Abstract
p38α mitogen-activated protein (MAP) kinase is a broadly expressed signaling molecule that participates in the regulation of cellular responses to stress as well as in the control of proliferation and survival of many cell types. We have used cell lines derived from p38α knockout mice to study the role of this signaling pathway in the regulation of apoptosis. Here, we show that cardiomyocytes and fibroblasts lacking p38α are more resistant to apoptosis induced by different stimuli. The reduced apoptosis of p38α-deficient cells correlates with decreased expression of the mitochondrial proapoptotic protein Bax and the apoptosis-inducing receptor Fas/CD-95. Cells lacking p38α also have increased extracellular signal-regulated kinase (ERKs) MAP kinase activity, and the up-regulation of this survival pathway seems to be at least partially responsible for the reduced levels of apoptosis in the absence of p38α. Phosphorylation of the transcription factor STAT3 on Ser-727, mediated by the extracellular signal-regulated kinase MAP kinase pathway, may contribute to the decrease in both Bax and Fas expression in p38α-/- cells. Thus, p38α seems to sensitize cells to apoptosis via both up-regulation of proapoptotic proteins and down-regulation of survival pathways.
INTRODUCTION
The family of p38 mitogen-activated protein kinases (MAPKs) are strongly activated by stress and inflammatory cytokines, but nonstressful stimuli can also activate p38 MAPKs, leading to the regulation of cellular functions such as proliferation, differentiation, and survival. Four different p38 MAPK family members have been identified, p38α, β, γ, and δ, also known as stress-activated kinase (SAPK)2a, SAPK2b, SAPK3, and SAPK4, respectively, which may have both overlapping and specific functions (reviewed by Cohen, 1997; Nebreda and Porras, 2000; Ono and Han, 2000; Kyriakis and Avruch, 2001). p38α is broadly expressed and is also the most abundant p38 family member present in most cell types. Targeted inactivation of the mouse p38α gene results in embryonic death due to a placental defect (Adams et al., 2000; Mudgett et al., 2000; Tamura et al., 2000). Studies using cell lines have suggested an important role for p38 MAPKs in the regulation of cardiomyocyte differentiation, hypertrophy, and apoptosis (Wang et al., 1998; Zechner et al., 1998; Mackay and Mochly-Rosen, 1999; Saurin et al., 2000; Stephanou et al., 2000). However, p38α does not seem to play a critical role in the development of the embryonic heart in mice (Adams et al., 2000), although heterozygous p38α+/- mice have been reported to be less sensitive to myocardial cell death caused by ischemia-reperfusion (Otsu et al., 2003). In addition, cardiac-specific expression of dominant negative forms of p38α and their activators MKK3 and MKK6 enhances cardiac hypertrophy in transgenic mice and/or makes the mice resistant to cardiac fibrosis (Braz et al., 2003; Zhang et al., 2003b).
The role of p38 MAPKs in apoptosis depends on the cell type and the stimuli (reviewed by Nebreda and Porras, 2000). Most of the data available are based on the effect of either chemical inhibitors such as SB203580 and SB202190 (which can inhibit both p38α and p38β) or the overexpression of mutant forms of the p38 MAPKs and their activators.
Anti-apoptotic roles of p38 MAPKs have been described in cardiomyocytes (Zechner et al., 1998), DNA-damaged fibroblasts (Héron-Milhavet et al., 2002), endothelial cells exposed to anoxia-reoxygenation (Zhang et al., 2003a), differentiating neurons (Okamato et al., 2000), and activated macrophages (Park et al., 2002). In some cases, prevention of apoptosis by p38 MAPKs has been associated with the regulation of transcription factor activity (Okamato et al., 2000; Park et al., 2002). On the other hand, there is also good evidence for a role of p38 MAPKs as mediators of apoptosis, for example, in neurons (Le-Niculescu et al., 1999; Ghatan et al., 2000; Ciesielski-Treska et al., 2001; DeZutter and Davis, 2001) and cardiac cells (Wang et al., 1998; Mackay and Mochly-Rosen, 1999; Saurin et al., 2000). In other cell types, p38 MAPKs can also have proapoptotic roles, for example, upon stimulation with tumor necrosis factor-α (Valladares et al., 2000), transforming growth factor-β (Edlund et al., 2003), or in response to oxidative stress (Zhuang et al., 2000). The mechanisms by which p38α can contribute to an enhanced apoptotic response include the phosphorylation and/or translocation of proteins from the Bcl-2 family, which leads to the release of cytochrome c from the mitochondria (Ghatan et al., 2000; Zhuang et al., 2000; Torcia et al., 2001), the transforming growth factor-β-induced activation of caspase 8 (Schrantz et al., 2001) as well as the regulation of membrane blebbing and nuclear condensation (Deschesnes et al., 2001). At the transcriptional level, expression of monoamine oxidase (DeZutter and Davis, 2001) or growth arrest and DNA damage (GADD)-inducible genes (Sarkar et al., 2002) have both been shown to mediate proapoptotic effects of p38 MAPKs.
We have investigated the apoptotic response in different types of p38α-deficient cells: primary fibroblasts and immortalized cardiomyocytes and fibroblasts. We have found that p38α-deficient cells were all more resistant to apoptosis induced by different stimuli. Reduced apoptosis in the absence of p38α correlates with the down-regulation of the proapoptotic proteins Fas and Bax as well as enhanced activity of the extracellular signal-regulated kinase (ERK) MAPKs survival pathway.
MATERIALS AND METHODS
Cell Lines and Culture Conditions
Mouse embryonic fibroblasts (MEFs) were isolated from E9.5 embryos, trypsinized, plated, grown in DMEM containing 10% fetal bovine serum (Invitrogen, Carlsbad, CA), and immortalized either by retroviral infection (Brown et al., 1986) or by transfection with simian virus 40 (SV40) large T antigen constructs (Benito et al., 1993). Cells expressing large T were selected with G-418.
Cardiomyocytes were isolated from E9.5 embryos obtained by intercrossing animals heterozygous for both p38α and the immorto transgene allele, which drives expression of a temperature-sensitive large T under the control of the interferon-γ (IFN-γ)-inducible H-2K promoter (Jat et al., 1991). Cardiomyocytes were isolated by trypsinization of embryonic hearts as described previously (Adams et al., 2000). Cardiomyocytes were grown on collagen-coated tissue culture plates in DMEM containing 10% fetal bovine serum (Invitrogen), IFN-γ (10 U/ml; Sigma-Aldrich, St. Louis, MO), and cardiotrophin-1 (0.2 ng/ml; R&D Systems, Minneapolis, MN) at 33°C in a humidified atmosphere of 5% CO2 or, when indicated, at 37°C in the absence of IFN-γ.
Primary MEFs were derived from E11.5 and E12.5 embryos obtained by crossing heterozygous p38α+/- mice, as described previously (Ambrosino et al., 2003). For the rescue experiments, two rounds of retroviral infection were performed as described in Ambrosino et al. (2003).
Transfection of Bax and p38α Constructs
The mouse Bax and human p38α cDNAs were cloned into the EcoRI site of the pEFmlink expression vector. Transient transfections with the Bax and p38α constructs were performed using the MBS transfection kit (2003-88-51; Stratagene, La Jolla, CA), a modified calcium phosphate method. The protocol supplied by the manufacturer was followed using 10 μg of DNA per dish (1.5 × 106 cells). Cells were analyzed 24-48 h after transfection.
Induction of Apoptosis
To induce apoptosis, cells were treated as follows: UV stimulated for 10 min followed by 30 min - 3h in the 37°C incubator; serum deprived for 24-48 h; and incubated either with 1-2 μg/ml of the agonist anti-Fas antibody Jo2 (15401D; BD Biosciences PharMingen, San Diego, CA) or with 2 μM of the Ca2+ ionophore A23187 for different periods. Before these treatments, cardiomyocytes were transferred to 37°C and maintained without IFN-γ for 16-18 h.
For 4,6-diamidino-2-phenylindole (DAPI) staining, cells were washed with phosphate-buffered saline (PBS), fixed in 1.5% paraformaldehyde (prepared in PBS at pH 7.2) for 20-30 min, washed with PBS, and stained with 1 μM DAPI for 15 min in the dark. After washing with PBS, nuclear morphology was analyzed by fluorescence microscopy.
Apoptotic cells were quantified by flow cytometry. Cells were trypsinized, washed with PBS, and fixed with cold ethanol (70% vol/vol). The cells were then washed and resuspended in PBS and incubated with RNase (25 μg/106 cells) for 30 min at 37°C. After addition of 0.05% propidium iodide, cells were analyzed in the cytometer.
Fluorometric Analysis of Caspase 3 Activity
Caspase 3 activity was determined by fluorometric quantification of the fluorogenic substrate Ac-DEVD-AMC (66081; BD Biosciences PharMingen). Cells were lysed by incubation for 10 min at 4°C in a buffer (125 μl/6-cm plate) containing 10 mM Tris (pH 7.5), 130 mM NaCl, 1% Triton X-100, and 10 mM Na F. Cell lysates (2-10 μg of total protein) were incubated at 37°C for 1.5 h in a buffer containing 20 mM HEPES (pH 7.5), 10% glycerol, 2 mM dithiothreitol, and 20 μM Ac-DEVD-AMC. A blank without lysate was prepared in parallel. The fluorescent AMC liberated by caspase 3 activity was quantified in a fluorometer with a fixed excitation wavelength of 380 nm and an emission wavelength range of 430-460 nm.
