Abstract
While the diversity of neocortical and hippocampal GABAergic interneurons is recognized in terms of their anatomical, molecular, and functional properties, principal cells are usually assumed to constitute homogenous populations. However, even within a single layer, subpopulations of principal cells can often be differentiated by their distinct long-range projection targets. Such subpopulations of principal cells can have different local connection properties and excitatory inputs, forming subnetworks that may serve as separate information-processing channels. Interestingly, as reviewed here, recent evidence has revealed specific instances where interneuron cell types selectively innervated distinct subpopulations of principal cells, targeting only those with particular long-distance projection targets. This organization represents a novel form of interneuron specialization, providing interneurons with the potential to selectively regulate specific information-processing streams.
Introduction
In the cerebral cortex, interneuron cell types are specialized to deliver GABA at particular times to select spatial domains along the somato-dendritic axis of principal cells (PC). In area CA1 of the hippocampus alone, there are at least 21 recognized types of inhibitory interneurons, which are distinguished by their anatomical features (e.g., domain-specific innervation of postsynaptic cells), molecular characteristics (e.g., expression of calcium binding proteins, neuropeptides, and transcription factors), and functional properties (e.g., intrinsic electrophysiological properties and the phase-specific firing during hippocampal network oscillations) [1–3] (Box 1). In contrast to this well-recognized interneuronal diversity, excitatory PCs are often tacitly regarded as a de facto homogenous population, where the differences between the cells are assumed to be relatively subtle and functionally inconsequential, especially in terms of the integration of the PCs with the local interneurons into cortical microcircuits.
Box 1. Introduction to Interneuron Diversity & Classification.
GABAergic interneurons can be classified by their anatomical, molecular, and functional properties [1,104]. Interneurons target distinct regions along the axo-somato-dendritic axes of PCs, and can be classified anatomically into three broad categories: 1) perisomatically targeting (including basket and axoaxonic cells; for reviews see [47,87]), 2) dendritically targeting (an especially diverse category; see [14] for a review), and 3) interneuron targeting (which do not target PCs) [44–45]. Interneurons can also be divided by the molecular markers they express, such as parvalbumin (PV), cholecystokinin (CCK), neuropeptide Y (NPY), nitric oxide synthase (NOS), vasoactive intestinal peptide (VIP), calbindin, calretinin, and reelin. Intrinsic electrophysiological properties (including firing patterns in response to depolarizing current steps) and phase-specific firing during network oscillations are also used to distinguish interneuron cell types [1–3]. Figure I illustrates some of these characteristics for a small subset of interneuron cell types. Importantly, different interneurons also differ in the receptors for various neuromodulators, including opioids and endocannabinoids.
However, there is accumulating evidence and a concomitantly emerging recognition that, even within a single cortical or hippocampal layer, PCs can be in fact surprisingly diverse, as illustrated by the heterogeneous expression of specific cellular markers (see below) and the different long-distance axonal projection targets. Importantly, recent data also indicate that such subpopulations of PCs with different long-distance axonal projection patterns may also differ in their local connectivity [4–9] and in the excitatory inputs that they receive [9–13]. Such findings suggest that these subpopulations of PCs may form distinct excitatory subnetworks that participate in functionally different information-processing streams.
But how do such excitatory subnetworks formed by PCs with different projection targets integrate with local GABAergic microcircuits? Interestingly, recent evidence discussed below indicates that at least some interneuron cell types in certain brain areas are capable of selectively innervating a subset of glutamatergic cells from the available pool of PCs, targeting only those with specific long-distance projection targets. In this way, such local GABAergic cells show selectivity not only in where along the axo-somato-dendritic axis of the postsynaptic PCs they synapse and at which preferred temporal window they release GABA during hippocampal oscillations [14], but also in the distinct subpopulations of postsynaptic PCs they actually innervate, and, consequently, which excitatory long-distance projections they may selectively regulate.
The latter form of interneuronal selectivity represents a unique, previously unrecognized, form of GABAergic microcircuit specialization. Furthermore, by selectively innervating subpopulations of PCs defined by their long-range project targets, distinct interneurons may selectively regulate specific subnetworks and information-processing channels. In addition, such hitherto unrecognized interneuronal organization selective for PC subcircuits within a given layer or area would have consequences not only for normal circuit functions, but also for the various neurobiological disorders in which interneurons are altered [15]. Here, we discuss the evidence for interneuronal cell type specific regulation of subpopulations of PCs defined by their long-distance projection targets, and consider the functional implications of the selective innervation of PC subnetworks by specific interneuronal subtypes.
Quo vadis, principal cell?
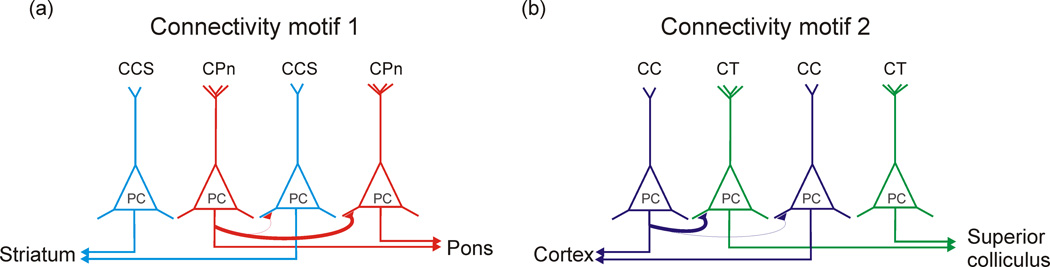
Based on the presently available data, there appear to be two main variations on local connection selectivity between subpopulations of PCs with distinct long-distance projection targets (Figure 1). First, PCs may preferentially partner with members of the same subpopulation (i.e., with cells that have similar long-distance axonal projection target areas). Second, cells of one PC population can innervate other PCs within the same layer or area that project to a different long-range target, rather than preferentially synapsing on members of their own subpopulation. An example of the first scenario has been reported to take place in layer 5 of the frontal cortex, where pyramidal neurons projecting to the pons provide strong local excitatory input to cells which also project to the pons, while avoiding neighboring cells which project bilaterally to the striatum [4–5] (Figure 1a). Importantly, the excitatory input to these layer 5 PCs from layer 2/3 also segregates based on the long-distance projection targets of the PCs. Namely, a given layer 2/3 excitatory cell is more likely to innervate two layer 5 PCs if those PCs are themselves connected [10,16].
Figure 1. Two different major motifs of selective local excitatory connections between subpopulations of principal neurons defined by their long-distance projection targets.
(a) In the first connectivity motif, principal cells preferentially innervate other principal cells with the same long-distance projection targets. An example of this connectivity motif is illustrated. In layer 5 of the frontal cortex, principal cells which project to the pons (CPn, shown in red) preferentially target principal cells (PC) which also project to the pons, while largely avoiding neighboring pyramidal cells which instead project bilaterally to the striatum (CCS, shown in light blue) [4–5]. For clarity, connections between CCS and CPn and other CCS cells are not shown. (b) In the second connectivity motif, one subpopulation of principal cells instead preferentially connects to principal cells with a different long-distance projection target. An example of this second connectivity motif from layer 5 of the visual cortex is illustrated. Pyramidal cells projecting to the contralateral cortex (CC, shown in dark blue) preferentially target neighboring cells which project to the tectum (CT, shown in green), rather than other CC neurons [6]. Note that both schemes suggest that local glutamatergic connectivity between principal cells depends on the long-distance projection targets of both the pre- and post-synaptic cell.
Regarding the second organizational motif of selective local excitatory connectivity, a recently reported and particularly striking example comes from experiments performed in layer 5 of the visual cortex, where PCs projecting to the contralateral cortex preferentially target neighboring cells which project to the tectum, rather than other corticocortical cells [6] (Figure 1b). While these two examples of excitatory connection schemes illustrated in Figure 1 differ regarding which population of PCs is preferentially targeted, they are both examples of local excitatory connectivity between PCs being determined by the long-distance projection targets of both the pre- and post-synaptic cells. This may be a recurring theme in excitatory circuitry organization, as indicating by similar examples uncovered in a variety of layers and brain areas [7–9,11,13].
