenesis in the moth Manduca sexta. Development. 1998b;125(2):269–277. doi: 10.1242/dev.125.2.269. [DOI] [PubMed] [Google Scholar]
- Cherbas P, Cherbas L, Savakis C, Koehler MMD. Ecdysteroid-responsive genes in a Drosophila cell line. Integrative and Comparative Biology. 1981;21(3):743–750. [Google Scholar]
- Connolly K, Cook R. Rejection responses by female Drosophila melano-gaster: their ontogeny, causality and effects upon the behaviour of the courting male. Behaviour. 1973;44:142–167. [Google Scholar]
- Clyne JD, Miesenböck G. Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell. 2008;133(2):354–363. doi: 10.1016/j.cell.2008.01.050. [DOI] [PubMed] [Google Scholar]
- Dalton JE, Lebo MS, Sanders LE, Sun F, Arbeitman MN. Ecdysone receptor acts in fruitless- expressing neurons to mediate Drosophila courtship behaviors. Current Biology. 2009;19(17):1447–1452. doi: 10.1016/j.cub.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir E, Dickson BJ. fruitless splicing specifies male courtship behavior in Drosophila. Cell. 2005;121(5):785–794. doi: 10.1016/j.cell.2005.04.027. [DOI] [PubMed] [Google Scholar]
- Ferveur J-F. Drosophila female courtship and mating behaviors: sensory signals, genes, neural structures and evolution. Current Opinion in Neurobiology. 2010;20:764–769. doi: 10.1016/j.conb.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Gailey DA, Hall JC. Behavior and cytogenetics of fruitless in Drosophila melanogaster: different courtship defects caused by separate, closely linked lesions. Genetics. 1989;121(4):773–785. doi: 10.1093/genetics/121.4.773. Erratum in: Genetics: 122(2) 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganter GK, Merriman J, Salmon M, Walton K, Kravitz EA. EcR deficiency results in male-male courtship in adult flies. Behavior Genetics. 2007;37(3):507–512. doi: 10.1007/s10519-006-9140-1. [DOI] [PubMed] [Google Scholar]
- Ganter GK, Panaitiu AE, Desilets JB, Davis-Heim JA, Fisher EA, Tan LCH, Heinrich R, Buchanan EB, Brooks KM, Kenney MT, Verde MG, Downey J, Adams AM, Grenier JS, Maddula S, Shah P, Kincaid KM, O'Brien JRM. Drosophila male courtship behavior is modulated by ecdysteroids. Journal of Insect Physiology. 2011;57(9):1179–1184. doi: 10.1016/j.jinsphys.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garen A, Kauvar L, Lepesant JA. Roles of ecdysone in Drosophila development. Proceedings of the National Academy of Sciences of the United States of America. 1977;74(11):5099–5103. doi: 10.1073/pnas.74.11.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler AM. Ecdysteroid titers during pupal and adult development in Drosophila melanogaster. Developmental Biology. 1982;93(1):73–82. doi: 10.1016/0012-1606(82)90240-8. [DOI] [PubMed] [Google Scholar]
- Henley CL, Nunez AA, Clemens LG. Hormones of choice: The neuroendocrinology of partner preference in animals. Frontiers in Neuroendocrinology. 2011;32(2):146–154. doi: 10.1016/j.yfrne.2011.02.010. [DOI] [PubMed] [Google Scholar]
- Ishimoto H, Kitamoto T. The steroid molting hormone ecdysone regulates sleep in adult Drosophila melanogaster. Genetics. 2010;185(1):269–281. doi: 10.1534/genetics.110.114587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimoto H, Sakai T, Kitamoto T. Ecdysone signaling regulates the formation of long-term courtship memory in adult Drosophila melanogaster. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(15):6381–6386. doi: 10.1073/pnas.0810213106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koganezawa M, Haba D, Matsuo T, Yamamoto D. The shaping of male courtship posture by lateralized gustatory inputs to male-specific interneurons. Current Biology. 2010;20:1–8. doi: 10.1016/j.cub.2009.11.038. [DOI] [PubMed] [Google Scholar]
- Lasbleiz C, Ferveur J-F, Everaerts C. Courtship behaviour of Drosophila melanogaster revisited. Animal Behaviour. 2006;72(5):1001–1012. [Google Scholar]
- Li H, Harrison D, Jones G, Jones D, Cooper RL. Alterations in development, behavior, and physiology in Drosophila larva that have reduced ecdysone production. Journal of Neurophysiology. 2001;85:98–104. doi: 10.1152/jn.2001.85.1.98. [DOI] [PubMed] [Google Scholar]
- Manoli DS, Foss M, Villella A, Taylor BJ, Hall JC, Baker BS. Male-specific fruitless specifies the neural substrates of Drosophila courtship behaviour. Nature. 2005;436(7049):395–400. doi: 10.1038/nature03859. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Ball GF. Chapter 7: The Neuroendocrine Control of Sex Specific Behavior in Vertebrates. Lessons from Mammals and Birds. Current Topics in Developmental Biology. 2008;83:213–248. doi: 10.1016/S0070-2153(08)00407-9. [DOI] [PubMed] [Google Scholar]
- Paillette M, Ikeda H, Jallon J-M. A new acoustic signal of the fruit-flies Drosophila simulans and D. melanogaster. Bioacoustics. 1991;3(4):247–254. [Google Scholar]
- Pan Y, Robinett CC, Baker BS. Turning males on: Activation of male courtship behavior in Drosophila melanogaster. PLoS One. 2011;6(6):e21144. doi: 10.1371/journal.pone.0021144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel DJ, Milner MJ. The response of Drosophila imaginal disc cell lines to ecdysteroids. Roux's Archives of Developmental Biology. 1992;202(1):23–35. doi: 10.1007/BF00364594. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. Hormone receptors and the regulation of insect metamorphosis. Receptor. 1993;3(3):203–209. [PubMed] [Google Scholar]
- Satokangas P, Liimatainen JO, Hoikkala A. Songs produced by the females of the Drosophila virilis group of species. Behavior Genetics. 1994;24(3):263–272. doi: 10.1007/BF01067193. [DOI] [PubMed] [Google Scholar]
- Siwicki KK, Kravitz EA. Fruitless, doublesex and the genetics of social behavior in Drosophila melanogaster. Current Opinion in Neurobiology. 2009;19(2):200–206. doi: 10.1016/j.conb.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, Yu EJ, Kennedy K, Chatwin H, Reale V, Hamon M, Smith T, Evans PD. Rapid, nongenomic responses to ecdysteroids and catecholamines mediated by a novel Drosophila G-protein-coupled receptor. The Journal of Neuroscience. 2005;25(26):6145–6155. doi: 10.1523/JNEUROSCI.1005-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger P, Kvitsiani D, Rotkopf S, Tirián L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121(5):795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- von Phillipsborn AC, Liu T, Yu J, Masser C, Bidaye S, Dickson BJ. Neuronal control of Drosophila courtship song. Neuron. 2011;69:509–522. doi: 10.1016/j.neuron.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Von Schilcher F. The function of pulse song and sine song in the courtship of Drosophila melanogaster. Animal Behaviour. 1976;24(3):622–625. [Google Scholar]
- Wicker C, Jallon JM. Hormonal control of sex pheromone biosynthesis in Drosophila melanogaster. Journal of Insect Physiology. 1995;41(1):65–70. [Google Scholar]