Western Blot Analysis
Western blot analysis was carried out as described previously (Adams et al., 2000). Proteins were separated by electrophoresis by using Anderson gels and transferred to nitrocellulose membranes that were probed with the following antibodies: active cleaved caspase 3 (9661; Cell Signaling Technology, Beverley, MA), Bax (sc-526; Santa Cruz Biotechnology, Santa Cruz, CA), BclxL (sc-634; Santa Cruz Biotechnology), Bid (AF860; R&D Systems), p38α (sc-535; Santa Cruz Biotechnology), p53 (antibody-1 0P03; Calbiochem, San Diego, CA), phospho-Akt (9271; Cell Signaling Technology), phospho-ERK1/2 (9101S; Cell Signaling Technology), STAT1 (sc-464; Santa Cruz Biotechnology), phospho-Ser727-STAT3 (sc-8001R; Santa Cruz Biotechnology), and phospho-Tyr705-STAT3 (9131; Cell Signaling Technology).
Total cell extracts were obtained as described previously (Adams et al., 2000) except for the analysis of the levels of active caspase 3 and Bid proteolytic fragment, which were prepared as described above for the fluorometric analysis of caspase 3 activity. Nuclear extracts were prepared as described previously (Valladares et al., 2000).
Cytometric Quantification of Fas Expression
Fas expression in cardiomyocytes was quantified by flow cytometric analysis. Cells were grown at 33°C, and trypsinized, washed, resuspended in PBS (1-1.5 × 106 cells per assay), and incubated for 30 min at 4°C with 2.5 μg of the anti-Fas antibody Jo2 conjugated with fluorescein isothiocyanate (15404D; BD Biosciences PharMingen). After washing, cells were incubated with propidium iodide (0.005%) and analyzed in the cytometer. The percentage of cells labeled with the anti-Fas antibody was considered positive for Fas expression, whereas the relative fluorescence intensity was recorded as a measure of the amount of Fas expression per cell.
Semiquantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Northern Blot
Total RNA (2 μg) was isolated with the TRIzol reagent (15596-026; Invitrogen) and used for cDNA synthesis with the SuperScript II RT kit (1098-018; Invitrogen). The following primers were used for PCR: Fas-5′ ATC CGA GCT AGG AGG CGG GTT CAT GAA AC and Fas-3′ GGT TCT AGA TTC AGG GTC ATC CTG (Hsu et al., 1999); p38α-5′ TGC ATA ATT TTC TGA ATT TTG and p38α-3′ TCC TAT GGC ATA CCA GAT TAC; and GAPDH-5′ CAG TAT GAC TCC ACT CAC GGC and GAPDH-3′ GAG GGG CCA TCC ACA GTC TTC.
Total RNA was analyzed by Northern blotting as described previously (Valladares et al., 2000).
Statistical Analysis
Statistical analysis was carried out by Student's t test.
RESULTS
Apoptosis Induced by Serum Withdrawal Is Greatly Reduced in Cells Lacking p38α
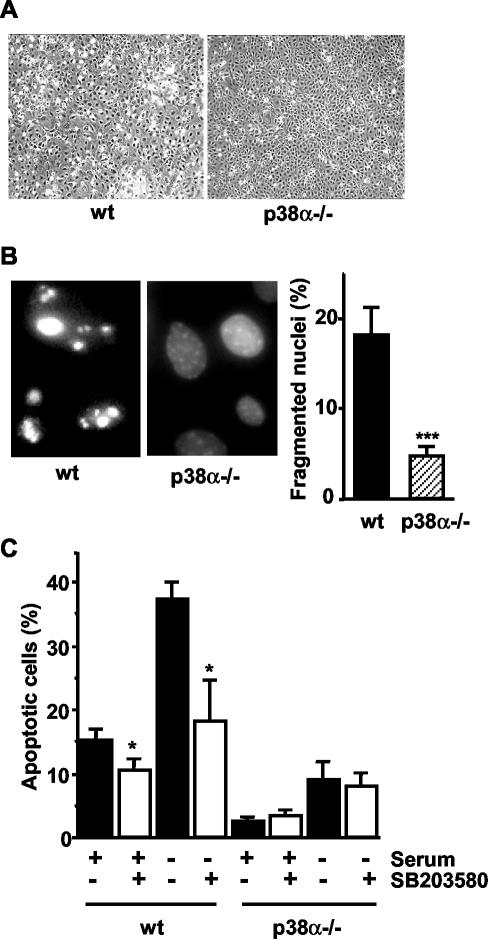
To investigate the role of p38α in apoptosis, we first compared the effect of serum withdrawal in wild-type (wt) and p38α-deficient cardiomyocytic cell lines. After 48 h of serum deprivation, phase-contrast microscopy evidenced the presence of a high number of wild-type (wt) cardiomyocytes with the characteristic morphology of apoptotic cells. This included the loss of cellular contacts, appearance of cellular blebbing, and finally detachment of many cells from the plate (Figure 1A, left). In contrast, most of the p38α-/- cells remained attached to the plate, and the apoptotic phenotype was only observed in a few cells (Figure 1A, right). Analysis of nuclear morphology by fluorescence microscopy after DAPI staining also indicated the presence of a higher number of condensed and/or fragmented nuclei in wt cardiomyocytes (18%) than in p38α-/- cells (5%) (Figure 1B). In addition, the percentage of cells with DNA content lower than 2C (as determined by flow cytometry analysis) was also higher in wt than in p38α-deficient cardiomyocytes, either serum deprived or maintained in 10% serum (Figure 1C). Treatment with the p38 MAPK inhibitor SB203580 strongly reduced the number of apoptotic wt cells, in particular upon serum withdrawal, whereas having little effect on p38α-/- cells. These results support a connection between p38α and the increased levels of apoptosis observed in wt cells.
Figure 1.
Apoptosis induced by serum withdrawal is decreased in cardiomyocytes lacking p38α. Cardiomyocytes were serum deprived for 48 h and then analyzed for cellular and nuclear morphology. (A) Representative phase-contrast microscopy images of wt and p38α-deficient cardiomyocytes. (B) Representative nuclear morphology images (left) of wt and p38α-deficient cardiomyocytes after staining of DNA with DAPI and analysis by fluorescence microscopy. A high number of condensed and/or fragmented nuclei can be visualized in wt cells. The percentage of fragmented nuclei was estimated by counting 200 cells (histogram, right). Results shown are the mean ± SEM. Statistical analysis was carried out by Student's t test by comparison with wt cells ***p < 0.001. (C) Flow cytometric analysis of the percentage of cells with DNA lower than 2C (apoptotic cells). Inhibition of p38 with SB203580 (5 μM) highly reduced the number of apoptotic cells in wt cardiomyocytes, either maintained in the absence or in the presence of serum for 48 h. Results shown are the mean ± SEM (n = 3). Statistical analysis was carried out by Student's t test by comparison of cells treated with SB203580 with the nontreated; *p < 0.05.
p38α-deficient Cells Are More Resistant to Apoptosis Induced by Different Stimuli
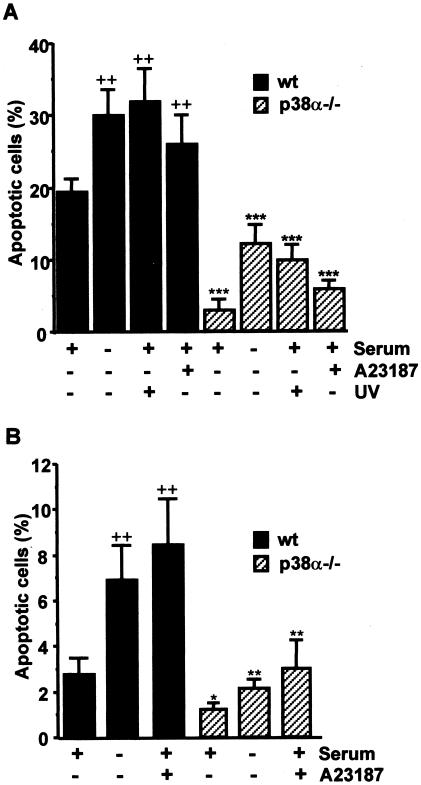
The reduced susceptibility of p38α-lacking cardiomyocytes to apoptosis upon serum deprivation prompted us to investigate other proapoptotic stimuli. Quantification by cytometry of the percentage of cells with DNA content lower than 2C also showed that treatment with UV or the Ca2+ ionophore A23187 also induced a higher number of apoptotic cells in wt than in p38α-/- cardiomyocytes (Figure 2A). p38α-/- cells were also more resistant to other proapoptotic stimuli such as tumor necrosis factor-α or staurosporine (our unpublished data). Together, these data indicate that the presence of p38α sensitizes cardiomyocytes to apoptosis, both under basal conditions and in response to different apoptotic stimuli. It should be mentioned, however, that basal apoptosis varied depending on cell density, being significantly higher (around twofold) in wt confluent cells than in cells maintained at a lower density (our unpublished data).
Figure 2.
Effect of p38α deficiency on the response of cardiomyocytes and MEFs to different apoptotic stimuli. (A) Cardiomyocytes were transferred to 37°C in the absence of IFN-γ and either maintained in 10% serum or challenged with different apoptotic stimuli: UV (10 min plus 3 h of incubation), Ca2+ ionophore A23187 (2 μM for 20 h) or serum deprivation for 48 h. Analysis of the percentage of cells with DNA content lower than 2C (apoptotic cells) by flow cytometric is shown. Results are the mean ± SEM of three independent experiments. (B) MEFs were maintained in 10% serum, stimulated with Ca2+ ionophore A23187 (2 μM for 20 h), or serum deprived for 48 h and then analyzed by flow cytometry. Statistical analysis by Student's t test was carried out by comparing either p38α-deficient cells with wt cells given the same treatment (*p < 0.05, **p < 0.01, ***p < 0.001) or treated with untreated (maintained with 10% serum) wt cells (++p < 0.01).