As noted above, in addition to long-distance projection targets, subpopulations of excitatory projection neurons within a given layer or area may also be distinguished by their anatomical, molecular, and functional properties [17–19]. For example, pyramidal cells in area CA1 of the hippocampus are known to differ in their calbindin expression [20–21] and zinc content [22], and more recent data indicate that CA1 pyramidal cells display distinct behavioral state-dependent firing properties during hippocampal oscillations [23]. Interestingly, developmental origin and time of birth may be an important determinant of excitatory subnetworks. This is illustrated by the fact that radially aligned sister cells (excitatory neurons from the same progenitor mother cell) form connections across cortical layers with their sister cells, rather than with non-sibling cells [24], even when the non-sibling cells show dendritic overlap with the connected sister cell. In the hippocampal formation, a similar form of subnetworks has been recently described [25], where the relative time of the cell’s birth directs connectivity and dictates which excitatory granule cells in the dentate connect to which pyramidal cells in CA3, and in turn which pyramidal cells in CA1 these CA3 cells project to. These subpopulations of cells in the hippocampus also showed distinct molecular profiles, with unique gene expression patterns. Future studies will need to integrate these sister cell-related and time of birth-based subnetworks within the connectivity schemes based on PC long-distance projection targets, as well as to understand the role of activity-dependent refinement of connections, and the integration of adult born neurons into existing neuronal networks [26].
Is there a different local interneuronal microcircuit for each PC subclass defined by long-distance projection targets?
In contrast to the growing evidence on the extent and importance of excitatory subnetworks, the relationship of the local GABAergic connections to these connectivity schemes remains largely unexplored. How do GABAergic interneurons fit into such glutamatergic subnetworks? What sort of selectivity do interneurons show in their connections with heterogeneous postsynaptic PC populations? The unstated but widely held assumption is that different classes of interneurons, while highly specialized in terms of their innervation of distinct segments of the axo-somato-dendritic axes of the postsynaptic PCs, form GABAergic synapses with individual PCs within their axonal arbors without any apparent selectivity. However, there is increasing evidence that at least some GABAergic neurons in some brain areas show selectivity for specific PCs defined by their long-distance projection targets, with unique interneuronal subtypes specialized to modulate select glutamatergic subnetworks.
Perhaps the most unambiguous example of such a novel form of interneuronal specialization is that which has been recently reported to occur in the medial entorhinal cortex (MEC) [18]. Specifically, in layer 2 of the MEC there are two, seemingly intermingled, distinct populations of PCs defined by their long-distance projection targets [18] (Figure 2). One population of glutamatergic PCs gives rise to the classical perforant path (that is, they project to the ipsilateral hippocampal formation) and expresses reelin. The second, considerably less appreciated or studied population, projects to the contralateral EC and expresses calbindin. Importantly, the two populations of PCs in the MEC defined by their long-distance projection targets were also found to differ in their electrophysiological properties [18], and may correspond to the morphologically defined stellate and non-stellate cells [27–28] that have been shown to possess differences in their excitatory inputs [12]. It remains to be determined how these cell populations relate to functionally defined MEC cell populations in layer 2 (i.e., grid [29] and border cells [30]). Taken together, layer 2 of MEC represents an example of seemingly intermingled PC types that differ in 1) electrophysiological properties, 2) morphology, 3) neurochemical markers, 4) excitatory input, and, importantly, 5) their long-distance projection targets.
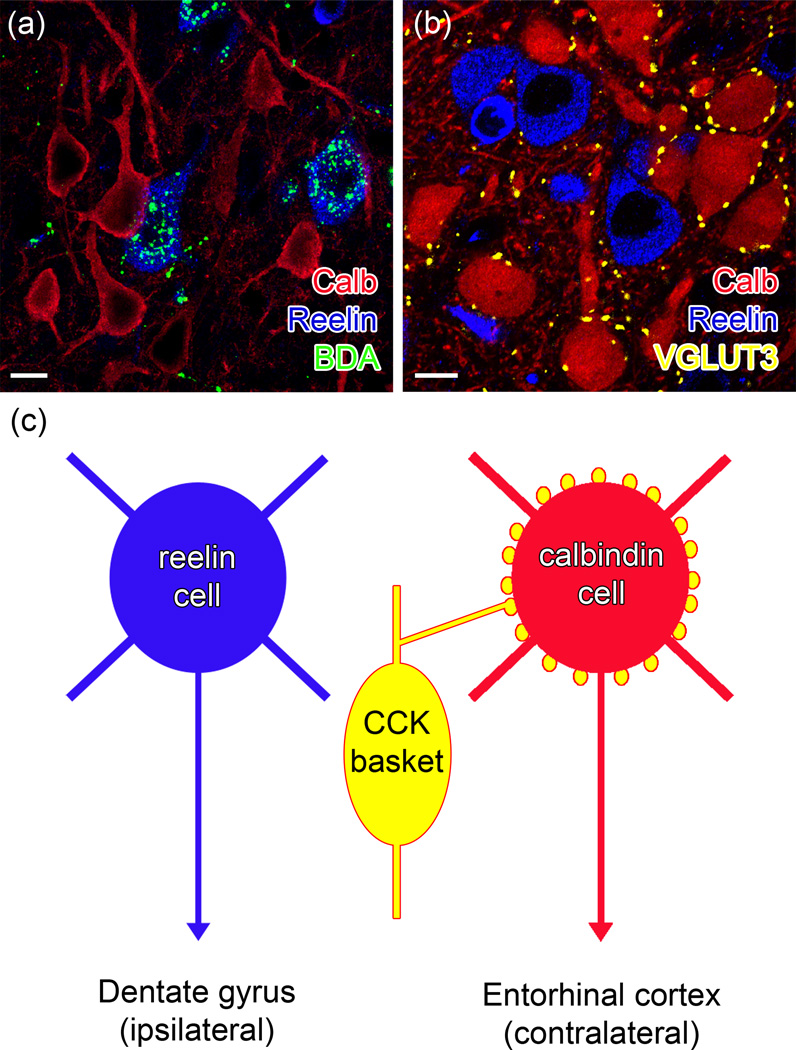
Figure 2. An example of highly specialized inhibitory input to apparently spatially intermingled, but differently projecting principal cells can be found in the layer 2 of the medial entorhinal cortex.
(a) Only the reelin positive cells (blue) are labeled after retrograde tracer biotinylated dextrane amine (BDA, green) injection into the ipsilateral hippocampus (left in panel c). Calbindin (Calb) positive cells (red) instead project to the contralateral entorhinal cortex (not shown). (b) Importantly, only the calbindin positive projection cells are perisomatically inhibited by CCK+ basket cells. Note that CCK+ basket cell terminals were visualized using vesicular glutamate transporter 3 (VGLUT3, yellow) as an alternative marker, as it allows sharper visualization of these terminals [103]. Scale bars: 10 µm. Reproduced, with permission, from [18]. (c) A schematic diagram illustrating a CCK+ basket cell (yellow) selectively innervating a principal cell projecting to the contralateral entorhinal cortex (calbindin+, red), but not a principal cell projecting to the ipsilateral hippocampal formation (reelin+, blue).
Remarkably, it was found that cholecystokinin-expressing (CCK+) GABAergic basket cells (BCs), one of the major subtypes of perisomatically projecting interneurons (Box 1), selectively innervate those principal cells in layer 2 of the MEC that project to the contralateral EC, while avoiding neighboring cells which project to the ipsilateral dentate gyrus [18]. Interestingly, CCK+ BC terminals are known to express high levels of cannabinoid type-1 (CB1) receptors, which regulate the release of GABA in both tonic and activity-dependent manners [31–35]. As expected, brief depolarization (used to trigger the synthesis and release of endocannabinoids [36–37]) of calbindin positive, contralaterally projecting, but not reelin positive, ipsilaterally projecting layer 2 MEC PCs resulted in the transient inhibition GABA-release from the CCK+/CB1+ BC terminals [18]. Due to the selective innervation by CCK+ BCs of the calbindin positive PCs projecting to the contralateral cortex, activation of CB1 receptors in the MEC cortex under physiological conditions in vivo (e.g., during grid cell activation characterized by repetitive firing of layer 2 cells during locomotion [29]) would be expected to preferentially modulate information transfer to the contralateral EC. Although the latter specific prediction will need to be rigorously tested by future experiments, the organization of the selective CCK+ BC innervation of subnetworks of PCs indicate that specific interneurons may produce selective modulation of specific information-processing streams.
