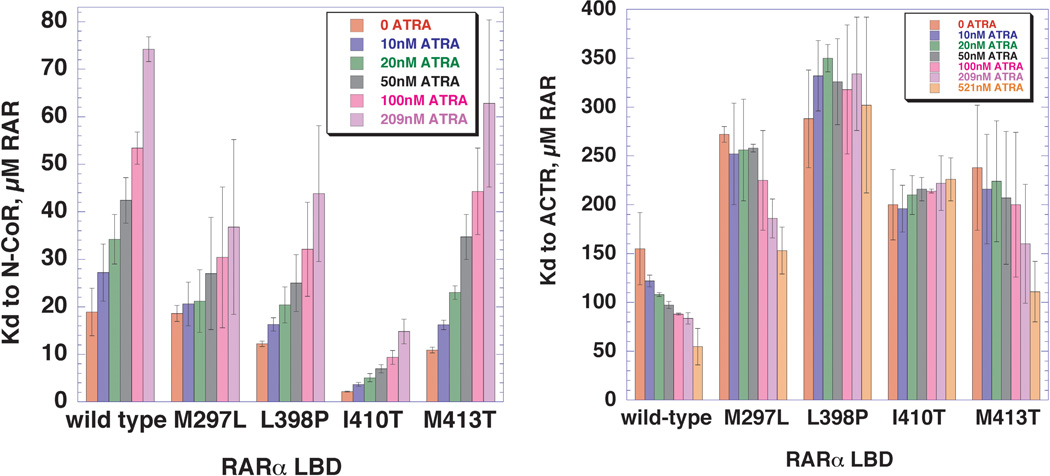
Figure 6. Variation of the dissociations constant for binding of N-CoR and ACTR peptides to wild type and mutant RARα LBDs, as a function of increasing ATRA.
A, Bar graph representation of the equilibrium dissociation constants (Kds) for N-CoR co-repressor binding to wild type and mutant RARα LBDs, determined over a range of ATRA concentrations. Each equilibrium dissociation constant was determined from a binding isotherm of the type shown in Figure 2, performed as described in Experimental Procedures. The error bars represent standard error of the mean. B, Bar graph representation of the equilibrium dissociation constants (KDs) for ACTR co-activator binding to wild type and mutant RARα LBDs, determined over a range of ATRA concentrations.