Similar results were observed by comparing wt and p38α-/- immortalized MEFs. The percentage of apoptotic cells, as determined by flow cytometry analysis of DNA content, was always higher in wt immortalized MEFs, either maintained in 10% serum or upon serum withdrawal or incubation with A23187 (Figure 2B). Therefore, the proapoptotic effect of p38α seems to be neither cell type nor stimulus specific.
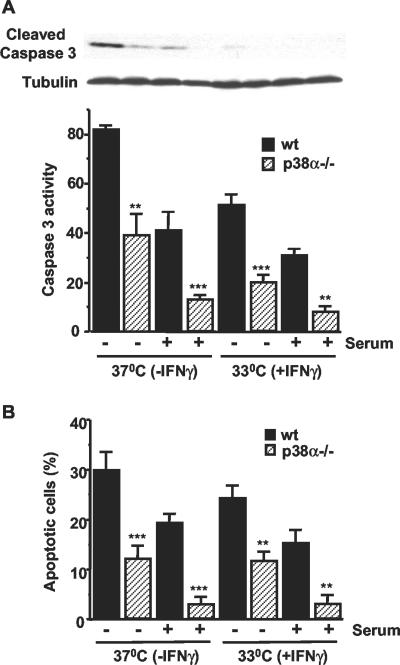
Cardiomyocytes were usually grown at 33°C with IFN-γ to induce large T expression. To investigate the effect of different culture conditions, cardiomyocytes were grown at 37°C in the absence of IFN-γ for 18 h before the induction of apoptosis. Quantification of caspase 3 activity by using a fluorogenic substrate showed that it was approximately twofold higher in wt than in p38α-/- cells maintained in the absence of serum for 48 h (Figure 3A, histogram). As expected, the levels of caspase 3 activity were reduced in cells maintained in 10% serum, but this activity was also ∼2-3 times higher in wt than in p38α-/- cells. In cells maintained at 33°C, caspase 3 activity was generally reduced, but the differences between wt and p38α-/- cells were also maintained. These results were confirmed by immunoblots with an antibody that specifically detected active (cleaved) caspase 3 (Figure 3A, top) and cytometric quantification of the percentage of hypodiploid cells (Figure 3B). Therefore, the reduced levels of apoptosis in cardiomyocytes lacking p38α compared with wt cells is affected neither by the presence of IFN-γ nor by the growth temperature. MEFs lacking p38α also show reduced levels of caspase 3 activity (measured by using a fluorometric substrate) upon treatment with UV or A23187 (our unpublished data).
Figure 3.
Cardiomyocytes lacking p38α are more resistant to apoptosis independent of the culture conditions. Cardiomyocytes were grown at 33°C with IFN-γ and were then either maintained under the same conditions or transferred to 37°C without IFN-γ in the presence or absence of 10% serum, as indicated. (A) Lysates were analyzed by Western blot with cleaved caspase 3 antibodies (top). Caspase 3 activity was also determined by a fluorometric assay using Ac-DEVD-AMC as a substrate. Results (mean ± SEM) are indicated as arbitrary units per microgram of total protein. (B) Percentage of cells with DNA content lower than 2C, as determined by flow cytometry analysis. Results shown are the mean ± SEM (n = 3). Statistical analysis was carried out by Student's t test by comparison of p38α-deficient cells with wt cells with the same treatment (**p < 0.01, ***p < 0.001).
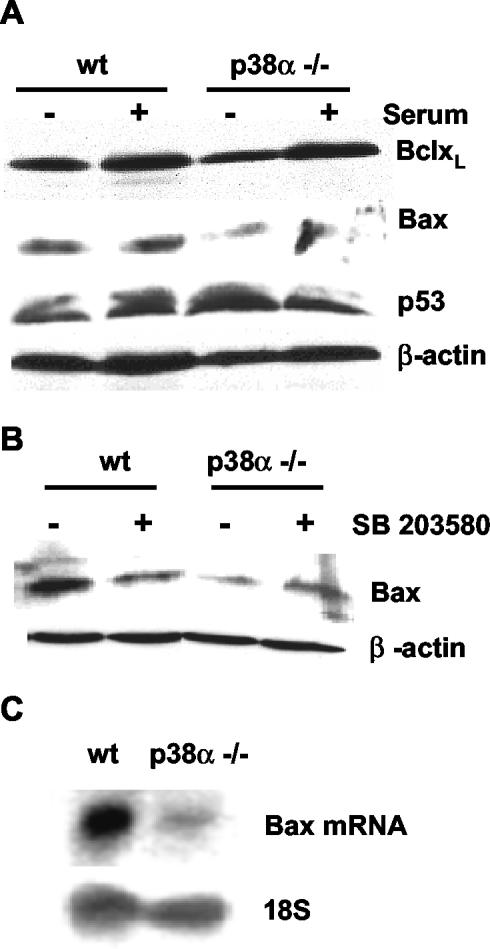
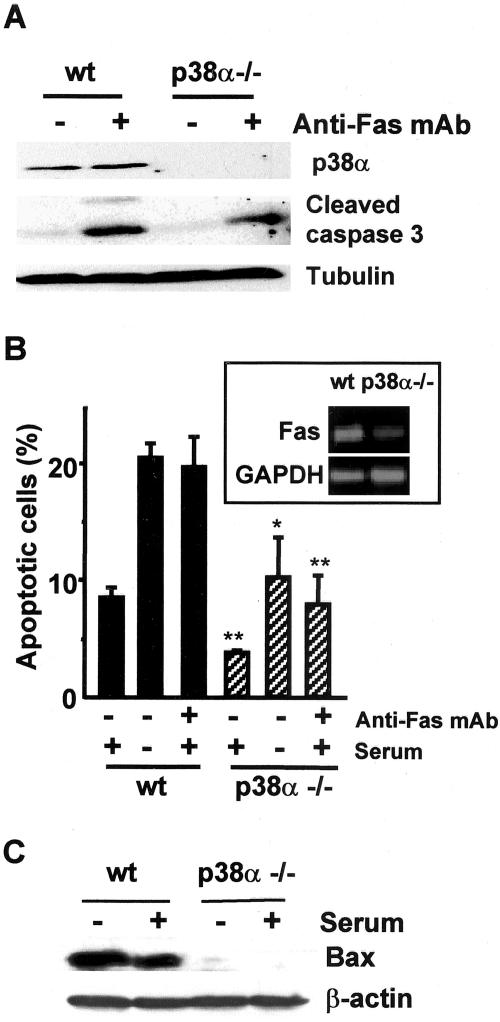
Down-Regulation of Bax Expression in p38α-deficient Cells
To investigate the molecular basis for the reduced apoptosis observed in cells lacking p38α, we analyzed the expression levels of several pro- and antiapoptotic proteins. We first studied these differences in cardiomyocytes, where apoptosis levels were higher than in MEFs. No differences were found in the expression levels of BclxL and p53 proteins, either in the presence or absence of serum for 48 h (Figure 4A). Likewise, Bcl-2 and phospho-Bad levels were similar in wt and p38α-/- cells (our unpublished data), whereas Bim protein expression was detected neither in wt nor in p38α-deficient cells. In contrast, the levels of the proapoptotic protein Bax were higher in wt cells, either with or without serum deprivation (Figure 4A). Consistent with the idea that p38α positively regulates Bax expression, incubation with the p38 inhibitor SB203580 decreased Bax protein levels in wt cells (Figure 4B). Moreover, Bax mRNA expression was also increased in wt cells compared with p38α-deficient cells (Figure 4C), suggesting that p38α up-regulates Bax gene expression, either at the level of transcription or mRNA stability.
Figure 4.
Bax expression is down-regulated in p38α-deficient cardiomyocytes. Cardiomyocytes were grown at 33°C with IFN-γ as described in text, transferred to 37°C without IFN-γ, and maintained either in the presence or absence of 10% serum. (A) Total levels of BclxL, Bax, and p53 were determined by Western blot. (B) Effect of the p38 inhibitor SB203580 (5 μM) on Bax expression level analyzed by Western blot in total extracts of cardiomyocytes maintained with serum. Normalization of blots was done with β-actin. (C) Northern blot analysis of Bax mRNA expression in cells maintained with 10% serum. Blots were normalized by reprobing with a 18S rRNA probe.
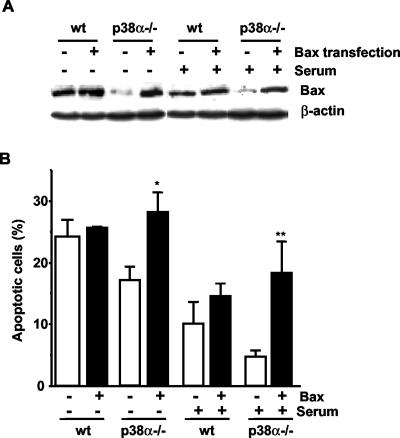
To characterize the effect that different levels of Bax expression might have on apoptosis, wt and p38α-deficient MEFs were transiently transfected with a Bax expression construct. We found that forced expression of Bax in p38α-deficient cells, to a similar level of that in wt cells (Figure 5A), increased the number of apoptotic cells, either in the absence or in the presence of serum, up to a similar level as in wt cells (Figure 5B). These results correlate low expression levels of Bax protein with reduced apoptosis and place p38α upstream of Bax in the proapoptotic pathway.
Figure 5.
Resistance to apoptosis of p38α-deficient cells is lost upon Bax expression. Transient transfection of MEFs with a wt Bax construct. (A) Analysis of Bax expression determined by Western blot. Normalization was done with β-actin. (B) Flow cytometric analysis of the percentage of cells with DNA lower than 2C (apoptotic cells). Results shown are the mean ± SEM (n = 3). Statistical analysis was carried out by Student's t test by comparison of Bax transfected cells with those transfected with the empty vector under the same treatment (*p < 0.05. **p < 0.01).