But just how widespread is the phenomenon of interneurons selectively innervating only one subgroup, and not the other subgroups, of PCs as differentiated by their long-distance projection patterns? Do subnetworks in other areas also have such dedicated interneuron cell types? Do interneuron cell types other than CCK+ BCs selectively innervate subpopulations of PCs in the MEC and beyond? While this is clearly a field that will require future studies, there are already interesting indications that various degrees of interneuronal selectivity for subgroups of heterogeneous PCs may in fact be a general phenomenon.
First, in the visual cortex, corticocortical cells have been reported to receive over 8 times as many inputs onto their axon initial segments from axoaxonic (chandelier) cells than do corticothalamic cells [38]. While not quite as stark of a difference as indicated by the findings in the MEC discussed above, the apparently preferential targeting of a subpopluation of PCs defined by their long-distance projection targets by axoaxonic cells indicates that this form of interneuronal specialization may not be unique to CCK+ BCs (as axoaxonic cells may also preferentially innervate subpopulations of PCs) or to the MEC (as it is observed in the visual cortex as well). There are also additional indications of this novel manifestation of interneuronal specialization in other brain areas [8,39–43]. For example, differences in GABAergic inhibition of excitatory subnetworks have been noted in layer 5 of rat somatosensory cortex, where stimulation of a PC projecting to subcortical regions was found to produce disynaptic inhibition in a second PC with the same long-distance projection [8]. Importantly, however, disynaptic inhibition was not observed when dual recordings were made instead from two cells projecting to the contralateral hemisphere; this could reflect differences in the inhibitory innervation of these PCs and/or differences in input to the inhibitory neurons themselves.
It should also be noted that while the concept of interneurons selectively innervating subpopulations of PCs based on their long-distance projection targets is novel, interneurons clearly show cell type-level target selectivity more generally. Beyond where on a postsynaptic cell interneurons form input synapses (e.g., somatic versus distal dendritic) (Figure 3), some interneurons are also known to show selectivity regarding which postsynaptic cells they target, especially when it comes to the innervation of other interneurons. The most salient of these are the connections formed by interneuron-selective interneurons [44–45]. As their name suggests, these interneurons selectively target other interneurons, rather than excitatory neurons. Another case of interneurons showing synapse selectivity can be seen in cortical low-threshold spiking (LTS) cells, which inhibit PCs and fast- spiking (FS) interneurons, but only rarely other LTS cells [46]. While these are both examples of target selectivity when the target is also an inhibitory interneuron, they are clear indications that interneurons are capable of selectively innervating subsets of cells from a population of neurons, even when diverse cell types are physically intermingled.
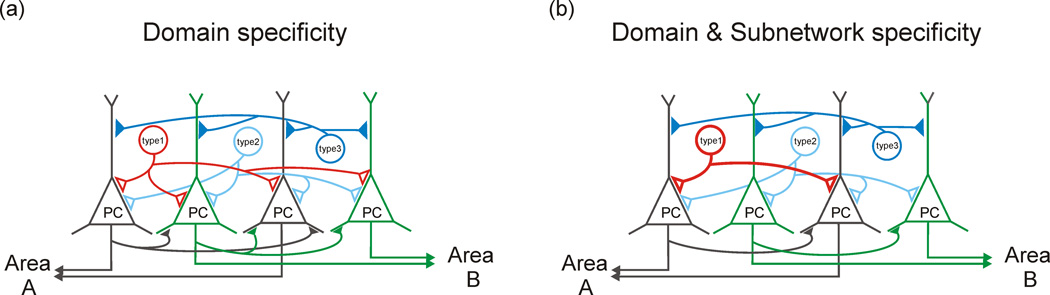
Figure 3. In addition to domain specificity, some interneuron cell-types may show selectivity regarding which principal subpopulations they target.
(a) Interneurons target distinct domains along the axo-somato-dendritic domain of principal cells (PC). Illustrated are two hypothetical populations of perisomatically targeting, basket, interneuron cell types (type1, shown in red and type2, shown in light blue), and a dendritically targeting interneuronal cell type (type3, shown in dark blue). (b) Some interneuron cell types may also show connection specificity (type1, red), regulating only particular subpopulations of principal cells defined by their long-distance projection targets. This selectivity parallels the selectivity seen for local excitatory connections between principal cells. Other interneuron types (type2 and type3, light and dark blue, respectively) may show less specificity, innervating all principal neurons equally.
A spectrum of interneuronal target selectivity for PC subpopulations?
The examples above support the possibility that the finding of interneurons targeting discrete subpopulations of PCs may extend beyond the MEC and CCK+ BCs. However, it is likely that only some interneuron cell types will selectively inhibit PCs with a particular long-distance projection target. Other interneurons may show less selectivity, and instead provide inhibition to diverse post-synaptic populations. In the CA1 region, for example, a form of inhibition that was most likely mediated by FS, parvalbumin (PV) expressing BCs (the other major type of BCs, that show sharply distinct properties with respect to the CCK+ BCs [47]) was found to be homogenous across neighboring PCs [48]. In general, FS BCs show high connectivity rates [49–50], which may at first glance suggest limited selectivity in their connections, although it does not necessarily rule it out. Indeed, a study of FS neurons in layer 2/3 of rat visual cortex found that FS cells preferentially connected to PCs that excited them (forming reciprocal connections) [43]. Moreover, these cells with reciprocal connections also shared additional excitatory inputs, and can thus be viewed as being part of the same information-processing stream. Therefore, even when an interneuron type shows high connectivity rates, it does not exclude their preferential involvement in particular fine-scale subnetworks. Additionally, there may be regional differences in target selectivity. For example, FS cells in the striatum preferentially target medium spiny neurons (MSNs) projecting to the substantia nigra pars reticulata (SNr) over MSNs which project instead to the globus pallidus [51]. In contrast, it was recently reported that in layer 2/3 of mouse frontal cortex, PV+ FS cells show a greater than 75% connection rate with PCs within 200µm, possibly indicating nonspecific, or blanket, inhibition [50]. These regional differences in FS target selectivity extend beyond the striatum versus superficial frontal cortex; in the latter study, PV+ FS to PC connection rates were also studied in additional cortical layers and areas, and significant differences in connection rates were found [50].
It is also important to note that a high rate of connectivity with PCs is not seen for all interneuron types. A study that directly compared the connection rate between either an FS cell or a CB1+ cell and PCs in layer 2/3 found that FS cells formed connections with PCs at a significantly higher rate than did CB1+ cells [52]. Not only did individual FS cells inhibit nearly twice as many PCs as CB1+ cells did, FS cells also received substantially more local excitatory input than did CB1+ cells (25% connection rate for PCs to FS cells, versus a mere 4.5% connection rate for PCs to CB1+ cells). Similarly, in the hippocampus, PV+ BCs receive more excitatory inputs than CCK+ BCs [53–54], although unexpected differences in the strength of the excitatory inputs to these two types of BCs have also been reported [55]. Taken together, these data suggest not only selectivity in terms of precisely which PCs CB1+ cells actually target, but also which PCs provide input to these INs.
At the far end of the spectrum of specificity, it seems that neurogliaform cells (NGF) may be the most likely candidates for a relatively non-specific circuit element, inhibiting cells regardless of their long-range projection targets. Indeed, NGFs have been proposed to exert inhibition at least partially via volume transmission [56], evoking postsynaptic responses seemingly in all cells with processes coursing through their characteristic, dense axonal clouds, resulting in unusually high connection rates in paired recordings from presynaptic NGFs and neighboring PCs [57]. Furthermore, in the hippocampal formation, axons from NGFs in CA1 and NGFs in the dentate gyrus cross the hippocampal fissure, inhibiting two clearly distinct populations of PCs (pyramidal cells in CA1 and granule cells in the dentate gyrus) [58–59]. NGFs are also uniquely non-selective in their electrical synapses, forming gap junctions in a promiscuous manner with a variety of other interneuron cell types [58,60–61]. Therefore, NGFs may be the prime counterexample of selectivity for PC subgroups, potentially influencing a wide range of possible partners through electrical and chemical means of inter-cellular communication.
