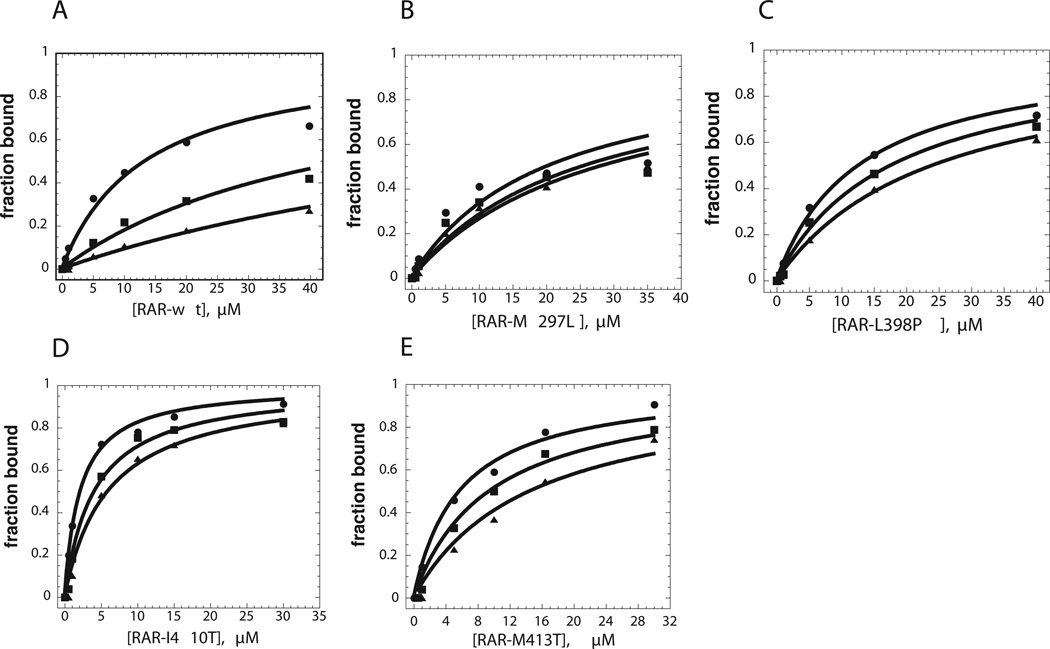
Figure 5. Binding of co-repressor peptides to wild type and mutant RARα as measured by fluorescence anisotropy.
The measurements were performed at 20 °C and pH 8.1, and the fraction of bound was determined as described in “Materials and Methods”. All measurements were repeated using at least two independent protein preparations, with representative plots shown. Binding experiments were carried out for the wild-type LBD and each of the four mutants, in the presence of different ATRA concentrations as described in “Materials and Methods”. A, wild type; B, M297L C, L398P; D, I410T; E., M413T. ➂, Binding isotherms for N-CoR peptide in the absence of ATRA, plotted as a function of LBD concentration. ➄, Binding isotherms for N-CoR peptide binding in the presence of 10 nM ATRA, plotted as a function of LBD concentration. ➉, Binding isotherms for N-CoR peptide binding in the presence of 20 nM ATRA, plotted as a function of LBD concentration.