Down-Regulation of Fas Expression in p38α-deficient Cells
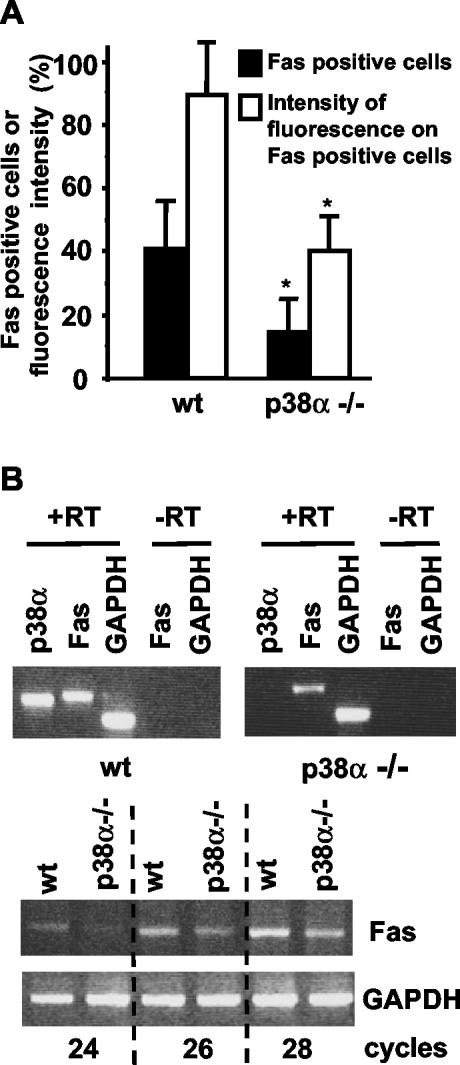
Another important proapoptotic molecule is the receptor Fas/CD-95, which can be induced by p38 MAPKs (Hsu et al., 1999; Stephanou et al., 2001) and can mediate cardiomyocyte apoptosis (Jeremias et al., 2000; Stephanou et al., 2001). We therefore quantified Fas expression on the cell surface of wt and p38α-/- cardiomyocytes by flow cytometry by using a fluorescein isothiocyanate-labeled anti-Fas antibody. As shown in Figure 6A, the percentage of cells positive for Fas expression was more than double in wt than in p38α-/- cardiomyocytes under basal conditions (in the presence of serum). In addition, the anti-Fas fluorescence intensity was also higher in wt cells (Figure 6A), indicating the presence of more Fas protein per cell in wt cardiomyocytes than in those deficient in p38α. Therefore, not only the percentage of cardiomyocytes expressing Fas but also the number of Fas molecules per cell is reduced in the absence of p38α.
Figure 6.
Fas expression is reduced in p38α-deficient cardiomyocytes. (A) Cardiomyocytes were subjected to cytometric analysis of cell surface Fas expression. The percentage of cells detected as positive for Fas expression after binding to the anti-Fas antibody (black bars) as well as the anti-Fas fluorescence intensity (white bars) are represented. Results are expressed as relative values and are means ± SEM of three independent experiments. Statistical analysis was carried out by Student's t test by comparison of p38α-deficient cells with wt cells (*p < 0.05). (B) RT-PCR analysis of Fas mRNA expression in cardiomyocytes. Top, cDNA was amplified with specific oligos for Fas, p38α, and GAPDH for 30 cycles. RNA samples without reverse transcriptase (-RT) treatment were used as controls. Bottom, cDNA was amplified with specific oligos for Fas and GAPDH by using the indicated number of PCR cycles.
The differences in Fas expression at the cell surface might be due to changes in the cellular distribution of the receptor or to changes in Fas gene expression. To address these possibilities, the levels of Fas mRNA were analyzed by RT-PCR. The results revealed reduced expression levels of Fas mRNA in p38α-/- cardiomyocytes (Figure 6B).
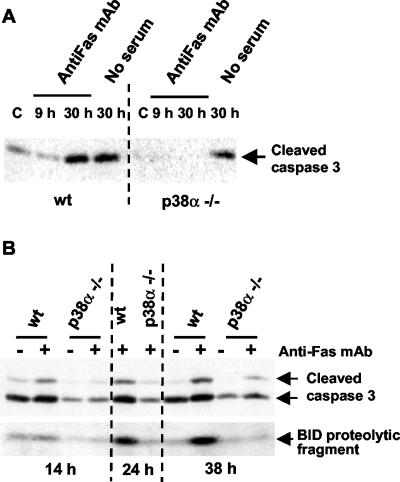
Fas has been implicated in apoptosis induced by many stimuli, suggesting that differences in Fas expression could also explain the different levels of apoptosis found in p38α-/- cells under basal conditions. Moreover, it is expected that apoptosis induced by FasL, which interacts with and activates Fas receptor, should be also reduced in p38α-deficient cardiomyocytes. To confirm this hypothesis, wt and p38α-/- cardiomyocytes were incubated with the anti-Fas antibody Jo2, which mimics the effect of FasL. Figure 7A shows that incubation with Jo2 strongly increased the levels of active caspase 3 in wt cardiomyocytes, similar to that induced by serum withdrawal. In contrast, active cleaved caspase 3 was undetectable in p38α-deficient cardiomyocytes treated with anti-Fas antibody, and serum deprivation also induced a lower increase in cleaved caspase 3 than in wt cells. Therefore, cardiomyocytes lacking p38α are more resistant to Fas-induced apoptosis.
Figure 7.
Cardiomyocytes lacking p38α are more resistant to apoptosis induced by anti-Fas antibody than wt cells. Cardiomyocytes were grown at 33°C with IFN-γ and transferred to 37°C without IFN-γ and either serum-deprived or treated with anti-Fas antibody Jo2 (1-2 μg/ml) for the indicated periods. Cell extracts were analyzed by Western blot for the levels of cleaved caspase 3 (A and B) and the Bid proteolytic fragment of 15 kDa (B, bottom). In some experiments, two cleaved caspase 3 cross-reacting bands of 17 and 19 kDa, respectively, are observed (B).
To further characterize the differences in Fas-induced apoptosis between wt and p38α-/- cardiomyocytes, a time-course study was performed. In this experiment, high levels of active caspase 3 were already present in wt cardiomyocytes maintained with serum but were further increased upon treatment with anti-Fas (Figure 7B). In contrast, p38α-/- cells presented a low basal level of cleaved caspase 3 that only slightly increased after 38 h of treatment with anti-Fas (Figure 7B). We also found that caspase 3 activation correlated with the appearance of the proteolytic fragment of Bid in wt cells (Figure 7B, bottom). In p38α-/- cardiomyocytes, the level of the proteolytic fragment of Bid was very low and did not significantly increase with time, which correlates well with the low levels of cleaved caspase 3 and the decreased apoptosis. The results suggest a possible role for Bid cleavage in Fas-induced apoptosis of these cells, although it is unclear whether Bid cleavage is produced before the first peak of caspase 3 activation or later, leading to the amplification of the apoptotic response. In either case, cleaved Bid is important as it facilitates the proapoptotic effect of Bax.
Reintroduction of p38α Sensitizes p38α-/- Cardiomyocytes to Apoptosis Induced by Fas and Serum Withdrawal
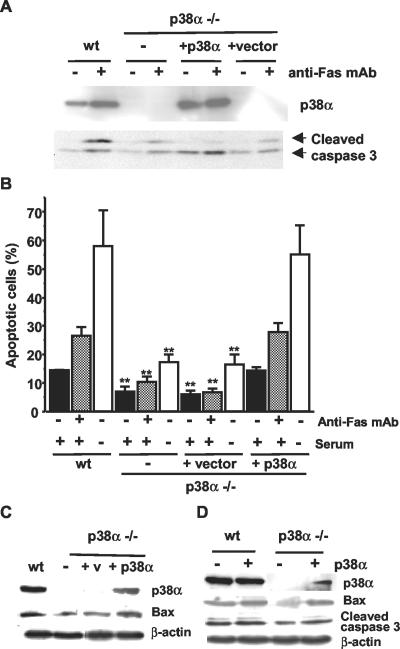
The data presented above suggest that p38α sensitizes cells to apoptosis by a mechanism involving enhanced Bax and Fas expression. To confirm that the reduced apoptosis of p38α-/- was directly due to the absence of p38α, we infected these cells with a p38α-expressing retrovirus. Figure 8A shows that the level of active caspase 3 induced by anti-Fas treatment was increased upon reintroduction of p38α in the p38α-/- cardiomyocytes, to a similar level as in wt cells. Accordingly, the number of apoptotic cells determined by flow cytometry was also higher in the rescued cardiomyocytes, either treated with anti-Fas or serum deprived, compared with cardiomyocytes deficient in p38α (Figure 8B). The increased levels of apoptosis in rescued cells correlated with the level of p38α expression and are consistent with the idea that p38α sensitizes cells to Fas and serum withdrawal-induced apoptosis. The increased level of basal apoptosis (no induction) normally seen in wt cells was also observed in the rescued p38α-/- cells expressing ectopic p38α. Bax expression was also up-regulated upon reintroduction of p38α in p38α-deficient cardiomyocytes (Figure 8C).
Figure 8.
Reintroduction of p38α sensitizes p38α-/- cells to apoptosis. wt or p38α-/- cardiomyocytes were infected with either a retrovirus expressing p38α or the vector alone and grown at 33°C with IFN-γ. Cells were then transferred to 37°C without IFN-γ and either serum deprived or treated with anti-Fas antibody Jo2 (2 μg/ml) for 24 h as indicated. (A) Effect of anti-Fas antibody on caspase 3 activity. Cell lysates were analyzed by Western blot with p38α (top) and cleaved caspase 3 (bottom) antibodies. (B) Flow cytometric analysis of the percentage of cells with DNA content lower than 2C (apoptotic cells). Results are means ± SEM of three experiments. Statistical analysis was carried out by Student's t test by comparison of p38α-/- cardiomyocytes either uninfected or infected with a retrovirus expressing p38α or the vector alone with wt cells treated in the same way (**p < 0.01). (C) Western blot analysis of Bax upon p38α rescue of p38α-/- cardiomyocytes. (D) Western blot analysis of Bax and cleaved caspase 3 levels in p38α-/- MEFs transiently transfected with either a p38α construct or the empty vector. Normalization of Western blots was done with β-actin.