In addition to interneuron cell types which show preferential targeting of subpopulations of PCs, and interneuron cell types which may show little or no selectivity, there is also a third (for the moment, only theoretical) possibility, namely, some cells may show selectivity at the single cell level, rather than the cell-class level. That is, as a group, interneurons of a particular cell type may inhibit all PCs in a brain area, but a given single cell from that class of interneurons may still preferentially target PCs with a particular long-distance projection. Alternatively, one can also imagine an interneuronal subtype, or an individual interneuron within a given subtype, that preferentially targets PC subnetworks distinguished in some manner other than their long-distance projections, such as birthdates [25] or neuronal lineage [24]. However, it is important to note that recent evidence indicates that the long-distance projections of PCs is indeed a major, developmental determinant of interneuronal selectivity. Specifically, new results show that it is the long-range projection targets of PCs, rather than strictly their time of birth, that may determine which interneurons migrate towards them during development [62], highlighting the central role of long-range PC projections for local interneuronal circuits. The precise developmental mechanisms behind interneuron target selectivity remain to be elucidated.
Functional implications and outlook
As noted above, different excitatory subnetworks may carry different information to their respective long-distance targets, and, in this way, the concept of subnetworks can be expanded to the idea of distinct information-processing streams. For example, in the visual cortex, the local connection probability between cells is correlated with the similarity of stimulus preference of those cells [63]. Additionally, in primary somatosensory cortex, cells projecting to primary motor cortex have larger receptive fields than neighboring cells projecting to the secondary somatosensory area [64]. Note that the differences in receptive field size could be achieved by different excitatory input [65], or alternatively, by different inhibition [66], potentially provided by different classes of interneurons. It will be important to determine to what extent the different receptive field sizes seen in different subpopulations of PCs is due to the regulation of these information-processing streams by subnetwork-specific interneurons.
In cases where excitatory subnetworks have dedicated interneurons, the output of these subnetworks may be then modulated by such interneurons selectively, without globally affecting all circuits in the region. Alternatively, in those cases where the interneuron receives input instead from a different network than the one it inhibits, dedicated interneurons may also allow direct control of one subnetwork by another subnetwork. Take for example the case of layer 2 of the MEC discussed above, where CCK+ BCs selectively innervate PCs that project to the contralateral EC [18]. If future data indicate that the CCK+ BCs receive input from the same sources as the contralaterally-projecting, calbindin positive cells that they inhibit (or even from the calbindin positive cells themselves), CCK+ BCs would be expected to provide a mechanism for controlling the information flow to the contralateral EC, without directly affecting the relay of information from the MEC to the ipsilateral hippocampus. However, if the CCK+ BCs instead receive local excitatory inputs from the reelin positive PCs which project to the ipsilateral hippocampus, the reelin positive cells would both directly control layer 2 MEC output to the hippocampus (by virtue of their axons forming the perforant pathway) and indirectly (disynaptically, via CCK+ BCs) regulate communication with the contralateral EC. That is, the dedicated interneuron cell type (CCK+ basket cells) would allow one subnetwork (in this example, the reelin-expressing PCs) to regulate another subnetwork (the calbindin-expressing PCs). Future studies will need to address the input to these CCK+ BCs, and thus clarify their role in information processing in MEC. Although our understanding of the nature of the selective excitatory inputs to interneurons have dramatically increased within the last years [11,42–43,67–70], additional research efforts will be needed to determine the specificity of excitatory control of interneurons dedicated to the regulation of distinct excitatory subnetworks with different long-distance projection targets.
Furthermore, modulation of such dedicated interneurons provides a mechanism for the select neuromodulation of specific subnetworks, as may be predicted from the recent report on the state-dependent changes observed for discrete subpopulations of PCs [23]. Importantly, the selective modulation of interneuron cell types is known to occur through various local and ascending neuromodulatory systems. For example, as mentioned above, CCK+ BCs, but not PV+ BCs, are inhibited by endocannabinoids [35,71], and, as a result, those PCs that are not innervated by CCK+ BCs, but instead only by PV+ BCs, do not show endocannabinoid-mediated depolarization-induced suppression of inhibition (DSI) [18,40]. Another example of a select modulator of interneurons is the neuropeptide CCK itself; CCK enhances output from PV+ basket cells, while inhibiting GABA release from CCK+ BCs [34,72–74]. In this way, CCK is a molecular switch, determining the source of perisomatic inhibition to PCs [72]. Moreover, given the selective innervation by CCK+ BCs in MEC, CCK may even act as a molecular switch at a larger level, deciding which PCs, and therefore which information-processing streams, may predominate in terms of relative activity levels at a given time. In addition, neuromodulators may not only directly regulate interneurons themselves in a specific manner, but may also act selectively on the excitatory inputs to some, but not all, interneuronal subtypes [75]. It is also possible that specific subgroups of PCs are regulated differentially by neuromodulatory systems, as indicated by, for example, a recent report that PCs in the somatosensory cortex can markedly differ in their ability for endocannabinoid mediated slow self-inhibition [76].
Regulation of subnetworks of PCs by dedicated interneurons also provides a means to experimentally modulate select information-processing streams, even when the subnetworks are spatially intermingled. Modulation of specific types of INs, and thus the specific subnetworks they regulate, could be achieved by 1) optogenetic techniques (e.g., using specific promoters to drive opsin expression in subsets of interneurons), 2) pharmacology, when interneuron cell-type differences are known (e.g., mu opioid receptors are found on PV+ BCs, but only rarely on CCK+ BCs [32,77]), or 3) genetic manipulation (e.g., use of mice expressing tetanus toxin in cells defined by two parameters [78]). New techniques are also emerging which may allow direct manipulation of cells defined by their long-distance projection targets, including modified rabies virus [79–80], transfection via whole-cell recordings [81], and wheat germ agglutinin (WGA)-Cre that allows for topology-based optogenetics [82]. Importantly, these techniques will also allow further exploration of long-range GABAergic projection neurons with axons connecting distinct brain areas [55,83–86] and their integration with the specific excitatory subnetworks discussed above.
Finally, long-term alterations of specific interneuronal subtypes may occur following neuronal injury and in neurological or psychiatric disorders (for reviews, see [15,87–90]). For example, alterations of specific interneurons may be involved in autism spectrum disorders [91–93], schizophrenia [94–96], and epilepsy [87,97–101]. As to what extent such pathological long-lasting modifications may affect interneurons with selective connections to specific excitatory subnetworks is not yet understood, but selective changes to particular PC – interneuron subnetworks would be expected to have major implications for disease mechanisms (Figure 4). Therefore, in light of the possible heterogeneity of PCs and their dedicated interneuron partners, it may be necessary to reassess injury-related cell loss or persistent pathological plasticity at the level of specific subnetworks associated with distinct long-distance projection targets, as opposed to the current practice of averaging such changes across the neuronal population within a given layer or brain area. For example, as noted above, in control conditions, FS cells in the striatum preferentially target MSNs projecting to the SNr [51]. However, after dopamine depletion, FS cells display axonal sprouting and FS input (as measured by the connection rate) to MSNs projecting to the globus pallidus is selectively increased (while input to MSNs projecting to SNr is unchanged) [102]. Moreover, this alteration of target selectivity may be sufficient to enhance synchrony in these networks [102].
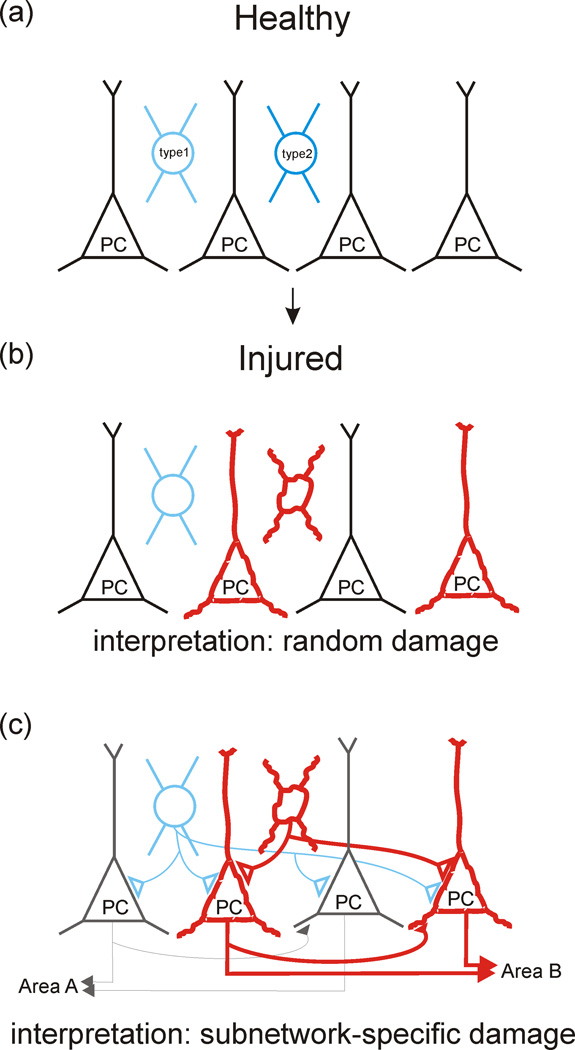
Figure 4. Potential for subnetwork-specific alterations in neurological disorders.