In addition, transient transfection of p38α into p38α-/- MEFs was able to increase both Bax expression and the basal level of apoptosis of these cells (as determined by cleaved caspase 3 levels) up to a similar level as in wt cells (Figure 8D).
Fas and Serum Withdrawal-induced Apoptosis Are Reduced in Primary p38α-/- MEFs
To investigate whether the resistance to Fas-induced apoptosis in p38α-deficient cardiomyocytes was cell type specific, we used primary MEFs. Both the Fas-induced activation of caspase 3 and the percentage of apoptotic cells, as determined by flow cytometry, were lower in p38α-/- MEFs (Figure 9A). This also correlated with higher expression of Fas mRNA in wt cells (Figure 9B, inset). Therefore, the decreased sensitivity of p38α-deficient cells to Fas-induced apoptosis is not particular to the cardiomyocytic cell line.
Figure 9.
Apoptosis induced by anti-Fas antibody and serum-deprivation is reduced in p38α-/- primary MEFs. MEFs maintained in primary culture were either treated with anti-Fas antibody Jo2 (2 μg/ml) for 24 h or serum deprived, as indicated. (A) Western blot analysis of p38α (top), cleaved caspase 3 (middle), and tubulin (bottom). (B) Flow cytometric analysis of the percentage of cells with DNA content lower than 2C (apoptotic cells). Results are the mean ± SEM of three experiments. Inset, analysis of Fas mRNA expression by RT-PCR (30 cycles). (C) Western blot analysis of Bax. The same blot was reprobed with β-actin.
To exclude the possibility that some of the results described above could be a consequence of large T expression, we also measured the apoptotic response and the expression of Bax protein in primary MEFs. As shown in Figure 9B, both the basal level of apoptosis and the apoptosis induced upon serum deprivation were higher in wt than in p38α-deficient primary MEFs. Bax protein levels were also higher in wt MEFs and were further increased in cells maintained without serum for 48 h (Figure 9C). Therefore, similarly to the cardiomyocyte-derived cell lines, there is also a correlation between Bax protein expression and apoptosis in primary MEFs.
Up-Regulation of ERK MAPKs and Increased Levels of Nuclear Phospho-Ser-STAT3 in p38α-/- Cardiomyocytes
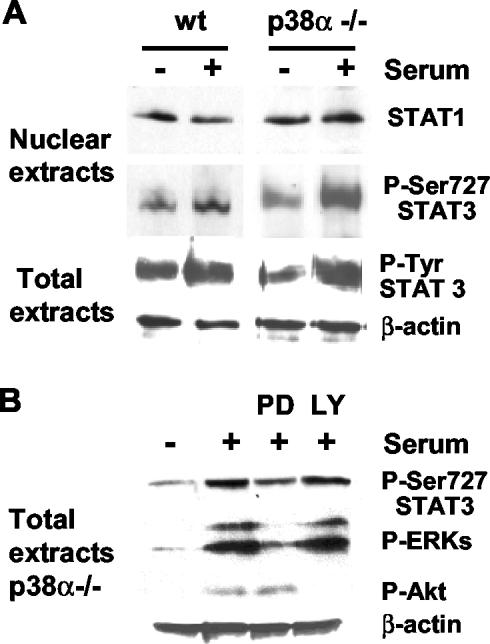
Searching for potential mechanisms that could account for the proapoptotic effect of p38α, we analyzed the activation state and nuclear levels of the transcription factors STAT1 and STAT3, which are potential candidates to regulate Fas and Bax expression (Naka et al., 1998; Ivanov et al., 2001; Stephanou et al., 2001). As shown in Figure 10A, treatment of p38α-deficient cardiomyocytes with 10% serum strongly increased the nuclear levels of STAT3 phosphorylated on Ser-727, the active form of STAT3. However, the nuclear levels of STAT1 were not significantly modified. We did not observe significant changes in the total level of tyrosine-phosphorylated STAT3 between wt and p38α-/- cells (Figure 10A).
Figure 10.
Nuclear phospho-Ser727 STAT3 is up-regulated in p38α-deficient cardiomyocytes. Cardiomyocytes were grown at 33°C with IFN-γ, transferred to 37°C without IFN-γ, and serum starved overnight before stimulation with 10% serum plus cardiotrophin-1 and IFN-γ for 15 min. Similar results were obtained when cells were only treated with 10% serum for 5-30 min. Where indicated, cells were preincubated for 30 min with the MEK inhibitor PD98059 (20 μM) or the phosphatidylinositol 3-kinase inhibitor LY294002 (10 μM). (A) Western blot analysis of STAT1 and phospho-Ser727-STAT3 in nuclear extracts and of phospho-Tyr705-STAT3 and actin in total cell extracts. (B) Western blot analysis of phospho-Ser-727-STAT3, phospho-ERK1/2, and phospho-Akt in total cell extracts from p38α-deficient cardiomyocytes. Normalization was done with β-actin.
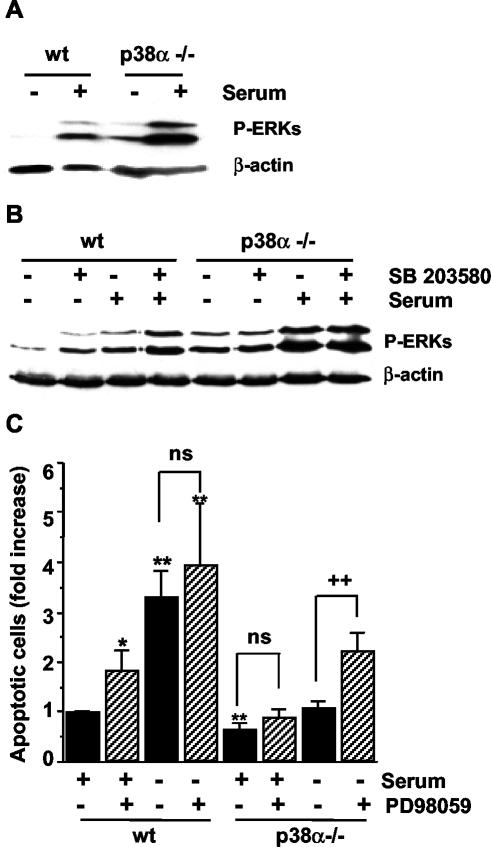
STAT transcription factors can be phosphorylated by the MAPKs ERK and p38 (Kovarik et al., 1999; Stephanou et al., 2001; Zhang et al., 2001; Ramsauer et al., 2002) and by other protein kinases such as Akt (Nguyen et al., 2001). We found that incubation with the mitogen-activated protein kinase kinase (MEK) inhibitor PD98059 strongly inhibited STAT3 phosphorylation on Ser-727, whereas the phosphatidylinositol 3-kinase inhibitor LY29400 abolished Akt activation but did not affect STAT3 phosphorylation (Figure 10B). Moreover, the levels of active ERK were found to be higher in p38α-deficient cells either in serum-deprived medium or after serum stimulation for 10 min (Figure 11A). In addition, treatment with the p38 inhibitor SB203580 also induced an up-regulation of the active form of ERKs in wt cells (Figure 11B), indicating that p38α negatively regulates ERK activity. Thus, increased ERK activity may account for the increased phosphorylation of Ser-727-STAT3, which in turn could participate in the negative regulation of Bax and Fas, leading to increased survival of p38α-deficient cells. In agreement with this hypothesis, inhibition of ERK activity with PD98059 increased the number of apoptotic cells (Figure 11C). This effect of ERK inhibition was particularly important in serum-deprived p38α-deficient cells.
Figure 11.
ERK up-regulation contributes to increased survival of p38α-deficient cardiomyocytes. Cardiomyocytes were grown at 33°C with IFN-γ, transferred to 37°C without IFN-γ, and serum deprived overnight before stimulation with 10% serum for 10 min (A and B). When indicated, cells were pretreated with SB203580 (5 μM) for 30 min. (A and B) Western blot analysis of phospho-ERK1/2. Normalization was done with β-actin. (C) Flow cytometric analysis of the percentage of cells with DNA content lower than 2C (apoptotic cells) in cardiomyocytes maintained either in the presence or absence of 10% serum for 24 h. Where indicated, cells were treated with PD98059 (20 μM). Results are the mean ± SEM of three experiments. Statistical analysis by Student's t test was carried out by comparing wt cells maintained with 10% serum (*p < 0.05, **p < 0.01) or with cells nonpretreated with PD98059 (++p < 0.01).
The up-regulation of ERK activity described above does not seem to be stimulus specific, because it was also observed upon serum deprivation for 24 h and during Fas-induced apoptosis (our unpublished data).
DISCUSSION
We have found that cardiomyocytes and fibroblasts lacking p38α are more resistant to apoptosis induced by different stimuli (including activators of both the death receptor pathway and the mitochondrial pathway) and also have reduced levels of basal apoptosis under normal proliferating conditions. In contrast, a previous study reported no differences in apoptosis between wt and p38α-deficient embryonic stem cells (Allen et al., 2000). This confirms that the effect of p38 MAPKs on apoptosis depends on the cellular context, as suggested by previous studies using chemical inhibitors or mutated components of the p38 MAPK pathway (see INTRODUCTION). Our results also indicate that p38α can sensitize cells to apoptosis through the positive regulation of Fas/CD-95 and Bax expression.
Cardiomyocytes lacking p38α are particularly resistant to Fas-induced apoptosis, and this correlates with decreased expression of the receptor in these cells. Fas receptor has been implicated in apoptosis induced not only by its ligand (FasL) but also by other stimuli such as UV (Leverkus et al., 1997), survival factor withdrawal (Le-Nicolescu et al., 1998), ischemia (Jeremias et al., 2000; Stephanou et al., 2001), and cisplatin treatment (Yuan et al., 2003). Therefore, higher cell surface expression of Fas receptor may sensitize wt cells to apoptosis induced by several treatments.
Fas receptor is constitutively present in a variety of cell types (Leithauser et al., 1993), and its expression can be increased by several extracellular signals such as UV (Leverkus et al., 1997), hypoxia (Tanaka et al., 1994), or cytokines (Stephanou et al., 2001) through the regulation of different transcription factors. p38 MAPKs have been implicated in the regulation of Fas expression, although their precise role is still controversial. In human melanoma cells, for example, p38 down-regulates Fas expression through inhibition of nuclear factor-κB (Ivanov and Ronai, 2000). In contrast, p38α was shown to contribute to the anti-CD3-induced up-regulation of Fas and FasL expression in T cells, although p38α alone was unable to increase Fas expression (Hsu et al., 1999). In cardiac myocytes exposed to ischemia/reperfusion, p38α activation also mediates Fas expression through phosphorylation of STAT1 in Ser-727 (Stephanou et al., 2001). Thus, Fas expression could be induced in wt cardiomyocytes by a p38α-dependent mechanism. Factors present in the serum and/or autocrine or paracrine signals could be responsible for the stimulation of p38α leading to Fas expression, whereas the absence of p38α would impair this mechanism. It should be noted that cardiomyocytes are normally grown in the presence of IFN-γ, which might also trigger the induction of Fas expression (Ruiz-Ruiz et al., 2000; Stephanou et al., 2001). However, wt primary MEFs or cardiomyocytes that are grown in the absence of IFN-γ still show an enhanced response to Fas-induced apoptosis compared with p38α-deficient cells. A possible transcription factor candidate to account for the differences in apoptosis and Fas expression produced by p38α deficiency is p53, which can be phosphorylated on different residues by p38 MAPKs (Bulavin et al., 1999; Huang et al., 1999; Sánchez-Prieto et al., 2000; Bulavin et al., 2002; Kwon et al., 2002) and can induce Fas expression (Munsch et al., 2000). In addition, the increased levels of nuclear STAT3 phosphorylated on Ser-727 that we have found in p38α-deficient cardiomyocytes could play a role because it has been shown to down-regulate Fas expression through cooperation with c-Jun (Ivanov et al., 2001).
The reduced levels of proapoptotic protein Bax in p38α-deficient cells may also contribute to the resistance of these cells to Fas-induced apoptosis (Ruffolo et al., 2000; Wei et al., 2001; Roth and Reed, 2002). Hepatocytes from Bax/Bak double knockout mice are resistant to death receptor-induced apoptosis in vivo (Wei et al., 2001). This is probably due to the cooperation between Bid and Bax. The activation of caspase 8 upon death receptor engagement could lead to the generation of Bid proteolytic fragment, which in turn would promote the insertion of Bax into mitochondria (Ruffolo et al., 2000). A similar situation may be occurring in our wt cell lines, because we found that Bid cleavage is produced upon Fas engagement, probably through caspase 8 activation, as it has been described for other cell types (Yin et al., 1999). In addition, down-regulation of Bax protein in p38α-deficient cells might also explain the resistance of these cells to other proapoptotic stimuli as well as their lower level of basal apoptosis. In agreement with this idea, increased expression of Bax (by transfection) in p38α-deficient cells can rescue serum withdrawal-induced and basal apoptosis to about the same level as in wt cells. Thus, a certain level of Bax seems to be required for apoptosis as it has been shown for c-Myc-induced apoptosis, where Bax and c-Myc cooperate to induce the release of cytochrome c from mitochondria (Juin et al., 2002). A further possible mechanism by which p38 MAPK facilitates apoptosis may be via the transmission of the Fas-triggered apoptotic signal through activation of Rb/E2F1 (Hou et al., 2002).
Regulation of Bax activity and, in particular, Bax protein levels by p38 MAPKs is not well understood. p38 MAPK can mediate both activating conformational changes in Bax (Yuan et al., 2003) and its translocation to mitochondria (Ghatan et al., 2000; Deacon et al., 2003). Recent data also suggest that activation of p38 MAPKs could mediate morphine-induced macrophage apoptosis through up-regulation of Bax, p53, and Fas (Singhal et al., 2002). However, no direct effect of p38α on Bax expression has been reported so far, although this could be mediated by p53, which has been proposed to have a positive role in Bax gene expression (Thornborrow and Manfredi, 2001; Pyrzynska et al., 2002). We have found that p53 protein is expressed to similar levels in wt and p38α-deficient cardiomyocytes; the levels of p53 are high due to the expression of SV40 large T. However, as discussed above, we cannot rule out that the phosphorylation of p53 by p38α might contribute to the changes in Bax expression levels. We have preliminary results suggesting that p53 phosphorylation on Ser15 may be reduced in p38α-/- cells and that incubation with the p53 inhibitor pifithrin-α (Lorenzo et al., 2002) can reduce Bax protein levels, at least in cardiomyocytes. On the other hand, because STAT3 phosphorylated on Ser727 is up-regulated in p38α-deficient cardiomyocytes and it is an inhibitor of Bax expression (Naka et al., 1998), it could play a role in Bax down-regulation. Hence, ERK up-regulation in p38α-deficient cardiomyocytes would mediate the increase in phospho-Ser-727 STAT3, leading to decreased Bax expression. In addition, experiments done with the MEK inhibitor PD98059 indicate that upregulation of the ERK pathway in p38α-/- cells is involved in cell survival, which together with the down-regulation of proapoptotic proteins would lead to reduced levels of apoptosis in p38α-/- cells. In agreement with our findings, Li et al. (2003) recently showed that the proapoptotic effect of the p38 MAPK pathway relies on the dephosphorylation of MEK1/2, perhaps through PP2A, which would also result in increased ERK activity in the absence of p38α.
It should be noted that up-regulation of ERKs in p38α-deficient cardiomyocytes could participate in other survival mechanisms, in addition to the proposed regulation of Fas and Bax expression through STAT3. The Ras/ERK/Rsk pathway can mediate cell survival through phosphorylation of the transcription factor cAMP response element-binding protein and the proapoptotic protein Bad (Bonni et al., 1999) The ERK pathway can also increase the levels of antiapoptotic proteins such as Bcl-2, Mcl-1, Bclx-L, and c-FLIP (Boucher et al., 2000; Wang et al., 2002).
In summary, our results demonstrate that cells lacking p38α MAPK are more resistant to apoptosis induced by different stimuli, indicating that p38α is a positive regulator of apoptosis. We found that the absence of p38α correlate with lower levels of both Fas and Bax proteins, which can explain the resistance of p38α-/- cells to Fas-induced apoptosis. The proapoptotic role of p38α via regulation of the expression of Bax and probably Fas is also likely to confer a higher sensitivity to other apoptotic stimuli such as serum deprivation. Up-regulation of ERK MAPKs may also contribute to the reduced apoptosis of p38α-deficient cells.
Acknowledgments
We thank Dr. D. Livingston for providing SV40 large T retrovirus-producing cells and A. Vázquez for the flow cytometry analysis. This work was partially supported by European Molecular Biology Organization and Federation of European Biochemical Societies short-term fellowships to A.P. and by grant CAM-08.4/0035/2001-N°10109 from Comunidad deMadrid (Spain) and the European Molecular Biology Laboratory. A.V. and S.Z. are recipients of predoctoral fellowships from the Ministerio de Educación y Cultura, Spain.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-08-0592. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-08-0592.