(a) A schematic of a healthy network, containing principal cells (PC, black) and interneuron cell types (blue). (b) Subsets of principal cells and select interneuron cell types may be altered following injury or in neurological disorders (shown in red). If principal cells are viewed as a homogenous population, these alterations to select cells may seem to be sporadic, with no apparent pattern, and therefore specific functional consequences may be difficult to discern. (c) However, when the heterogeneity of principal cells - and in particular their distinct long-range projection targets - are recognized, a clearer image may emerge. Furthermore, consideration of the potential for interneuronal cell types to selectively regulate particular subpopulations of principal cells may reveal additional specificity in the alterations seen in neurological disorders. In the theoretical situation illustrated, damage or pathological plasticity is not random, but is instead selective to a particular subnetwork of excitatory neurons and the interneuronal cell type which regulates that subnetwork.
In summary, some interneuron cell types may selectively target specific subsets of PCs, rather than providing blanket inhibition to all PCs regardless of their long-range projection targets. In doing so, these interneurons may provide tools to selectively alter physically intermingled information processing streams. Ultimately, understanding cortical networks, and associated pathological alterations, will require greater knowledge of interneuron target cell specificity.
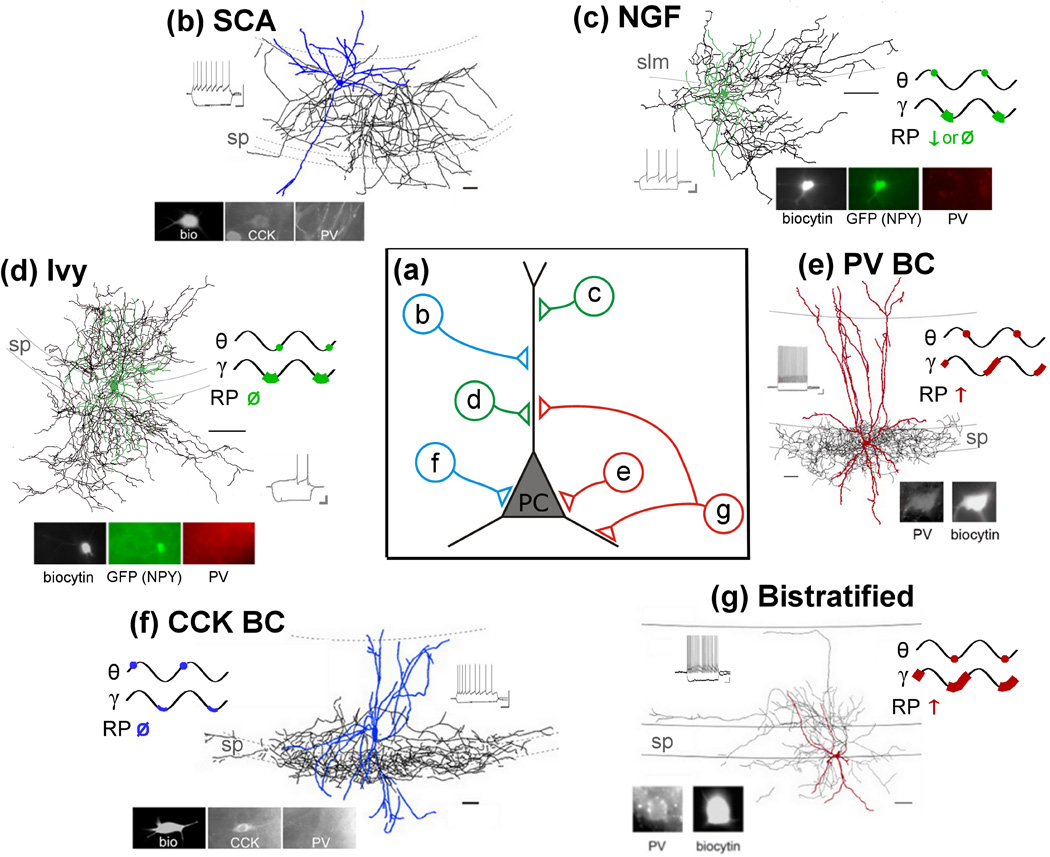
Box 1. Figure I. An abundance of interneuron cell types.
(a) In CA1 alone, there are at least 21 different types of interneurons [3], including axoaxonic (not illustrated), Schaffer collateral associated (SCA; b) cells, neurogliaform (NGF; c) cells, Ivy cells (d), basket cells (BC; e–f), bistratified (g), and O-LM cells (not illustrated). Note that only a few of these cell types are illustrated here. These cells can express different molecular markers, including CCK (blue), PV (red), and NPY (green), and target distinct regions along the axo-somato-dendritic axis of postsynaptic principal cells (PC). Note that these cell types can be further subdivided, for example by additional molecular markers or developmental origin. (b) SCA cells are dendritically targeting, display an adapting firing pattern in response to a depolarizing current step (inset), are CCK+ (inset) [105–106], express CB1 receptors, and are indirectly inhibited by the neuropeptide CCK [33–34]. It remains to be established how these cells fire in relation to in vivo network oscillations. (c) NGFs target distal dendrites, have a dense axonal plexus, and often express NPY but not PV [107] (inset). NGFs additionally express μ opioid receptors (MORs) [108], and preferentially fire just after the peak of theta (θ; the dot indicates the relative time of peak firing rates), and show strong gamma modulation (γ; depth of gamma modulation ‘r’ greater than 0.2, thick green line roughly indicates the range observed across NGFs), firing at the trough of locally recorded gamma oscillations [59]. The firing rate of NGFs either decreases or remains unchanged during sharp wave-associated ripples (RP) [59]. (Note that for all other example cell types illustrated, gamma and theta are both relative to striatum pyramidale. Note also that in each panel, reconstructions, firing pattern, and immunocytochemistry are from one example cell recorded in vitro, while in vivo firing preferences are represented for the interneuron class, rather than a specific example cell). (d) Ivy cells target proximal dendrites, and, similar to NGFs, have a dense axonal plexus, display a late spiking firing pattern (inset), and express MORs [108] and NPY, but not PV [109]. Ivy cells are strongly gamma modulated, and fire near the trough of both gamma and theta oscillations [109]. Firing rates of Ivy cells are unchanged during sharp wave-associated ripples [109]. (e) PV+ BCs target principal cells perisomatically, display a non-adapting high-frequency train of action potentials in response to a depolarizing current step (fast spiking, FS, inset), express MORs but not CB1 receptors [77,108], and are directly depolarized (excited) by CCK via Gi/o coupled CCK2 receptors [72,74]. PV+ BCs preferentially fire just after the peak of theta, and are moderately modulated by gamma, preferentially firing during the rising phase of gamma (depth of gamma modulation ‘r’ between 0.1 and 0.2, indicated by the moderately thick red line) [3,110]. PV+ BCs show an increase in firing rate during ripples. (f) CCK+ BCs display an adapting, low-frequency, regular spiking firing pattern (RS, inset). Unlike PV+ BCs, CCK+ BCs express CB1 receptors, but rarely MORs [31–32,35,77]. CCK+ BCs are indirectly inhibited (via endocannabinoids) by the neuropeptide CCK [31–34,72–73]. Some CCK+ BCs express the vesicular glutamate transporter VGLUT3 (not illustrated here, but see Figure 2) [18,103]. CCK+ BCs preferentially fire just prior to the peak of theta [3], are modulated by gamma, though less so than are some other interneurons (depth of gamma modulation ‘r’ less than 0.1, thin blue line), and fire near the trough of gamma oscillations [110]. CCK+ BCs do not show increased firing rates during ripples. (g) Bistratified cells are so called because their axons target pyramidal cell dendrites in both stratum radiatum and stratum oriens. Bistratified cells express PV (in addition to other molecular markers, including NPY, not illustrated [1,14]) and display an FS firing pattern. Unlike PV+ BCs, bistratified cells do not express functional CCK2 receptors [74]. Note that the axons of bistratified cells produce a less dense plexus than seen for Ivy cells [109]. Bistratified cells fire near the trough of theta, and highly modulated by gamma, firing on the rising phase of gamma [3,110]. Bistratified cells increase their firing during ripples. (b–g) Reconstructions, in vitro firing patterns, and immunocytochemical images adapted, with permission, from [33] (b,f), [74] (e,g), [108] (c,d). Reconstructions: soma and dendrites color-coded to match schematic in a; axons in black; scale bars: 50µm. Note that examples b, e–f are from rat [33,74], and examples c–d are from mouse [108]. Calibration: b, f) 50mV, 200ms; c–d) 20mV, 200ms; e, g) 10mV, 250ms.