References
- Adams, R.H., Porras, A., Alonso, G., Jones, M., Vintersten, K., Panelli, S., Valladares, A., Perez, L., Klein, R., and Nebreda, A.R. (2000). Essential role of p38alpha MAP kinase in placental but not embryonic cardiovascular development. Mol. Cell 6, 109-116. [PubMed] [Google Scholar]
- Allen, M., Svensson, L., Roach, M., Hambor, J., McNeish, J., and Gabel, C.A. (2000). Deficiency of the stress kinase p38alpha results in embryonic lethality: characterization of the kinase dependence of stress responses of enzyme-deficient embryonic stem cells. J. Exp. Med. 191, 859-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosino, C., Mace, G., Galban, S., Fritsch, C., Vintersten, K., Black, E., Gorospe, M., and Nebreda, A.R. (2003). Negative feed-back regulation of MKK6 mRNA stability by p38α MAP kinase. Mol. Cell Biol. 23, 370-381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito, M., Porras, A., and Santos, E. (1993). Establishment of permanent brown adipocyte cell lines achieved by transfection with SV40 large T antigen and ras genes. Exp. Cell Res. 209, 248-254. [DOI] [PubMed] [Google Scholar]
- Bonni, A., Brunet, A., West, A.E., Datta, S.R., Takasu, M.A., and Greenberg, M.E. (1999). Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 286, 1358-1362. [DOI] [PubMed] [Google Scholar]
- Boucher, M.J., Morisset, J., Vachon, P.H., Reed, J.C., Laine, J., and Rivard, N. (2000). MEK/ERK signaling pathway regulates the expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of human pancreatic cancer cells. J. Cell Biochem. 79, 355-369. [PubMed] [Google Scholar]
- Braz, J.C., Bueno, O.F., Liang, Q., Wilkins, B.J., Dai, Y.S., Parsons, S., Braunwart, J., Glascock, B.J., Klevitsky, R., Kimball, T.F., Hewett, T.E., and Molkentin, J.D. (2003). Targeted inhibition of p38 MAPK promotes hypertrophic cardiomyopathy through upregulation of calcineurin-NFAT signaling. J. Clin. Invest. 111, 1475-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, M., McCormack, M., Zinn, K.G., Farrell, M.P., Bikel, I., and Livingston, D.M. (1986). A recombinant murine retrovirus for simian virus 40 large T cDNA transforms mouse fibroblasts to anchorage-independent growth. J. Virol. 60, 290-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulavin, D.V., Saito, S., Hollander, M.C., Sakaguchi, K., Anderson, C.W., Appella, E., and Fornace, A.J., Jr. (1999). Phosphorylation of human p53 by p38 kinase coordinates N-terminal phosphorylation and apoptosis in response to UV radiation. EMBO J. 18, 6845-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulavin, D.V., et al. (2002). Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat. Genet. 31, 210-215. [DOI] [PubMed] [Google Scholar]
- Ciesielski-Treska, J., Ulrich, G., Chasserot-Golaz, S., Zwiller, J., Revel, M.O., Aunis, D., and Bader, M.F. (2001). Mechanisms underlying neuronal death induced by chromogranin A-activated microglia. J. Biol. Chem. 276, 13113-13120. [DOI] [PubMed] [Google Scholar]
- Cohen, P. (1997). The search for physiological substrates of MAP and SAP kinases in mammalian cells. Trends Cell Biol. 7, 353-361. [DOI] [PubMed] [Google Scholar]
- Deacon, K., Mistry, P., Chernoff, J., Blank, J.L., and Patel, R. (2003). p38 Mitogen-Activated Protein Kinase Mediates Cell Death and p21-Activated Kinase Mediates Cell Survival during Chemotherapeutic Drug-induced Mitotic Arrest. Mol. Biol. Cell 14, 2071-2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschesnes, R.G., Huot, J., Valerie, K., and Landry, J. (2001). Involvement of p38 in apoptosis-associated membrane blebbing and nuclear condensation. Mol. Biol. Cell 2, 1569-1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Zutter, G.S., and Davis, R.J. (2001). Pro-apoptotic gene expression mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Proc. Natl. Acad. Sci. USA 98, 6168-6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund, S., Bu, S., Schuster, N., Aspenstrom, P., Heuchel, R., Heldin, N.E., Ten Dijke, P., Heldin, C.H., and Landstrom, M. (2003). Transforming Growth Factor-beta1 (TGF-beta)-induced Apoptosis of Prostate Cancer Cells Involves Smad7-dependent Activation of p38 by TGF-beta-activated Kinase 1 and Mitogen-activated Protein Kinase Kinase 3. Mol. Biol. Cell 14, 529-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatan, S., Larner, S., Kinoshita, Y., Hetman, M., Patel, L., Xia, Z., Youle, R.J., and Morrison, R.S. (2000). p38 MAP kinase mediates bax translocation in nitric oxide-induced apoptosis in neurons. J. Cell Biol. 150, 335-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh, K.C., Haque, S.J., and Williams, B.R. (1999). p38 MAP kinase is required for STAT1 serine phosphorylation and transcriptional activation induced by interferons. EMBO J. 18, 5601-5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron-Milhavet, L., and LeRoith, D. (2002). Insulin-like growth factor I induces MDM2-dependent degradation of p53 via the p38 MAPK pathway in response to DNA damage. J. Biol. Chem. 277, 15600-15606. [DOI] [PubMed] [Google Scholar]
- Hou, S.T., Xie, X., Baggley, A., Park, D.S., Chen, G., and Walker, T. (2002). Activation of the Rb/E2F1 pathway by the non-proliferative p38 MAP kinase during Fas (APO1/CD95)-mediated neuronal apoptosis. J. Biol. Chem. 277, 48764-48770. [DOI] [PubMed] [Google Scholar]
- Hsu, S.C., Gavrilin, M.A., Tsai, M.H., Han, J., and Lai, M.Z. (1999). p38 mitogen-activated protein kinase is involved in Fas ligand expression. J. Biol. Chem. 274, 25769-25776. [DOI] [PubMed] [Google Scholar]
- Huang, C., Ma, W.Y., Maxiner, A., Sun, Y., and Dong, Z. (1999). p38 kinase mediates UV-induced phosphorylation of p53 protein at serine 389. J. Biol. Chem. 274, 12229-12235. [DOI] [PubMed] [Google Scholar]
- Ivanov, V.N., and Ronai, Z. (2000). p38 protects human melanoma cells from UV-induced apoptosis through down-regulation of NF-kappaB activity and Fas expression. Oncogene 19, 3003-3012. [DOI] [PubMed] [Google Scholar]
- Ivanov, V.N., Bhoumik, A., Krasilnikov, M., Raz, R., Owen-Schaub, L.B., Levy, D., Horvath, C.M., and Ronai, Z. (2001). Cooperation between STAT3 and c-jun suppresses Fas transcription. Mol. Cell 7, 517-528. [DOI] [PubMed] [Google Scholar]
- Jat, P.S., Noble, M.D., Ataliotis, P., Tanaka, Y., Yannoutsos, N., Larsen, L., and Kioussis, D. (1991). Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc. Natl. Acad. Sci. USA 88, 5096-5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeremias, I., Kupatt, C., Martin-Villalba, A., Habazettl, H., Schenkel, J., Boekstegers, P., and Debatin, K.M. (2000). Involvement of CD95/Apo1/Fas in cell death after myocardial ischemia. Circulation 102, 915-920. [DOI] [PubMed] [Google Scholar]
- Juin, P., Hunt, A., Littlewood, T., Griffiths, B., Swigart, L.B., Korsmeyer, S., and Evan, G. (2002). c-Myc functionally cooperates with Bax to induce apoptosis. Mol. Cell Biol. 22, 6158-6169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovarik, P., Stoiber, D., Eyers, P.A., Menghini, R., Neininger, A., Gaestel, M., Cohen, P., and Decker, T. (1999). Stress-induced phosphorylation of STAT1 at Ser727 requires p38 mitogen-activated protein kinase whereas IFN-gamma uses a different signaling pathway. Proc. Natl. Acad. Sci. USA 96, 13956-13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon, Y.W., Ueda, S., Ueno, M., Yodoi, J., and Masutani, H. (2002). Mechanism of p53-dependent apoptosis induced by 3-methylcholanthrene: involvement of p53 phosphorylation and p38 MAPK. J. Biol. Chem. 277, 1837-1844. [DOI] [PubMed] [Google Scholar]
- Kyriakis, J.M., and Avruch, J. (2001). Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 81, 807-869. [DOI] [PubMed] [Google Scholar]
- Leithauser, F., Dhein, J., Mechtersheimer, G., Koretz, K., Bruderlein, S., Henne, C., Schmidt, A., Debatin, K.M., Krammer, P.H., and Moller, P. (1993). Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab. Investig. 69, 415-429. [PubMed] [Google Scholar]
- Le-Niculescu, H., Bonfoco, E., Kasuya, Y., Claret, F.X., Green, D.R., and Karin, M. (1999). Withdrawal of survival factors results in activation of the JNK pathway in neuronal cells leading to Fas ligand induction and cell death. Mol. Cell Biol. 19, 751-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverkus, M., Yaar, M., and Gilchrest, B.A. (1997). Fas/Fas ligand interaction contributes to UV-induced apoptosis in human keratinocytes. Exp. Cell Res. 232, 255-262. [DOI] [PubMed] [Google Scholar]
- Li, S.P., Junttila, M., R., Han, J., Kahari, V.M., and Westermarck, J. (2003). p38 Mitogen-activated protein kinase pathway suppresses cell survival by inducing dephosphorylation of mitogen-activated protein/extracellular signal-regulated kinase kinase1, 2. Cancer Res. 63, 3473-3477. [PubMed] [Google Scholar]
- Lorenzo, E., Ruiz-Ruiz, C., Quesada, A.J., Hernandez, G., Rodriguez, A., Lopez-Rivas, A., and Redondo, J.M. (2002). Doxorubicin induces apoptosis and CD95 gene expression in human primary endothelial cells through a p53-dependent mechanism. J. Biol. Chem. 277, 10883-10892. [DOI] [PubMed] [Google Scholar]
- Mackay, K., and Mochly-Rosen, D. (1999). An inhibitor of p38 mitogen-activated protein kinase protects neonatal cardiac myocytes from ischemia. J. Biol. Chem. 274, 6272-6279. [DOI] [PubMed] [Google Scholar]
- Mudgett, J.S., Ding, J., Guh-Siesel, L., Chartrain, N.A., Yang, L., Gopal, S., and Shen, M.M. (2000). Essential role for p38alpha mitogen-activated protein kinase in placental angiogenesis. Proc. Natl. Acad. Sci. USA 97, 10454-10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsch, D., Watanabe-Fukunaga, R., Bourdon, J.C., Nagata, S., May, E., Yonish-Rouach, E., and Reisdorf, P. (2000). Human and mouse Fas (APO-1/CD95) death receptor genes each contain a p53-responsive element that is activated by p53 mutants unable to induce apoptosis. J. Biol. Chem. 275, 3867-3872. [DOI] [PubMed] [Google Scholar]
- Naka, T., Matsumoto, T., Narazaki, M., Fujimoto, M., Morita, Y., Ohsawa, Y., Saito, H., Nagasawa, T., Uchiyama, Y., and Kishimoto, T. (1998). Accelerated apoptosis of lymphocytes by augmented induction of Bax in SSI-1 (STAT-induced STAT inhibitor-1) deficient mice. Proc. Natl. Acad. Sci. USA 95, 15577-15582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebreda, A.R., and Porras, A. (2000). p38 MAP kinases: beyond the stress response. Trends Biochem. Sci. 25, 257-260. [DOI] [PubMed] [Google Scholar]
- Nguyen, H., Ramana, C.V., Bayes, J., and Stark, G.R. (2001). Roles of phosphatidylinositol 3-kinase in interferon-gamma-dependent phosphorylation of STAT1 on serine 727 and activation of gene expression. J. Biol. Chem. 276, 33361-33368. [DOI] [PubMed] [Google Scholar]
- Okamoto, S., Krainc, D., Sherman, K., and Lipton, S.A. (2000). Antiapoptotic role of the p38 mitogen-activated protein kinase-myocyte enhancer factor 2 transcription factor pathway during neuronal differentiation. Proc. Natl. Acad. Sci. USA 97, 7561-7566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono, K., and Han, J. (2000). The p38 signal transduction pathway: activation and function. Cell Signal 12, 1-13. [DOI] [PubMed] [Google Scholar]
- Otsu, K., et al. (2003). Disruption of a single copy of the p38α MAP kinase gene leads to cardioprotection against ischemia-reperfusion. Biochem. Biophys. Res. Commun. 302, 56-60. [DOI] [PubMed] [Google Scholar]
- Park, J.M., Greten, F.R., Li, Z.W., and Karin, M. (2002). Macrophage apoptosis by anthrax lethal factor through p38 MAP kinase inhibition. Science 297, 2048-2051. [DOI] [PubMed] [Google Scholar]
- Pyrzynska, B., Serrano, M., Martinez, A.C., and Kaminska, B. (2002). Tumor suppressor p53 mediates apoptotic cell death triggered by cyclosporin A. J. Biol. Chem. 277, 14102-14108. [DOI] [PubMed] [Google Scholar]
- Ramsauer, K., Sadzak, I., Porras, A., Pilz, A., Nebreda, A.R., Decker, T., and Kovarik, P. (2002). p38 MAPK enhances STAT1-dependent transcription independently of Ser-727 phosphorylation. Proc. Natl. Acad. Sci. USA 99, 12859-12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, W., and Reed, J.C. (2002). Apoptosis and cancer: when BAX is TRAILing away. Nat. Med. 8, 216-218. [DOI] [PubMed] [Google Scholar]
- Ruffolo, S.C., Breckenridge, D.G., Nguyen, M., Goping, I.S., Gross, A., Korsmeyer, S.J., Li, H., Yuan, J., and Shore, G.C. (2000). BID-dependent and BID-independent pathways for BAX insertion into mitochondria. Cell Death Differ. 7, 1101-1108. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ruiz, C., Muñoz-Pinedo, C., and Lopez-Rivas, A. (2000). Interferon-gamma treatment elevates caspase-8 expression and sensitizes human breast tumor cells to a death receptor-induced mitochondria-operated apoptotic program. Cancer Res. 60, 5673-5680. [PubMed] [Google Scholar]
- Sanchez-Prieto, R., Rojas, J.M., Taya, Y., and Gutkind, J.S. (2000). A role for the p38 mitogen-activated protein kinase pathway in the transcriptional activation of p53 on genotoxic stress by chemotherapeutic agents. Cancer Res. 60, 2464-2472. [PubMed] [Google Scholar]
- Sarkar, D., Su, Z.Z., Lebedeva, I.V., Sauane, M., Gopalkrishnan, R.V., Valerie, K., Dent, P., and Fisher, P.B. (2002). mda-7 (IL-24) Mediates selective apoptosis in human melanoma cells by inducing the coordinated overexpression of the GADD family of genes by means of p38 MAPK. Proc. Natl. Acad. Sci. USA 99, 10054-10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saurin, A.T., Martin, J.L., Heads, R.J., Foley, C., Mockridge, J.W., Wright, M.J., Wang, Y., and Marber, M.S. (2000). The role of differential activation of p38-mitogen-activated protein kinase in preconditioned ventricular myocytes. FASEB J. 14, 2237-2246. [DOI] [PubMed] [Google Scholar]
- Schrantz, N., Bourgeade, M.F., Mouhamad, S., Leca, G., Sharma, S., and Vazquez, A. (2001). p38-mediated regulation of an Fas-associated death domain protein-independent pathway leading to caspase-8 activation during TGFbeta-induced apoptosis in human Burkitt lymphoma B cells BL41. Mol. Biol. Cell 12, 3139-3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal, P.C., Bhaskaran, M., Patel, J., Patel, K., Kasinath, B.S., Duraisamy, S., Franki, N., Reddy, K., and Kapasi, A.A. (2002). Role of p38 mitogen-activated protein kinase phosphorylation and Fas-Fas ligand interaction in morphine-induced macrophage apoptosis. J. Immunol. 168, 4025-4033. [DOI] [PubMed] [Google Scholar]
- Stephanou, A., Scarabelli, T.M., Brar, B.K., Nakanishi, Y., Matsumura, M., Knight, R.A., and Latchman, D.S. (2001). Induction of apoptosis and Fas receptor/Fas ligand expression by ischemia/reperfusion in cardiac myocytes requires serine 727 of the STAT-1 transcription factor but not tyrosine 701. J. Biol. Chem. 276, 28340-28347. [DOI] [PubMed] [Google Scholar]
- Tamura, K., Sudo, T., Senftleben, U., Dadak, A.M., Johnson, R., and Karin, M. (2000). Requirement for p38alpha in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell 102, 221-231. [DOI] [PubMed] [Google Scholar]
- Tanaka, M., Ito, H., Adachi, S., Akimoto, H., Nishikawa, T., Kasajima, T., Marumo, F., and Hiroe, M. (1994). Hypoxia induces apoptosis with enhanced expression of Fas antigen messenger RNA in cultured neonatal rat cardiomyocytes. Circ. Res. 75, 426-433. [DOI] [PubMed] [Google Scholar]
- Thornborrow, E.C., and Manfredi, J.J. (2001). The tumor suppressor protein p53 requires a cofactor to activate transcriptionally the human BAX promoter. J. Biol. Chem. 276, 15598-15608. [DOI] [PubMed] [Google Scholar]
- Torcia, M., et al. (2001). Nerve growth factor inhibits apoptosis in memory B lymphocytes via inactivation of p38 MAPK, prevention of Bcl-2 phosphorylation, and cytochrome c release. J. Biol. Chem. 276, 39027-39036. [DOI] [PubMed] [Google Scholar]
- Valladares, A., Alvarez, A.M., Ventura, J.J., Roncero, C., Benito, M., and Porras, A. (2000). p38 mitogen-activated protein kinase mediates tumor necrosis factor-alpha-induced apoptosis in rat fetal brown adipocytes. Endocrinology 141, 4383-4395. [DOI] [PubMed] [Google Scholar]
- Wang, Y., Huang, S., Sah, V.P., Ross, J. Jr., Brown, J.H., Han, J., and Chien, K.R. (1998). Cardiac muscle cell hypertrophy and apoptosis induced by distinct members of the p38 mitogen-activated protein kinase family. J. Biol. Chem. 273, 2161-2168. [DOI] [PubMed] [Google Scholar]
- Wang, W., Prince, C.Z., Mou, Y., and Pollman, M.J. (2002). Notch3 signaling in vascular smooth muscle cells induces c-FLIP expression via ERK/MAPK activation. Resistance to Fas ligand-induced apoptosis. J. Biol. Chem. 277, 21723-21729. [DOI] [PubMed] [Google Scholar]
- Wei, M.C., Zong, W.X., Cheng, E.H., Lindsten, T., Panoutsakopoulou, V., Ross, A.J., Roth, K.A., MacGregor, G.R., Thompson, C.B., and Korsmeyer, S.J. (2001). Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, X.-M., Wang, K., Gross, A., Zhao, Y., Zinkel, S., Klocke, B., Roth, K.A., and Korsmeyer, S.J. (1999). Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400, 886-891. [DOI] [PubMed] [Google Scholar]
- Yuan, Z.Q., Feldman, R.I., Sussman, G.E., Coppola, D., Nicosia, S.V., and Cheng, J.Q. (2003). AKT2 inhibition of cisplatin-induced JNK/p38 and Bax activation by phosphorylation of ASK 1, implication of AKT2 in chemoresistance. J. Biol. Chem. 278, 23432-23440. [DOI] [PubMed] [Google Scholar]
- Zechner, D., Craig, R., Hanford, D.S., McDonough, P.M., Sabbadini, R.A., and Glembotski, C.C. (1998). MKK6 activates myocardial cell NF-kappaB and inhibits apoptosis in a p38 mitogen-activated protein kinase-dependent manner. J. Biol. Chem. 273, 8232-8239. [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Liu, G., and Dong, Z. (2001). MSK1 and JNKs mediate phosphorylation of STAT3 in UVA-irradiated mouse epidermal JB6 cells. J. Biol. Chem. 276, 42534-42542. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Shan, P., Alam, J., Davis, R.J., Flavell, R.A., and Lee, P.J. (2003a). Carbon monoxide modulates Fas/Fas ligand, caspases, and Bcl-2 family proteins via the p38alpha mitogen-activated protein kinase pathway during ischemia-reperfusion lung injury. J. Biol. Chem. 278, 22061-22070. [DOI] [PubMed] [Google Scholar]
- Zhang, S., Weinheimer, C., Courtois, M., Kovacs, A., Zhang, C.E., Cheng, A.M., Wang, Y., and Muslin, A.J. (2003b). The role of the Grb2-p38 MAPK signaling pathway in cardiac hypertrophy and fibrosis. J. Clin. Invest. 111, 833-841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang, S., Demirs, J.T., and Kochevar, I.E. (2000). p38 mitogen-activated protein kinase mediates bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen but not by hydrogen peroxide. J. Biol. Chem. 275, 25939-25948. [DOI] [PubMed] [Google Scholar]