Acknowledgements
This work was supported by the George E. Hewitt Foundation for Medical Research (E.K.-M.), the US National Institutes of Health grants NS35915, NS074702, and NS74432 (I.S.), and the Epilepsy Foundation (C.V.) including the generous support of the Eric W. Lothman Training Fellowship (S.-H.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6(4):347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 2.Ascoli GA, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9(7):557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321(5885):53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morishima M, Kawaguchi Y. Recurrent connection patterns of corticostriatal pyramidal cells in frontal cortex. J Neurosci. 2006;26(16):4394–4405. doi: 10.1523/JNEUROSCI.0252-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morishima M, et al. Highly Differentiated Projection-Specific Cortical Subnetworks. J Neurosci. 2011;31(28):10380–10391. doi: 10.1523/JNEUROSCI.0772-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown SP, Hestrin S. Intracortical circuits of pyramidal neurons reflect their long-range axonal targets. Nature. 2009;457(7233):1133–1136. doi: 10.1038/nature07658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schubert D, et al. Mapping functional connectivity in barrel-related columns reveals layer- and cell type-specific microcircuits. Brain Struct Funct. 2007;212(2):107–119. doi: 10.1007/s00429-007-0147-z. [DOI] [PubMed] [Google Scholar]
- 8.Le Be JV, et al. Morphological, electrophysiological, and synaptic properties of corticocallosal pyramidal cells in the neonatal rat neocortex. Cereb Cortex. 2007;17(9):2204–2213. doi: 10.1093/cercor/bhl127. [DOI] [PubMed] [Google Scholar]
- 9.Kampa BM, et al. Cortical feed-forward networks for binding different streams of sensory information. Nat Neurosci. 2006;9(12):1472–1473. doi: 10.1038/nn1798. [DOI] [PubMed] [Google Scholar]
- 10.Otsuka T, Kawaguchi Y. Firing-pattern-dependent specificity of cortical excitatory feed-forward subnetworks. J Neurosci. 2008;28(44):11186–11195. doi: 10.1523/JNEUROSCI.1921-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zarrinpar A, Callaway EM. Local connections to specific types of layer 6 neurons in the rat visual cortex. J Neurophysiol. 2006;95(3):1751–1761. doi: 10.1152/jn.00974.2005. [DOI] [PubMed] [Google Scholar]
- 12.Beed P, et al. Analysis of excitatory microcircuitry in the medial entorhinal cortex reveals cell-type-specific differences. Neuron. 2010;68(6):1059–1066. doi: 10.1016/j.neuron.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 13.Anderson CT, et al. Sublayer-specific microcircuits of corticospinal and corticostriatal neurons in motor cortex. Nat Neurosci. 2010;13(6):739–744. doi: 10.1038/nn.2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klausberger T. GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. Eur J Neurosci. 2009;30(6):947–957. doi: 10.1111/j.1460-9568.2009.06913.x. [DOI] [PubMed] [Google Scholar]
- 15.Santhakumar V, Soltesz I. Plasticity of interneuronal species diversity and parameter variance in neurological diseases. Trends Neurosci. 2004;27(8):504–510. doi: 10.1016/j.tins.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Otsuka T, Kawaguchi Y. Cell diversity and connection specificity between callosal projection neurons in the frontal cortex. J Neurosci. 2011;31(10):3862–3870. doi: 10.1523/JNEUROSCI.5795-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molnar Z, Cheung AF. Towards the classification of subpopulations of layer V pyramidal projection neurons. Neurosci Res. 2006;55(2):105–115. doi: 10.1016/j.neures.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Varga C, et al. Target-selective GABAergic control of entorhinal cortex output. Nat Neurosci. 2010;13(7):822–824. doi: 10.1038/nn.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slomianka L, et al. Hippocampal pyramidal cells: the reemergence of cortical lamination. Brain Struct Funct. 2011;216(4):301–317. doi: 10.1007/s00429-011-0322-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baimbridge KG, et al. Bursting response to current-evoked depolarization in rat CA1 pyramidal neurons is correlated with lucifer yellow dye coupling but not with the presence of calbindin-D28k. Synapse. 1991;7(4):269–277. doi: 10.1002/syn.890070404. [DOI] [PubMed] [Google Scholar]
- 21.Seress L, et al. Distribution, morphological features, and synaptic connections of parvalbumin- and calbindin D28k-immunoreactive neurons in the human hippocampal formation. J Comp Neurol. 1993;337(2):208–230. doi: 10.1002/cne.903370204. [DOI] [PubMed] [Google Scholar]
- 22.Slomianka L. Neurons of origin of zinc-containing pathways and the distribution of zinc-containing boutons in the hippocampal region of the rat. Neuroscience. 1992;48(2):325–352. doi: 10.1016/0306-4522(92)90494-m. [DOI] [PubMed] [Google Scholar]
- 23.Mizuseki K, et al. Hippocampal CA1 pyramidal cells form functionally distinct sublayers. Nat Neurosci. 2011;14(9):1174–1181. doi: 10.1038/nn.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu YC, et al. Specific synapses develop preferentially among sister excitatory neurons in the neocortex. Nature. 2009;458(7237):501–504. doi: 10.1038/nature07722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deguchi Y, et al. Temporally matched subpopulations of selectively interconnected principal neurons in the hippocampus. Nat Neurosci. 2011;14(4):495–504. doi: 10.1038/nn.2768. [DOI] [PubMed] [Google Scholar]
- 26.Yasuda M, et al. Multiple forms of activity-dependent competition refine hippocampal circuits in vivo. Neuron. 2011;70(6):1128–1142. doi: 10.1016/j.neuron.2011.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alonso A, Klink R. Differential electroresponsiveness of stellate and pyramidal-like cells of medial entorhinal cortex layer II. J Neurophysiol. 1993;70(1):128–143. doi: 10.1152/jn.1993.70.1.128. [DOI] [PubMed] [Google Scholar]
- 28.Klink R, Alonso A. Morphological characteristics of layer II projection neurons in the rat medial entorhinal cortex. Hippocampus. 1997;7(5):571–583. doi: 10.1002/(SICI)1098-1063(1997)7:5<571::AID-HIPO12>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 29.Hafting T, et al. Microstructure of a spatial map in the entorhinal cortex. Nature. 2005;436(7052):801–806. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- 30.Solstad T, et al. Representation of geometric borders in the entorhinal cortex. Science. 2008;322(5909):1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- 31.Foldy C, et al. Presynaptic, activity-dependent modulation of cannabinoid type 1 receptor-mediated inhibition of GABA release. J Neurosci. 2006;26(5):1465–1469. doi: 10.1523/JNEUROSCI.4587-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neu A, et al. Postsynaptic origin of CB1-dependent tonic inhibition of GABA release at cholecystokinin-positive basket cell to pyramidal cell synapses in the CA1 region of the rat hippocampus. J Physiol. 2007;578(Pt 1):233–247. doi: 10.1113/jphysiol.2006.115691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee SH, et al. Distinct endocannabinoid control of GABA release at perisomatic and dendritic synapses in the hippocampus. J Neurosci. 2010;30(23):7993–8000. doi: 10.1523/JNEUROSCI.6238-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SH, Soltesz I. Requirement for CB1 but not GABAB receptors in the cholecystokinin mediated inhibition of GABA release from cholecystokinin expressing basket cells. J Physiol. 2011;589(Pt 4):891–902. doi: 10.1113/jphysiol.2010.198499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katona I, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19(11):4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson RI, et al. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31(3):453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- 37.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410(6828):588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 38.Farinas I, DeFelipe J. Patterns of synaptic input on corticocortical and corticothalamic cells in the cat visual cortex. II. The axon initial segment. J Comp Neurol. 1991;304(1):70–77. doi: 10.1002/cne.903040106. [DOI] [PubMed] [Google Scholar]
- 39.Krook-Magnuson EI, et al. Tonically active inhibition selectively controls feedforward circuits in mouse barrel cortex. J Neurophysiol. 2008;100(2):932–944. doi: 10.1152/jn.01360.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bodor AL, et al. Endocannabinoid signaling in rat somatosensory cortex: laminar differences and involvement of specific interneuron types. J Neurosci. 2005;25(29):6845–6856. doi: 10.1523/JNEUROSCI.0442-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Viviani D, et al. Oxytocin selectively gates fear responses through distinct outputs from the central amygdala. Science. 2011;333(6038):104–107. doi: 10.1126/science.1201043. [DOI] [PubMed] [Google Scholar]
- 42.Otsuka T, Kawaguchi Y. Cortical inhibitory cell types differentially form intralaminar and interlaminar subnetworks with excitatory neurons. J Neurosci. 2009;29(34):10533–10540. doi: 10.1523/JNEUROSCI.2219-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshimura Y, Callaway EM. Fine-scale specificity of cortical networks depends on inhibitory cell type and connectivity. Nat Neurosci. 2005;8(11):1552–1559. doi: 10.1038/nn1565. [DOI] [PubMed] [Google Scholar]
- 44.Acsady L, et al. Different populations of vasoactive intestinal polypeptide-immunoreactive interneurons are specialized to control pyramidal cells or interneurons in the hippocampus. Neuroscience. 1996;73(2):317–334. doi: 10.1016/0306-4522(95)00609-5. [DOI] [PubMed] [Google Scholar]
- 45.Gulyas AI, et al. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci. 1996;16(10):3397–3411. doi: 10.1523/JNEUROSCI.16-10-03397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibson JR, et al. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402(6757):75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 47.Freund TF. Interneuron Diversity series: Rhythm and mood in perisomatic inhibition. Trends Neurosci. 2003;26(9):489–495. doi: 10.1016/S0166-2236(03)00227-3. [DOI] [PubMed] [Google Scholar]
- 48.Pouille F, et al. Input normalization by global feedforward inhibition expands cortical dynamic range. Nat Neurosci. 2009;12(12):1577–1585. doi: 10.1038/nn.2441. [DOI] [PubMed] [Google Scholar]
- 49.Holmgren C, et al. Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J Physiol. 2003;551(Pt 1):139–153. doi: 10.1113/jphysiol.2003.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci. 2011;31(37):13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gittis AH, et al. Distinct roles of GABAergic interneurons in the regulation of striatal output pathways. J Neurosci. 2010;30(6):2223–2234. doi: 10.1523/JNEUROSCI.4870-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Galarreta M, et al. Cannabinoid sensitivity and synaptic properties of 2 GABAergic networks in the neocortex. Cereb Cortex. 2008;18(10):2296–2305. doi: 10.1093/cercor/bhm253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matyas F, et al. Convergence of excitatory and inhibitory inputs onto CCK-containing basket cells in the CA1 area of the rat hippocampus. Eur J Neurosci. 2004;19(5):1243–1256. doi: 10.1111/j.1460-9568.2004.03225.x. [DOI] [PubMed] [Google Scholar]
- 54.Gulyas AI, et al. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19(22):10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szabadics J, Soltesz I. Functional specificity of mossy fiber innervation of GABAergic cells in the hippocampus. J Neurosci. 2009;29(13):4239–4251. doi: 10.1523/JNEUROSCI.5390-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olah S, et al. Regulation of cortical microcircuits by unitary GABA-mediated volume transmission. Nature. 2009;461(7268):1278–1281. doi: 10.1038/nature08503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szabadics J, et al. Different transmitter transients underlie presynaptic cell type specificity of GABAA,slow and GABAA,fast. Proc Natl Acad Sci U S A. 2007;104(37):14831–14836. doi: 10.1073/pnas.0707204104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Armstrong C, et al. Neurogliaform cells in the molecular layer of the dentate gyrus as feed-forward gamma-aminobutyric acidergic modulators of entorhinal-hippocampal interplay. J Comp Neurol. 2011;519(8):1476–1491. doi: 10.1002/cne.22577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fuentealba P, et al. Expression of COUP-TFII nuclear receptor in restricted GABAergic neuronal populations in the adult rat hippocampus. J Neurosci. 2010;30(5):1595–1609. doi: 10.1523/JNEUROSCI.4199-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simon A, et al. Gap-junctional coupling between neurogliaform cells and various interneuron types in the neocortex. J Neurosci. 2005;25(27):6278–6285. doi: 10.1523/JNEUROSCI.1431-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zsiros V, Maccaferri G. Electrical coupling between interneurons with different excitable properties in the stratum lacunosum-moleculare of the juvenile CA1 rat hippocampus. J Neurosci. 2005;25(38):8686–8695. doi: 10.1523/JNEUROSCI.2810-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lodato S, et al. Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron. 2011;69(4):763–779. doi: 10.1016/j.neuron.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ko H, et al. Functional specificity of local synaptic connections in neocortical networks. Nature. 2011;473(7345):87–91. doi: 10.1038/nature09880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato TR, Svoboda K. The functional properties of barrel cortex neurons projecting to the primary motor cortex. J Neurosci. 2010;30(12):4256–4260. doi: 10.1523/JNEUROSCI.3774-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schubert D, et al. Cell type-specific circuits of cortical layer IV spiny neurons. J Neurosci. 2003;23(7):2961–2970. doi: 10.1523/JNEUROSCI.23-07-02961.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chowdhury SA, Rasmusson DD. Comparison of receptive field expansion produced by GABA(B) and GABA(A) receptor antagonists in raccoon primary somatosensory cortex. Exp Brain Res. 2002;144(1):114–121. doi: 10.1007/s00221-002-1035-7. [DOI] [PubMed] [Google Scholar]
- 67.Staiger JF, et al. Local circuits targeting parvalbumin-containing interneurons in layer IV of rat barrel cortex. Brain Struct Funct. 2009;214(1):1–13. doi: 10.1007/s00429-009-0225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.White EL, Keller A. Intrinsic circuitry involving the local axon collaterals of corticothalamic projection cells in mouse SmI cortex. J Comp Neurol. 1987;262(1):13–26. doi: 10.1002/cne.902620103. [DOI] [PubMed] [Google Scholar]
- 69.West DC, et al. Layer 6 cortico-thalamic pyramidal cells preferentially innervate interneurons and generate facilitating EPSPs. Cereb Cortex. 2006;16(2):200–211. doi: 10.1093/cercor/bhi098. [DOI] [PubMed] [Google Scholar]
- 70.Dantzker JL, Callaway EM. Laminar sources of synaptic input to cortical inhibitory interneurons and pyramidal neurons. Nat Neurosci. 2000;3(7):701–707. doi: 10.1038/76656. [DOI] [PubMed] [Google Scholar]
- 71.Eggan SM, et al. Relationship of cannabinoid CB1 receptor and cholecystokinin immunoreactivity in monkey dorsolateral prefrontal cortex. Neuroscience. 2010;169(4):1651–1661. doi: 10.1016/j.neuroscience.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foldy C, et al. Cell type-specific gating of perisomatic inhibition by cholecystokinin. Nat Neurosci. 2007;10(9):1128–1130. doi: 10.1038/nn1952. [DOI] [PubMed] [Google Scholar]
- 73.Lee SY, Soltesz I. Cholecystokinin: a multi-functional molecular switch of neuronal circuits. Dev Neurobiol. 2011;71(1):83–91. doi: 10.1002/dneu.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee SY, et al. Cell-Type-Specific CCK2 Receptor Signaling Underlies the Cholecystokinin-Mediated Selective Excitation of Hippocampal Parvalbumin-Positive Fast-Spiking Basket Cells. J Neurosci. 2011;31(30):10993–11002. doi: 10.1523/JNEUROSCI.1970-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Winterer J, et al. Cell-type-specific modulation of feedback inhibition by serotonin in the hippocampus. J Neurosci. 2011;31(23):8464–8475. doi: 10.1523/JNEUROSCI.6382-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marinelli S, et al. Self-modulation of neocortical pyramidal neurons by endocannabinoids. Nat Neurosci. 2009;12(12):1488–1490. doi: 10.1038/nn.2430. [DOI] [PubMed] [Google Scholar]
- 77.Glickfeld LL, et al. Complementary modulation of somatic inhibition by opioids and cannabinoids. J Neurosci. 2008;28(8):1824–1832. doi: 10.1523/JNEUROSCI.4700-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim JC, et al. Linking genetically defined neurons to behavior through a broadly applicable silencing allele. Neuron. 2009;63(3):305–315. doi: 10.1016/j.neuron.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wall NR, et al. Monosynaptic circuit tracing in vivo through Cre-dependent targeting and complementation of modified rabies virus. Proc Natl Acad Sci U S A. 2010;107(50):21848–21853. doi: 10.1073/pnas.1011756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Weible AP, et al. Transgenic targeting of recombinant rabies virus reveals monosynaptic connectivity of specific neurons. J Neurosci. 2010;30(49):16509–16513. doi: 10.1523/JNEUROSCI.2442-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rancz EA, et al. Transfection via whole-cell recording in vivo: bridging single-cell physiology, genetics and connectomics. Nat Neurosci. 2011;14(4):527–532. doi: 10.1038/nn.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gradinaru V, et al. Molecular and cellular approaches for diversifying and extending optogenetics. Cell. 2010;141(1):154–165. doi: 10.1016/j.cell.2010.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Freund TF, Gulyas AI. GABAergic interneurons containing calbindin D28K or somatostatin are major targets of GABAergic basal forebrain afferents in the rat neocortex. J Comp Neurol. 1991;314(1):187–199. doi: 10.1002/cne.903140117. [DOI] [PubMed] [Google Scholar]
- 84.Gulyas AI, et al. Septal GABAergic neurons innervate inhibitory interneurons in the hippocampus of the macaque monkey. Neuroscience. 1991;47(2–3):381–390. doi: 10.1016/0306-4522(91)90334-k. [DOI] [PubMed] [Google Scholar]
- 85.Takacs VT, et al. Types and synaptic connections of hippocampal inhibitory neurons reciprocally connected with the medial septum. Eur J Neurosci. 2008;28(1):148–164. doi: 10.1111/j.1460-9568.2008.06319.x. [DOI] [PubMed] [Google Scholar]
- 86.Muller M, et al. Synaptology of ventral CA1 and subiculum projections to the basomedial nucleus of the amygdala in the mouse: relation to GABAergic interneurons. Brain Struct Funct. 2011 doi: 10.1007/s00429-011-0326-9. [DOI] [PubMed] [Google Scholar]
- 87.Howard A, et al. Lighting the chandelier: new vistas for axo-axonic cells. Trends Neurosci. 2005;28(6):310–316. doi: 10.1016/j.tins.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 88.Coulter DA. Chronic epileptogenic cellular alterations in the limbic system after status epilepticus. Epilepsia. 40 Suppl 1:S23–S33. doi: 10.1111/j.1528-1157.1999.tb00875.x. discussion S40-1. [DOI] [PubMed] [Google Scholar]
- 89.Jones MW. Errant ensembles: dysfunctional neuronal network dynamics in schizophrenia. Biochem Soc Trans. 2010;38(2):516–521. doi: 10.1042/BST0380516. [DOI] [PubMed] [Google Scholar]
- 90.Prince DA, et al. Epilepsy following cortical injury: cellular and molecular mechanisms as targets for potential prophylaxis. Epilepsia. 2009;50 Suppl 2:30–40. doi: 10.1111/j.1528-1167.2008.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Paluszkiewicz SM, et al. Impaired inhibitory control of cortical synchronization in fragile x syndrome. J Neurophysiol. 2011 doi: 10.1152/jn.00421.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wills S, et al. Further characterization of autoantibodies to GABAergic neurons in the central nervous system produced by a subset of children with autism. Mol Autism. 2011;2:5. doi: 10.1186/2040-2392-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lawrence YA, et al. Parvalbumin-, calbindin-, and calretinin-immunoreactive hippocampal interneuron density in autism. Acta Neurol Scand. 2010;121(2):99–108. doi: 10.1111/j.1600-0404.2009.01234.x. [DOI] [PubMed] [Google Scholar]
- 94.Beneyto M, et al. Lamina- and cell-specific alterations in cortical somatostatin receptor 2 mRNA expression in schizophrenia. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fung SJ, et al. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167(12):1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- 96.Konradi C, et al. Hippocampal interneurons are abnormal in schizophrenia. Schizophr Res. 2011;131(1–3):165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen K, et al. Long-term plasticity of endocannabinoid signaling induced by developmental febrile seizures. Neuron. 2003;39(4):599–611. doi: 10.1016/s0896-6273(03)00499-9. [DOI] [PubMed] [Google Scholar]
- 98.Neu A, Soltesz I. How good vibes turn bad: rise of ictal events from oscillatory network activity. Focus on "transient depression of excitatory synapses on interneurons contributes to epileptogenesis during gamma oscillations in the mouse hippocampal slice". J Neurophysiol. 2005;94(2):905–906. doi: 10.1152/jn.00210.2005. [DOI] [PubMed] [Google Scholar]
- 99.Zhang W, et al. Surviving hilar somatostatin interneurons enlarge, sprout axons, and form new synapses with granule cells in a mouse model of temporal lobe epilepsy. J Neurosci. 2009;29(45):14247–14256. doi: 10.1523/JNEUROSCI.3842-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Toth Z, et al. Instantaneous perturbation of dentate interneuronal networks by a pressure wave-transient delivered to the neocortex. J Neurosci. 1997;17(21):8106–8117. doi: 10.1523/JNEUROSCI.17-21-08106.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wyeth MS, et al. Selective reduction of cholecystokinin-positive basket cell innervation in a model of temporal lobe epilepsy. J Neurosci. 2010;30(26):8993–9006. doi: 10.1523/JNEUROSCI.1183-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gittis AH, et al. Rapid target-specific remodeling of fast-spiking inhibitory circuits after loss of dopamine. Neuron. 2011;71(5):858–868. doi: 10.1016/j.neuron.2011.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Somogyi J, et al. GABAergic basket cells expressing cholecystokinin contain vesicular glutamate transporter type 3 (VGLUT3) in their synaptic terminals in hippocampus and isocortex of the rat. Eur J Neurosci. 2004;19(3):552–569. doi: 10.1111/j.0953-816x.2003.03091.x. [DOI] [PubMed] [Google Scholar]
- 104.Soltesz I. Diversity in the Neuronal Machine. Oxford University Press; 2005. [Google Scholar]
- 105.Cope DW, et al. Cholecystokinin-immunopositive basket and Schaffer collateral-associated interneurones target different domains of pyramidal cells in the CA1 area of the rat hippocampus. Neuroscience. 2002;109(1):63–80. doi: 10.1016/s0306-4522(01)00440-7. [DOI] [PubMed] [Google Scholar]
- 106.Pawelzik H, et al. Physiological and morphological diversity of immunocytochemically defined parvalbumin- and cholecystokinin-positive interneurones in CA1 of the adult rat hippocampus. J Comp Neurol. 2002;443(4):346–367. doi: 10.1002/cne.10118. [DOI] [PubMed] [Google Scholar]
- 107.Price CJ, et al. Neurogliaform neurons form a novel inhibitory network in the hippocampal CA1 area. J Neurosci. 2005;25(29):6775–6786. doi: 10.1523/JNEUROSCI.1135-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Krook-Magnuson E, et al. Ivy and Neurogliaform Interneurons Are a Major Target of μ-Opioid Receptor Modulation. J Neurosci. 2011;31(42):14861–14870. doi: 10.1523/JNEUROSCI.2269-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fuentealba P, et al. Ivy cells: a population of nitric-oxide-producing, slow-spiking GABAergic neurons and their involvement in hippocampal network activity. Neuron. 2008;57(6):917–929. doi: 10.1016/j.neuron.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tukker JJ, et al. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci. 2007;27(31):8184–8189. doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]